Polyvinyl Chloride Nanoparticles Affect Cell Membrane Integrity by Disturbing the Properties of the Multicomponent Lipid Bilayer in Arabidopsis thaliana
Abstract
:1. Introduction
2. Results
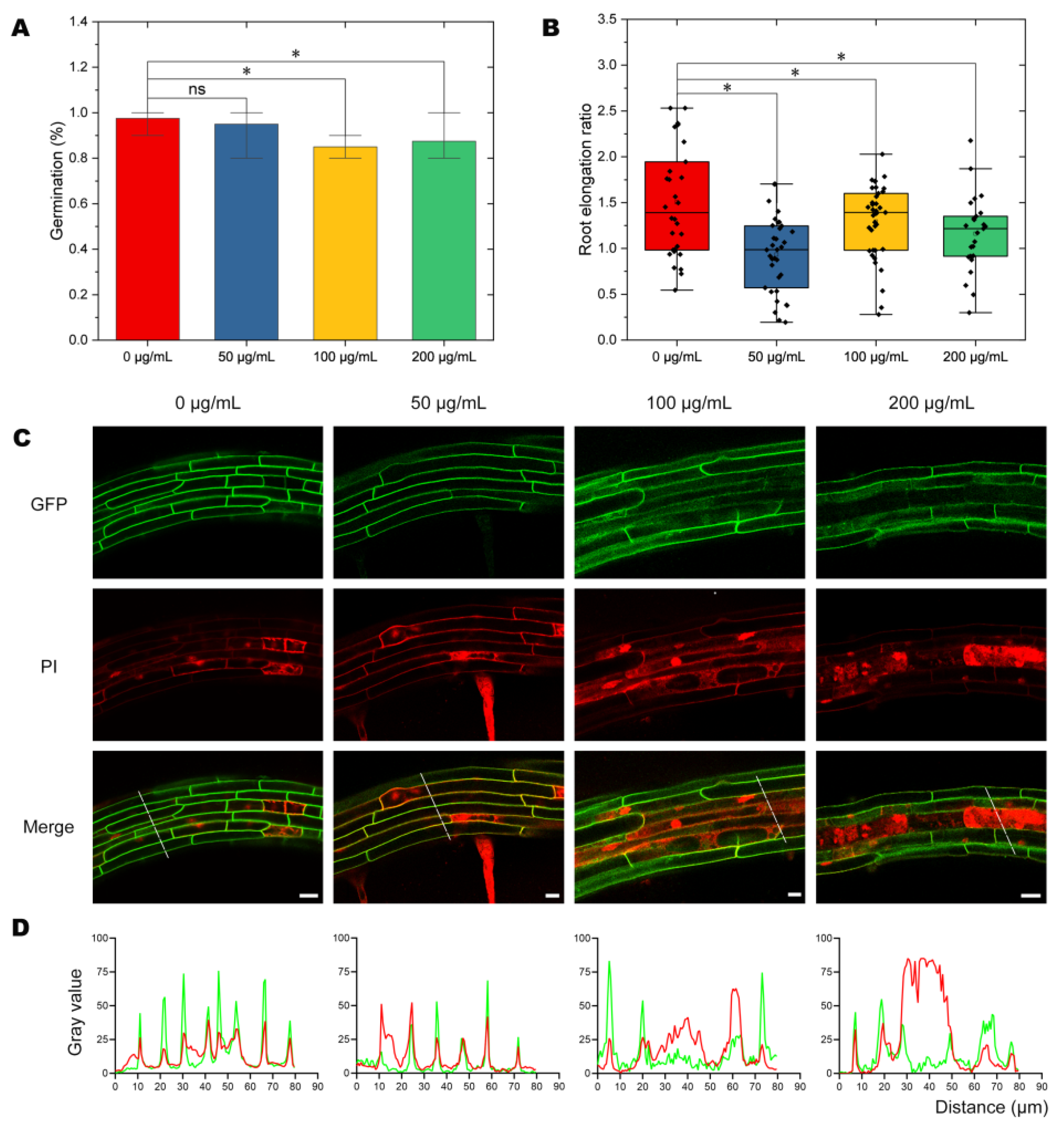
2.1. Effects of NPs on Seed Germination and Plant Growth
2.2. Analysis of Cellular Integrity after NP Treatment
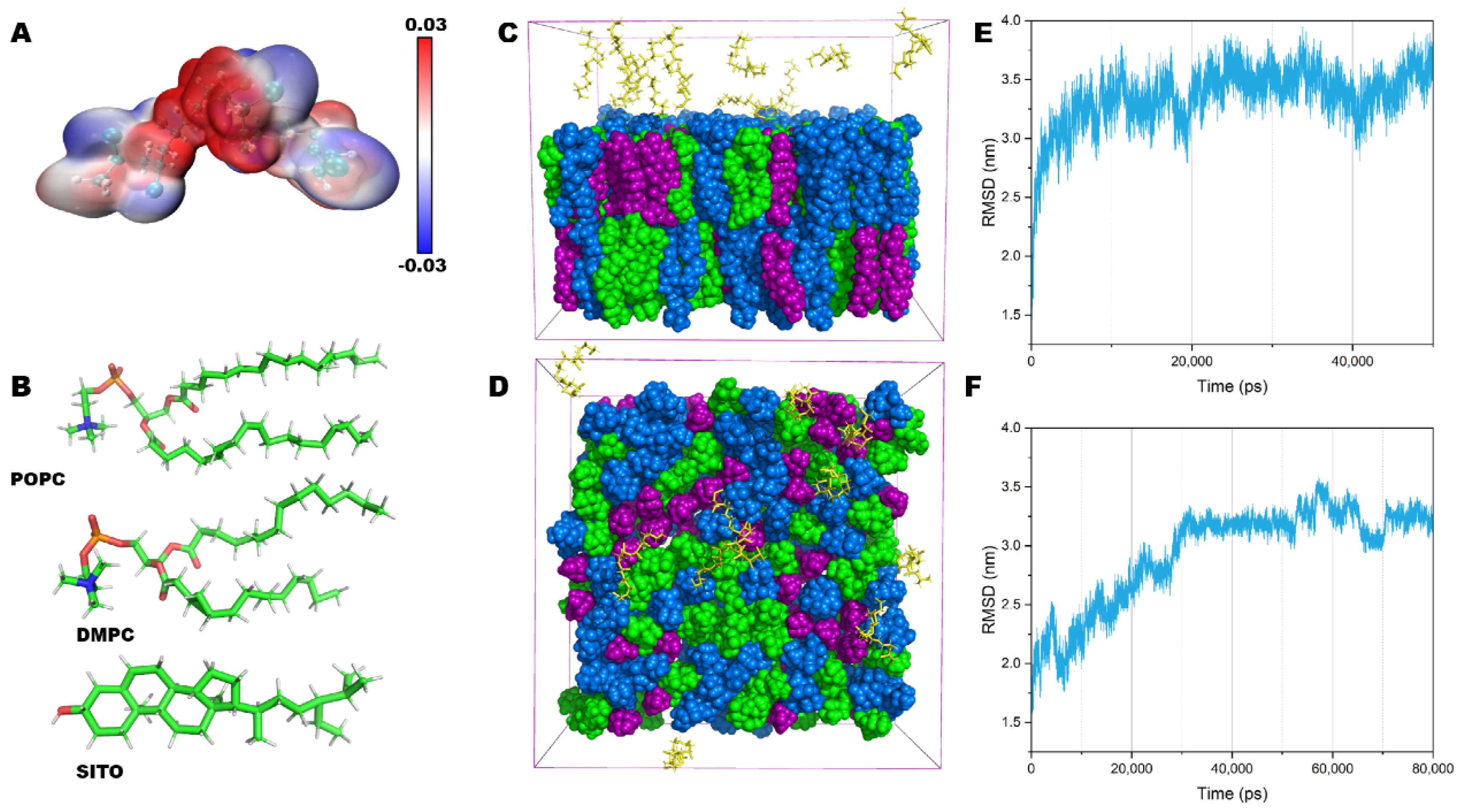
2.3. Structural and Conformational Properties of the Lipid Bilayer
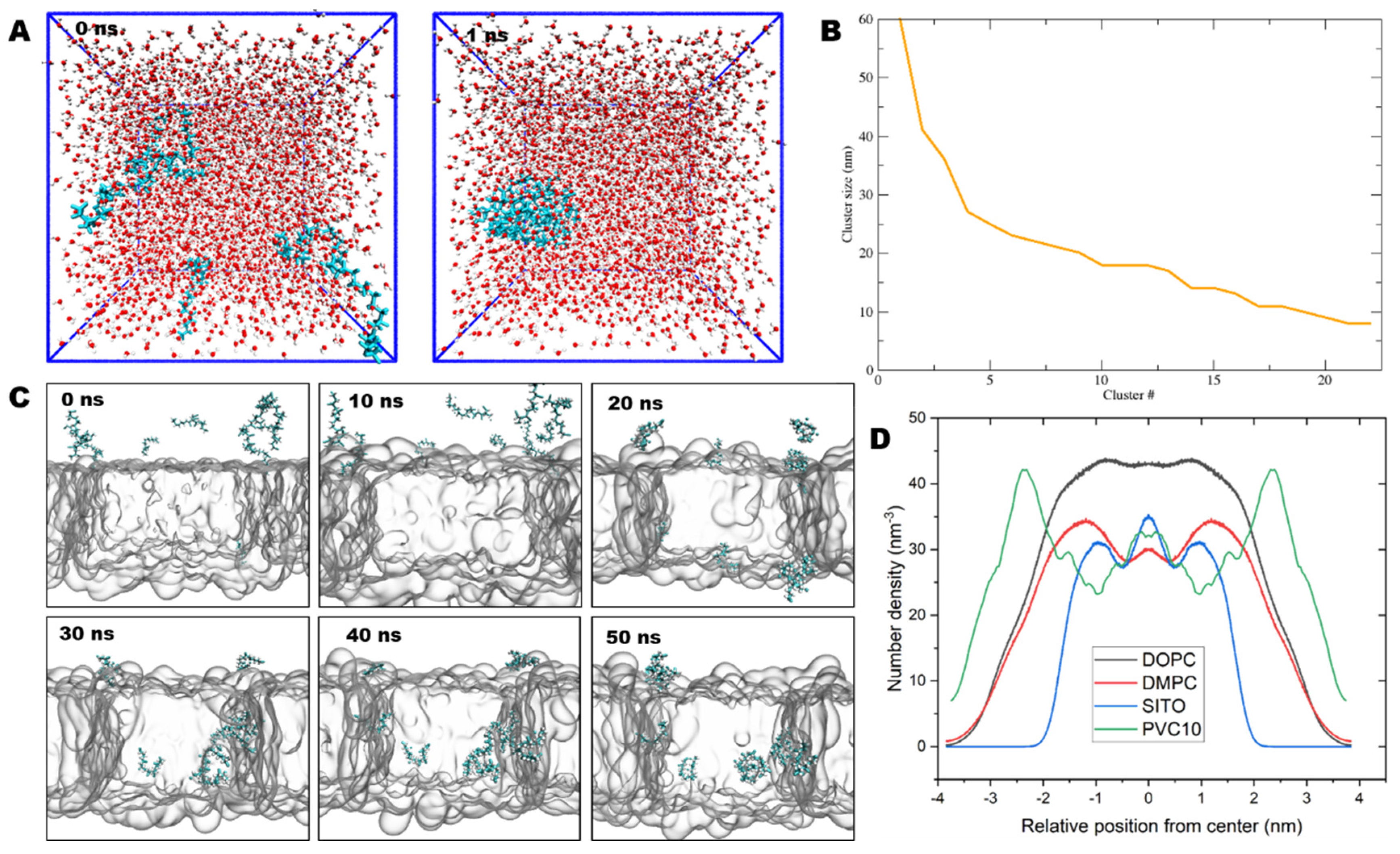
2.4. NPs Insert into the Lipid Bilayer in Numerous Simulations
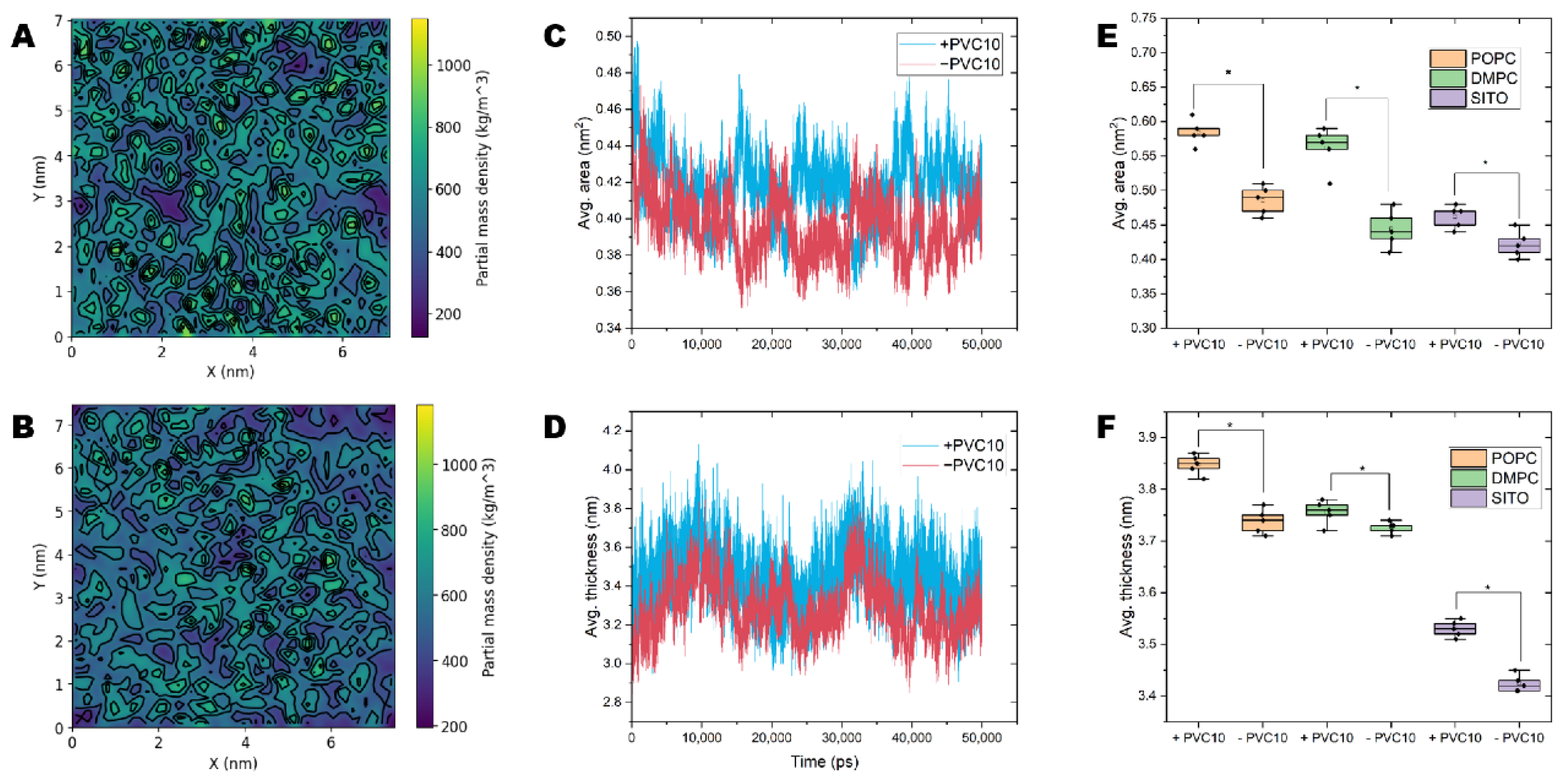
2.5. PVC Chains Alter the Homeostasis of Model Membranes
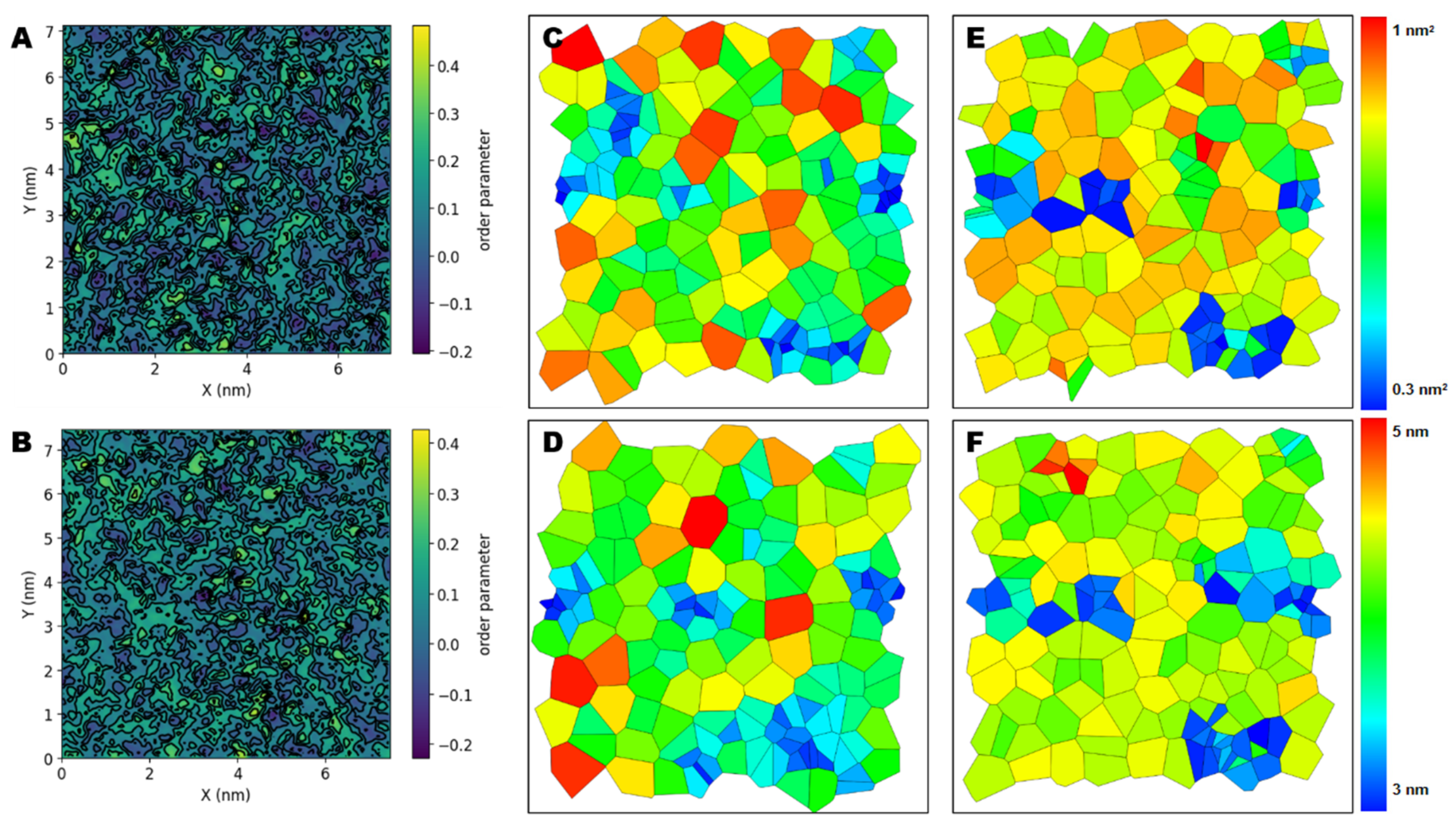
2.6. NPs Affect the Lateral Organization of Multicomponent Membranes
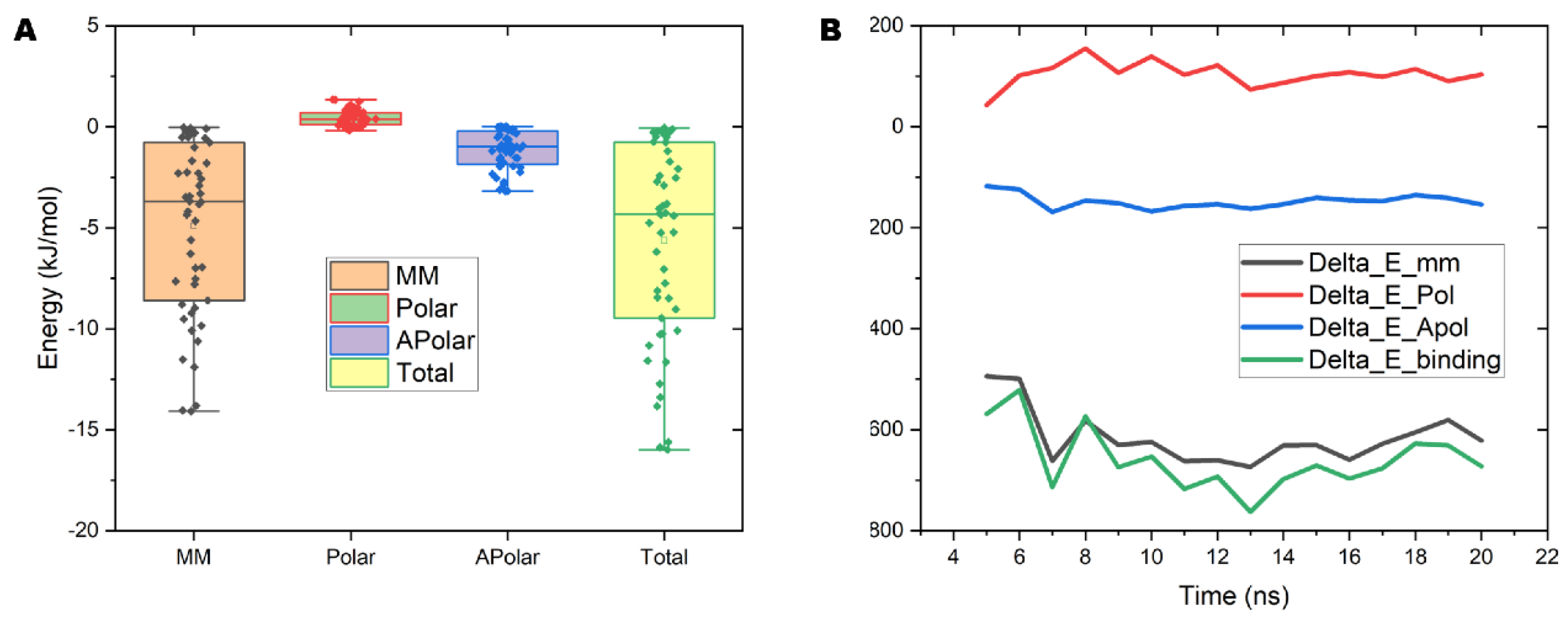
2.7. The Interaction Energy between PVC10 and the Lipid Bilayer
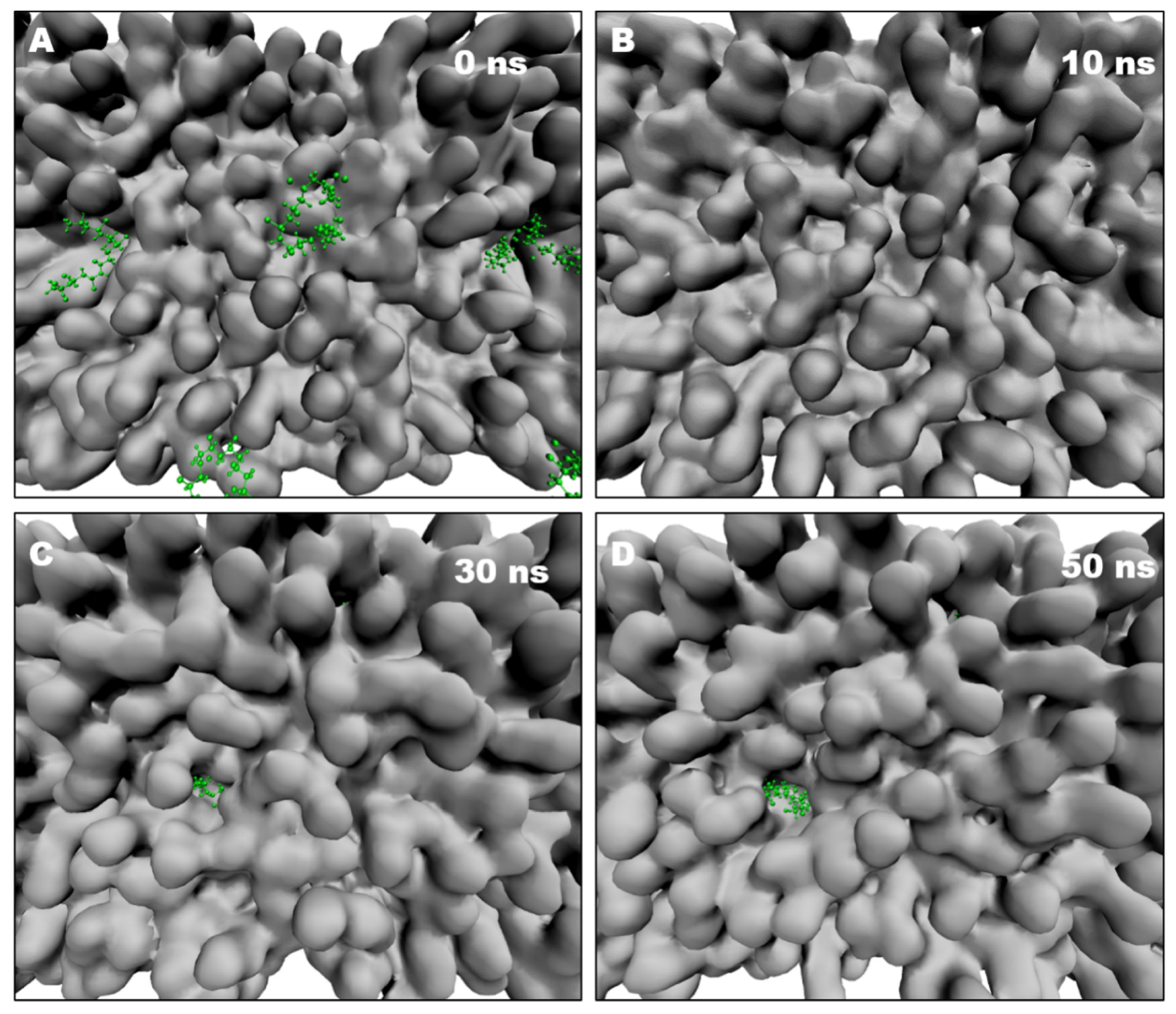
2.8. NPs Induce Pore Formation in the Lipid Bilayer
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Plant Cell Labeling and Treatment with PVC NPs
4.3. Confocal Microscopy Detection
4.4. Atomistic Molecular Dynamics Simulation
4.5. Molecular Dynamics Simulation Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef]
- Mateos-Cárdenas, A.; van Pelt, F.N.A.M.; O’Halloran, J.; Jansen, M.A.K. Adsorption, uptake and toxicity of micro- and nanoplastics: Effects on terrestrial plants and aquatic macrophytes. Environ. Pollut. 2021, 284, 117183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, G.; Wei, W. Simulation of nanoparticles interacting with a cell membrane: Probing the structural basis and potential biomedical application. NPG Asia Mater. 2021, 13, 52. [Google Scholar] [CrossRef]
