Protection of Erythrocytes and Microvascular Endothelial Cells against Oxidative Damage by Fragaria vesca L. and Rubus idaeus L. Leaves Extracts—The Mechanism of Action
Abstract
:1. Introduction
2. Results
2.1. Phenolic Content
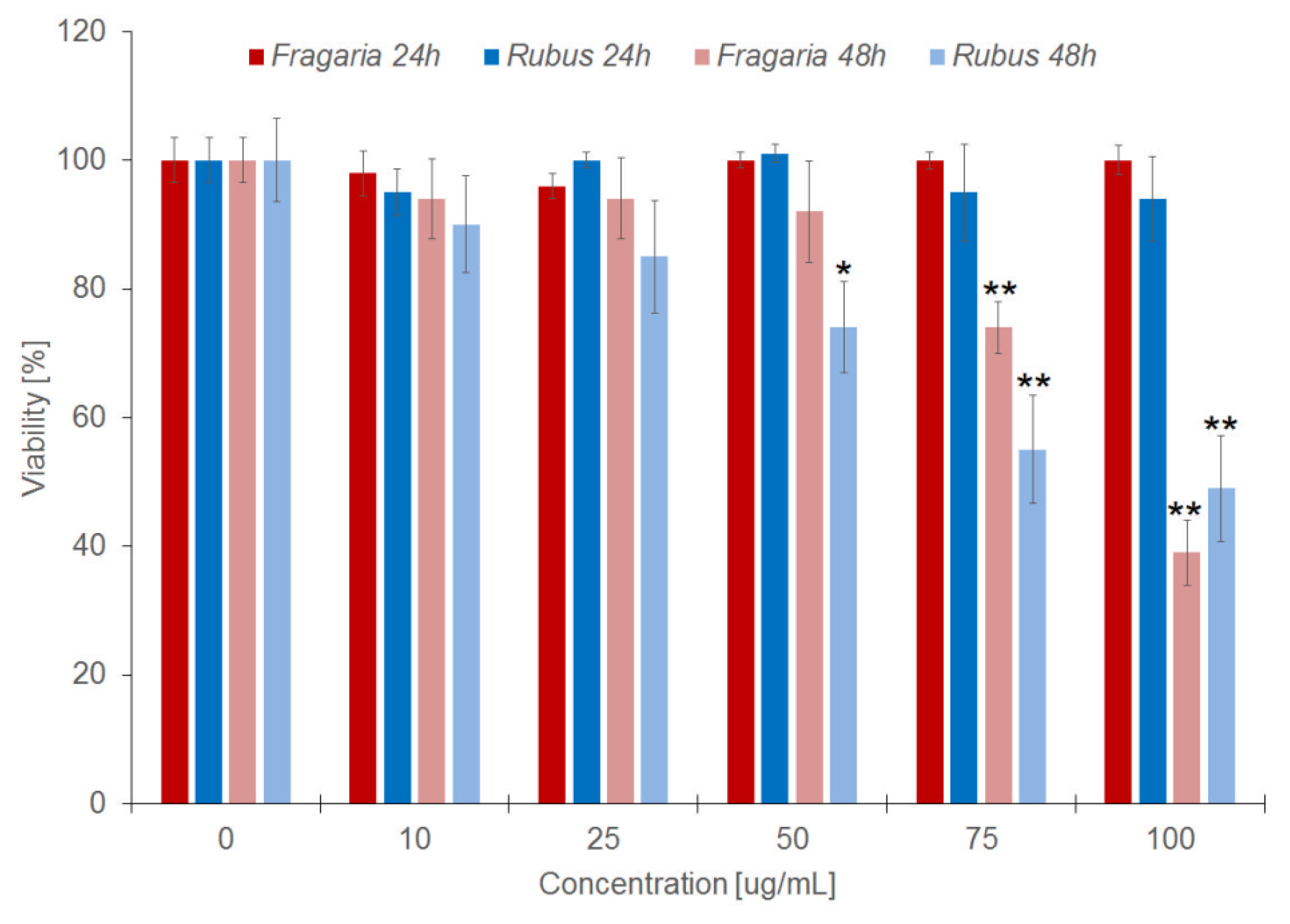
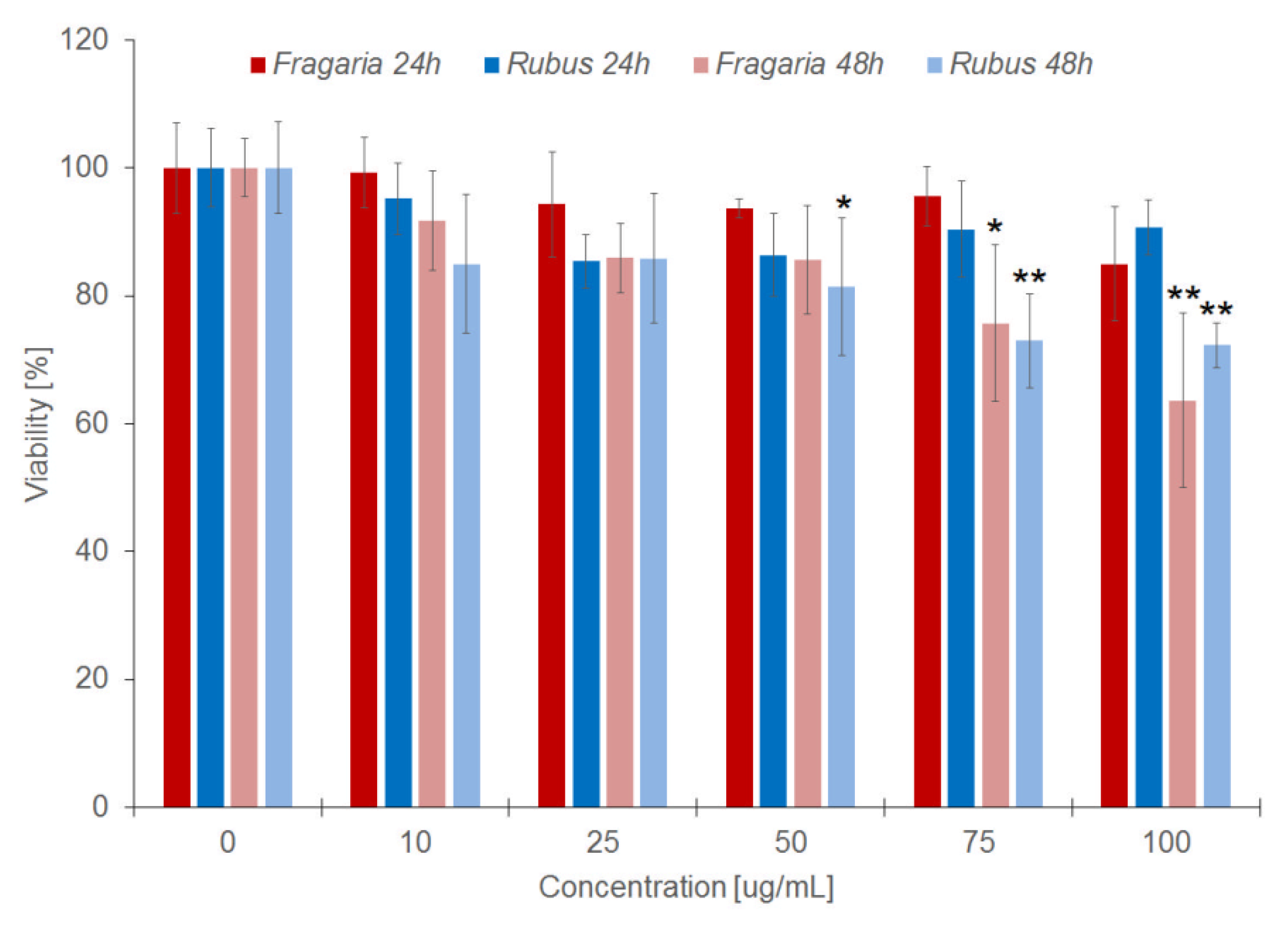
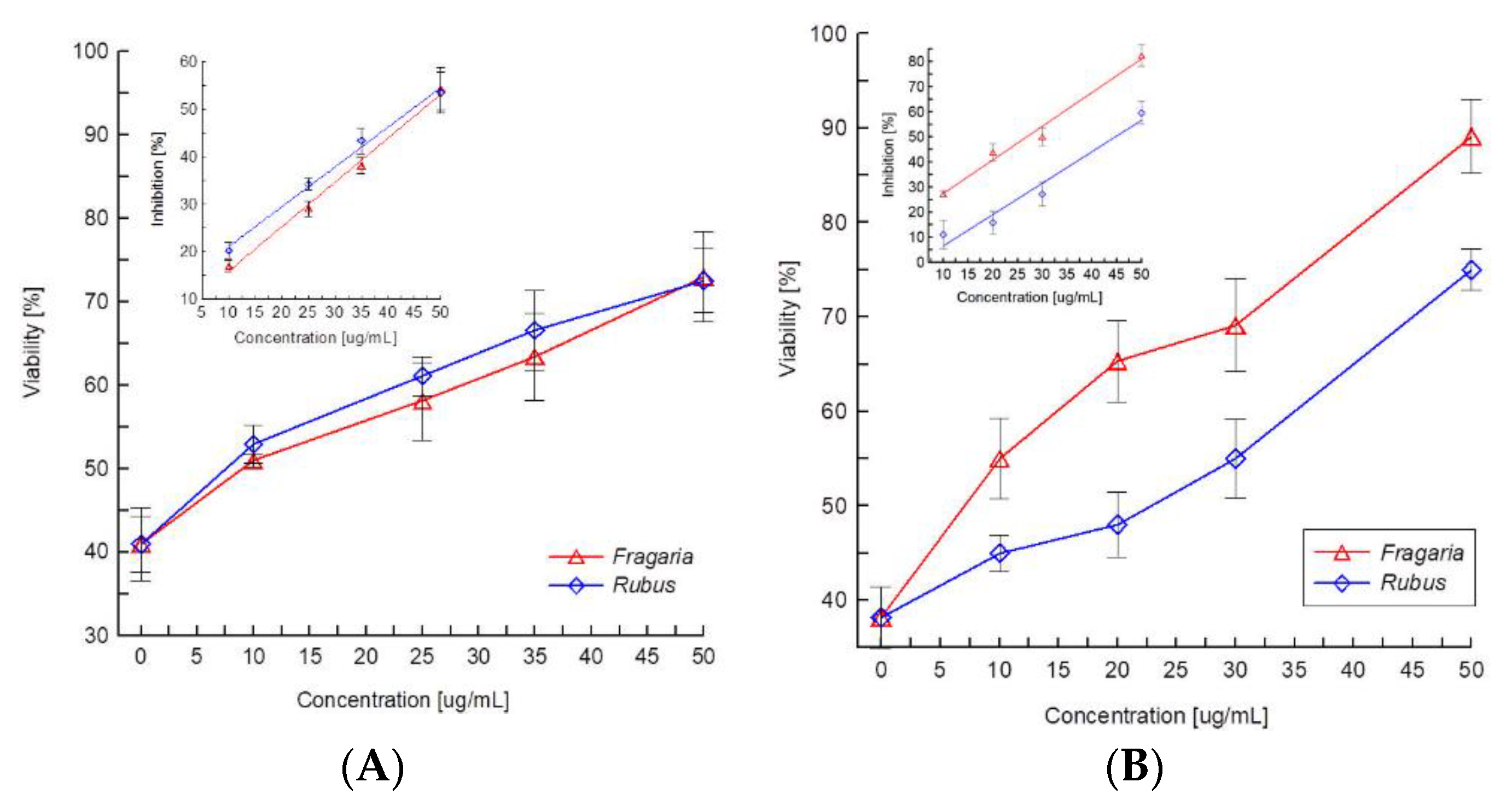
2.2. Toxicity of Extracts
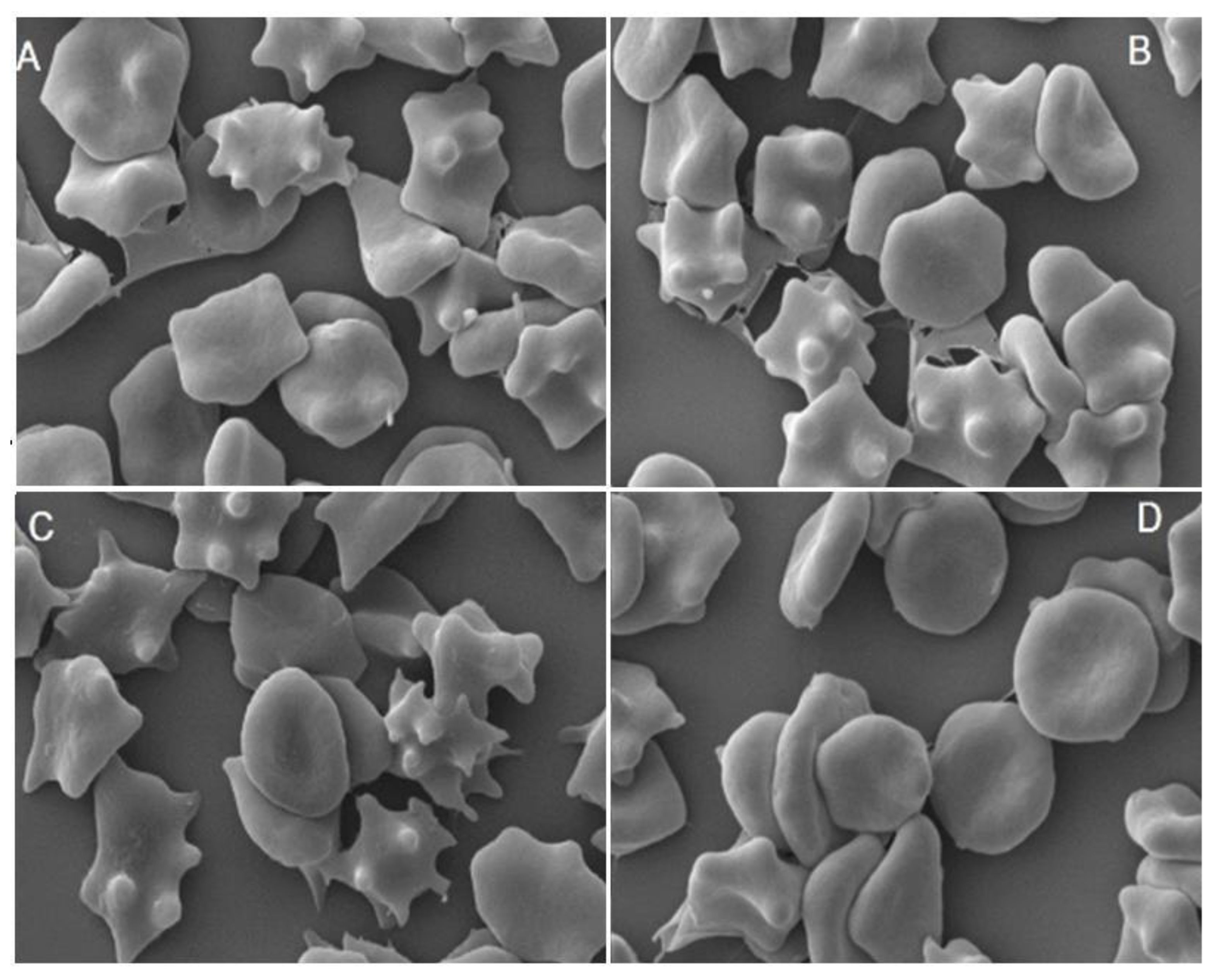
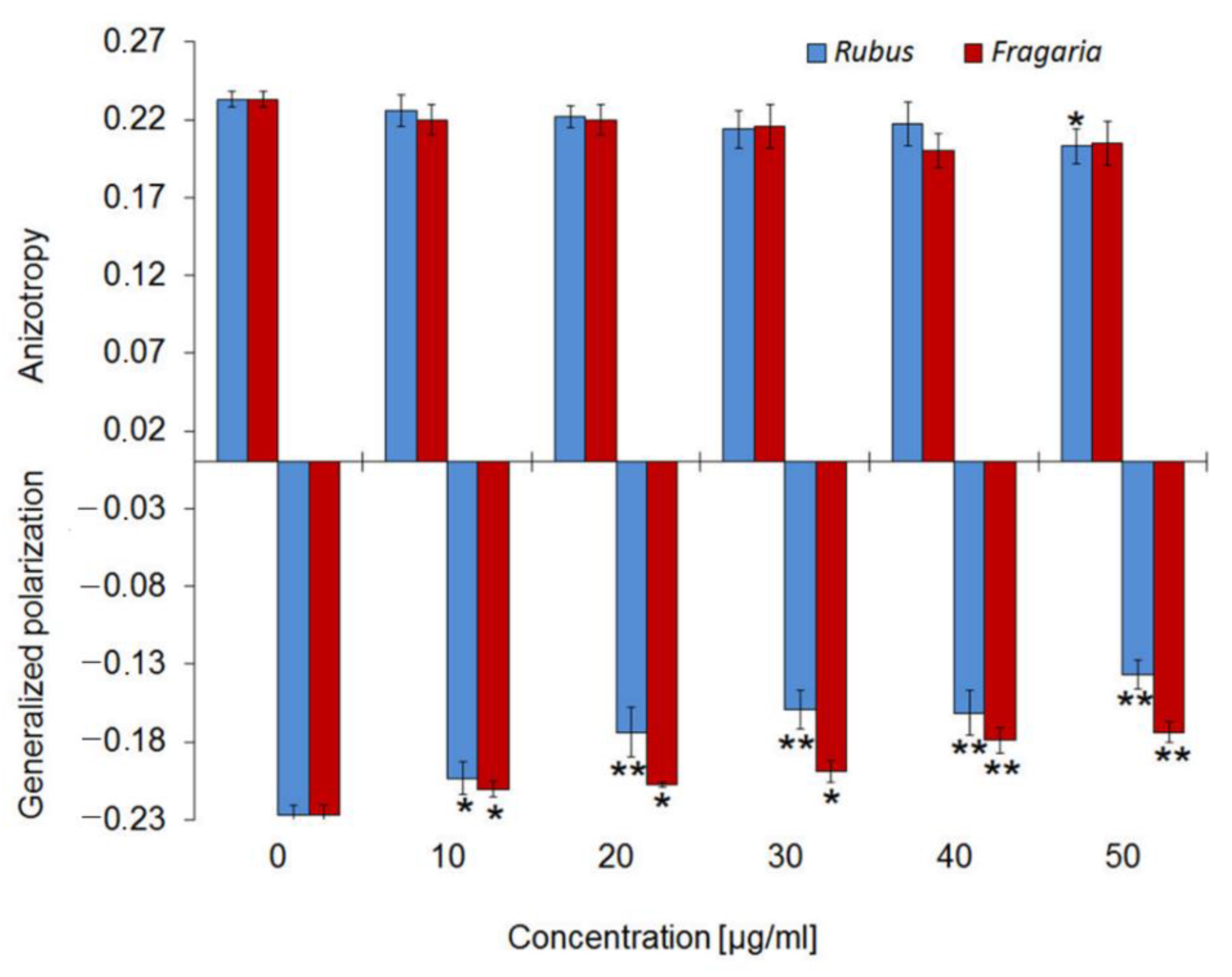
2.3. Influence of Extracts on the Physical Properties of Membrane
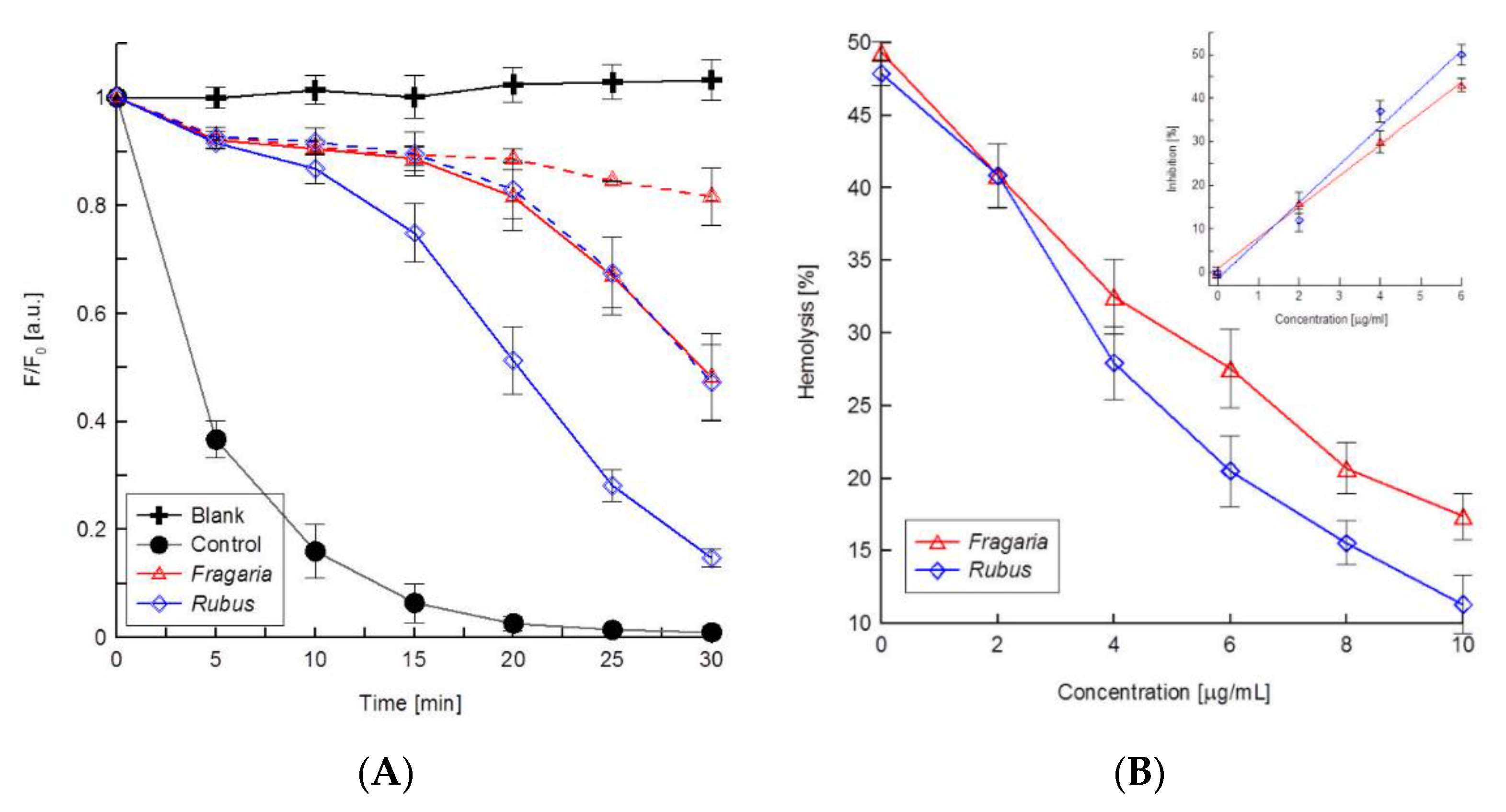
2.4. Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Preparation and Phenolic Content of the Extracts
4.2. Cells and Membranes
4.3. Fluorescence Probes, Reagents
4.4. Toxicity of Extracts
4.4.1. Haemolytic Assay
4.4.2. Viability Assays
4.5. Influence of Extracts on the Physical Properties of Membrane
4.5.1. Osmotic Resistance
4.5.2. Shape of Erythrocytes
4.5.3. Fluidity and Mobility/Hydration of the Polar Head of the Membrane Lipids
4.6. Antioxidant Activity
4.6.1. Erythrocyte Membranes
4.6.2. Erythrocytes
4.6.3. HMEC-1 Cells
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kaur, J.; Mahajan, J.; Kumar, R.; Arora, S. Oxidative stress-implication, source and its prevention. Environ. Sci. Pollut. Res. 2014, 21, 1599–1613. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, A.R. The genomics of oxidative DNA damage, repair, and resulting mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.L.; Swenberg, J.A.; Rusyn, I. Expression of base excision DNA repair genes as biomarker of oxidative DNA damage. Cancer Lett. 2005, 229, 1–11. [Google Scholar]
- Alfarisi, H.A.H.; Mohamed, Z.B.H.; Ibrahim, M.B. Basic pathogenic mechanisms of atherosclerosis. Egypt. J. Basic Appl. Sci. 2020, 7, 116–125. [Google Scholar] [CrossRef]
