Indane-1,3-Dione: From Synthetic Strategies to Applications
Abstract
1. Introduction
2. Chemical Modification of the Indane-1,3-Dione Core
2.1. Synthesis of Indane-1,3-Dione
2.2. Chemical Engineering around the Ketone Groups
2.2.1. Functionalization with Cyano Groups
2.2.2. Self-Condensation of Indane-1,3-Dione: The Bindone Adduct
2.2.3. Formation of bis-Thiazoles and bis-Thiazolidinone
2.3. Chemical Engineering around the Aromatic Groups
2.3.1. Polyaromatic Structures
2.3.2. Halogenated Indane-1,3-Diones
2.3.3. Introduction of Various Electron-Withdrawing Groups on Aromatic Ring
Nitration
2.3.4. Cyanation
2.3.5. Introduction of Alkoxy-Carbonyl Groups
2.4. Chemical Engineering around the Methylene Group
2.4.1. Knoevenagel Reaction
2.4.2. Oxidation Reaction
2.4.3. Halogenation
2.4.4. Cyanation
2.4.5. Nitration
3. Indane-1,3-Diones as Reagents for Various Chemical Transformations
3.1. Synthesis of Cyclophanes
3.2. Synthesis of Crown Ether Derivatives of Indane-1,3-Dione
3.3. Synthesis of Tetracycline Heterocyclic Analogues
3.4. Synthesis of Indane-1,3-Dione Derivatives via Tert-Butylisocyanide Insertion
3.5. Copper-Catalyzed Sulfonamidation of Benzylic C-H Bonds
3.6. Michael Addition on β-Substituted meso-Tetraphenylporphyrins
3.7. Synthesis of 1,4-Isochromandione
3.8. Synthesis of Benzofurans by Electrooxidation of Hydroquinone Derivatives
3.9. Combination of Knoevenagel Condensation and Michael Addition Reactions
3.10. Indane-1,3-Dione: Versatile Building Block for Spirocyclic Compounds Synthesis
3.10.1. Synthesis of Spiroindanediones by Cycloaddition
3.10.2. Synthesis of Spiro-Indane-1,3-Diones by Domino Reaction
3.10.3. Synthesis of Spiro-Indane-1,3-Diones by MCR
3.10.4. Synthesis of Spiro-Indane-1,3-Diones by Miscellaneous Way
4. Applications of Indane-1,3-Dione-Based Structures
4.1. Photopolymerization
4.2. Non-Linear Optical Properties
4.3. Fluorescent Chemosensors and Chemodosimeters
4.4. Solar Cells
5. Biological Applications
5.1. Indane-1,3-Dione as Antimicrobial Agent
5.2. Indane-1,3-Diones as Anticancer Agents
5.3. Indane-1,3-Dione as Building Block for Bioimaging Agents
5.4. Indane-1,3-Dione in Neurology Drugs
5.5. Indane-1,3-Dione as Anticoagulant Drugs
6. Conclusions-Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Patil, S.A.; Patil, R.; Patil, S.A. Recent Developments in Biological Activities of Indanones. Eur. J. Med. Chem. 2017, 138, 182–198. [Google Scholar] [CrossRef] [PubMed]
- Turek, M.; Szczęsna, D.; Koprowski, M.; Bałczewski, P. Synthesis of 1-Indanones with a Broad Range of Biological Activity. Beilstein J. Org. Chem. 2017, 13, 451–494. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.C.J.M.D.S. Arylidene Indanone Scaffold: Medicinal Chemistry and Structure–Activity Relationship View. RSC Adv. 2017, 7, 9357–9372. [Google Scholar] [CrossRef]
- Costanzo, P.; Cariati, L.; Desiderio, D.; Sgammato, R.; Lamberti, A.; Arcone, R.; Salerno, R.; Nardi, M.; Masullo, M.; Oliverio, M. Design, Synthesis, and Evaluation of Donepezil-Like Compounds as AChE and BACE-1 Inhibitors. ACS Med. Chem. Lett. 2016, 7, 470–475. [Google Scholar] [CrossRef]
- Vacca, J.P.; Dorsey, B.D.; Schleif, W.A.; Levin, R.B.; McDaniel, S.L.; Darke, P.L.; Zugay, J.; Quintero, J.C.; Blahy, O.M.; Roth, E. L-735,524: An Orally Bioavailable Human Immunodeficiency Virus Type 1 Protease Inhibitor. Proc. Natl. Acad. Sci. USA 1994, 91, 4096. [Google Scholar] [CrossRef]
- Luo, H.-F.; Zhang, L.-P.; Hu, C.-Q. Five Novel Oligostilbenes from the Roots of Caragana Sinica. Tetrahedron 2001, 57, 4849–4854. [Google Scholar] [CrossRef]
- Nagle, D.G.; Zhou, Y.-D.; Park, P.U.; Paul, V.J.; Rajbhandari, I.; Duncan, C.J.G.; Pasco, D.S. A New Indanone from the Marine Cyanobacterium Lyngbya Majuscula That Inhibits Hypoxia-Induced Activation of the VEGF Promoter in Hep3B Cells. J. Nat. Prod. 2000, 63, 1431–1433. [Google Scholar] [CrossRef]
- Kim, J.; Kim, I. Design and Synthesis of a Hybrid Framework of Indanone and Chromane: Total Synthesis of a Homoisoflavanoid, Brazilane. Org. Biomol. Chem. 2018, 16, 89–100. [Google Scholar] [CrossRef]
- Yang, Y.; Philips, D.; Pan, S. A Concise Synthesis of Paucifloral F and Related Indanone Analogues via Palladium-Catalyzed α-Arylation. J. Org. Chem. 2011, 76, 1902–1905. [Google Scholar] [CrossRef]
- Buckingham, J. Dictionary of Natural Products, Supplement 1, 1st ed.; Taylor & Francis: New York, NY, USA, 1994; ISBN 978-1-00-305992-9. [Google Scholar]
- Winzenberg, K.N.; Kemppinen, P.; Scholes, F.H.; Collis, G.E.; Shu, Y.; Birendra Singh, T.; Bilic, A.; Forsyth, C.M.; Watkins, S.E. Indan-1,3-Dione Electron-Acceptor Small Molecules for Solution-Processable Solar Cells: A Structure–Property Correlation. Chem. Commun. 2013, 49, 6307–6309. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Bui, T.-T.; Péralta, S.; Gigmes, D.; Nechab, M.; Dumur, F. Push-Pull Chromophores Based on the Naphthalene Scaffold: Potential Candidates for Optoelectronic Applications. Materials 2019, 12, 1342. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Giles, D.; Basavarajaswamy, G.; Sreedhar, C.; Patel, A. Synthesis, Pharmacological Evaluation and Molecular Docking Studies of Indanone Derivatives. Med. Chem. Res. 2012, 21, 4403–4411. [Google Scholar] [CrossRef]
- Karthik, R.; Jasmin Sajni, R.; Sasikumar, S.; Kalyan, S.B.; Christina, A.J.M.; Jagan, A.; Sundara Saravanan, K. Evaluation of Anti-Tubercular Activity of Some Synthesised Benz Spiro-Oxirane Derivatives of Indane-1,3-Dione. Pharmacologyonline 2008, 2, 176–191. [Google Scholar]
- Liu, J.; Hu, K.-F.; Qu, J.-P.; Kang, Y.-B. Organopromoted Selectivity-Switchable Synthesis of Polyketones. Org. Lett. 2017, 19, 5593–5596. [Google Scholar] [CrossRef]
- Mardani, H.R.; Golchoubian, H. Selective and Efficient C-H Oxidation of Alkanes with Hydrogen Peroxide Catalyzed by a Manganese(III) Schiff Base Complex. J. Mol. Catal. Chem. 2006, 259, 197–200. [Google Scholar] [CrossRef]
- Delaval, N.; Bouquillon, S.; Hénin, F.; Muzart, J. Use of Benzotrifluoride as Solvent for Chromium-Catalysed Oxidations with Sodium Percarbonate. J. Chem. Res. Synop. 1999, 4, 286–287. [Google Scholar] [CrossRef]
- He, G.; Wu, C.; Zhou, J.; Yang, Q.; Zhang, C.; Zhou, Y.; Zhang, H.; Liu, H. A Method for Synthesis of 3-Hydroxy-1-Indanones via Cu-Catalyzed Intramolecular Annulation Reactions. J. Org. Chem. 2018, 83, 13356–13362. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, P.; Joshi, P.; Rawat, D.S. IBX-TfOH Mediated Oxidation of Alcohols to Aldehydes and Ketones under Mild Reaction Conditions. Tetrahedron Lett. 2020, 61, 151749. [Google Scholar] [CrossRef]
- Marvi, O.; Giahi, M. Montmorillonite KSF Clay as Novel and Recyclable Heterogeneous Catalyst for the Microwave Mediated Synthesis of Indan-1,3-Diones. Bull. Korean Chem. Soc. 2009, 30, 2918–2920. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, N.; Tang, X.; Mao, Z.; Zhang, X.; Yan, M.; Xuan, Y. Cyclopropanation of Active Methylene Compounds with β-Alkoxycarbonyl Vinylsulfonium Salts. Chin. Chem. Lett. 2019, 30, 406–408. [Google Scholar] [CrossRef]
- Berezina, G.R.; Vorob’ev, Y.G.; Mukhanova, O.N. Macroheterocyclic Compounds Based on Nitro- and Chloro-1,3-Indandiones. Russ. J. Gen. Chem. 2005, 75, 1594–1598. [Google Scholar] [CrossRef]
- Li, S.; Ye, L.; Zhao, W.; Zhang, S.; Mukherjee, S.; Ade, H.; Hou, J. Energy-Level Modulation of Small-Molecule Electron Acceptors to Achieve over 12% Efficiency in Polymer Solar Cells. Adv. Mater. 2016, 28, 9423–9429. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ren, H.; Yu, J.; Wang, Z.; Qian, G. An Indanone-Based Alkoxysilane Dye with Second Order Nonlinear Optical Properties. Dyes Pigments 2009, 81, 53–57. [Google Scholar] [CrossRef]
- Abdelrazek, F.M.; Metz, P.; Jaeger, A. Some Reactions with Indane-1,3-Dione: A Facile Synthesis of Pentacycline Heterocyclic Analogues. J. Heterocycl. Chem. 2019, 56, 1939–1945. [Google Scholar] [CrossRef]
- Tirelli, N.; Amabile, S.; Cellai, C.; Pucci, A.; Regoli, L.; Ruggeri, G.; Ciardelli, F. New Terthiophene Derivatives for Ultrahigh Molecular Weight Polyethylene-Based Absorption Polarizers. Macromolecules 2001, 34, 2129–2137. [Google Scholar] [CrossRef]
- Shang, Y.; Wen, Y.; Li, S.; Du, S.; He, X.; Cai, L.; Li, Y.; Yang, L.; Gao, H.; Song, Y. A Triphenylamine-Containing Donor−Acceptor Molecule for Stable, Reversible, Ultrahigh Density Data Storage. J. Am. Chem. Soc. 2007, 129, 11674–11675. [Google Scholar] [CrossRef]
- Planells, M.; Robertson, N. Naphthyl Derivatives Functionalised with Electron Acceptor Units–Synthesis, Electronic Characterisation and DFT Calculations. Eur. J. Org. Chem. 2012, 2012, 4947–4953. [Google Scholar] [CrossRef]
- Yang, X.; Fox, T.; Berke, H. Synthetic and Mechanistic Studies of Metal-Free Transfer Hydrogenations Applying Polarized Olefins as Hydrogen Acceptors and Amine Borane Adducts as Hydrogen Donors. Org. Biomol. Chem. 2012, 10, 852–860. [Google Scholar] [CrossRef]
- Morales, A.R.; Frazer, A.; Woodward, A.W.; Ahn-White, H.-Y.; Fonari, A.; Tongwa, P.; Timofeeva, T.; Belfield, K.D. Design, Synthesis, and Structural and Spectroscopic Studies of Push–Pull Two-Photon Absorbing Chromophores with Acceptor Groups of Varying Strength. J. Org. Chem. 2013, 78, 1014–1025. [Google Scholar] [CrossRef]
- Matsui, M.; Tanaka, N.; Funabiki, K.; Haishima, Y.; Manseki, K.; Jin, J.; Inoue, Y.; Higashijima, S.; Kubota, Y. Application of Indoline Dyes Attached with Strongly Electron-Withdrawing Carboxylated Indan-1,3-Dione Analogues Linked with a Hexylthiophene Ring to Dye-Sensitized Solar Cells. Tetrahedron 2018, 74, 3498–3506. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, F.; Zhang, Q.; Lau, T.-K.; Li, T.; Liu, K.; Ling, Q.; Wang, C.; Lu, X.; You, W.; et al. Fused Nonacyclic Electron Acceptors for Efficient Polymer Solar Cells. J. Am. Chem. Soc. 2017, 139, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, S.; Liu, X.; Yao, H.; Wang, F.; Zhang, S.; Sun, M.; Hou, J. Subtle Side-Chain Tuning on Terminal Groups of Small Molecule Electron Acceptors for Efficient Fullerene-Free Polymer Solar Cells. J. Mater. Chem. A 2017, 5, 15175–15182. [Google Scholar] [CrossRef]
- Lai, H.; Chen, H.; Zhou, J.; Qu, J.; Wang, M.; Xie, W.; Xie, Z.; He, F. 3D Interpenetrating Network for High-Performance Nonfullerene Acceptors via Asymmetric Chlorine Substitution. J. Phys. Chem. Lett. 2019, 10, 4737–4743. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, T.J.; Matta, M.; Zhu, W.; Swick, S.M.; Stern, C.L.; Schatz, G.C.; Facchetti, A.; Melkonyan, F.S.; Marks, T.J. Fluorination Effects on Indacenodithienothiophene Acceptor Packing and Electronic Structure, End-Group Redistribution, and Solar Cell Photovoltaic Response. J. Am. Chem. Soc. 2019, 141, 3274–3287. [Google Scholar] [CrossRef]
- Swick, S.M.; Zhu, W.; Matta, M.; Aldrich, T.J.; Harbuzaru, A.; Lopez Navarrete, J.T.; Ponce Ortiz, R.; Kohlstedt, K.L.; Schatz, G.C.; Facchetti, A.; et al. Closely Packed, Low Reorganization Energy π-Extended Postfullerene Acceptors for Efficient Polymer Solar Cells. Proc. Natl. Acad. Sci. USA 2018, 115, E8341. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, C.; Yao, H.; Zhu, J.; Wang, Y.; Jia, G.; Gao, F.; Hou, J. Efficient Semitransparent Organic Solar Cells with Tunable Color Enabled by an Ultralow-Bandgap Nonfullerene Acceptor. Adv. Mater. 2017, 29, 1703080. [Google Scholar] [CrossRef]
- Yao, H.; Cui, Y.; Yu, R.; Gao, B.; Zhang, H.; Hou, J. Design, Synthesis, and Photovoltaic Characterization of a Small Molecular Acceptor with an Ultra-Narrow Band Gap. Angew. Chem. Int. Ed. 2017, 56, 3045–3049. [Google Scholar] [CrossRef]
- Bürckstümmer, H.; Tulyakova, E.V.; Deppisch, M.; Lenze, M.R.; Kronenberg, N.M.; Gsänger, M.; Stolte, M.; Meerholz, K.; Würthner, F. Efficient Solution-Processed Bulk Heterojunction Solar Cells by Antiparallel Supramolecular Arrangement of Dipolar Donor–Acceptor Dyes. Angew. Chem. Int. Ed. 2011, 50, 11628–11632. [Google Scholar] [CrossRef]
- Wilbuer, J.; Schnakenburg, G.; Esser, B. Syntheses, Structures and Optoelectronic Properties of Spiroconjugated Cyclic Ketones. Eur. J. Org. Chem. 2016, 2016, 2404–2412. [Google Scholar] [CrossRef]
- Sloop, J.C.; Boyle, P.D.; Fountain, A.W.; Gomez, C.; Jackson, J.L.; Pearman, W.F.; Schmidt, R.D.; Weyand, J. Novel Fluorinated Indanone, Tetralone and Naphthone Derivatives: Synthesis and Unique Structural Features. Appl. Sci. 2012, 2, 61–99. [Google Scholar] [CrossRef]
- Nikulin, V.I.; Lugovskaya, N.Y.; Sveshnikov, N.N.; Pisarenko, L.M.; D’yachkovskaya, R.F. Synthesis and Antitumor Activity of 2-Arylindane-1,3-Dione Derivatives with an Alkylating Fragment. Pharm. Chem. J. 1994, 28, 146–149. [Google Scholar] [CrossRef]
- Abdel-Latif, F.F.; Mashaly, M.M.; El-Gawish, E.H. ChemInform Abstract: Synthesis of Heterocycles Through Reactions of Nucleophiles with Acrylonitriles. Part 15. Synthesis of Some New Functionalized Benzo(b) Pyrans and Indeno(1,2-b)Pyrans of Potential Biological Activity. ChemInform 1995, 26, 42. [Google Scholar] [CrossRef]
- Becker, H.-D. 2-[p-(Phenylsulfonyl) Phenyl]-1, 3-Indanedione and Its Tautomer. U.S. Patent 3,356,732, 25 December 1967. [Google Scholar]
- Liu, Y.; Saldivar, A.; Bess, J.; Solomon, L.; Chen, C.-M.; Tripathi, R.; Barrett, L.; Richardson, P.L.; Molla, A.; Kohlbrenner, W.; et al. Investigating the Origin of the Slow-Binding Inhibition of HCV NS3 Serine Protease by a Novel Substrate Based Inhibitor. Biochemistry 2003, 42, 8862–8869. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Ahuja, P.; Ahsan, W.; Pandeya, S.N.; Shamsher Alam, M. Thiadiazoles: Progress Report on Biological Activities. J. Chem. Pharm. Res. 2009, 1, 19–30. [Google Scholar]
- Salgın-Gökşen, U.; Gökhan-Kelekçi, N.; Göktaş, Ö.; Köysal, Y.; Kılıç, E.; Işık, Ş.; Aktay, G.; Özalp, M. 1-Acylthiosemicarbazides, 1,2,4-Triazole-5(4H)-Thiones, 1,3,4-Thiadiazoles and Hydrazones Containing 5-Methyl-2-Benzoxazolinones: Synthesis, Analgesic-Anti-Inflammatory and Antimicrobial Activities. Bioorg. Med. Chem. 2007, 15, 5738–5751. [Google Scholar] [CrossRef] [PubMed]
- Schenone, S.; Brullo, C.; Bruno, O.; Bondavalli, F.; Ranise, A.; Filippelli, W.; Rinaldi, B.; Capuano, A.; Falcone, G. New 1,3,4-Thiadiazole Derivatives Endowed with Analgesic and Anti-Inflammatory Activities. Bioorg. Med. Chem. 2006, 14, 1698–1705. [Google Scholar] [CrossRef]
- Wei, M.-X.; Feng, L.; Li, X.-Q.; Zhou, X.-Z.; Shao, Z.-H. Synthesis of New Chiral 2,5-Disubstituted 1,3,4-Thiadiazoles Possessing γ-Butenolide Moiety and Preliminary Evaluation of in Vitro Anticancer Activity. Eur. J. Med. Chem. 2009, 44, 3340–3344. [Google Scholar] [CrossRef]
- Mavrova, A.T.; Wesselinova, D.; Tsenov, Y.A.; Denkova, P. Synthesis, Cytotoxicity and Effects of Some 1,2,4-Triazole and 1,3,4-Thiadiazole Derivatives on Immunocompetent Cells. Eur. J. Med. Chem. 2009, 44, 63–69. [Google Scholar] [CrossRef]
- Siddiqui, N.; Ali, S.; Khan, S.; Drabu, S.; Rana, A.; Alam, M. Synthesis of 3-Arylamino-4-Aryl-5-(N-Arylthiocarbonylimino)-4, 5-Dihydro-1, 2, 4-Thiadiazoles as Anticonvulsant Agents. Indian J. Heterocycl. Chem. 2004, 14, 159–160. [Google Scholar]
- Kuş, C.; Ayhan-Kılcıgil, G.; Özbey, S.; Kaynak, F.B.; Kaya, M.; Çoban, T.; Can-Eke, B. Synthesis and Antioxidant Properties of Novel N-Methyl-1,3,4-Thiadiazol-2-Amine and 4-Methyl-2H-1,2,4-Triazole-3(4H)-Thione Derivatives of Benzimidazole Class. Bioorg. Med. Chem. 2008, 16, 4294–4303. [Google Scholar] [CrossRef]
- Cressier, D.; Prouillac, C.; Hernandez, P.; Amourette, C.; Diserbo, M.; Lion, C.; Rima, G. Synthesis, Antioxidant Properties and Radioprotective Effects of New Benzothiazoles and Thiadiazoles. Bioorg. Med. Chem. 2009, 17, 5275–5284. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Misra, G.P.; Sainy, J.; Chaturvedi, S.C. Synthesis and Biological Evaluation of 2-Amino-5-Sulfanyl-1,3,4-Thiadiazole Derivatives as Antidepressant, Anxiolytics and Anticonvulsant Agents. Med. Chem. Res. 2011, 20, 245–253. [Google Scholar] [CrossRef]
- Muhammad, Z.A.; Masaret, G.S.; Amin, M.M.; Abdallah, M.A.; Farghaly, T.A. Anti-Inflammatory, Analgesic and Anti-Ulcerogenic Activities of Novel Bis-Thiadiazoles, Bis-Thiazoles and Bis-Formazanes. Med. Chem. 2017, 13, 226–238. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Peralta, S.; Duval, S.; Nechab, M.; Gigmes, D.; Dumur, F. Unprecedented Nucleophilic Attack of Piperidine on the Electron Acceptor during the Synthesis of Push-Pull Dyes by a Knoevenagel Reaction. Helv. Chim. Acta 2019, 102, e1900229. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Peralta, S.; Duval, S.; Bui, T.-T.; Aubert, P.-H.; Nechab, M.; Gigmes, D.; Dumur, F. New Push-Pull Dyes Based on 2-(3-Oxo-2,3-Dihydro-1H-Cyclopenta[b]Naphthalen-1-Ylidene)Malononitrile: An Amine-Directed Synthesis. Dyes Pigments 2020, 175, 108182. [Google Scholar] [CrossRef]
- Feng, H.; Qiu, N.; Wang, X.; Wang, Y.; Kan, B.; Wan, X.; Zhang, M.; Xia, A.; Li, C.; Liu, F.; et al. An A-D-A Type Small-Molecule Electron Acceptor with End-Extended Conjugation for High Performance Organic Solar Cells. Chem. Mater. 2017, 29, 7908–7917. [Google Scholar] [CrossRef]
- Li, R.; Liu, G.; Xiao, M.; Yang, X.; Liu, X.; Wang, Z.; Ying, L.; Huang, F.; Cao, Y. Non-Fullerene Acceptors Based on Fused-Ring Oligomers for Efficient Polymer Solar Cells via Complementary Light-Absorption. J. Mater. Chem. A 2017, 5, 23926–23936. [Google Scholar] [CrossRef]
- Sanguinet, L.; Williams, J.C.; Yang, Z.; Twieg, R.J.; Mao, G.; Singer, K.D.; Wiggers, G.; Petschek, R.G. Synthesis and Characterization of New Truxenones for Nonlinear Optical Applications. Chem. Mater. 2006, 18, 4259–4269. [Google Scholar] [CrossRef]
- Knoevenagel, E. Ueber Eine Darstellungsweise Des Benzylidenacetessigesters. Berichte Dtsch. Chem. Ges. 1896, 29, 172–174. [Google Scholar] [CrossRef]
- Landmesser, T.; Linden, A.; Hansen, H.-J. A Novel Route to 1-Substituted 3-(Dialkylamino)-9-Oxo-9H-Indeno[2,1-c]Pyridine-4-Carbonitriles. Helv. Chim. Acta 2008, 91, 265–284. [Google Scholar] [CrossRef]
- Helmy, S.; Oh, S.; Leibfarth, F.A.; Hawker, C.J.; Read de Alaniz, J. Design and Synthesis of Donor–Acceptor Stenhouse Adducts: A Visible Light Photoswitch Derived from Furfural. J. Org. Chem. 2014, 79, 11316–11329. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Su, H.; Lin, Y.; Ling, X.; Li, S.; Qin, A.; Tang, B.Z. Photoactivatable Aggregation-Induced Emission Probes for Lipid Droplets-Specific Live Cell Imaging. Chem. Sci. 2017, 8, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, A.; Esposito, A.; Caruso, T.; Borbone, F.; Carella, A.; Centore, R.; Peluso, A. Tuning Wavefunction Mixing in Push–Pull Molecules: From Neutral to Zwitterionic Compounds. Eur. J. Org. Chem. 2012, 2012, 2980–2989. [Google Scholar] [CrossRef]
- Bello, K.A.; Cheng, L.; Griffiths, J. Near-Infrared Absorbing Methine Dyes Based on Dicyanovinyl Derivatives of Indane-1,3-Dione. J. Chem. Soc. Perkin Trans. 1987, 2, 815–818. [Google Scholar] [CrossRef]
- Singh, A.; Lim, C.-K.; Lee, Y.-D.; Maeng, J.; Lee, S.; Koh, J.; Kim, S. Tuning Solid-State Fluorescence to the Near-Infrared: A Combinatorial Approach to Discovering Molecular Nanoprobes for Biomedical Imaging. ACS Appl. Mater. Interfaces 2013, 5, 8881–8888. [Google Scholar] [CrossRef] [PubMed]
- Zilinskaite, V.; Gudeika, D.; Grazulevicius, J.V.; Hladka, I. Synthesis and Cationic Polymerization of Oxyranyl-Functionalized Indandiones. Polym. Bull. 2016, 1, 229–239. [Google Scholar] [CrossRef]
- Gudeika, D.; Zilinskaite, V.; Grazulevicius, J.V.; Lytvyn, R.; Rutkis, M.; Tokmakov, A. 4-(Diethylamino)Salicylaldehyde-Based Twin Compounds as NLO-Active Materials. Dyes Pigments 2016, 134, 244–250. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Lashgari, N.; Azimian, F.; Kruger, H.G.; Gholamzadeh, P. Ninhydrin in Synthesis of Heterocyclic Compounds. ARKIVOC 2015, 2015, 1–139. [Google Scholar] [CrossRef]
- Marminon, C.; Nacereddine, A.; Bouaziz, Z.; Nebois, P.; Jose, J.; Le Borgne, M. Microwave-Assisted Oxidation of Indan-1-Ones into Ninhydrins. Tetrahedron Lett. 2015, 56, 1840–1842. [Google Scholar] [CrossRef]
- Jong, J.A.W.; Moret, M.-E.; Verhaar, M.C.; Hennink, W.E.; Gerritsen, K.G.F.; van Nostrum, C.F. Effect of Substituents on the Reactivity of Ninhydrin with Urea. ChemistrySelect 2018, 3, 1224–1229. [Google Scholar] [CrossRef]
- Taherpour, A.; Kamal, S. 1,2,3-Trione Compounds Synthesis by Oxidation 1,3-Diketones. Asian J. Chem. 2007, 19, 4107–4109. [Google Scholar]
- Prakash, O.; Sharma, P.K.; Saini, N. ChemInform Abstract: A New Synthesis of Ninhydrin and Its Ketals Using Iodobenzene Diacetate. ChemInform 1995, 26, 39. [Google Scholar] [CrossRef]
- Tatsugi, J.; Izawa, Y. A Facile One-Pot Synthesis of Vicinal Di- and Tri-Ketones from α-Methylene Ketones by N-Bromosuccinimide-Dimethyl Sulphoxide Oxidation. J. Chem. Res. Synop. Print 1988, 11, 356–357. [Google Scholar]
- Vickery, B.; Kaberia, F. Reactions of Sodium Hypochlorite with Some Compounds Having Reactive Methylene Groups. Experientia 1979, 35, 299. [Google Scholar] [CrossRef]
- Wasserman, H.H.; Pickett, J.E. The Fluoride Ion Effect in the Reactions of Singlet Oxygen with Enols. Tetrahedron 1985, 41, 2155–2162. [Google Scholar] [CrossRef]
- Gao, S.; Bethel, T.K.; Kakeshpour, T.; Hubbell, G.E.; Jackson, J.E.; Tepe, J.J. Substrate Controlled Regioselective Bromination of Acylated Pyrroles Using Tetrabutylammonium Tribromide (TBABr3). J. Org. Chem. 2018, 83, 9250–9255. [Google Scholar] [CrossRef]
- Wengryniuk, S.E.; Weickgenannt, A.; Reiher, C.; Strotman, N.A.; Chen, K.; Eastgate, M.D.; Baran, P.S. Regioselective Bromination of Fused Heterocyclic N-Oxides. Org. Lett. 2013, 15, 792–795. [Google Scholar] [CrossRef]
- Pathak, T.P.; Miller, S.J. Site-Selective Bromination of Vancomycin. J. Am. Chem. Soc. 2012, 134, 6120–6123. [Google Scholar] [CrossRef]
- Shi, X.; Dai, L. Mild Halogenation of Stabilized Ester Enolates by Cupric Halides. J. Org. Chem. 1993, 58, 4596–4598. [Google Scholar] [CrossRef]
- Yang, D.; Yan, Y.-L.; Lui, B. Mild α-Halogenation Reactions of 1,3-Dicarbonyl Compounds Catalyzed by Lewis Acids. J. Org. Chem. 2002, 67, 7429–7431. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T.; Nishida, Y.; Satsumabayashi, K.; Horaguchi, T. A Mild and Efficient Procedure for α-Bromination of Ketones Using N-Bromosuccinimide Catalysed by Ammonium Acetate. Chem. Commun. 2004, 4, 470–471. [Google Scholar] [CrossRef]
- Arbuj, S.S.; Waghmode, S.B.; Ramaswamy, A.V. Photochemical α-Bromination of Ketones Using N-Bromosuccinimide: A Simple, Mild and Efficient Method. Tetrahedron Lett. 2007, 48, 1411–1415. [Google Scholar] [CrossRef]
- Wang, G.-W.; Gao, J. Solvent-Free Bromination Reactions with Sodium Bromide and Oxone Promoted by Mechanical Milling. Green Chem. 2012, 14, 1125–1131. [Google Scholar] [CrossRef]
- Meshram, H.M.; Reddy, P.N.; Vishnu, P.; Sadashiv, K.; Yadav, J.S. A Green Approach for Efficient α-Halogenation of β-Dicarbonyl Compounds and Cyclic Ketones Using N-Halosuccinimides in Ionic Liquids. Tetrahedron Lett. 2006, 47, 991–995. [Google Scholar] [CrossRef]
- Zou, L.-H.; Li, Y.-C.; Li, P.-G.; Zhou, J.; Wu, Z. Solvent-Controlled α-Monobromination, α,α-Dibromination or Imidation of 1,3-Diketones with N-Bromosuccinimide. Eur. J. Org. Chem. 2018, 2018, 5639–5643. [Google Scholar] [CrossRef]
- Saikia, I.; Borah, A.J.; Phukan, P. Use of Bromine and Bromo-Organic Compounds in Organic Synthesis. Chem. Rev. 2016, 116, 6837–7042. [Google Scholar] [CrossRef]
- Luo, L.; Meng, L.; Sun, Q.; Ge, Z.; Li, R. Novel Synthesis of Thiazolo/Thienoazepine-5,8-Diones from Dihalo Cyclic 1,3-Diketones and Mercaptonitrile Salts. RSC Adv. 2014, 4, 6845–6849. [Google Scholar] [CrossRef]
- Mishra, A.K.; Nagarajaiah, H.; Moorthy, J.N. Trihaloisocyanuric Acids as Atom-Economic Reagents for Halogenation of Aromatics and Carbonyl Compounds in the Solid State by Ball Milling. Eur. J. Org. Chem. 2015, 2015, 2733–2738. [Google Scholar] [CrossRef]
- Macharla, A.K.; Chozhiyath Nappunni, R.; Marri, M.R.; Peraka, S.; Nama, N. Oxidative Bromination of Ketones Using Ammonium Bromide and Oxone®. Tetrahedron Lett. 2012, 53, 191–195. [Google Scholar] [CrossRef]
- Kim, J.-J.; Kweon, D.-H.; Cho, S.-D.; Kim, H.-K.; Lee, S.-G.; Yoon, Y.-J. Conversion of Nucleophilic Halides to Electrophilic Halides: Efficient and Selective Halogenation of Azinones, Amides, and Carbonyl Compounds Using Metal Halide/Lead Tetraacetate. Synlett 2006, 2006, 194–200. [Google Scholar] [CrossRef]
- Košmrlj, J.; Kočevar, M.; Polanc, S. A New Convenient Bromination with KBrO3/KBr/Dowex®. Synth. Commun. 1996, 26, 3583–3592. [Google Scholar] [CrossRef]
- Spitulnik, M.J. Synthesis of 1-Aryl-4-Halo-2-Pyrazolin-5-Ones by Ascorbic Acid Reduction of 1-Aryl-4,4-Dihalo-2-Pyrazolin-5-Ones. Synthesis 2002, 1985, 299–300. [Google Scholar] [CrossRef]
- Stavber, G.; Zupan, M.; Jereb, M.; Stavber, S. Selective and Effective Fluorination of Organic Compounds in Water Using Selectfluor F-TEDA-BF4. Org. Lett. 2004, 6, 4973–4976. [Google Scholar] [CrossRef]
- Matarlo, J.S.; Evans, C.E.; Sharma, I.; Lavaud, L.J.; Ngo, S.C.; Shek, R.; Rajashankar, K.R.; French, J.B.; Tan, D.S.; Tonge, P.J. Mechanism of MenE Inhibition by Acyl-Adenylate Analogues and Discovery of Novel Antibacterial Agents. Biochemistry 2015, 54, 6514–6524. [Google Scholar] [CrossRef] [PubMed]
- Sloop, J.C.; Churley, M.; Guzman, A.; Moseley, S.; Stalker, S.; Weyand, J.; Yi, J. Synthesis and Reactivity of Fluorinated Cyclic Ketones: Initial Findings. Am. J. Org. Chem. 2014, 4, 1–10. [Google Scholar]
- Nyffeler, P.T.; Durón, S.G.; Burkart, M.D.; Vincent, S.P.; Wong, C.-H. Selectfluor: Mechanistic Insight and Applications. Angew. Chem. Int. Ed. 2005, 44, 192–212. [Google Scholar] [CrossRef]
- Yi, H.; Zhang, G.; Wang, H.; Huang, Z.; Wang, J.; Singh, A.K.; Lei, A. Recent Advances in Radical C-H Activation/Radical Cross-Coupling. Chem. Rev. 2017, 117, 9016–9085. [Google Scholar] [CrossRef]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef]
- Wang, C.-S.; Dixneuf, P.H.; Soulé, J.-F. Photoredox Catalysis for Building C-C Bonds from C(Sp2)–H Bonds. Chem. Rev. 2018, 118, 7532–7585. [Google Scholar] [CrossRef]
- Li, A.Y.; Moores, A. Carbonyl Reduction and Biomass: A Case Study of Sustainable Catalysis. ACS Sustain. Chem. Eng. 2019, 7, 10182–10197. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, R.; Wang, Y.; Shi, Z.; Lu, T.; Feng, J. Visible-Light-Promoted α,α-Dibromination in Minutes: Efficient Route for Construction of Quaternary Carbon Centers. ACS Sustain. Chem. Eng. 2019, 7, 18542–18546. [Google Scholar] [CrossRef]
- Buckle, D.R.; Cantello, B.C.C.; Smith, H.; Spicer, B.A. 2-Cyano-1,3-Dicarbonyl Compounds with Antiallergic Activity. J. Med. Chem. 1977, 20, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Paquette, L.A.; Crich, D.; Fuchs, P.L.; Molander, G.A. Encyclopedia of Reagents for Organic Synthesis; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The Influence of Natural Products upon Drug Discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Fürstner, A. Cover Picture: Total Syntheses and Biological Assessment of Macrocyclic Glycolipids (Eur. J. Org. Chem. 5/2004). Eur. J. Org. Chem. 2004, 2004, 933. [Google Scholar] [CrossRef]
- Layton, M.E.; Morales, C.A.; Shair, M.D. Biomimetic Synthesis of (−)-Longithorone A. J. Am. Chem. Soc. 2002, 124, 773–775. [Google Scholar] [CrossRef]
- Yoshinari, T.; Ohmori, K.; Schrems, M.G.; Pfaltz, A.; Suzuki, K. Total Synthesis and Absolute Configuration of Macrocidin A, a Cyclophane Tetramic Acid Natural Product. Angew. Chem. Int. Ed. 2010, 49, 881–885. [Google Scholar] [CrossRef]
- Huang, M.; Song, L.; Liu, B. Construction of the Cyclophane Core of the Hirsutellones via a RCM Strategy. Org. Lett. 2010, 12, 2504–2507. [Google Scholar] [CrossRef]
- Diederich, F.; Lutter, H.D. Catalytic Cyclophanes. 4. Supramolecular Catalysis of Benzoin Condensations by a Thiazolium Cyclophane. J. Am. Chem. Soc. 1989, 111, 8438–8446. [Google Scholar] [CrossRef]
- Driggers, E.M.; Hale, S.P.; Lee, J.; Terrett, N.K. The Exploration of Macrocycles for Drug Discovery—An Underexploited Structural Class. Nat. Rev. Drug Discov. 2008, 7, 608–624. [Google Scholar] [CrossRef]
- Rajakumar, P.; Mohammed Abdul Rasheed, A.; Iman Rabia, A.; Chamundeeswari, D. Synthesis and Study of Anti-Inflammatory Activity of Some Novel Cyclophane Amides. Bioorg. Med. Chem. Lett. 2006, 16, 6019–6023. [Google Scholar] [CrossRef]
- Rajakumar, P.; Mohammed Abdul Rasheed, A.; Balu, P.M.; Murugesan, K. Synthesis, Characterization, and Anti-Bacterial Efficacy of Some Novel Cyclophane Amide. Bioorg. Med. Chem. 2006, 14, 7458–7467. [Google Scholar] [CrossRef] [PubMed]
- Seel, C.; Vögtle, F. Molecules with Large Cavities in Supramolecular Chemistry. Angew. Chem. Int. Ed. Engl. 1992, 31, 528–549. [Google Scholar] [CrossRef]
- Zakarian, J.E.; El-Azizi, Y.; Collins, S.K. Exploiting Quadrupolar Interactions in the Synthesis of the Macrocyclic Portion of Longithorone C. Org. Lett. 2008, 10, 2927–2930. [Google Scholar] [CrossRef] [PubMed]
- Kotha, S.; Meshram, M.; Tiwari, A. Advanced Approach to Polycyclics by a Synergistic Combination of Enyne Metathesis and Diels–Alder Reaction. Chem. Soc. Rev. 2009, 38, 2065–2092. [Google Scholar] [CrossRef]
- Kotha, S.; Krishna, N.G.; Halder, S.; Misra, S. A Synergistic Approach to Polycyclics via a Strategic Utilization of Claisen Rearrangement and Olefin Metathesis. Org. Biomol. Chem. 2011, 9, 5597–5624. [Google Scholar] [CrossRef]
- Jimenez, L.; Diederich, F. Catalytic Cyclophanes: A Highly Efficient Model for Pyruvate Oxidase. Tetrahedron Lett. 1989, 30, 2759–2762. [Google Scholar] [CrossRef]
- Gleiter, R.; Hopf, H. Modern Cyclophane Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weiheim, Germany, 2004; ISBN 978-3-527-60396-1. [Google Scholar]
- Weber, E. Cyclophanes; Springer: Berlin/Heidelberg, Germany, 1994; Volume 172. [Google Scholar]
- Vögtle, F. Cyclophane Chemistry: Synthesis, Structures, and Reactions; John Wiley and Sons: Chichester, UK, 1993. [Google Scholar]
- Diederich, F. Cyclophanes; Royal Society of Chemistry: Cambridge, UK, 1991. [Google Scholar]
- Takemura, H. Cyclophane Chemistry for the 21st Century; Research Signpost: Trivandrum, India, 2002. [Google Scholar]
- Davis, F.; Higson, S. Macrocycles: Construction, Chemistry and Nanotechnology Applications; John Wiley and Sons Ltd.: Chichester, UK, 2011. [Google Scholar]
- Kotha, S.; Shirbhate, M.E.; Waghule, G.T. Selected Synthetic Strategies to Cyclophanes. Beilstein J. Org. Chem. 2015, 11, 1274–1331. [Google Scholar] [CrossRef]
- Cram, D.J.; Steinberg, H. Macro Rings. I. Preparation and Spectra of the Paracyclophanes. J. Am. Chem. Soc. 1951, 73, 5691–5704. [Google Scholar] [CrossRef]
- Cram, D.J.; Helgeson, R.C. Macro Rings. XXXIV. A Ring Expansion Route to the Higher Paracyclophanes, and Spectra-Structure Correlations of Their Derived Ketones1. J. Am. Chem. Soc. 1966, 88, 3515–3521. [Google Scholar] [CrossRef]
- Krois, D.; Lehner, H. [4.2]- and [4.3]-Metacyclophanes. J. Chem. Soc. Perkin 1 1982, 2369–2372. [Google Scholar] [CrossRef]
- Tamao, K.; Kodama, S.; Nakatsuka, T.; Kiso, Y.; Kumada, M. One-Step Preparation of Metacyclophanes and (2,6)Pyridinophanes by Nickel-Catalzyed Grignard Cyclocoupling. J. Am. Chem. Soc. 1975, 97, 4405–4406. [Google Scholar] [CrossRef]
- Errede, L.A.; Gregorian, R.S.; Hoyt, J.M. The Chemistry of Xylylenes. VI. The Polymerization of p-Xylylene2. J. Am. Chem. Soc. 1960, 82, 5218–5223. [Google Scholar] [CrossRef]
- Pechlivanidis, Z.; Hopf, H.; Ernst, L. Paracyclophanes: Extending the Bridges. Synthesis. Eur. J. Org. Chem. 2009, 2009, 223–237. [Google Scholar] [CrossRef]
- Allinger, N.L.; Cram, D.J. Macro Rings. IV. The Preparation of Three New Paracyclophanes1. J. Am. Chem. Soc. 1954, 76, 2362–2367. [Google Scholar] [CrossRef]
- Carbonnelle, A.-C.; Zhu, J. A Novel Synthesis of Biaryl-Containing Macrocycles by a Domino Miyaura Arylboronate Formation: Intramolecular Suzuki Reaction. Org. Lett. 2000, 2, 3477–3480. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.B.; Hill, D.E.; Cropp, T.A.; Walsh, R.D.; Cartrette, D.; Hipps, S.; Shachter, A.M.; Pennington, W.T.; Kwochka, W.R. Synthesis of [n]- and [n.n]Cyclophanes by Using Suzuki−Miyaura Coupling. J. Org. Chem. 2002, 67, 5333–5337. [Google Scholar] [CrossRef]
- Bodwell, G.J.; Li, J. Concise Synthesis and Transannular Inverse Electron Demand Diels−Alder Reaction of [3](3,6)Pyridazino[3](1,3)Indolophane. Rapid Access to a Pentacyclic Indoloid System. Org. Lett. 2002, 4, 127–130. [Google Scholar] [CrossRef]
- Bodwell, G.J.; Li, J. A Concise Formal Total Synthesis of (±)-Strychnine by Using a Transannular Inverse-Electron-Demand Diels–Alder Reaction of a [3](1,3)Indolo[3](3,6)Pyridazinophane. Angew. Chem. Int. Ed. 2002, 41, 3261–3262. [Google Scholar] [CrossRef]
- Smith, A.B.; Adams, C.M.; Kozmin, S.A. On the Reversible Nature of the Olefin Cross Metathesis Reaction. J. Am. Chem. Soc. 2001, 123, 990–991. [Google Scholar] [CrossRef]
- Locke, A.J.; Jones, C.; Richards, C.J. A Rapid Approach to Ferrocenophanes via Ring-Closing Metathesis. J. Organomet. Chem. 2001, 637–639, 669–676. [Google Scholar] [CrossRef]
- Fürstner, A.; Stelzer, F.; Rumbo, A.; Krause, H. Total Synthesis of the Turrianes and Evaluation of Their DNA-Cleaving Properties. Chem. Eur. J. 2002, 8, 1856–1871. [Google Scholar] [CrossRef]
- Martinez, V.; Blais, J.-C.; Astruc, D. A Fast Organometallic Route from P-Xylene, Mesitylene, and p-Diisopropylbenzene to Organoiron and Polycyclic Aromatic Cyclophanes, Capsules and Polymers. Angew. Chem. Int. Ed. 2003, 42, 4366–4369. [Google Scholar] [CrossRef] [PubMed]
- Tae, J.; Yang, Y.-K. Efficient Synthesis of Macrocyclic Paracyclophanes by Ring-Closing Metathesis Dimerization and Trimerization Reactions. Org. Lett. 2003, 5, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.D.; Jäckel, F.; Severin, N.; Rabe, J.P.; Müllen, K. A Hexa-Peri-Hexabenzocoronene Cyclophane: An Addition to the Toolbox for Molecular Electronics. J. Am. Chem. Soc. 2004, 126, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Blais, J.-C.; Bravic, G.; Astruc, D. Coupling Multiple Benzylic Activation of Simple Arenes by CpFe+ with Multiple Alkene Metathesis Using Grubbs Catalysts: An Efficient Carbon−Carbon Bond Formation Strategy Leading to Polycycles, Cyclophanes, Capsules, and Polymeric Compounds and Their CpFe+ Complexes. Organometallics 2004, 23, 861–874. [Google Scholar] [CrossRef]
- Branowska, D.; Buczek, I.; Kalińska, K.; Nowaczyk, J.; Rykowski, A. S-Transalkylation/Ring Closing Metathesis as a Route to Azathiamacrocycles Incorporating 2,2′-Bipyridine Subunits. Tetrahedron Lett. 2005, 46, 8539–8541. [Google Scholar] [CrossRef]
- Ueda, T.; Kanomata, N.; Machida, H. Synthesis of Planar-Chiral Paracyclophanes via Samarium(II)-Catalyzed Intramolecular Pinacol Coupling. Org. Lett. 2005, 7, 2365–2368. [Google Scholar] [CrossRef]
- Branowska, D.; Rykowski, A. Ring-Closing Metathesis Approach to Symmetrical and Unsymmetrical Cycloakeno[c]Fused 2,2′-Bipyridine-Based Cyclophanes. Tetrahedron 2005, 61, 10713–10718. [Google Scholar] [CrossRef]
- Kotha, S.; Chavan, A.S.; Shaikh, M. Diversity-Oriented Approach to Macrocyclic Cyclophane Derivatives by Suzuki–Miyaura Cross-Coupling and Olefin Metathesis as Key Steps. J. Org. Chem. 2012, 77, 482–489. [Google Scholar] [CrossRef]
- Alfimov, M.V.; Fedorova, O.A.; Gromov, S.P. Photoswitchable Molecular Receptors. Photoreact. Control Photofunct. Mater. II 2003, 158, 183–198. [Google Scholar] [CrossRef]
- Li, J.; Yim, D.; Jang, W.-D.; Yoon, J. Recent Progress in the Design and Applications of Fluorescence Probes Containing Crown Ethers. Chem. Soc. Rev. 2017, 46, 2437–2458. [Google Scholar] [CrossRef] [PubMed]
- Gokel, G.W.; Leevy, W.M.; Weber, M.E. Crown Ethers: Sensors for Ions and Molecular Scaffolds for Materials and Biological Models. Chem. Rev. 2004, 104, 2723–2750. [Google Scholar] [CrossRef] [PubMed]
- Ahmedova, A.; Burdzhiev, N.; Ciattini, S.; Stanoeva, E.; Mitewa, M. Synthesis, Structure, Spectral and Coordination Properties of a Crown Ether Derivative of 1,3-Indandione. A New Structural Evidence for the Versatile Reactivity of 2-Acetyl-1,3-Indandione. Comptes Rendus Chim. 2010, 13, 1269–1277. [Google Scholar] [CrossRef]
- Sartori, G.; Casnati, G.; Bigi, F.; Baraldi, D. Friedel-Crafts Coordinated Processes: Selective Cyclooligomerization of Acyl Chlorides. Tetrahedron Lett. 1991, 32, 2153–2156. [Google Scholar] [CrossRef]
- Kilgore, L.B.; Ford, J.H.; Wolfe, W.C. Insecticidal Properties of 1,3-Indandiones. Ind. Eng. Chem. 1942, 34, 494–497. [Google Scholar] [CrossRef]
- Teotonio, E.E.S.; Brito, H.F.; Viertler, H.; Faustino, W.M.; Malta, O.L.; de Sá, G.F.; Felinto, M.C.F.C.; Santos, R.H.A.; Cremona, M. Synthesis and Luminescent Properties of Eu3+-Complexes with 2-Acyl-1,3-Indandionates (ACIND) and TPPO Ligands: The First X-Ray Structure of Eu–ACIND Complex. Polyhedron 2006, 25, 3488–3494. [Google Scholar] [CrossRef]
- Griffin, M.O.; Fricovsky, E.; Ceballos, G.; Villarreal, F. Tetracyclines: A Pleitropic Family of Compounds with Promising Therapeutic Properties. Review of the Literature. Am. J. Physiol.-Cell Physiol. 2010, 299, C539–C548. [Google Scholar] [CrossRef]
- Li, M.; Lv, X.-L.; Wen, L.-R.; Hu, Z.-Q. Direct Solvent-Free Regioselective Construction of Pyrrolo[1,2-a][1,10]Phenanthrolines Based on Isocyanide-Based Multicomponent Reactions. Org. Lett. 2013, 15, 1262–1265. [Google Scholar] [CrossRef]
- Pirrung, M.C.; Sarma, K.D. Aqueous Medium Effects on Multi-Component Reactions. Multicomponent React. 2005, 61, 11456–11472. [Google Scholar] [CrossRef]
- Wang, S.-X.; Wang, M.-X.; Wang, D.-X.; Zhu, J. Catalytic Enantioselective Passerini Three-Component Reaction. Angew. Chem. Int. Ed. 2008, 47, 388–391. [Google Scholar] [CrossRef]
- Kłossowski, S.; Wiraszka, B.; Berłożecki, S.; Ostaszewski, R. Model Studies on the First Enzyme-Catalyzed Ugi Reaction. Org. Lett. 2013, 15, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Andreana, P.R.; Liu, C.C.; Schreiber, S.L. Stereochemical Control of the Passerini Reaction. Org. Lett. 2004, 6, 4231–4233. [Google Scholar] [CrossRef] [PubMed]
- Kusebauch, U.; Beck, B.; Messer, K.; Herdtweck, E.; Dömling, A. Massive Parallel Catalyst Screening: Toward Asymmetric MCRs. Org. Lett. 2003, 5, 4021–4024. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A.; Ugi, I. Multicomponent Reactions with Isocyanides. Angew. Chem. Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Jiang, X.; Tang, T.; Wang, J.-M.; Chen, Z.; Zhu, Y.-M.; Ji, S.-J. Palladium-Catalyzed One-Pot Synthesis of Quinazolinones via Tert-Butyl Isocyanide Insertion. J. Org. Chem. 2014, 79, 5082–5087. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Jiang, X.; Wang, J.-M.; Sun, Y.-X.; Zhu, Y.-M. Divergent Synthesis of 6H-Isoindolo[2,1-a]Indol-6-Ones and Indenoindolones: An Investigation of Pd-Catalyzed Isocyanide Insertion. Tetrahedron 2014, 70, 2999–3004. [Google Scholar] [CrossRef]
- Duan, H.; Chen, Z.; Han, L.; Feng, Y.; Zhu, Y.; Yang, S. Palladium-Catalyzed Chemoselective Synthesis of Indane-1,3-Dione Derivatives via Tert-Butyl Isocyanide Insertion. Org. Biomol. Chem. 2015, 13, 6782–6788. [Google Scholar] [CrossRef]
- Wan, J.-P.; Jing, Y. Recent Advances in Copper-Catalyzed C-H Bond Amidation. Beilstein J. Org. Chem. 2015, 11, 2209–2222. [Google Scholar] [CrossRef]
- Liang, C.; Collet, F.; Robert-Peillard, F.; Müller, P.; Dodd, R.H.; Dauban, P. Toward a Synthetically Useful Stereoselective C−H Amination of Hydrocarbons. J. Am. Chem. Soc. 2008, 130, 343–350. [Google Scholar] [CrossRef]
- Bryant, J.R.; Mayer, J.M. Oxidation of C−H Bonds by [(Bpy)2(Py)RuIVO]2+ Occurs by Hydrogen Atom Abstraction. J. Am. Chem. Soc. 2003, 125, 10351–10361. [Google Scholar] [CrossRef]
- Pelletier, G.; Powell, D.A. Copper-Catalyzed Amidation of Allylic and Benzylic CH Bonds. Org. Lett. 2006, 8, 6031–6034. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Hupp, C.D.; Allen, K.L.; Slough, G.A. Catalytic Allylic Amination versus Allylic Oxidation: A Mechanistic Dichotomy. Organometallics 2005, 24, 1747–1755. [Google Scholar] [CrossRef]
- Lu, H.; Subbarayan, V.; Tao, J.; Zhang, X.P. Cobalt(II)-Catalyzed Intermolecular Benzylic C−H Amination with 2,2,2-Trichloroethoxycarbonyl Azide (TrocN3). Organometallics 2010, 29, 389–393. [Google Scholar] [CrossRef]
- Badiei, Y.M.; Dinescu, A.; Dai, X.; Palomino, R.M.; Heinemann, F.W.; Cundari, T.R.; Warren, T.H. Copper–Nitrene Complexes in Catalytic C-H Amination. Angew. Chem. Int. Ed. 2008, 47, 9961–9964. [Google Scholar] [CrossRef] [PubMed]
- Huard, K.; Lebel, H. N-Tosyloxycarbamates as Reagents in Rhodium-Catalyzed C-H Amination Reactions. Chem. Eur. J. 2008, 14, 6222–6230. [Google Scholar] [CrossRef] [PubMed]
- Kalita, B.; Lamar, A.A.; Nicholas, K.M. Hydrous Zinc Halide-Catalyzed Aminosulfonation of Hydrocarbons. Chem. Commun. 2008, 36, 4291–4293. [Google Scholar] [CrossRef]
- Li, Z.; Capretto, D.A.; Rahaman, R.; He, C. Silver-Catalyzed Intermolecular Amination of C-H Groups. Angew. Chem. Int. Ed. 2007, 46, 5184–5186. [Google Scholar] [CrossRef]
- Harden, J.D.; Ruppel, J.V.; Gao, G.-Y.; Zhang, X.P. Cobalt-Catalyzed Intermolecular C-H Amination with Bromamine-T as Nitrene Source. Chem. Commun. 2007, 44, 4644–4646. [Google Scholar] [CrossRef]
- Bhuyan, R.; Nicholas, K.M. Efficient Copper-Catalyzed Benzylic Amidation with Anhydrous Chloramine-T. Org. Lett. 2007, 9, 3957–3959. [Google Scholar] [CrossRef]
- Lebel, H.; Huard, K. De Novo Synthesis of Troc-Protected Amines: Intermolecular Rhodium-Catalyzed C−H Amination with N-Tosyloxycarbamates. Org. Lett. 2007, 9, 639–642. [Google Scholar] [CrossRef]
- Fiori, K.W.; Du Bois, J. Catalytic Intermolecular Amination of C−H Bonds: Method Development and Mechanistic Insights. J. Am. Chem. Soc. 2007, 129, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Fructos, M.R.; Trofimenko, S.; Díaz-Requejo, M.M.; Pérez, P.J. Facile Amine Formation by Intermolecular Catalytic Amidation of Carbon−Hydrogen Bonds. J. Am. Chem. Soc. 2006, 128, 11784–11791. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.P.; Davies, H.M.L. Dirhodium Tetracarboxylates Derived from Adamantylglycine as Chiral Catalysts for Enantioselective C−H Aminations. Org. Lett. 2006, 8, 5013–5016. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Robert-Peillard, F.; Fruit, C.; Müller, P.; Dodd, R.H.; Dauban, P. Efficient Diastereoselective Intermolecular Rhodium-Catalyzed C-H Amination. Angew. Chem. Int. Ed. 2006, 45, 4641–4644. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.K.-Y.; Tsui, W.-M.; Huang, J.-S.; Che, C.-M.; Liang, J.-L.; Zhu, N. Imido Transfer from Bis(Imido)Ruthenium(VI) Porphyrins to Hydrocarbons: Effect of Imido Substituents, C−H Bond Dissociation Energies, and RuVI/V Reduction Potentials. J. Am. Chem. Soc. 2005, 127, 16629–16640. [Google Scholar] [CrossRef]
- Yamawaki, M.; Tsutsui, H.; Kitagaki, S.; Anada, M.; Hashimoto, S. Dirhodium(II) Tetrakis[N-Tetrachlorophthaloyl-(S)-Tert-Leucinate]: A New Chiral Rh(II) Catalyst for Enantioselective Amidation of C-H Bonds. Tetrahedron Lett. 2002, 43, 9561–9564. [Google Scholar] [CrossRef]
- Kohmura, Y.; Katsuki, T. Mn(Salen)-Catalyzed Enantioselective C-H Amination. Tetrahedron Lett. 2001, 42, 3339–3342. [Google Scholar] [CrossRef]
- Albone, D.P.; Aujla, P.S.; Challenger, S.; Derrick, A.M. A Simple Copper Catalyst for Both Aziridination of Alkenes and Amination of Activated Hydrocarbons with Chloramine-T Trihydrate. J. Org. Chem. 1998, 63, 9569–9571. [Google Scholar] [CrossRef]
- Powell, D.A.; Fan, H. Copper-Catalyzed Amination of Primary Benzylic C−H Bonds with Primary and Secondary Sulfonamides. J. Org. Chem. 2010, 75, 2726–2729. [Google Scholar] [CrossRef]
- Andrus, M.B.; Chen, X. Catalytic Enantioselective Allylic Oxidation of Olefins with Copper(I) Catalysts and New Perester Oxidants. Tetrahedron 1997, 53, 16229–16240. [Google Scholar] [CrossRef]
- Kadish, K.M.; Smith, K.M.; Guilard, R. Handbook of Porphyrin Science; World Scientific Publishing: Singapore, 2010. [Google Scholar]
- Shy, H.; Mackin, P.; Orvieto, A.S.; Gharbharan, D.; Peterson, G.R.; Bampos, N.; Hamilton, T.D. The Two-Step Mechanochemical Synthesis of Porphyrins. Faraday Discuss. 2014, 170, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Boyle, R.W. Synthetic Routes to Porphyrins Bearing Fused Rings. Tetrahedron 2006, 62, 10039–10054. [Google Scholar] [CrossRef]
- Akhigbe, J.; Luciano, M.; Zeller, M.; Brückner, C. Mono- and Bisquinoline-Annulated Porphyrins from Porphyrin β,Β′-Dione Oximes. J. Org. Chem. 2015, 80, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Götz, D.C.G.; Gehrold, A.C.; Dorazio, S.J.; Daddario, P.; Samankumara, L.; Bringmann, G.; Brückner, C.; Bruhn, T. Indaphyrins and Indachlorins: Optical and Chiroptical Properties of a Family of Helimeric Porphyrinoids. Eur. J. Org. Chem. 2015, 2015, 3913–3922. [Google Scholar] [CrossRef]
- Aguiar, A.; Leite, A.; Silva, A.M.N.; Tomé, A.C.; Cunha-Silva, L.; de Castro, B.; Rangel, M.; Silva, A.M.G. Isoxazolidine-Fused Meso-Tetraarylchlorins as Key Tools for the Synthesis of Mono- and Bis-Annulated Chlorins. Org. Biomol. Chem. 2015, 13, 7131–7135. [Google Scholar] [CrossRef]
- Brückner, C. The Breaking and Mending of Meso-Tetraarylporphyrins: Transmuting the Pyrrolic Building Blocks. Acc. Chem. Res. 2016, 49, 1080–1092. [Google Scholar] [CrossRef]
- Jaquinod, L.; Gros, C.; Olmstead, M.M.; Antolovich, M.; Smith, K.M. First Syntheses of Fused Pyrroloporphyrins. Chem. Commun. 1996, 12, 1475–1476. [Google Scholar] [CrossRef]
- Jaquinod, L.; Gros, C.; Khoury, R.G.; Smith, K.M. A Convenient Synthesis of Functionalized Tetraphenylchlorins. Chem. Commun. 1996, 22, 2581–2582. [Google Scholar] [CrossRef]
- Shea, K.M.; Jaquinod, L. Dodecasubstituted Metallochlorins (Metallodihydroporphyrins). Chem. Commun. 1998, 7, 759–760. [Google Scholar] [CrossRef]
- Gros, C.P.; Jaquinod, L.; Khoury, R.G.; Olmstead, M.M.; Smith, K.M. Approaches to β-Fused Porphyrinoporphyrins: Pyrrolo- and Dipyrromethaneporphyrins. J. Porphyr. Phthalocyanines 1997, 01, 201–212. [Google Scholar] [CrossRef]
- Shea, K.M.; Jaquinod, L.; Smith, K.M. Dihydroporphyrin Synthesis: New Methodology. J. Org. Chem. 1998, 63, 7013–7021. [Google Scholar] [CrossRef] [PubMed]
- Shelnutt, J.A.; Song, X.-Z.; Ma, J.-G.; Jia, S.-L.; Jentzen, W.; Medforth, C.J. Nonplanar Porphyrins and Their Significance in Proteins. Chem. Soc. Rev. 1998, 27, 31–42. [Google Scholar] [CrossRef]
- Mandon, D.; Ochenbein, P.; Fischer, J.; Weiss, R.; Jayaraj, K.; Austin, R.N.; Gold, A.; White, P.S.; Brigaud, O. .beta.-Halogenated-Pyrrole Porphyrins. Molecular Structures of 2,3,7,8,12,13,17,18-Octabromo-5,10,15,20-Tetramesitylporphyrin, Nickel(II) 2,3,7,8,12,13,17,18-Octabromo-5,10,15,20-Tetramesitylporphyrin, and Nickel(II) 2,3,7,8,12,13,17,18-Octabromo-5,10,15,20-Tetrakis(Pentafluorophenyl)Porphyrin. Inorg. Chem. 1992, 31, 2044–2049. [Google Scholar] [CrossRef]
- Hodge, J.A.; Hill, M.G.; Gray, H.B. Electrochemistry of Nonplanar Zinc(II) Tetrakis(Pentafluorophenyl)Porphyrins. Inorg. Chem. 1995, 34, 809–812. [Google Scholar] [CrossRef]
- Kojima, T.; Nakanishi, T.; Harada, R.; Ohkubo, K.; Yamauchi, S.; Fukuzumi, S. Selective Inclusion of Electron-Donating Molecules into Porphyrin Nanochannels Derived from the Self-Assembly of Saddle-Distorted, Protonated Porphyrins and Photoinduced Electron Transfer from Guest Molecules to Porphyrin Dications. Chem. Eur. J. 2007, 13, 8714–8725. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, N.; Grover, N.; Sankar, M. Versatile Synthetic Route for β-Functionalized Chlorins and Porphyrins by Varying the Size of Michael Donors: Syntheses, Photophysical, and Electrochemical Redox Properties. Inorg. Chem. 2017, 56, 11532–11545. [Google Scholar] [CrossRef]
- Lindsey, J.S. De Novo Synthesis of Gem-Dialkyl Chlorophyll Analogues for Probing and Emulating Our Green World. Chem. Rev. 2015, 115, 6534–6620. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Synthetic Chlorins, Possible Surrogates for Chlorophylls, Prepared by Derivatization of Porphyrins. Chem. Rev. 2017, 117, 344–535. [Google Scholar] [CrossRef]
- Samankumara, L.P.; Dorazio, S.J.; Akhigbe, J.; Li, R.; Nimthong-Roldán, A.; Zeller, M.; Brückner, C. Indachlorins: Nonplanar Indanone-Annulated Chlorin Analogues with Panchromatic Absorption Spectra between 300 and 900 Nm. Chem. Eur. J. 2015, 21, 11118–11128. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Hyland, M.A.; Brückner, C. Synthesis of Indaphyrins: Meso-Tetraarylsecochlorin-Based Porphyrinoids Containing Direct o-Phenyl-to-β-Linkages. Org. Biomol. Chem. 2004, 2, 1484–1491. [Google Scholar] [CrossRef]
- Nishiyabu, R.; Anzenbacher, P. Sensing of Antipyretic Carboxylates by Simple Chromogenic Calix[4]Pyrroles. J. Am. Chem. Soc. 2005, 127, 8270–8271. [Google Scholar] [CrossRef] [PubMed]
- Nishiyabu, R.; Anzenbacher, P. 1,3-Indane-Based Chromogenic Calixpyrroles with Push−Pull Chromophores: Synthesis and Anion Sensing. Org. Lett. 2006, 8, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Hong, S.-J.; Yoo, J.; Kim, S.K.; Sessler, J.L.; Lee, C.-H. Strapped Calix[4]Pyrroles Bearing a 1,3-Indanedione at a β-Pyrrolic Position: Chemodosimeters for the Cyanide Anion. Org. Lett. 2009, 11, 3626–3629. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, N.; Grover, N.; Sankar, M. Nickel-Induced Skeletal Rearrangement of Free Base Trans-Chlorins into Monofused NiII-Porphyrins: Synthesis, Structural, Spectral, and Electrochemical Redox Properties. Inorg. Chem. 2018, 57, 11349–11360. [Google Scholar] [CrossRef] [PubMed]
- Mchardy, N.; Hudson, A.T.; Morgan, D.W.T.; Rae, D.G.; Dolan, T.T. Activity of 10 Naphthoquinones, Including Parvaquone (993C) and Menoctone, in Cattle Artificially Infected with Theileria Parva. Res. Vet. Sci. 1983, 35, 347–352. [Google Scholar] [CrossRef]
- Patil, P.C.; Akamanchi, K.G. Simple and Effective Route for Synthesis of Parvaquone, an Antiprotozoal Drug. RSC Adv. 2014, 4, 58214–58216. [Google Scholar] [CrossRef]
- Britton, H.; Catterick, D.; Dwyer, A.N.; Gordon, A.H.; Leach, S.G.; McCormick, C.; Mountain, C.E.; Simpson, A.; Stevens, D.R.; Urquhart, M.W.J.; et al. Discovery and Development of an Efficient Process to Atovaquone. Org. Process Res. Dev. 2012, 16, 1607–1617. [Google Scholar] [CrossRef]
- Holt, G.; Wall, D.K. Some Reactions of 2-Diazoindane-1,3-Dione. J. Chem. Soc. C Org. 1966, 857–858. [Google Scholar] [CrossRef]
- Spangler, R.J.; Kim, J.H.; Cava, M.P. Pyrolytic and Photochemical Wolff Rearrangement of Diazoindanones. Synthesis of 2-Carboalkoxybenzocyclobutenones. J. Org. Chem. 1977, 42, 1697–1703. [Google Scholar] [CrossRef]
- Regitz, M.; Heck, G. Synthesen Und Einige Umsetzungen Des 2-Diazo- Und Des 2-Hydroxy-Indandions-(1.3). Chem. Ber. 1964, 97, 1482–1501. [Google Scholar] [CrossRef]
- Rosenfeld, M.J.; Shankar, B.K.R.; Shechter, H. Rhodium(II) Acetate-Catalyzed Reactions of 2-Diazo-1,3-Indandione and 2-Diazo-1-Indanone with Various Substrates. J. Org. Chem. 1988, 53, 2699–2705. [Google Scholar] [CrossRef]
- Alloum, A.B.; Villemin, D. Potassium Fluoride on Alumina: An Easy Preparation of Diazocarbonyl Compounds. Synth. Commun. 1989, 19, 2567–2571. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W.; Huang, D.; Zeng, X.; Wang, X.; Hu, Y. Tandem Synthesis of α-Diazoketones from 1,3-Diketones. J. Org. Chem. 2017, 82, 9171–9174. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.; Kresge, A.J.; Zhu, Y. Kinetics and Mechanism of the Base–Catalyzed Cleavage of 2-Diazo-1,3-Indanedione and the Acid–Catalyzed Decomposition of Its Hydrolysis Product, 2-(Diazoacetyl)Benzoic Acid. ARKIVOC 2001, 2001, 108–115. [Google Scholar] [CrossRef]
- Reichel, L.; Hampel, W. Chemie Und Biochemie Der Pflanzenstoffe, XXI1) Über Das 1.4-Dioxo-Isochroman. Justus Liebigs Ann. Chem. 1968, 712, 152–154. [Google Scholar] [CrossRef]
- Asif, M. Mini Review on Important Biological Properties of Benzofuran Derivatives. J. Anal. Pharm. Res. 2016, 3, 11–12. [Google Scholar] [CrossRef][Green Version]
- Miao, Y.; Hu, Y.; Yang, J.; Liu, T.; Sun, J.; Wang, X. Natural Source, Bioactivity and Synthesis of Benzofuran Derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar] [CrossRef]
- Sun, W.; Sarma, J.S.M.; Singh, B.N. Electrophysiological Effects of Dronedarone (SR33589), a Noniodinated Benzofuran Derivative, in the Rabbit Heart. Circulation 1999, 100, 2276–2281. [Google Scholar] [CrossRef]
- Oter, O.; Ertekin, K.; Kirilmis, C.; Koca, M.; Ahmedzade, M. Characterization of a Newly Synthesized Fluorescent Benzofuran Derivative and Usage as a Selective Fiber Optic Sensor for Fe(III). Sens. Actuators B Chem. 2007, 122, 450–456. [Google Scholar] [CrossRef]
- Karatas, F.; Koca, M.; Kara, H.; Servi, S. Synthesis and Oxidant Properties of Novel (5-Bromobenzofuran-2-Yl)(3-Methyl-3-Mesitylcyclobutyl)Ketonethiosemicarbazone. Eur. J. Med. Chem. 2006, 41, 664–669. [Google Scholar] [CrossRef]
- Habermann, J.; Ley, S.V.; Smits, R. Three-Step Synthesis of an Array of Substituted Benzofurans Using Polymer-Supported Reagents. J. Chem. Soc. Perkin 1 1999, 17, 2421–2423. [Google Scholar] [CrossRef]
- Ameri, M.; Asghari, A.; Amoozadeh, A.; Bakherad, M.; Nematollahi, D. An Efficient, Simple, Non-Catalytic Electrosynthesis of New Polycyclic Benzofuran Derivatives. Tetrahedron Lett. 2015, 56, 2141–2144. [Google Scholar] [CrossRef]
- Allais, C.; Grassot, J.-M.; Rodriguez, J.; Constantieux, T. Metal-Free Multicomponent Syntheses of Pyridines. Chem. Rev. 2014, 114, 10829–10868. [Google Scholar] [CrossRef]
- Damavandi, S.; Sandaroos, R. Solvent-Free One Pot Synthesis of Indenoquinolinones Catalyzed by Iron(III) Triflate. Heterocycl. Commun. 2011, 17, 121–124. [Google Scholar] [CrossRef]
- Maleki, A.; Nooraie Yeganeh, N. Facile One-Pot Synthesis of a Series of 7-Aryl-8H-Benzo[h]Indeno[1,2-b]Quinoline-8-One Derivatives Catalyzed by Cellulose-Based Magnetic Nanocomposite. Appl. Organomet. Chem. 2017, 31, e3814. [Google Scholar] [CrossRef]
- Safajoo, N.; Mirjalili, B.B.F.; Bamoniri, A. A Facile and Clean Synthesis of Indenopyrido[2,3-d]Pyrimidines in the Presence of Fe3O4@NCs/Cu(II) as Bio-Based Magnetic Nano-Catalyst. Polycycl. Aromat. Compd. 2021, 41, 1241–1248. [Google Scholar] [CrossRef]
- Mamaghani, M.; Shirini, F.; Bassereh, E.; Hossein Nia, R. 1,2-Dimethyl-N-Butanesulfonic Acid Imidazolium Hydrogen Sulfate as Efficient Ionic Liquid Catalyst in the Synthesis of Indeno Fused Pyrido[2,3-d]Pyrimidines. J. Saudi Chem. Soc. 2016, 20, 570–576. [Google Scholar] [CrossRef]
- Polo, E.; Arce-Parada, V.; López-Cortés, X.A.; Sánchez-Márquez, J.; Morales-Bayuelo, A.; Forero-Doria, O.; Gutiérrez, M. Synthesis of Pyrazolo-Fused 4-Azafluorenones in an Ionic Liquid. Mechanistic Insights by Joint Studies Using DFT Analysis and Mass Spectrometry. Catalysts 2019, 9, 820. [Google Scholar] [CrossRef]
- Sheldon, R. Catalytic Reactions in Ionic Liquids. Chem. Commun. 2001, 23, 2399–2407. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L. Ionic Liquids: Perspectives for Organic and Catalytic Reactions. J. Mol. Catal. Chem. 2002, 182–183, 419–437. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic Liquids—New “Solutions” for Transition Metal Catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Gu, Y. Multicomponent Reactions in Unconventional Solvents: State of the Art. Green Chem. 2012, 14, 2091–2128. [Google Scholar] [CrossRef]
- Ahmed, K.; Dubey, B.; Nadeem, S.; Shrivastava, B.; Sharma, P. P-TSA-Catalyzed One-Pot Synthesis and Docking Studies of Some 5H-Indeno[1,2-b]Quinoline-9,11(6H,10H)-Dione Derivatives as Anticonvulsant Agents. Chin. Chem. Lett. 2016, 27, 721–725. [Google Scholar] [CrossRef]
- Verma, G.K.; Raghuvanshi, K.; Kumar, R.; Singh, M.S. An Efficient One-Pot Three-Component Synthesis of Functionalized Pyrimido[4,5-b]Quinolines and Indeno Fused Pyrido[2,3-d]Pyrimidines in Water. Tetrahedron Lett. 2012, 53, 399–402. [Google Scholar] [CrossRef]
- Bhaskaruni, S.V.H.S.; Maddila, S.; Van Zyl, W.E.; Jonnalagadda, S.B. Ag2O on ZrO2 as a Recyclable Catalyst for Multicomponent Synthesis of Indenopyrimidine Derivatives. Molecules 2018, 23, 1648. [Google Scholar] [CrossRef]
- Chupakhin, E.; Babich, O.; Prosekov, A.; Asyakina, L.; Krasavin, M. Spirocyclic Motifs in Natural Products. Molecules 2019, 24, 4165. [Google Scholar] [CrossRef]
- Donthi, R.; Reddy, V.R.; Reddy, S.N.; Chandra, R. Base Catalysed Diastereoselective Tamura Cycloaddition of Vinylidene Indanediones. Tetrahedron Lett. 2019, 60, 1–4. [Google Scholar] [CrossRef]
- Andrew Evans, P.; Brandt, T.A. Palladium Catalyzed Cross-Coupling Acylation Approach to the Antitumor Antibiotic Fredericamycin A. Tetrahedron Lett. 1996, 37, 1367–1370. [Google Scholar] [CrossRef]
- Pizzirani, D.; Roberti, M.; Recanatini, M. Domino Knoevenagel/Diels–Alder Sequence Coupled to Suzuki Reaction: A Valuable Synthetic Platform for Chemical Biology. Tetrahedron Lett. 2007, 48, 7120–7124. [Google Scholar] [CrossRef]
- Coldham, I.; Hufton, R. Intramolecular Dipolar Cycloaddition Reactions of Azomethine Ylides. Chem. Rev. 2005, 105, 2765–2810. [Google Scholar] [CrossRef]
- Kathiravan, S.; Raghunathan, R. Expedient Synthesis of Novel Ferrocenyl Spiropyrrolidines Through 1,3-Dipolar Cycloaddition Reaction. J. Heterocycl. Chem. 2014, 51, 906–910. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, J.; Yan, C.-G. Generation of New 1,3-Dipolar Azomethine Ylide via Reaction of Ethyl Glycinate with Dialkyl But-2-Ynedioate and Tandem 1,3-Dipolar Cycloaddition Reaction. ChemistrySelect 2017, 2, 10496–10500. [Google Scholar] [CrossRef]
- Qiu, G.; Kuang, Y.; Wu, J. N-Imide Ylide-Based Reactions: C-H Functionalization, Nucleophilic Addition and Cycloaddition. Adv. Synth. Catal. 2014, 356, 3483–3504. [Google Scholar] [CrossRef]
- Duan, J.; Cheng, J.; Cheng, Y.; Li, P. Synthesis of Dinitrogen-Fused Spirocyclic Heterocycles via Organocatalytic 1,3-Dipolar Cycloaddition of 2-Arylidene-1,3-Indandiones and an Azomethine Imine. Asian J. Org. Chem. 2016, 5, 477–480. [Google Scholar] [CrossRef]
- Yu, J.-K.; Chien, H.-W.; Lin, Y.-J.; Karanam, P.; Chen, Y.-H.; Lin, W. Diversity-Oriented Synthesis of Chromenopyrrolidines from Azomethine Ylides and 2-Hydroxybenzylidene Indandiones via Base-Controlled Regiodivergent (3+2) Cycloaddition. Chem. Commun. 2018, 54, 9921–9924. [Google Scholar] [CrossRef]
- Winter, M.; Faust, K.; Himmelsbach, M.; Waser, M. Synthesis of α-CF3-Proline Derivatives by Means of a Formal (3 + 2)-Cyclisation between Trifluoropyruvate Imines and Michael Acceptors. Org. Biomol. Chem. 2019, 17, 5731–5735. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Ganapuram, M.R.; Hsieh, K.-H.; Chen, K.-H.; Karanam, P.; Vagh, S.S.; Liou, Y.-C.; Lin, W. 3-Homoacyl Coumarin: An All Carbon 1,3-Dipole for Enantioselective Concerted (3+2) Cycloaddition. Chem. Commun. 2018, 54, 12702–12705. [Google Scholar] [CrossRef]
- Hu, F.; Wei, Y.; Shi, M. Enantioselective Synthesis of Spirocyclic Cyclopentenes: Asymmetric [3+2] Annulation of 2-Arylideneindane-1,3-Diones with MBH Carbonates Derivatives Catalyzed by Multifunctional Thiourea–Phosphines. Tetrahedron 2012, 68, 7911–7919. [Google Scholar] [CrossRef]
- Vetica, F.; Bailey, S.J.; Kumar, M.; Mahajan, S.; von Essen, C.; Rissanen, K.; Enders, D. Palladium-Catalyzed [3+2] Cycloaddition of Vinylaziridine and Indane-1,3-Diones: Diastereo- and Enantioselective Access to Spiro-Pyrrolidines. Synthesis 2020, 52, 2038–2044. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, X.; Jiang, F.; Shi, W.; Wang, W.; Wu, Y.; Zhang, C.; Shi, X.; Guo, H. Palladium-Catalyzed Asymmetric [3+2] Cycloaddition of Vinylethylene Carbonates with 2-Arylidene-1,3-Indandiones: Synthesis of Tetrahydrofuran-Fused Spirocyclic 1,3-Indandiones. Eur. J. Org. Chem. 2020, 2020, 4801–4804. [Google Scholar] [CrossRef]
- Wei, F.; Ren, C.-L.; Wang, D.; Liu, L. Highly Enantioselective [3+2] Cycloaddition of Vinylcyclopropane with Nitroalkenes Catalyzed by Palladium(0) with a Chiral Bis(Tert-Amine) Ligand. Chem. Eur. J. 2015, 21, 2335–2338. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Laugeois, M.; Ratovelomanana-Vidal, V.; Vitale, M.R. Palladium(0)-Catalyzed Diastereoselective (3+2) Cycloadditions of Vinylcyclopropanes with Sulfonyl-Activated Imines. Synlett 2018, 29, 2288–2292. [Google Scholar]
- Laugeois, M.; Ponra, S.; Ratovelomanana-Vidal, V.; Michelet, V.; Vitale, M.R. Asymmetric Preparation of Polysubstituted Cyclopentanes by Synergistic Pd(0)/Amine Catalyzed Formal [3+2] Cycloadditions of Vinyl Cyclopropanes with Enals. Chem. Commun. 2016, 52, 5332–5335. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.-Y.; Tang, X.-Y.; Shi, M. One-Pot Tandem Diastereoselective and Enantioselective Synthesis of Functionalized Oxindole-Fused Spiropyrazolidine Frameworks. Chem. Eur. J. 2014, 20, 13136–13142. [Google Scholar] [CrossRef]
- Yuan, Z.; Wei, W.; Lin, A.; Yao, H. Bifunctional Organo/Metal Cooperatively Catalyzed [3 + 2] Annulation of Para-Quinone Methides with Vinylcyclopropanes: Approach to Spiro[4.5]Deca-6,9-Diene-8-Ones. Org. Lett. 2016, 18, 3370–3373. [Google Scholar] [CrossRef]
- Corti, V.; Marcantonio, E.; Mamone, M.; Giungi, A.; Fochi, M.; Bernardi, L. Synergistic Palladium-Phosphoric Acid Catalysis in (3 + 2) Cycloaddition Reactions between Vinylcyclopropanes and Imines. Catalysts 2020, 10, 150. [Google Scholar] [CrossRef]
- Duan, J.; Cheng, Y.; Li, R.; Li, P. Synthesis of Spiro[Indane-1,3-Dione-1-Pyrrolines] via Copper-Catalyzed Heteroannulation of Ketoxime Acetates with 2-Arylideneindane-1,3-Diones. Org. Chem. Front. 2016, 3, 1614–1618. [Google Scholar] [CrossRef]
- Bdiri, B.; Zhou, Z.-M. Novel Asymmetric Synthesis of Spiroindene-1,3dione-Pyrrolidines via CoII/Amino Acids Complex Catalysed Asymmetric 1,3-Dipolar Cycloaddition of Azomethine Ylides and 2-Arylidenindane-1,3-Diones. Tetrahedron Lett. 2017, 58, 4600–4608. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Sun, J.; Sun, Q.; Yan, C.-G. Construction of Spiro[Indene-2,1′-Pyrrolo[2,1-a]Isoquinoline]s through a Visible-Light-Catalyzed Oxidative [3+2] Cycloaddition Reaction. Asian J. Org. Chem. 2017, 6, 862–866. [Google Scholar] [CrossRef]
- Domínguez, G.; Pérez-Castells, J. Recent Advances in [2+2+2] Cycloaddition Reactions. Chem. Soc. Rev. 2011, 40, 3430–3444. [Google Scholar] [CrossRef] [PubMed]
- Kotha, S.; Sreevani, G. Molybdenum Hexacarbonyl: Air Stable Catalyst for Microwave Assisted Intermolecular [2+2+2] Co-Trimerization Involving Propargyl Halides. Tetrahedron Lett. 2015, 56, 5903–5908. [Google Scholar] [CrossRef]
- Tran, C.; Haddad, M.; Ratovelomanana-Vidal, V. Ruthenium-Catalyzed [2+2+2] Cycloaddition of α,ω-Diynes and Selenocyanates: An Entry to Selenopyridine Derivatives. Synthesis 2019, 51, 2532–2541. [Google Scholar] [CrossRef]
- Ye, F.; Boukattaya, F.; Haddad, M.; Ratovelomanana-Vidal, V.; Michelet, V. Synthesis of 2-Aminopyridines via Ruthenium-Catalyzed [2+2+2] Cycloaddition of 1,6- and 1,7-Diynes with Cyanamides: Scope and Limitations. New J. Chem. 2018, 42, 3222–3235. [Google Scholar] [CrossRef]
- Ye, F.; Haddad, M.; Michelet, V.; Ratovelomanana-Vidal, V. Solvent-Free Ruthenium Trichloride-Mediated [2 + 2 + 2] Cycloaddition of α,ω-Diynes and Cyanamides: A Convenient Access to 2-Aminopyridines. Org. Chem. Front. 2017, 4, 1063–1068. [Google Scholar] [CrossRef]
- Tran, C.; Haddad, M.; Ratovelomanana-Vidal, V. Ruthenium-Catalyzed, Microwave-Mediated [2+2+2] Cycloaddition: A Useful Combination for the Synthesis of 2-Aminopyridines. Synlett 2019, 30, 1891–1894. [Google Scholar] [CrossRef]
- Ye, F.; Haddad, M.; Ratovelomanana-Vidal, V.; Michelet, V. Ruthenium-Catalyzed [2 + 2 + 2] Cycloaddition Reaction Forming 2-Aminopyridine Derivatives from α,ω-Diynes and Cyanamides. Org. Lett. 2017, 19, 1104–1107. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, B.; Wang, B. Cp*Co(III)-Catalyzed Regioselective Synthesis of Cyclopenta[b]Carbazoles via Dual C(Sp2)–H Functionalization of 1-(Pyridin-2-Yl)-Indoles with Diynes. Org. Lett. 2018, 20, 7884–7887. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Wang, B. RhIII-Catalyzed Synthesis of Cyclopenta[b]Carbazoles via Cascade C-H/C-C Bond Cleavage and Cyclization Reactions: Using Amide as a Traceless Directing Group. Org. Lett. 2020, 22, 83–87. [Google Scholar] [CrossRef]
- Avarvari, N.; Le Floch, P.; Ricard, L.; Mathey, F. 1,3,2-Diazaphosphinines and -Diazaarsinines as Precursors for Polyfunctional Phosphinines and Arsinines. Organometallics 1997, 16, 4089–4098. [Google Scholar] [CrossRef]
- Weemers, J.J.M.; van der Graaff, W.N.P.; Pidko, E.A.; Lutz, M.; Müller, C. Bulky Phosphinines: From a Molecular Design to an Application in Homogeneous Catalysis. Chem. Eur. J. 2013, 19, 8991–9004. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Takata, S.; Sakata, K.; Nishibayashi, Y. Synthesis of Phosphabenzenes by an Iron-Catalyzed [2+2+2] Cycloaddition Reaction of Diynes with Phosphaalkynes. Angew. Chem. Int. Ed. 2015, 54, 7597–7601. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Zhang, K.; Louie, J. An Expeditious Route to Eight-Membered Heterocycles By Nickel-Catalyzed Cycloaddition: Low-Temperature C-C Bond Cleavage. Angew. Chem. Int. Ed. 2012, 51, 8602–8606. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, M.; Xiang, J.; Xi, H.; Wu, A. Generation of O-Quinodimethanes (o-QDMs) from Benzo[c]Oxepines and the Synthetic Application for Polysubstituted Tetrahydronaphthalenes. Tetrahedron 2015, 71, 7687–7694. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, Y.; Du, W.; Chen, Y.-C. Asymmetric Cross [10+2] Cycloadditions of 2-Alkylidene-1-Indanones and Activated Alkenes under Phase-Transfer Catalysis. Chem. Eur. J. 2020, 26, 1754–1758. [Google Scholar] [CrossRef]
- Ramachary, D.B.; Anebouselvy, K.; Chowdari, N.S.; Barbas, C.F. Direct Organocatalytic Asymmetric Heterodomino Reactions: The Knoevenagel/Diels−Alder/Epimerization Sequence for the Highly Diastereoselective Synthesis of Symmetrical and Nonsymmetrical Synthons of Benzoannelated Centropolyquinanes. J. Org. Chem. 2004, 69, 5838–5849. [Google Scholar] [CrossRef]
- Duan, J.; Cheng, J.; Li, B.; Qi, F.; Li, P. Enantioselective Synthesis of Spiro[1,3-indanedione–Tetrahydrothiophene]s by Organocatalytic Sulfa-Michael/Michael Domino Reaction. Eur. J. Org. Chem. 2015, 2015, 6130–6134. [Google Scholar] [CrossRef]
- Mahajan, S.; Chauhan, P.; Blümel, M.; Puttreddy, R.; Rissanen, K.; Raabe, G.; Enders, D. Asymmetric Synthesis of Spiro Tetrahydrothiophene-Indan-1,3-Diones via a Squaramide-Catalyzed Sulfa-Michael/Aldol Domino Reaction. Synthesis 2016, 48, 1131–1138. [Google Scholar] [CrossRef]
- Yazdani, H.; Bazgir, A. Lewis Acid Catalyzed Regio- and Diastereoselective Synthesis of Spiroisoxazolines via One-Pot Sequential Knoevenagel Condensation/1,3-Dipolar Cycloaddition Reaction. Synthesis 2019, 51, 1669–1679. [Google Scholar]
- Aitha, A.; Yennam, S.; Behera, M.; Anireddy, J.S. Synthesis of Spiroindene-1,3-Dione Isothiazolines via a Cascade Michael/1,3-Dipolar Cycloaddition Reaction of 1,3,4-Oxathiazol-2-One and 2-Arylidene-1,3-Indandiones. Tetrahedron Lett. 2017, 58, 578–581. [Google Scholar] [CrossRef]
- Liang, L.; Li, E.; Xie, P.; Huang, Y. Phosphine-Initiated Domino Reaction: A Convenient Method for the Preparation of Spirocyclopentanones. Chem. Asian J. 2014, 9, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Li, Y.; Yang, S. Silver-Catalyzed Cascade Radical Cyclization for Stereoselective Synthesis of Exocyclic Phosphine Oxides. Chin. J. Chem. 2017, 35, 316–322. [Google Scholar] [CrossRef]
- Shi, W.; Mao, B.; Xu, J.; Wang, Q.; Wang, W.; Wu, Y.; Li, X.; Guo, H. Phosphine-Catalyzed Cascade Michael Addition/[4+2] Cycloaddition Reaction of Allenoates and 2-Arylidene-1,3-Indanediones. Org. Lett. 2020, 22, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Champetter, P.; Castillo-Aguilera, O.; Taillier, C.; Brière, J.-F.; Dalla, V.; Oudeyer, S.; Comesse, S. N-Alkoxyacrylamides in Domino Reactions: Catalytic and Stereoselective Access to δ-Lactams. Eur. J. Org. Chem. 2019, 2019, 7703–7710. [Google Scholar] [CrossRef]
- Yang, S.-M.; Tsai, Y.-L.; Reddy, G.M.; Möhlmann, L.; Lin, W. Chemo- and Diastereoselective Michael–Michael-Acetalization Cascade for the Synthesis of 1,3-Indandione-Fused Spiro[4.5]Decan Scaffolds. J. Org. Chem. 2017, 82, 9182–9190. [Google Scholar] [CrossRef]
- Ren, W.; Wang, X.-Y.; Li, J.-J.; Tian, M.; Liu, J.; Ouyang, L.; Wang, J.-H. Efficient Construction of Biologically Important Functionalized Polycyclic Spiro-Fused Carbocyclicoxindoles via an Asymmetric Organocatalytic Quadruple-Cascade Reaction. RSC Adv. 2017, 7, 1863–1868. [Google Scholar] [CrossRef]
- Banothu, J.; Basavoju, S.; Bavantula, R. Pyridinium Ylide Assisted Highly Stereoselective One-Pot Synthesis of Trans-2-(4-Chlorobenzoyl)-3-Aryl-Spiro[Cyclopropane-1,2′-Inden]-1′,3′-Diones and Their Antimicrobial and Nematicidal Activities. J. Heterocycl. Chem. 2015, 52, 853–860. [Google Scholar] [CrossRef]
- Nassiri, M.; Milani, F.J.; Hassankhani, A. Synthesis of Spiro Pyrrolobenzothiazole Derivatives via a Three-Component Reaction. J. Heterocycl. Chem. 2015, 52, 1162–1166. [Google Scholar] [CrossRef]
- Manjappa, K.B.; Peng, Y.-T.; Jhang, W.-F.; Yang, D.-Y. Microwave-Promoted, Catalyst-Free, Multi-Component Reaction of Proline, Aldehyde, 1,3-Diketone: One Pot Synthesis of Pyrrolizidines and Pyrrolizinones. Tetrahedron 2016, 72, 853–861. [Google Scholar] [CrossRef]
- Rajeswari, M.; Sindhu, J.; Singh, H.; Khurana, J.M. An Efficient, Green Synthesis of Novel Regioselective and Stereoselective Indan-1,3-Dione Grafted Spirooxindolopyrrolizidine Linked 1,2,3-Triazoles via a One-Pot Five-Component Condensation Using PEG-400. RSC Adv. 2015, 5, 39686–39691. [Google Scholar] [CrossRef]
- Firouzi-Haji, R.; Maleki, A. L-Proline-Functionalized Fe3O4 Nanoparticles as an Efficient Nanomagnetic Organocatalyst for Highly Stereoselective One-Pot Two-Step Tandem Synthesis of Substituted Cyclopropanes. ChemistrySelect 2019, 4, 853–857. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Han, Y.; Chen, C.-F. PH-Controlled Motions in Mechanically Interlocked Molecules. Mater. Chem. Front. 2020, 4, 12–28. [Google Scholar] [CrossRef]
- Kitson, P.J.; Parenty, A.D.C.; Richmond, C.J.; Long, D.-L.; Cronin, L. A New C-C Bond Forming Annulation Reaction Leading to PH Switchable Heterocycles. Chem. Commun. 2009, 27, 4067–4069. [Google Scholar] [CrossRef]
- Qi, J.; Zheng, J.; Cui, S. Facile Synthesis of Carbo- and Heterocycles via Fe(Iii)-Catalyzed Alkene Hydrofunctionalization. Org. Chem. Front. 2018, 5, 222–225. [Google Scholar] [CrossRef]
- Duan, J.; Mao, Y.; Zhang, L.; Zhu, N.; Fang, Z.; Guo, K. Copper-Catalyzed [3+2] Annulation of 2-Arylidene-1,3-Indandiones with N-Acetyl Enamides for the Synthesis of Spiropyrrolines. Adv. Synth. Catal. 2020, 362, 695–699. [Google Scholar] [CrossRef]
- Zhao, B.; Liang, H.-W.; Yang, J.; Yang, Z.; Wei, Y. Copper-Catalyzed Intermolecular Cyclization between Oximes and Alkenes: A Facile Access to Spiropyrrolines. ACS Catal. 2017, 7, 5612–5617. [Google Scholar] [CrossRef]
- Gupta, A.K.; Vaishanv, N.K.; Kant, R.; Mohanan, K. Rapid and Selective Synthesis of Spiropyrazolines and Pyrazolylphthalides Employing Seyferth–Gilbert Reagent. Org. Biomol. Chem. 2017, 15, 6411–6415. [Google Scholar] [CrossRef]
- Das, S. Annulations Involving 2-Arylidene-1,3-Indanediones: Stereoselective Synthesis of Spiro- and Fused Scaffolds. New J. Chem. 2020, 44, 17148–17176. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Dumur, F.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Visible Light Sensitive Photoinitiating Systems: Recent Progress in Cationic and Radical Photopolymerization Reactions under Soft Conditions. Prog. Polym. Sci. 2015, 41, 32–66. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Garra, P.; Schmitt, M.; Toufaily, J.; Hamieh, T.; Graff, B.; Fouassier, J.P.; Dumur, F.; Lalevée, J. 3-Hydroxyflavone and N-Phenylglycine in High Performance Photoinitiating Systems for 3D Printing and Photocomposites Synthesis. Macromolecules 2018, 51, 4633–4641. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Xiao, P.; Delgove, M.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Chalcone Derivatives as Highly Versatile Photoinitiators for Radical, Cationic, Thiol–Ene and IPN Polymerization Reactions upon Exposure to Visible Light. Polym. Chem. 2014, 5, 382–390. [Google Scholar] [CrossRef]
- Zivic, N.; Zhang, J.; Bardelang, D.; Dumur, F.; Xiao, P.; Jet, T.; Versace, D.-L.; Dietlin, C.; Morlet-Savary, F.; Graff, B.; et al. Novel Naphthalimide–Amine Based Photoinitiators Operating under Violet and Blue LEDs and Usable for Various Polymerization Reactions and Synthesis of Hydrogels. Polym. Chem. 2015, 7, 418–429. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Contal, E.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. New Insights into Radical and Cationic Polymerizations upon Visible Light Exposure: Role of Novel Photoinitiator Systems Based on the Pyrene Chromophore. Polym. Chem. 2013, 4, 1625–1634. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. N-[2-(Dimethylamino)Ethyl]-1,8-Naphthalimide Derivatives as Photoinitiators under LEDs. Polym. Chem. 2018, 9, 994–1003. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Contal, E.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Novel Highly Efficient Organophotocatalysts: Truxene–Acridine-1,8-Diones as Photoinitiators of Polymerization. Macromol. Chem. Phys. 2013, 214, 2189–2201. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Visible Light Metal-Based Photocatalysts for Polymerization under Low Light Intensity. Catalysts 2019, 9, 736. [Google Scholar] [CrossRef]
- Bonardi, A.-H.; Dumur, F.; Noirbent, G.; Lalevée, J.; Gigmes, D. Organometallic vs Organic Photoredox Catalysts for Photocuring Reactions in the Visible Region. Beilstein J. Org. Chem. 2018, 14, 3025–3046. [Google Scholar] [CrossRef]
- Zivic, N.; Bouzrati-Zerelli, M.; Kermagoret, A.; Dumur, F.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Photocatalysts in Polymerization Reactions. ChemCatChem 2016, 8, 1617–1631. [Google Scholar] [CrossRef]
- Lalevée, J.; Telitel, S.; Xiao, P.; Lepeltier, M.; Dumur, F.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P. Metal and Metal-Free Photocatalysts: Mechanistic Approach and Application as Photoinitiators of Photopolymerization. Beilstein J. Org. Chem. 2014, 10, 863–876. [Google Scholar] [CrossRef]
- Sun, K.; Pigot, C.; Zhang, Y.; Borjigin, T.; Morlet-Savary, F.; Graff, B.; Nechab, M.; Xiao, P.; Dumur, F.; Lalevée, J. Sunlight Induced Polymerization Photoinitiated by Novel Push–Pull Dyes: Indane-1,3-Dione, 1H-Cyclopenta[b]Naphthalene-1,3(2H)-Dione and 4-Dimethoxyphenyl-1-Allylidene Derivatives. Macromol. Chem. Phys. 2022, 223, 2100439. [Google Scholar] [CrossRef]
- Sun, K.; Liu, S.; Chen, H.; Morlet-Savary, F.; Graff, B.; Pigot, C.; Nechab, M.; Xiao, P.; Dumur, F.; Lalevée, J. N-Ethyl Carbazole-1-Allylidene-Based Push-Pull Dyes as Efficient Light Harvesting Photoinitiators for Sunlight Induced Polymerization. Eur. Polym. J. 2021, 147, 110331. [Google Scholar] [CrossRef]
- Sun, K.; Liu, S.; Pigot, C.; Brunel, D.; Graff, B.; Nechab, M.; Gigmes, D.; Morlet-Savary, F.; Zhang, Y.; Xiao, P.; et al. Novel Push–Pull Dyes Derived from 1H-Cyclopenta[b]Naphthalene-1,3(2H)-Dione as Versatile Photoinitiators for Photopolymerization and Their Related Applications: 3D Printing and Fabrication of Photocomposites. Catalysts 2020, 10, 1196. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Brunel, D.; Dumur, F. Recent Advances on Push–Pull Organic Dyes as Visible Light Photoinitiators of Polymerization. Eur. Polym. J. 2020, 133, 109797. [Google Scholar] [CrossRef]
- Mokbel, H.; Toufaily, J.; Hamieh, T.; Dumur, F.; Campolo, D.; Gigmes, D.; Fouassier, J.P.; Ortyl, J.; Lalevée, J. Specific Cationic Photoinitiators for near UV and Visible LEDs: Iodonium versus Ferrocenium Structures. J. Appl. Polym. Sci. 2015, 132, 42759. [Google Scholar] [CrossRef]
- Dumur, F.; Nasr, G.; Wantz, G.; Mayer, C.R.; Dumas, E.; Guerlin, A.; Miomandre, F.; Clavier, G.; Bertin, D.; Gigmes, D. Cationic Iridium Complex for the Design of Soft Salt-Based Phosphorescent OLEDs and Color-Tunable Light-Emitting Electrochemical Cells. Org. Electron. 2011, 12, 1683–1694. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. A Benzophenone-Naphthalimide Derivative as Versatile Photoinitiator of Polymerization under near UV and Visible Lights. J. Polym. Sci. Part Polym. Chem. 2015, 53, 445–451. [Google Scholar] [CrossRef]
- Xiao, P.; Frigoli, M.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Julolidine or Fluorenone Based Push–Pull Dyes for Polymerization upon Soft Polychromatic Visible Light or Green Light. Macromolecules 2014, 47, 106–112. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Push–Pull (Thio)Barbituric Acid Derivatives in Dye Photosensitized Radical and Cationic Polymerization Reactions under 457/473 Nm Laser Beams or Blue LEDs. Polym. Chem. 2013, 4, 3866–3875. [Google Scholar] [CrossRef]
- Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Organic Electronics: An El Dorado in the Quest of New Photocatalysts for Polymerization Reactions. Acc. Chem. Res. 2016, 49, 1980–1989. [Google Scholar] [CrossRef]
- Mokbel, H.; Dumur, F.; Telitel, S.; Vidal, L.; Xiao, P.; Versace, D.-L.; Tehfe, M.-A.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; et al. Photoinitiating Systems of Polymerization and in Situ Incorporation of Metal Nanoparticles into Polymer Matrices upon Exposure to Visible Light: Push–Pull Malonate and Malononitrile Based Dyes. Polym. Chem. 2013, 4, 5679–5687. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Blue-to-Red Light Sensitive Push–Pull Structured Photoinitiators: Indanedione Derivatives for Radical and Cationic Photopolymerization Reactions. Macromolecules 2013, 46, 3332–3341. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Vidal, L.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Structural Effects in the Indanedione Skeleton for the Design of Low Intensity 300–500 Nm Light Sensitive Initiators. Macromolecules 2014, 47, 26–34. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. New Push–Pull Dyes Derived from Michler’s Ketone For Polymerization Reactions Upon Visible Lights. Macromolecules 2013, 46, 3761–3770. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Kavalli, T.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. The 1,3-Bis(Dicyanomethylidene)Indane Skeleton as a (Photo) Initiator in Thermal Ring Opening Polymerization at RT and Radical or Cationic Photopolymerization. RSC Adv. 2014, 4, 15930–15936. [Google Scholar] [CrossRef]
- Sun, K.; Pigot, C.; Chen, H.; Nechab, M.; Gigmes, D.; Morlet-Savary, F.; Graff, B.; Liu, S.; Xiao, P.; Dumur, F.; et al. Free Radical Photopolymerization and 3D Printing Using Newly Developed Dyes: Indane-1,3-Dione and 1H-Cyclopentanaphthalene-1,3-Dione Derivatives as Photoinitiators in Three-Component Systems. Catalysts 2020, 10, 463. [Google Scholar] [CrossRef]
- Bakshiev, N.G. Universal Intermolecular Interactions and Their Effect on the Position of the Electronic Spectra of Molecules in Two Component Solutions. Opt. Spektrosk. 1964, 16, 821–832. [Google Scholar]
- Kawski, A. Der Wellenzahl von Elecktronenbanden Lumineszierenden Molecule. Acta Phys Pol. 1966, 29, 507–518. [Google Scholar]
- Lippert, E. Dipolmoment Und Elektronenstruktur von Angeregten Molekülen. Z. Für Naturforschung A 1955, 10, 541–545. [Google Scholar] [CrossRef]
- McRae, E.G. Theory of Solvent Effects on Molecular Electronic Spectra. Frequency Shifts. J. Phys. Chem. 1957, 61, 562–572. [Google Scholar] [CrossRef]
- Suppan, P. Solvent Effects on the Energy of Electronic Transitions: Experimental Observations and Applications to Structural Problems of Excited Molecules. J. Chem. Soc. Inorg. Phys. Theor. 1968, 3125–3133. [Google Scholar] [CrossRef]
- Steyrer, B.; Neubauer, P.; Liska, R.; Stampfl, J. Visible Light Photoinitiator for 3D-Printing of Tough Methacrylate Resins. Materials 2017, 10, 1445. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-Curing 3D Printing Technique and Its Challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Zhu, D.; Xiao, P. Yellow Triazine as an Efficient Photoinitiator for Polymerization and 3D Printing under LEDs. Macromol. Chem. Phys. 2019, 220, 1900315. [Google Scholar] [CrossRef]
- Mitterbauer, M.; Knaack, P.; Naumov, S.; Markovic, M.; Ovsianikov, A.; Moszner, N.; Liska, R. Acylstannanes: Cleavable and Highly Reactive Photoinitiators for Radical Photopolymerization at Wavelengths above 500 Nm with Excellent Photobleaching Behavior. Angew. Chem. Int. Ed. 2018, 57, 12146–12150. [Google Scholar] [CrossRef]
- Bouzrati-Zerelli, M.; Zivic, N.; Dumur, F.; Gigmes, D.; Graff, B.; Fouassier, J.P.; Lalevée, J. New Violet to Yellow Light Sensitive Diketo Pyrrolo–Pyrrole Photoinitiators: High Performance Systems with Unusual Bleaching Properties and Solubility in Water. Polym. Chem. 2017, 8, 2028–2040. [Google Scholar] [CrossRef]
- Li, J.; Hao, Y.; Zhong, M.; Tang, L.; Nie, J.; Zhu, X. Synthesis of Furan Derivative as LED Light Photoinitiator: One-Pot, Low Usage, Photobleaching for Light Color 3D Printing. Dyes Pigments 2019, 165, 467–473. [Google Scholar] [CrossRef]
- Dumur, F. Zinc Complexes in OLEDs: An Overview. Synth. Met. 2014, 195, 241–251. [Google Scholar] [CrossRef]
- Dumur, F.; Bertin, D.; Gigmes, D. Iridium (III) Complexes as Promising Emitters for Solid–State Light–Emitting Electrochemical Cells (LECs). Int. J. Nanotechnol. 2012, 9, 377–395. [Google Scholar] [CrossRef]
- Terenziani, F.; Mongin, O.; Katan, C.; Bhatthula, B.K.G.; Blanchard-Desce, M. Effects of Dipolar Interactions on Linear and Nonlinear Optical Properties of Multichromophore Assemblies: A Case Study. Chem. Eur. J. 2006, 12, 3089–3102. [Google Scholar] [CrossRef]
- Lou, A.J.-T.; Marks, T.J. A Twist on Nonlinear Optics: Understanding the Unique Response of π-Twisted Chromophores. Acc. Chem. Res. 2019, 52, 1428–1438. [Google Scholar] [CrossRef]
- Rutkis, M.; Tokmakovs, A.; Jecs, E.; Kreicberga, J.; Kampars, V.; Kokars, V. Indanedione Based Binary Chromophore Supramolecular Systems as a NLO Active Polymer Composites. Opt. Prop. Funct. Mater. Nanomater. 2010, 32, 796–802. [Google Scholar] [CrossRef]
- Acharya, S.; Krief, P.; Khodorkovsky, V.; Kotler, Z.; Berkovic, G.; Klug, J.T.; Efrima, S. Studies of Langmuir and Langmuir–Blodgett Films of NLO-Active Amphiphilic 1,3-Indanedione Derivatives. New J. Chem. 2005, 29, 1049–1057. [Google Scholar] [CrossRef]
- Schwartz, H.; Mazor, R.; Khodorkovsky, V.; Shapiro, L.; Klug, J.T.; Kovalev, E.; Meshulam, G.; Berkovic, G.; Kotler, Z.; Efrima, S. Langmuir and Langmuir−Blodgett Films of NLO Active 2-(p-N-Alkyl-N-Methylamino)Benzylidene-1,3-Indandioneπ/A Curves, UV−Vis Spectra, and SHG Behavior. J. Phys. Chem. B 2001, 105, 5914–5921. [Google Scholar] [CrossRef]
- Doddamani, R.V.; Tasaganva, R.G.; Inamdar, S.R.; Kariduraganavar, M.Y. Synthesis of Chromophores and Polyimides with a Green Chemistry Approach for Second-Order Nonlinear Optical Applications. Polym. Adv. Technol. 2018, 29, 2091–2102. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent Chemosensors: The Past, Present and Future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Wu, X.; Xu, B.; Tong, H.; Wang, L. Highly Selective and Sensitive Detection of Cyanide by a Reaction-Based Conjugated Polymer Chemosensor. Macromolecules 2011, 44, 4241–4248. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Water-Soluble Photoinitiators in Biomedical Applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef]
- Hu, J.-W.; Lin, W.-C.; Hsiao, S.-Y.; Wu, Y.-H.; Chen, H.-W.; Chen, K.-Y. An Indanedione-Based Chemodosimeter for Selective Naked-Eye and Fluorogenic Detection of Cyanide. Sens. Actuators B Chem. 2016, 233, 510–519. [Google Scholar] [CrossRef]
- Wang, L.; Du, J.; Cao, D. A Colorimetric and Fluorescent Probe Containing Diketopyrrolopyrrole and 1,3-Indanedione for Cyanide Detection Based on Exciplex Signaling Mechanism. Sens. Actuators B Chem. 2014, 198, 455–461. [Google Scholar] [CrossRef]
- Facchetti, A. π-Conjugated Polymers for Organic Electronics and Photovoltaic Cell Applications. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Bian, L.; Zhu, E.; Tang, J.; Tang, W.; Zhang, F. Recent Progress in the Design of Narrow Bandgap Conjugated Polymers for High-Efficiency Organic Solar Cells. Top. Issue Conduct. Polym. 2012, 37, 1292–1331. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; You, W. Rational Design of High Performance Conjugated Polymers for Organic Solar Cells. Macromolecules 2012, 45, 607–632. [Google Scholar] [CrossRef]
- Bloking, J.T.; Han, X.; Higgs, A.T.; Kastrop, J.P.; Pandey, L.; Norton, J.E.; Risko, C.; Chen, C.E.; Brédas, J.-L.; McGehee, M.D.; et al. Solution-Processed Organic Solar Cells with Power Conversion Efficiencies of 2.5% Using Benzothiadiazole/Imide-Based Acceptors. Chem. Mater. 2011, 23, 5484–5490. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Zhan, X. A Solution-Processable Electron Acceptor Based on Dibenzosilole and Diketopyrrolopyrrole for Organic Solar Cells. Adv. Energy Mater. 2013, 3, 724–728. [Google Scholar] [CrossRef]
- Patil, H.; Gupta, A.; Bilic, A.; Bhosale, S.V.; Bhosale, S.V. A Solution-Processable Electron Acceptor Based on Diketopyrrolopyrrole and Naphthalenediimide Motifs for Organic Solar Cells. Tetrahedron Lett. 2014, 55, 4430–4432. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Wang, J.; Hou, J.; Li, Y.; Zhu, D.; Zhan, X. A Star-Shaped Perylene Diimide Electron Acceptor for High-Performance Organic Solar Cells. Adv. Mater. 2014, 26, 5137–5142. [Google Scholar] [CrossRef]
- Cheng, P.; Ye, L.; Zhao, X.; Hou, J.; Li, Y.; Zhan, X. Binary Additives Synergistically Boost the Efficiency of All-Polymer Solar Cells up to 3.45%. Energy Environ. Sci. 2014, 7, 1351–1356. [Google Scholar] [CrossRef]
- Patil, H.; Zu, W.X.; Gupta, A.; Chellappan, V.; Bilic, A.; Sonar, P.; Rananaware, A.; Bhosale, S.V.; Bhosale, S.V. A Non-Fullerene Electron Acceptor Based on Fluorene and Diketopyrrolopyrrole Building Blocks for Solution-Processable Organic Solar Cells with an Impressive Open-Circuit Voltage. Phys. Chem. Chem. Phys. 2014, 16, 23837–23842. [Google Scholar] [CrossRef]
- Raynor, A.M.; Gupta, A.; Patil, H.; Bilic, A.; Bhosale, S.V. A Diketopyrrolopyrrole and Benzothiadiazole Based Small Molecule Electron Acceptor: Design, Synthesis, Characterization and Photovoltaic Properties. RSC Adv. 2014, 4, 57635–57638. [Google Scholar] [CrossRef]
- Lin, Y.; Cheng, P.; Li, Y.; Zhan, X. A 3D Star-Shaped Non-Fullerene Acceptor for Solution-Processed Organic Solar Cells with a High Open-Circuit Voltage of 1.18 V. Chem. Commun. 2012, 48, 4773–4775. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Z.; Ye, L.; Zhan, C.; Hou, J.; Zhang, S.; Jiang, B.; Zhao, Y.; Huang, J.; Zhang, S.; et al. A Potential Perylene Diimide Dimer-Based Acceptor Material for Highly Efficient Solution-Processed Non-Fullerene Organic Solar Cells with 4.03% Efficiency. Adv. Mater. 2013, 25, 5791–5797. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, X.; Long, G.; Wan, X.; Chen, Y.; Zhang, Q. A Perylene Diimide (PDI)-Based Small Molecule with Tetrahedral Configuration as a Non-Fullerene Acceptor for Organic Solar Cells. J. Mater. Chem. C 2015, 3, 4698–4705. [Google Scholar] [CrossRef]
- Chen, W.; Salim, T.; Fan, H.; James, L.; Lam, Y.M.; Zhang, Q. Quinoxaline-Functionalized C60 Derivatives as Electron Acceptors in Organic Solar Cells. RSC Adv. 2014, 4, 25291–25301. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, J.; Yin, Z.; Duong, H.M.; Qiao, F.; Boey, F.; Hu, X.; Zhang, H.; Wudl, F. Synthesis, Characterization, and Physical Properties of a Conjugated Heteroacene: 2-Methyl-1,4,6,7,8,9-Hexaphenylbenz(g)Isoquinolin-3(2H)-One (BIQ). Chem. Asian J. 2011, 6, 856–862. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Dai, S.; Li, Y.; Zhu, D.; Zhan, X. A Twisted Dimeric Perylene Diimide Electron Acceptor for Efficient Organic Solar Cells. Adv. Energy Mater. 2014, 4, 1400420. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Y.; Cheng, P.; Wang, J.; Wu, Y.; Hou, J.; Zhan, X. An Electron Acceptor Based on Indacenodithiophene and 1,1-Dicyanomethylene-3-Indanone for Fullerene-Free Organic Solar Cells. J. Mater. Chem. A 2015, 3, 1910–1914. [Google Scholar] [CrossRef]
- Zhan, X.; Tan, Z.; Domercq, B.; An, Z.; Zhang, X.; Barlow, S.; Li, Y.; Zhu, D.; Kippelen, B.; Marder, S.R. A High-Mobility Electron-Transport Polymer with Broad Absorption and Its Use in Field-Effect Transistors and All-Polymer Solar Cells. J. Am. Chem. Soc. 2007, 129, 7246–7247. [Google Scholar] [CrossRef]
- Holliday, S.; Ashraf, R.S.; Nielsen, C.B.; Kirkus, M.; Röhr, J.A.; Tan, C.-H.; Collado-Fregoso, E.; Knall, A.-C.; Durrant, J.R.; Nelson, J.; et al. A Rhodanine Flanked Nonfullerene Acceptor for Solution-Processed Organic Photovoltaics. J. Am. Chem. Soc. 2015, 137, 898–904. [Google Scholar] [CrossRef]
- Patil, H.; Gupta, A.; Alford, B.; Ma, D.; Privér, S.H.; Bilic, A.; Sonar, P.; Bhosale, S.V. Conjoint Use of Dibenzosilole and Indan-1,3-Dione Functionalities to Prepare an Efficient Non-Fullerene Acceptor for Solution-Processable Bulk-Heterojunction Solar Cells. Asian J. Org. Chem. 2015, 4, 1096–1102. [Google Scholar] [CrossRef]
- Srivani, D.; Gupta, A.; Bhosale, S.V.; Puyad, A.L.; Xiang, W.; Li, J.; Evans, R.A.; Bhosale, S.V. Non-Fullerene Acceptors Based on Central Naphthalene Diimide Flanked by Rhodanine or 1,3-Indanedione. Chem. Commun. 2017, 53, 7080–7083. [Google Scholar] [CrossRef]
- Chen, X.; Feng, H.; Lin, Z.; Jiang, Z.; He, T.; Yin, S.; Wan, X.; Chen, Y.; Zhang, Q.; Qiu, H. Impact of End-Capped Groups on the Properties of Dithienosilole-Based Small Molecules for Solution-Processed Organic Solar Cells. Dyes Pigments 2017, 147, 183–189. [Google Scholar] [CrossRef]
- Raynor, A.M.; Gupta, A.; Plummer, C.M.; Jackson, S.L.; Bilic, A.; Patil, H.; Sonar, P.; Bhosale, S.V. Significant Improvement of Optoelectronic and Photovoltaic Properties by Incorporating Thiophene in a Solution-Processable D–A–D Modular Chromophore. Molecules 2015, 20, 21787–21801. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Li, M.; Feng, H.; Ni, W.; Zhang, H.; Wan, X.; Chen, Y. Efficient Carbazole-Based Small-Molecule Organic Solar Cells with an Improved Fill Factor. RSC Adv. 2018, 8, 4867–4871. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Rana, T.; Looser, A.; Stolte, M.; Würthner, F.; Bäuerle, P.; Sharma, G.D. High Performance A–D–A Oligothiophene-Based Organic Solar Cells Employing Two-Step Annealing and Solution-Processable Copper Thiocyanate (CuSCN) as an Interfacial Hole Transporting Layer. J. Mater. Chem. A 2016, 4, 17344–17353. [Google Scholar] [CrossRef]
- Qi, X.; Lo, Y.-C.; Zhao, Y.; Xuan, L.; Ting, H.-C.; Wong, K.-T.; Rahaman, M.; Chen, Z.; Xiao, L.; Qu, B. Two Novel Small Molecule Donors and the Applications in Bulk-Heterojunction Solar Cells. Front. Chem. 2018, 6, 260. [Google Scholar] [CrossRef]
- Adhikari, T.; Solanke, P.; Pathak, D.; Wagner, T.; Bureš, F.; Reed, T.; Nunzi, J.-M. T-Shaped Indan-1,3-Dione Derivatives as Promising Electron Donors for Bulk Heterojunction Small Molecule Solar Cell. Opt. Mater. 2017, 69, 312–317. [Google Scholar] [CrossRef]
- Walker, T.K.; Suthers, A.J.; Roe, L.L.; Shaw, H. LXIX.—Syntheses of Antiseptic Derivatives of Indan-1: 3-Dione. Part II. Interaction of Alkylmalonyl Chlorides with p-Tolyl Methyl Ether. J. Chem. Soc. Resumed 1931, 514–520. [Google Scholar] [CrossRef]
- Robinson, F.A.; Suthers, A.J.; Walker, T.K. Some New Antiseptics Related to Indan-I:3-Dione. Biochem. J. 1932, 26, 1890–1901. [Google Scholar] [CrossRef]
- Wen, A.; Delaquis, P.; Stanich, K.; Toivonen, P. Antilisterial Activity of Selected Phenolic Acids. Food Microbiol. 2003, 20, 305–311. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol; American Society for Microbiology: Washington, DC, USA, 2009. [Google Scholar]
- Fairbrother, R.W. A Text-Book of Medical Bacteriology; William Heinemann: London, UK, 1937. [Google Scholar]
- Mohil, R.; Kumar, D.; Mor, S. Synthesis and Antimicrobial Activity of Some 1,3-Disubstituted Indeno[1,2-c]Pyrazoles. J. Heterocycl. Chem. 2014, 51, 203–211. [Google Scholar] [CrossRef]
- Mor, S.; Mohil, R.; Nagoria, S.; Kumar, A.; Lal, K.; Kumar, D.; Singh, V. Regioselective Synthesis, Antimicrobial Evaluation and QSAR Studies of Some 3-Aryl-1-Heteroarylindeno[1,2-c]Pyrazol-4(1H)-Ones. J. Heterocycl. Chem. 2017, 54, 1327–1341. [Google Scholar] [CrossRef]
- Alsharif, M.A.; Mukhtar, S.; Asiri, A.M.; Khan, S.A. One Pot Synthesis, Physicochemical and Photophysical Investigation of Biologically Active Pyridine-3-Carboxylate (ECPC) as Probe to Determine CMC of Surfactants in Organized Media. Colloids Surf. Physicochem. Eng. Asp. 2018, 543, 38–45. [Google Scholar] [CrossRef]
- Pandian, R.; Naushad, E.; Vijayakumar, V.; Peters, G.H.; Mondikalipudur Nanjappagounder, P. Synthesis and Crystal Structures of 2-Methyl-4-Aryl-5-Oxo-5H-Indeno [1,2-b] Pyridine Carboxylate Derivatives. Chem. Cent. J. 2014, 8, 34. [Google Scholar] [CrossRef]
- Gupta, R.; Chaudhary, R.P. Efficient Ionic Liquid-Catalysed Synthesis and Antimicrobial Studies of 4,6-Diaryl- and 4,5-Fused Pyrimidine-2-Thiones. J. Chem. Res. 2012, 36, 718–721. [Google Scholar] [CrossRef]
- Meena, K.; Kumari, S.; Khurana, J.M.; Malik, A.; Sharma, C.; Panwar, H. One Pot Three Component Synthesis of Spiro [Indolo-3,10′-Indeno[1,2-b] Quinolin]-2,4,11′-Triones as a New Class of Antifungal and Antimicrobial Agents. Chin. Chem. Lett. 2017, 28, 136–142. [Google Scholar] [CrossRef]
- Khanna, G.; Aggarwal, K.; Khurana, J. An Efficient and Confluent Approach for the Synthesis of Novel 3,4-Dihydro-2H-Naphtho[2,3-e][1,3]Oxazine-5,10-Dione Derivatives by a Three Component Reaction in Ionic Liquid. ChemInform 2015, 46, 46448–46454. [Google Scholar] [CrossRef]
- Saluja, P.; Khurana, J.M.; Sharma, C.; Aneja, K.R. An Efficient and Convenient Approach for the Synthesis of Novel Pyrazolo[1,2-a]Triazole-Triones and Evaluation of Their Antimicrobial Activities. Aust. J. Chem. 2014, 67, 867–874. [Google Scholar] [CrossRef]
- Singh, H.; Sindhu, J.; Khurana, J.M.; Sharma, C.; Aneja, K.R. Ultrasound Promoted One Pot Synthesis of Novel Fluorescent Triazolyl Spirocyclic Oxindoles Using DBU Based Task Specific Ionic Liquids and Their Antimicrobial Activity. Eur. J. Med. Chem. 2014, 77, 145–154. [Google Scholar] [CrossRef]
- Rajeswari, M.; Saluja, P.; Khurana, J.M. A Facile and Green Approach for the Synthesis of Spiro[Naphthalene-2,5′-Pyrimidine]-4-Carbonitrile via a One-Pot Three-Component Condensation Reaction Using DBU as a Catalyst. RSC Adv. 2016, 6, 1307–1312. [Google Scholar] [CrossRef]
- Avendano, C.; Menendez, J.C. Medicinal Chemistry of Anticancer Drugs; Elsevier Science: Amsterdam, The Netherlands, 1995; ISBN 978-0-444-62667-7. [Google Scholar]
- Kumar, D.; Jain, S.K. A Comprehensive Review of N-Heterocycles as Cytotoxic Agents. Curr. Med. Chem. 2016, 23, 4338–4394. [Google Scholar] [CrossRef]
- Lang, D.K.; Kaur, R.; Arora, R.; Saini, B.; Arora, S. Nitrogen-Containing Heterocycles as Anticancer Agents: An Overview. Anticancer Agents Med. Chem. 2020, 20, 2150–2168. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, M.; Morrell, A.; Ioanoviciu, A.; Antony, S.; Kohlhagen, G.; Agama, K.; Hollingshead, M.; Pommier, Y.; Cushman, M. Synthesis and Evaluation of Indenoisoquinoline Topoisomerase I Inhibitors Substituted with Nitrogen Heterocycles. J. Med. Chem. 2006, 49, 6283–6289. [Google Scholar] [CrossRef] [PubMed]
- Prachayasittikul, S.; Manam, P.; Chinworrungsee, M.; Isarankura-Na-Ayudhya, C.; Ruchirawat, S.; Prachayasittikul, V. Bioactive Azafluorenone Alkaloids from Polyalthia Debilis (Pierre) Finet & Gagnep. Molecules 2009, 14, 4414–4424. [Google Scholar] [CrossRef] [PubMed]
- Manpadi, M.; Uglinskii, P.Y.; Rastogi, S.K.; Cotter, K.M.; Wong, Y.-S.C.; Anderson, L.A.; Ortega, A.J.; Van slambrouck, S.; Steelant, W.F.A.; Rogelj, S.; et al. Three-Component Synthesis and Anticancer Evaluation of Polycyclic Indenopyridines Lead to the Discovery of a Novel Indenoheterocycle with Potent Apoptosis Inducing Properties. Org. Biomol. Chem. 2007, 5, 3865–3872. [Google Scholar] [CrossRef] [PubMed]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A Novel Assay for Apoptosis Flow Cytometric Detection of Phosphatidylserine Expression on Early Apoptotic Cells Using Fluorescein Labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Evdokimov, N.M.; Van slambrouck, S.; Heffeter, P.; Tu, L.; Le Calvé, B.; Lamoral-Theys, D.; Hooten, C.J.; Uglinskii, P.Y.; Rogelj, S.; Kiss, R.; et al. Structural Simplification of Bioactive Natural Products with Multicomponent Synthesis. 3. Fused Uracil-Containing Heterocycles as Novel Topoisomerase-Targeting Agents. J. Med. Chem. 2011, 54, 2012–2021. [Google Scholar] [CrossRef][Green Version]
- Abdel-Aziz, A.A.-M.; El-Azab, A.S.; Ceruso, M.; Supuran, C.T. Carbonic Anhydrase Inhibitory Activity of Sulfonamides and Carboxylic Acids Incorporating Cyclic Imide Scaffolds. Bioorg. Med. Chem. Lett. 2014, 24, 5185–5189. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Al-Said, M.S. Anticancer Activity of Novel Indenopyridine Derivatives. Arch. Pharm. Res. 2012, 35, 987–994. [Google Scholar] [CrossRef]
- Arnold, L.D.; Xu, Y.; Barlozzari, T.; Rafferty, P.; Hockley, M.; Turner, A. Indeno[1,2-C]Pyrazole Derivatives for Inhibiting Tyrosine Kinase Activity. U.S. Patent 6,534,655, 18 March 2003. [Google Scholar]
- Nugiel, D.A.; Vidwans, A.; Etzkorn, A.-M.; Rossi, K.A.; Benfield, P.A.; Burton, C.R.; Cox, S.; Doleniak, D.; Seitz, S.P. Synthesis and Evaluation of Indenopyrazoles as Cyclin-Dependent Kinase Inhibitors. 2. Probing the Indeno Ring Substituent Pattern. J. Med. Chem. 2002, 45, 5224–5232. [Google Scholar] [CrossRef]
- Yue, E.W.; Higley, C.A.; DiMeo, S.V.; Carini, D.J.; Nugiel, D.A.; Benware, C.; Benfield, P.A.; Burton, C.R.; Cox, S.; Grafstrom, R.H.; et al. Synthesis and Evaluation of Indenopyrazoles as Cyclin-Dependent Kinase Inhibitors. 3. Structure Activity Relationships at C3. J. Med. Chem. 2002, 45, 5233–5248. [Google Scholar] [CrossRef]
- Nugiel, D.A.; Carini, D.J.; Yue, E.W.; Dimeo, S.V. 5-Aminoindeno(1,2-c)Pyrazol-4-Ones as Anti-Cancer and Anti-Proliferative Agents. PCT/US1999/008616, 28 October 1999. [Google Scholar]
- Matsuo, T.; Nishida, S.; Takagi, T. Anticancer Agent. JPS60130521A, 12 July 1985. [Google Scholar]
- Heck, A.J.R.; Chandler, D.W. Imaging Techniques for the Study of Chemical Reaction Dynamics. Annu. Rev. Phys. Chem. 1995, 46, 335–372. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Tang, B.Z. AIE Luminogens for Bioimaging and Theranostics: From Organelles to Animals. Chem 2017, 3, 56–91. [Google Scholar] [CrossRef]
- Dai, Y.; He, F.; Ji, H.; Zhao, X.; Misal, S.; Qi, Z. Dual-Functional NIR AIEgens for High-Fidelity Imaging of Lysosomes in Cells and Photodynamic Therapy. ACS Sens. 2020, 5, 225–233. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Chen, W.; Huang, J.; Huang, C.; Sheng, J.; Song, X. A Lysosome-Targetable Fluorescent Probe for Simultaneously Sensing Cys/Hcy, GSH, and H2S from Different Signal Patterns. ACS Sens. 2018, 3, 2513–2517. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Su, H.; Li, S.; Lin, Y.; Ling, X.; Qin, A.; Tang, B.Z. An Easily Accessible Aggregation-Induced Emission Probe for Lipid Droplet-Specific Imaging and Movement Tracking. Chem. Commun. 2017, 53, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Daemen, S.; van Zandvoort, M.A.M.J.; Parekh, S.H.; Hesselink, M.K.C. Microscopy Tools for the Investigation of Intracellular Lipid Storage and Dynamics. Mol. Metab. 2016, 5, 153–163. [Google Scholar] [CrossRef]
- Zhai, J.; Zhang, Y.; Yang, C.; Xu, Y.; Qin, Y. A Long Wavelength Hydrophobic Probe for Intracellular Lipid Droplets. Analyst 2014, 139, 52–54. [Google Scholar] [CrossRef]
- Kim, E.; Lee, S.; Park, S.B. A Seoul-Fluor-Based Bioprobe for Lipid Droplets and Its Application in Image-Based High Throughput Screening. Chem. Commun. 2012, 48, 2331–2333. [Google Scholar] [CrossRef]
- Lee, J.H.; So, J.-H.; Jeon, J.H.; Choi, E.B.; Lee, Y.-R.; Chang, Y.-T.; Kim, C.-H.; Bae, M.A.; Ahn, J.H. Synthesis of a New Fluorescent Small Molecule Probe and Its Use for in Vivolipid Imaging. Chem. Commun. 2011, 47, 7500–7502. [Google Scholar] [CrossRef]
- Sharma, A.; Umar, S.; Kar, P.; Singh, K.; Sachdev, M.; Goel, A. A New Type of Biocompatible Fluorescent Probe AFN for Fixed and Live Cell Imaging of Intracellular Lipid Droplets. Analyst 2016, 141, 137–143. [Google Scholar] [CrossRef]
- Neef, A.B.; Schultz, C. Selective Fluorescence Labeling of Lipids in Living Cells. Angew. Chem. Int. Ed. 2009, 48, 1498–1500. [Google Scholar] [CrossRef] [PubMed]
- Eggert, D.; Rösch, K.; Reimer, R.; Herker, E. Visualization and Analysis of Hepatitis C Virus Structural Proteins at Lipid Droplets by Super-Resolution Microscopy. PLoS ONE 2014, 9, e102511. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile Red: A Selective Fluorescent Stain for Intracellular Lipid Droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef]
- Spandl, J.; White, D.J.; Peychl, J.; Thiele, C. Live Cell Multicolor Imaging of Lipid Droplets with a New Dye, LD540. Traffic 2009, 10, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Naskar, B.; Goswami, S.; Prodhan, C.; Chaudhuri, K.; Mukhopadhyay, C. A Quick Accelerating Microwave-Assisted Sustainable Technique: Permutated Spiro-Casing for Imaging Experiment. Mol. Divers. 2020, 24, 93–106. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Schapira, A.H. Non-Motor Symptoms of Parkinson’s Disease: Dopaminergic Pathophysiology and Treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef]
- Weintraub, D.; Comella, C.L.; Horn, S. Parkinson’s Disease—Part 2: Treatment of Motor Symptoms. Am. J. Manag. Care 2008, 14, S49–S58. [Google Scholar]
- Shook, B.C.; Rassnick, S.; Osborne, M.C.; Davis, S.; Westover, L.; Boulet, J.; Hall, D.; Rupert, K.C.; Heintzelman, G.R.; Hansen, K.; et al. In Vivo Characterization of a Dual Adenosine A2A/A1 Receptor Antagonist in Animal Models of Parkinson’s Disease. J. Med. Chem. 2010, 53, 8104–8115. [Google Scholar] [CrossRef]
- Shook, B.C.; Rassnick, S.; Hall, D.; Rupert, K.C.; Heintzelman, G.R.; Hansen, K.; Chakravarty, D.; Bullington, J.L.; Scannevin, R.H.; Magliaro, B.; et al. Methylene Amine Substituted Arylindenopyrimidines as Potent Adenosine A2A/A1 Antagonists. Bioorg. Med. Chem. Lett. 2010, 20, 2864–2867. [Google Scholar] [CrossRef]
- Atack, J.R.; Shook, B.C.; Rassnick, S.; Jackson, P.F.; Rhodes, K.; Drinkenburg, W.H.; Ahnaou, A.; te Riele, P.; Langlois, X.; Hrupka, B.; et al. JNJ-40255293, a Novel Adenosine A2A/A1 Antagonist with Efficacy in Preclinical Models of Parkinson’s Disease. ACS Chem. Neurosci. 2014, 5, 1005–1019. [Google Scholar] [CrossRef]
- Lim, H.-K.; Chen, J.; Sensenhauser, C.; Cook, K.; Preston, R.; Thomas, T.; Shook, B.; Jackson, P.F.; Rassnick, S.; Rhodes, K.; et al. Overcoming the Genotoxicity of a Pyrrolidine Substituted Arylindenopyrimidine As a Potent Dual Adenosine A2A/A1 Antagonist by Minimizing Bioactivation to an Iminium Ion Reactive Intermediate. Chem. Res. Toxicol. 2011, 24, 1012–1030. [Google Scholar] [CrossRef] [PubMed]
- Heintzelman, G.R.; Bullington, J.L.; Rupert, K.C. Arylindenopyridines and Arylindenopyrimidines and Related Therapeutic and Prophylactic Methods. U.S. 20050267138, 1 December 2005. [Google Scholar]
- Gijsen, H.J.M.; Berthelot, D.; De Cleyn, M.A.J.; Geuens, I.; Brône, B.; Mercken, M. Tricyclic 3,4-Dihydropyrimidine-2-Thione Derivatives as Potent TRPA1 Antagonists. Bioorg. Med. Chem. Lett. 2012, 22, 797–800. [Google Scholar] [CrossRef]
- Edwards, P.J.; Sturino, C. Managing the Liabilities Arising from Structural Alerts: A Safe Philosophy for Medicinal Chemists. Curr. Med. Chem. 2011, 18, 3116–3135. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Boas, W.V.E.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic Seizures and Epilepsy: Definitions Proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef]
- Picot, M.-C.; Baldy-Moulinier, M.; Daurès, J.-P.; Dujols, P.; Crespel, A. The Prevalence of Epilepsy and Pharmacoresistant Epilepsy in Adults: A Population-Based Study in a Western European Country. Epilepsia 2008, 49, 1230–1238. [Google Scholar] [CrossRef]
- Rashid, U.; Ansari, F.L. Chapter 2—Challenges in Designing Therapeutic Agents for Treating Alzheimer’s Disease-from Serendipity to Rationality. In Drug Design and Discovery in Alzheimer’s Disease; Atta-ur-Rahman, Choudhary, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 40–141. ISBN 978-0-12-803959-5. [Google Scholar]
- LaFerla, F.M.; Green, K.N.; Oddo, S. Intracellular Amyloid-β in Alzheimer’s Disease. Nat. Rev. Neurosci. 2007, 8, 499–509. [Google Scholar] [CrossRef]
- Tanoli, S.T.; Ramzan, M.; Hassan, A.; Sadiq, A.; Jan, M.S.; Khan, F.A.; Ullah, F.; Ahmad, H.; Bibi, M.; Mahmood, T.; et al. Design, Synthesis and Bioevaluation of Tricyclic Fused Ring System as Dual Binding Site Acetylcholinesterase Inhibitors. Bioorganic Chem. 2019, 83, 336–347. [Google Scholar] [CrossRef]
- Campagna, F.; Palluotto, F.; Carotti, A.; Maciocco, E. Synthesis, Central and Peripheral Benzodiazepine Receptor Affinity of Pyrazole and Pyrazole-Containing Polycyclic Derivatives. Il Farm. 2004, 59, 849–856. [Google Scholar] [CrossRef]
- Catto, M.; Aliano, R.; Carotti, A.; Cellamare, S.; Palluotto, F.; Purgatorio, R.; De Stradis, A.; Campagna, F. Design, Synthesis and Biological Evaluation of Indane-2-Arylhydrazinylmethylene-1,3-Diones and Indol-2-Aryldiazenylmethylene-3-Ones as β-Amyloid Aggregation Inhibitors. Eur. J. Med. Chem. 2010, 45, 1359–1366. [Google Scholar] [CrossRef]
- Shapiro, S.L.; Geiger, K.; Freedman, L. Indandione Anticoagulants. J. Org. Chem. 1960, 25, 1860–1865. [Google Scholar] [CrossRef]
- Murphy, M.J. Chapter 46—Anticoagulant Rodenticides. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 583–612. ISBN 978-0-12-811410-0. [Google Scholar]
- Daveluy, A.; Milpied, B.; Barbaud, A.; Lebrun-Vignes, B.; Gouraud, A.; Laroche, M.-L.; Ciobanu, E.; Bégaud, B.; Moore, N.; Miremont-Salamé, G.; et al. Fluindione and Drug Reaction with Eosinophilia and Systemic Symptoms: An Unrecognised Adverse Effect? Eur. J. Clin. Pharmacol. 2012, 68, 101–105. [Google Scholar] [CrossRef]
- Wehling, M.; Collins, R.; Gil, V.M.; Hanon, O.; Hardt, R.; Hoffmeister, M.; Monteiro, P.; Quinn, T.J.; Ropers, D.; Sergi, G.; et al. Appropriateness of Oral Anticoagulants for the Long-Term Treatment of Atrial Fibrillation in Older People: Results of an Evidence-Based Review and International Consensus Validation Process (OAC-FORTA 2016). Drugs Aging 2017, 34, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Long, D.; Chen, P.; Shi, H.; Li, H.; Fang, R.; Wang, X.; Xie, X.; She, X. Synthesis of 2,3-Disubstituted Indoles via a Tandem Reaction. Org. Chem. Front. 2020, 7, 2689–2695. [Google Scholar] [CrossRef]
- Murdock, K.C. Triacylhalomethanes: 2-Halo-2-Acyl-1,3-Indandiones. J. Org. Chem. 1959, 24, 845–849. [Google Scholar] [CrossRef]
- Larsen, B.J.; Rosano, R.J.; Ford-Hutchinson, T.A.; Reitz, A.B.; Wrobel, J.E. A Method for C2 Acylation of 1,3-Indandiones. Tetrahedron 2018, 74, 2762–2768. [Google Scholar] [CrossRef]
- Regnery, J.; Friesen, A.; Geduhn, A.; Göckener, B.; Kotthoff, M.; Parrhysius, P.; Petersohn, E.; Reifferscheid, G.; Schmolz, E.; Schulz, R.S.; et al. Rating the Risks of Anticoagulant Rodenticides in the Aquatic Environment: A Review. Environ. Chem. Lett. 2019, 17, 215–240. [Google Scholar] [CrossRef]
- Bates, N. Anticoagulant Rodenticide Toxicosis. Companion Anim. 2016, 21, 466–471. [Google Scholar] [CrossRef]

