Connexin Expression in Human Minor Salivary Glands: An Immunohistochemical Microscopy Study
Abstract
:1. Introduction
2. Results and Discussion
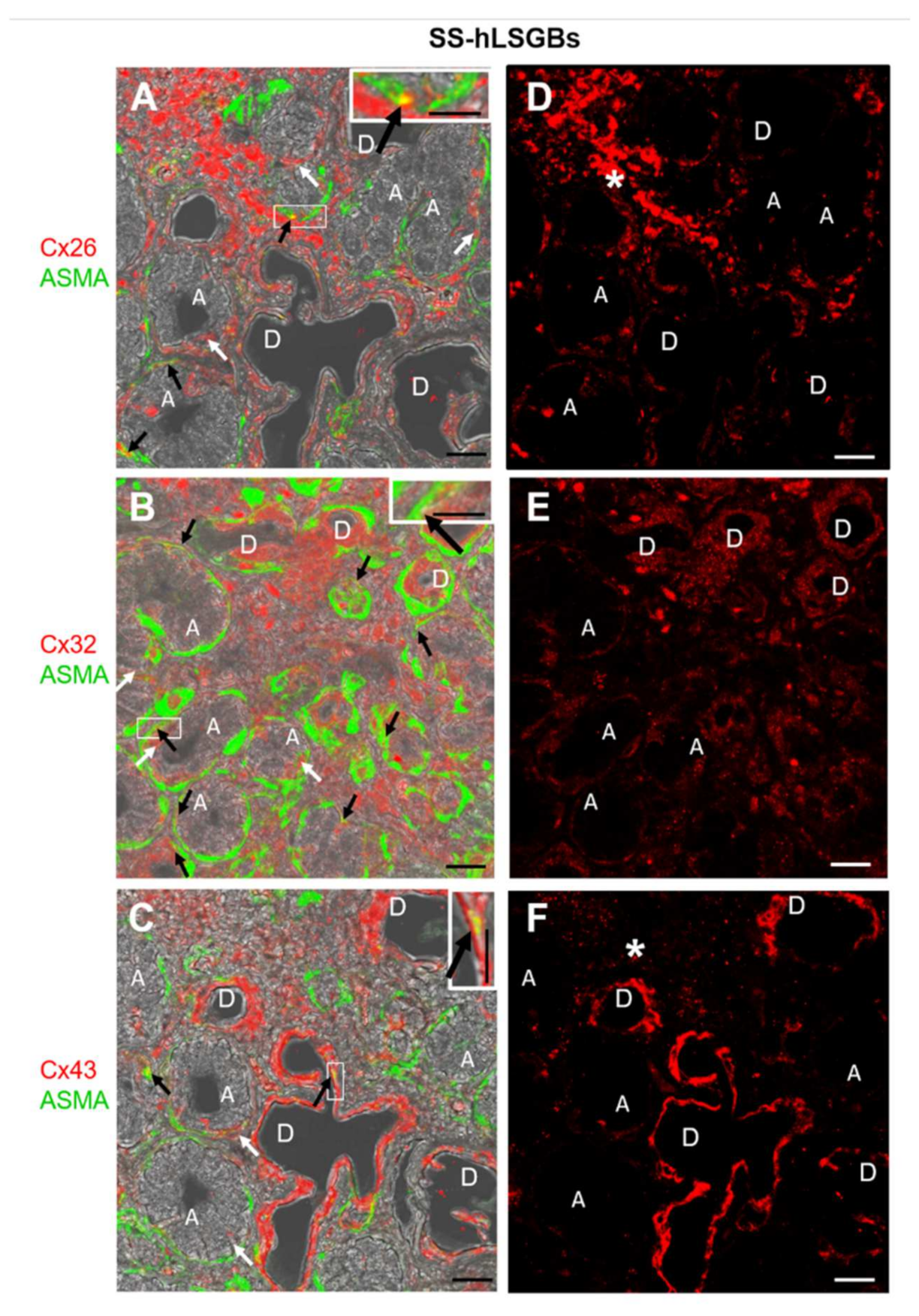
2.1. Cx Protein IF Analysis
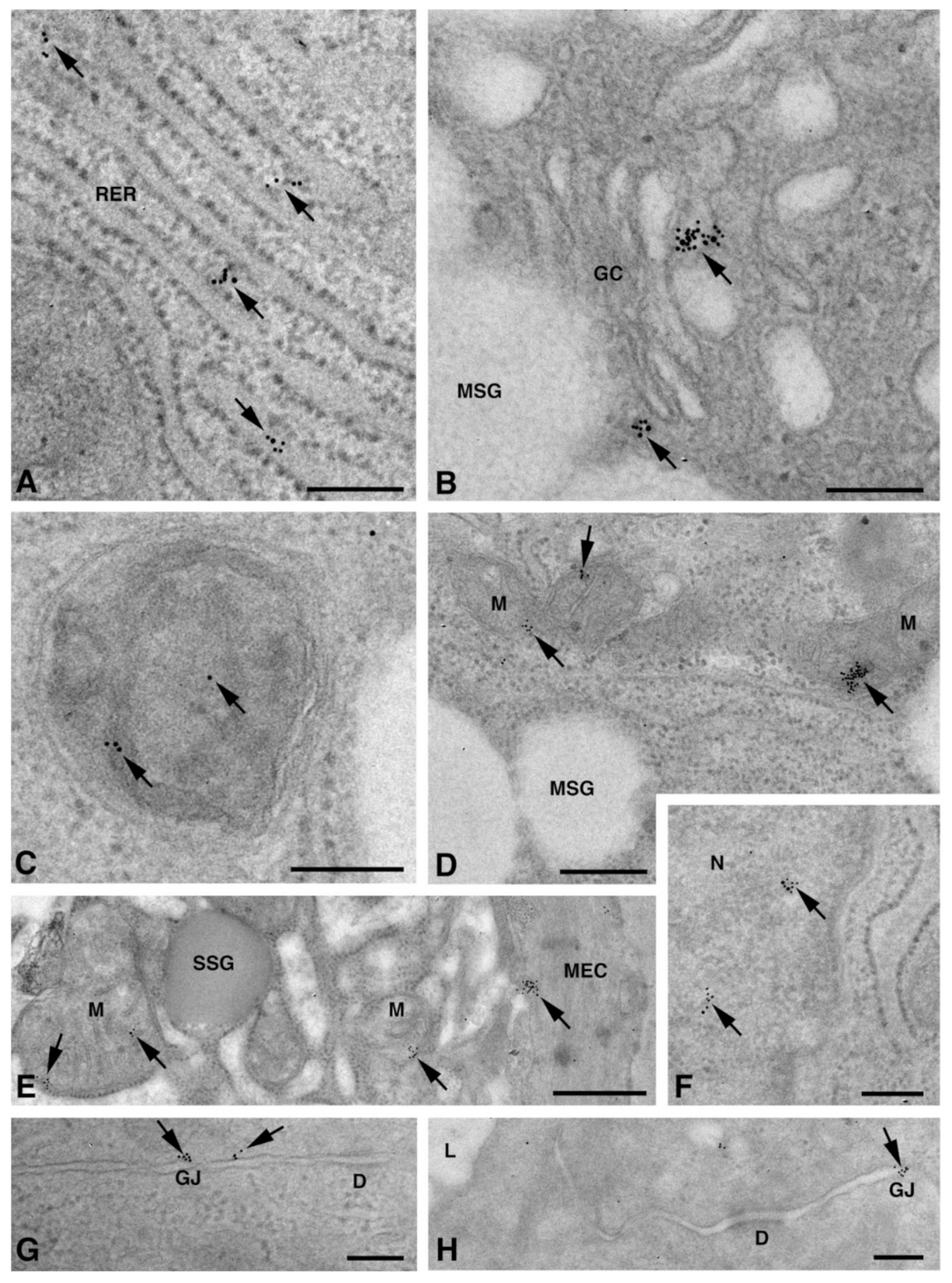
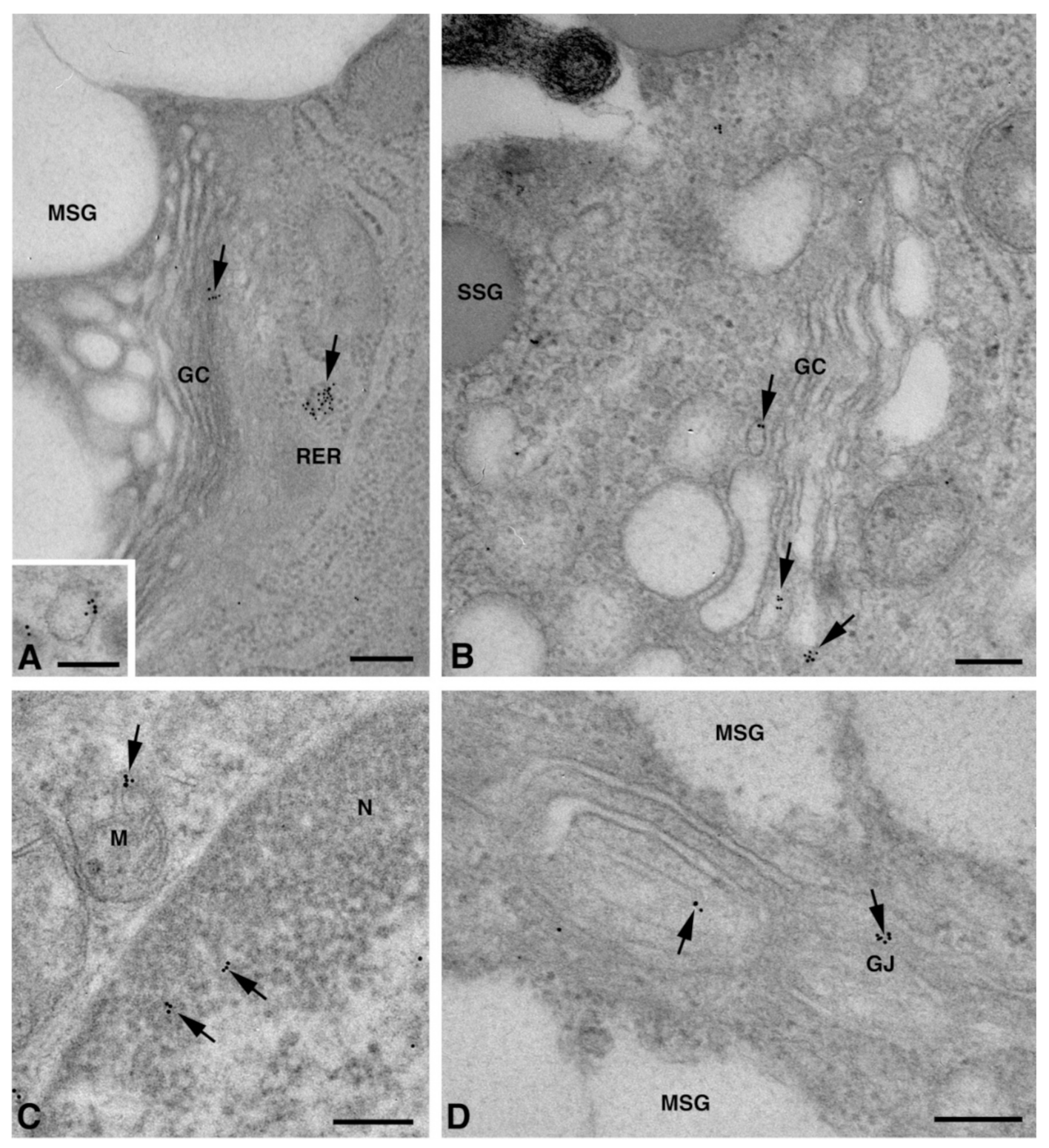
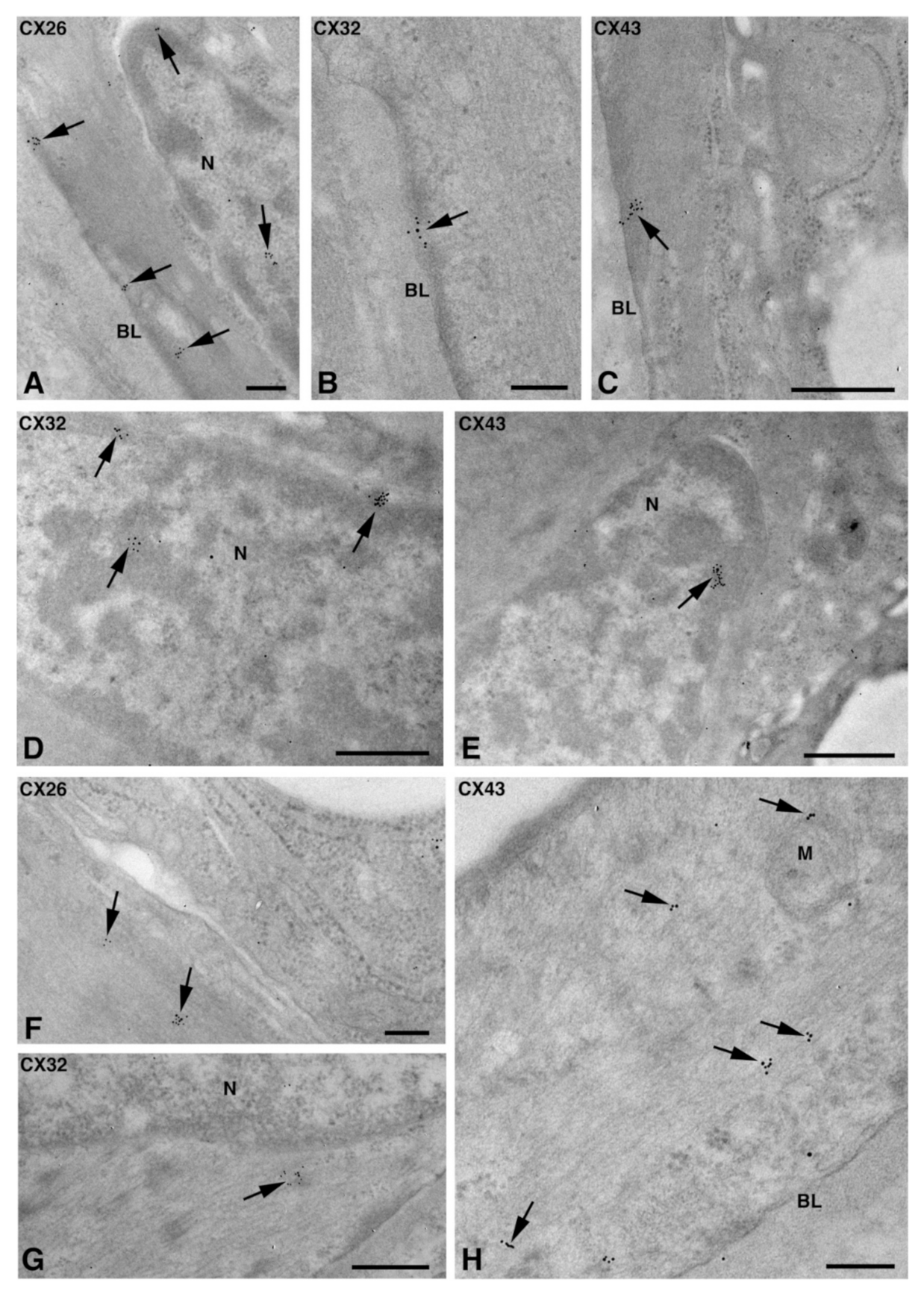
2.2. Cx Protein IE Microscopy
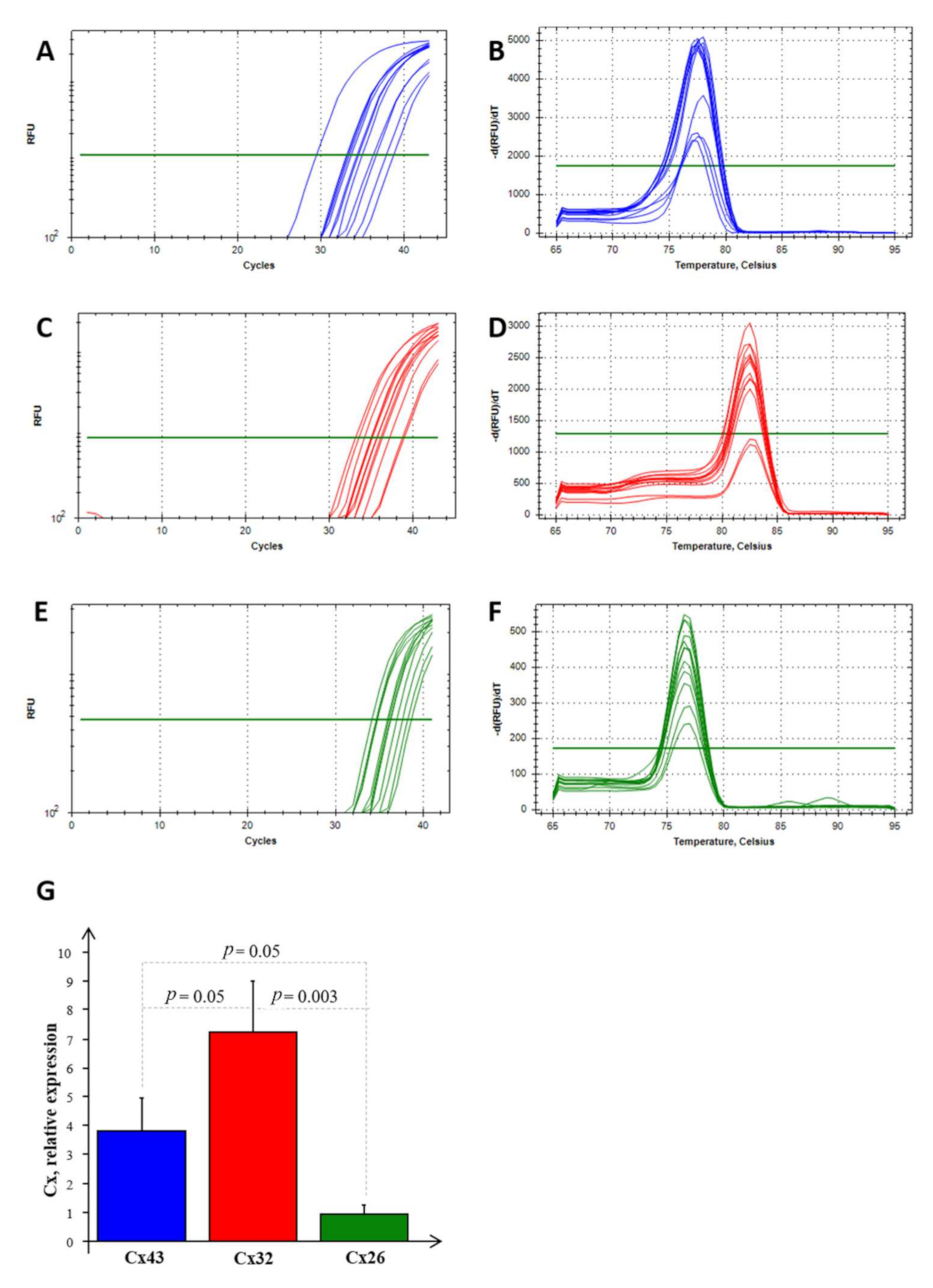
2.3. Cx mRNA Expression Analysis
3. Materials and Methods
3.1. Salivary Gland Collection
3.2. Immunohistochemical Analyses
3.2.1. Immunofluorescence (IF) Microscopy
3.2.2. Immunoelectron (IE) Microscopy Post-Embedding Technique
3.3. RNA Extraction and Real Time PCR Analysis
Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laird, D.W. Life cycle of connexins in health and disease. Biochem. J. 2006, 394, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Söhl, G.; Willecke, K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun. Adhes. 2003, 10, 173–180. [Google Scholar] [CrossRef]