- Sumpio, B.E.; Riley, J.T.; Dardik, A. Cells in focus: Endothelial cell. Int. J. Biochem. Cell Biol. 2002, 34, 1508–1512. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial Dysfunction in Cardiovascular Diseases: The Role of Oxidant Stress. Circ. Res. 2020, 87, 840–844. [Google Scholar] [CrossRef]
- Clemens, M.R.; Waller, H.D. Lipid peroxidation in erythrocytes. Chem. Phys. Lipids 1987, 45, 251–268. [Google Scholar] [CrossRef]
- Stolze, K.; Nohl, H. Free radical formation and erythrocyte membrane alterations during MetHb formation induced by the BHA metabolite, tert-butylhydroquinone. Free Radic. Res. 1999, 30, 295–303. [Google Scholar] [CrossRef]
- Sinha, A.; Chu, T.; Dao, M.; Chandramohanadas, R. Single-cell evaluation of red blood cell bio-mechanical and nano-structural alterations upon chemically induced oxidative stress. Sci. Rep. 2015, 5, 9768. [Google Scholar] [CrossRef]
- Tyurina, Y.Y.; Shvedova, A.A.; Kawai, K.; Tyurin, V.A.; Kommineni, C.; Quinn, P.J.; Schor, N.F.; Fabisiak, J.P.; Kagan, V.E. Phospholipid signalling in apoptosis: Peroxidation and externization of phosphatidylserine. Toxicology 2000, 148, 93–101. [Google Scholar] [CrossRef]
- Garcia, J.J.; Reiter, R.J.; Karbownik, M.; Calvo, J.R.; Ortiz, G.G.; Tan, D.X.; Martinez-Ballarin, E.; Acuna-Castroviejo, D. N-acetylserotonin suppresses hepatic microsomal membrane rigidity associated with lipid peroxidation. Eur. J. Pharmacol. 2001, 428, 169–175. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Dvaranauskaite, A.; Labokas, J. Radical scavenging activity and composition of raspberry (R. idaeus) leaves from different locations in Lithuania. Fitoterapia 2007, 78, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, R.C.; Temocico, G.; Fierascu, I.; Ortan, A.; Babeanu, N.E. Fragaria Genus: Chemical Composition and Biological Activities. Molecules 2020, 25, 498. [Google Scholar] [CrossRef]