- Larue, C.; Sarret, G.; Castillo-Michel, H.; Pradas del Real, A.E. A critical review on the impacts of nanoplastics and microplastics on aquatic and terrestrial photosynthetic organisms. Small 2021, 17, e2005834. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and nano-plastics in marine environment: Source, distribution and threats—A review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Qiu, Z.; Cui, Z.; Li, N.; Li, X.; Wang, Y.; Zhang, H.; Zhao, C. Effects of polyethylene microplastics on cell membranes: A combined study of experiments and molecular dynamics simulations. J. Hazard. Mater. 2022, 429, 128323. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Qiu, W.; Song, Z. Effects of microplastic on arsenic accumulation in Chlamydomonas reinhardtii in a freshwater environment. J. Hazard. Mater. 2021, 405, 124232. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus Edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Li, H.; Ye, X.; Guo, X.; Geng, Z.; Wang, G. Effects of surface ligands on the uptake and transport of gold nanoparticles in rice and tomato. J. Hazard. Mater. 2016, 314, 188–196. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, W.; Yuan, Y.; Li, Y.; Liu, X.; Wang, X.; Fan, Z. Growth inhibition, toxin production and oxidative stress caused by three microplastics in Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2021, 208, 111575. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Ershov, D.; Islam, M.A.; Kämpfer, A.M.; Maslowska, K.A.; van der Gucht, J.; Alink, G.M.; Marcelis, A.T.M.; Zuilhof, H.; Rietjens, I.M.C.M. Role of membrane disturbance and oxidative stress in the mode of action underlying the toxicity of differently charged polystyrene nanoparticles. RSC Adv. 2014, 4, 19321–19330. [Google Scholar] [CrossRef]