- Sosinsky, G.E.; Nicholson, B.J. Structural organization of gap junction channels. Biochim. Biophys. Acta 2005, 1711, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Meda, P.; Pepper, M.S.; Traub, O.; Willecke, K.; Groß, D.; Beyer, E.; Nicholson, B.; Paul, D.; Orci, L. Differential expression of gap junction connexins in endocrine and exocrine glands. Endocrinology 1993, 133, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Meda, P.; Chanson, M.; Pepper, M.; Giordano, E.; Bosco, D.; Traub, O.; Willecke, K.; El Aoumari, A.; Gros, D.; Beyer, E.C.; et al. In vivo modulation of connexin 43 gene expression and junctional coupling of pancreatic B-cells. Exp. Cell Res. 1991, 192, 469–480. [Google Scholar] [CrossRef]
- Walcott, B.; Moore, L.C.; Birzgalis, A.; Claros, N.; Valiunas, V.; Ott, T.; Willecke, K.; Brink, P.R. Role of gap junctions in fluid secretion of lacrimal glands. Am. J. Physiol. Cell. Physiol. 2002, 282, C501–C507. [Google Scholar] [CrossRef]
- Shimono, M.; Young, L.C.; Matsuzaki, H.; Ishikawa, H.; Inoue, T.; Hashimoto, S.; Muramatsu, T. Connexins in salivary glands. Eur. J. Morphol. 2000, 38, 257–261. [Google Scholar] [CrossRef]
- Kidder, M.G.; Winterhager, E. Physiological roles of connexins in labour and lactation. Reproduction 2015, 150, R129–R136. [Google Scholar] [CrossRef]
