Quantitative Analysis and Stability Study on Iridoid Glycosides from Seed Meal of Eucommia ulmoides Oliver
Abstract
:1. Introduction
2. Results and Discussion
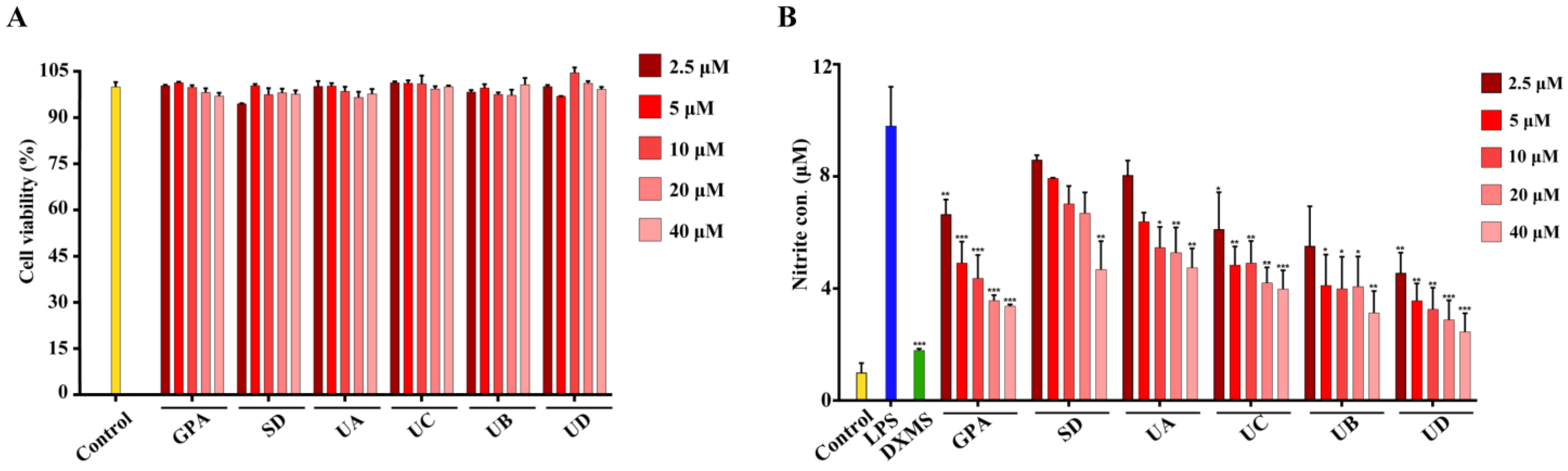
2.1. Evaluation of Anti-Inflammatory Activity for Six Compounds from Seed Meal of E. ulmoides Oliv.
2.2. Optimization of Extraction Conditions for Six Tested Compounds

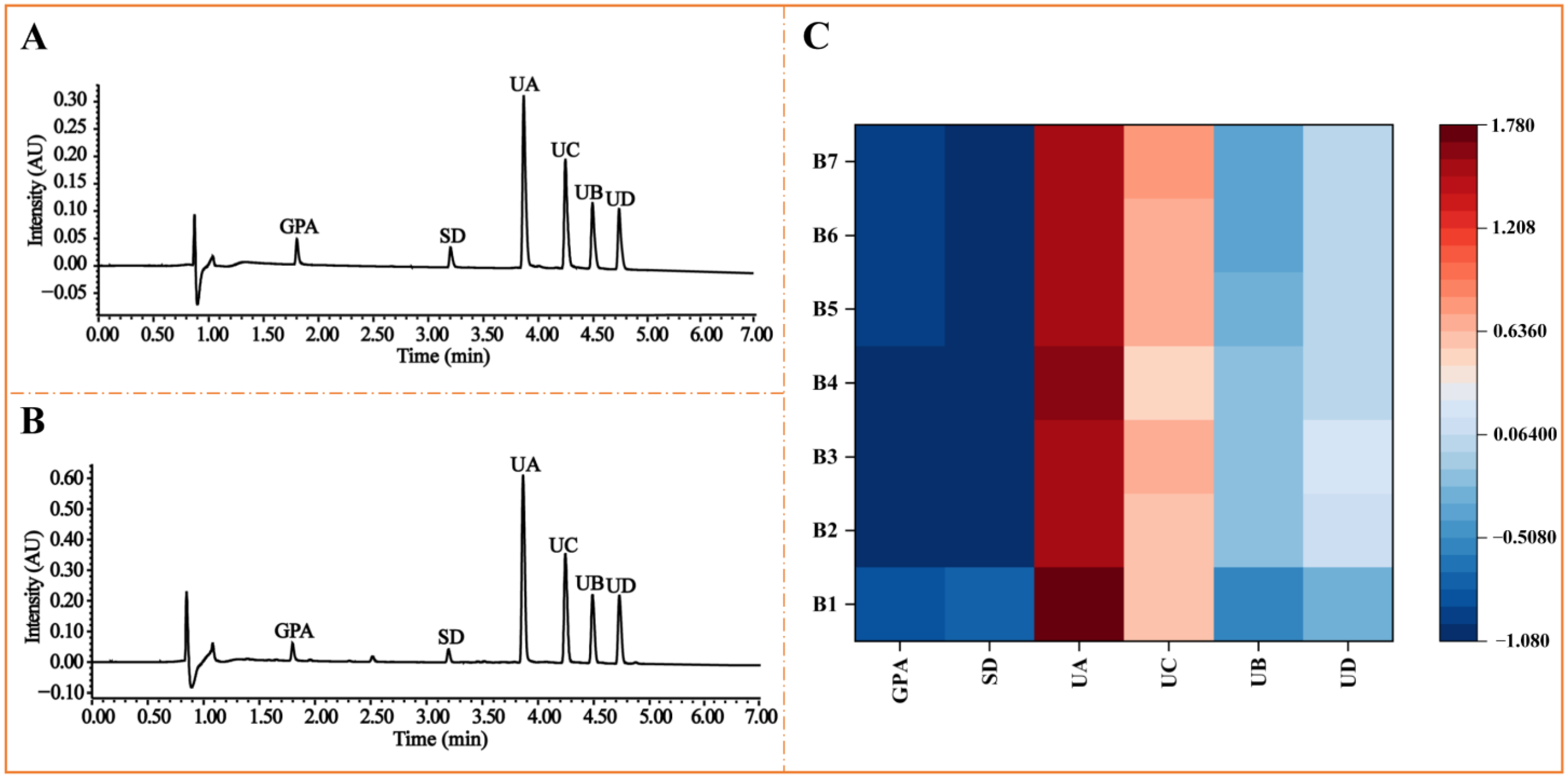
2.3. Methodological Validation of the Quantitative Analysis and Quantificaiton of Six Tested Compounds in Seed Meal of E. ulmoides Oliv.
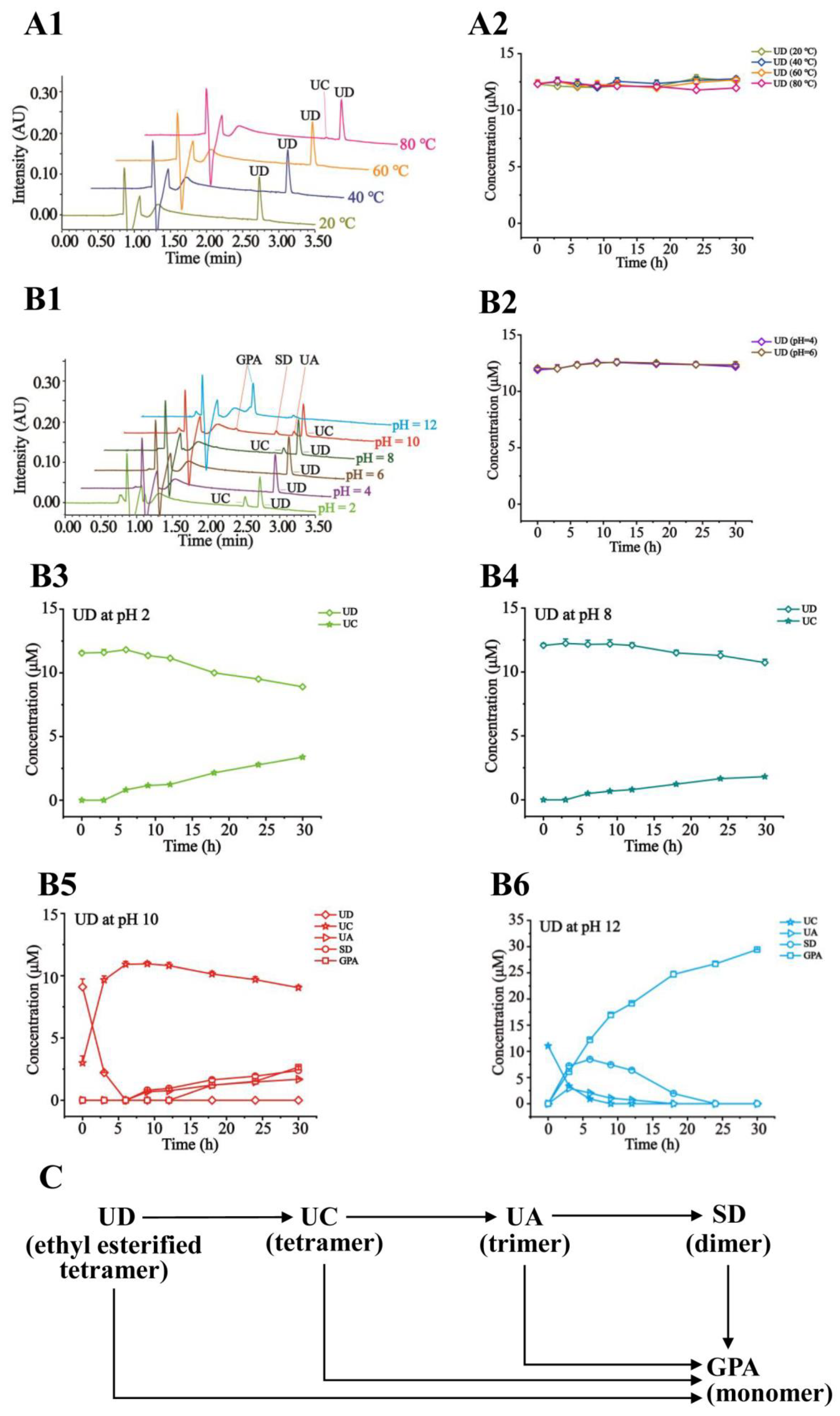
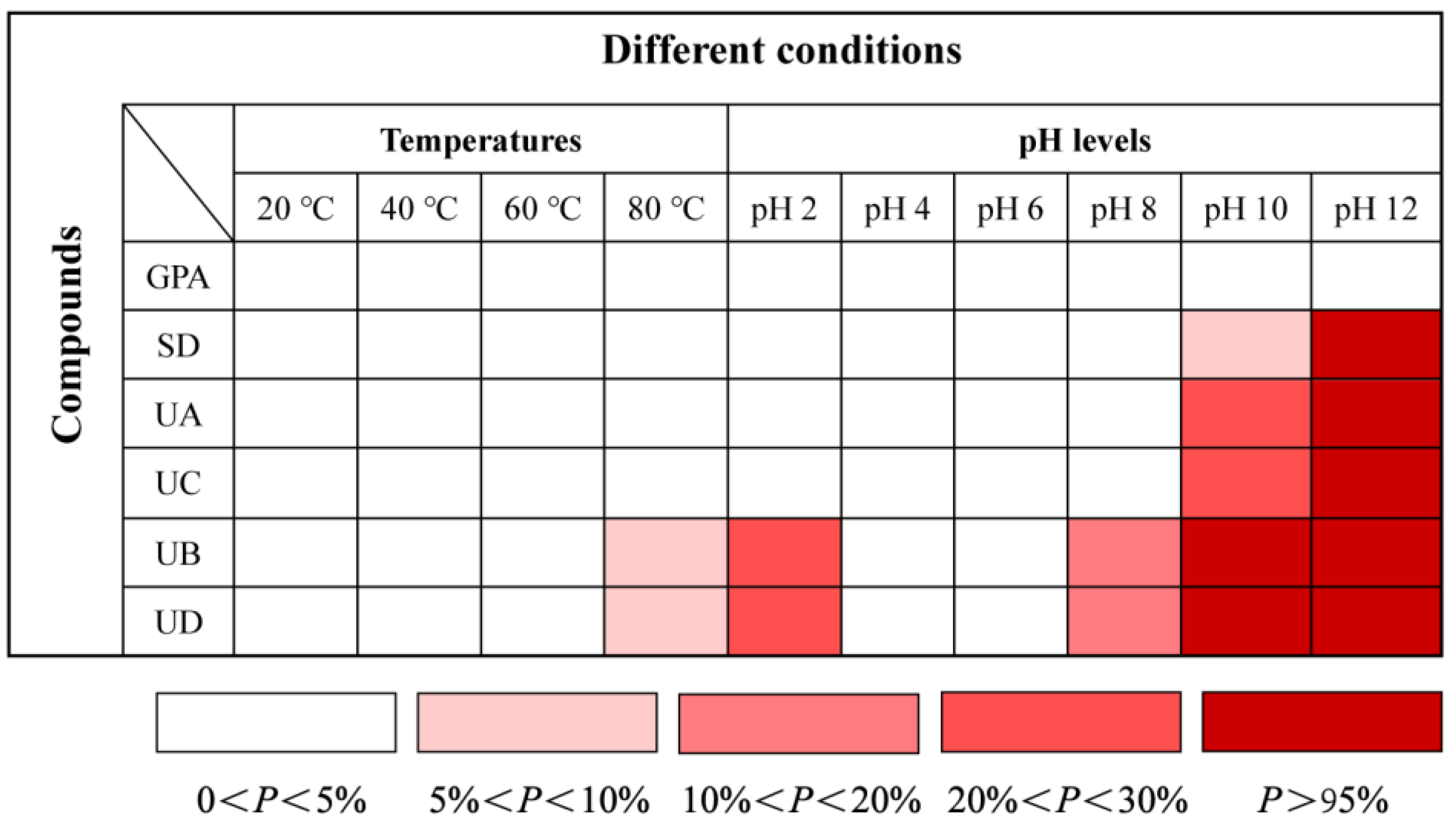
2.4. Effects of Temperatures and pH on Stability of GPA, SD, UA, UC, UB, and UD
3. Materials and Methods
3.1. Reagents and Materials
3.2. Cell Viability Assay
3.3. Measurement of Nitrite Levels
3.4. Preparation of Standard Solution
3.5. Preparation of Sample Solution
3.6. UPLC-PDA Analysis
3.7. Methodological Validation
3.8. Stability Study of the Tested Compounds under Different Temperatures and pH Levels
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| E. ulmoides Oliv. | Eucommia ulmoides Oliver |
| GPA | Geniposidic acid |
| SD | Scyphiphin D |
| UA | Ulmoidoside A |
| UC | Ulmoidoside C |
| UB | Ulmoidoside B |
| UD | Ulmoidoside D |
| UPLC-PDA | Ultra-performance liquid chromatography coupled with a photo-diode array |
| LPS | Lipopolysaccharide |
| DXMS | Dexamethasone |
| CCK8 | Cell counting kit-8 |
| DMEM | Dulbecco’s modified Eagle medium |
| FBS | Fetal bovine serum |
| PS | Penicillin-streptomycin |
| DMSO | Dimethyl sulfoxide |
References