- Magrì, D.; Sánchez-Moreno, P.; Caputo, G.; Gatto, F.; Veronesi, M.; Bardi, G.; Catelani, T.; Guarnieri, D.; Athanassiou, A.; Pompa, P.P.; et al. Laser ablation as a versatile tool to mimic polyethylene terephthalate nanoplastic pollutants: Characterization and toxicology assessment. ACS Nano 2018, 12, 7690–7700. [Google Scholar] [CrossRef] [PubMed]
- van Weert, S.; Redondo-Hasselerharm, P.E.; Diepens, N.J.; Koelmans, A.A. Effects of nanoplastics and microplastics on the growth of sediment-rooted macrophytes. Sci. Total Environ. 2019, 654, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Huerta Lwanga, E.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic Shape, Polymer type, and concentration affect soil properties and plant biomass. Front. Plant Sci. 2021, 12, 616645. [Google Scholar] [CrossRef]
- Miralles, P.; Church, T.L.; Harris, A.T. Toxicity, Uptake, and Translocation of engineered nanomaterials in vascular plants. Environ. Sci. Technol. 2012, 46, 9224–9239. [Google Scholar] [CrossRef]
- Gopinath, P.M.; Saranya, V.; Vijayakumar, S.; Mythili Meera, M.; Ruprekha, S.; Kunal, R.; Pranay, A.; Thomas, J.; Mukherjee, A.; Chandrasekaran, N. Assessment on interactive prospectives of nanoplastics with plasma proteins and the toxicological impacts of virgin, coronated and environmentally released-nanoplastics. Sci. Rep. 2019, 9, 8860. [Google Scholar] [CrossRef]