- Actis, A.B.; Lampe, P.D.; Eynard, A.R. Cellular basis and clinical implications of biological markers in salivary tissues: Their topological distribution in murine submandibular gland. Oral Oncol. 2002, 38, 441–449. [Google Scholar] [CrossRef]
- Ihara, A.; Muramatsu, T.; Shimono, M. Expression of connexin 32 and 43 in developing rat submandibular salivary glands. Arch. Oral Biol. 2000, 45, 227–235. [Google Scholar] [CrossRef]
- Mattii, L.; Polizzi, E.; Ferro, F.; Donati, V.; Cecchettini, A.; Baldini, C. Involvment of gap junctional comunication in salivary gland dysfunction related to primary Sjögren’s Syndrome. Clin. Exp. Rheumatol. 2018, 112, S-308. [Google Scholar]
- Shen, D.; Ono, K.; Do, Q.; Ohyama, H.; Nakamura, K.; Obata, K.; Ibaragi, S.; Watanabe, K.; Tubbs, R.S.; Iwanaga, J. Clinical anatomy of the inferior labial gland: A narrative review. Gland Surg. 2021, 10, 2284–2292. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, B.; Maes, M.; Pereira, I.V.A.; Willebrords, J.; Da Silva, T.C.; Yanguas, S.C.; Vinken, M. Immunohisto- and Cytochemistry Analysis of Connexins. Methods Mol Biol. 2016, 1437, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-S.; Yeh, H.