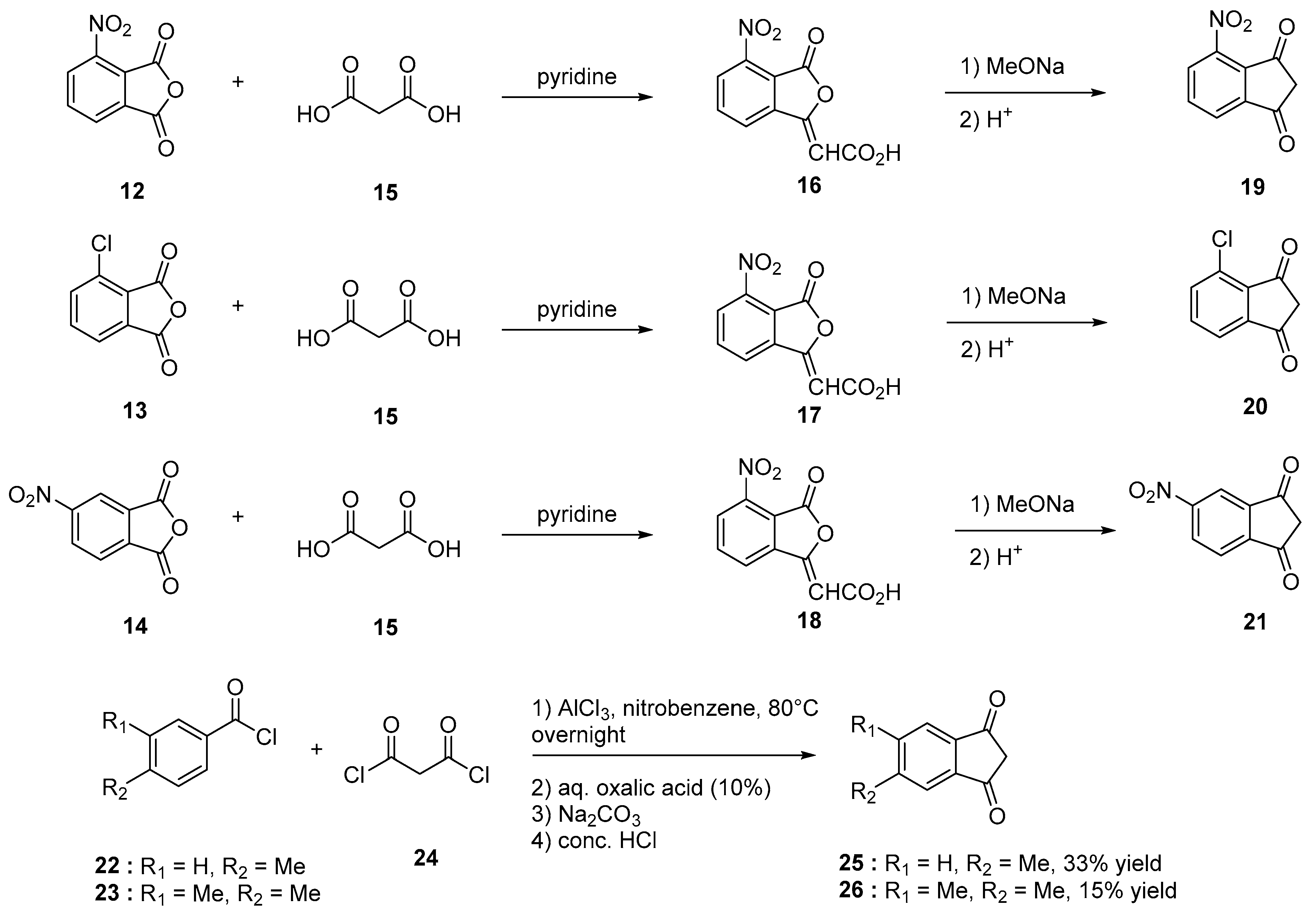
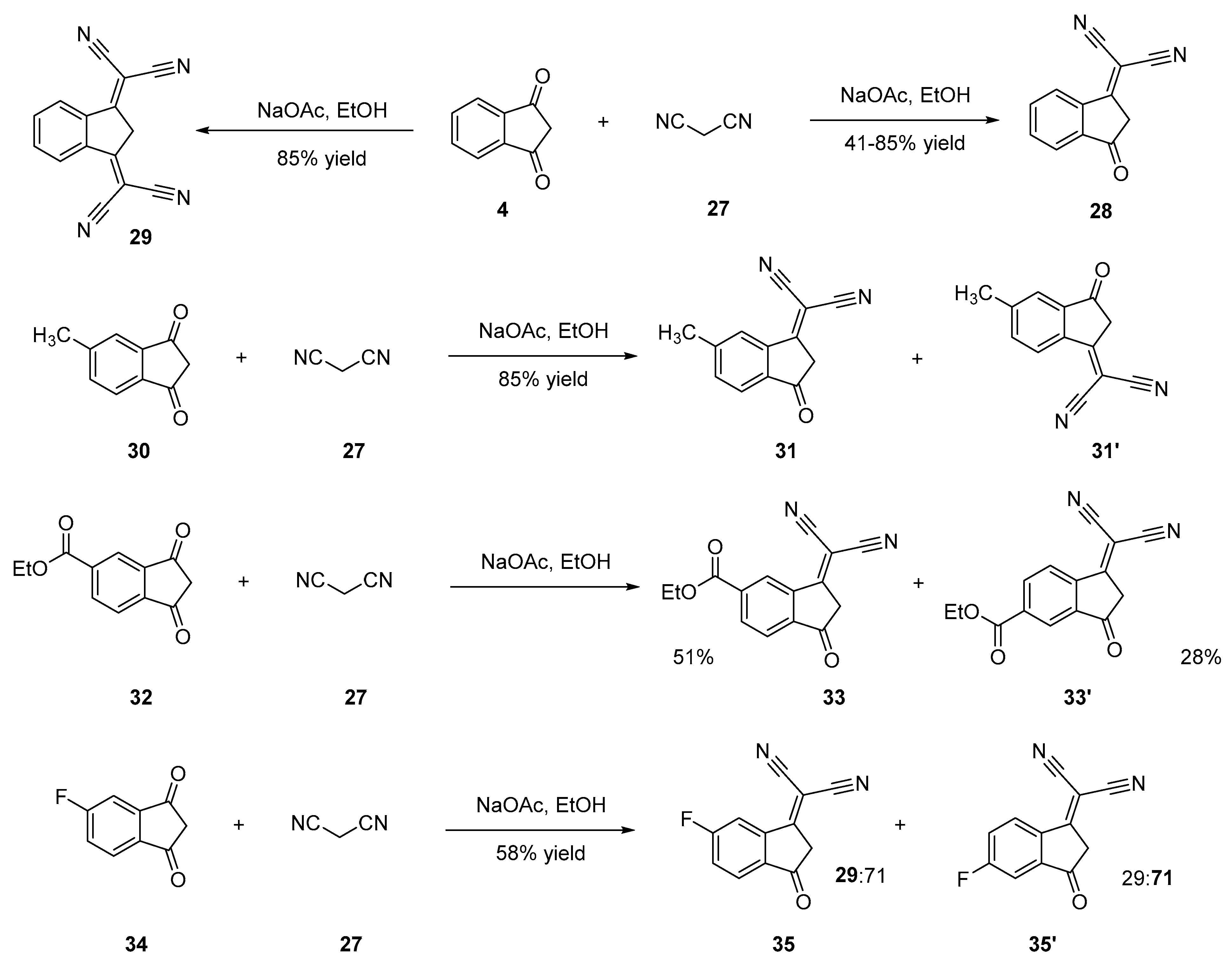
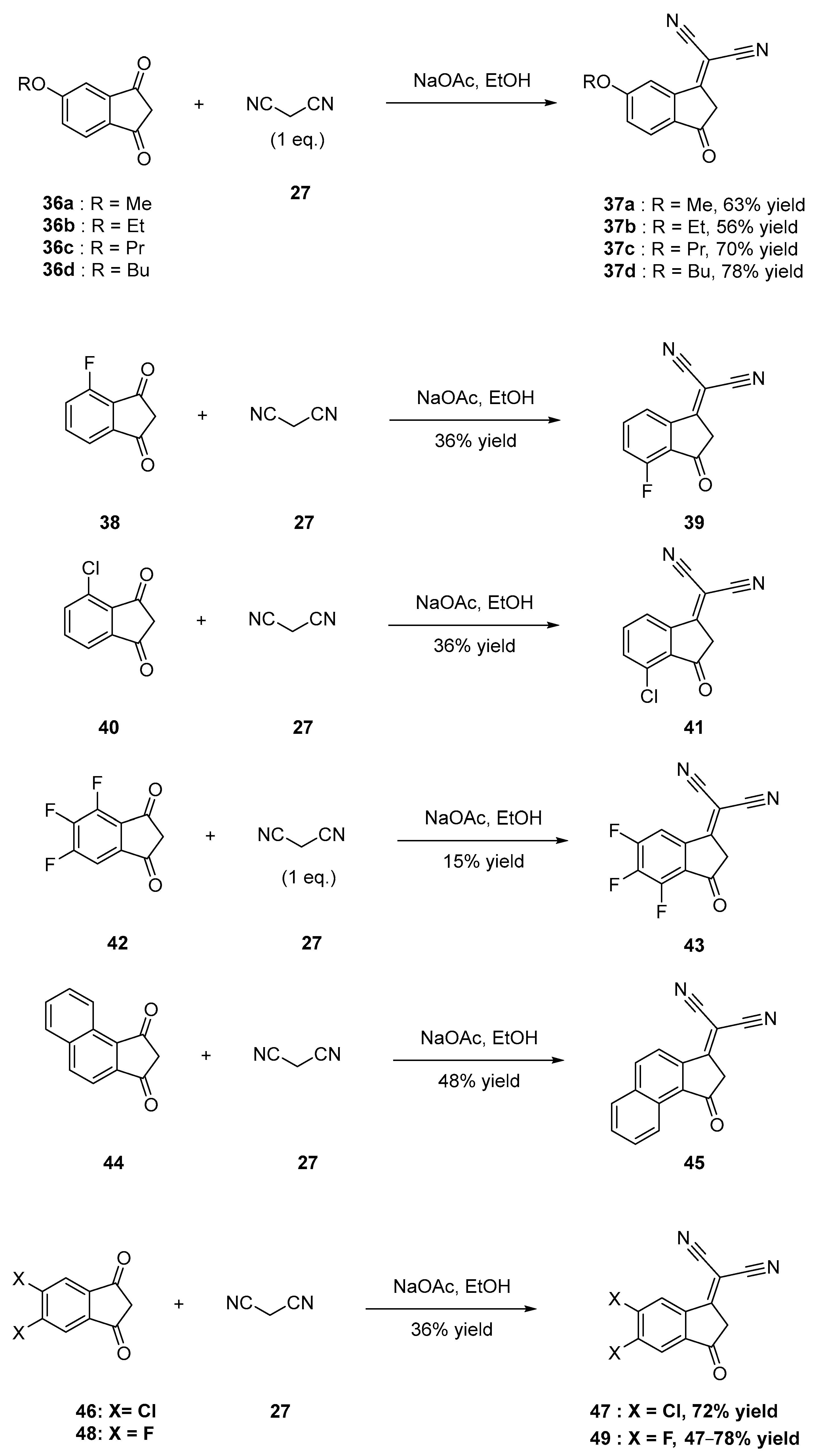
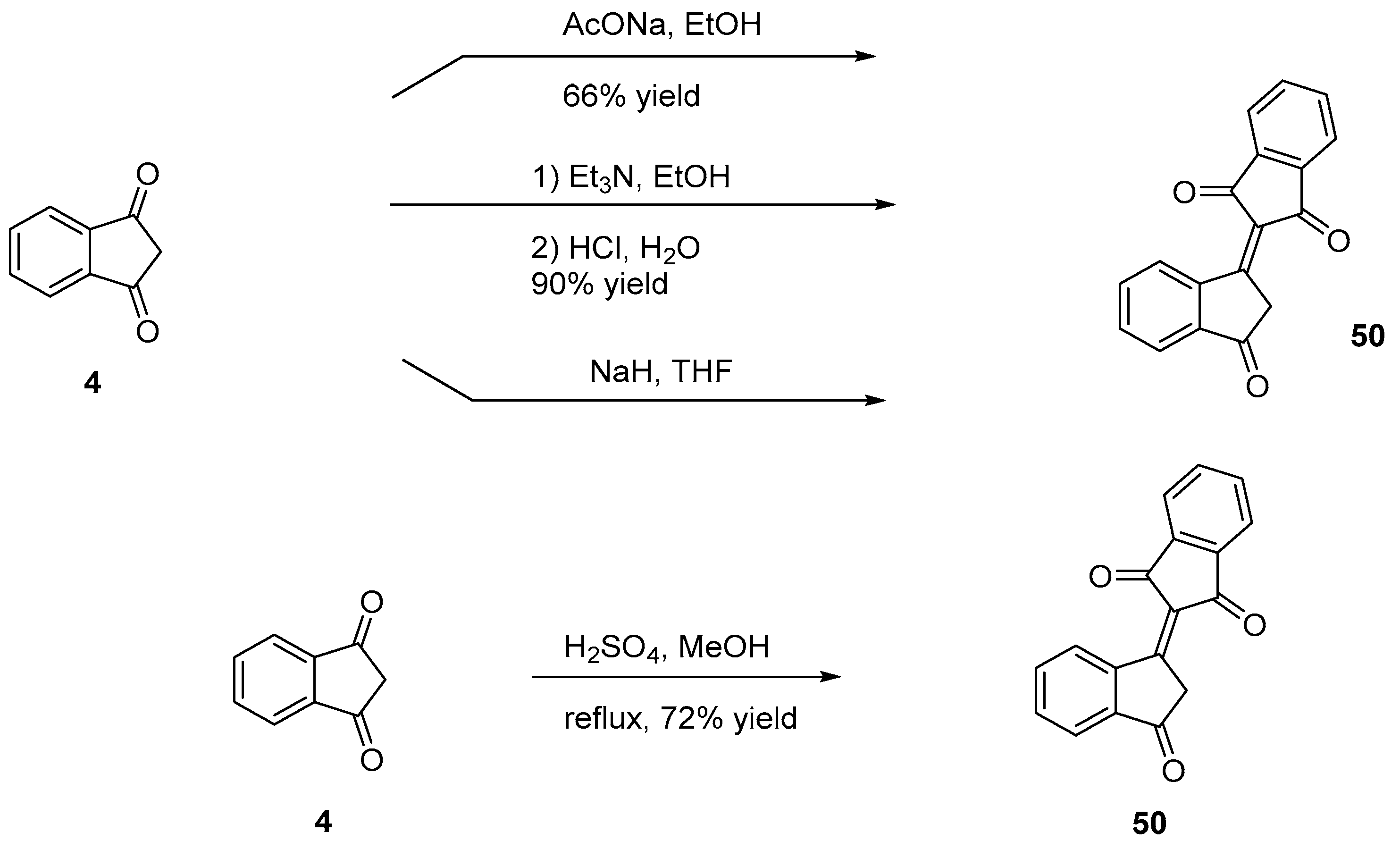