- Taylor, S.E.; Pearce, C.I.; Sanguinet, K.A.; Hu, D.; Chrisler, W.B.; Kim, Y.-M.; Wang, Z.; Flury, M. Polystyrene nano- and microplastic accumulation at Arabidopsis and wheat root cap cells, but no evidence for uptake into roots. Environ. Sci. Nano 2020, 7, 1942–1953. [Google Scholar] [CrossRef]
- Bandmann, V.; Müller, J.D.; Köhler, T.; Homann, U. Uptake of fluorescent nano beads into BY2-cells involves clathrin-dependent and clathrin-independent endocytosis. FEBS Lett. 2012, 586, 3626–3632. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, L.; Spano, C.; Muccifora, S.; Bottega, S.; Barbieri, F.; Bellani, L.; Ruffini Castiglione, M. Exploring the interaction between polystyrene nanoplastics and Allium cepa during germination: Internalization in root cells, induction of toxicity and oxidative stress. Plant Physiol. Biochem. 2020, 149, 170–177. [Google Scholar] [CrossRef]
- Rossi, G.; Barnoud, J.; Monticelli, L. Polystyrene nanoparticles perturb lipid membranes. J. Phys. Chem. Lett. 2014, 5, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Hollóczki, O.; Gehrke, S. Can nanoplastics alter cell membranes? Chemphy Schem. Eur. J. Chem. Phys. Phys. Chem. 2019, 21, 9–12. [Google Scholar] [CrossRef]
- Wang, X.; Song, K.; Li, Y.; Tang, L.; Deng, X. Single-molecule imaging and computational microscopy approaches clarify the mechanism of the dimerization and membrane interactions of green fluorescent protein. Int. J. Mol. Sci. 2019, 20, 1410. [Google Scholar] [CrossRef]
- Tang, L.; Li, Y.; Zhong, C.; Deng, X.; Wang, X. Plant sterol clustering correlates with membrane microdomains as revealed by optical and computational microscopy. Membranes 2021, 11, 747. [Google Scholar] [CrossRef]
