The Antiviral Effects of 2-Deoxy-D-glucose (2-DG), a Dual D-Glucose and D-Mannose Mimetic, against SARS-CoV-2 and Other Highly Pathogenic Viruses
Abstract
1. SARS-CoV-2 Pandemic and Current COVID-19 Treatment
2. Metabolic Shift in Host Cells during Viral Infection
3. The Importance of Host Glycosylation Process for Viral Replication
4. 2-DG in Antiviral Research
4.1. 2-DG Molecule and Its Intracellular Effects
4.2. Antiviral Action of 2-DG
4.2.1. SARS-CoV-2 and Other Coronaviruses
4.2.2. Papillomaviruses
4.2.3. Rhinoviruses
4.2.4. Noroviruses
4.2.5. Hepatitis B Virus
4.2.6. Zika Virus
4.2.7. Herpes Simplex Virus 1
5. 2-DG in Clinical Trials
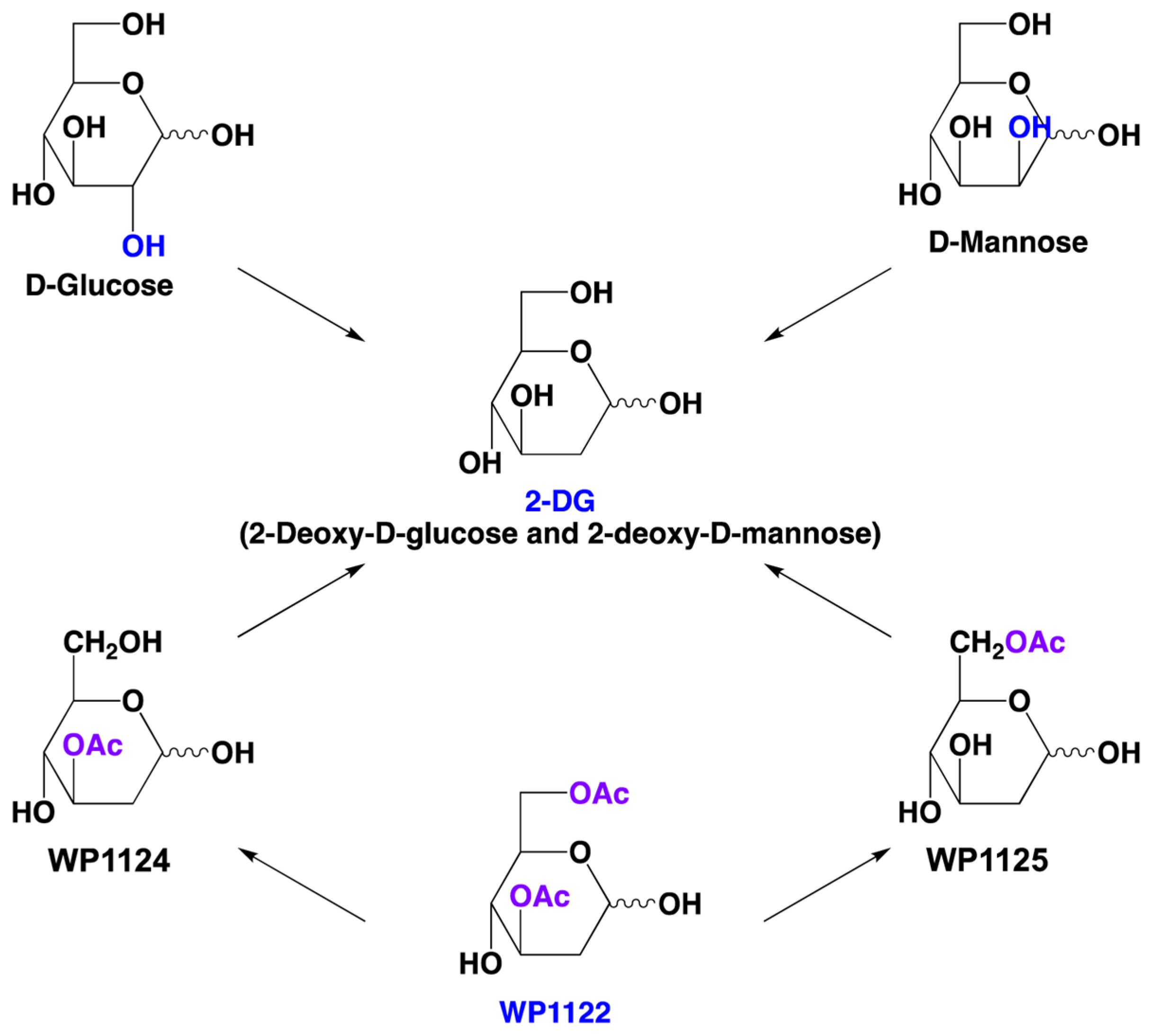
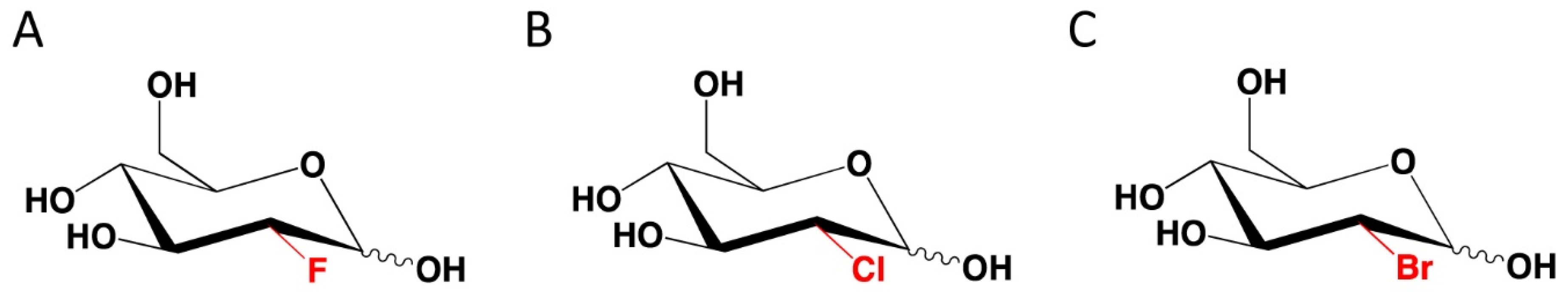
6. Novel 2-DG Analogs and Their Potential for Antiviral Therapy
7. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID Pandemic Data. Available online: https://en.wikipedia.org/wiki/Template:COVID-19_pandemic_data (accessed on 14 July 2022).
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The incubation period of coronavirus disease 2019 (COVID-19) from publicity reported confirmed cases: Estimation and application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef]
- Grant, R.A.; Morales-Nebreda, L.; Markov, N.S.; Swaminathan, S.; Querrey, M.; Guzman, E.R.; Abbott, D.A.; Donnelly, H.K.; Donayre, A.; Goldberg, I.A.; et al. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature 2021, 590, 635–641. [Google Scholar]