-I.; Rothery, S.; Dupont, E.; Coppen, S.R.; Severs, N.J. Connexin Make-up of Endothelial Gap Junctions in the Rat Pulmonary Artery as Revealed by Immunoconfocal Microscopy and Triple-label Immunogold Electron Microscopy. J. Histochem. Cytochem. 1999, 47, 683–692. [Google Scholar] [CrossRef]
- Moscato, S.; Cabiati, M.; Bianchi, F.; Vaglini, F.; Morales, M.A.; Burchielli, S.; Botta, L.; Sabbatini, A.R.M.; Falleni, A.; Del Ry, S.; et al. Connexin 26 Expression in Mammalian Cardiomyocytes. Sci. Rep. 2018, 8, 13975. [Google Scholar] [CrossRef]
- Moscato, S.; Cabiati, M.; Bianchi, F.; Panetta, D.; Burchielli, S.; Massimetti, G.; Del Ry, S.; Mattii, L. Heart and liver connexin expression related to the first stage of aging: A study on naturally aged animals. Acta Histochem. 2020, 122, 151651. [Google Scholar] [CrossRef]
- Shah, A.A.K.; Mulla, A.F.; Mayank, M. Pathophysiology of myoepithelial cells in salivary glands. J. Oral Maxillofac. Pathol. 2016, 20, 480–490. [Google Scholar] [CrossRef]
- Mroue, R.; Inman, J.; Mott, J.; Budunova, I.; Bissell, M.J. Asymmetric expression of connexins between luminal epithelial- and myoepithelial- cells is essential for contractile function of the mammary gland. Dev. Biol. 2015, 399, 15–26. [Google Scholar] [CrossRef]