- Feng, Q.N.; De Rycke, R.; Dagdas, Y.; Nowack, M.K. Autophagy promotes programmed cell death and corpse clearance in specific cell types of the Arabidopsis root cap. Curr. Biol. 2022, 32, 2110–2119.e3. [Google Scholar] [CrossRef]
- Huysmans, M.; Buono, R.A.; Skorzinski, N.; Radio, M.C.; De Winter, F.; Parizot, B.; Mertens, J.; Karimi, M.; Fendrych, M.; Nowack, M.K. NAC Transcription factors ANAC087 and ANAC046 control distinct aspects of programmed cell death in the Arabidopsis columella and lateral Root Cap. Plant Cell 2018, 30, 2197–2213. [Google Scholar] [CrossRef]
- Lee, W.S.; Cho, H.-J.; Kim, E.; Huh, Y.H.; Kim, H.-J.; Kim, B.; Kang, T.; Lee, J.-S.; Jeong, J. Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos. Nanoscale 2019, 11, 3173–3185. [Google Scholar] [CrossRef]
- Yee, M.S.; Hii, L.-W.; Looi, C.K.; Lim, W.-M.; Wong, S.-F.; Kok, Y.-Y.; Tan, B.-K.; Wong, C.-Y.; Leong, C.-O. Impact of microplastics and nanoplastics on human health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Akhatova, F.; Ishmukhametov, I.; Fakhrullina, G.; Fakhrullin, R. Nanomechanical Atomic force microscopy to probe cellular microplastics uptake and distribution. Int. J. Mol. Sci. 2022, 23, 806. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385, 121620. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Rodrigo, M.A.M.; Moulick, A.; Heger, Z.; Kopel, P.; Zítka, O.; Adam, V.; Lukatkin, A.S.; Duarte, A.C.; Pereira, E.; et al. Transport phenomena of nanoparticles in plants and animals/humans. Environ. Res. 2016, 151, 233–243. [Google Scholar] [CrossRef]