- Nielsen, M.C.; Reynoso, D.; Ren, P. The Brief Case: A fatal case of SARS-CoV-2 coinfection with Coccidioides in Texas—Another challenge we face. J. Clin. Microbiol. 2021, 59, e0016321. [Google Scholar] [CrossRef]
- Wei, Q.; Lin, H.; Wei, R.G.; Chen, N.; He, F.; Zou, D.H.; Wei, J.R. Tocilizumab treatment for COVID-19 patients: A systematic review and meta-analysis. Infect. Dis. Poverty 2021, 10, 71. [Google Scholar] [CrossRef]
- Zhou, Y.; Gammeltoft, K.A.; Ryberg, L.A.; Pham, L.V.; Fahnøe, U.; Binderup, A.; Rene, C.; Hernandez, D.; Offersgaard, A.; Fernendez-Antunez, C.; et al. Nirmatrelvir resistant SARS-CoV-2 variants with high fitness in vitro. BioRxiv 2022. [Google Scholar] [CrossRef]
- Jochmans, D.; Liu, C.; Donckers, K.; Stoycheva, A.; Boland, S.; Stevens, S.K.; de Vita, C.; Vanmechelen, B.; Maes, P.; Trüeb, B.; et al. The substitutions L50F, E166A and L167F in SARS-CoV-2 3CLpro are selected by a protease inhibitor in vitro and confer resistance to nirmatrelvir. BioRxiv 2022. [Google Scholar] [CrossRef]
- Ministry of Defense, India. Available online: https://pib.gov.in/PressReleasePage.aspx?PRID=1717007 (accessed on 14 July 2022).
- Borana, R. Dr Reddy’s Revealed More about 2-DG, and Its Approval Is More Confusing Now. Available online: https://science.thewire.in/health/dr-reddys-doc-vidya-webinar-2-dg-clinical-trials-primary-endpoints-problems/ (accessed on 14 July 2022).