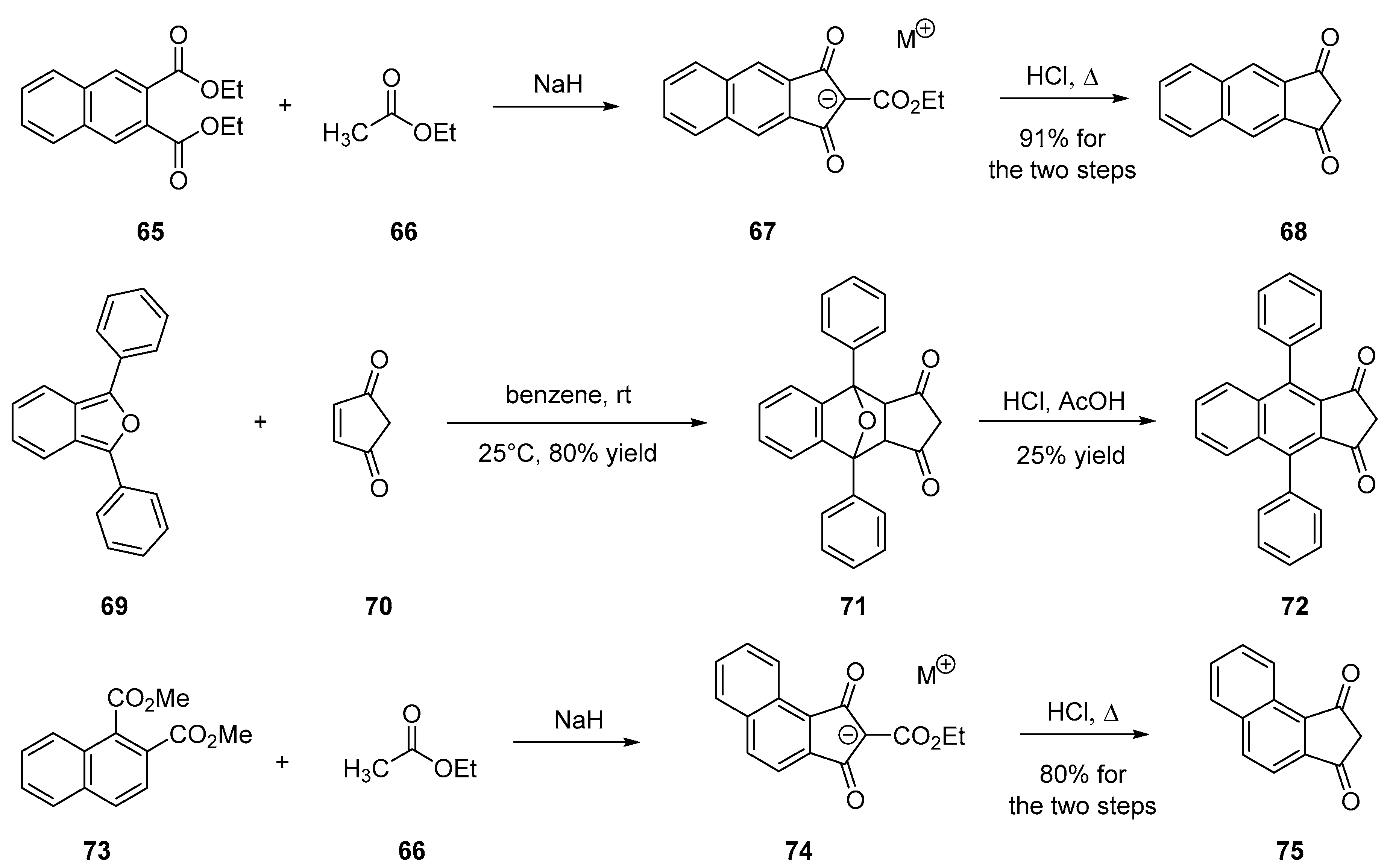

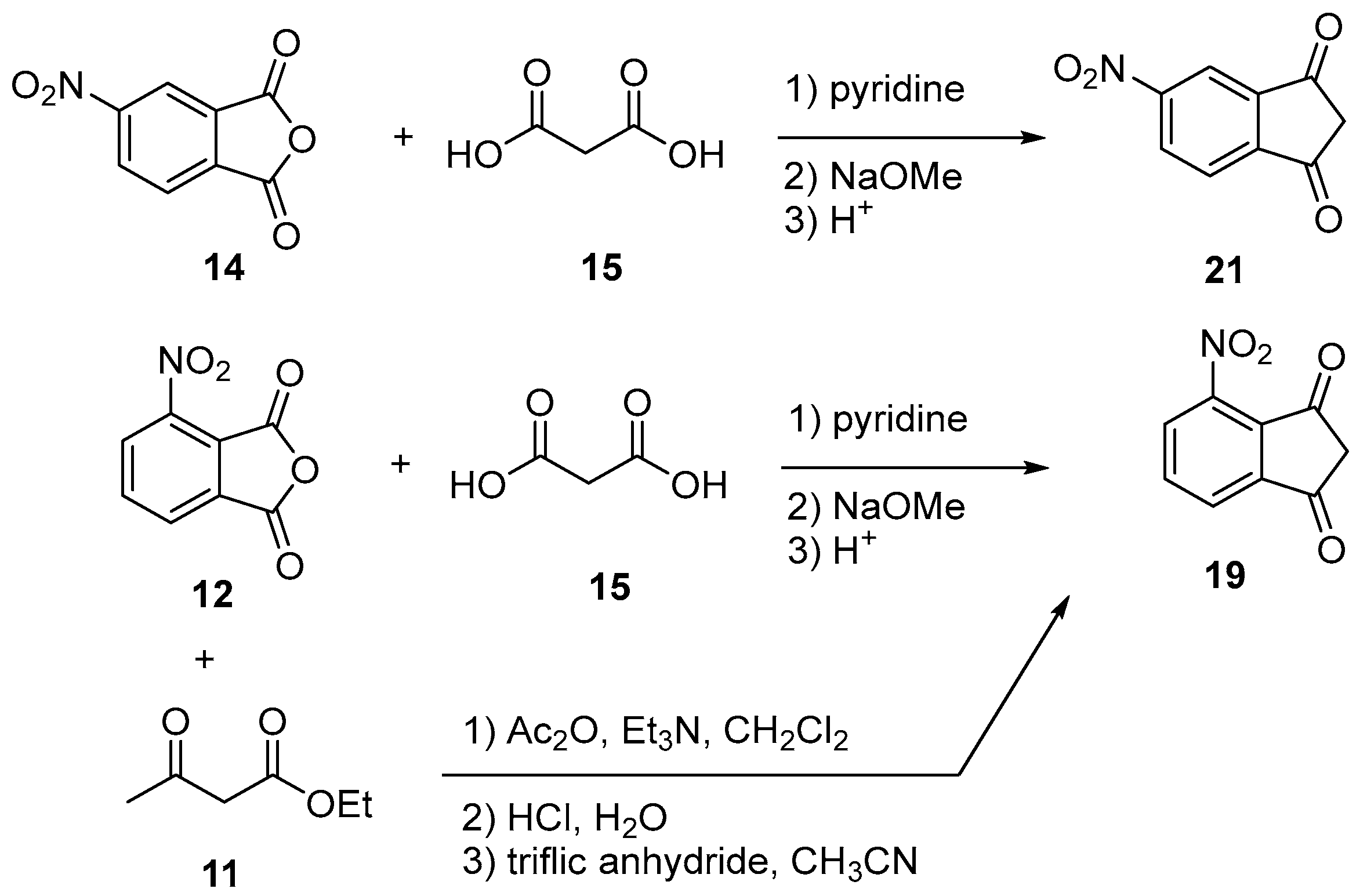



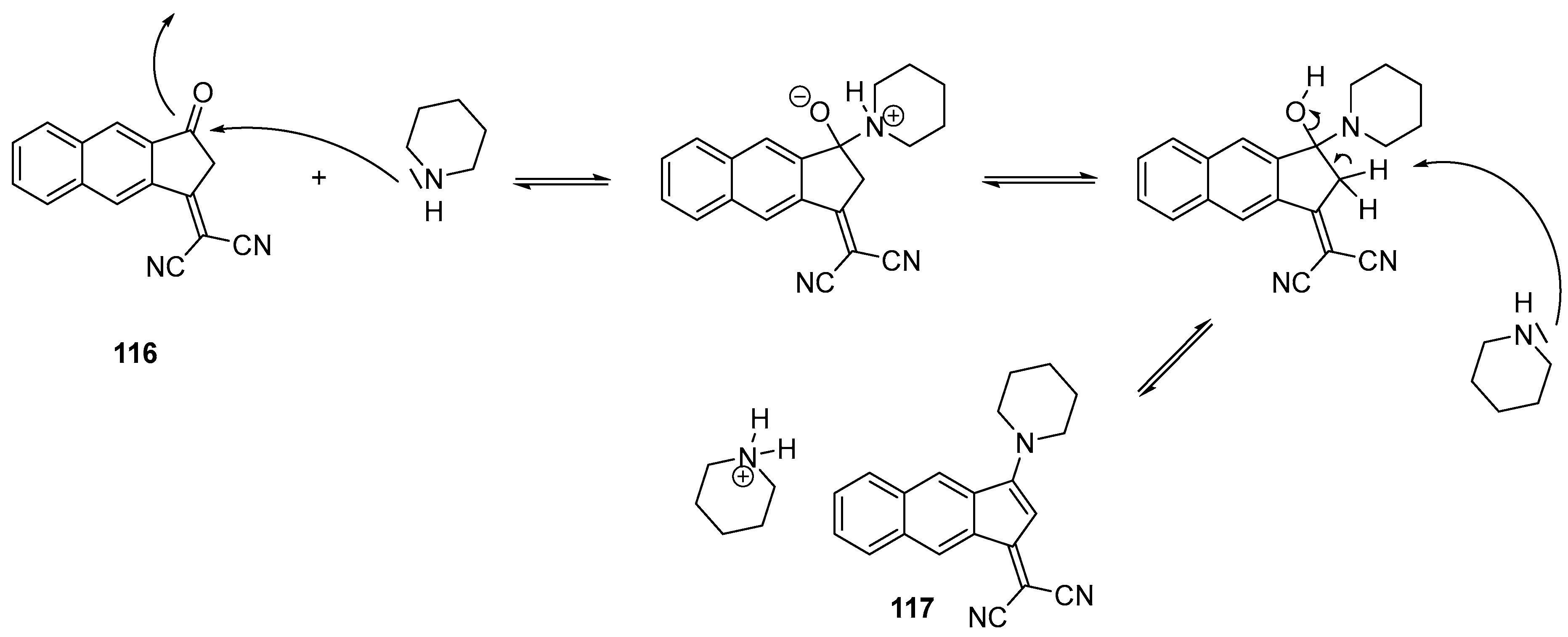
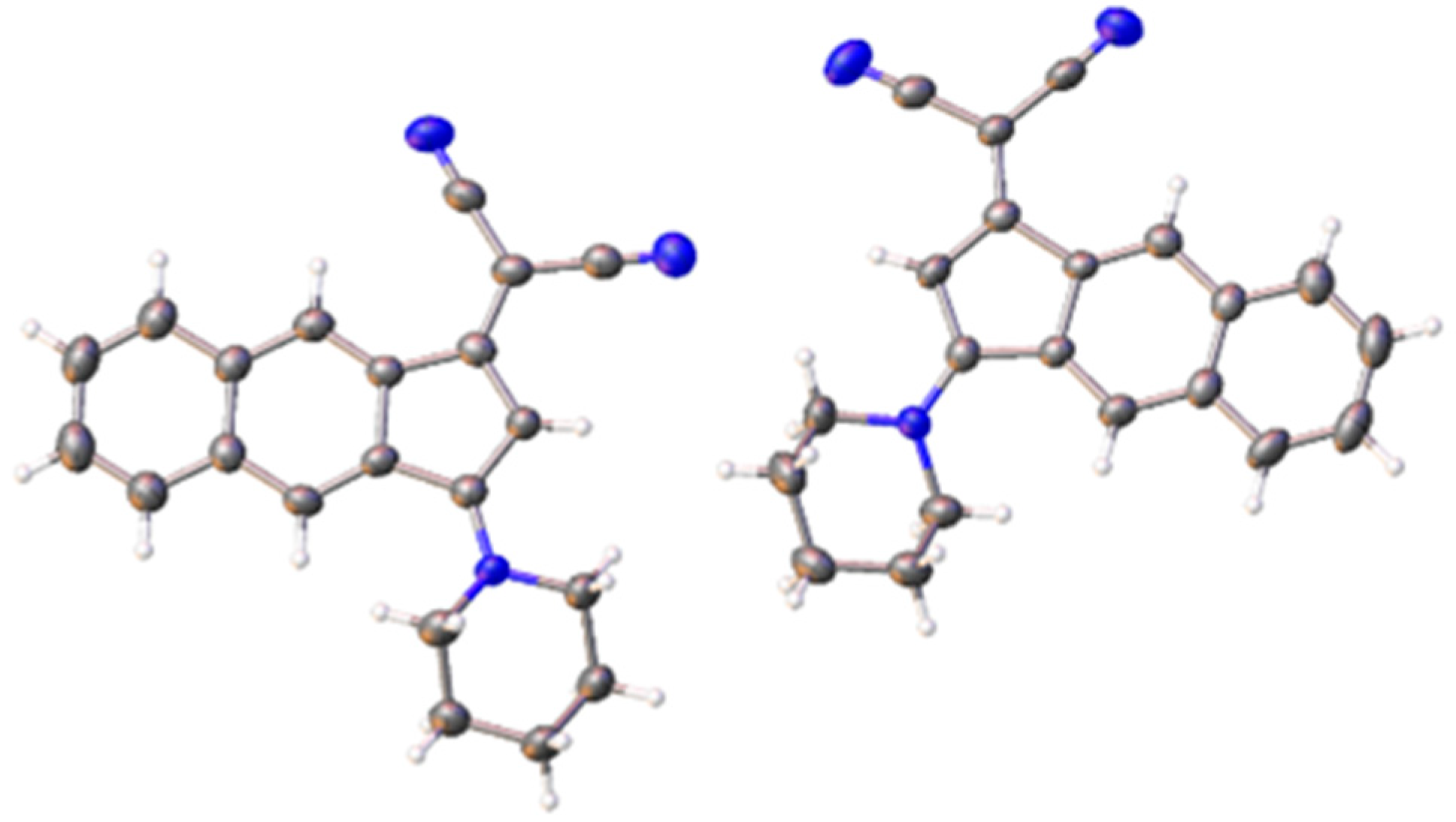
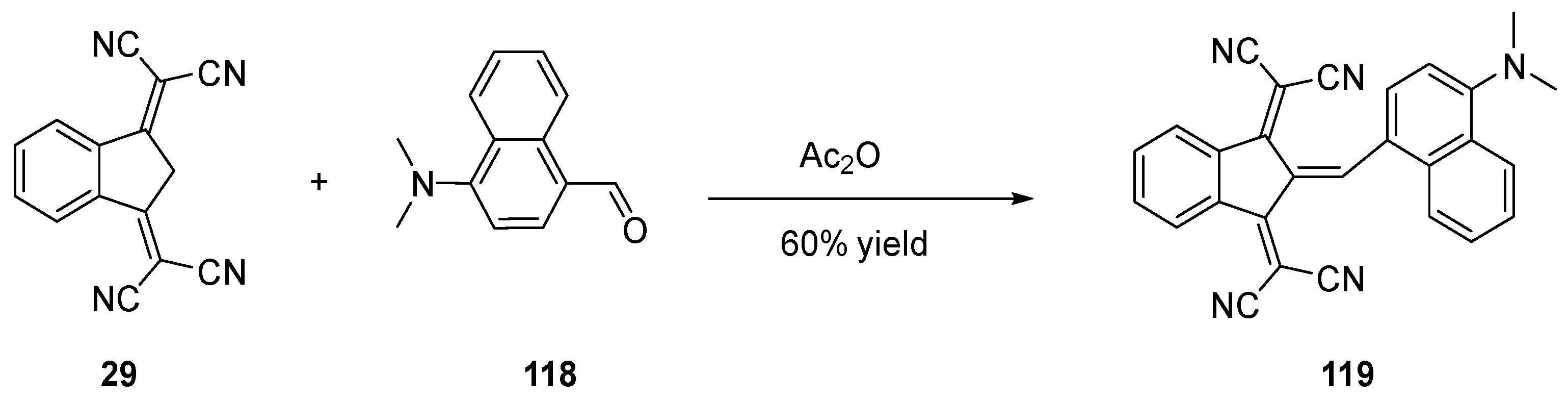

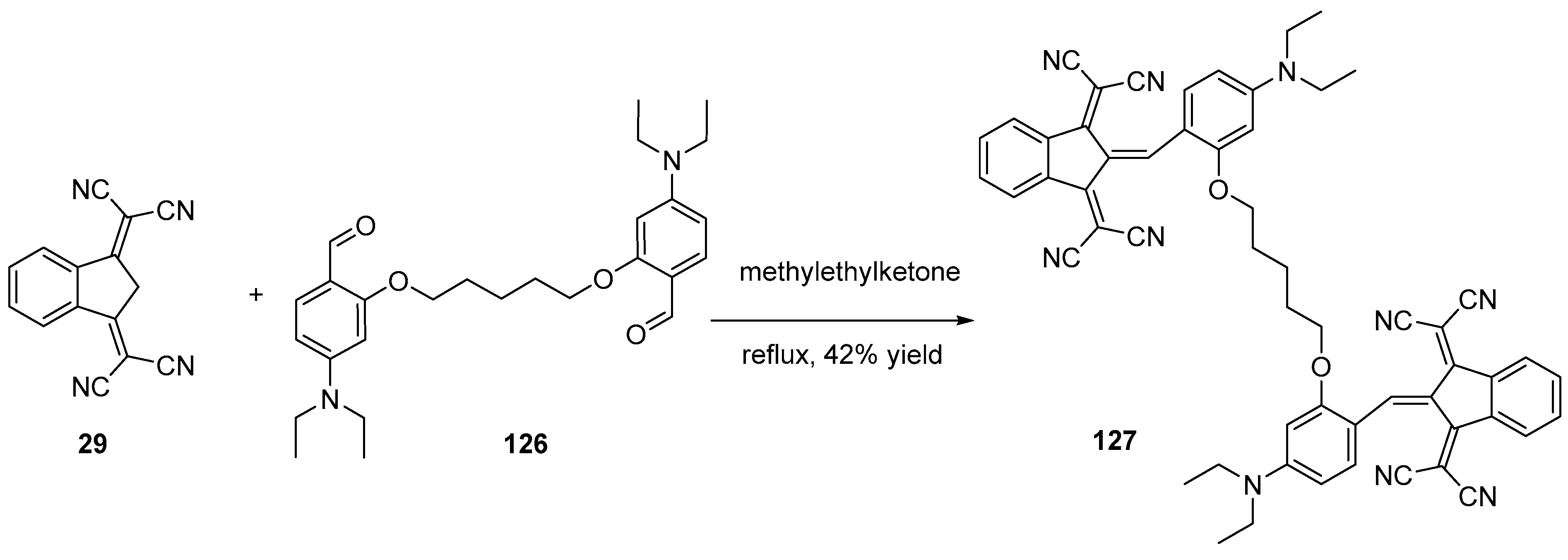
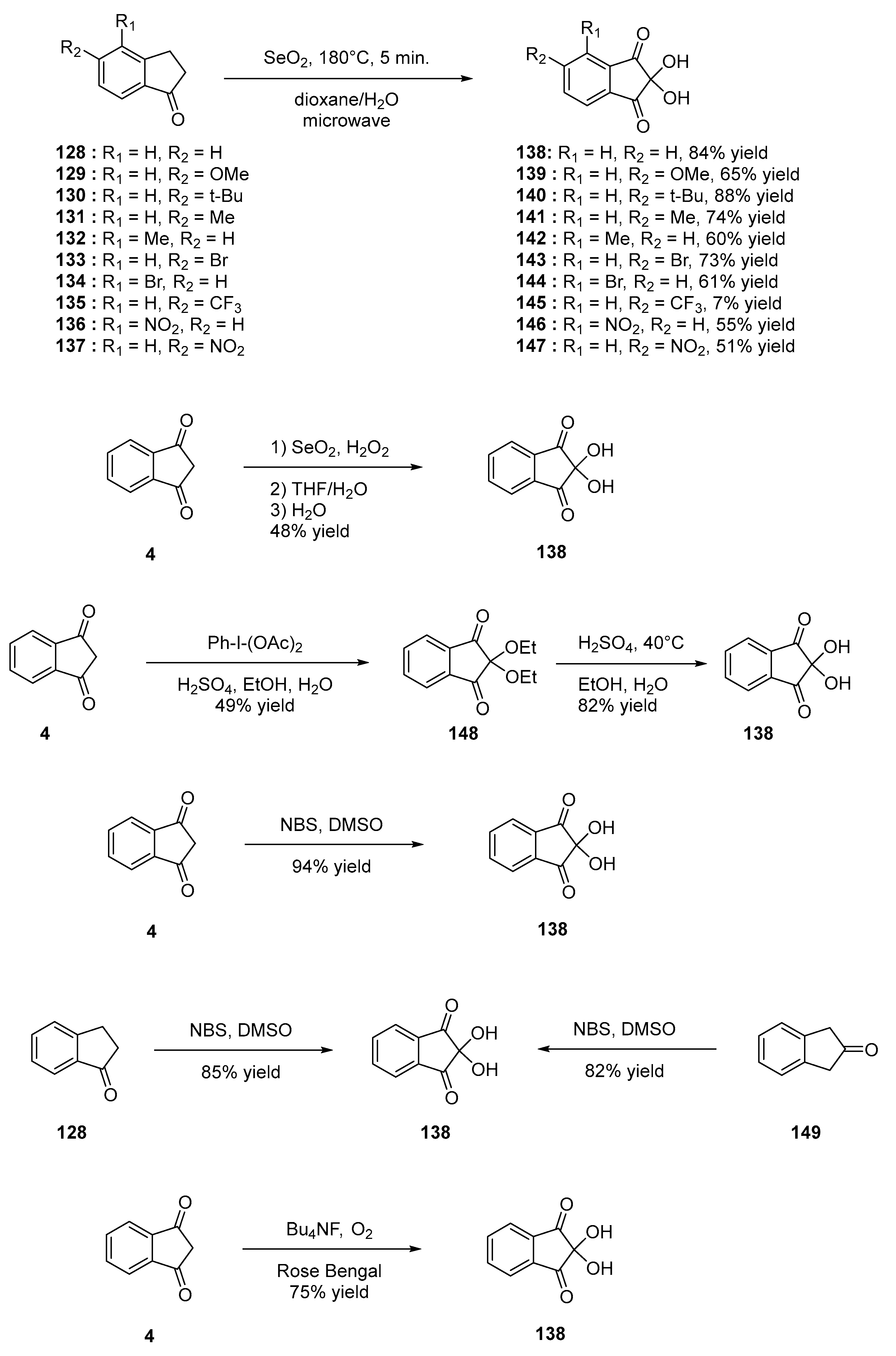
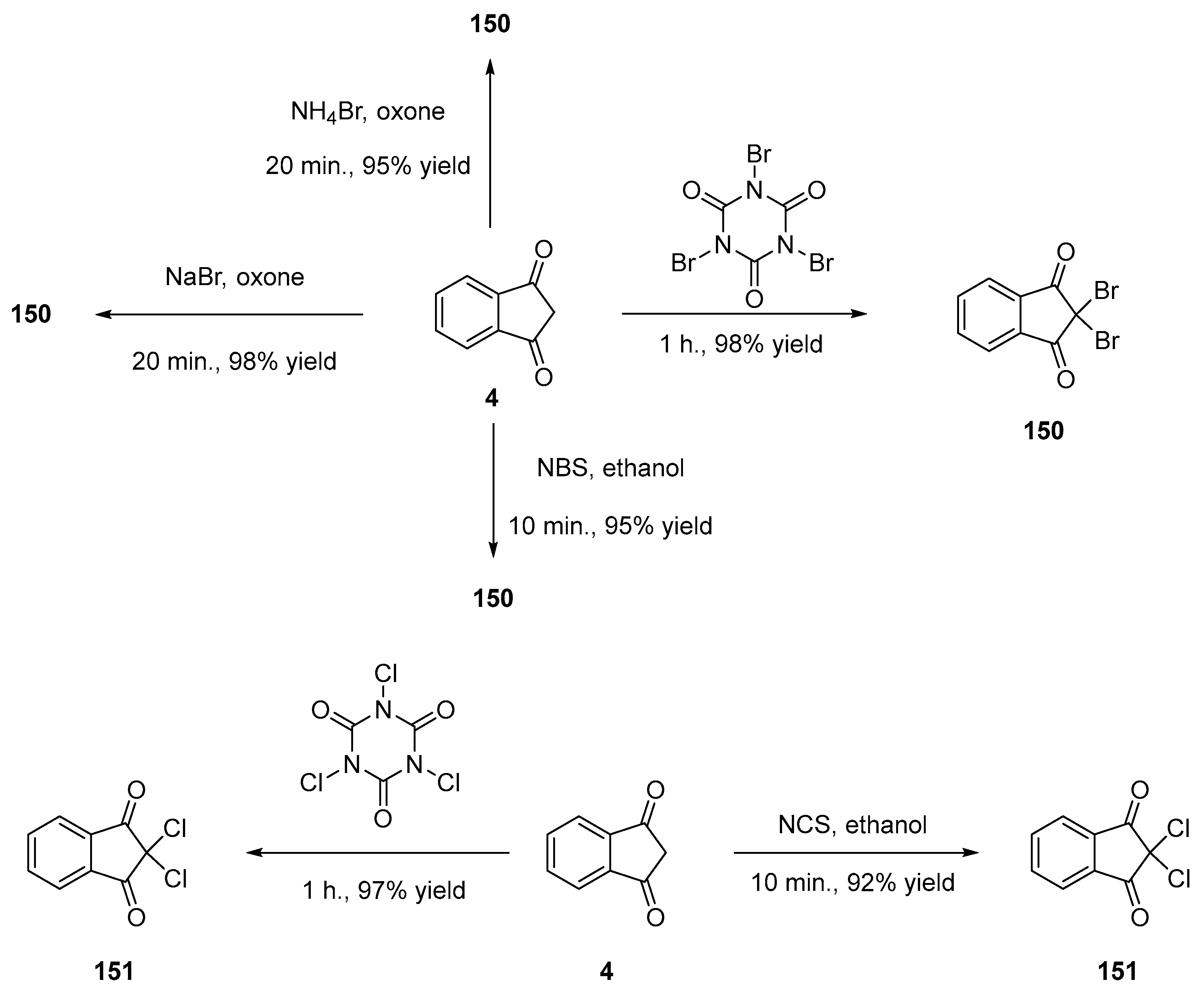
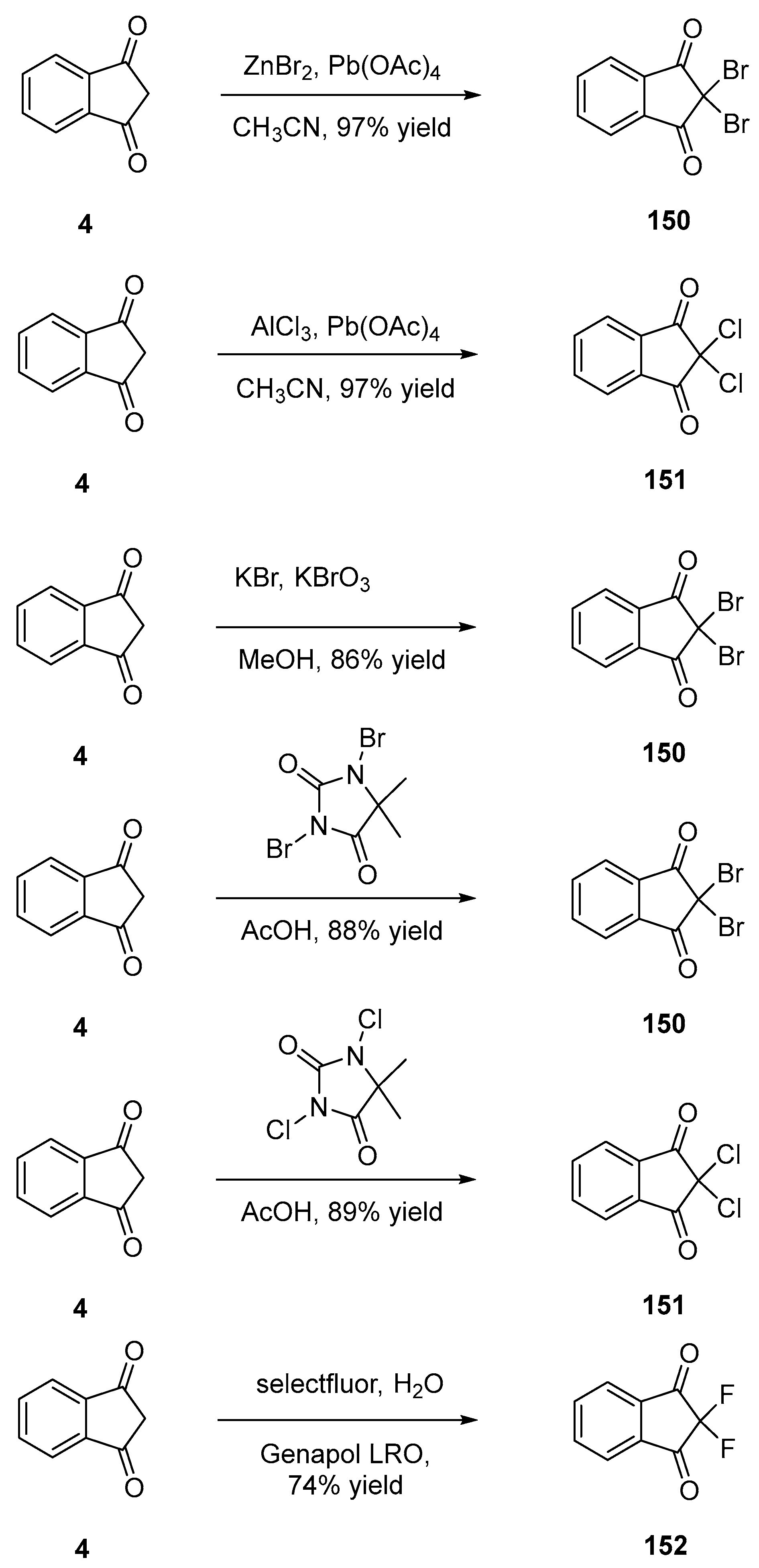