- Martin, P.; Evans, W. Incorporation of connexins into plasma membranes and gap junctions. Cardiovasc. Res. 2004, 62, 378–387. [Google Scholar] [CrossRef]
- Smyth, J.W.; Shaw, R.M. Autoregulation of Connexin43 Gap Junction Formation by Internally Translated Isoforms. Cell Rep. 2013, 5, 611–618. [Google Scholar] [CrossRef]
- Falleni, A.; Moscato, S.; Sabbatini, A.R.M.; Bernardeschi, M.; Bianchi, F.; Cecchettini, A.; Mattii, L. Subcellular Localization of Connexin 26 in Cardiomyocytes and in Cardiomyocyte-Derived Extracellular Vesicles. Molecules 2021, 26, 6726. [Google Scholar] [CrossRef] [PubMed]
- Gadicherla, A.K.; Wang, N.; Bulic, M.; Agullo-Pascual, E.; Lissoni, A.; De Smet, M.; Delmar, M.; Bultynck, G.; Krysko, D.V.; Camara, A.; et al. Mitochondrial Cx43 hemichannels contribute to mitochondrial calcium entry and cell death in the heart. Basic Res. Cardiol. 2017, 112, 27. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Shao, Q.; Thomas, T.; Kalra, J.; Alaoui-Jamali, M.A.; Laird, D.W. Connexin26 regulates the expression of angiogenesis-related genes in human breast tumor cells by both GJIC-dependent and -independent mechanisms. Cell. Commun. Adhes. 2003, 10, 387–393. [Google Scholar] [CrossRef]