- Thaker, S.K.; Chang, J.; Christfolk, H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019, 17, 59. [Google Scholar] [CrossRef]
- Eisenreich, W.; Rudel, T.; Heesemann, J.; Goebel, W. How viral and intracellular bacterial pathogens reprogram the metabolism of host cells to allow their intracellular replication. Front. Cell. Infect. Microbiol. 2019, 9, 42. [Google Scholar] [CrossRef]
- Munger, J.; Bennett, B.D.; Parikh, A.; Feng, X.J.; McArdle, J.; Rabitz, H.A.; Shenk, T.; Rabinowitz, J.D. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 2008, 26, 1179–1186. [Google Scholar] [CrossRef]
- Vastag, L.; Koyuncu, E.; Grady, S.L.; Shenk, T.E.; Rabinowitz, J.D. Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog. 2011, 7, e1002124. [Google Scholar] [CrossRef]
- Thai, M.; Graham, N.A.; Braas, D.; Nehil, M.; Komisopoulou, E.; Kurdistani, S.K.; McCormick, F.; Graeber, T.G.; Christfolk, H.R. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 2014, 19, 694–701. [Google Scholar] [CrossRef]
- Mayer, K.A.; Stockl, J.; Zlabinger, G.J.; Gualdoni, G.A. Hijacking the supplies: Metabolism as a novel facet of virus-host interaction. Front. Immunol. 2019, 10, 1533. [Google Scholar]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.R.; Sun, M.X.; Ni, B.; Huan, C.; Huang, L.; Li, C.; Fan, H.J.; Ren, X.F.; Mao, X. Triggering unfolded protein response by 2-Deoxy-D-glucose inhibits porcine epidemic diarrhea virus propagation. Antivir. Res. 2014, 106, 33–41. [Google Scholar] [CrossRef]
- Icard, P.; Lincet, H.; Wu, Z.; Coquerel, A.; Forgez, P.; Alifano, M.; Fournel, L. The key role of Warburg effect in SARS-CoV-2 replication and associated inflammatory response. Biochimie 2021, 180, 169–177. [Google Scholar] [CrossRef]
- Gibelini, L.; De Biasi., S.; Paolini, A.; Borella, R.; Boraldi, F.; Mattioli, M.; Lo Tartaro, D.; Fidanza, L.; Caro-Maldonado, A.; Quaglino, D.; et al. Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Mol. Med. 2020, 12, e13001. [Google Scholar] [CrossRef]
- Passalacqua, K.D.; Lu, J.; Goodfellow, I.; Kolawole, A.O.; Arche, J.R.; Maddox, R.J.; Carnahan, K.E.; O’Riordan, M.X.; Wobus, C.E. Glycolysis is an intrinsic factor for optimal replication of a norovirus. mBio 2019, 10, e02175-18. [Google Scholar] [CrossRef]
- Fontain, K.A.; Sanchez, E.L.; Camarda, R.; Lagunoff, M. Dengue virus induces and requires glycolysis for optimal replication. J. Virol. 2015, 89, 2358–2366. [Google Scholar] [CrossRef]
- Yu, Y.; Maguire, T.G.; Alwine, J.C. Human cytomegalovirus activates glucose transporter 4 expression to increase glucose uptake during infection. J. Virol. 2011, 85, 1573–1580. [Google Scholar] [CrossRef]
- Ramiere, C.; Rodriguez, J.; Enache, L.S.; Lotteau, V.; Andre, P.; Diaz, O. Activity of hexokinase is increased by its interaction with hepatitis C virus protein NS5A. J. Virol. 2014, 88, 3246–3254. [Google Scholar] [CrossRef]
- Abrantes, J.L.; Alves, C.M.; Costa, J.; Almeida, F.C.L.; Sola-Penna, M.; Fontes, C.F.L.; Souza, T.M.L. Herpes simplex type 1 activates glycolysis through engagement of the enzyme 6-phoshofructo-1-kinase (PFK-1). Biochim. Biophys. Acta 2012, 1822, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Manel, N.; Kim, F.J.; Kinet, S.; Taylor, N.; Sitbon, M.; Battini, J.L. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 2003, 115, 449–459. [Google Scholar] [CrossRef]
- Mansouri, K.; Rastegari-Pouyani, M.; Ghanbri-Movahed, M.; Safarzadeh, M.; Kiani, S.; Ghanbari-Movahed, Z. Can a metabolism-targeted therapeutic intervention successfully subjugate SARS-COV-2? A scientific rationale. Biomed. Pharm. 2020, 131, 110694. [Google Scholar]