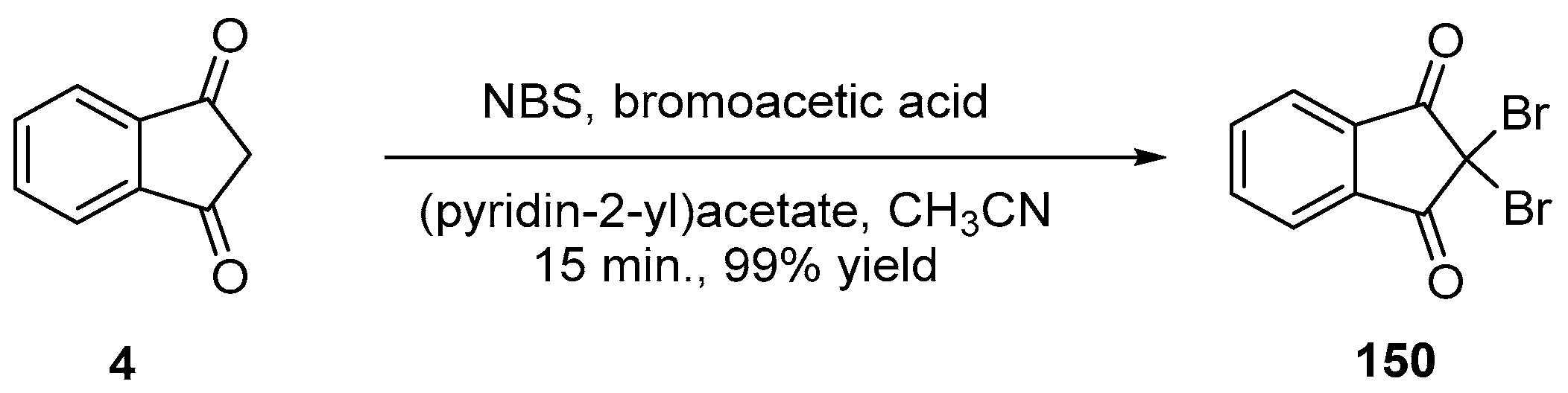



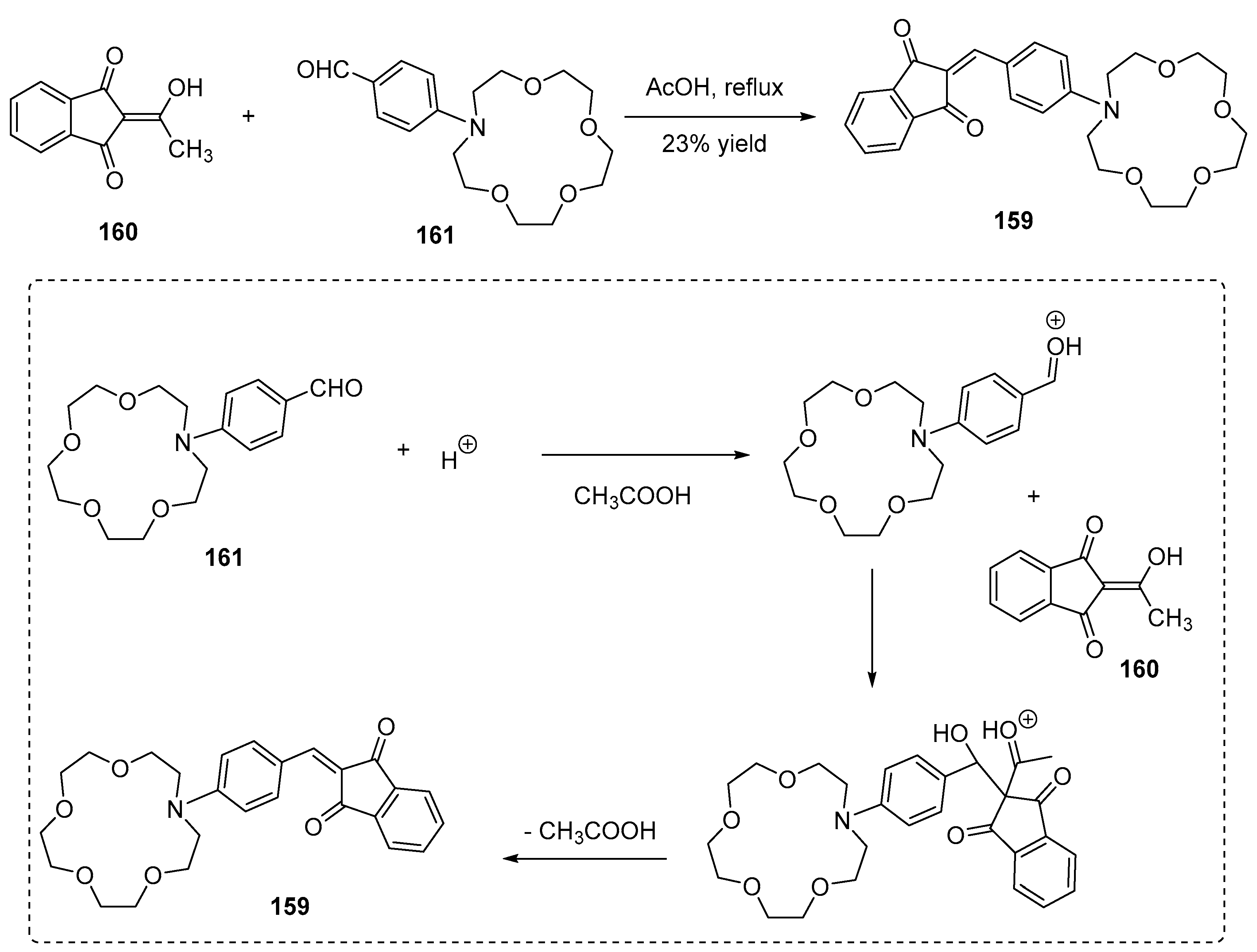
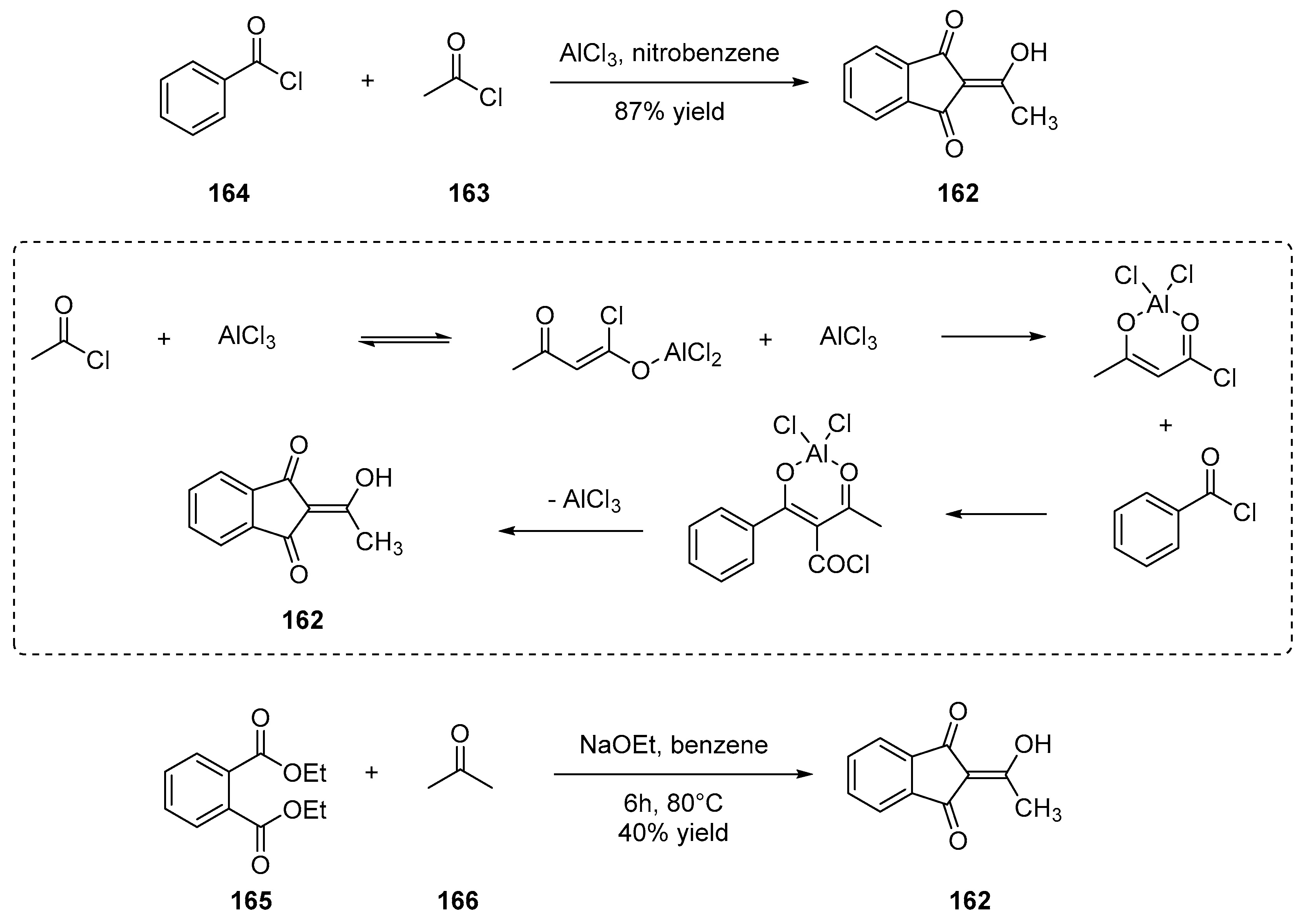
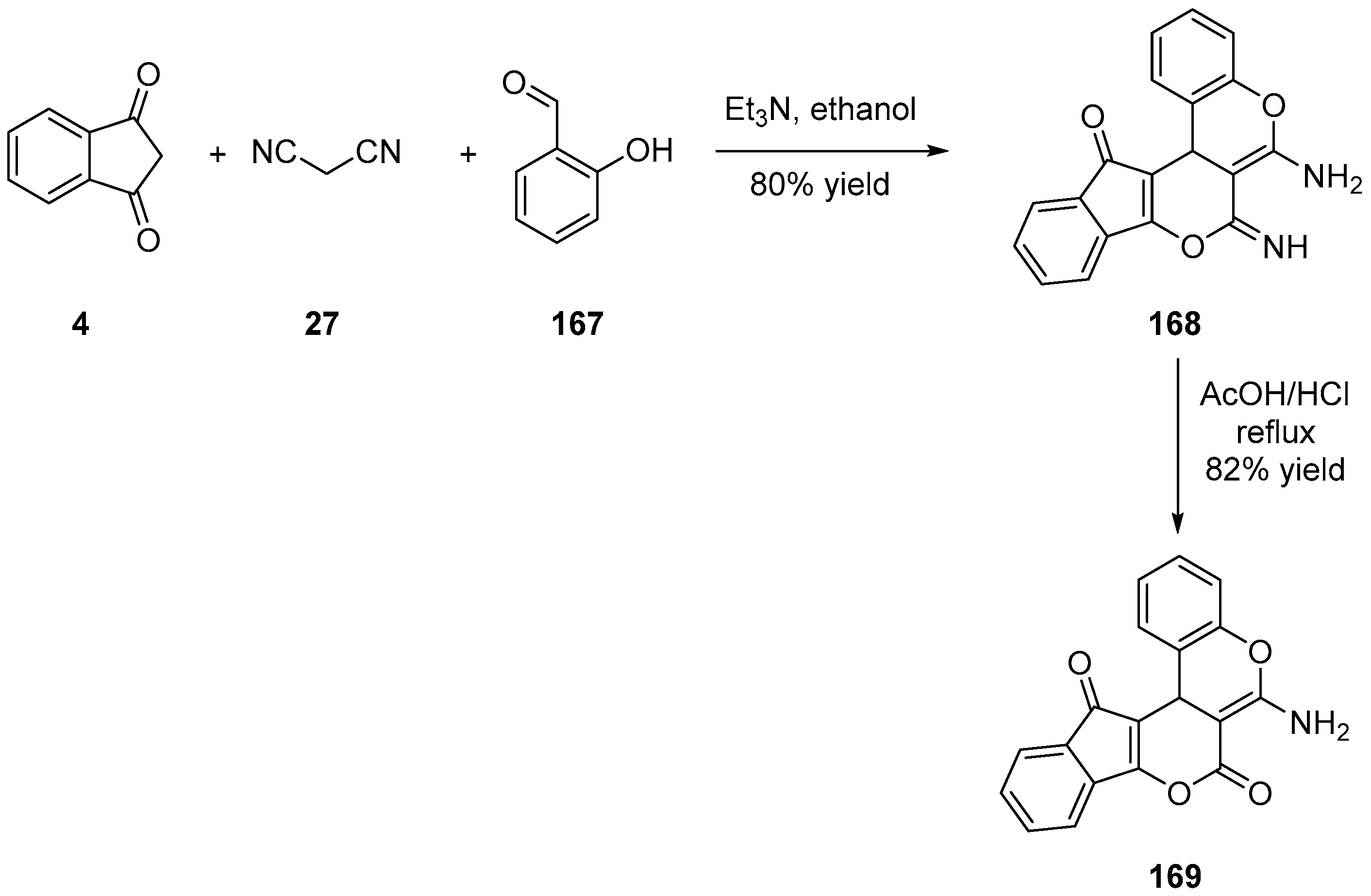


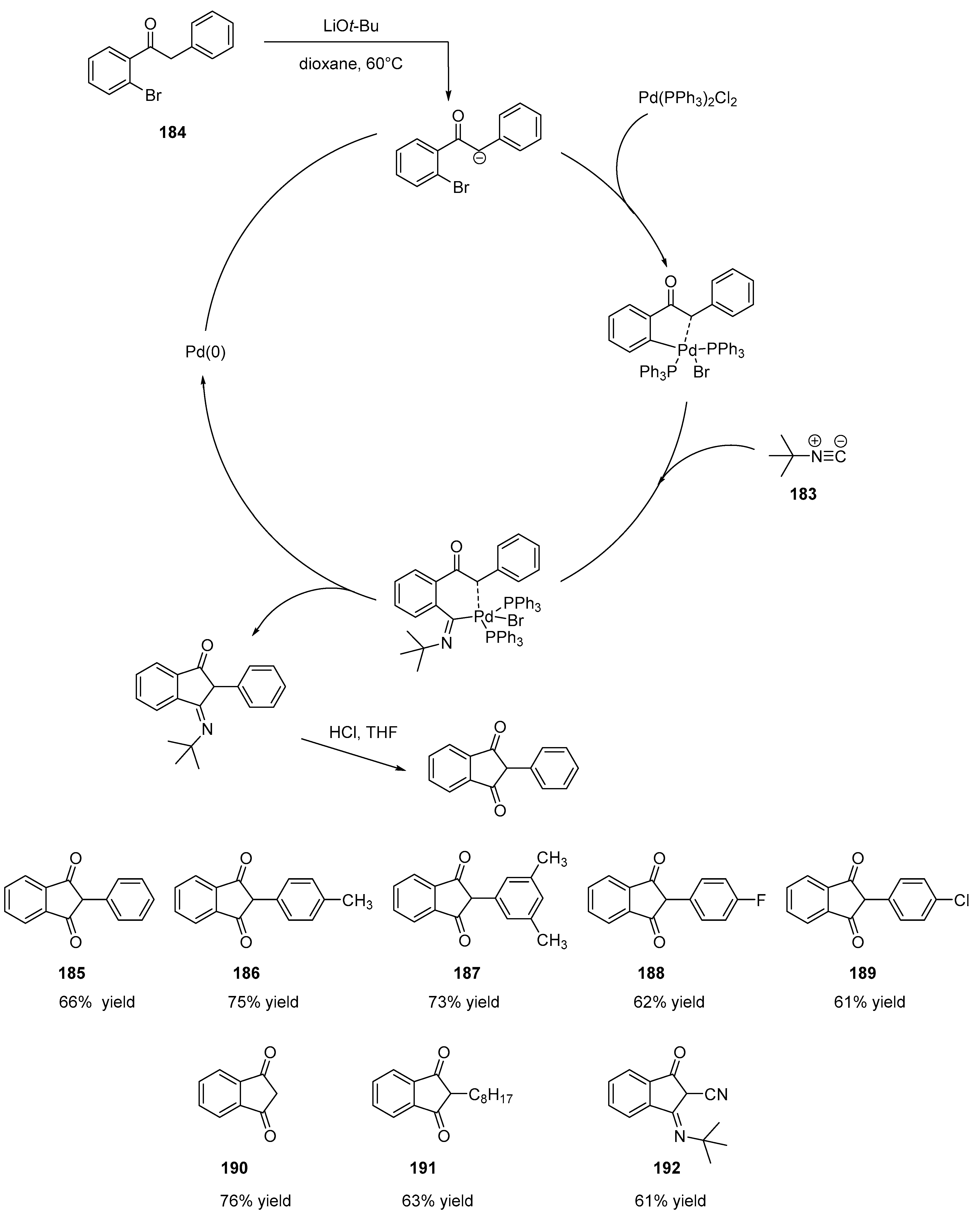
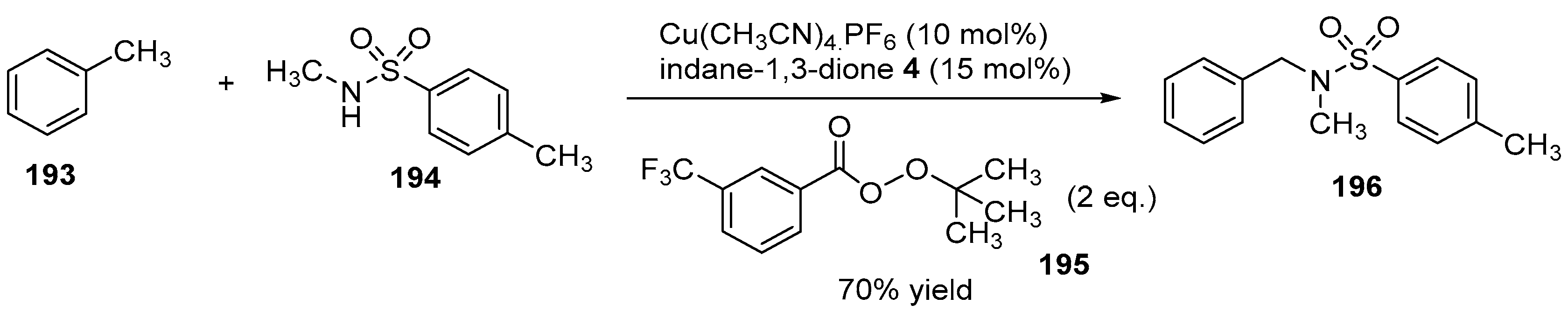

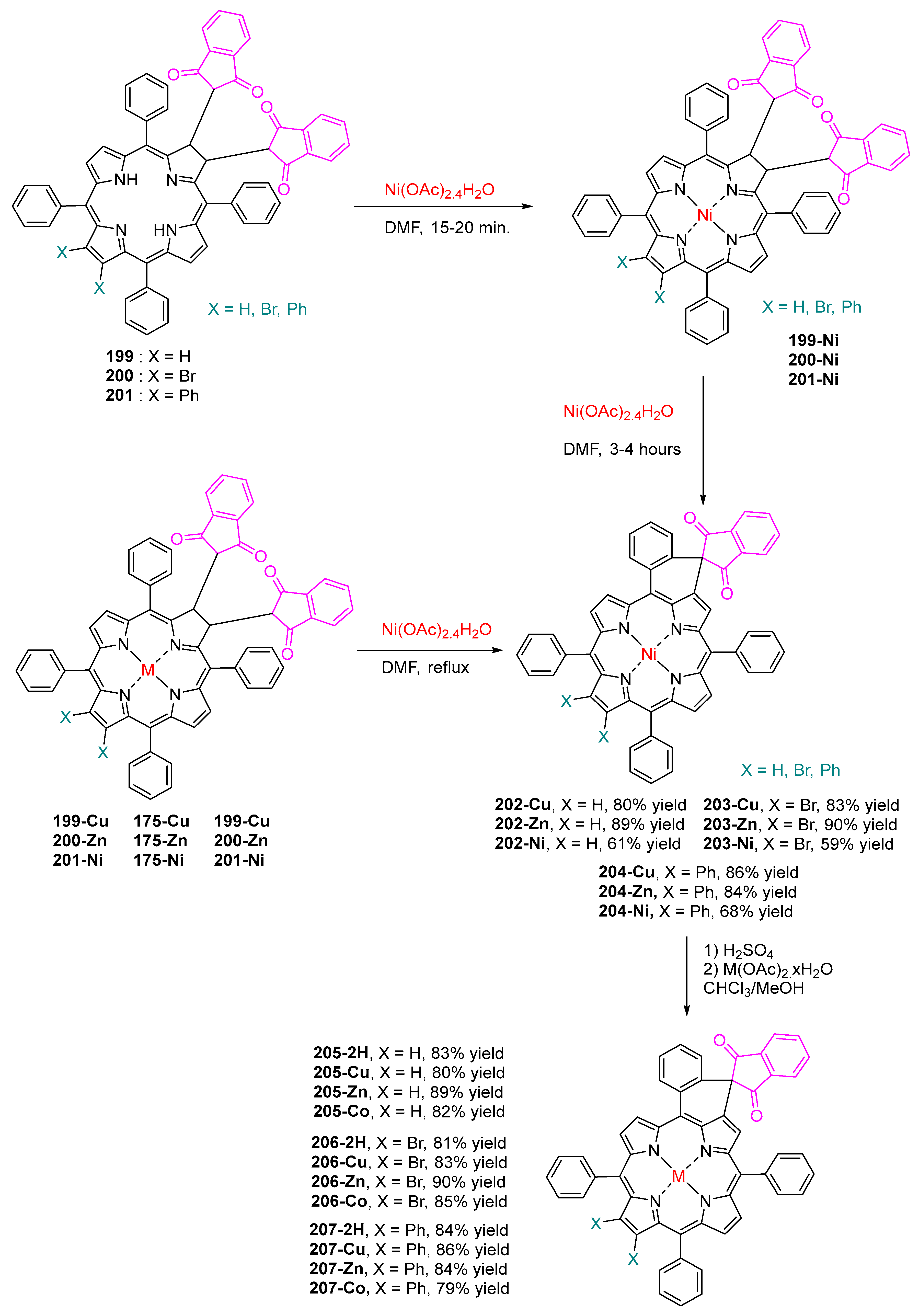

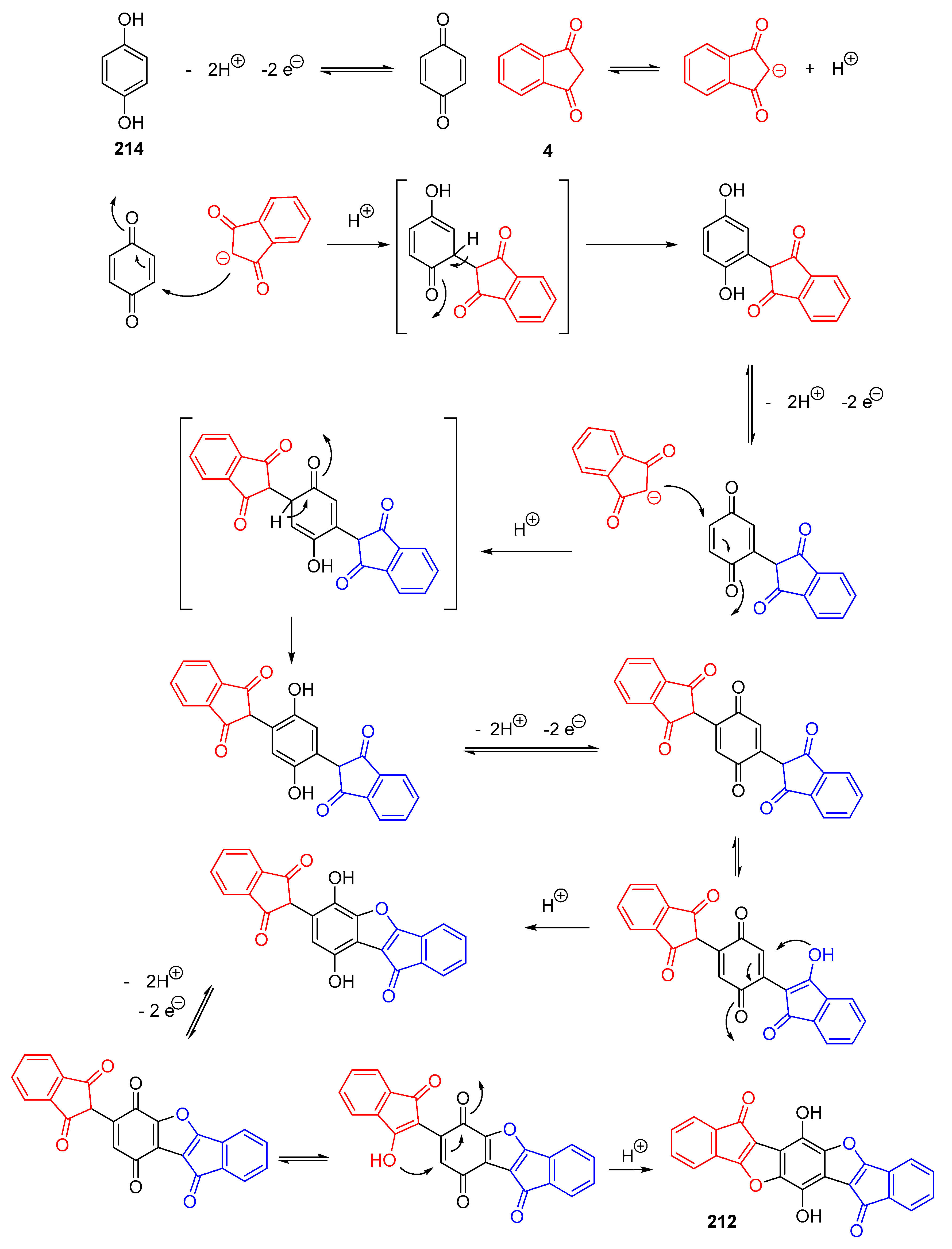
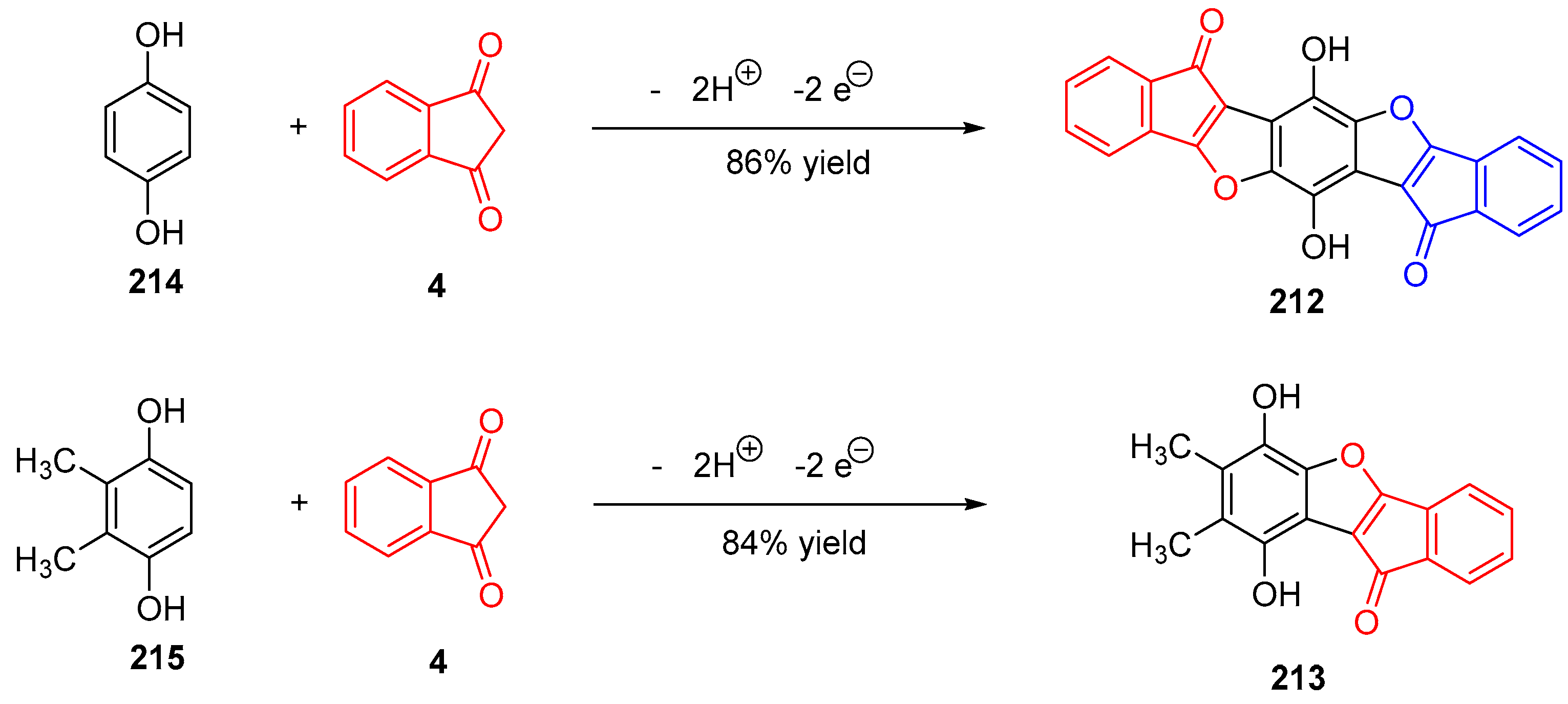
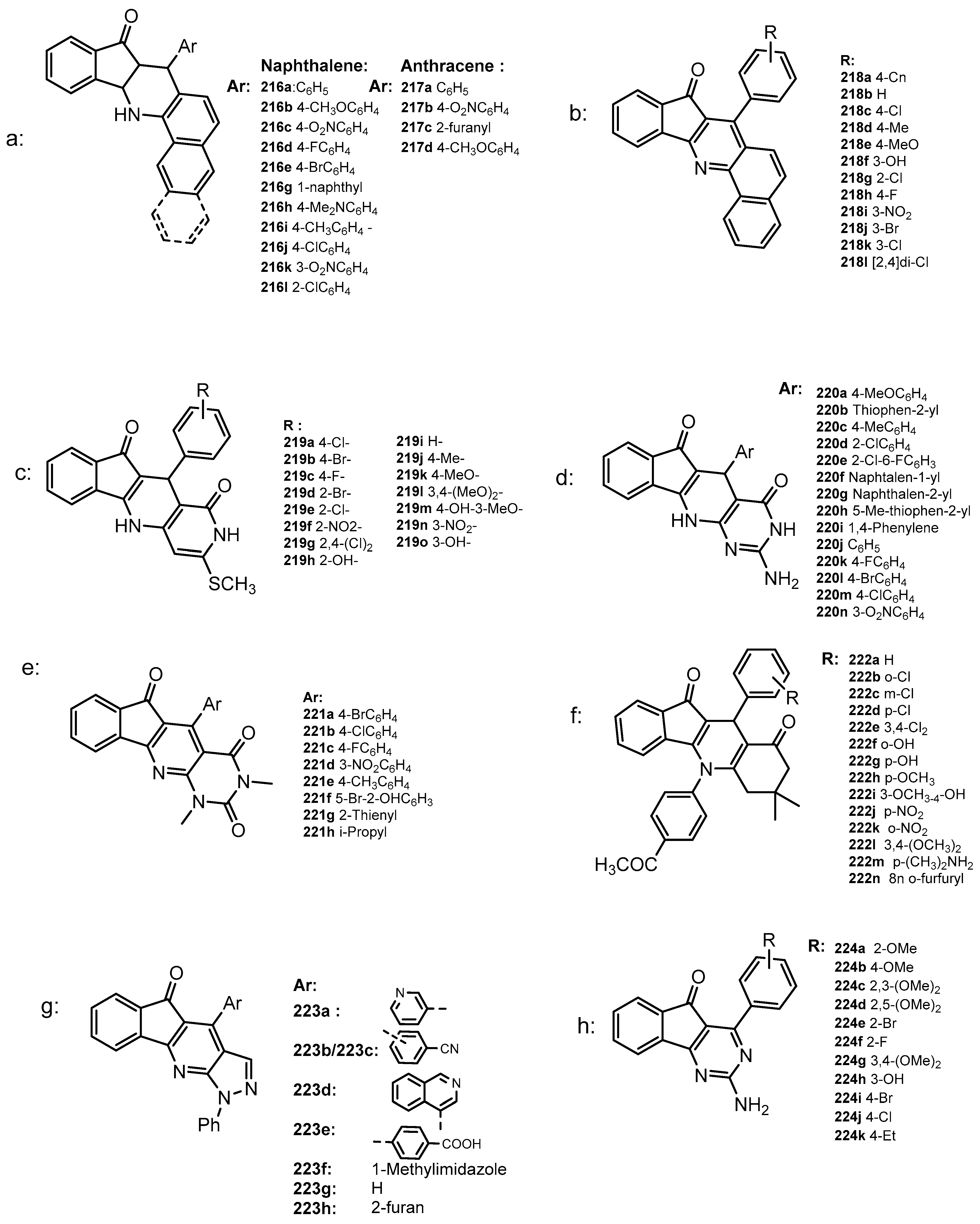
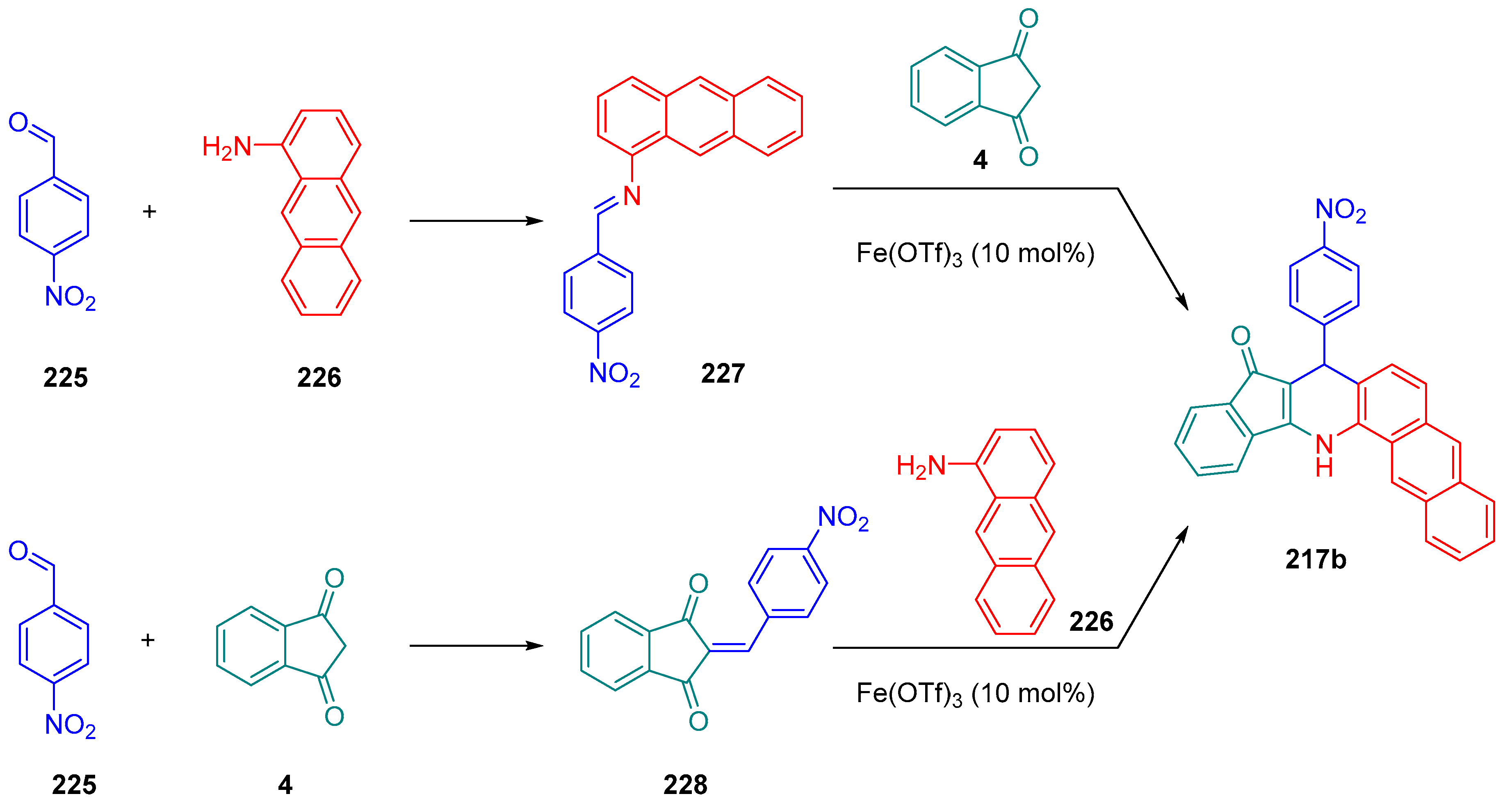
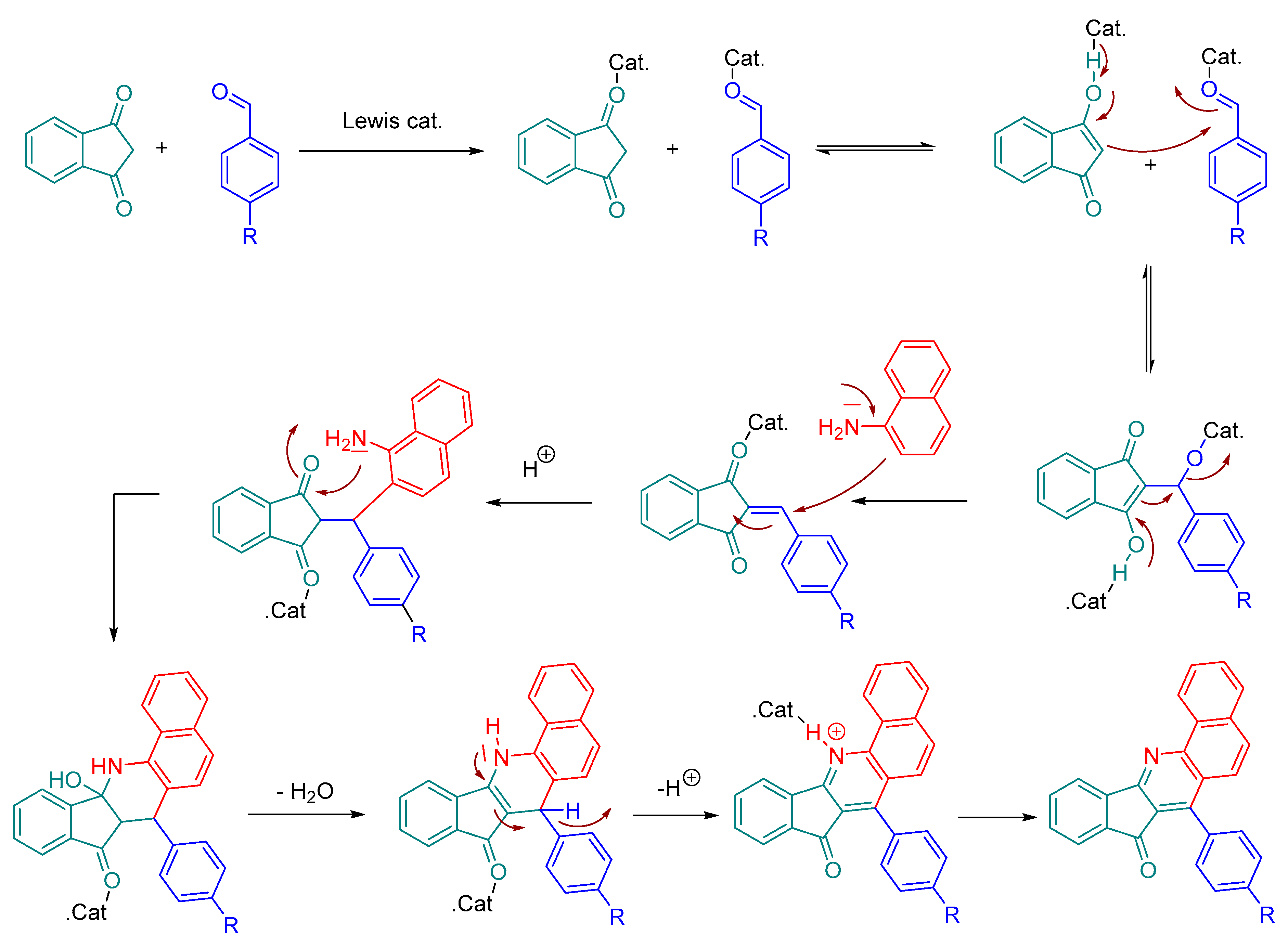
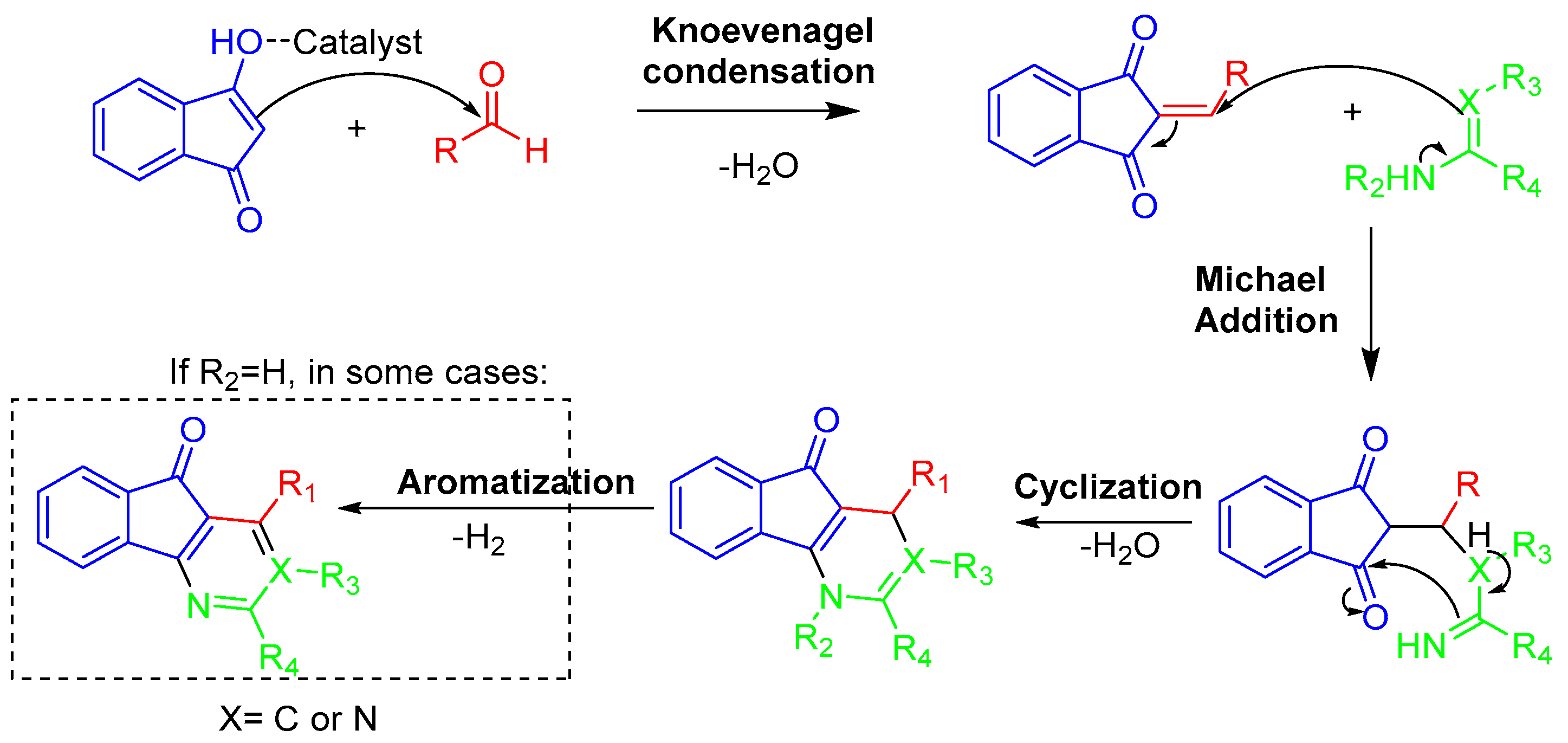

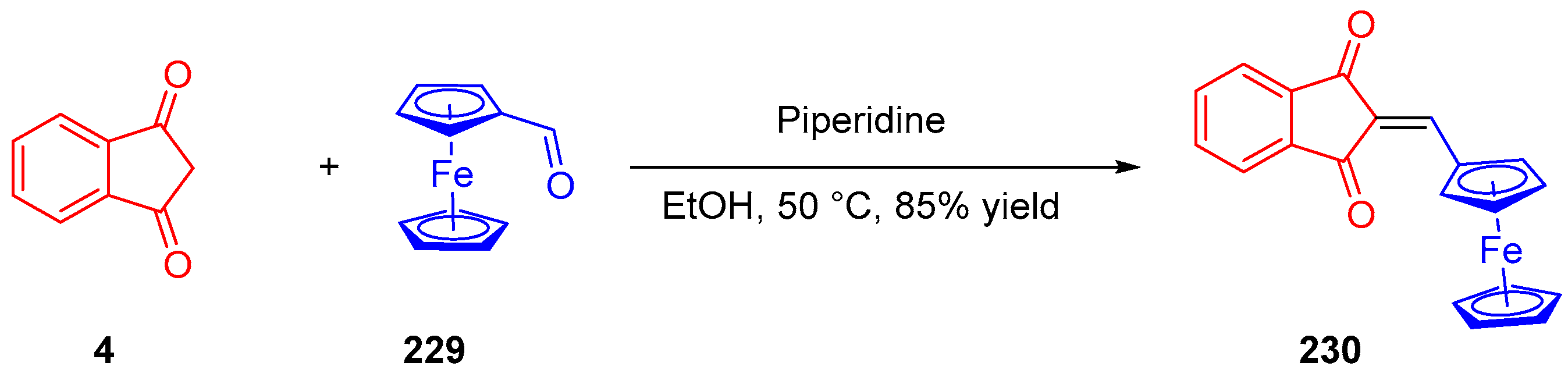
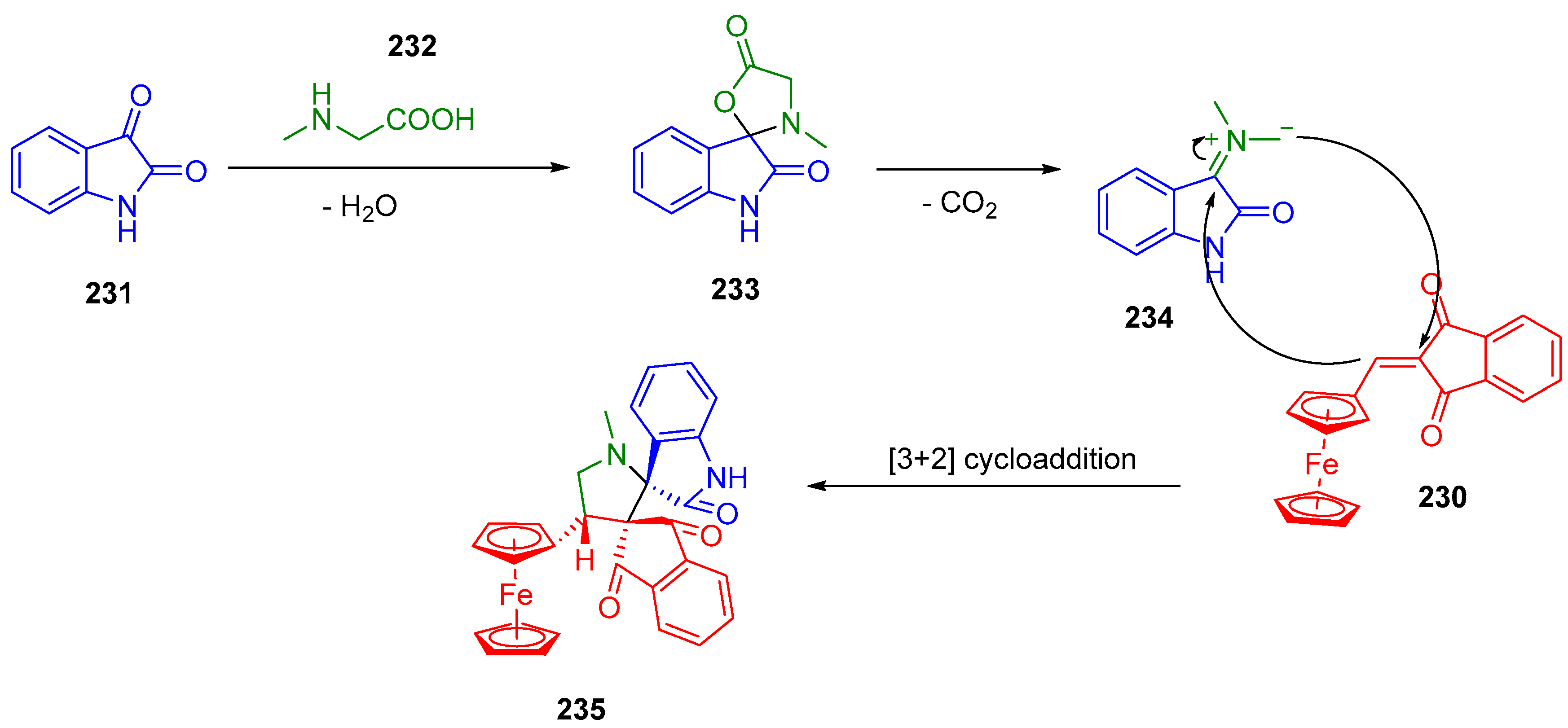

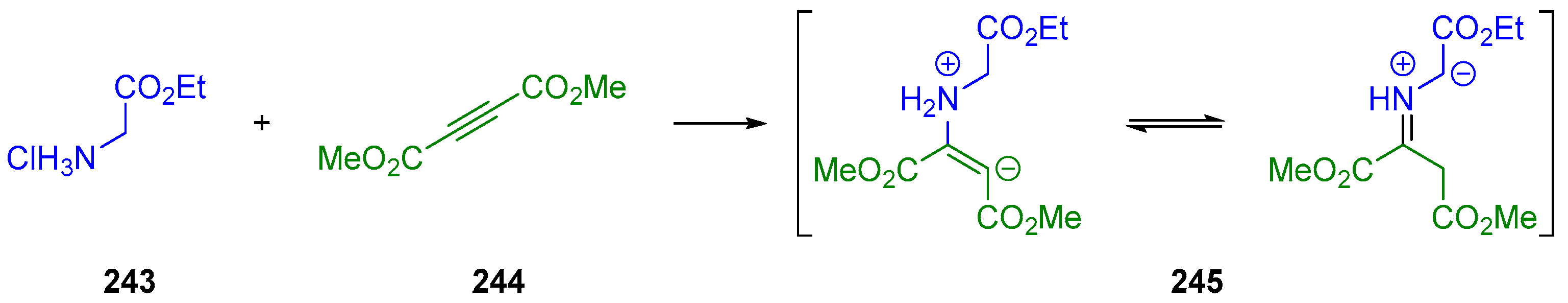
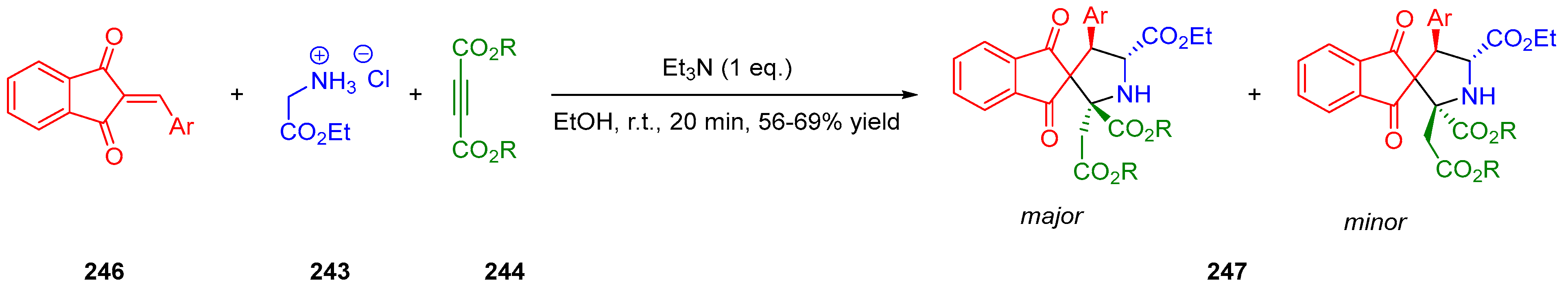
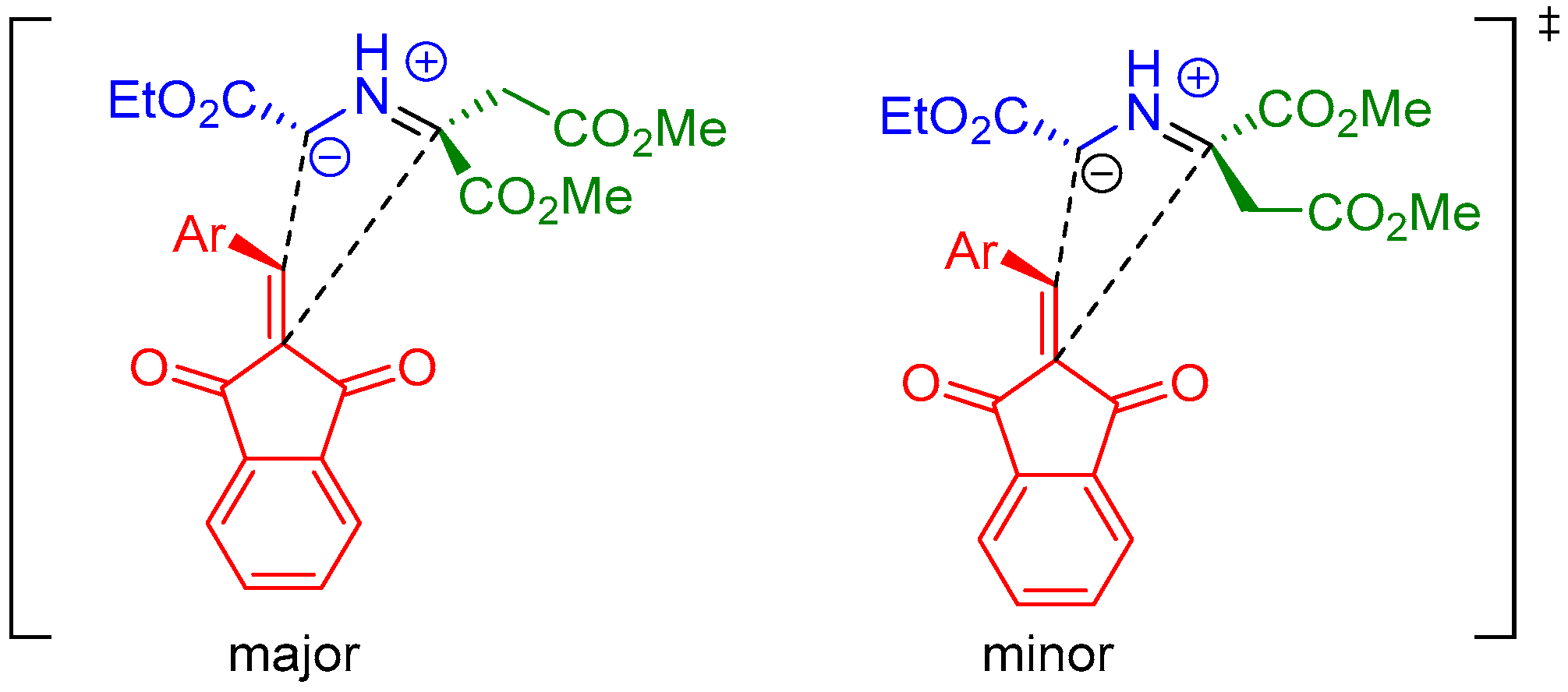
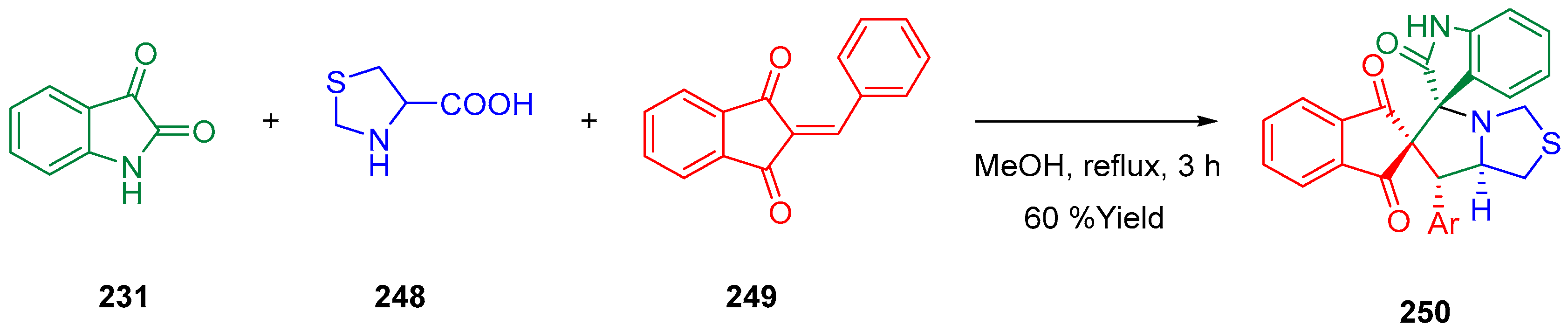
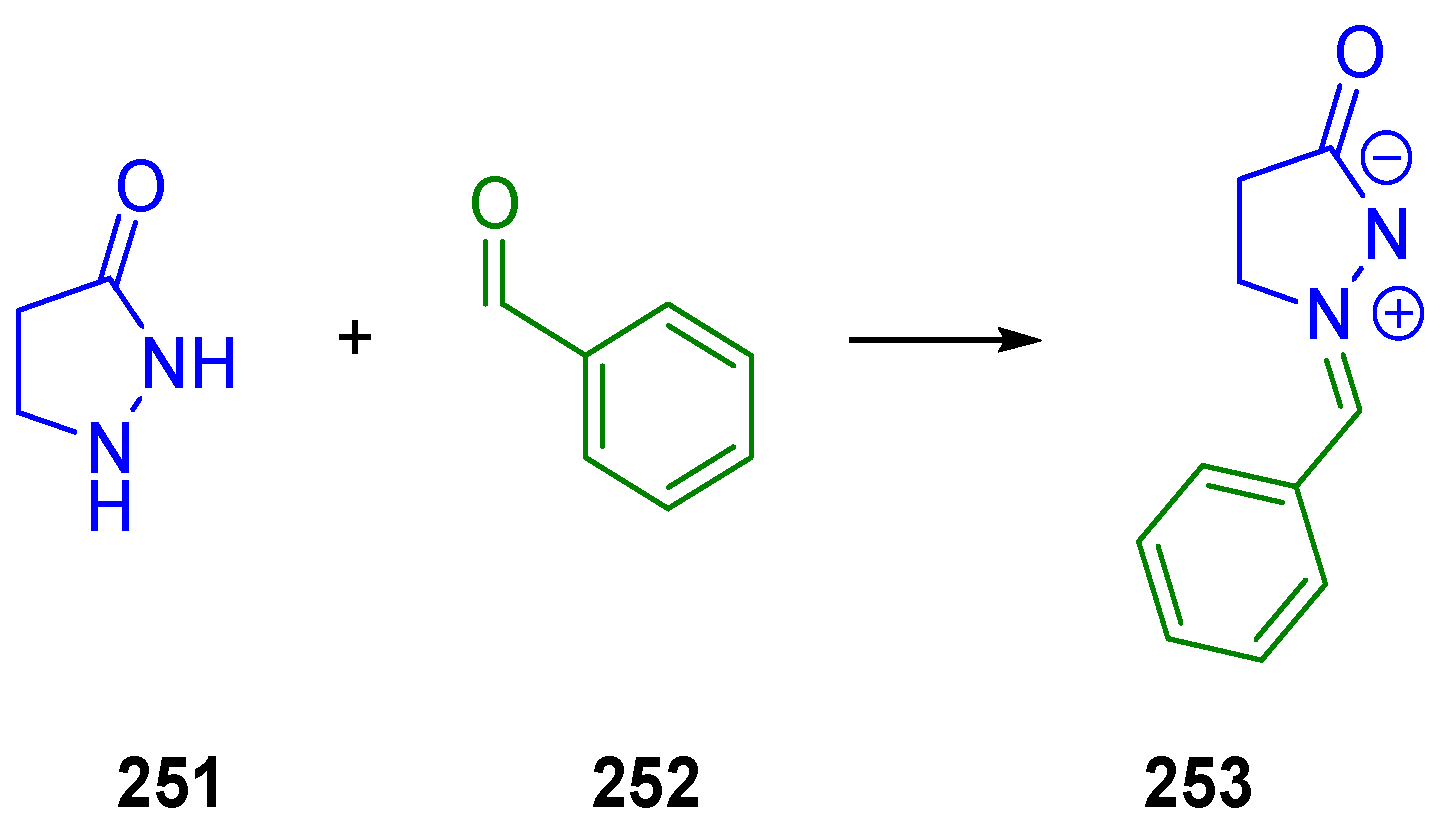