- Wang, C.Y.; Tang, L.; He, J.W.; Li, J.; Wang, Y.Z. Ethnobotany, phytochemistry and pharmacological properties of Eucommia ulmoides: A Review. Am. J. Chin. Med. 2019, 47, 259–300. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Lyu, Q.; Zheng, W.Y.; Yang, Q.; Cao, G. Traditional application and modern pharmacological research of Eucommia ulmoides Oliv. Chin. Med. 2021, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.F.; He, D.; Wang, Y.; Zeng, W.; Zhang, C.; Lu, Y.; Su, N.; Kong, Y.H.; Xing, X.H. Chemical constituents, biological functions and pharmacological effects for comprehensive utilization of Eucommia ulmoides Oliver. Food Sci. Hum. Wellness 2019, 8, 177–188. [Google Scholar] [CrossRef]
- Peng, M.F.; Tian, S.; Song, Y.G.; Li, C.X.; Miao, M.S.; Ren, Z.; Li, M. Effects of total flavonoids from Eucommia ulmoides Oliv. leaves on polycystic ovary syndrome with insulin resistance model rats induced by letrozole combined with a high-fat diet. J. Ethnopharmacol. 2021, 273, 113947. [Google Scholar] [CrossRef]
- Wu, D.; Yu, D.M.; Zhang, Y.J.; Dong, J.E.; Li, D.W.; Wang, D.M. Metabolite profiles, bioactivity, and HPLC fingerprint of different varieties of Eucommia ulmoides Oliv.: Towards the utilization of medicinal and commercial Chinese endemic tree. Molecules 2018, 23, 1898. [Google Scholar] [CrossRef]
- Yao, L.N.; Su, Y.F.; Yin, Z.Y.; Qin, N.; Li, T.X.; Si, C.L.; Liu, E.W.; Gao, X.M. A new phenolic glucoside and flavonoids from the bark of Eucommia ulmoides Oliv. Holzforschung 2010, 64, 571–575. [Google Scholar] [CrossRef]