- Kindrachuk, J.; Ork, B.; Hart, B.J.; Mazur, S.; Holbrook, M.R.; Frieman, M.B.; Traynor, D.; Johnson, R.F.; Dyall, J.; Kuhn, J.H.; et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agents Chemother. 2015, 59, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Ripoli, M.; D’Aprile, A.; Quarato, G.; Sarasin-Filipowicz, M.; Gouttenoire, J.; Scrima, R.; Cela, O.; Boffoli, D.; Heim, M.H.; Moradpour, D.; et al. Hepatitic C virus-linked mitochondrial dysfunction promotes hypoxia-inducible factor 1 alpha-mediated glycolytic adaptation. J. Virol. 2010, 84, 647–660. [Google Scholar] [CrossRef]
- Masson, J.J.; Billings, H.W.; Palmer, C.S. Metabolic reprogramming during hepatitis B disease progression offers novel diagnostic and therapeutic opportunities. Antivir. Chem. Chemother. 2017, 25, 53–57. [Google Scholar] [CrossRef]
- Wu, Y.H.; Yang, Y.; Chen, C.H.; Hsiao, C.J.; Li, T.N.; Liao, K.J.; Watashi, K.; Chen, B.S.; Wang, L.H.C. Aerobic glycolysis supports hepatitis B virus protein synthesis through interaction between viral surface antigen and pyruvate kinase isoform M2. PLoS Pathog. 2021, 17, e1008866. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y.; Kemper, T.; Chen, J.; Yuan, Z.; Liu, S.; Zhu, Y.; Broering, R.; Lu, M. AMPK and Akt/mTOR signalling pwathways participate in glucose-mediated regulation of the hepatitis B virus replication and cellular autophagy. Cell Microbiol. 2019, 22, e13131. [Google Scholar]
- Valle-Casuso, J.C.; Angin, M.; Volant, S.; Passaes, C.; Monceaux, V.; Mikhailova, A.; Bourdic, K.; Sitbon, M.; Lambotte, O.; Thoulouze, M.I.; et al. Cellular metabolism is a major determinant of HIV-1 reservoir seeding in CD4+ T cells and offers an opportunity to tackle infection. Cell Metab. 2019, 29, 611–626. [Google Scholar] [CrossRef]
- Palmer, C.S.; Ostrowski, M.; Gouillou, M.; Tsai, L.; Zhou, J.; Henstridge, D.C.; Maisa, A.; Hearps, A.C.; Lewin, S.R.; Landay, A.; et al. Increased glucose metabolic activity is associated with CD4+ T-cell activation and depletion during chronic HIV infection. AIDS 2014, 28, 297–309. [Google Scholar] [CrossRef]
- El-Bacha, T.; Menezes, M.M.; Azevedo e Silva, M.C.; Sola-Penna, M.; Da Poian, A.T. Mayaro virus infection alters glucose metabolism in cultured cells through activation of the enzyme 6-phosphofructo 1-kinase. Mol. Cell. Biochem. 2004, 266, 191–198. [Google Scholar] [PubMed]
- Guo, X.; Wu, S.; Li, N.; Lin, Q.; Liu, L.; Liang, H.; Huang, Z.; Fu, X. Accelerated metabolite levels of aerobic glycolysis and the pentose phosphate pathway are required for efficient replication of infectious spleen and kidney necrosis virus in Chinese perch brain cells. Biomolecules 2019, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.; Zhao, H.; Pettersson, U.; Bergstrom Lind, S. Time-resolved proteomics od adenovirus infected cells. PLoS ONE 2018, 13, e204522. [Google Scholar] [CrossRef]
- Prusinkiewicz, M.A.; Mymryk, J.S. Metabolic reprogramming of the host cell by human adenovirus infection. Viruses 2019, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.L.; Pulliam, T.H.; Dimaio, T.A.; Thalhofer, A.B.; Delgado, T.; Lagunoff, M. Glycolysis, glutaminolysis, and fatty acid synthesis are required for distinct stages of Kaposi’s sarcoma-associated herpesvirus lytic replication. J. Virol. 2017, 91, e02237-16. [Google Scholar] [CrossRef] [PubMed]
- Gualdoni, G.A.; Mayer, K.A.; Kapsch, A.M.; Kreuzberg, K.; Puck, A.; Kienzl, P.; Oberndorfer, F.; Fruwirth, K.; Winkler, S.; Blaas, D.; et al. Rhinovirus induces an anabolic reprogramming in host cells metabolism essential for viral replication. Proc. Natl. Acad. Sci. USA 2018, 115, E7158–E7165. [Google Scholar] [CrossRef]
- Ritter, J.B.; Wahl, A.S.; Freund, S.; Genzel, Y.; Reichl, U. Metabolic effects of influenza virus infection in cultured animal cells; intra- and extracellular metabolite profiling. BMC Syst. Biol. 2010, 4, 61. [Google Scholar]