- Likhachev, I.V.; Balabaev, N.K.; Galzitskaya, O.V. Is it possible to find an antimicrobial peptide that passes the membrane bilayer with minimal force resistance? An attempt at a predictive approach by molecular dynamics simulation. Int. J. Mol. Sci. 2022, 23, 5997. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, Y.D.; Lee, G.Y. Identification of bacterial membrane selectivity of Romo1-derived antimicrobial peptide AMPR-22 via molecular dynamics. Int. J. Mol. Sci. 2022, 23, 7404. [Google Scholar] [CrossRef]
- Fleetwood, O.; Carlsson, J.; Delemotte, L. Identification of ligand-specific G protein-coupled receptor states and prediction of downstream efficacy via data-driven modeling. eLife 2021, 10, e60715. [Google Scholar] [CrossRef]
- Souza, P.C.T.; Thallmair, S.; Conflitti, P.; Ramírez-Palacios, C.; Alessandri, R.; Raniolo, S.; Limongelli, V.; Marrink, S.J. Protein–ligand binding with the coarse-grained Martini model. Nat. Commun. 2020, 11, 3714. [Google Scholar] [CrossRef]
- James, J.R.; McColl, J.; Oliveira, M.I.; Dunne, P.D.; Huang, E.; Jansson, A.; Nilsson, P.; Sleep, D.L.; Goncalves, C.M.; Morgan, S.H.; et al. The T cell receptor triggering apparatus is composed of monovalent or monomeric proteins. J. Biol. Chem. 2011, 286, 31993–32001. [Google Scholar] [CrossRef]
- Lee, J.; Patel, D.S.; Ståhle, J.; Park, S.-J.; Kern, N.R.; Kim, S.; Lee, J.; Cheng, X.; Valvano, M.A.; Holst, O.; et al. CHARMM-GUI membrane builder for complex biological membrane simulations with glycolipids and lipoglycans. J. Chem. Theory Comput. 2019, 15, 775–786. [Google Scholar] [CrossRef] [Green Version]
- Pronk, S.; Pall, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Castillo, N.; Monticelli, L.; Barnoud, J.; Tieleman, D.P. Free energy of WALP23 dimer association in DMPC, DPPC, and DOPC bilayers. Chem. Phys. Lipids 2013, 169, 95–105. [Google Scholar] [CrossRef]