- Sun, Z.Q.; Li, F.D.; Du, H.Y.; Zhu, J.L.; Wang, Y.P. A novel silvicultural model for increasing biopolymer production from Eucommia ulmoides Oliver trees. Ind. Crops Prod. 2013, 42, 216–222. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.Z.; Kang, K.; Sun, G.T.; Zhu, M.Q. Review on extraction, characteristic, and engineering of the Eucommia ulmodies rubber for industrial application. Ind. Crops Prod. 2022, 180, 114733. [Google Scholar] [CrossRef]
- Li, D.H.; Yang, C.; Huang, Y.Q.; Li, L.; Han, W.C.; Kang, H.L.; Yang, F.; Fang, Q.H. Novel green resource material: Eucommia ulmoides gum. Resour. Chem. Mater. 2022, 1, 114–128. [Google Scholar] [CrossRef]
- Zhang, J.C.; Xue, Z.H.; Yan, R.F. Damping performance of Eucommia ulmoides gum. Chin. J. Polym. Sci. 2011, 29, 157–163. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Liu, Y.L.; Che, L.M. Characterization of a new alpha-linolenic acid-rich oil: Eucommia ulmoides seed oil. J. Food Sci. 2018, 83, 617–623. [Google Scholar] [CrossRef]
- Du, Q.X.; Wang, L.; Liu, P.F.; Qing, J.; Sun, C.W.; Sun, Z.Q.; Du, H.Y. Fatty acids variation in seed of Eucommia ulmoides populations collected from different regions in China. Forests 2018, 9, 505. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Zhou, Q.; Chen, G.X.; Shen, X.X.; Hu, J.Y. Research status and progress on comprehensive development and utilization of samara of Eucommia ulmoides Oliver. Chin. Wild Plant Resour. 2015, 34, 53–59. [Google Scholar] [CrossRef]
- Tang, J.J.; Zhao, N.; Gao, Y.Q.; Han, R.; Wang, X.Y.; Tian, J.M.; Gao, J.M. Phytosterol profiles and iridoids of the edible Eucommia ulmoides Oliver seeds and their anti-inflammatory potential. Food Biosci. 2021, 43, 101295. [Google Scholar] [CrossRef]