- Martin-Vincente, M.; Gonzalez-Riano, C.; Barbas, C.; Jimenez-Sousa, M.A.; Brochado-Kith, O.; Resino, S.; Martinez, I. Metabolic changes during respiratory syncytial virus infection of epithelial cells. PLoS ONE 2020, 15, e0230844. [Google Scholar]
- Singh, S.; Singh, P.K.; Suhail, H.; Arumugaswami, V.; Pellett, P.E.; Giri, S.; Kumar, A. AMP-activated protein kinase restricts Zika virus replication in endothelial cells by potentiating innate antiviral responses and inhibiting glycolysis. J. Immunol. 2020, 204, 1810–1824. [Google Scholar] [CrossRef]
- Vigerust, D.J.; Shepherd, V.L. Virus glycosylation: Role in virulence and immune interactions. Trends Microbiol. 2007, 15, 211–218. [Google Scholar] [CrossRef]
- Mantlo, E.K.; Maruyama, J.; Manning, J.T.; Wanninger, T.G.; Huang, C.; Smith, J.N.; Petterson, M.; Paessler, S.; Komab, T. Machupo virus with mutations in the transmembrane domain and glycosylation sites of the glycoprotein is attenuated and immunogenic in animal models of bolivian hemorrhagic fever. J. Virol. 2022, 96, e0020922. [Google Scholar] [CrossRef] [PubMed]
- Pieren, M.; Galli, C.; Denzel, A.; Molinari, M. The use of calnexin and calreticulin by cellular and viral glycoproteins. J. Biol. Chem. 2005, 31, 28265–28271. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, D.; Wang, Y.; Su, W.; Liu, G.; Dong, W. The importance of glycans of viral and host proteins in enveloped virus infection. Front. Immunol. 2021, 21, 638573. [Google Scholar] [CrossRef]
- Koma, T.; Huang, C.; Coscia, A.; Hallam, S.; Manning, J.T.; Maruyama, J.; Walker, A.G.; Miller, M.; Smith, J.N.; Patterson, M.; et al. Glycoprotein N-linked glycans play a critical role in arenavirus pathogenicity. PLoS Pathog. 2021, 17, e1009356. [Google Scholar] [CrossRef]
- Kim, P.; Jang, Y.H.; Kwon, S.B.; Lee, C.M.; Han, G.; Seong, B.L. Glycosylation of hemagglutinin and neuraminidase of influenza virus as signature for ecological spillover and adaptation among influenza reservoirs. Viruses 2018, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, C.; Feyznezhad, R.; Cao, L.; Chan, K.W.; Liu, K.; Yang, W.; Zhang, H.; Yolitz, J.; Arthos, J.; Nadas, A.; et al. Signal peptide of HIV-1 envelope modulates glycosylation impacting exposure of V1V2 and other epitopes. PLoS Pathog. 2020, 16, e1009185. [Google Scholar]
- Sharma, V.; Freeze, H.H. Mannose efflux from the cells: A potential source of mannose in blood. J. Biol. Chem. 2011, 286, 10193–10200. [Google Scholar] [CrossRef]
- Yeom, S.J.; Kim, Y.S.; Lim, Y.R.; Jeong, K.W.; Lee, J.Y.; Kim, Y.; Oh, D.K. Molecular characterization of a novel thermostable mannose-6-phosphate isomerase from Thermus thermophilus. Biochimie 2011, 93, 1659–1667. [Google Scholar] [CrossRef]
- Navale, A.M.; Paranjape, A.N. Glucose transporters: Physiological and pathological roles. Biophys. Rev. 2016, 8, 59. [Google Scholar] [CrossRef]
- Cura, A.J.; Carruthers, A. Role of monosaccharide transport proteins in carbohydrate assimilation, distribution, metabolism, and homeostasis. Compr. Physiol. 2012, 2, 863–914. [Google Scholar]
- Pajak, B.; Siwiak, E.; Sołtyka, M.; Priebe, A.; Zieliński, R.; Fokt, I.; Ziemniak, M.; Jaśkiewicz, A.; Borowski, R.; Domoradzki, T.; et al. 2-deoxy-D-glucose and its analogs: From diagnostic to therapeutic agents. Int. J. Mol. Sci. 2020, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Berthe, A.; Zano, M.; Muller, C.; Foulquier, F.; Houdou, M.; Schulz, C.; Bost, F.; De Fay, E.; Mazerbourg, S.; Flament, S. Protein N-glycosylation alteration and glycolysis inhibition both contribute to the antiproliferative action of 2-deoxyglucose in breast cancer cells. Breast Cancer Res. Treat. 2018, 171, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Chang, Y.; Lu, W.; Sheng, X.; Wang, S.; Xu, H.; Ma, J. Regulation of autophagy by glycolysis in cancer. Cancer Manag. Res. 2020, 12, 13259–13271. [Google Scholar] [CrossRef] [PubMed]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Thorburn, A. Apoptosis and autophagy: Regulatory connections between two supposedly different processes. Apoptosis 2008, 13, 1–9. [Google Scholar] [CrossRef]