- Durgo, K.; Belscak-Cvitanovic, A.; Stancic, A.; Franekic, J.; Komes, D. The bioactive potential of red raspberry (Rubus idaeus L.) leaves in exhibiting cytotoxic and cytoprotective activity on human laryngeal carcinoma and colon adenocarcinoma. J. Med. Food 2012, 15, 258–268. [Google Scholar] [CrossRef]
- Rojas-Vera, J.; Patel, A.V.; Dacke, C.G. Relaxant activity of raspberry (R. idaeus) leaf extract in guinea-pig ileum in vitro. Phytother. Res. 2002, 16, 665–668. [Google Scholar] [CrossRef]
- Liu, Z.; Schwimer, J.; Liu, D.; Lewis, J.; Greenway, F.L.; York, D.A.; Woltering, E. Gallic acid is partially responsible for the antiangiogenic activities of Rubus leaf extract. Phytother. Res. 2006, 20, 806–813. [Google Scholar] [CrossRef]
- Liberal, J.; Francisco, V.; Costa, G.; Figueirinha, A.; Amaral, M.T.; Marques, C.; Girão, H.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Bioactivity of Fragaria vesca leaves through inflammation, proteasome and autophagy modulation. J. Ethnopharmacol. 2014, 158, 113–122. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Li, X.; Abubaker, M.A.; Liu, X.; Li, Z.; Wang, X.; Zhu, X.; Zhang, J.; Chen, X. Comparative Study of Three Raspberry Cultivar (Rubus idaeus L.) Leaves Metabolites: Metabolome Profiling and Antioxidant Activities. Appl. Sci. 2022, 12, 990. [Google Scholar] [CrossRef]
- Liberal, J.; Costa, G.; Carmo, A.; Vitorino, R.; Marques, C.; Domingues, M.R.; Domingues, P.; Goncalves, A.C.; Alves, R.; Sarmento-Ribeiro, A.B.; et al. Chemical characterization and cytotoxic potential of an ellagitannin-enriched fraction from Fragaria vesca leaves. Arab. J. Chem. 2015, 12, 3652–3666. [Google Scholar] [CrossRef]