- Zhu, Y. Gap Junction-Dependent and -Independent Functions of Connexin43 in Biology. Biology 2022, 11, 283. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. International Sjögren’s Syndrome Criteria Working Group. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol 2017, 69, 35–45. [Google Scholar] [PubMed]
- Lenzi, P.; Ferese, R.; Biagioni, F.; Fulceri, F.; Busceti, C.; Falleni, A.; Gambardella, S.; Frati, A.; Fornai, F. Rapamycin Ameliorates Defects in Mitochondrial Fission and Mitophagy in Glioblastoma Cells. Int. J. Mol. Sci. 2021, 22, 5379. [Google Scholar] [CrossRef] [PubMed]
- Cabiati, M.; Sapio, A.; Salvadori, C.; Burchielli, S.; Carlucci, L.; Mattii, L.; Del Ry, S. Evaluation of transcriptional levels of the natriuretic peptides, endothelin-1, adrenomedullin, their receptors and long non-coding RNAs in rat cardiac tissue as cardiovascular biomarkers of aging. Peptides 2020, 123, 170173. [Google Scholar] [CrossRef]
- Cabiati, M.; Salvadori, C.; Sapio, A.; Burchielli, S.; Carlucci, L.; Moscato, S.; Sabatino, L.; Caselli, C.; Mattii, L.; Del Ry, S. Aging and biomarkers: Transcriptional levels evaluation of Osteopontin/miRNA-181a axis in hepatic tissue of rats in different age ranges. Exp. Gerontol. 2020, 133, 110879. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [Green Version]


| Gene | Primer Sequence | GenBank Accession Number | Amplicon Length | Ta, °C |
|---|---|---|---|---|
| Cx26 | Hs_GJB2_1_SG QuantiTect Primer Assay (QIAGEN, Hilden, Germany)—Blind sequence | NM_004004 | -------------- | 60 |
| Cx43 | F: CTCAACAACCTGGCTGCGAAA R: GGTGGGCACAGACACGAATAT | NM_012567.2 | 60 bp | 60 |
| Cx32 | F: TTGCCTTGCTGCCTGCTA R: ACTCTGATTTATCTGCCTGCTTCT | NM_017251 | 86 bp | 60 |
| ACTB | F: GTCGTACCACTGGCATTGTG R: CTCTCAGCTGTGGTGGTGAA | NM_031144 | 181 bp | 60 |
| RPL13a | F: CGCCCTACGACAAGAAAAAG R: CCGTAGCCTCATGAGCTGTT | NM_012423 | 206 | 60 |
| RPS4X | F: GATCCCCTCATCAAGGTGAA R: GCCCTTGCCAATAACAAAAA | NM_002046 | 243 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falleni, A.; Moscato, S.; Fulvio, G.; Polizzi, E.; Bernardeschi, M.; Bianchi, F.; Donati, V.; Cabiati, M.; Ippolito, C.; Del Ry, S.; et al. Connexin Expression in Human Minor Salivary Glands: An Immunohistochemical Microscopy Study. Molecules 2022, 27, 5926. https://doi.org/10.3390/molecules27185926