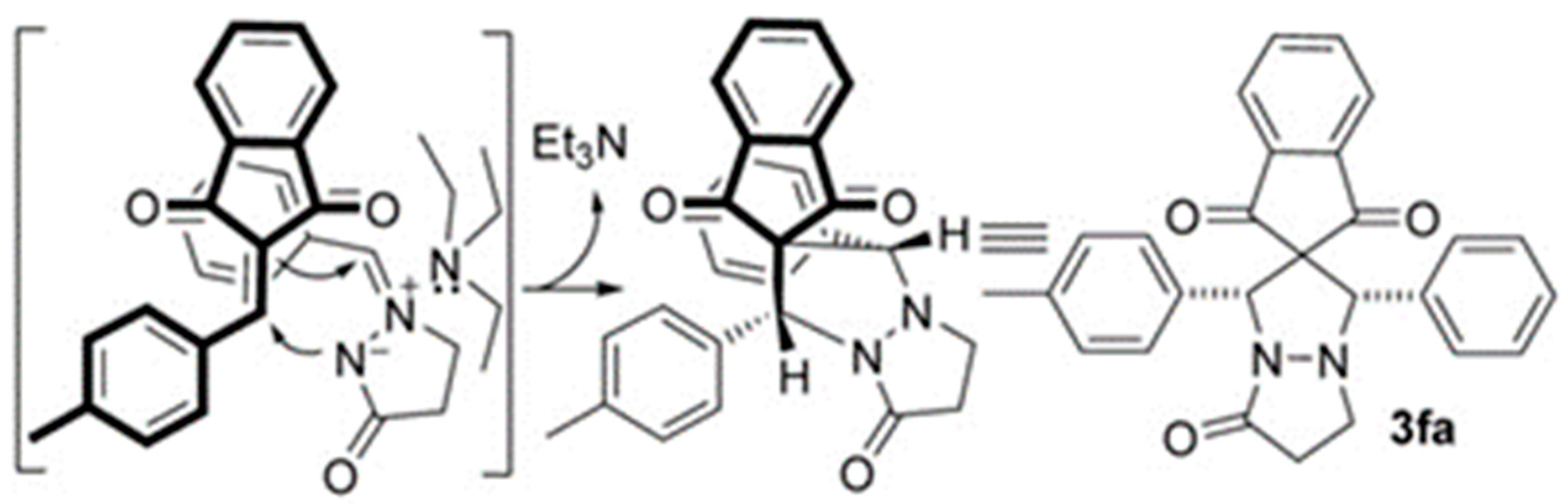
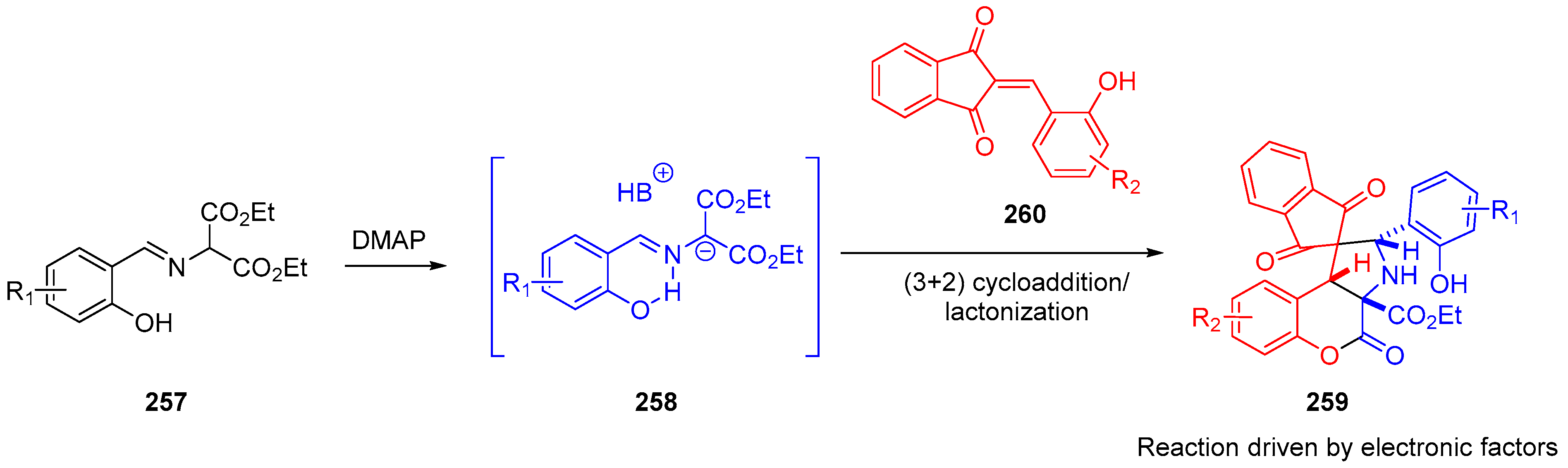


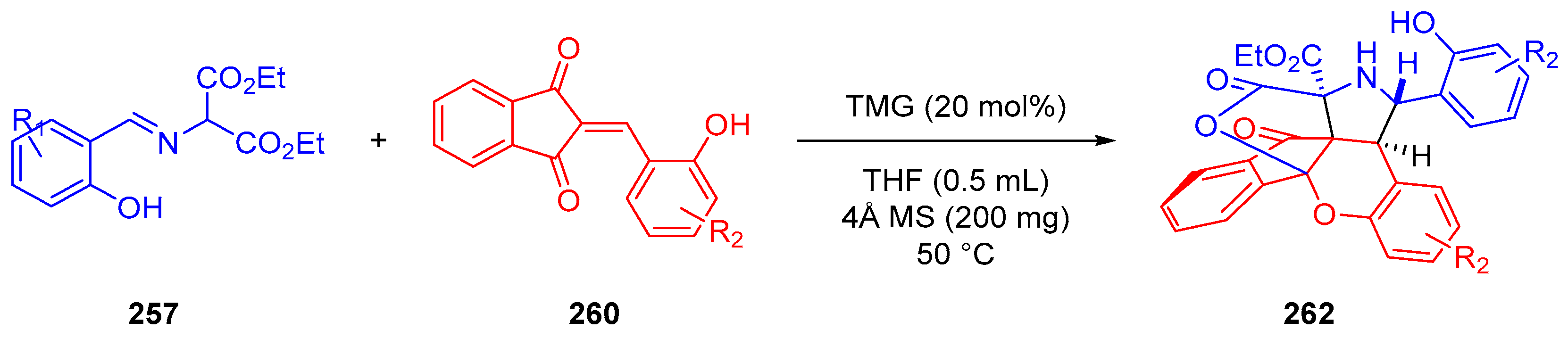
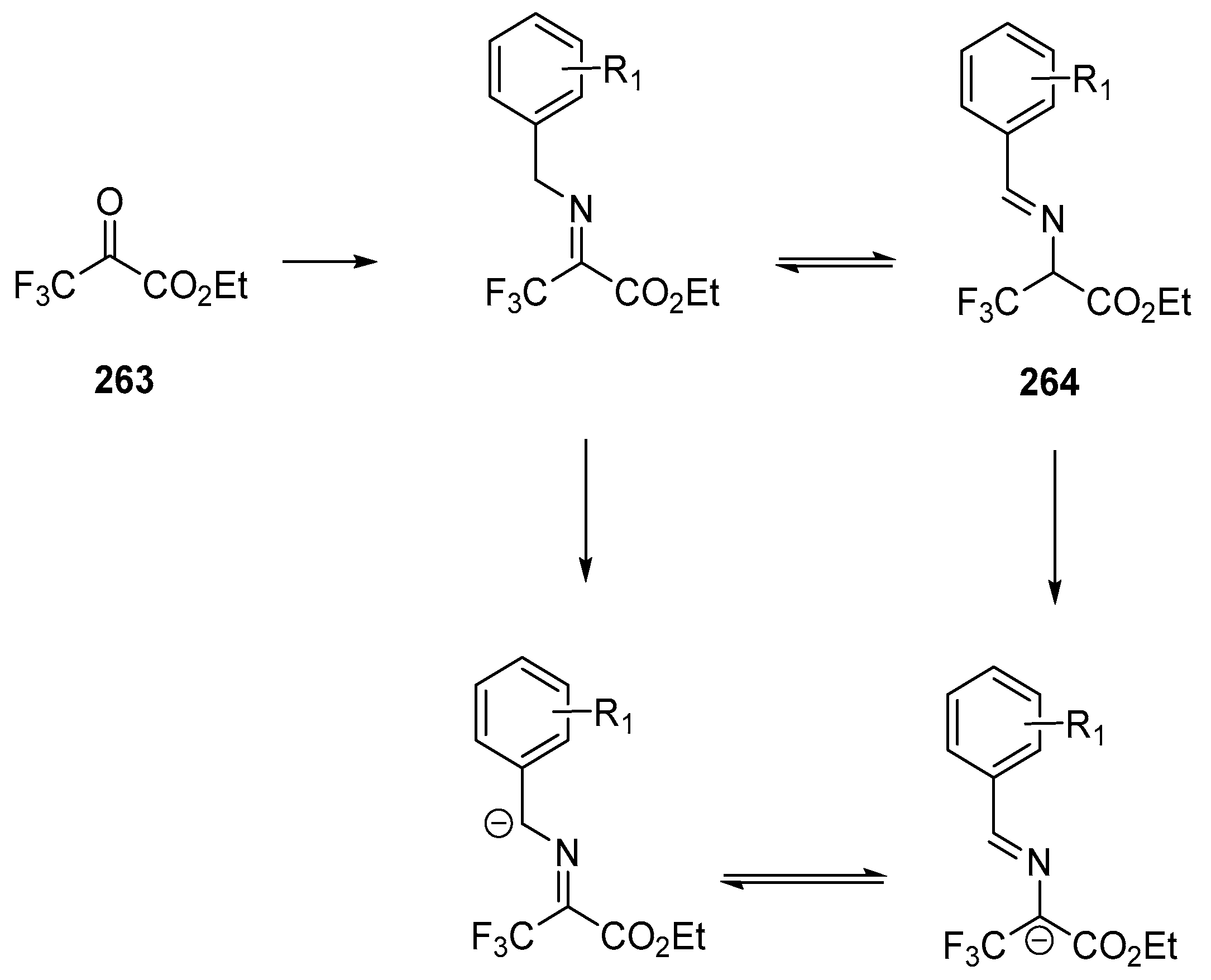
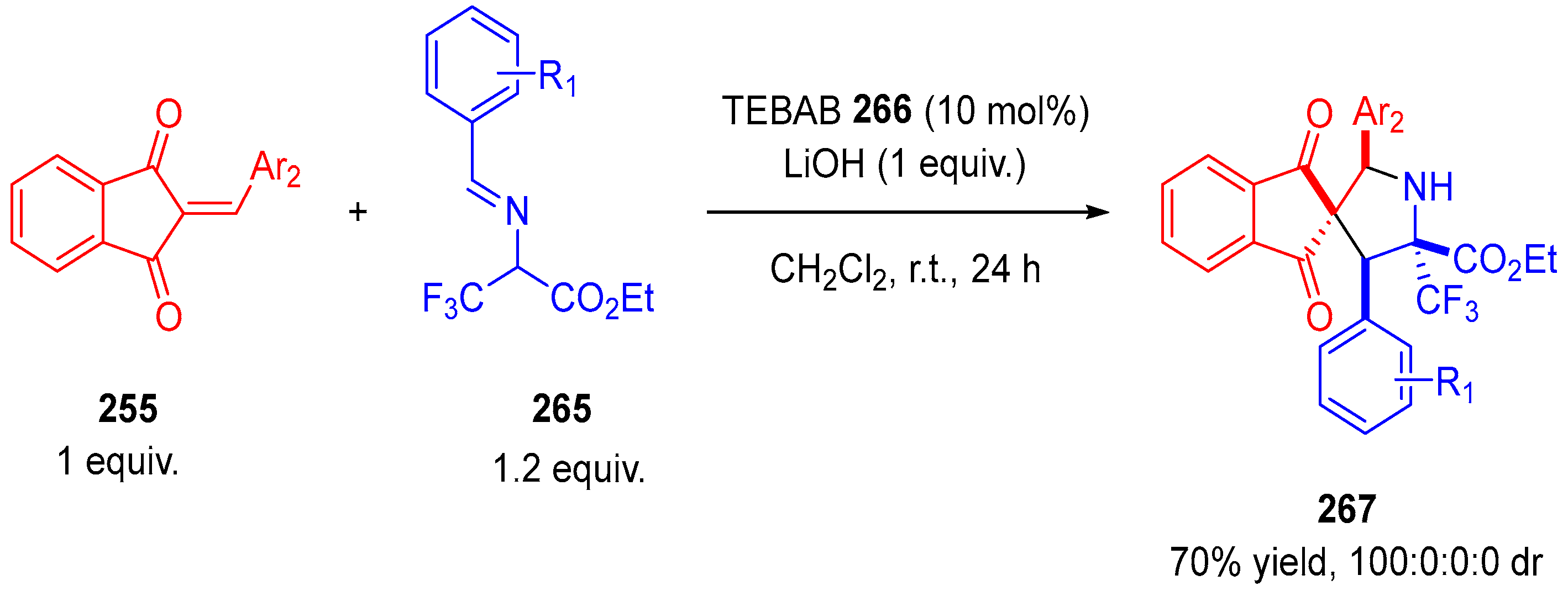
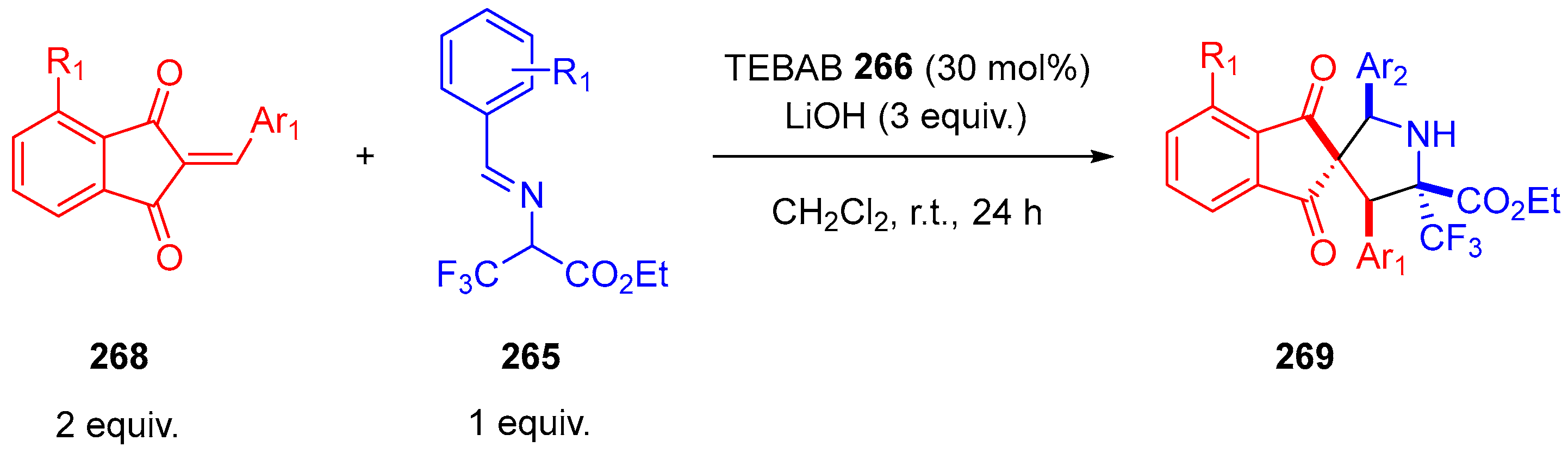
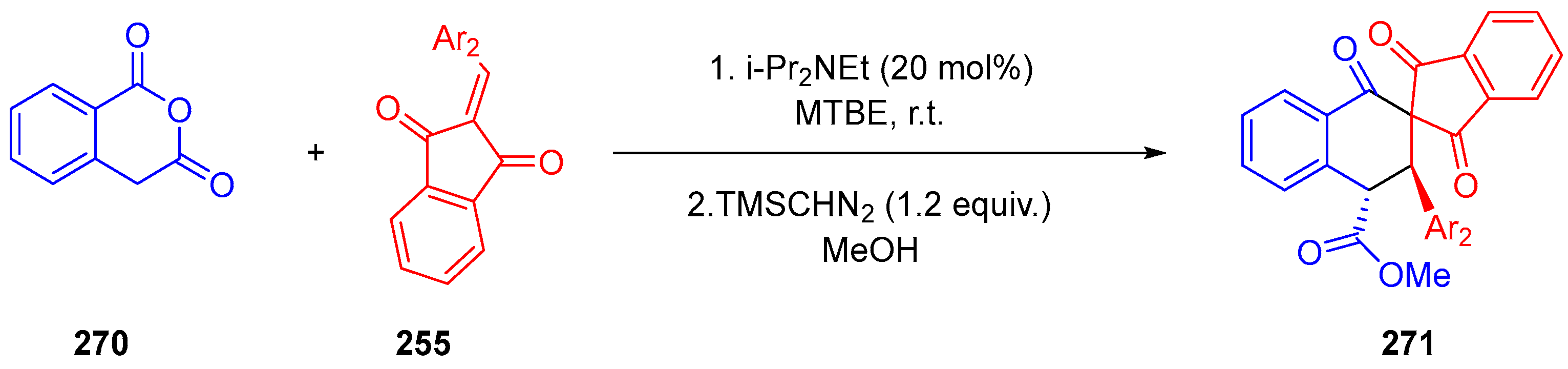
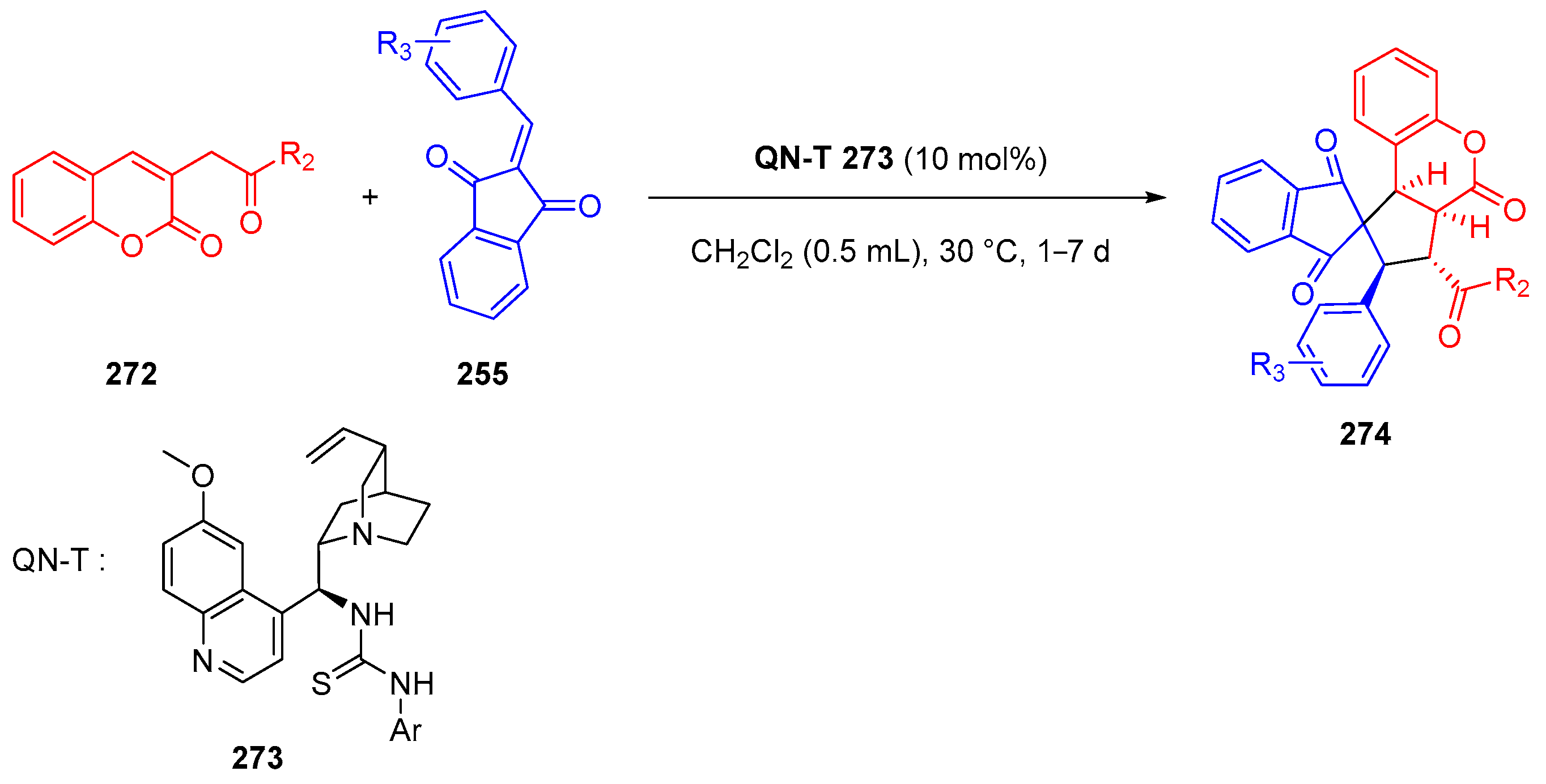
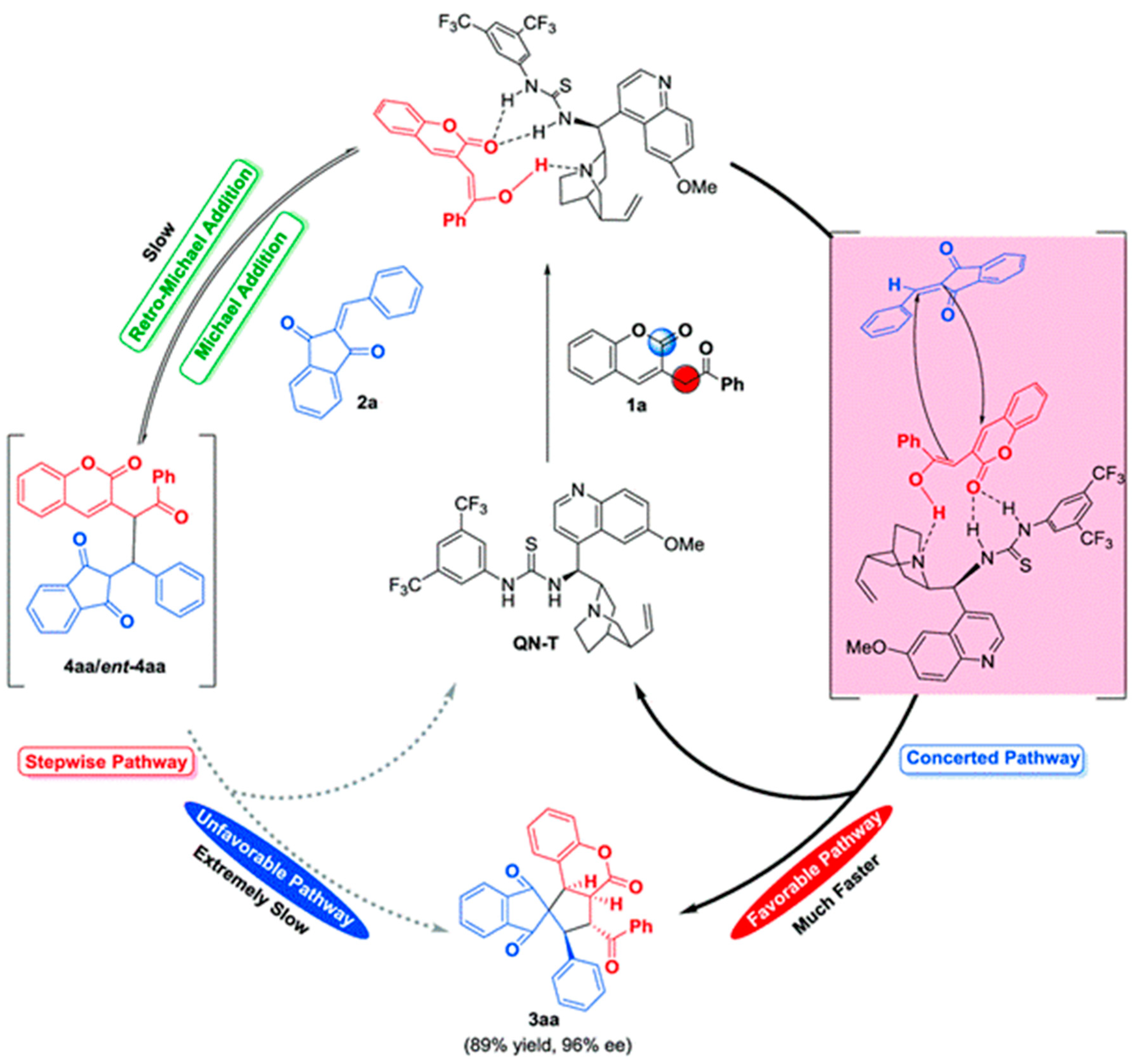
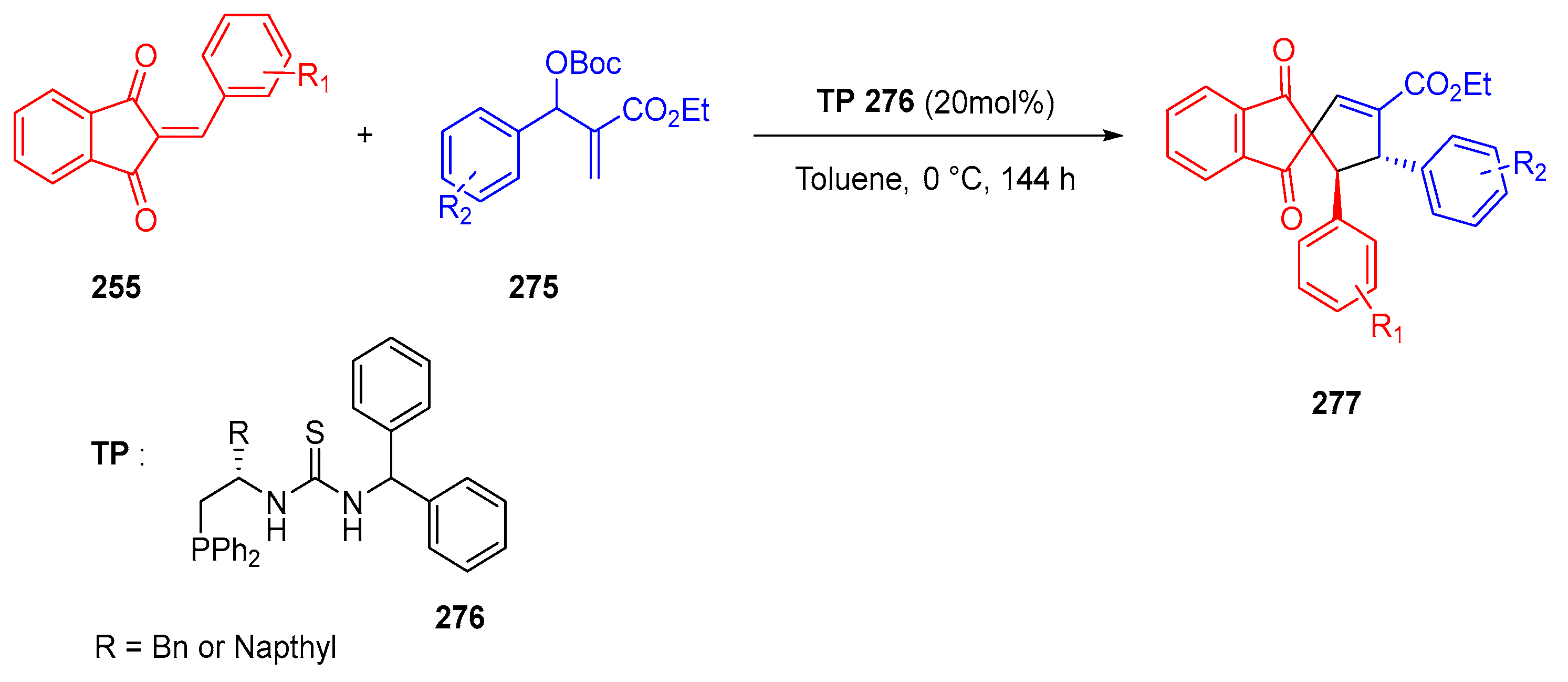
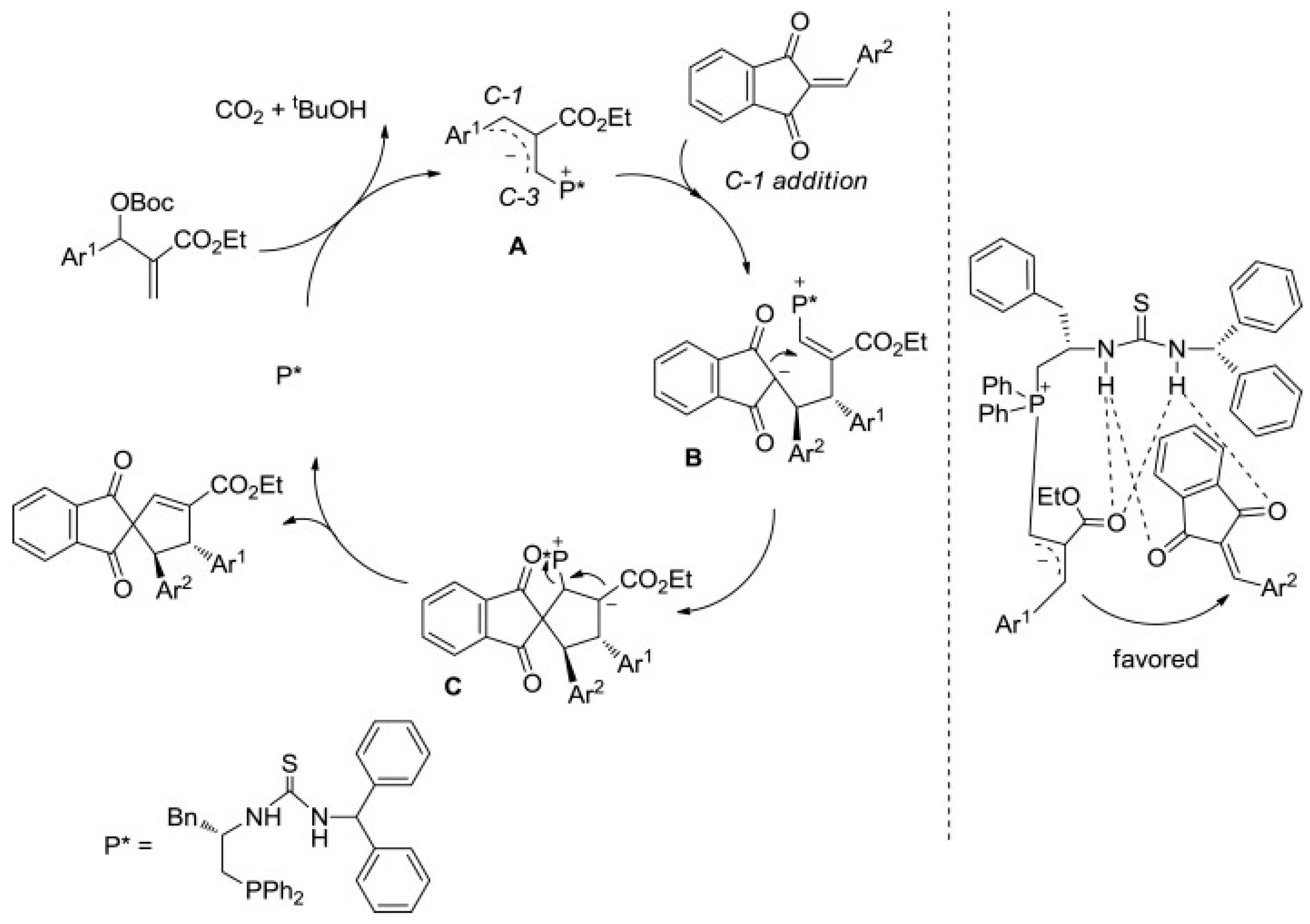
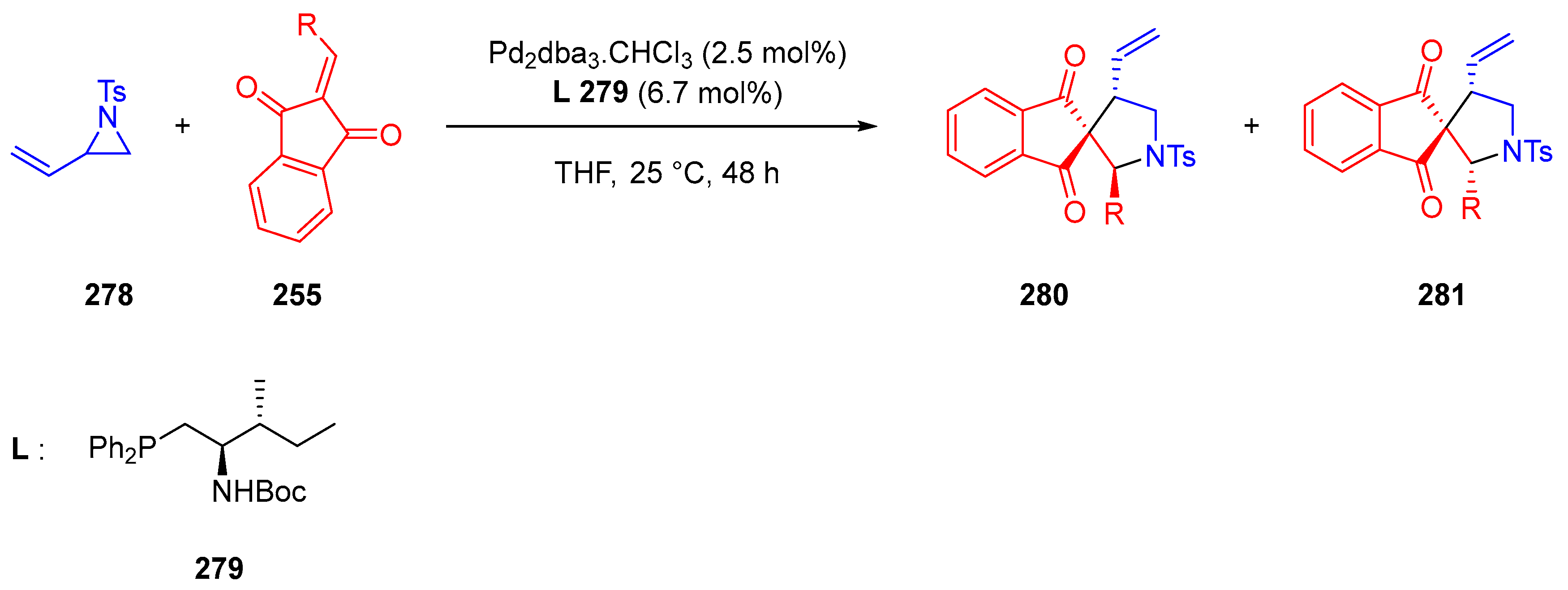
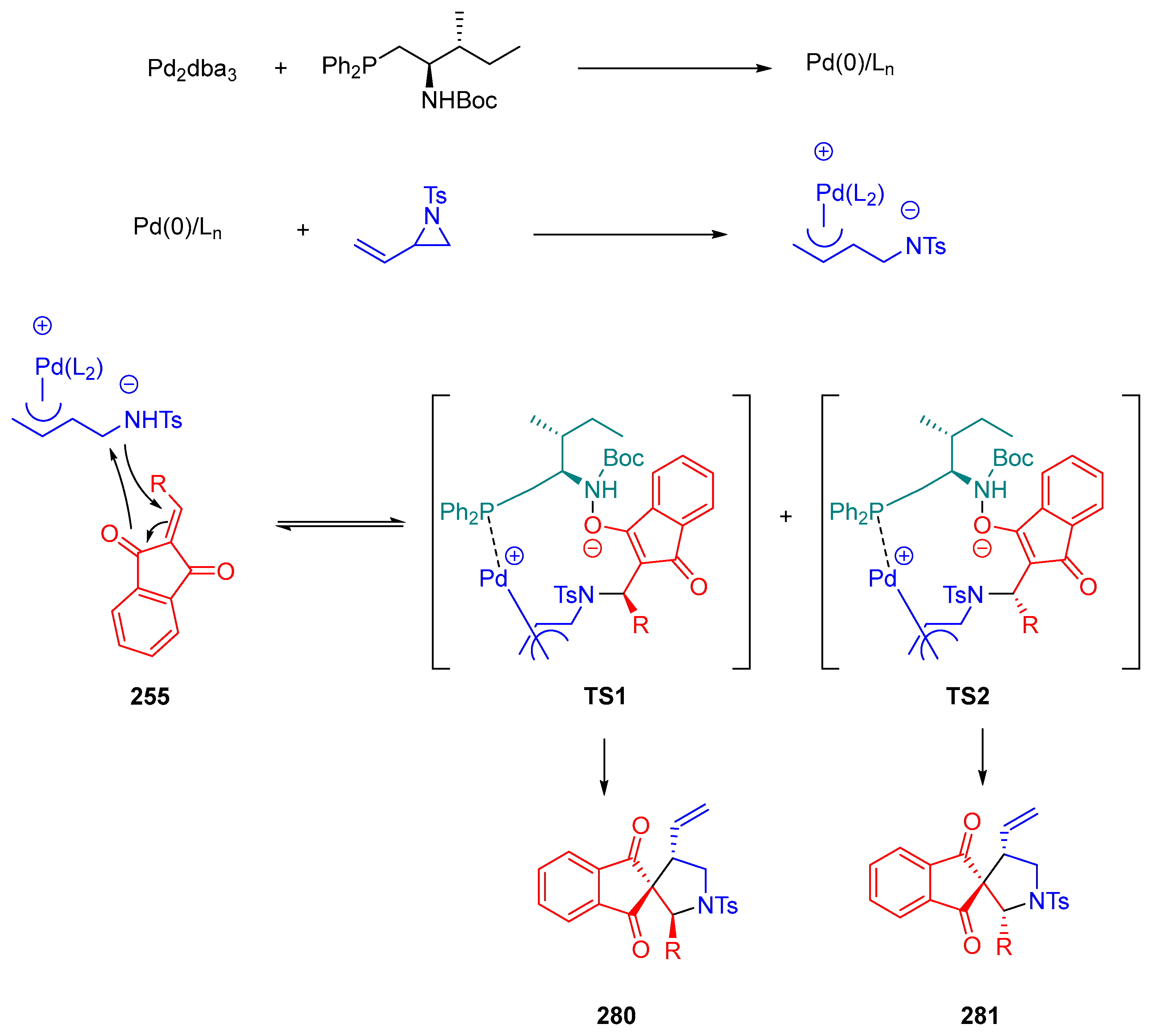
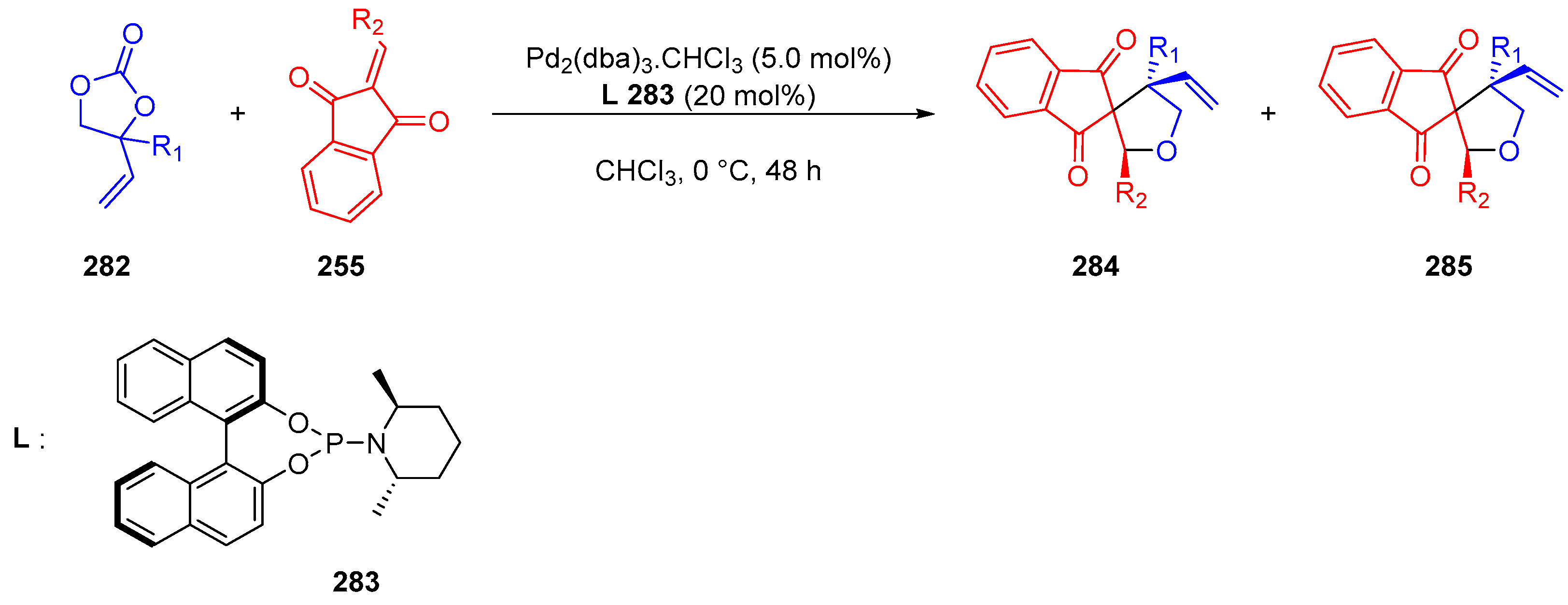

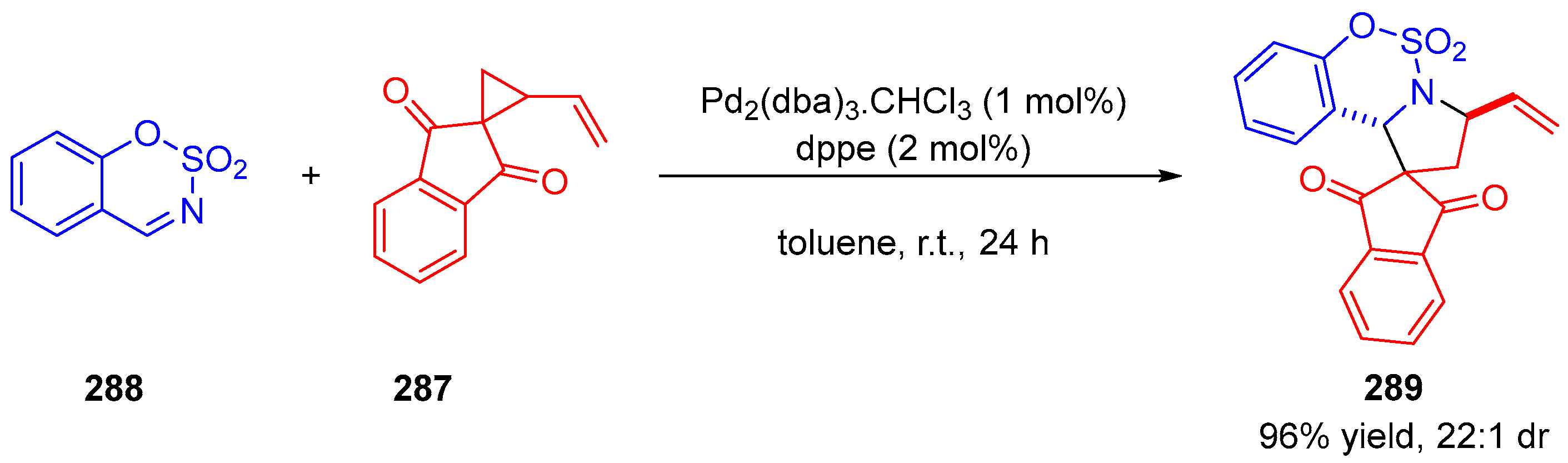

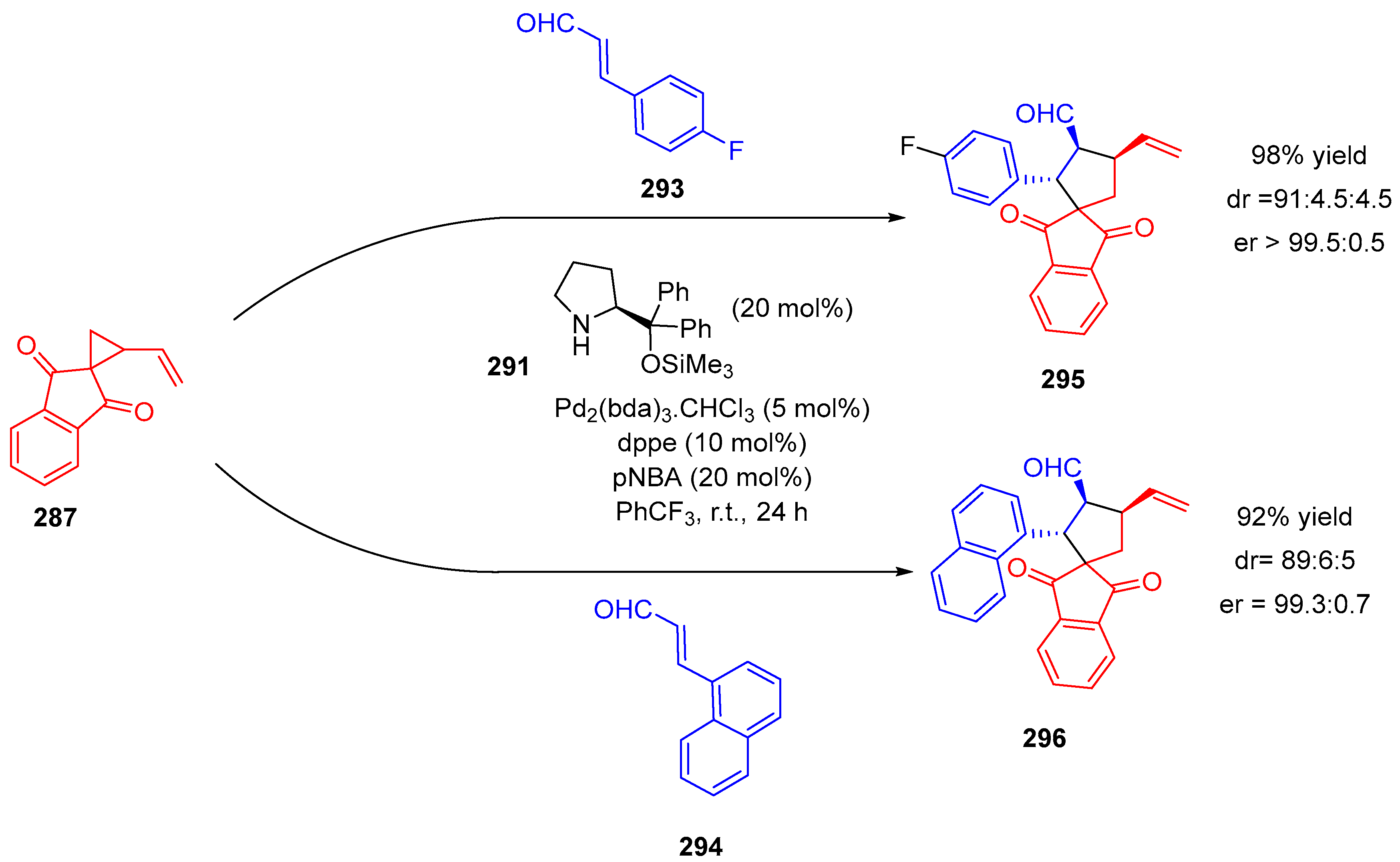
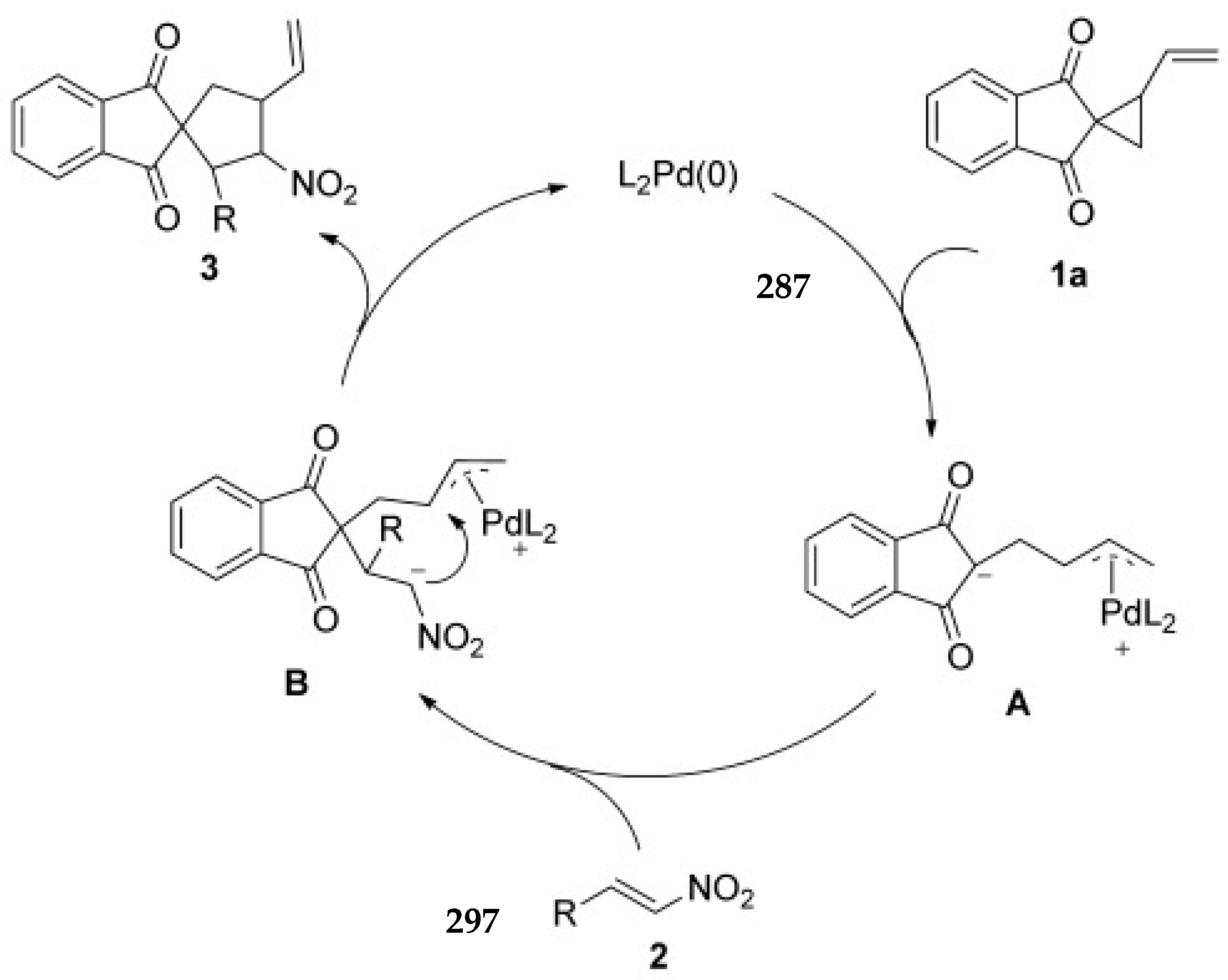