- Ouyang, H.; Li, S.L.; Peng, W.X.; Xiao, Z.P.; Zhang, Y.K. Optimization protocol and bioactivity assessment for the microwave-assisted extraction of flavonoids from Eucommia ulmoides Oliver seed meal using response surface methodology. Bioresources 2021, 16, 7366–7377. [Google Scholar] [CrossRef]
- Niu, X.L.; Xu, D.R.; Luo, J.; Kong, L. Main iridoid glycosides and HPLC/DAD-Q-TOF-MS/MS profile of glycosides from the antioxidant extract of Eucommia ulmoides Oliver seeds. Ind. Crops Prod. 2016, 79, 160–169. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.J.; Li, S.; Zhang, Y.M.; Zhao, Y.; Xie, R.M.; Sun, W.J. Effect of total glycosides from Eucommia ulmoides seed on bone microarchitecture in rats. Phytother. Res. 2011, 25, 1895–1897. [Google Scholar] [CrossRef]
- Yu, H.; Yang, J.; Ding, J.; He, Y.; Jiang, Z.; Chai, X.; Wang, Y. Stability study and identification of degradation products of caffeoylgluconic acid derivatives from Fructus Euodiae. Molecules 2018, 23, 1975. [Google Scholar] [CrossRef]
- Pfleiderer, M. Stability of vaccines-Bridging from stability data to continuous safety and efficacy throughout shelf life-An always reliable approach? Biologicals 2009, 37, 364–368. [Google Scholar] [CrossRef]
- Bansal, G.; Suthar, N.; Kaur, J.; Jain, A. Stability testing of herbal drugs: Challenges, regulatory compliance and perspectives. Phytother. Res. 2016, 30, 1046–1058. [Google Scholar] [CrossRef]
- Zeng, Y.B.; Mei, W.L.; Wang, H.; Li, X.N.; Dai, H.F. Scyphiphin D, a new iridoid glucoside dimer from Scyphiphora hydrophyllacea. J. Asian Nat. Prod. Res. 2010, 12, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Yahara, S.; Kato, K.; Nakazawa, Y.; Toda, Y.; Nohara, T. New iridoid trimers and tetramers from seeds of Eucommia ulmoided. Chem. Pharm. Bull. 1990, 38, 267–269. [Google Scholar] [CrossRef]