- Shutt, D.C.; O’Dorisio, S.M.; Aykin-Burns, N.; Spitz, D.R. 2-deoxy-D-glucose induces oxidative stress and cell killing in human neuroblastoma cells. Cancer Biol. Ther. 2010, 9, 853–861. [Google Scholar] [CrossRef]
- Adrestani, A.; Azizi, Z. Targeting glucose metabolism for treatment of COVID-19. Signal Transduct. Target. Ther. 2021, 6, 112. [Google Scholar]
- Bhatt, A.N.; Kumar, A.; Rai, Y.; Kumari, N.; Vedagiri, D.; Harshan, K.H.; Kumar, V.C.; Chandna, S. Glycolytic inhibitor 2-Deoxy-D-glucose attenuates SARS-CoV-2 multiplication in host cells and weakens the infective potential of progeny virions. Life Sci. 2022, 295, 120411. [Google Scholar] [CrossRef]
- Bojkova, D.; Klann, K.; Koch, B.; Widera, M.; Krause, M.; Ciesek, S.; Cinatl, J.; Munch, C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature 2020, 583, 469–472. [Google Scholar] [CrossRef]
- Codo, A.C.; Davanzo, G.G.; de Brito Monteiro, L.; de Souza, G.F.; Muraro, S.P.; Virgilio-da-Silva, J.V.; Prondoff, J.S.; Carregari, V.C.; Oliviera de Biagi Junior, C.A.; Crunfli, F.; et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020, 32, 437–446. [Google Scholar] [CrossRef]
- Maehama, T.; Patzelt, A.; Lengert, M.; Hutter, J.; Kanazawa, K.; Hausen, H.; Rosl, F. Selective down-regulation of human papillomavirus transcription by 2-deoxyglucose. Int. J. Cancer 1998, 76, 639–646. [Google Scholar] [CrossRef]
- Kang, H.T.; Ju, J.W.; Cho, J.W.; Hwang, E.S. Down-regulation of Sp1 activity through modulation of O-glycosylation by treatment with a low glucose mimetic, 2-deoxyglucose. J. Biol. Chem. 2003, 278, 51223–51231. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Huang, Y.; Song, S. Inhibiting the HPV16 oncogene-mediated glycolysis sensitizes human cervical carcinoma cells to 5-fluorouracil. OncoTargets Ther. 2019, 12, 6711–6720. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lasche, M.; Urban, H.; Gallwas, J.; Grundker, C. HPV and other microbiota; who’s good and who’s bad: Effects of the microbial environment on the development of cervical cancer—A non-systematic review. Cells 2021, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Belov, L.; Tapparel, C. Rhinoviruses and respiratory enteroviruses: Not as simple as ABC. Viruses 2016, 8, 16. [Google Scholar]
- Shawa, I.T. Hepatitis B and C viruses; Rodrigo, L., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Liang, T.J. Hepatitis B: The virus and disease. Hepatology 2009, 49, S13–S21. [Google Scholar] [CrossRef]
- Lin, S.C.; Chen, M.C.; Liu, S.; Callahan, V.M.; Bracci, N.R.; Lehman, C.W.; Dahal, B.; de la Fuente, C.; Lin, C.C.; Wang, T.T.; et al. Phloretin inhibits Zika virus infection by interfering with cellular glucose utilization. Int. J. Antimicrob. Agents 2019, 54, 80–84. [Google Scholar] [CrossRef]
- Pang, H.; Jiang, Y.; Li, J.; Wang, Y.; Nie, M.; Xiao, N.; Wang, S.; Song, Z.; Ji, F.; Chang, Y.; et al. Aberrant NAD+ metabolism underlines Zika virus-induced microcephaly. Nature Metab. 2021, 3, 11109–11124. [Google Scholar] [CrossRef]
- Subak-Sharpe, J.H.; Dargan, D.J. HSV molecular biology: General aspects of herpes simplex virus molecular biology. Virus Genes 1998, 16, 239–251. [Google Scholar] [CrossRef]
- WHO Herpes Simplex Virus Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus (accessed on 17 July 2022).