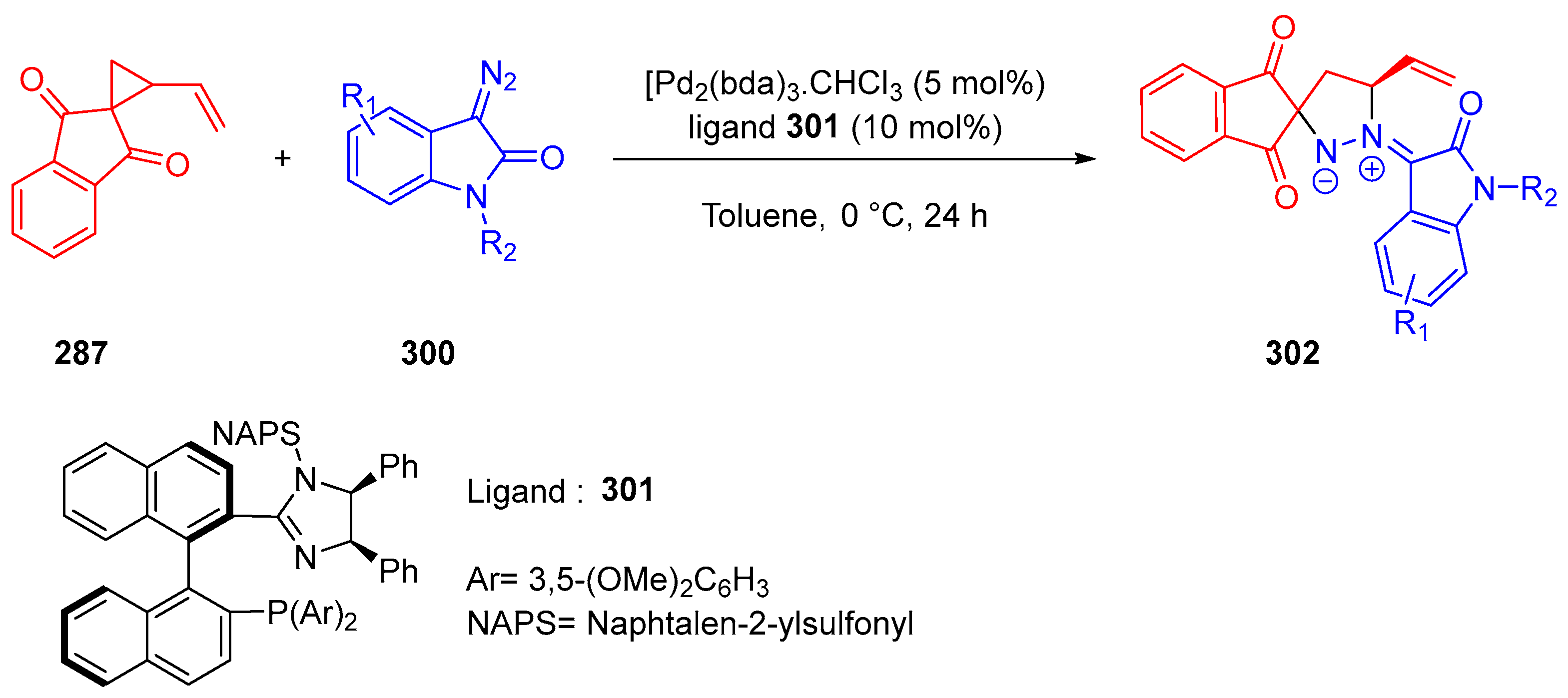
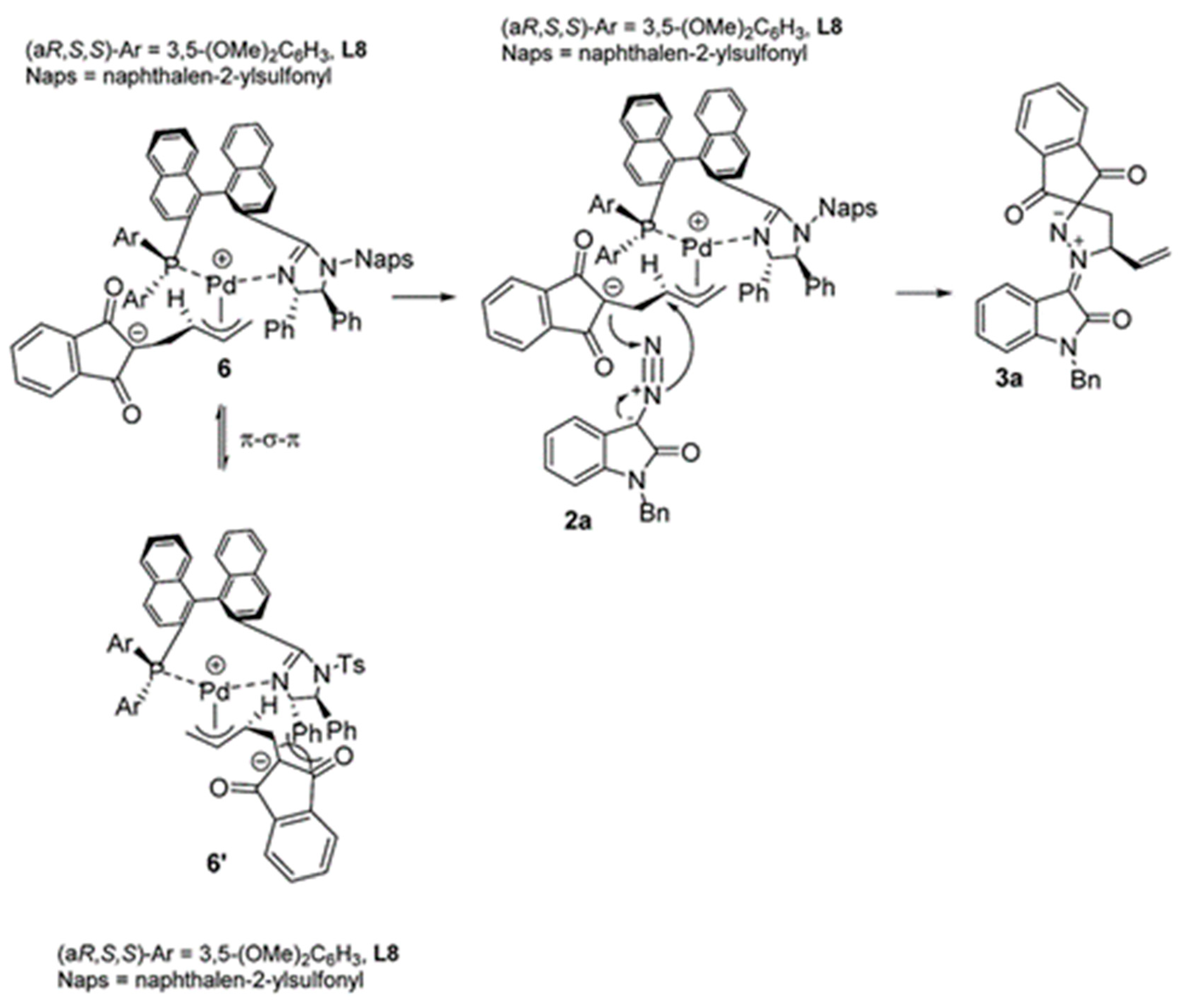
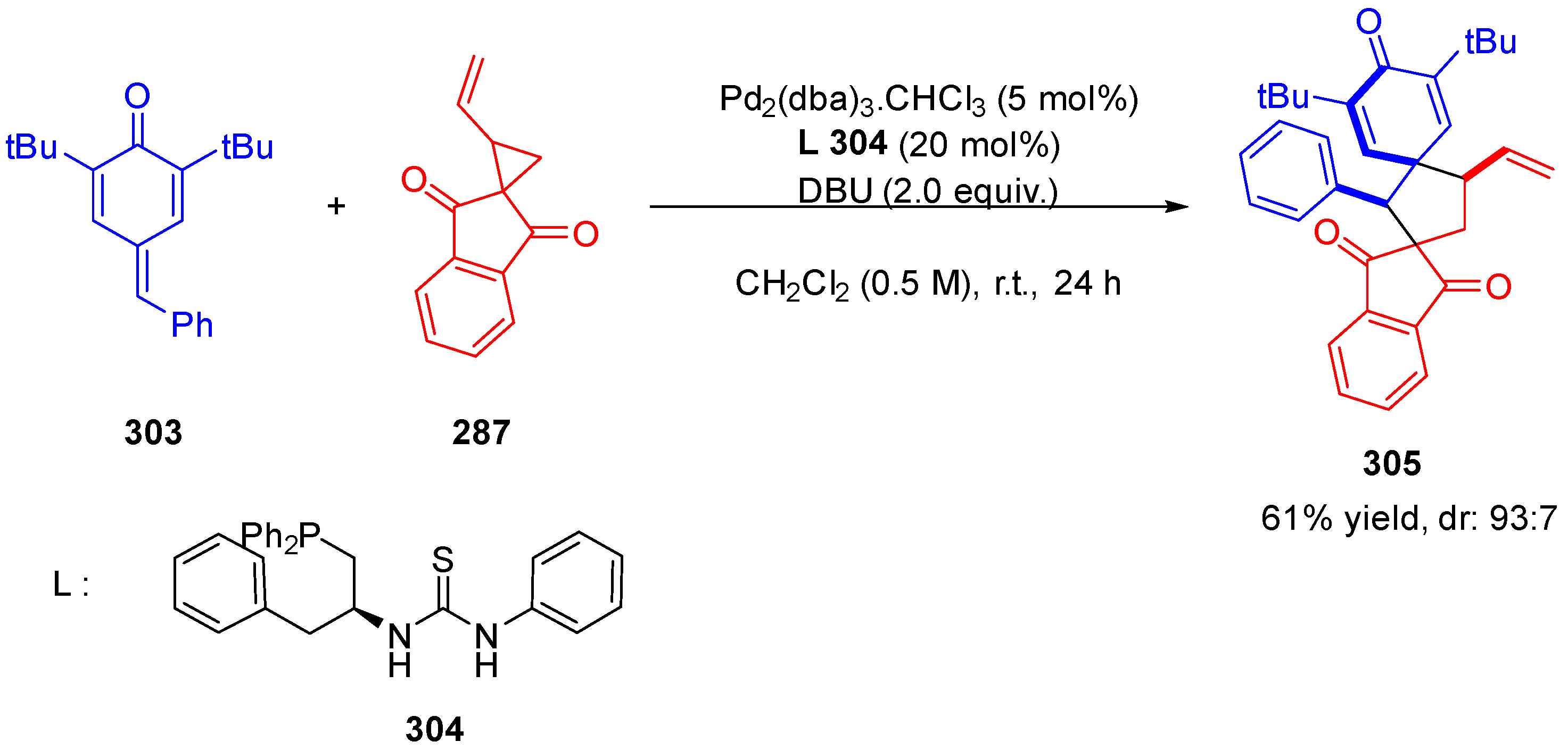
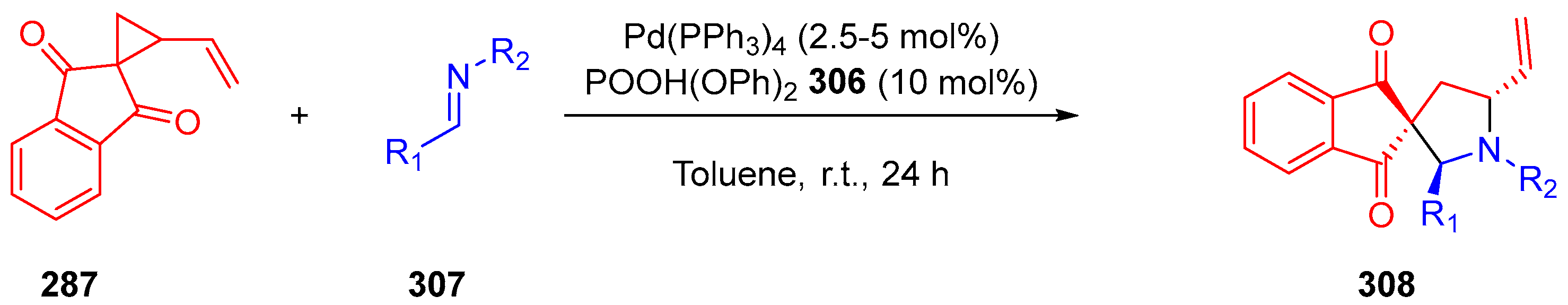
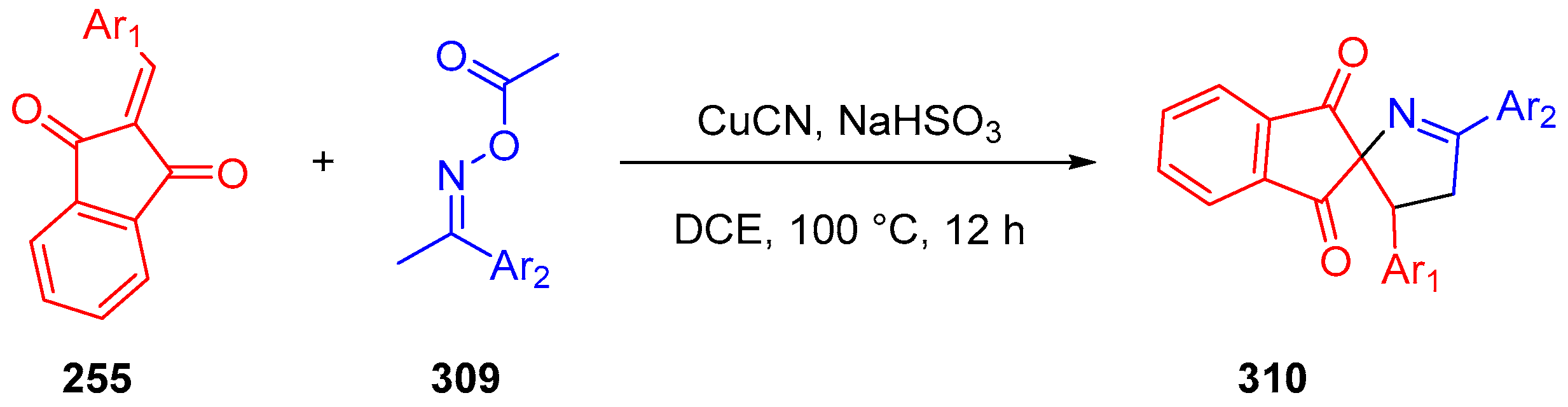
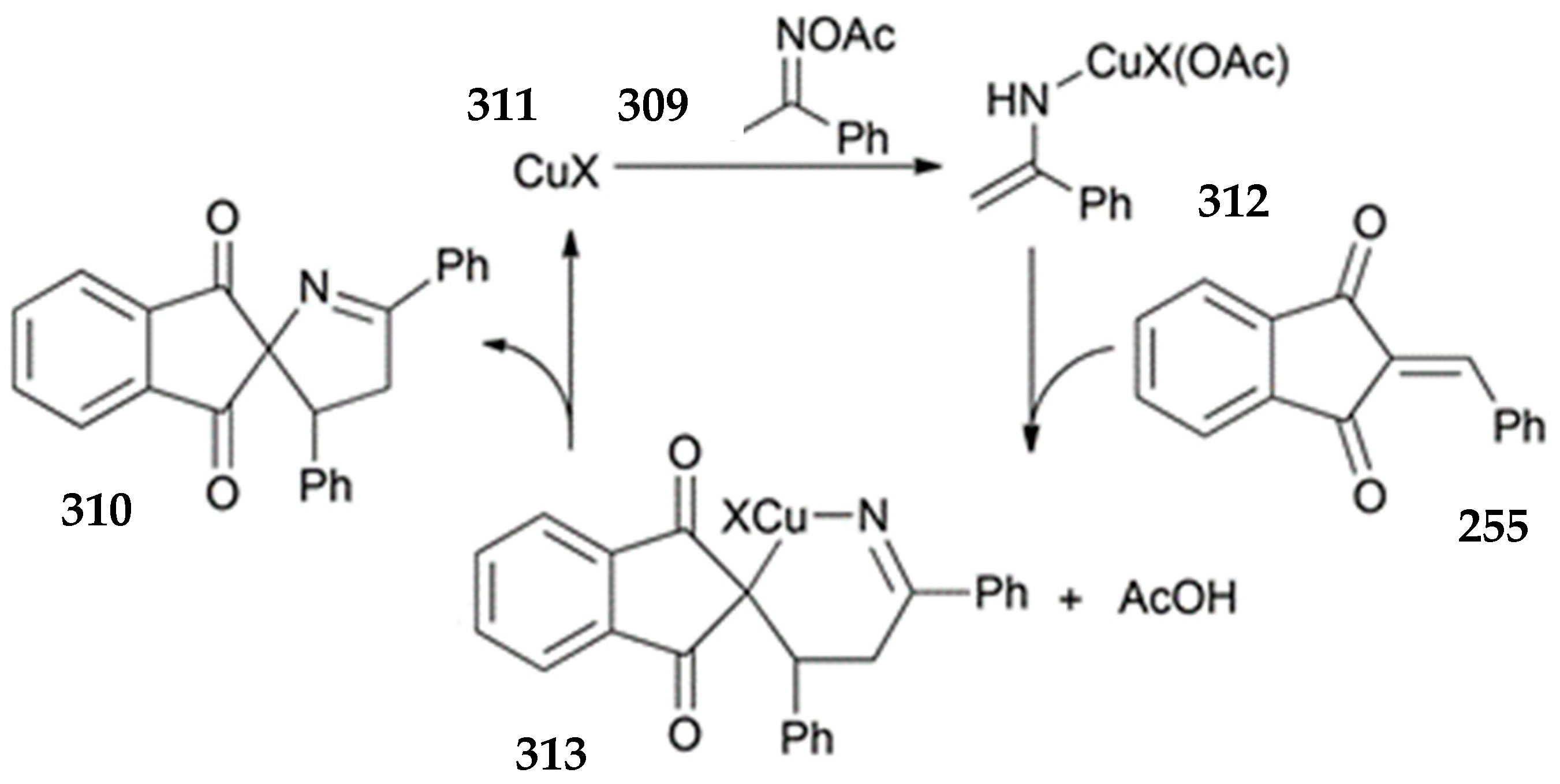
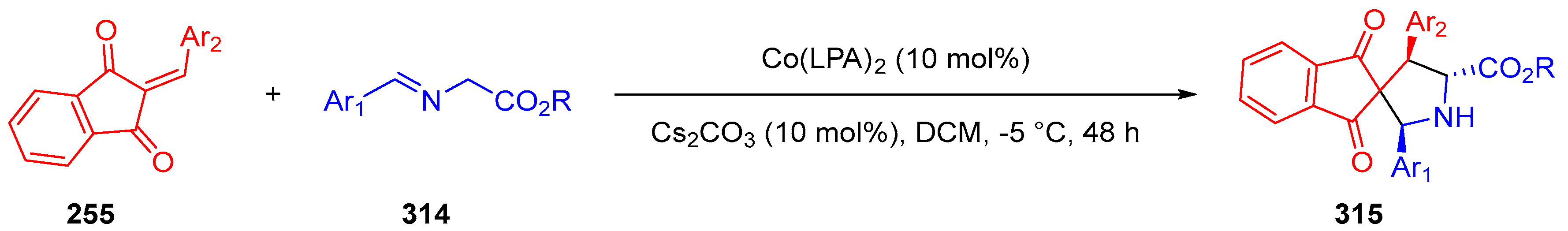


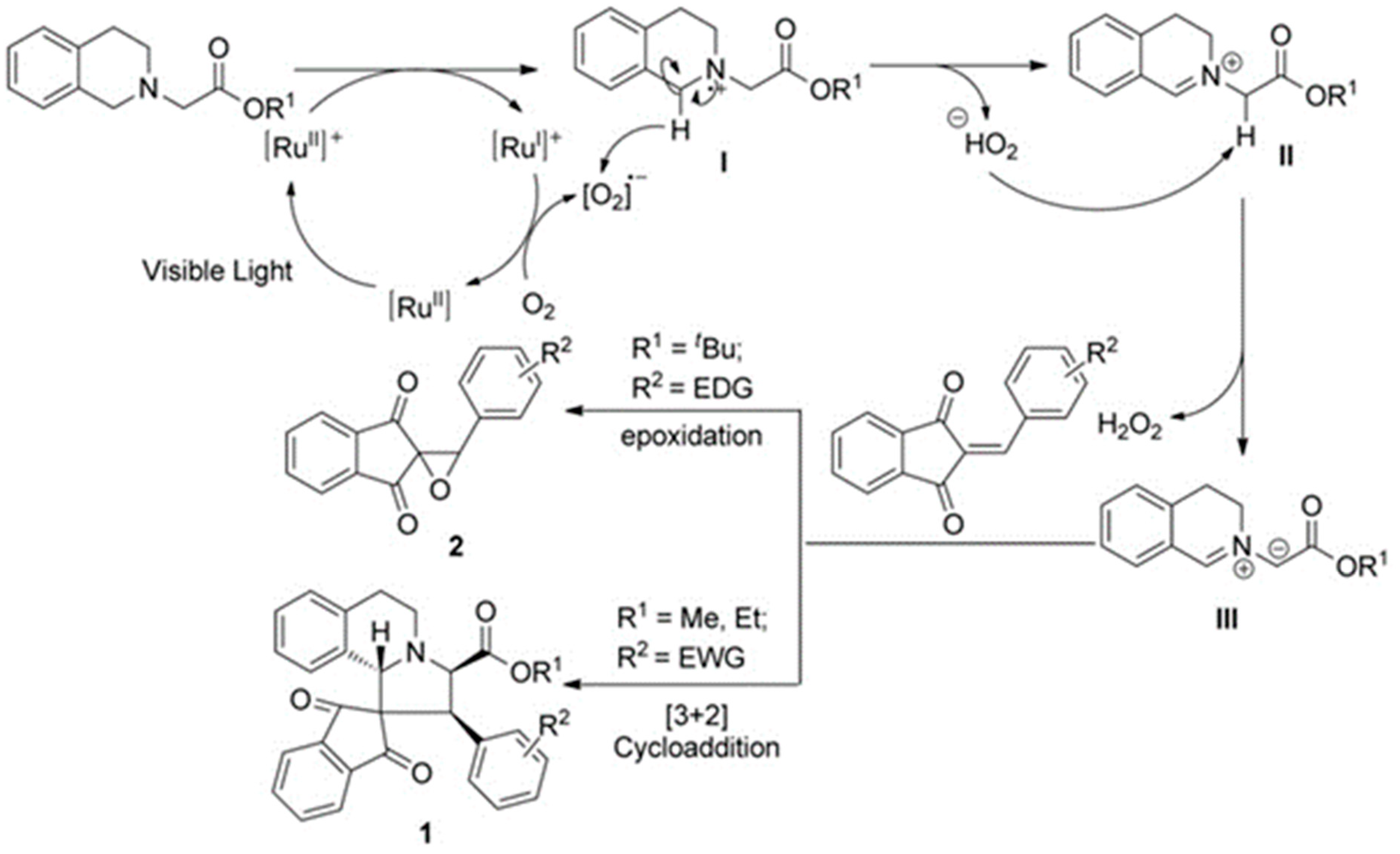
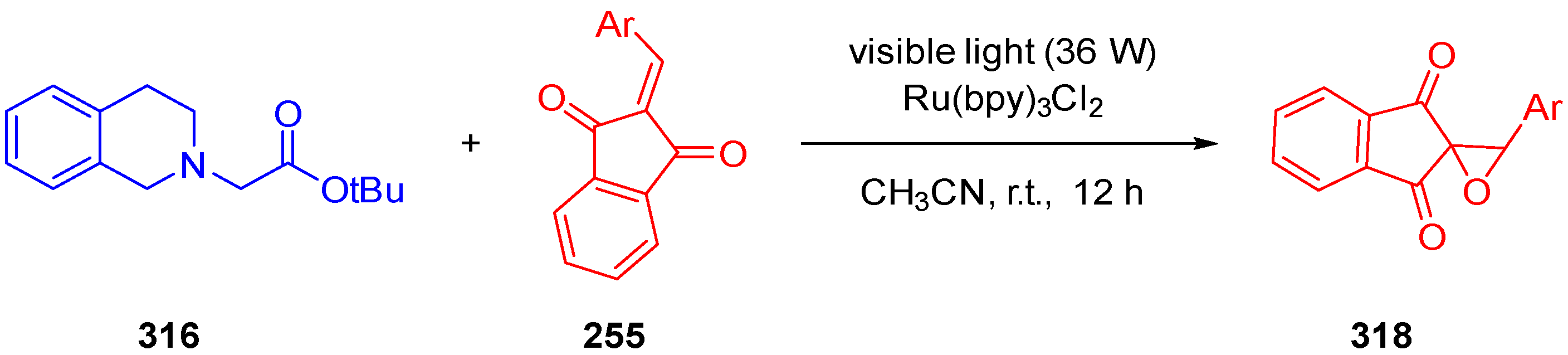
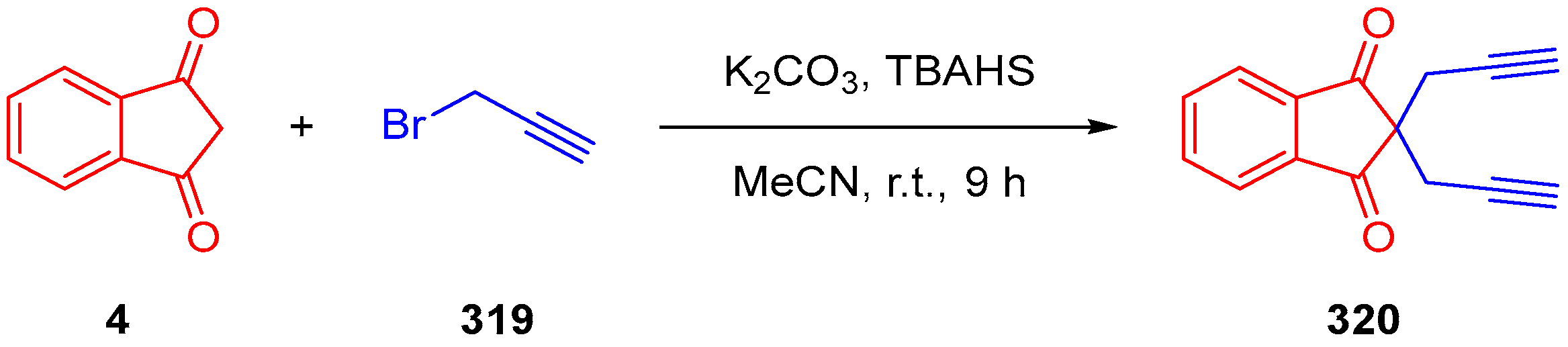
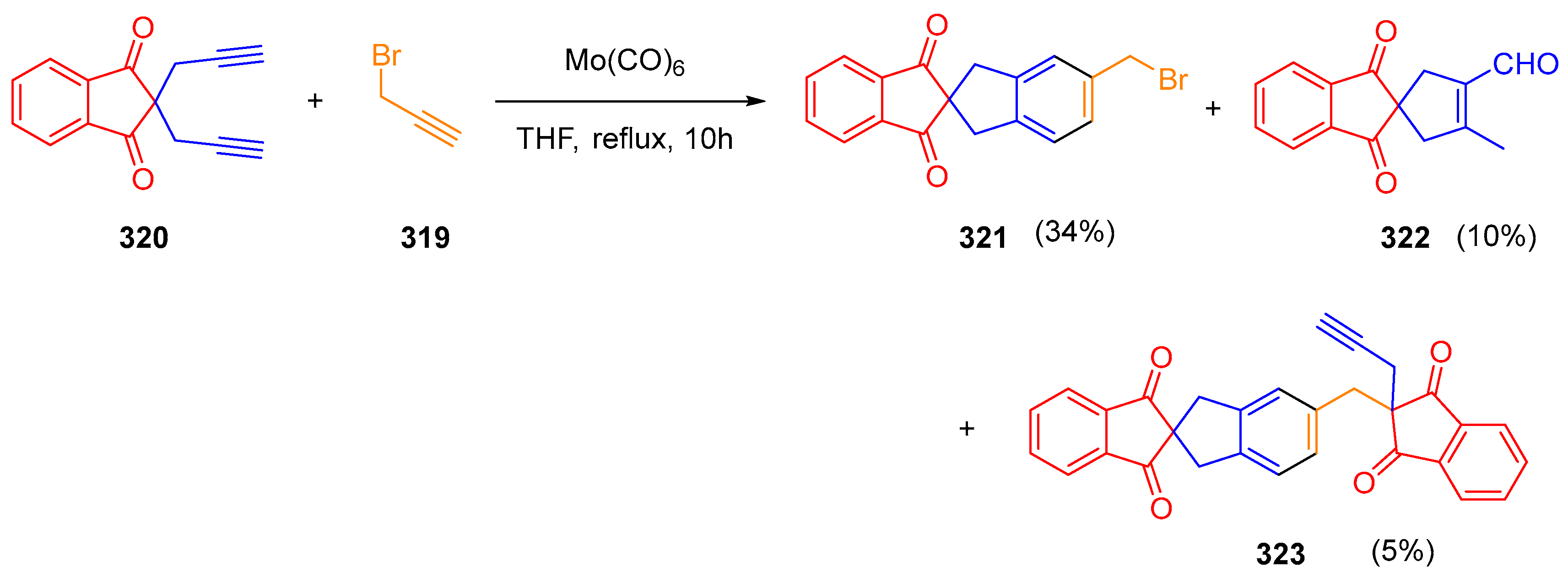
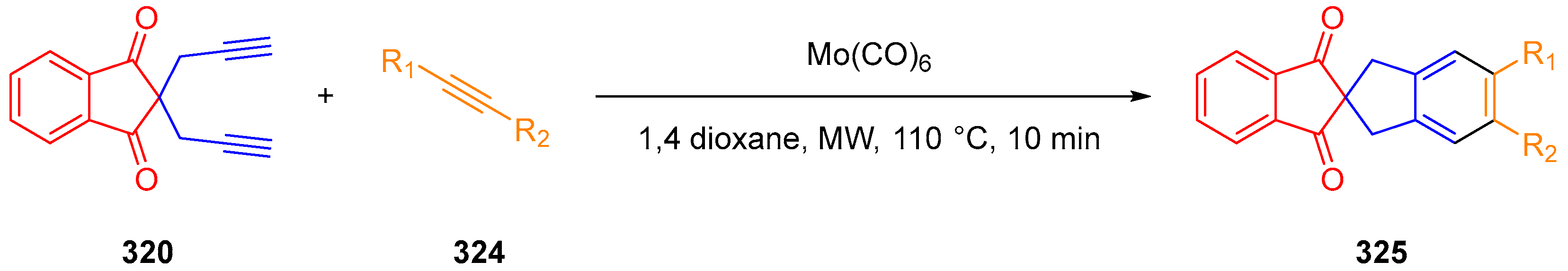


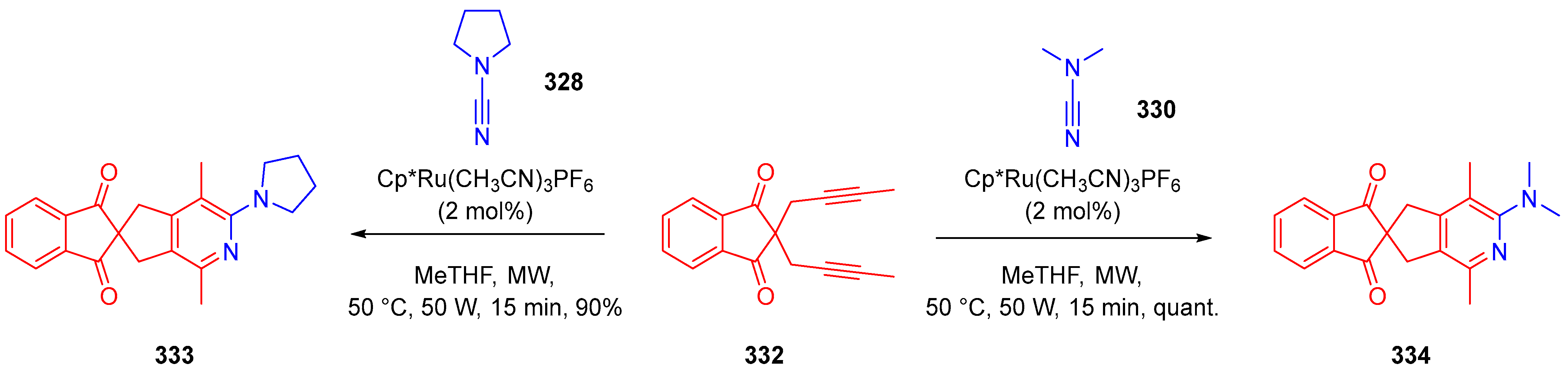
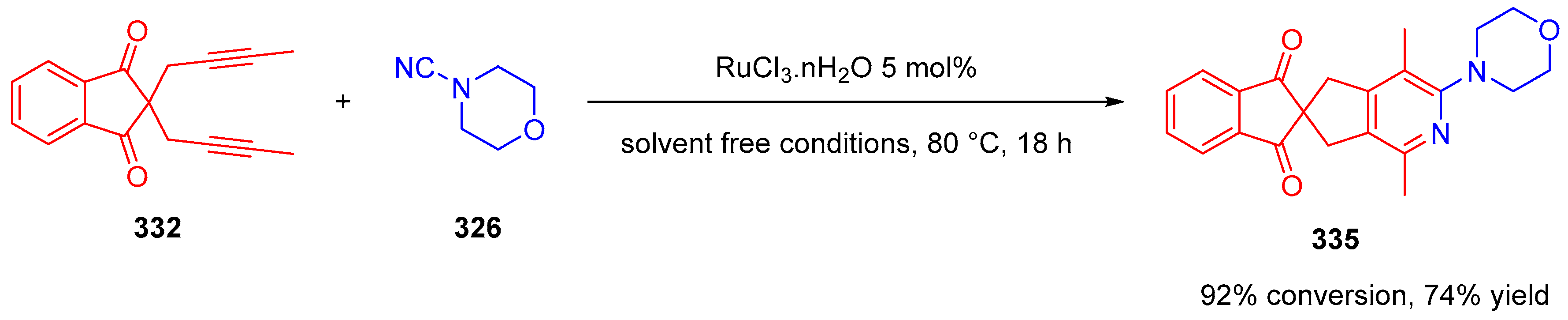
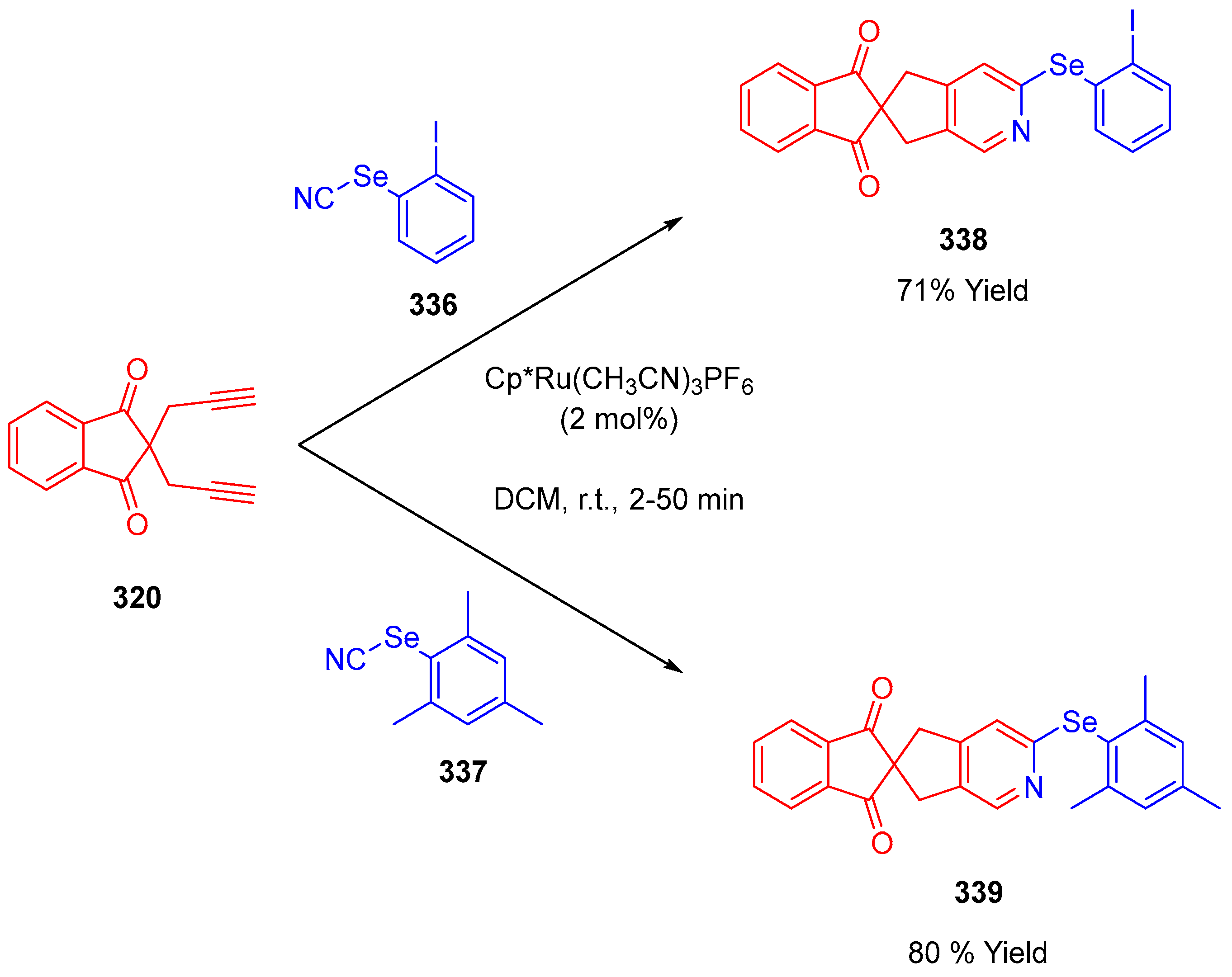
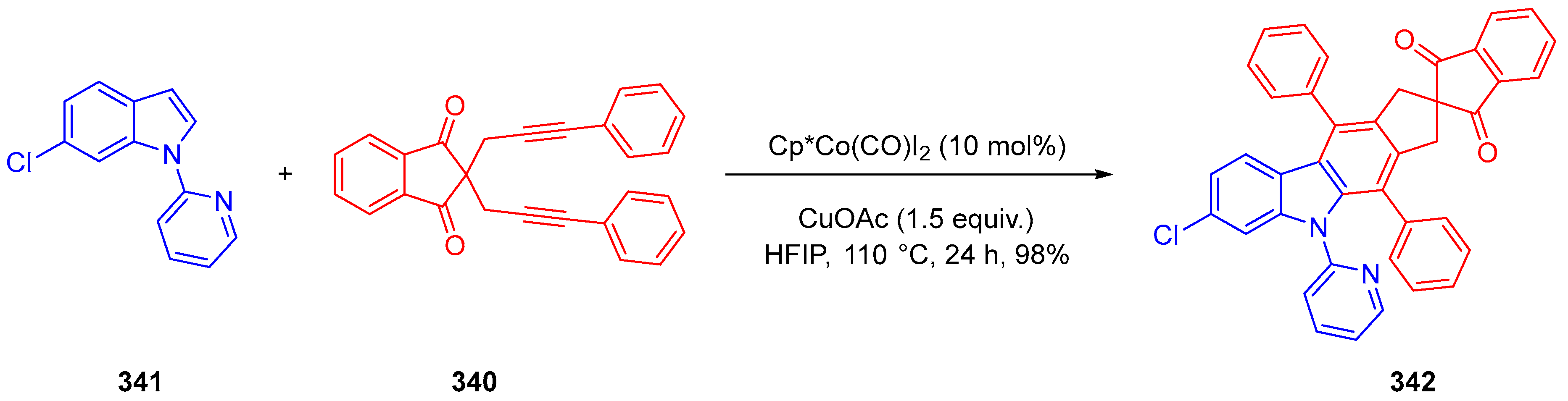
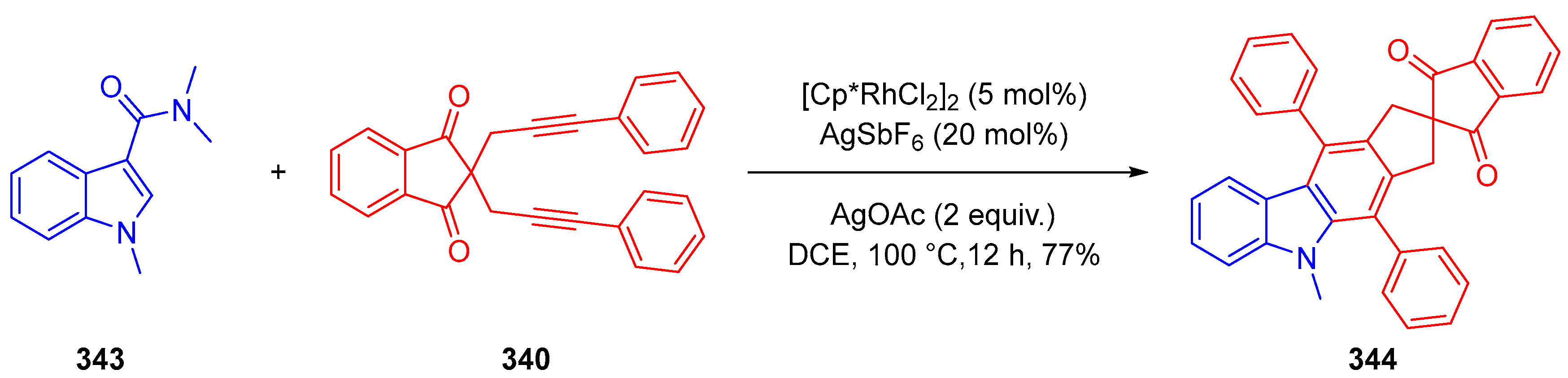
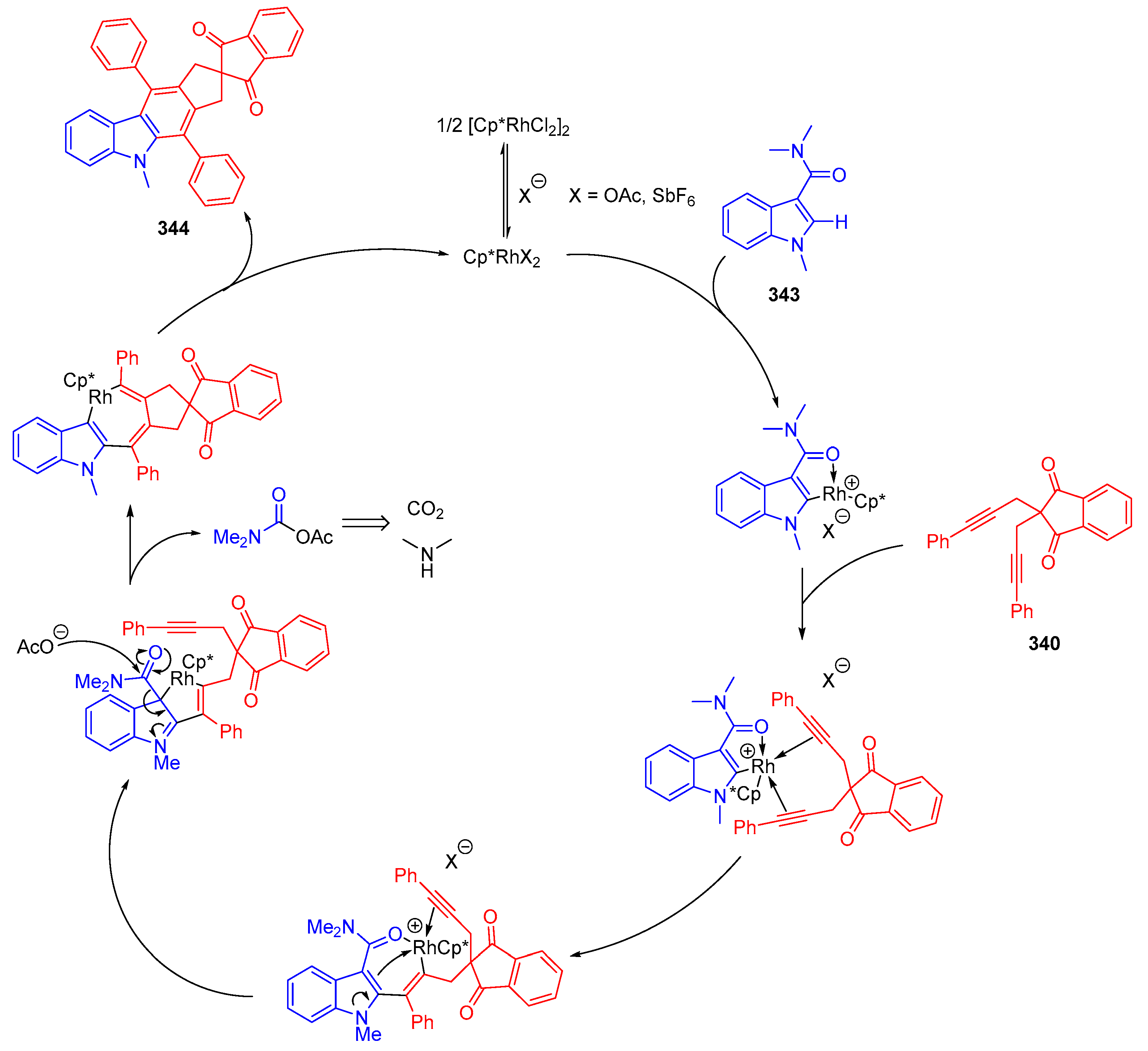
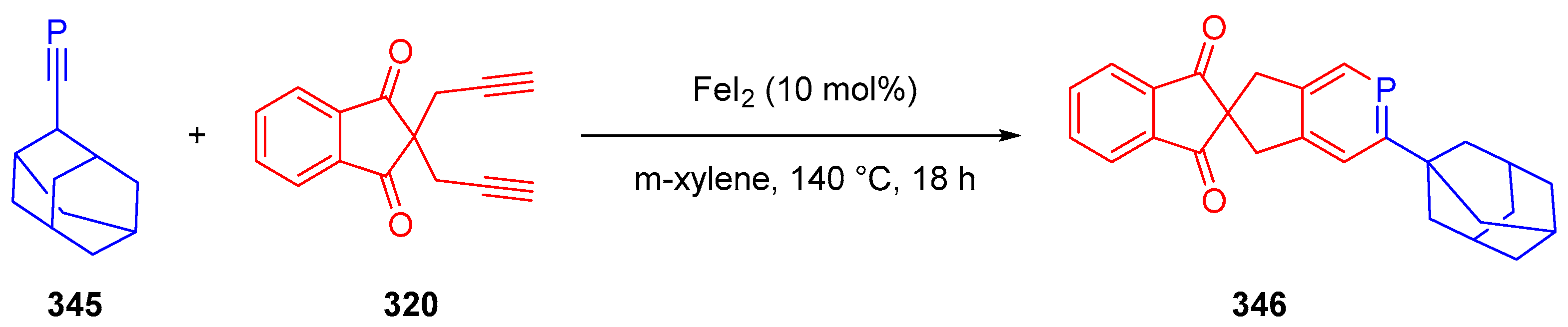
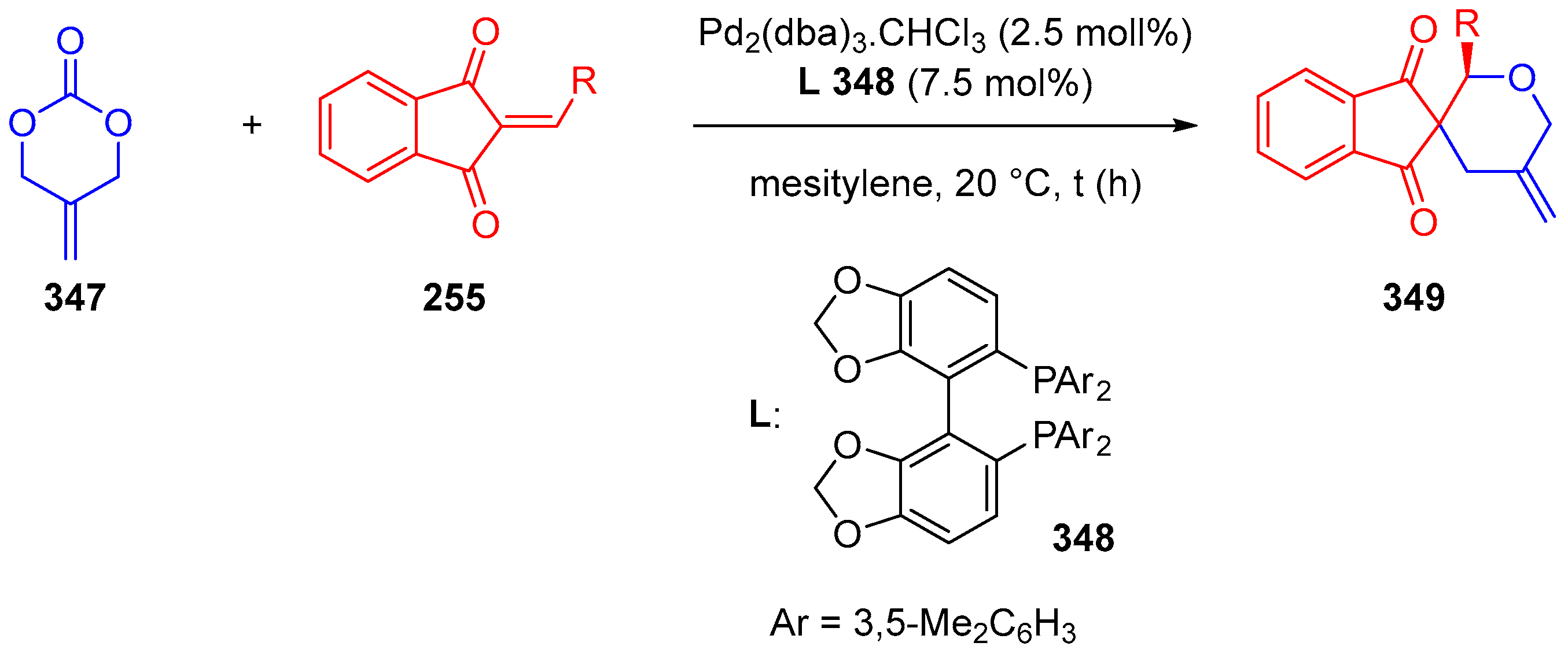
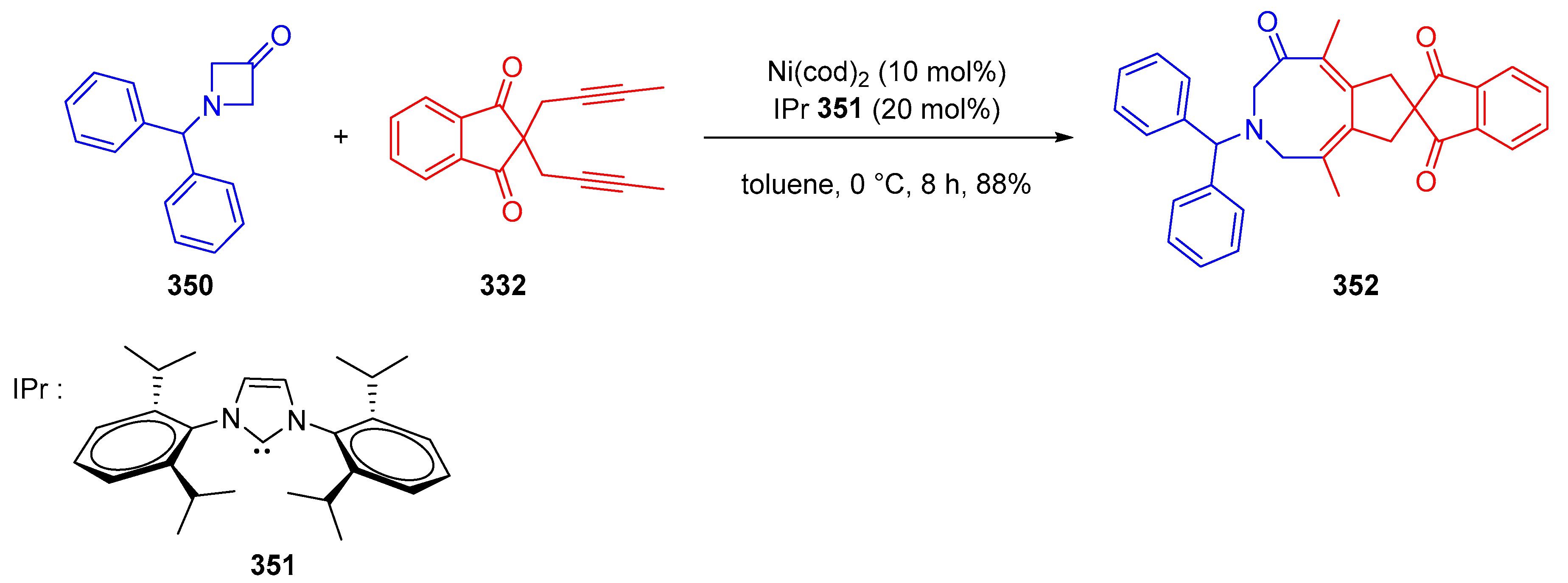
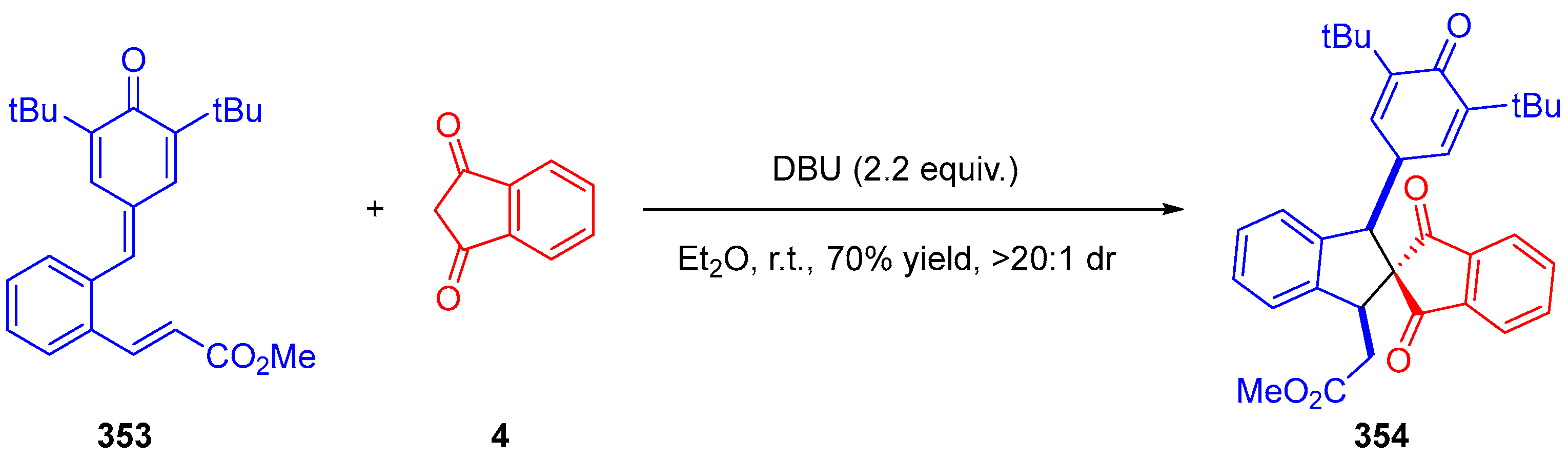
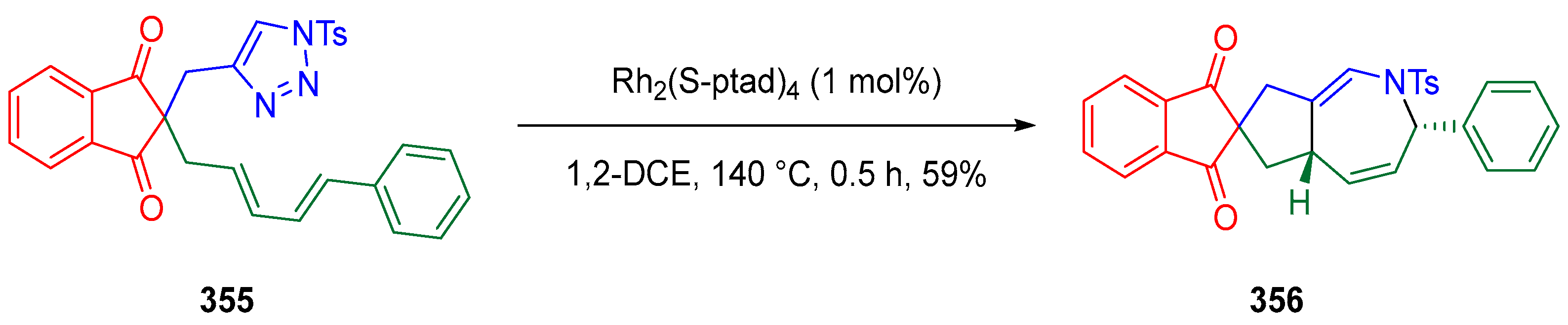

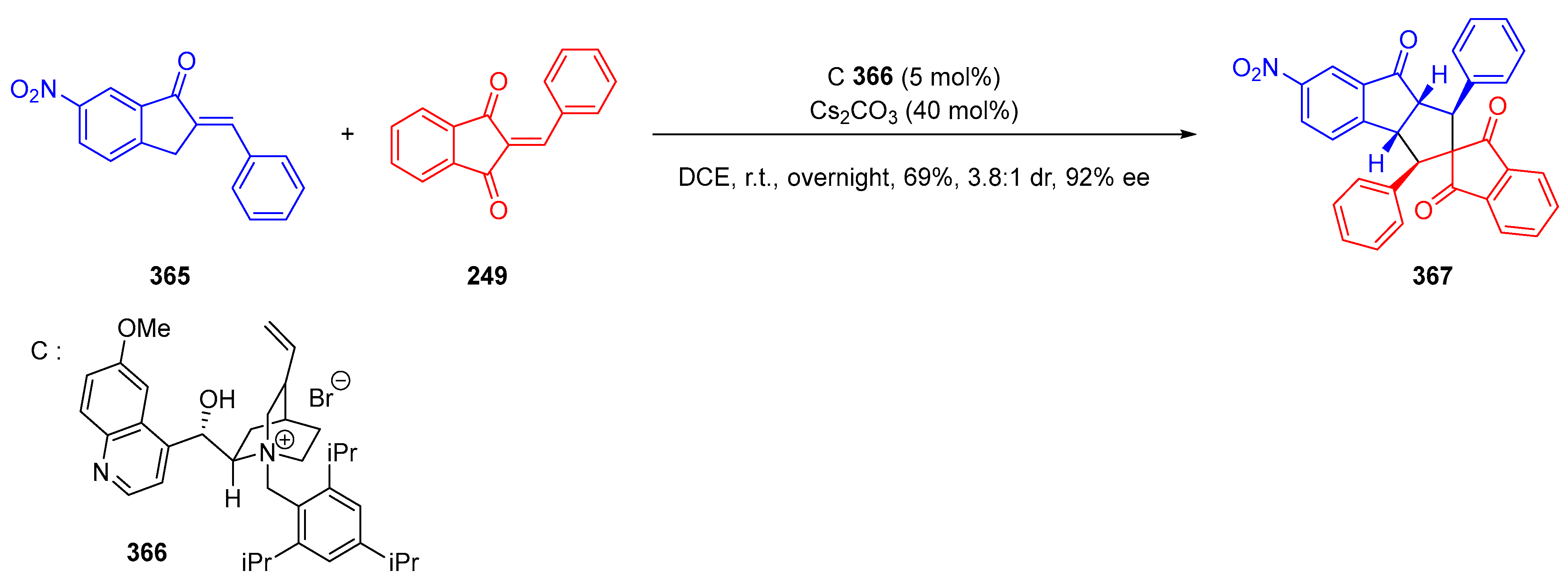
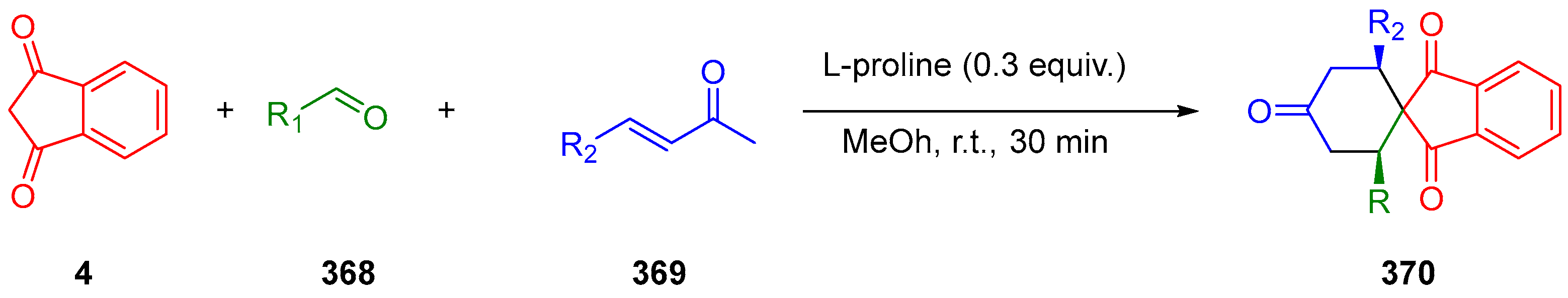

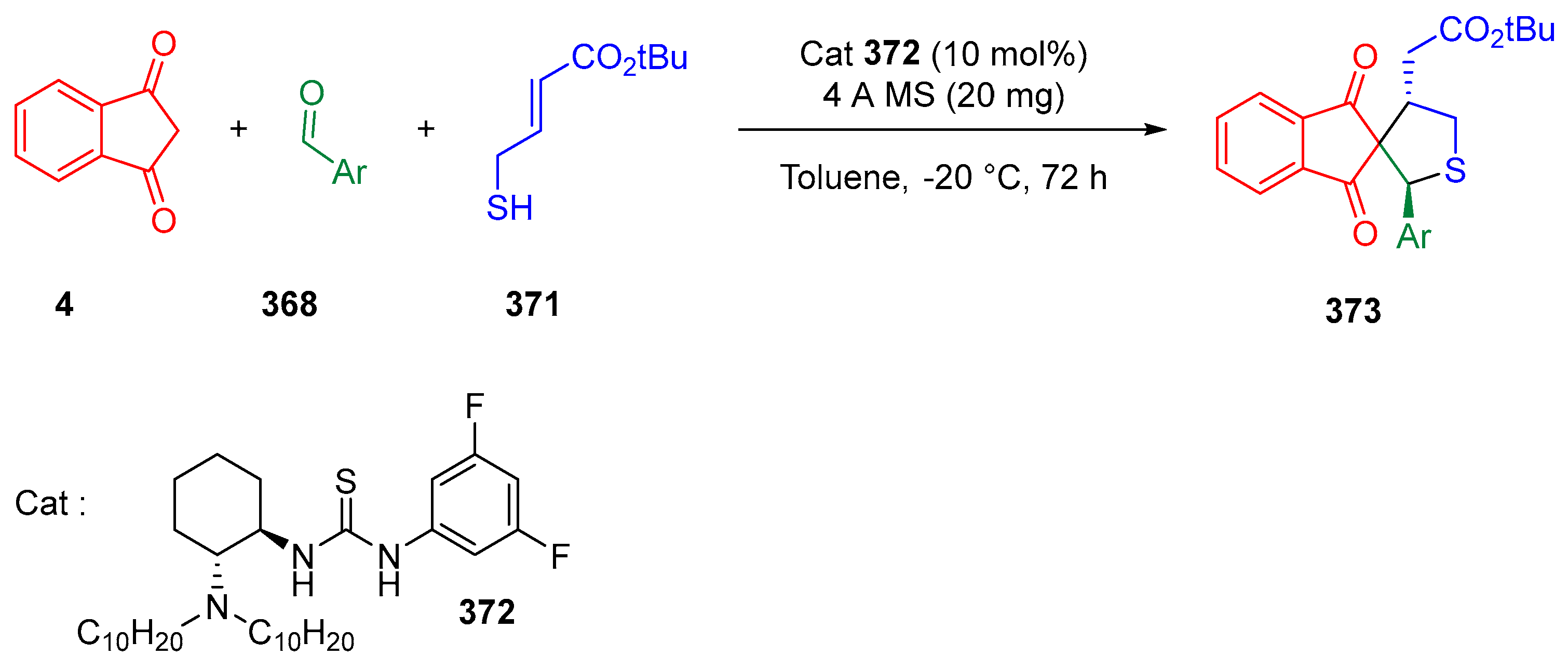
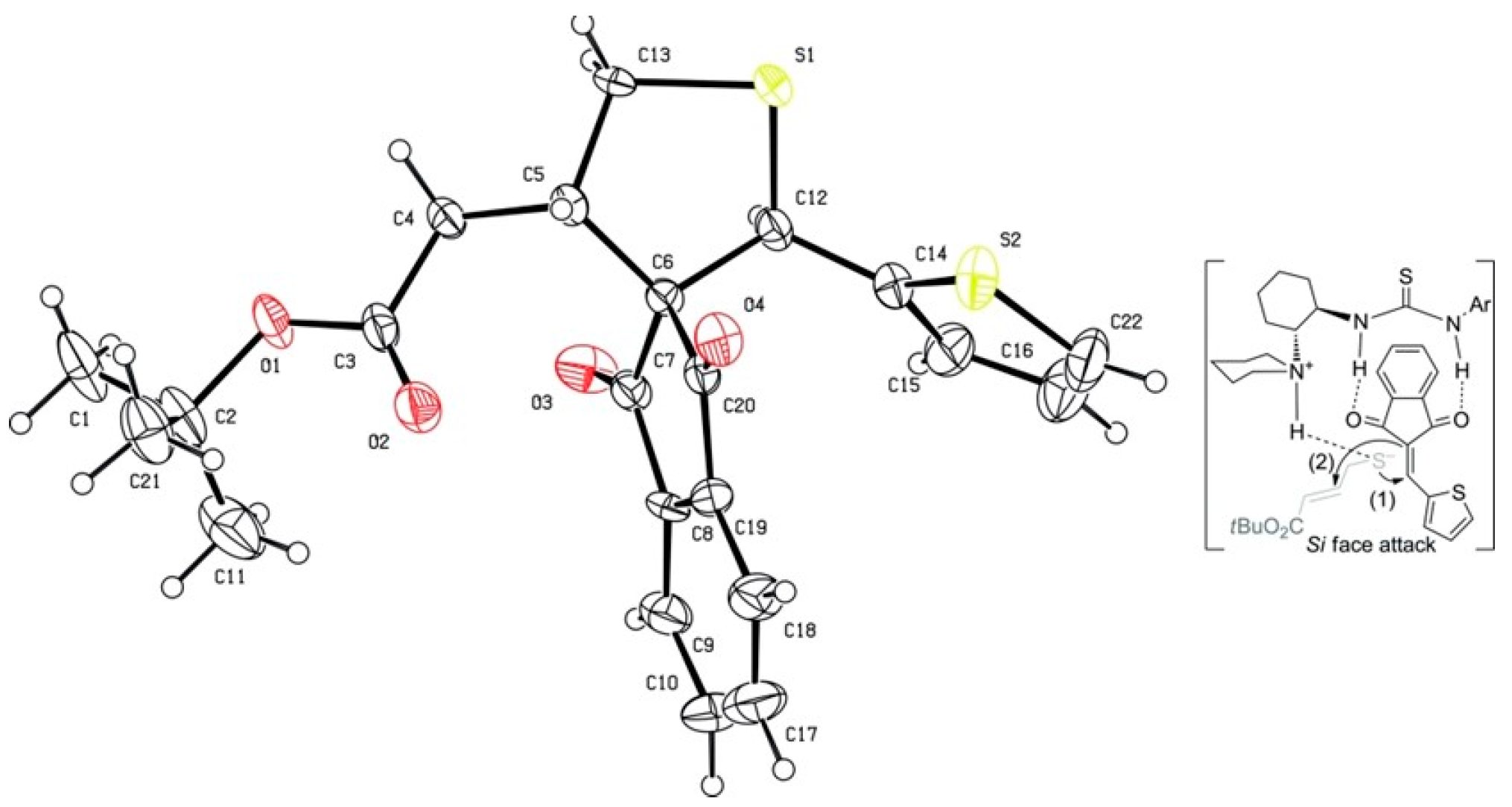
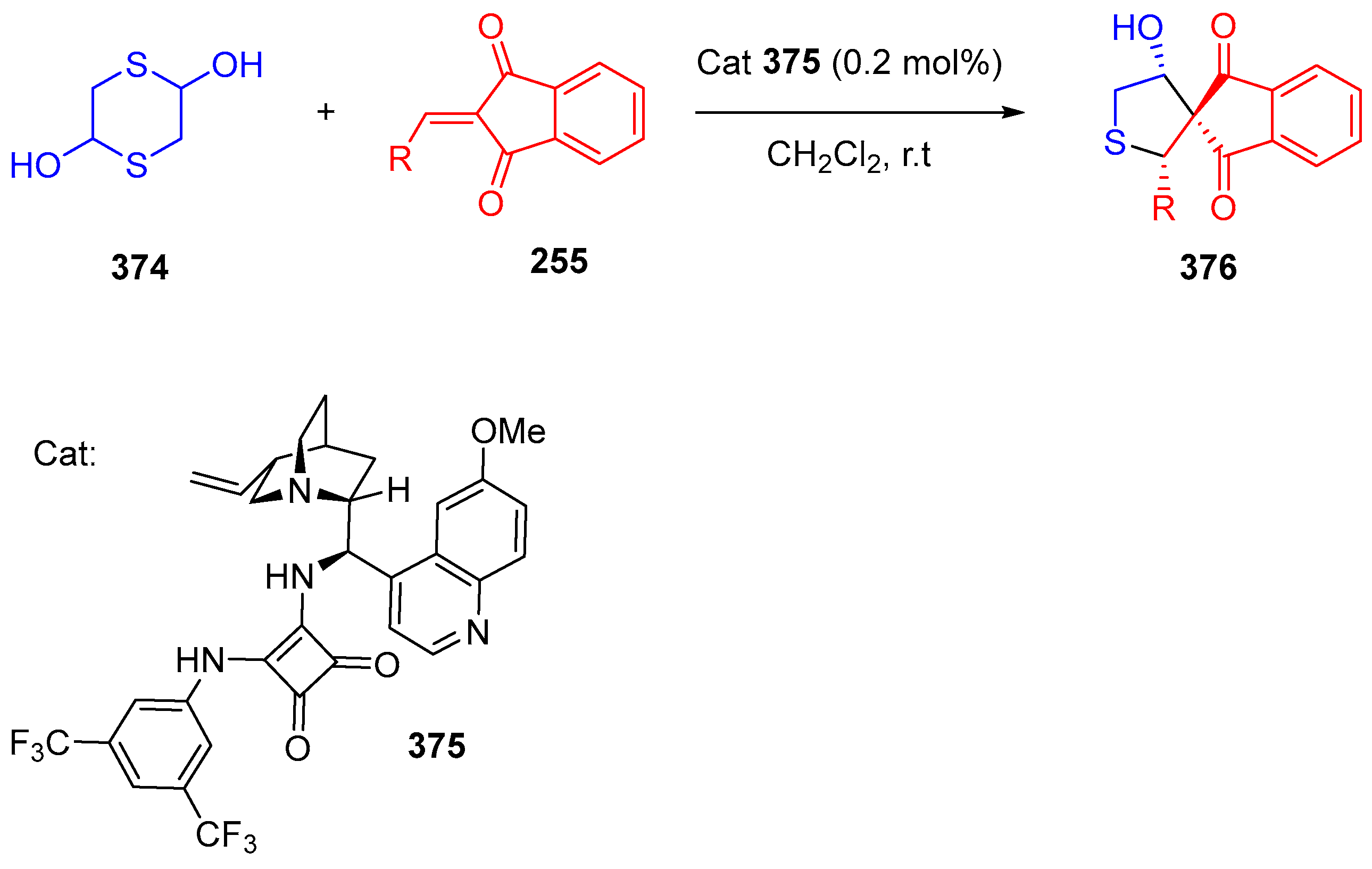