- Swanson, J.M.J.; Henchman, R.H.; McCammon, J.A. Revisiting free energy calculations: A theoretical connection to MM/PBSA and direct calculation of the association free energy. Biophys. J. 2004, 86, 67–74. [Google Scholar] [CrossRef]
- Lukat, G.; Kruger, J.; Sommer, B. APL@Voro: A Voronoi-based membrane analysis tool for GROMACS trajectories. J. Chem. Inf. Model. 2013, 53, 2908–2925. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Deng, X.; Luu, D.T.; Maurel, C.; Lin, J. Single-molecule fluorescence imaging to quantify membrane protein dynamics and oligomerization in living plant cells. Nat. Protoc. 2015, 10, 2054–2063. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Li, X.; Wang, X. Intracellular trafficking and imaging methods of membrane-bound transcription factors in plants. Crit. Rev. Plant Sci. 2020, 39, 418–430. [Google Scholar] [CrossRef]
- Jin, D.; Xi, P.; Wang, B.; Zhang, L.; Enderlein, J.; van Oijen, A.M. Nanoparticles for super-resolution microscopy and single-molecule tracking. Nat. Methods 2018, 15, 415–423. [Google Scholar] [CrossRef]
- Hao, X.; Allgeyer, E.S.; Lee, D.R.; Antonello, J.; Watters, K.; Gerdes, J.A.; Schroeder, L.K.; Bottanelli, F.; Zhao, J.; Kidd, P.; et al. Three-dimensional adaptive optical nanoscopy for thick specimen imaging at sub-50-nm resolution. Nat. Methods 2021, 18, 688–693. [Google Scholar] [CrossRef] [PubMed]
| Membrane in the Presence of NPs | ||||||
|---|---|---|---|---|---|---|
| Upside Layer | Downside Layer | |||||