- Varanasi, S.K.; Donohoe, D.; Jaggi, U.; Rouse, B.T. Manipulating glucose metabolism during different stages of viral pathogenesis can have either detrimental or beneficial effects. J. Immunol. 2017, 199, 1748–1761. [Google Scholar] [CrossRef] [PubMed]
- Knowles, R.W.; Person, S. Effects of 2-deoxyglucose, glucosamine and mannose on cell fusion and the glycoprotein of herpes simplex virus. J. Virol. 1976, 18, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Kern, E.R.; Glasgow, L.A.; Klein, R.J.; Friedman-Kien, A.E. Failure of 2-deoxy-D-glucose in the treatment of experimental cutaneous and genital infections due to herpes simplex virus. J. Infect. Dis. 1982, 146, 159–166. [Google Scholar] [CrossRef]
- Shannon, W.M.; Arnett, G.; Drennen, D.J. Lack of efficacy of 2-deoxy-D-glucose in the treatment of experimental herpes genitalis in guinea pigs. Antimicrob. Agents Chemother. 1982, 21, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Diao, D.; Zhang, H.; Guo, Q.; Wu, X.; Song, Y.; Dang, C. High glucose-induced resistance to 5-fluorouracil in pancreatic cancer cells alleviated by 2-deoxy-d-glucose. Biomed. Rep. 2014, 2, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.L.; Levy, M.M.; Kerr, D.S. The 2-deoxyglucose test as a supplement to fasting for detection of childhood hypoglycemia. Pediatric Res. 1984, 18, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, A.Y.; Pienimaki, T.; Pitkala, K.H.; Tilvis, R.S.; Salomaa, V.V.; Strandberg, T.E. Comparison of normal fasting and one-hour glucose levels as predictors of future diabetes during a 34-year follow-up. Ann. Med. 2013, 45, 336–340. [Google Scholar] [CrossRef]
- Stein, M.; Lin, H.; Jeyamohan, C.; Dvorzhinski, D.; Gounder, M.; Bray, K.; Eddy, S.; Goodin, S.; White, E.; DiPaola, R.S. Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate 2010, 70, 1388–1394. [Google Scholar] [CrossRef]
- Burckhardt, D.; Stalder, G.A. Cardiac changes during 2-deoxy-D-glucose test. A study in patients with selective vagotomy and pyloroplasty. Digestion 1975, 12, 1–8. [Google Scholar] [CrossRef]
- Stalder, G.A.; Schultheiss, H.R.; Allgower, M. Use of 2-deoxy-D-glucose for testing completeness of vagotomy in man. Gastroenterology 1972, 63, 552–556. [Google Scholar] [CrossRef]
- Raez, L.E.; Papadopoulos, K.; Ricart, A.D.; Chiorean, E.G.; Dipaola, R.S.; Stein, M.N.; Rocha Lima, C.M.; Schlesselman, J.J.; Tolba, K.; Langmuir, V.K.; et al. A phase I dose-escalation trial of 2-deoxy-d-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2013, 71, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Priebe, W.; Zielinski, R.; Fokt, I.; Felix, E.; Radjendirane, V.; Arumugam, J.; TaiKhuong, M.; Krasinski, M.; Skora, S. EXTH-07. Design and evaluation of WP1122, in inhibitor of glycolysis with increased CNS uptake. Neuro-Oncology 2018, 20, vi86. [Google Scholar] [CrossRef][Green Version]
- Keith, M.; Zielinski, R.; Walker, C.M.; Le Roux, L.; Priebe, W.; Bankson, J.A.; Schellingerhout, D. Hyperpolarized pyruvate MR spectroscopy depicts glycolytic inhibition in a mouse model of glioma. Radiology 2019, 293, 168–173. [Google Scholar]
- WP1122 Phase 1a Study. Available online: https://moleculin.com/ongoing-phase-1a-study-in-covid-19/ (accessed on 26 July 2022).