- Mudnic, I.; Modun, D.; Brizic, I.; Vukovic, J.; Generalic, I.; Katalinic, V.; Bilusic, T.; Ljubenkov, I.; Boban, M. Cardiovascular effects in vitro of aqueous extract of wild strawberry (Fragaria vesca, L.) leaves. Phytomedicine 2009, 16, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, E.; Vassilieff, V.S. Hypnotic, anticonvulsant and muscle relaxant effects of R. brasiliensis. Involvement of GABAA-system. J. Ethnopharmacol. 2000, 70, 275–280. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Assessment of cytotoxicity and antioxidant properties of berry leaves as by-products with potential application in cosmetic and pharmaceutical products. Sci. Rep. 2021, 11, 3240. [Google Scholar] [CrossRef] [PubMed]
- Couto, J.; Figueirinha, A.; Batista, M.T.; Paranhos, A.; Nunes, C.; Gonçalves, L.M.; Marto, J.; Fitas, M.; Pinto, P.; Ribeiro, H.M.; et al. Fragaria vesca L. Extract: A Promising Cosmetic Ingredient with Antioxidant Properties. Antioxidants 2020, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Szejk-Arendt, M.; Czubak-Prowizor, K.; Macieja, A.; Poplawski, T.; Olejnik, A.K.; Pawlaczyk-Graja, I.; Gancarz, R.; Zbikowska, H.M. Polyphenolic-polysaccharide conjugates from medicinal plants of Rosaceae/Asteraceae family protect human lymphocytes but not myeloid leukemia K562 cells against radiation-induced death. Int. J. Biol. Macromol. 2020, 156, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Szejka, M.; Poplawski, T.; Czubatka-Bienkowska, A.; Olejnik, A.K.; Pawlaczyk-Graja, I.; Gancarz, R.; Zbikowska, H.M. A comparative study on the radioprotective potential of the polyphenolic glycoconjugates from medicinal plants of Rosaceae and Asteraceae families versus their aglycones. J. Photochem. Photobiol. B Biol. 2017, 171, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, I.; Ellory, J.C. Red Cell Membrane Transport in Health and Disease; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Tzouwara-Karayanni, S.M.; Philianos, S.M. Isolation of quercetin and kaempferol from R. ulmifolius and their fluorometric assay. Microchem. J. 1982, 27, 144–161. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Determination of phenolic composition and antioxidant activity in fruits, rhizomes and leaves of the white strawberry (Fragaria chiloensis spp. chiloensis form chiloensis) using HPLC-DAD–ESI-MS and free radical quenching techniques. J. Food Comp. Anal. 2010, 23, 545–553. [Google Scholar] [CrossRef]
- Gudej, J.; Tomczyk, M. Determination of flavonoids, tannins and ellagic acid in leaves from Rubus L. Species. Arch. Pharmacal. Res. 2004, 27, 1114–1119. [Google Scholar] [CrossRef]
- Raudoniūtė, I.; Rovira, J.; Petras, J.; Venskutonis, R.; Damašius, J.; Rivero-Pérez, M.D.; González-SanJosé, M.L. Antioxidant properties of garden strawberry leaf extract and its effect on fish oil oxidation. Int. J. Food Sci. Technol. 2011, 46, 935–943. [Google Scholar] [CrossRef]
- Gudej, J. Kaempferol and quercetin glycosides from Idaeus L. leaves. Acta Pol. Pharm. 2003, 60, 313–316. [Google Scholar] [PubMed]
- Oszmiański, J.; Wojdyło, A.; Nowicka, P.; Teleszko, M.; Cebulak, T.; Wolanin, M. Determination of phenolic compounds and antioxidant activity in leaves from wild Rubus L. spaces. Molecules 2015, 20, 4951–4966. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Visioli, F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: Review of human evidence. Phytomedicine 2016, 23, 1145–1174. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, D.; Biffi, L.; Nicolini, G.; Garavello, W. Flavonoids in oral cancer prevention and therapy. Eur. J. Cancer Prev. 2015, 24, 517–528. [Google Scholar] [CrossRef]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights (Review). Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Nawwar, M.A.; Ayoub, N.A.; El-Rai, M.A.; Bassyouny, F.; Mostafa, E.S.; Al-Abd, A.M.; Harms, M.; Wende, K.; Lindequist, U.; Linscheid, M.W. Cytotoxic ellagitannins from Reaumuria vermiculata. Fitoterapia 2012, 83, 1256–1266. [Google Scholar] [CrossRef]