- Jiang, Z.Z.; Yang, J.; Wang, Y.F. Discrimination and identification of Q-markers based on ‘Spider-web’ mode for quality control of traditional Chinese medicine. Phytomedicine 2018, 44, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yu, H.; Chai, X.; Peng, S.; Yang, J.; Wu, D.; Du, J.; Wang, Y. Illumination on “Reserving phloem and discarding xylem” and quality evaluation of Radix polygalae by determining oligosaccharide esters, saponins, and xanthones. Molecules 2018, 23, 836. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chai, X.; Wang, L.; Yang, J.; Wang, Y. Study of the variation of phenolic acid and flavonoid content from fresh Artemisiae argyi Folium to moxa wool. Molecules 2019, 24, 4603. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. ChP 2020; China Medical Science Press: Beijing, China, 2020; Volume Ⅳ. [Google Scholar]
- Satheesh, B.; Pulluru, S.K.; Nitin, K.; Saravanan, D. Simultaneous determination of eprosartan, hydrochlorothiazide, and their related compounds in pharmaceutical dosage forms by UPLC. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1885–1900. [Google Scholar] [CrossRef]
- Ahn, S.; Siddiqi, M.H.; Noh, H.Y.; Kim, Y.J.; Kim, Y.J.; Jin, C.G.; Yang, D.C. Anti-inflammatory activity of ginsenosides in LPS-stimulated RAW 264.7 cells. Sci. Bull. 2015, 60, 773–784. [Google Scholar] [CrossRef] [Green Version]

| Compounds | Linear Regression | LODs (μg/mL) | LOQs (μg/mL) | Precision (RSD, %) | Repeatability (n = 6, RSD, %) | Stability (n = 6, RSD, %) | Recovery (n = 6, Mean ± SD, %) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Regression Equation | r2 | Linear Range (μg/mL) | Intra-Day (n = 6) | Inter-Day (n = 3) | ||||||