- Lampidis, T.J.; Kurtoglu, M.; Maher, J.C.; Liu, H.; Krishan, A.; Sheft, V.; Szymanski, S.; Fokt, I.; Rudnicki, W.R.; Ginalski, K.; et al. Efficacy of 2-halogen substituted D-glucose analogs in blocking glycolysis and killing “hypoxic tumor cells”. Cancer Chemother. Pharmacol. 2006, 58, 725. [Google Scholar] [CrossRef]
- Ziemniak, M.; Zawadzka-Kazimierczuk, A.; Pawlędzio, S.; Malinska, M.; Sołtyka, M.; Trzybinski, D.; Kożmiński, W.; Skóra, S.; Zieliński, R.; Fokt, I.; et al. Experimental and computational studies on structure and energetic properties of halogen derivatives of 2-deoxy-D-glucose. Int. J. Mol. Sci. 2021, 22, 3720. [Google Scholar] [CrossRef]

| Virus | Mechanism of Glycolysis Upregulation | References |
|---|---|---|
| Murine Norovirus (MNV) | Upregulation of Akt signaling that stimulates glycolysis and glucose metabolism | [20] |
| SARS-CoV-2 | Upregulation of PI3K/Akt signaling and GLUT1 expression | [18,26] |
| MERS-CoV | PI3K/Akt and MAPK/ERK signaling pathways upregulation | [27] |
| Porcine epidemic diarrhea virus (PEDV) | Not specified | [17] |
| Dengue virus | GLUT1 and HK upregulation | [21] |
| Hepatitis C virus (HCV) | Upregulated HK activity via direct interaction with viral NS5A protein; downregulation of mitochondrial activity, and HIF-1 level upregulation | [23,28] |
| Hepatitis B virus (HBV) | Upregulated PI3K/Akt/mTOR pathway and GLUT1 expression; interaction with pyruvate kinase isoform M2 | [29,30,31] |
| Human immunodeficiency virus (HIV) | Upregulated GLUT1 expression | [32,33] |
| Herpes simplex type 1 virus (HSV-1) | Upregulated PFK-1 activity | [24] |
| Human cytomegalovirus (HCMV) | Upregulation of GLUT4 expression | [22] |
| Mayaro virus | Enhanced PFK-1 activity and fructose 2,6-biphosphate level | [34] |
| Infectious spleen and kidney necrosis virus (ISKNV) | Upregulation of glycolytic enzymes expression | [35] |
| Human T cell leukemia virus (HTLV) | GLUT1 transporter-mediated virus entry | [25] |
| Human adenovirus type 2 (Ad2) | Viral oncoprotein E4ORF6 upregulates glycolysis pathway proteins expression | [36,37] |
| Kaposi’s sarcoma-associated herpesvirus (KSHV) | Viral protein ORF45 regulates transcription of glycolysis proteins | [38] |
| Rhinovirus (RV) | Activation of PI3K/Akt pathway and GLUT 1 expression | [39] |
| Influenza A virus (AIV) | Increased glucose uptake, glycolytic enzymes activity, and lactate synthesis; detailed mechanism not described | [40] |
| Human respiratory syncytial virus (HRSV) | Diminished TCA activity and upregulated glycolysis | [41] |
| Zika virus (ZIKV) | Downregulation of AMPK, upregulation of GLUT1, HK, and other glycolytic genes | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pająk, B.; Zieliński, R.; Manning, J.T.; Matejin, S.; Paessler, S.; Fokt, I.; Emmett, M.R.; Priebe, W. The Antiviral Effects of 2-Deoxy-D-glucose (2-DG), a Dual D-Glucose and D-Mannose Mimetic, against SARS-CoV-2 and Other Highly Pathogenic Viruses. Molecules 2022, 27, 5928. https://doi.org/10.3390/molecules27185928
Pająk B, Zieliński R, Manning JT, Matejin S, Paessler S, Fokt I, Emmett MR, Priebe W. The Antiviral Effects of 2-Deoxy-D-glucose (2-DG), a Dual D-Glucose and D-Mannose Mimetic, against SARS-CoV-2 and Other Highly Pathogenic Viruses. Molecules. 2022; 27(18):5928. https://doi.org/10.3390/molecules27185928
Chicago/Turabian StylePająk, Beata, Rafał Zieliński, John Tyler Manning, Stanislava Matejin, Slobodan Paessler, Izabela Fokt, Mark R. Emmett, and Waldemar Priebe. 2022. "The Antiviral Effects of 2-Deoxy-D-glucose (2-DG), a Dual D-Glucose and D-Mannose Mimetic, against SARS-CoV-2 and Other Highly Pathogenic Viruses" Molecules 27, no. 18: 5928. https://doi.org/10.3390/molecules27185928
APA StylePająk, B., Zieliński, R., Manning, J. T., Matejin, S., Paessler, S., Fokt, I., Emmett, M. R., & Priebe, W. (2022). The Antiviral Effects of 2-Deoxy-D-glucose (2-DG), a Dual D-Glucose and D-Mannose Mimetic, against SARS-CoV-2 and Other Highly Pathogenic Viruses. Molecules, 27(18), 5928. https://doi.org/10.3390/molecules27185928