| Tested Lipids | Avg. Area (nm2) | Avg. Thickness (nm) | Sum. Area (nm2) | Avg. Area (nm2) | Avg. Thickness (nm) | Sum. Area (nm2) |
| POPC | 0.58 | 3.87 | 21.49 | 0.56 | 3.83 | 21.08 |
| DMPC | 0.57 | 3.78 | 19.18 | 0.59 | 3.81 | 19.92 |
| SITO | 0.45 | 3.53 | 11.98 | 0.44 | 3.52 | 12.71 |
| PVC10 | 0.16 | 1.84 | 7.87 | 0.42 | 3.88 | 9.58 |
| Membrane Not Containing NPs | ||||||
| POPC | 0.47 | 3.77 | 17.37 | 0.45 | 3.84 | 16.72 |
| DMPC | 0. 46 | 3.75 | 15.27 | 0.49 | 3.95 | 16.39 |
| SITO | 0.37 | 3.45 | 10.69 | 0.36 | 3.51 | 10.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhang, Y.; Li, C.; Lin, J.; Li, X. Polyvinyl Chloride Nanoparticles Affect Cell Membrane Integrity by Disturbing the Properties of the Multicomponent Lipid Bilayer in Arabidopsis thaliana. Molecules 2022, 27, 5906. https://doi.org/10.3390/molecules27185906
Li M, Zhang Y, Li C, Lin J, Li X. Polyvinyl Chloride Nanoparticles Affect Cell Membrane Integrity by Disturbing the Properties of the Multicomponent Lipid Bilayer in Arabidopsis thaliana. Molecules. 2022; 27(18):5906. https://doi.org/10.3390/molecules27185906
Chicago/Turabian StyleLi, Mingyang, Yuan Zhang, Changyuan Li, Jinxing Lin, and Xiaojuan Li. 2022. "Polyvinyl Chloride Nanoparticles Affect Cell Membrane Integrity by Disturbing the Properties of the Multicomponent Lipid Bilayer in Arabidopsis thaliana" Molecules 27, no. 18: 5906. https://doi.org/10.3390/molecules27185906
APA StyleLi, M., Zhang, Y., Li, C., Lin, J., & Li, X. (2022). Polyvinyl Chloride Nanoparticles Affect Cell Membrane Integrity by Disturbing the Properties of the Multicomponent Lipid Bilayer in Arabidopsis thaliana. Molecules, 27(18), 5906. https://doi.org/10.3390/molecules27185906