| GPA | y = 9209.7 x − 631.79 | 0.9999 | 2.031–65.00 | 0.063 | 0.254 | 0.3 | 0.1 | 0.8 | 0.5 | 95.27 ± 1.40 |
| SD | y = 8883.6 x + 3985.4 | 0.9996 | 1.876–60.02 | 0.059 | 0.234 | 0.5 | 0.9 | 0.3 | 0.3 | 90.84 ± 0.31 |
| UA | y = 9343.4 x + 18,053.79 | 0.9996 | 16.02–512.5 | 0.063 | 0.250 | 0.2 | 0.9 | 0.1 | 0.2 | 96.35 ± 1.63 |
| UC | y = 9802.2 x − 13,433.35 | 0.9999 | 10.31–330.0 | 0.081 | 0.322 | 0.2 | 1.0 | 0.5 | 0.2 | 115.3 ± 0.65 |
| UB | y = 9785.1 x − 6293.28 | 0.9999 | 6.344–203.0 | 0.099 | 0.198 | 0.2 | 1.4 | 0.6 | 0.2 | 101.4 ± 1.02 |
| UD | y = 9103.4 x − 8611.40 | 0.9999 | 6.516–208.5 | 0.102 | 0.204 | 0.1 | 1.5 | 0.6 | 0.2 | 106.5 ± 2.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Meng, N.; Liu, B.; Wang, C.; Chai, X.; Huang, S.; Yu, H.; Wang, Y. Quantitative Analysis and Stability Study on Iridoid Glycosides from Seed Meal of Eucommia ulmoides Oliver. Molecules 2022, 27, 5924. https://doi.org/10.3390/molecules27185924
Ma L, Meng N, Liu B, Wang C, Chai X, Huang S, Yu H, Wang Y. Quantitative Analysis and Stability Study on Iridoid Glycosides from Seed Meal of Eucommia ulmoides Oliver. Molecules. 2022; 27(18):5924. https://doi.org/10.3390/molecules27185924
Chicago/Turabian StyleMa, Lulu, Ning Meng, Benyu Liu, Changjian Wang, Xin Chai, Shan Huang, Huijuan Yu, and Yuefei Wang. 2022. "Quantitative Analysis and Stability Study on Iridoid Glycosides from Seed Meal of Eucommia ulmoides Oliver" Molecules 27, no. 18: 5924. https://doi.org/10.3390/molecules27185924