- Nawwar, M.A.; Youb, N.A.; El-Raey, M.A.; Zaghloul, S.S.; Hashem, A.M.; Mostafa, E.S.; Eldahshan, O.; Werner, V.; Becker, A.; Haertel, B.; et al. Polyphenols in Ammania auriculata: Structures, antioxidative activity and cytotoxicity. Pharmazie 2014, 69, 860–864. [Google Scholar]
- Barrajón-Catalána, E.; Fernández-Arroyo, S.; Saura, D.; Guillén, E.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food Chem. Toxicol. 2010, 48, 2273–2282. [Google Scholar] [CrossRef]
- Sheetz, M.P.; Singer, S.J. Biological Membranes as Bilayer Couples. A Molecular Mechanism of Drug-Erythrocyte Interactions. Proc. Natl. Acad. Sci. USA 1974, 71, 4457–4461. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Fluorescence Polarization in Principles of Fluorescence Spectroscopy; Plenum Press: New York, NY, USA, 2006; pp. 353–382. [Google Scholar]
- Karonen, M. Insights into Polyphenol–Lipid Interactions: Chemical Methods, Molecular Aspects and Their Effects on Membrane Structures. Plants 2022, 11, 1809. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Dmitrenko, V.V. Pitfalls of the MTT assay: Direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene 2015, 754, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Serraino, I.; Dugo, L.; Dugo, P.; Mondello, L.; Mazzon, E.; Dugo, G.; Caputi, A.P.; Cuzzocrea, S. Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci. 2003, 73, 1097–1114. [Google Scholar] [CrossRef]
- Gąsiorowski, K.; Szyba, K.; Brokos, B.; Kołaczyńska, B.; Jankowiak-Włodarczyk, M.; Oszmiański, J. Antimutagenic activity of anthocyanins isolated from Aronia melanocarpa fruits. Cancer Lett. 1997, 119, 37–46. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Gorzelany, J.; Kapusta, I. Identification and characterization of low molecular weight polyphenols in berry leaf extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.T.; Mitchell, C.; Hanahan, D.J. The preparation and chemical characteristics of hemoglobin-free ghosts of erythrocytes. Arch. Biochem. 1963, 100, 119–130. [Google Scholar] [CrossRef]