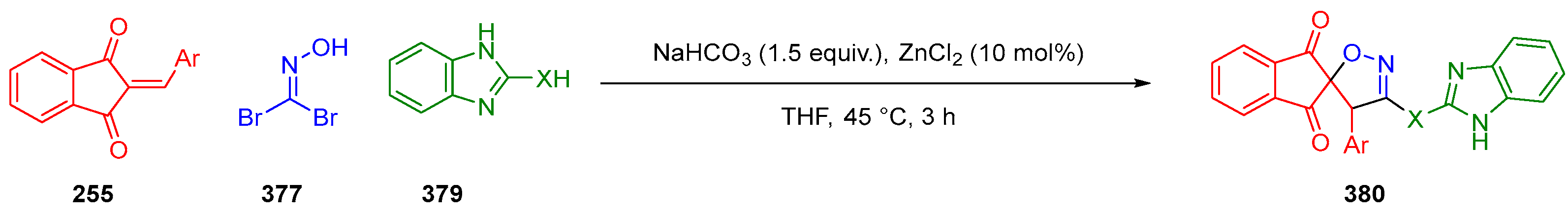
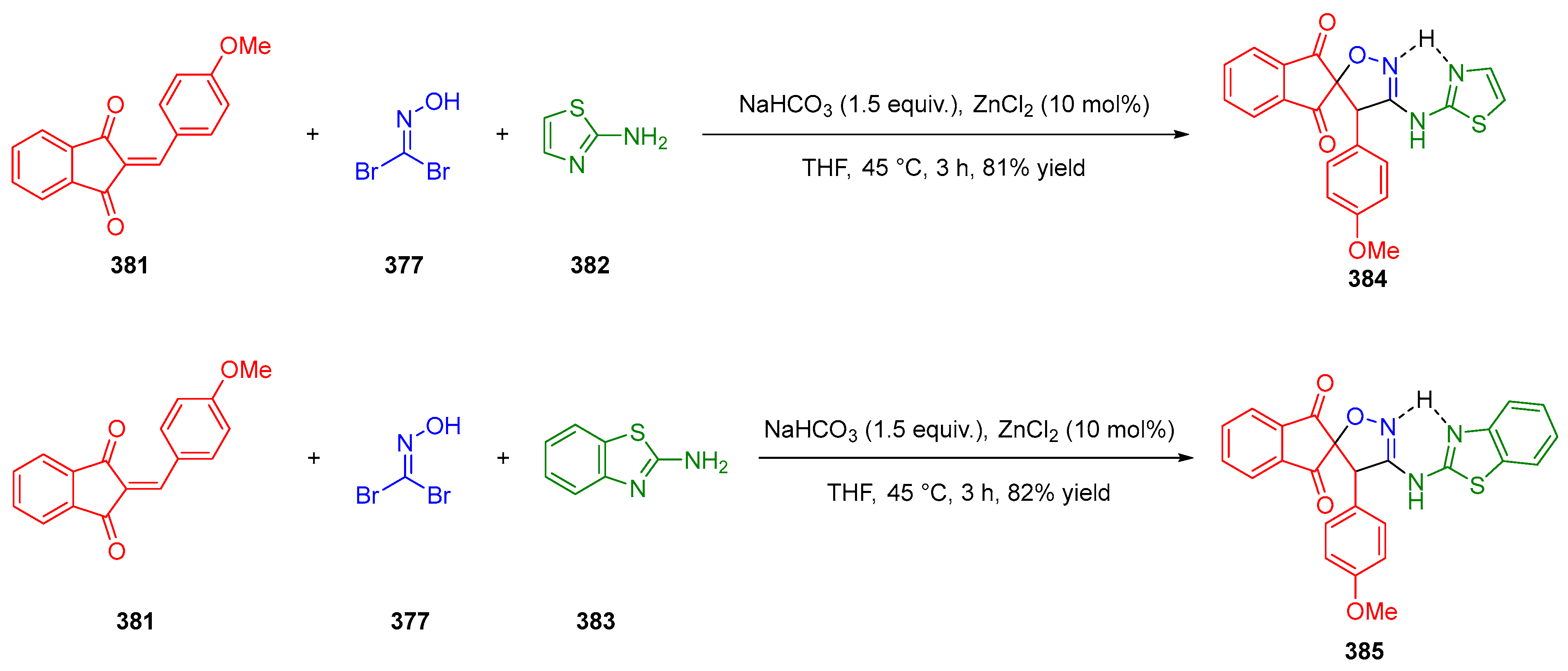
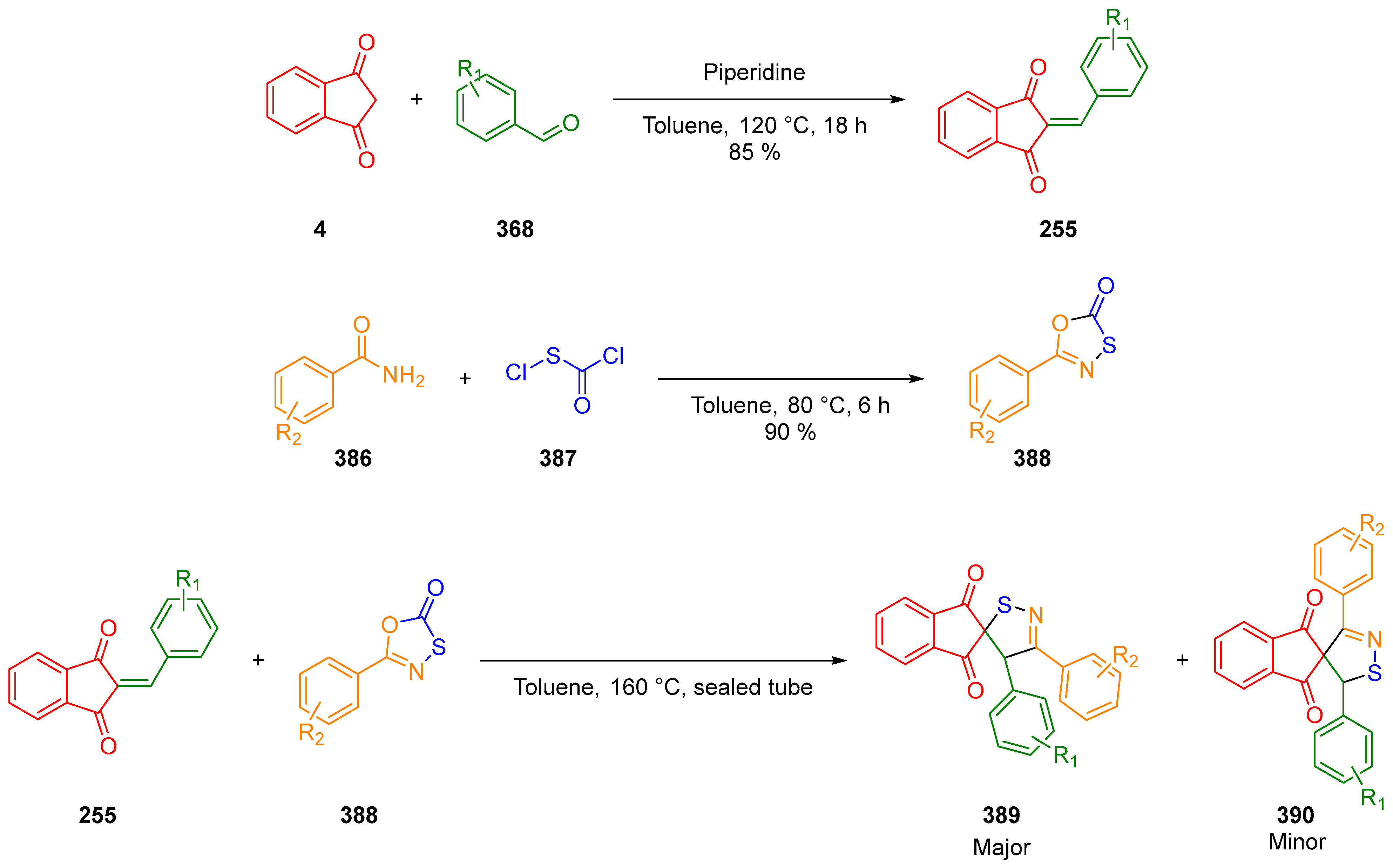

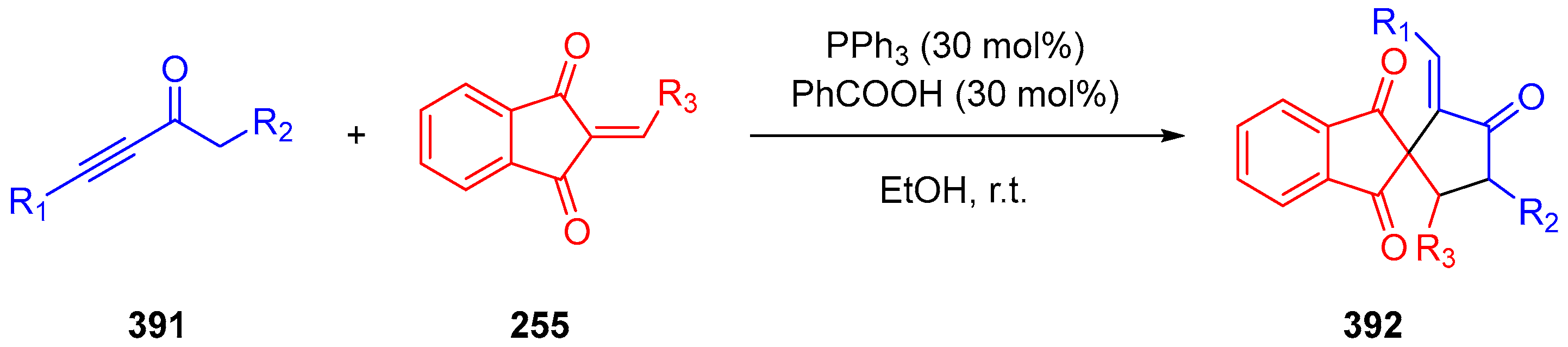

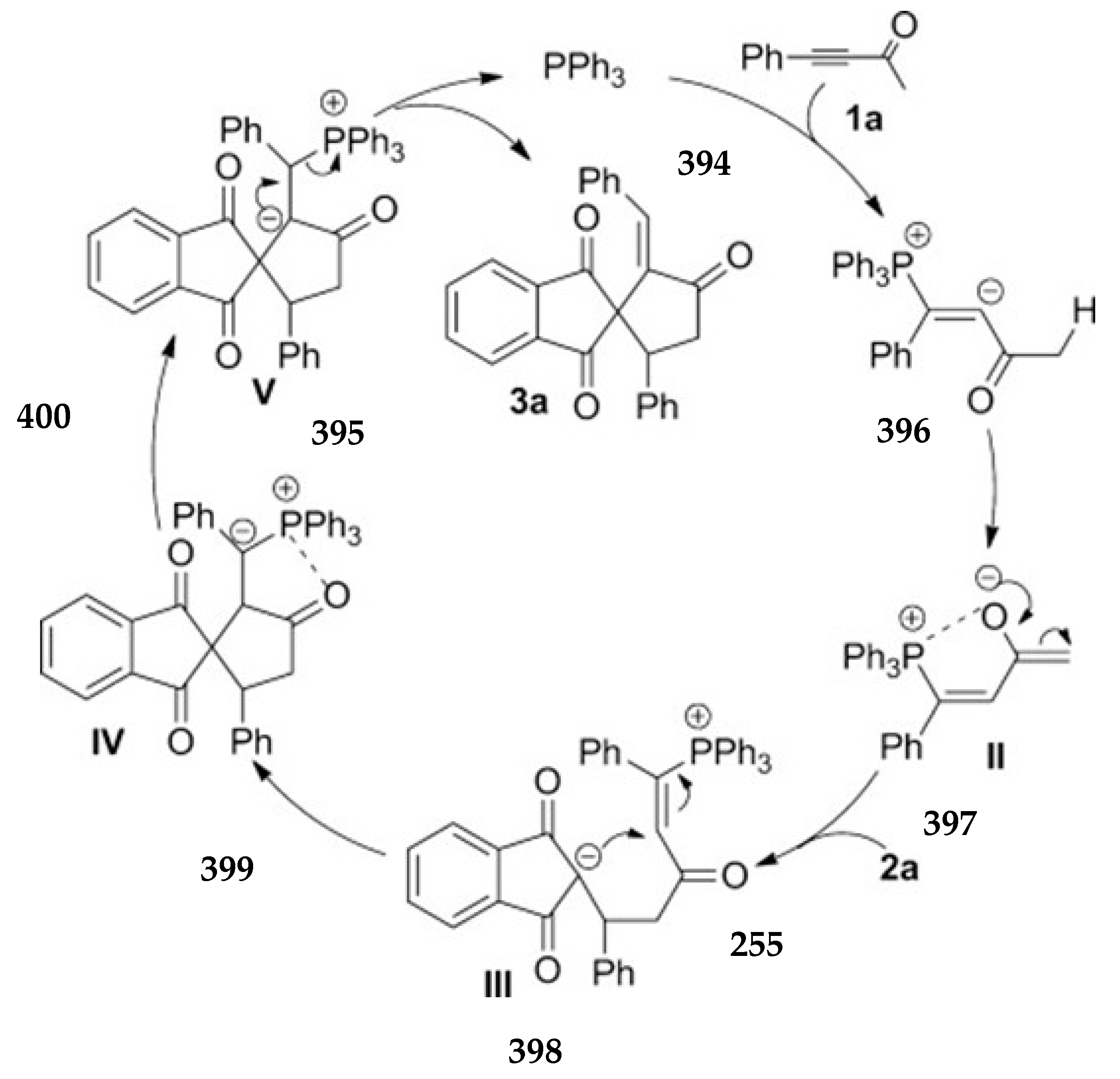
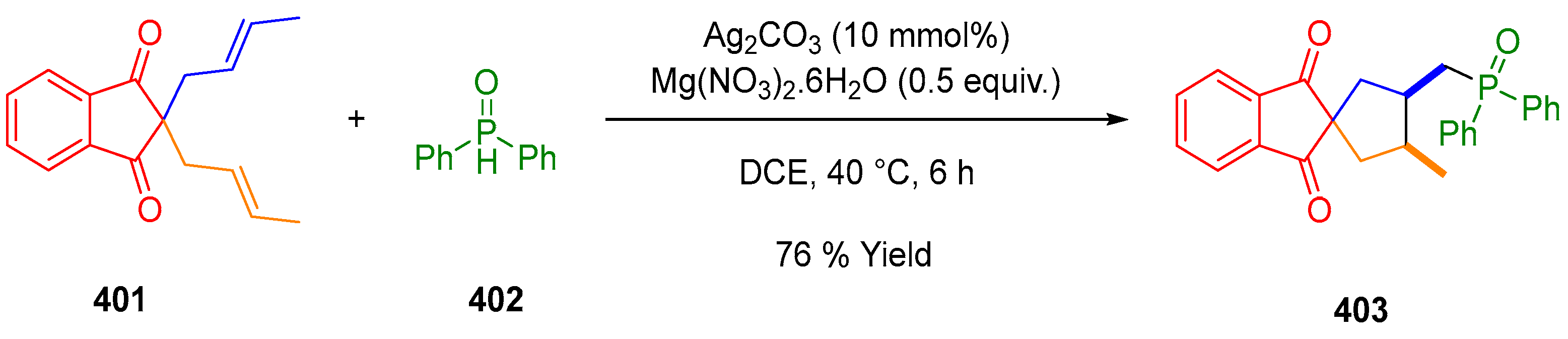
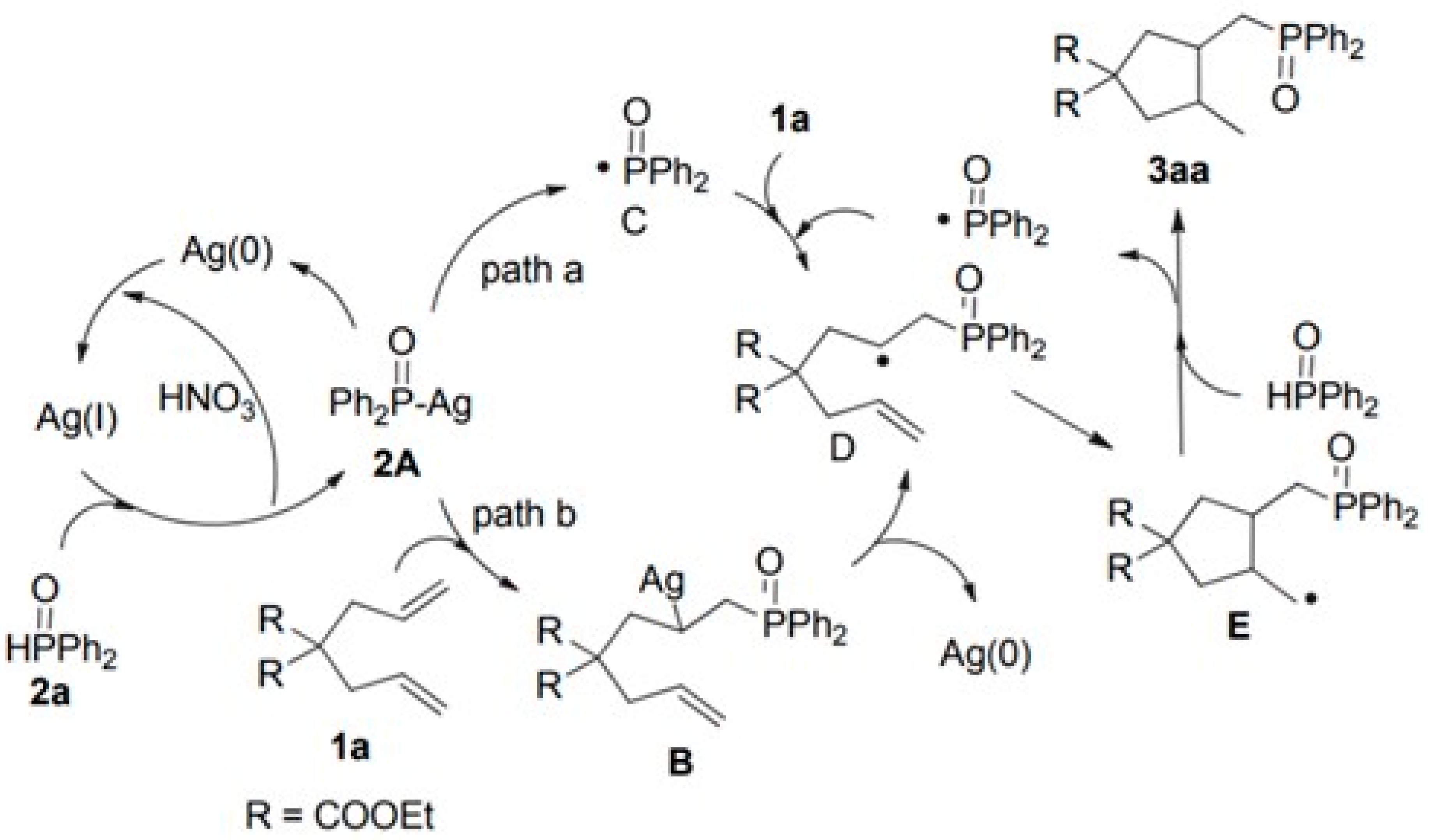
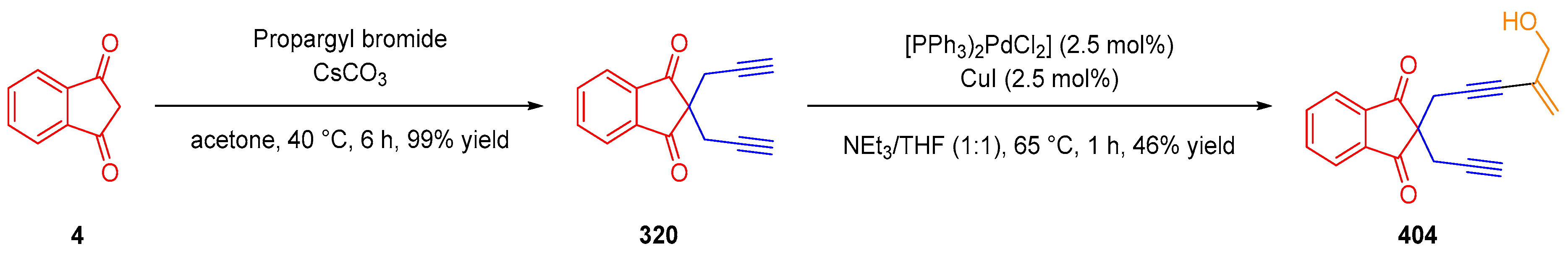
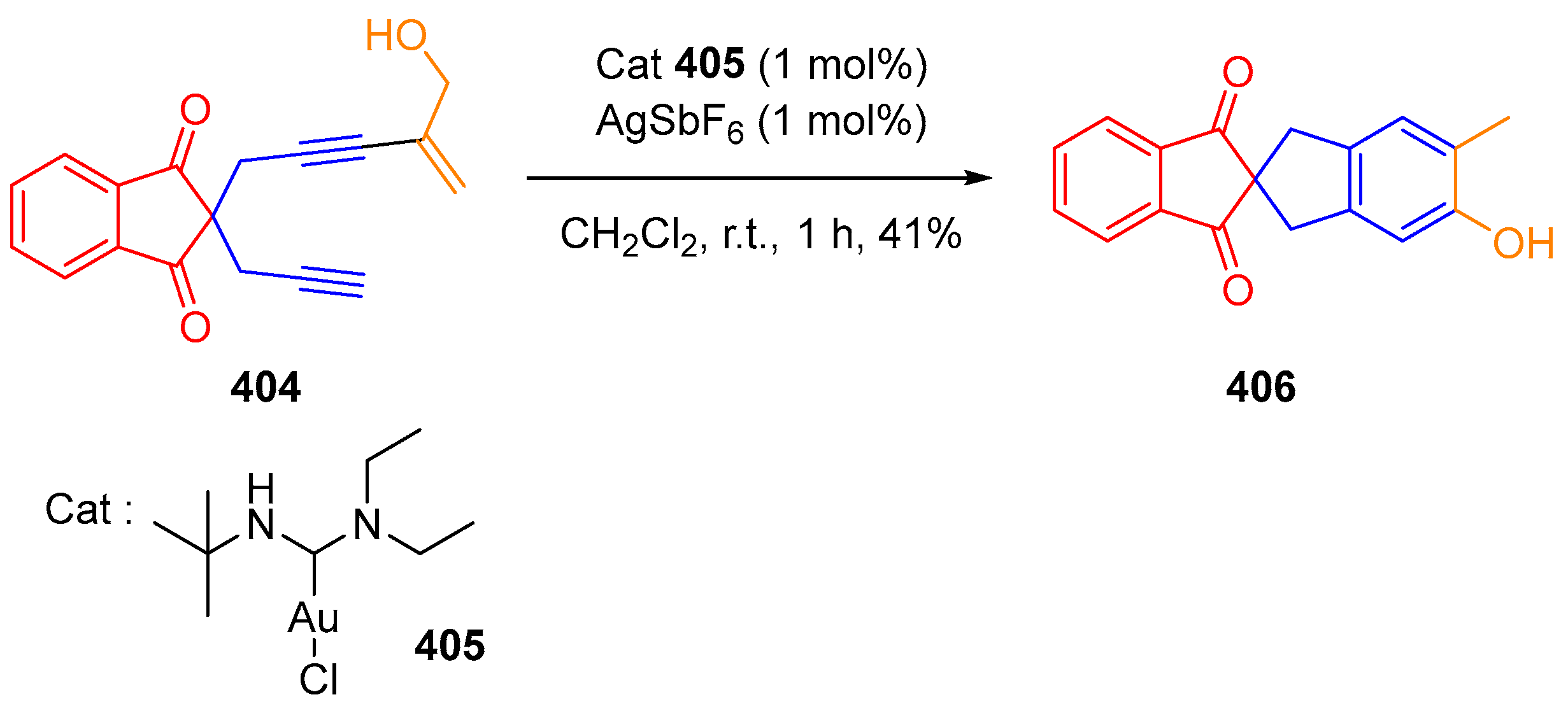
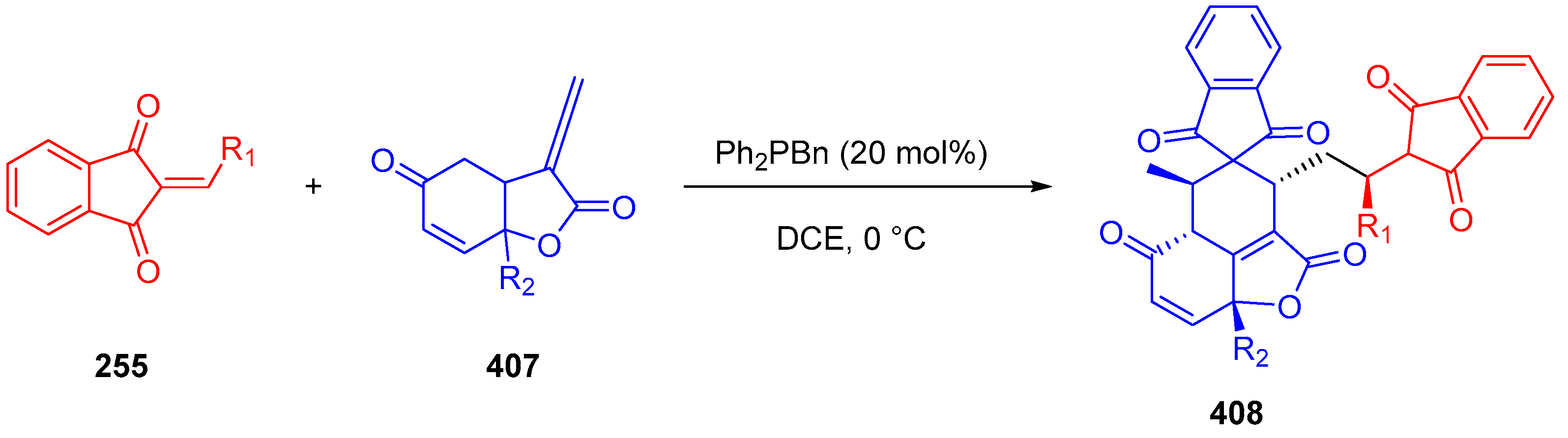
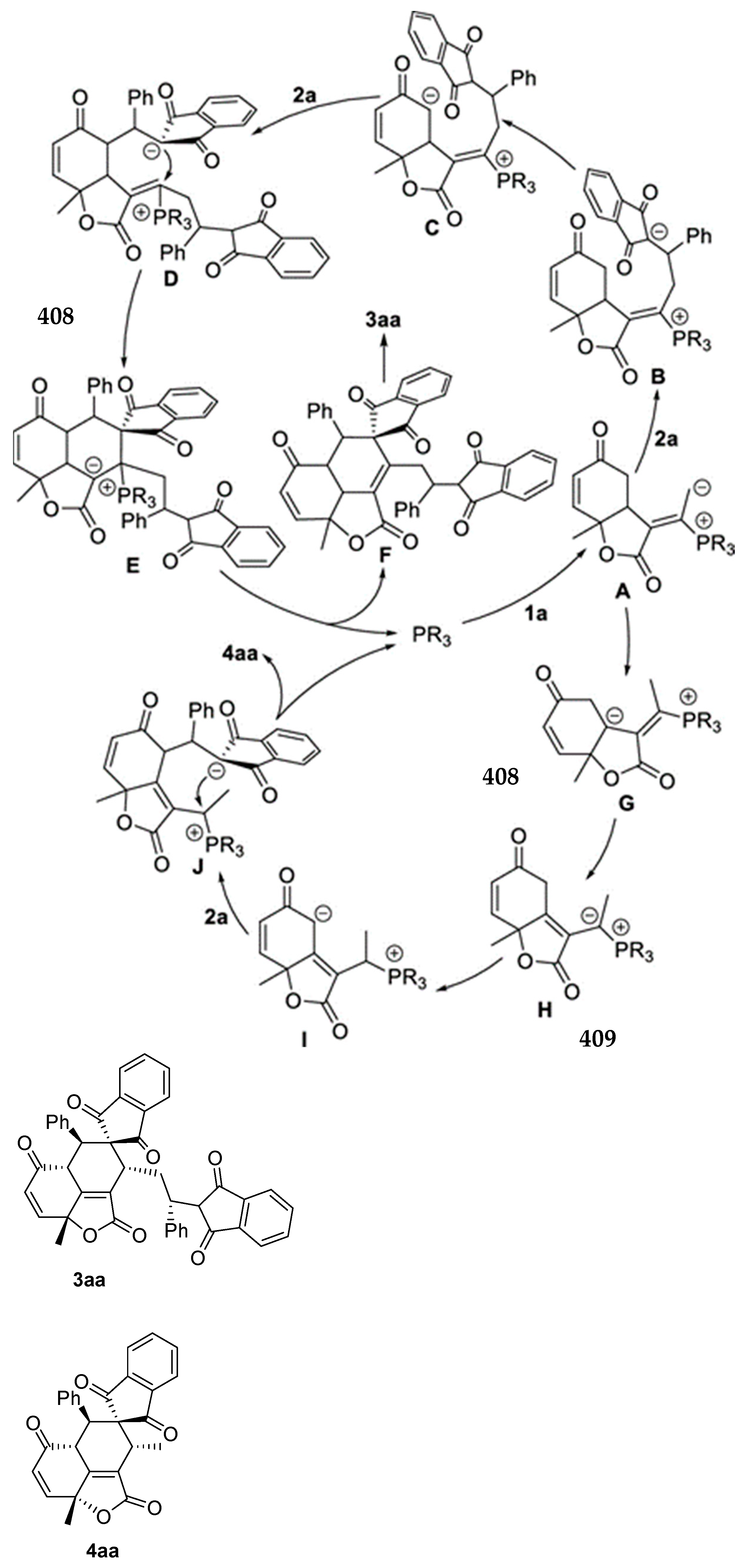

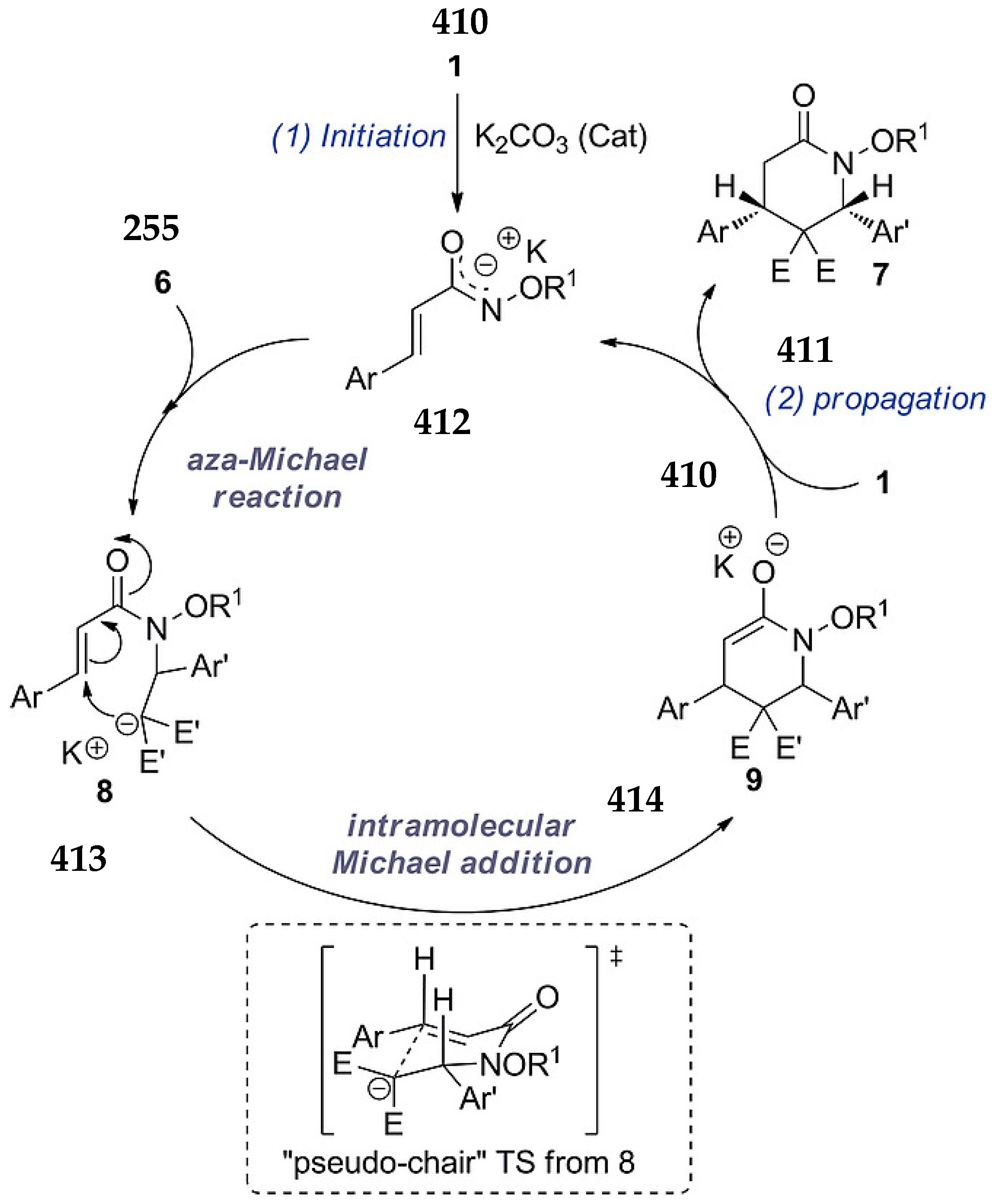
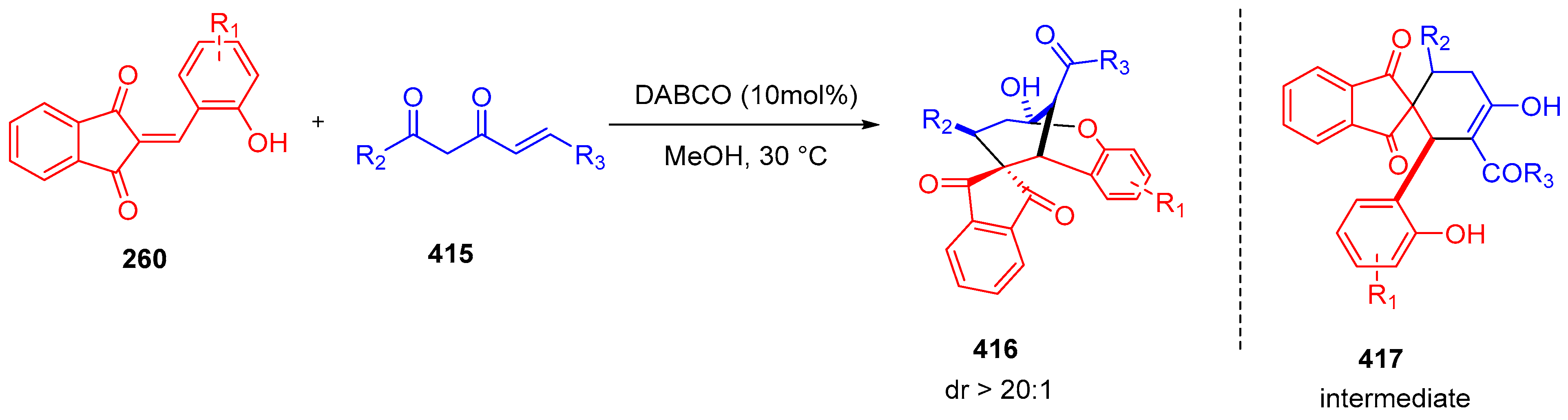

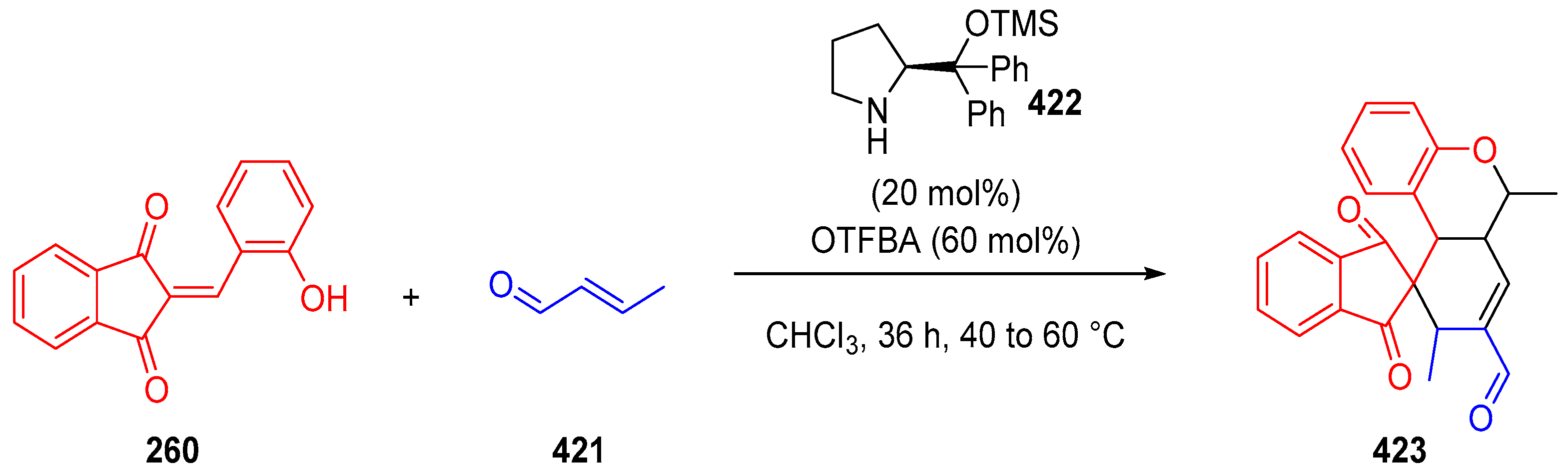
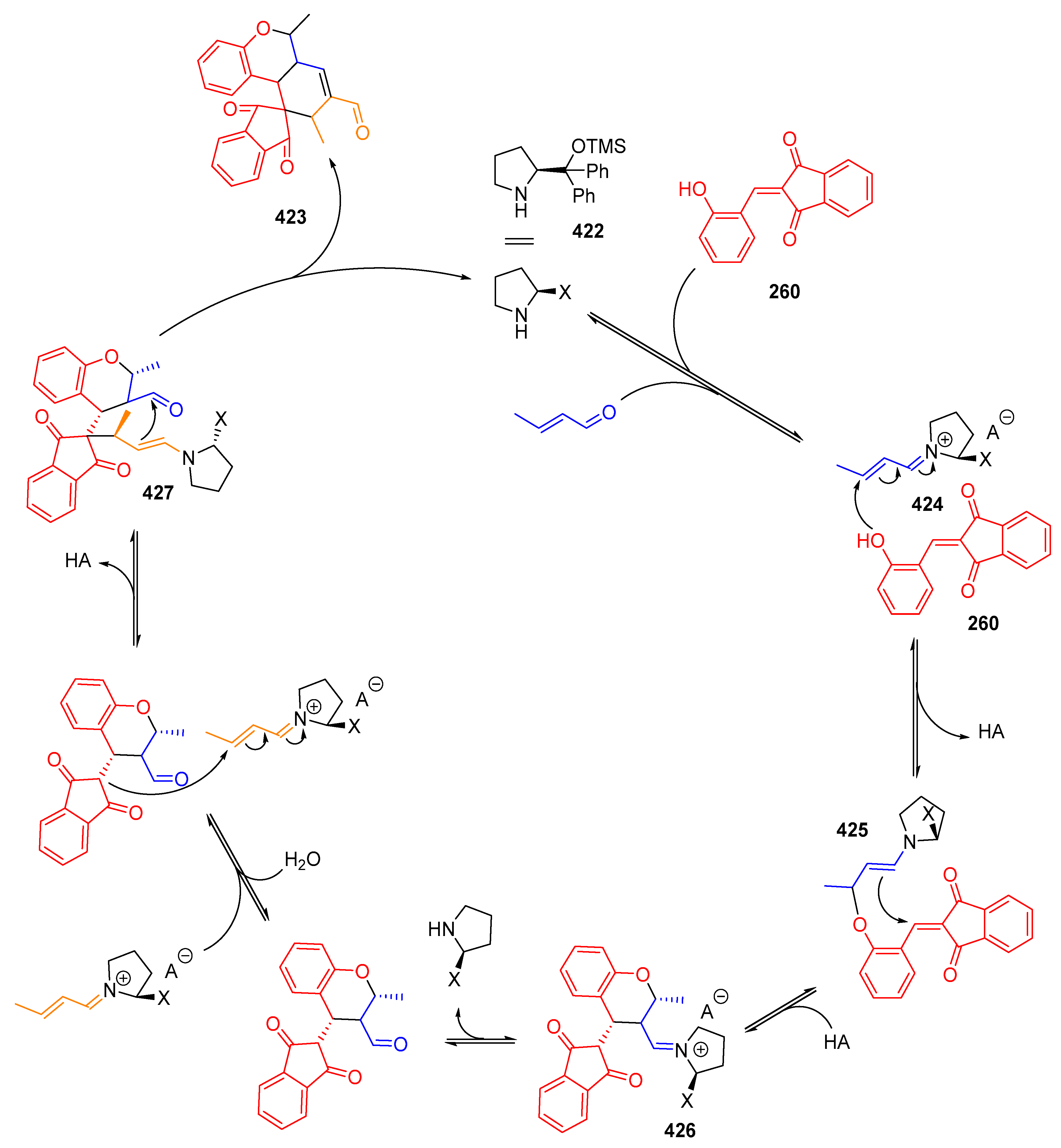
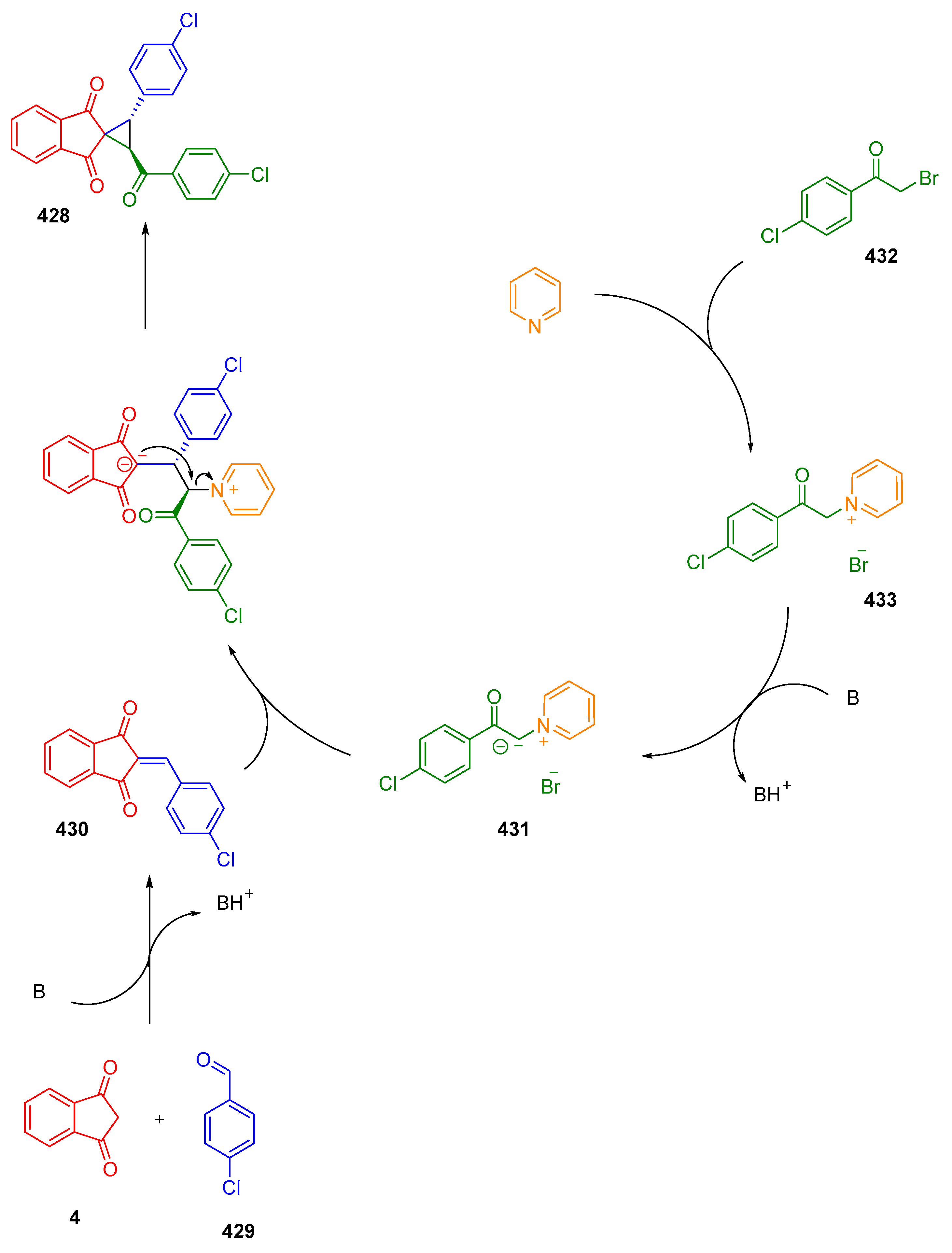
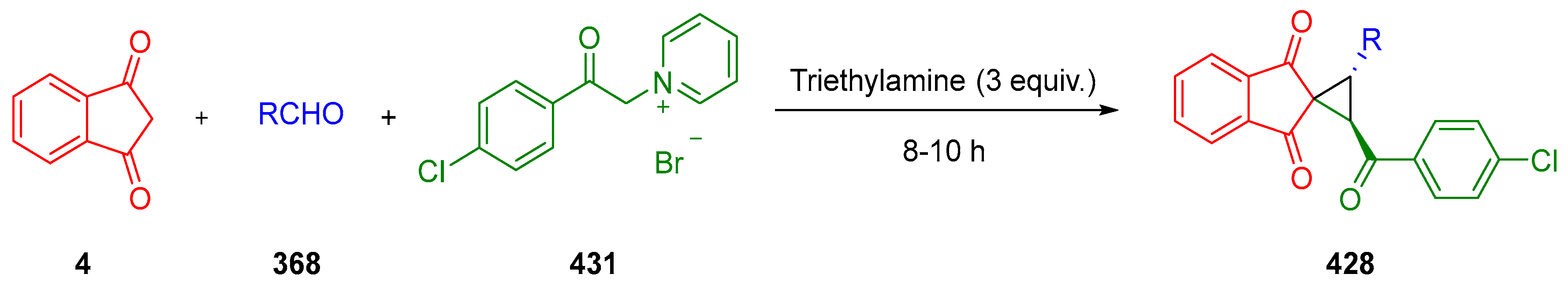
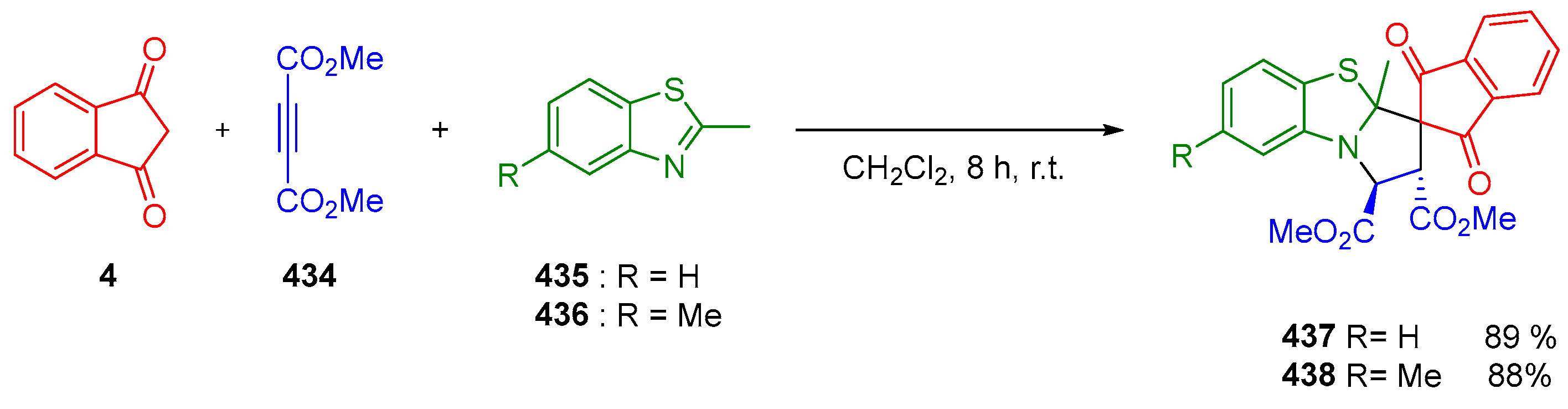
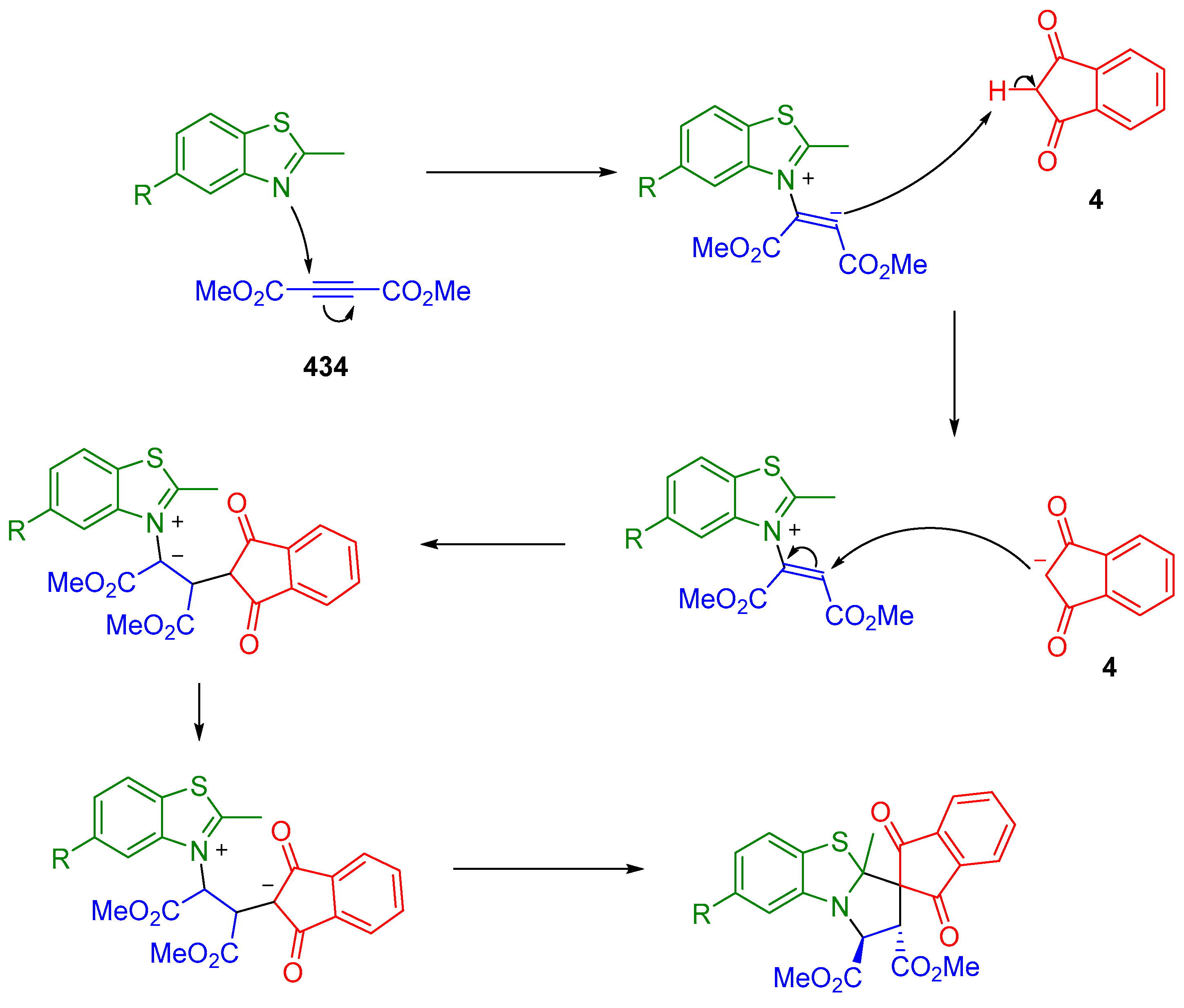


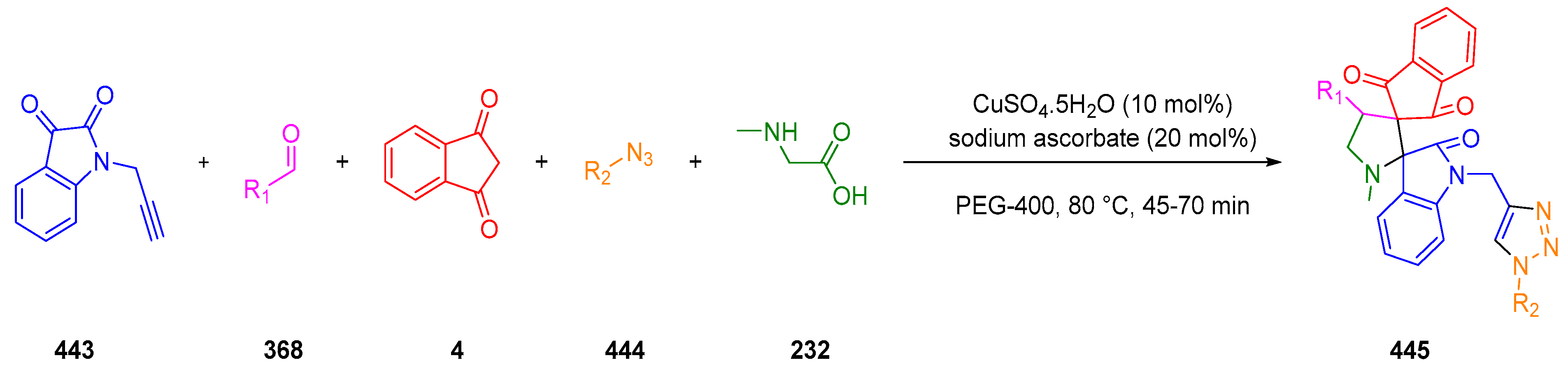
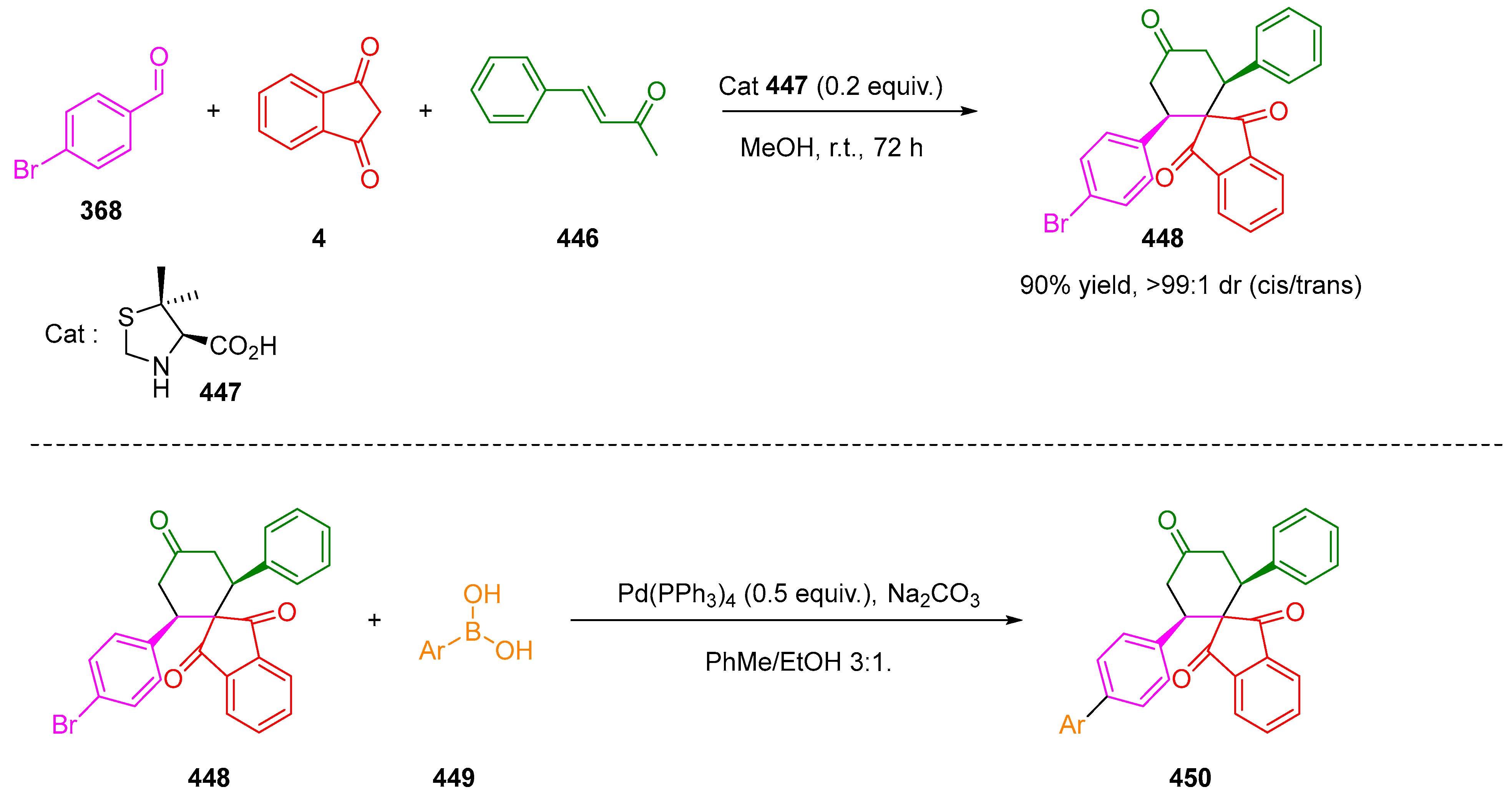
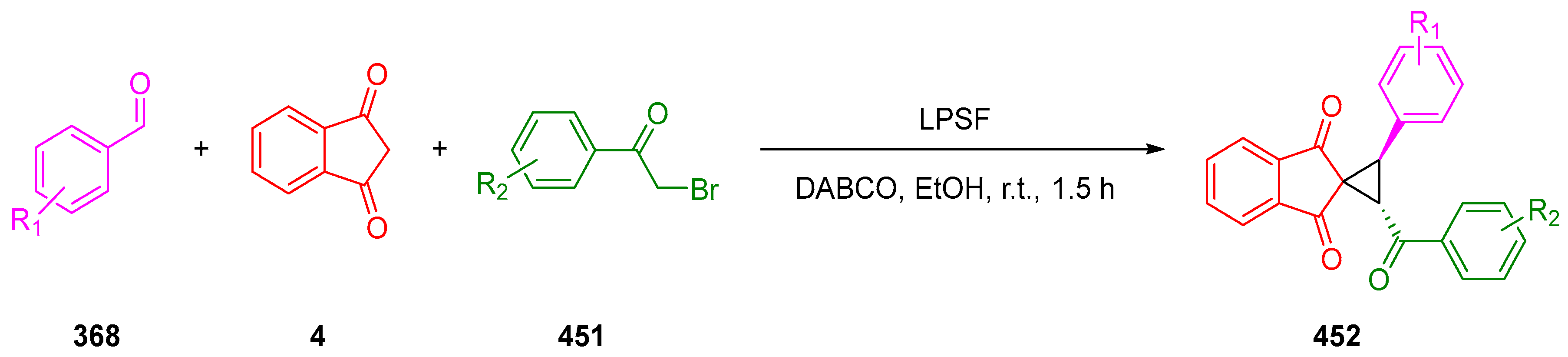