Falleni A, Moscato S, Fulvio G, Polizzi E, Bernardeschi M, Bianchi F, Donati V, Cabiati M, Ippolito C, Del Ry S, et al. Connexin Expression in Human Minor Salivary Glands: An Immunohistochemical Microscopy Study. Molecules. 2022; 27(18):5926. https://doi.org/10.3390/molecules27185926
Chicago/Turabian StyleFalleni, Alessandra, Stefania Moscato, Giovanni Fulvio, Enza Polizzi, Margherita Bernardeschi, Francesco Bianchi, Valentina Donati, Manuela Cabiati, Chiara Ippolito, Silvia Del Ry, and et al. 2022. "Connexin Expression in Human Minor Salivary Glands: An Immunohistochemical Microscopy Study" Molecules 27, no. 18: 5926. https://doi.org/10.3390/molecules27185926
APA StyleFalleni, A., Moscato, S., Fulvio, G., Polizzi, E., Bernardeschi, M., Bianchi, F., Donati, V., Cabiati, M., Ippolito, C., Del Ry, S., Baldini, C., & Mattii, L. (2022). Connexin Expression in Human Minor Salivary Glands: An Immunohistochemical Microscopy Study. Molecules, 27(18), 5926. https://doi.org/10.3390/molecules27185926