- Peterson, G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]
- Cyboran-Mikołajczyk, S.; Solarska-Ściuk, K.; Mieszała, K.; Glatzel-Plucińska, N.; Matczak, K.; Kleszczyńska, H. The Impact of O-Glycosylation on Cyanidin Interaction with RBCs and HMEC-1 Cells—Structure–Activity Relationships. Int. J. Mol. Sci. 2019, 20, 1928. [Google Scholar] [CrossRef] [Green Version]

| Compounds | Rubus [mg/g] | Fragaria [mg/g] |
|---|---|---|
| Neochlorogenic acid | 0 | 5.05 |
| Chlorogenic acid | 1.22 | 0 |
| Ellagic acid | 2.21 | 3 |
| p-Coumaroylquinc acid | 0.41 | 0 |
| Ellagitannins Lambertianin C | 0 | 267.98 |
| Ellagitannins hex (casuarinin) | 483.33 | 592.55 |
| Quercetin-3-O-rutinoside | 0 | 8.78 |
| Quercetin-3-O-glucoside- glucuronide | 3.44 | 0 |
| Quercetin-3-O-rutinoside | 26.62 | 2.25 |
| Quercetin-3-O-galactoside | 0 | 2.2 |
| Quercetin -3-O-glucuronide | 5.37 | 15.12 |
| Quercetin-3-O-glucoside | 0 | 6.6 |
| Kaempferol-3-O-rutinoside | 37.92 | 9.19 |
| Kaempferol -3-O-glucoside- glucuronide | 2.32 | 0 |
| Kaempferol-3-O-rhamnoside-7-O-galacturonide | 18.03 | 0 |
| Luteolino-3-O-glucoronide | 0 | 2.74 |
| Kaempferol-3-O-glucoside | 0 | 1.68 |
| Kaempferol-3-O-glucoside-rhamnoside-7-O-rhamnoside | 0 | 1.34 |
| Kaempferol-3-O-glucoside-7-O-rhamnoside | 0 | 1.47 |
| Kaempferol-3-O-glucuronide | 0.27 | 2.24 |
| Quercetin-3-O-6-acetylglucoside | 0 | 1.36 |
| Apigenin-3-O-glucoronide | 0 | 7.88 |
| Isorhamnetin-3-O-rhamnoside | 0.74 | 0 |
| Total | 581.88 | 931.43 |
| Object | Test | Fragaria [µg/mL] | Rubus [µg/mL] | Ascorbic Acid [µg/mL] |
|---|---|---|---|---|
| RBC membrane | fluorimetric | 3.15 ± 0.16 | 7.30 ± 0.51 | 20.5 ± 1.78 |
| RBC | spectrophotometric | 6.05 ± 0.61 | 5.63 ± 0.68 | 32.57 ± 3.16 |
| HMEC-1 | MTT | 46.0 ± 6.6 | 45.4 ± 5.8 | >100 |
| Hoechst 33342 | 26.9 ± 2.7 | 45.5 ± 3.5 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cyboran-Mikołajczyk, S.; Męczarska, K.; Solarska-Ściuk, K.; Ratajczak-Wielgomas, K.; Oszmiański, J.; Jencova, V.; Bonarska-Kujawa, D. Protection of Erythrocytes and Microvascular Endothelial Cells against Oxidative Damage by Fragaria vesca L. and Rubus idaeus L. Leaves Extracts—The Mechanism of Action. Molecules 2022, 27, 5865. https://doi.org/10.3390/molecules27185865
Cyboran-Mikołajczyk S, Męczarska K, Solarska-Ściuk K, Ratajczak-Wielgomas K, Oszmiański J, Jencova V, Bonarska-Kujawa D. Protection of Erythrocytes and Microvascular Endothelial Cells against Oxidative Damage by Fragaria vesca L. and Rubus idaeus L. Leaves Extracts—The Mechanism of Action. Molecules. 2022; 27(18):5865. https://doi.org/10.3390/molecules27185865
Chicago/Turabian StyleCyboran-Mikołajczyk, Sylwia, Katarzyna Męczarska, Katarzyna Solarska-Ściuk, Katarzyna Ratajczak-Wielgomas, Jan Oszmiański, Vera Jencova, and Dorota Bonarska-Kujawa. 2022. "Protection of Erythrocytes and Microvascular Endothelial Cells against Oxidative Damage by Fragaria vesca L. and Rubus idaeus L. Leaves Extracts—The Mechanism of Action" Molecules 27, no. 18: 5865. https://doi.org/10.3390/molecules27185865
APA StyleCyboran-Mikołajczyk, S., Męczarska, K., Solarska-Ściuk, K., Ratajczak-Wielgomas, K., Oszmiański, J., Jencova, V., & Bonarska-Kujawa, D. (2022). Protection of Erythrocytes and Microvascular Endothelial Cells against Oxidative Damage by Fragaria vesca L. and Rubus idaeus L. Leaves Extracts—The Mechanism of Action. Molecules, 27(18), 5865. https://doi.org/10.3390/molecules27185865