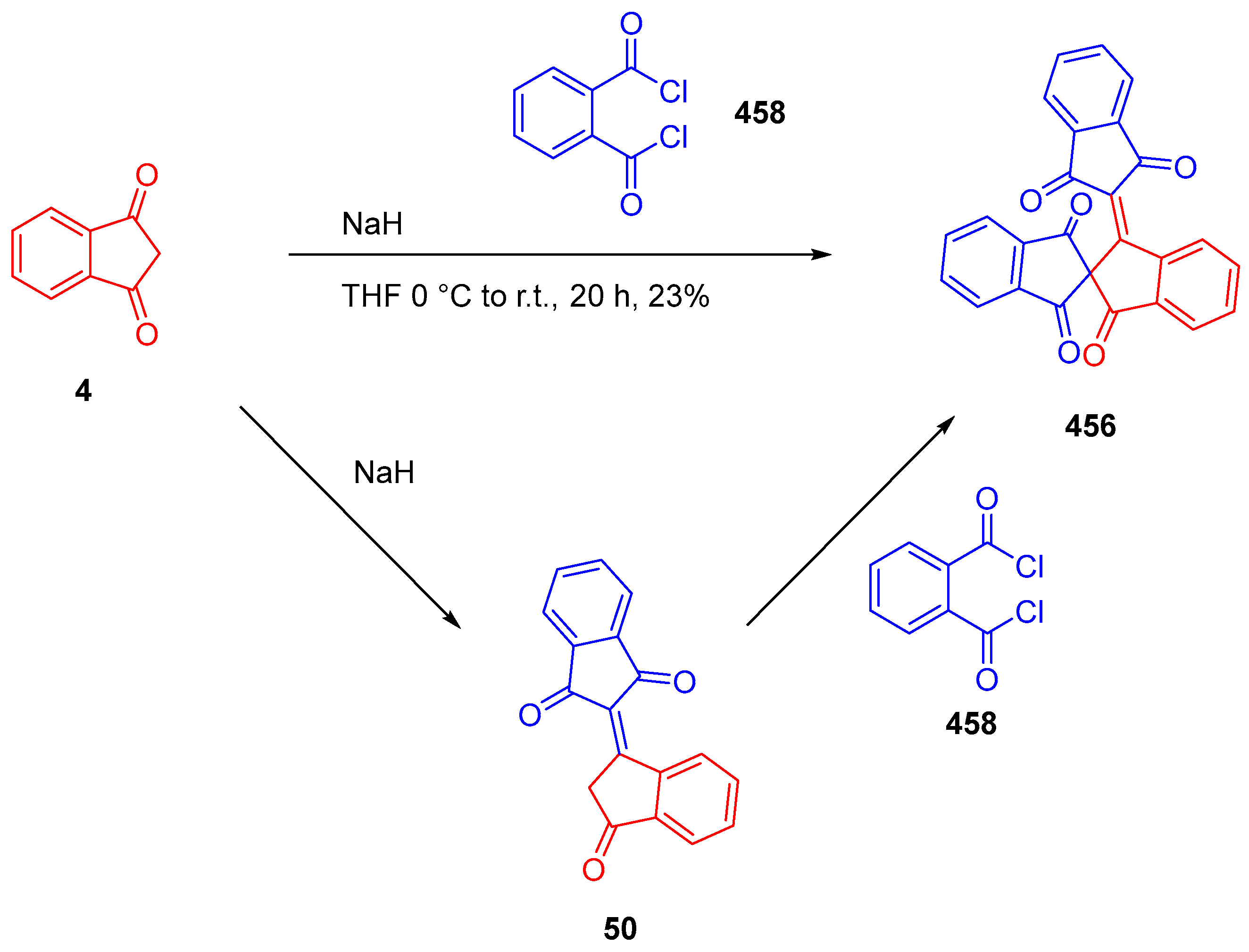


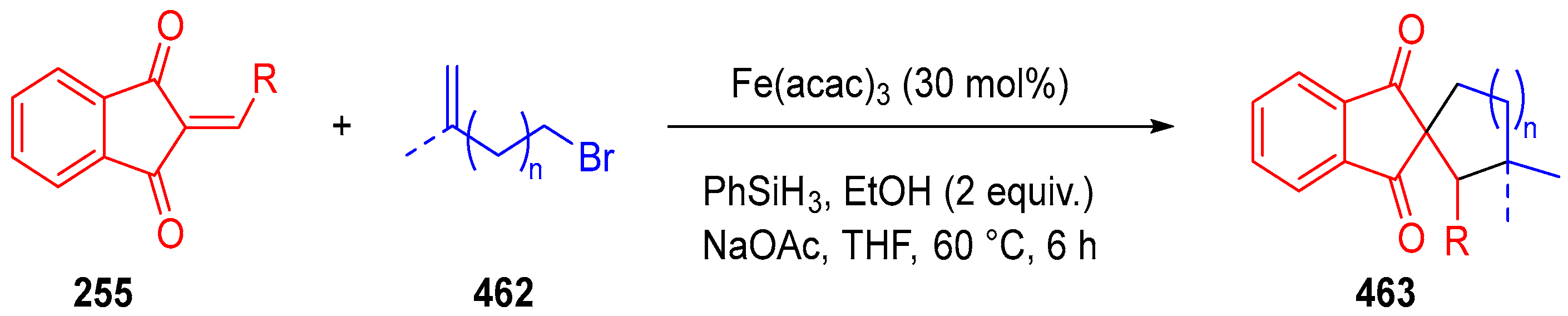

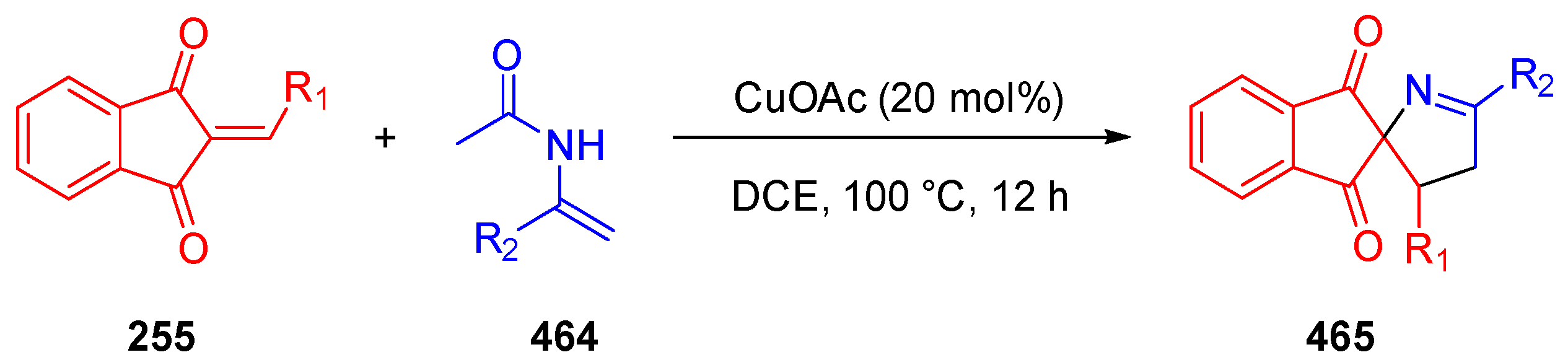
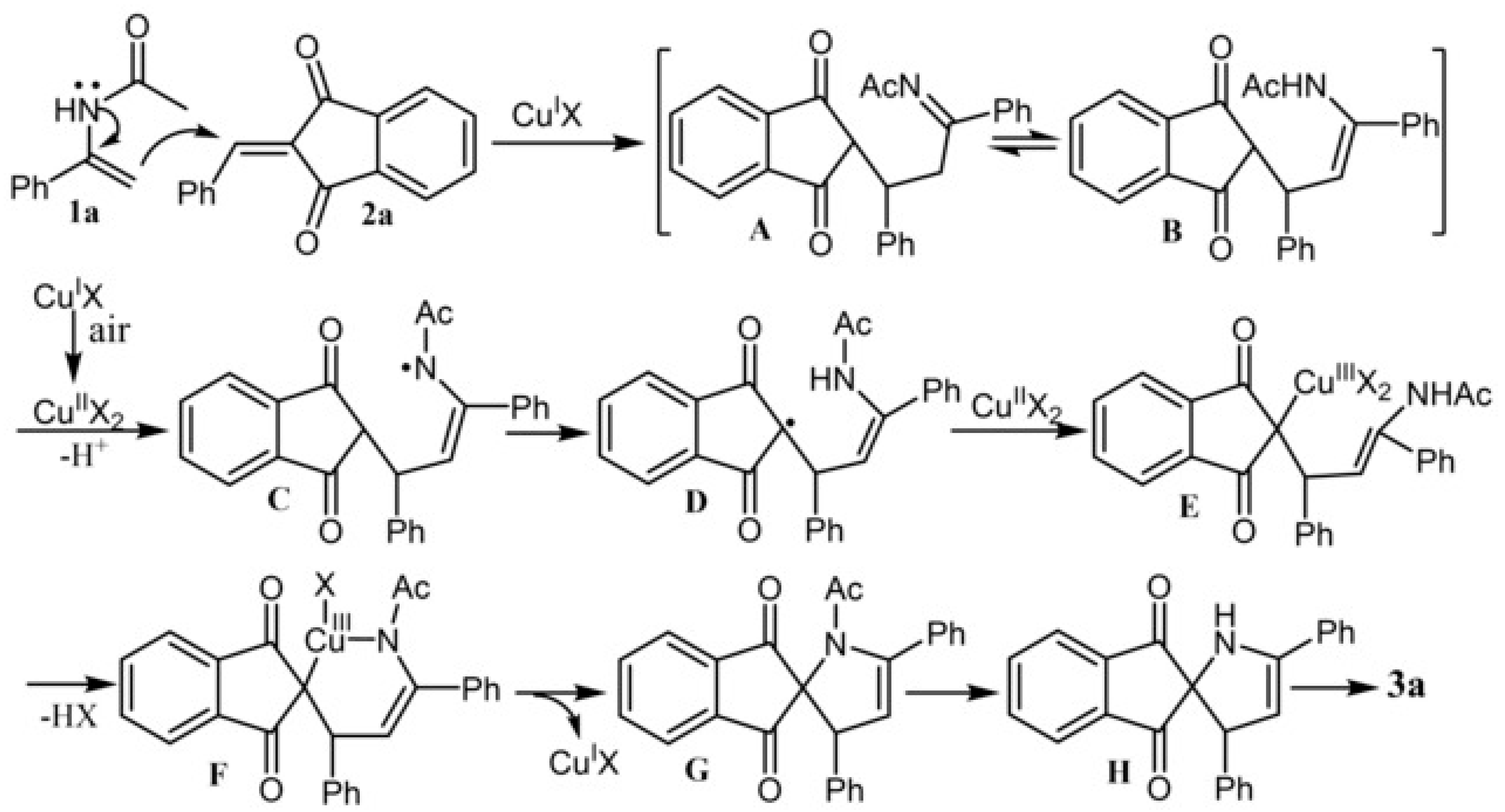
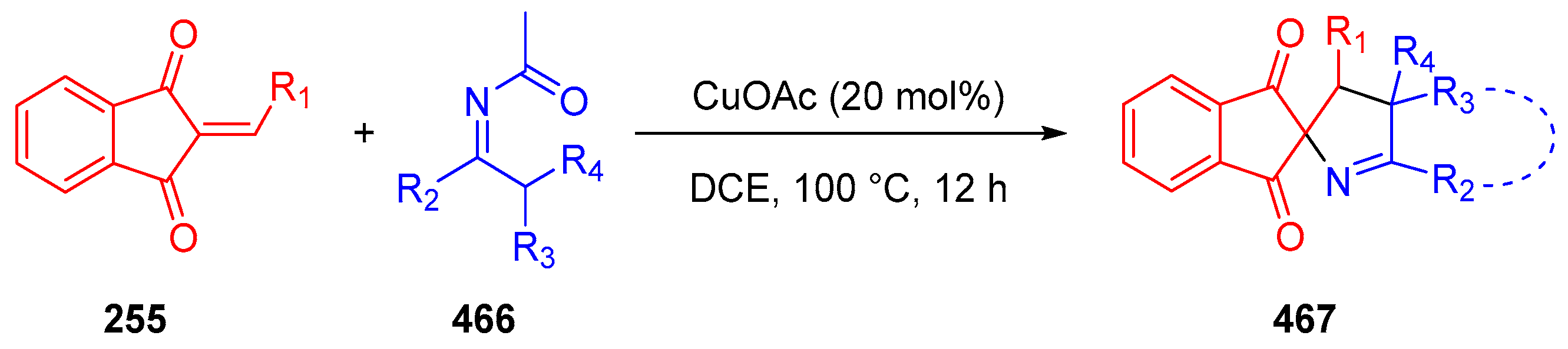
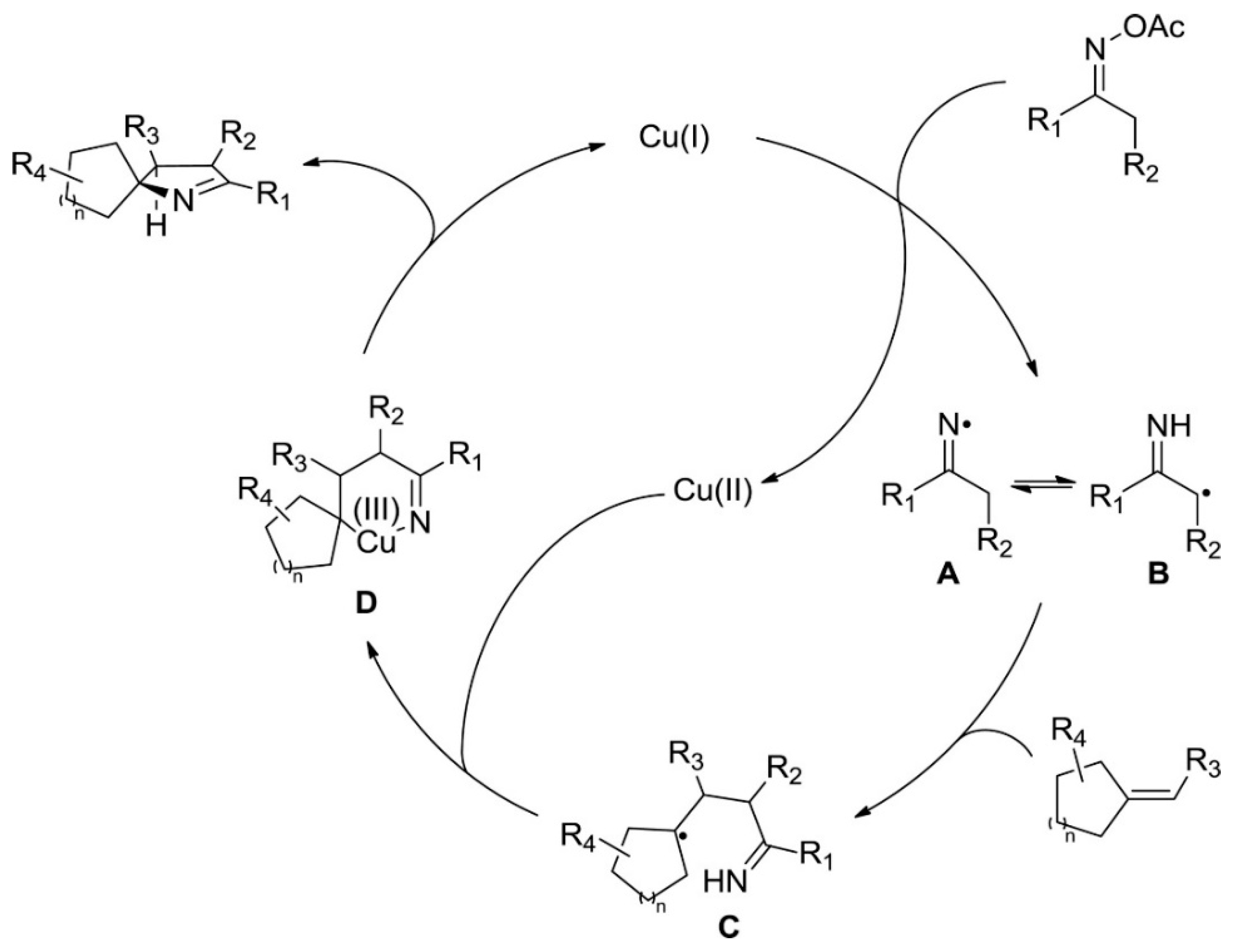
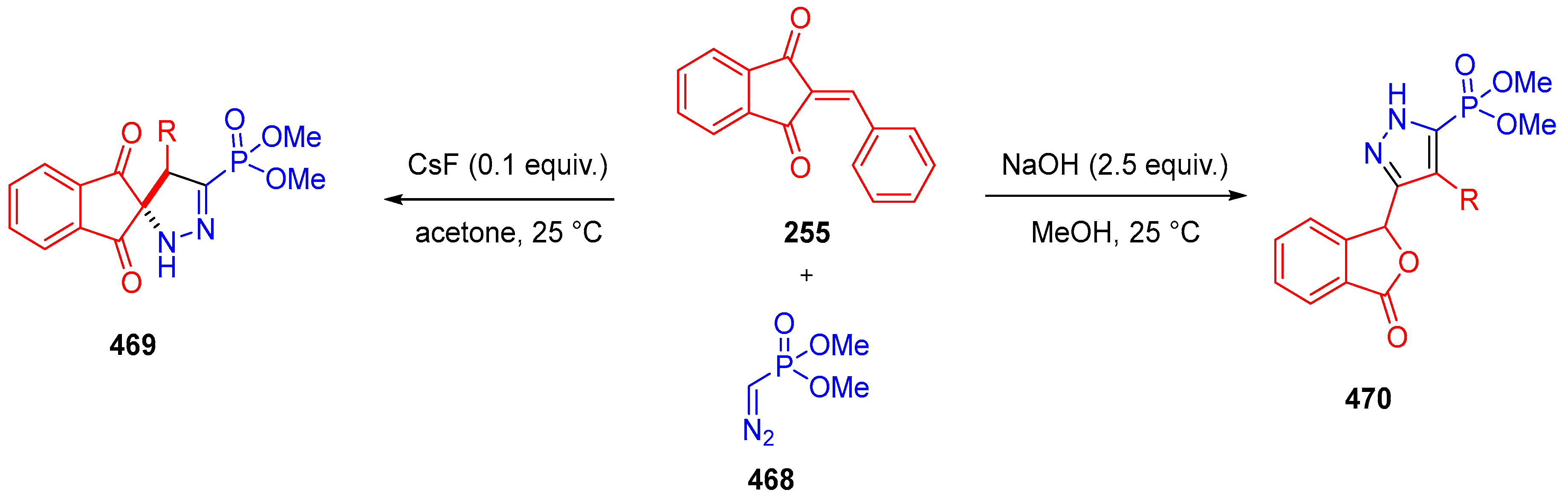
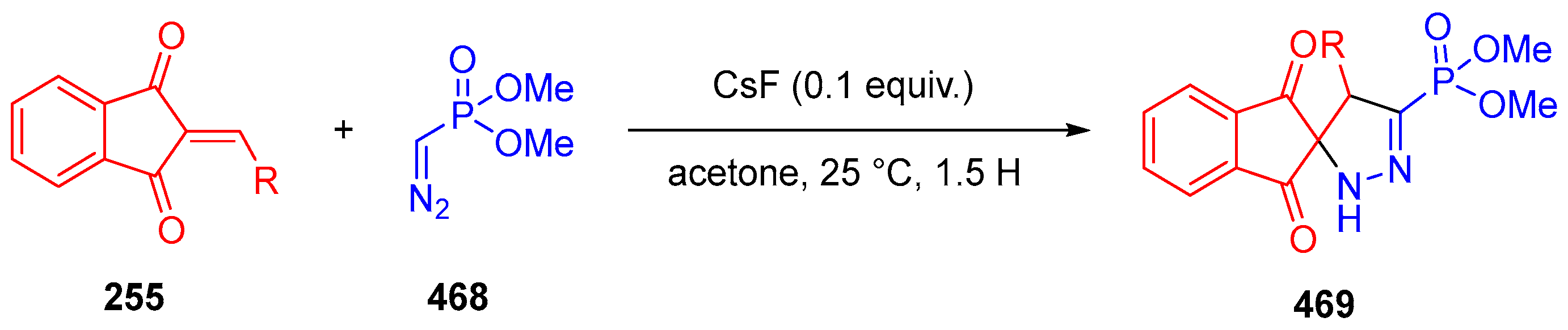

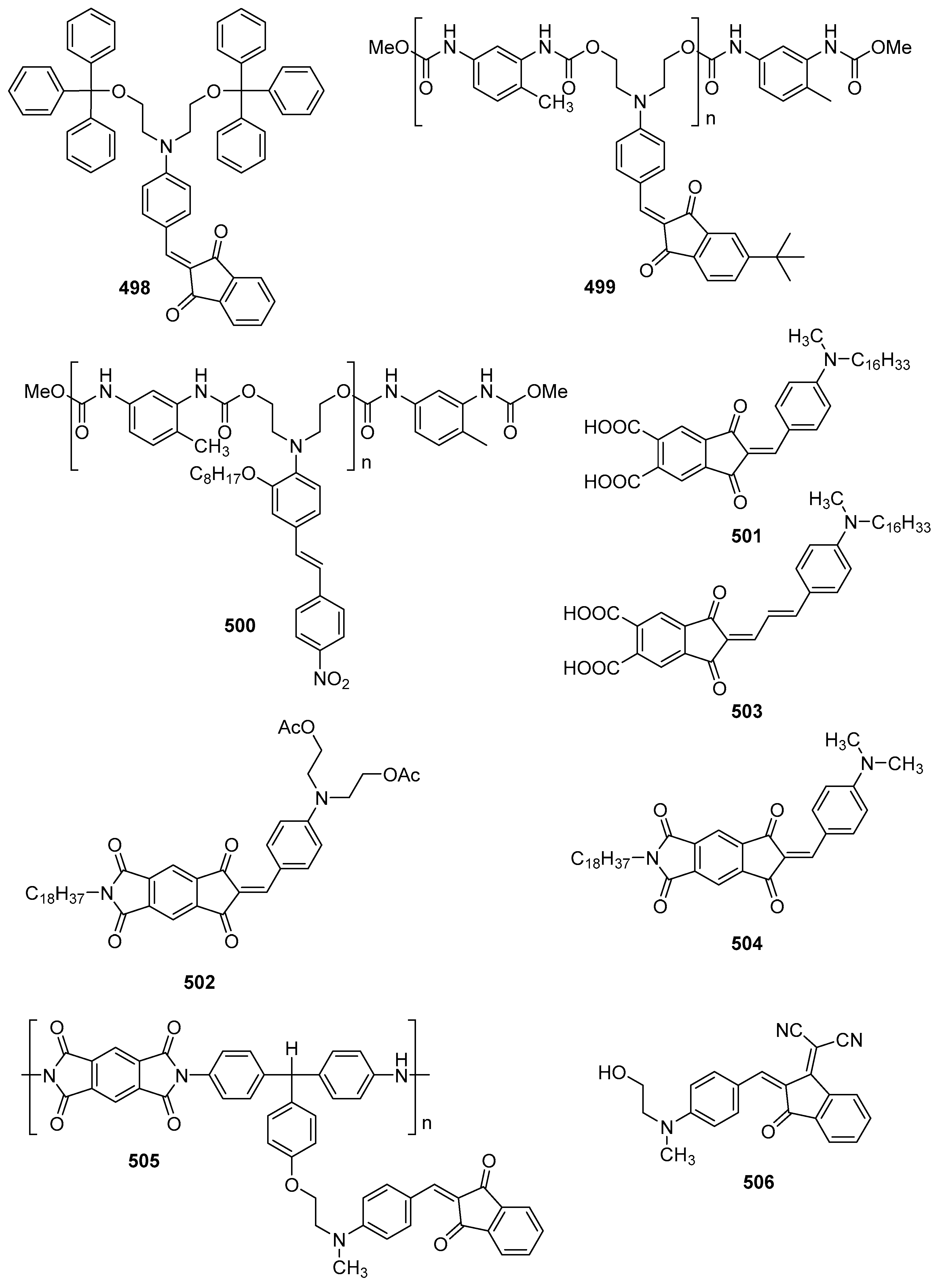
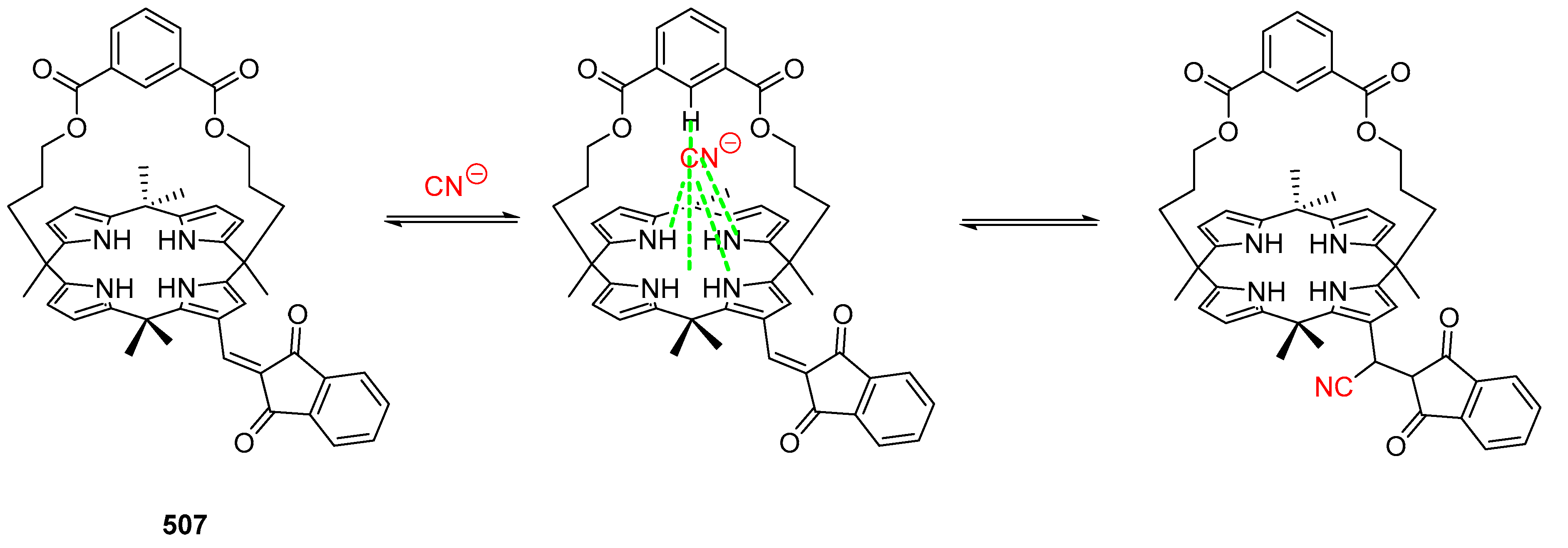
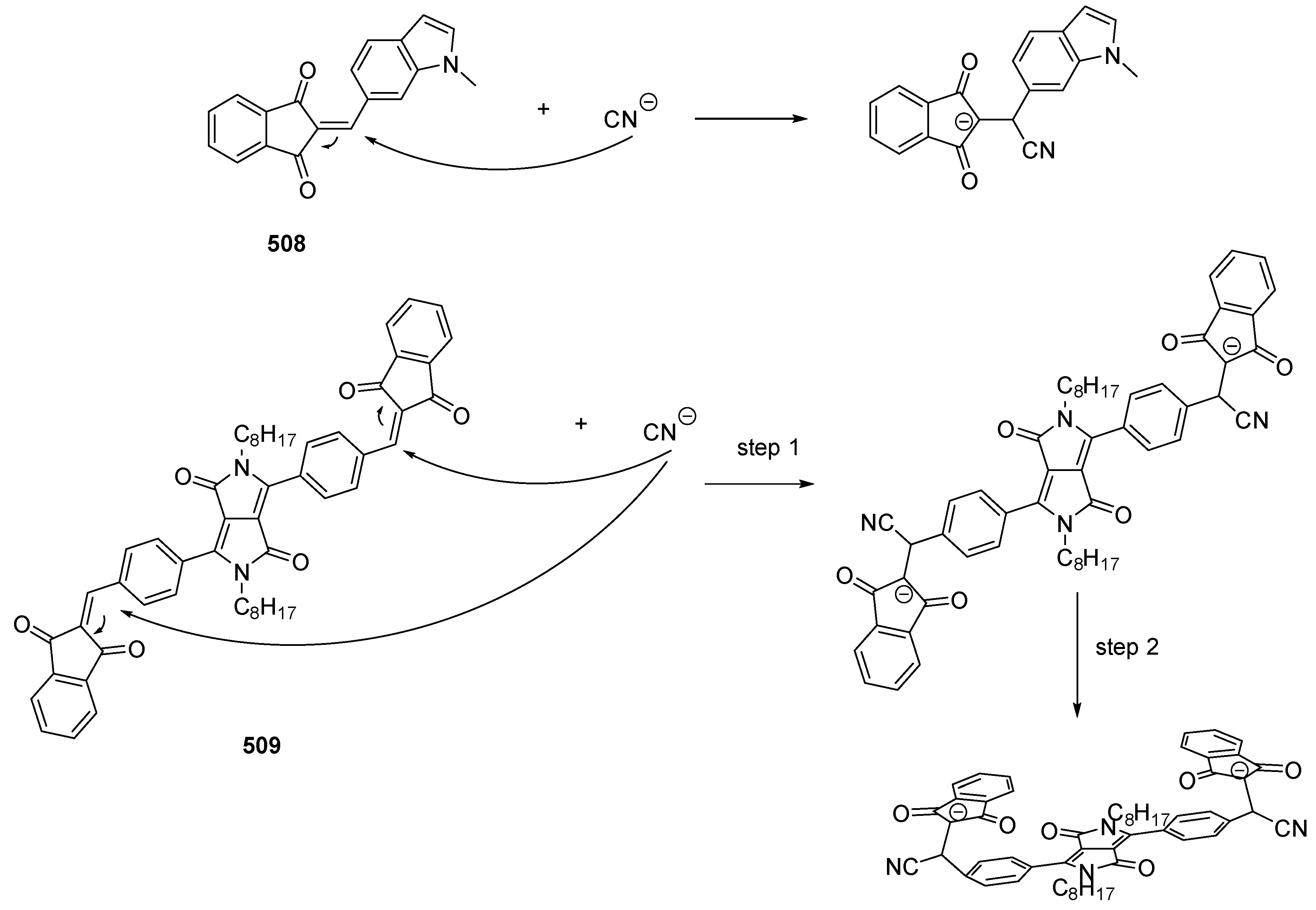

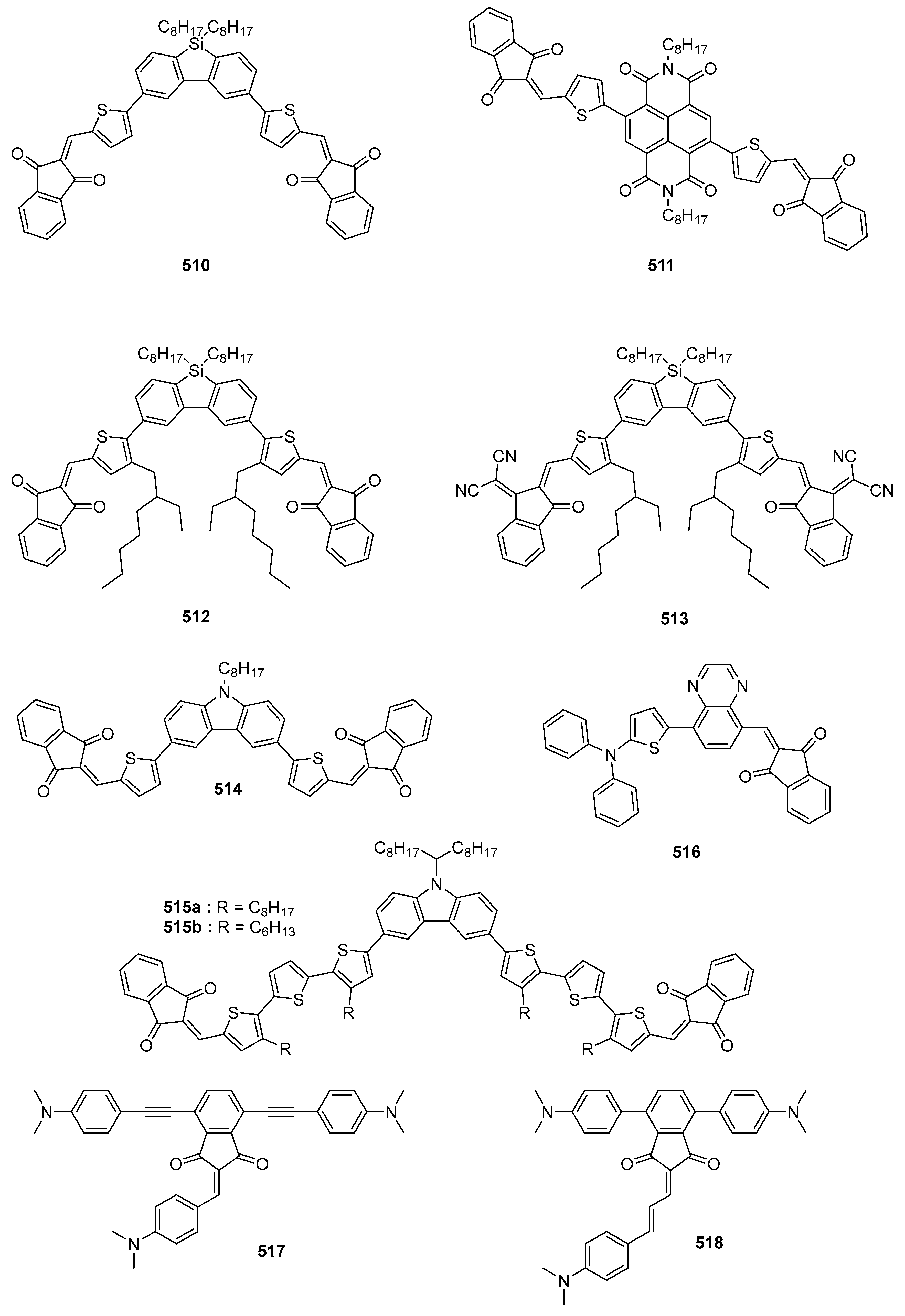
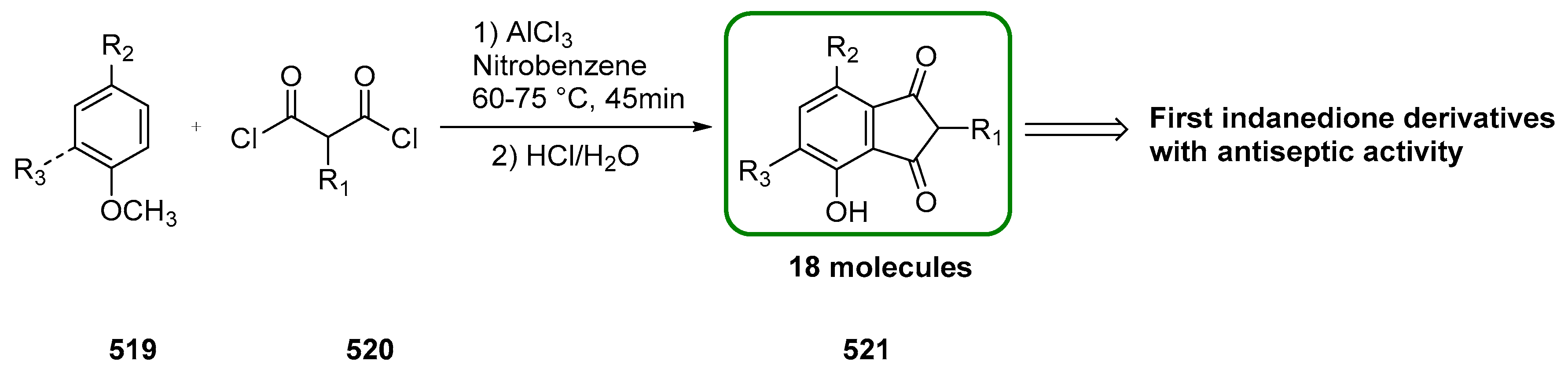
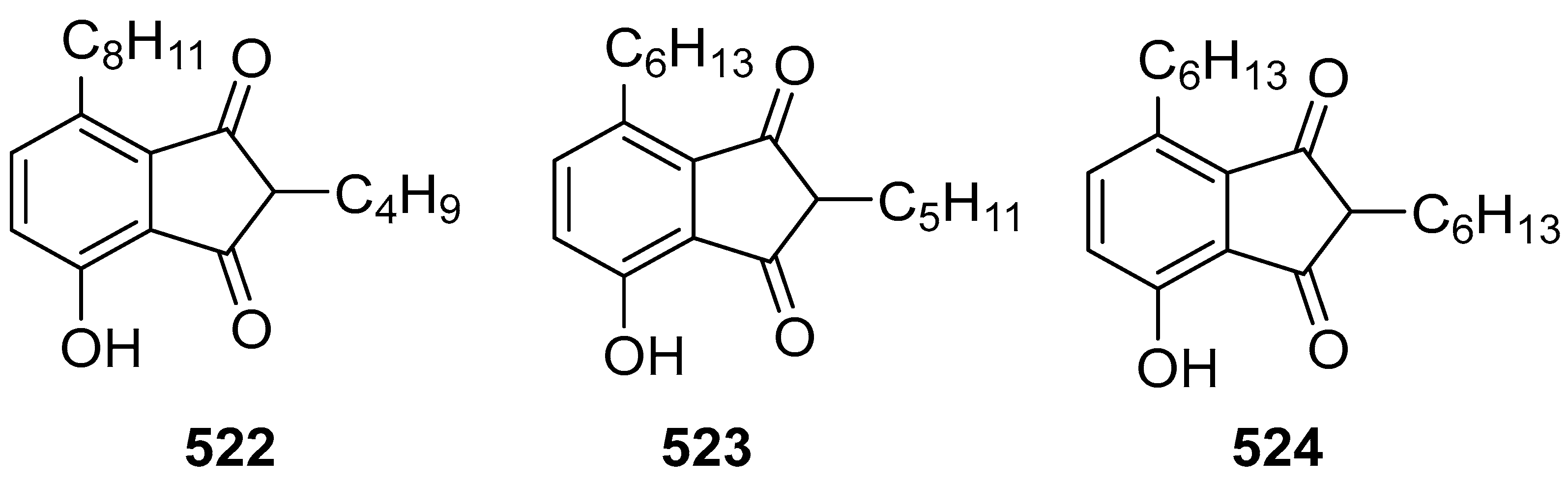
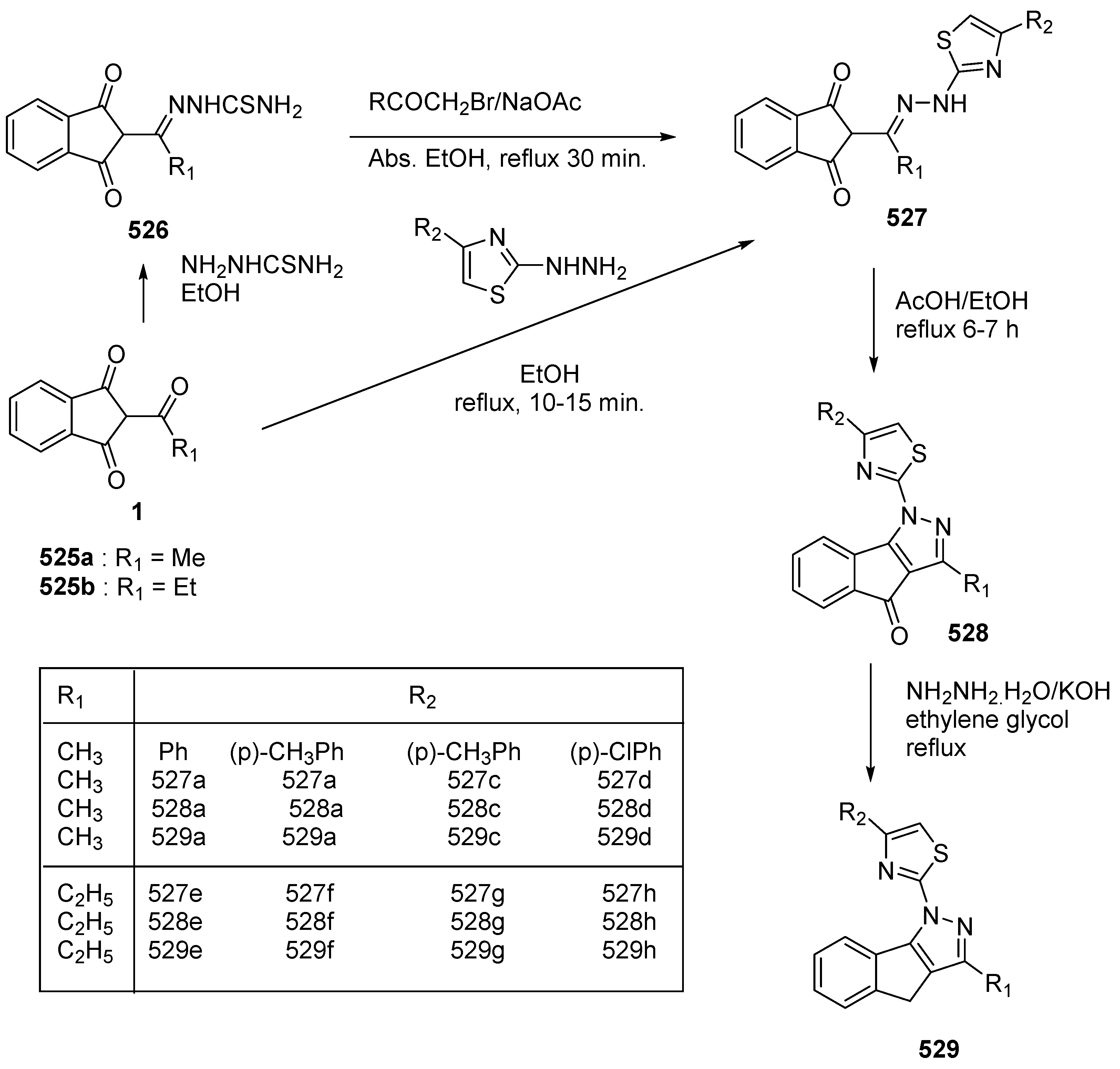
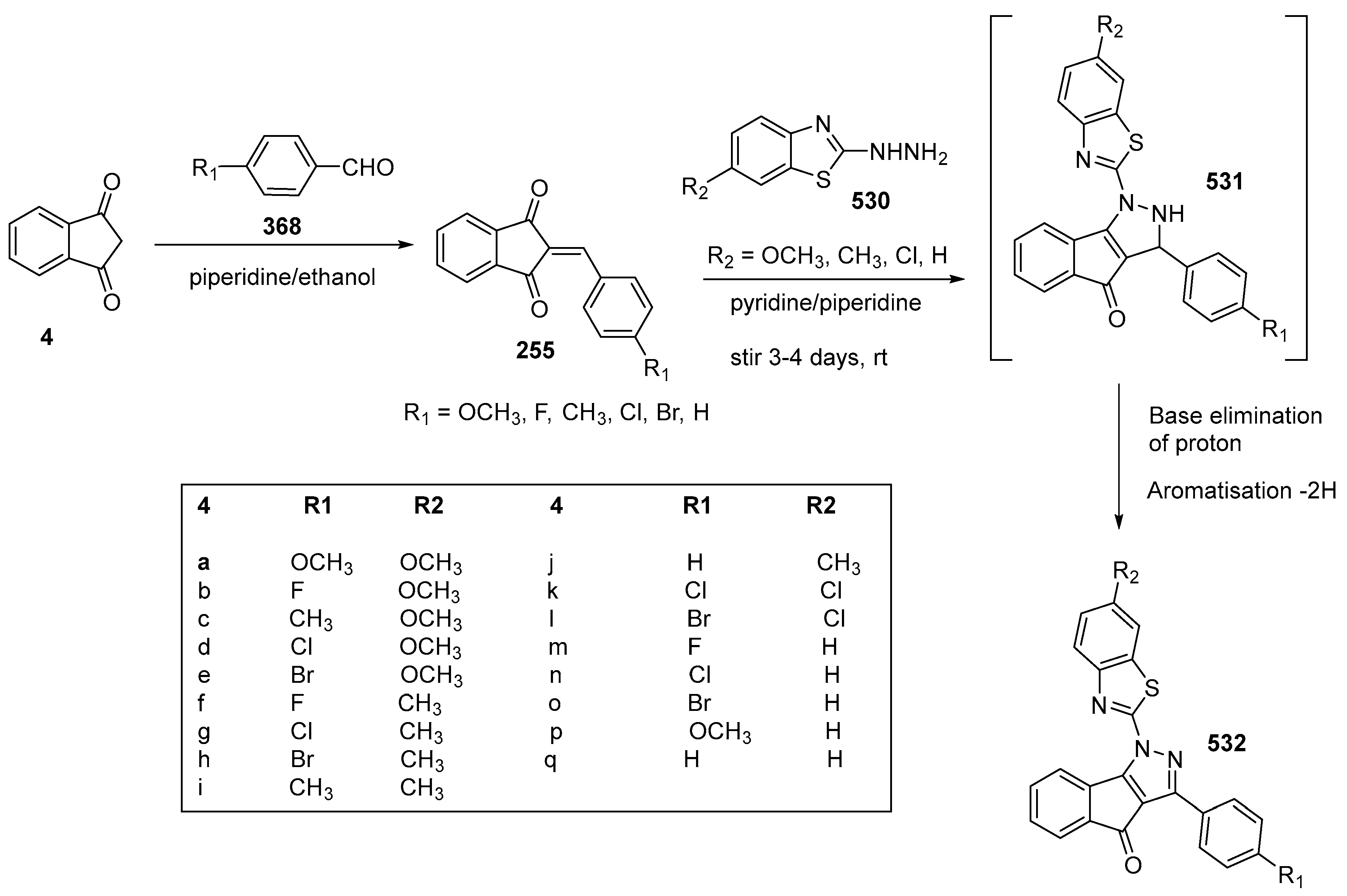

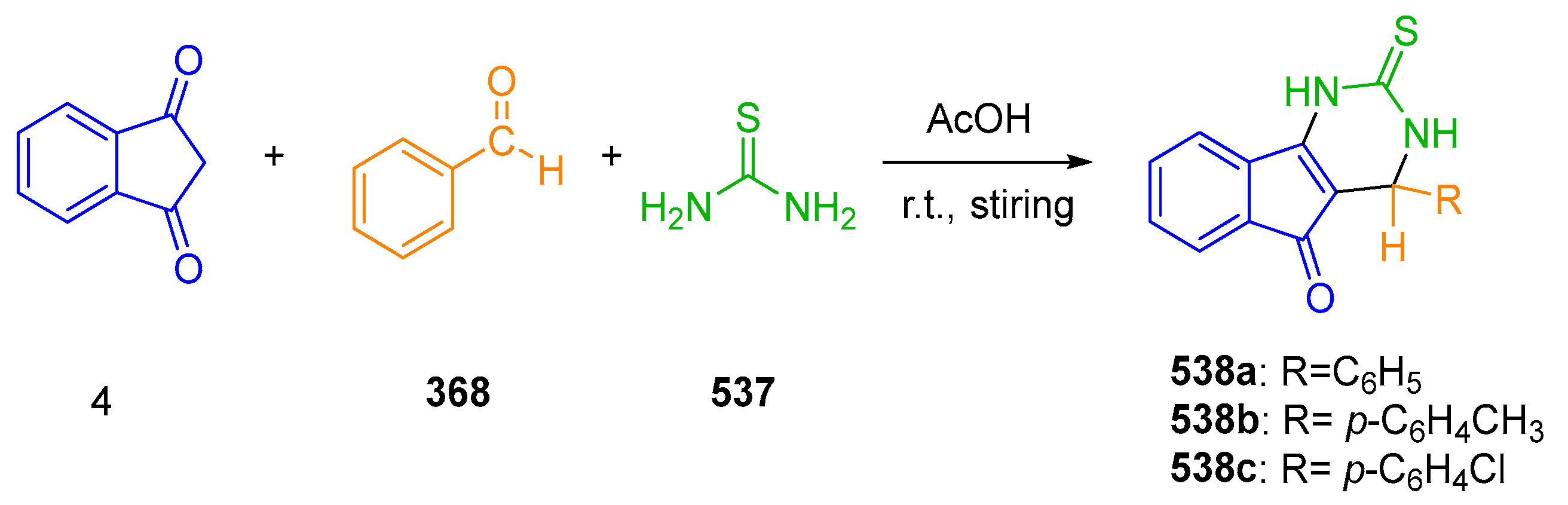



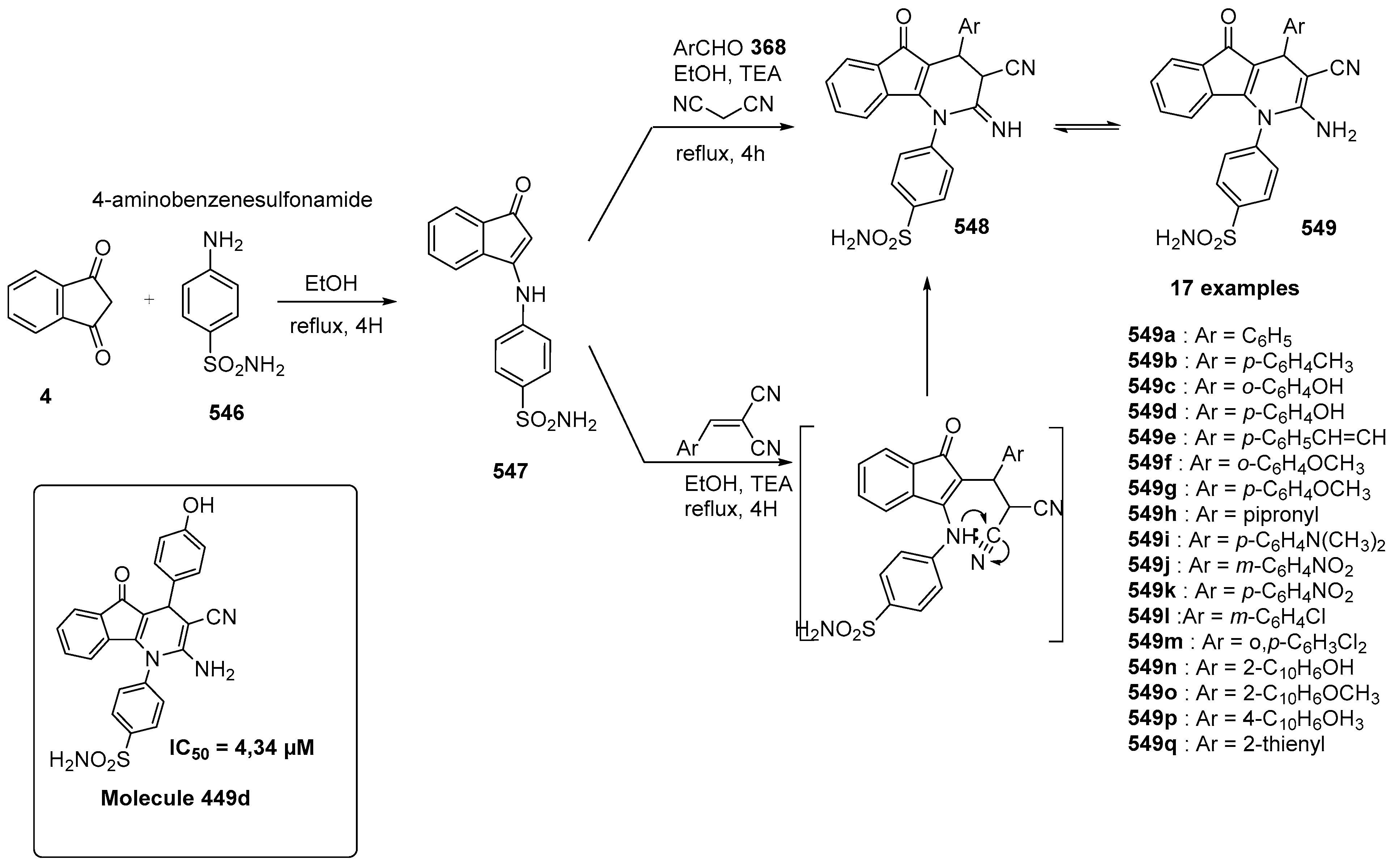
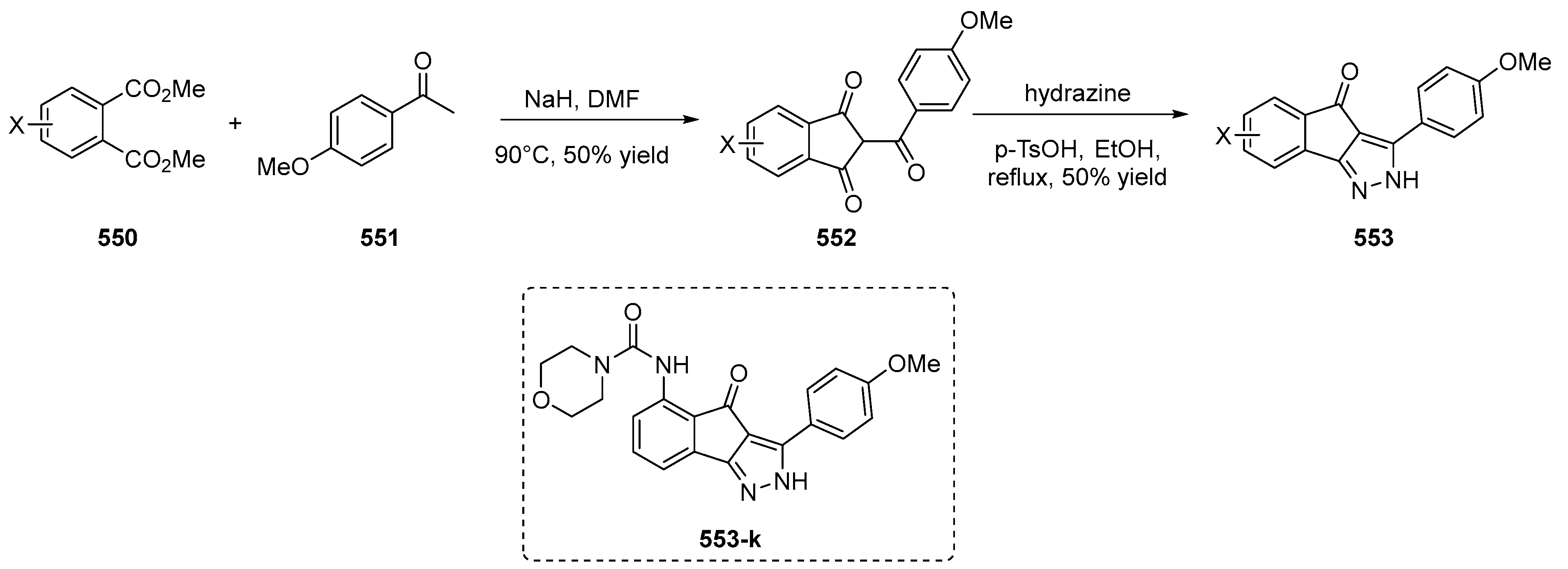
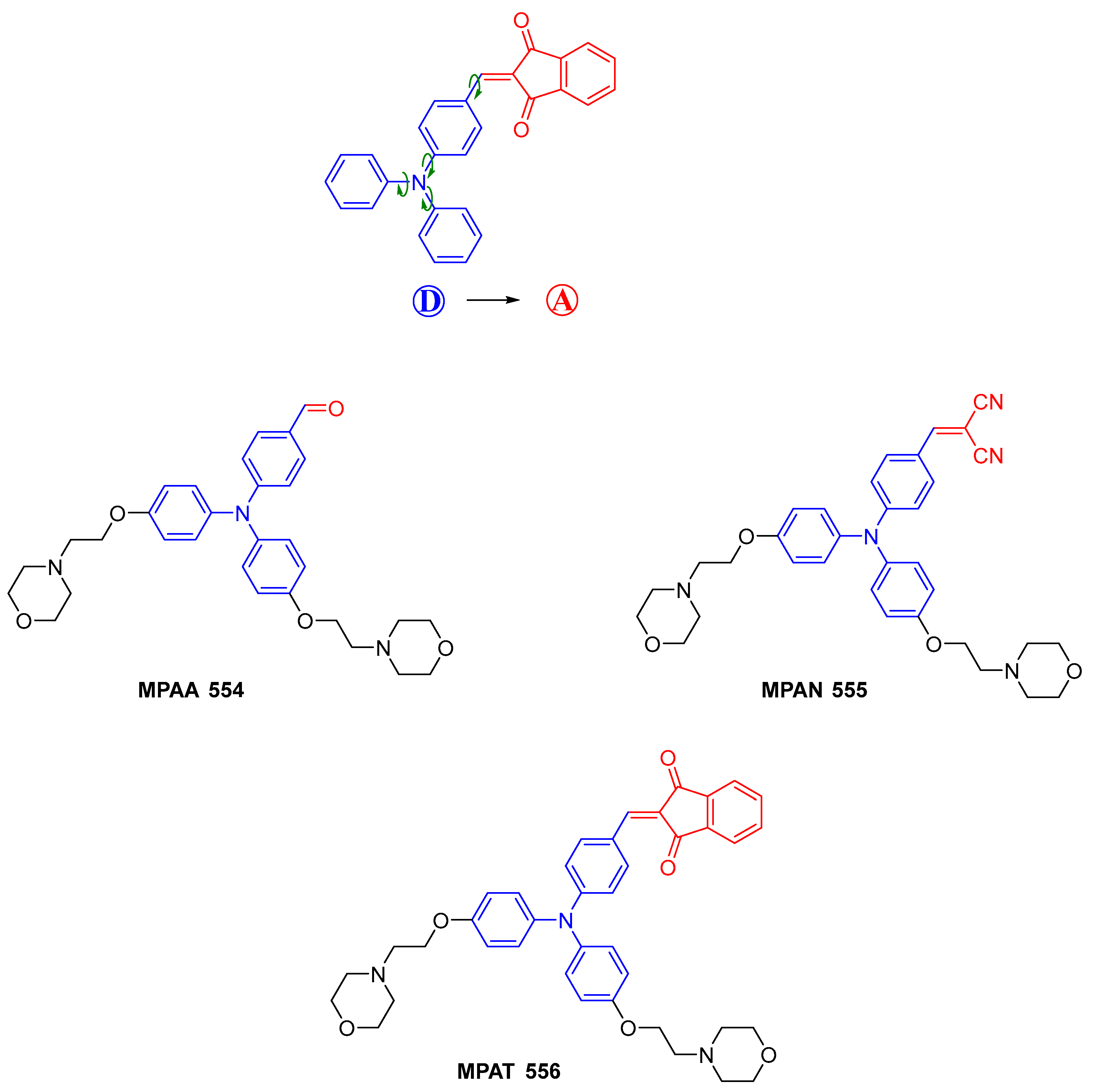
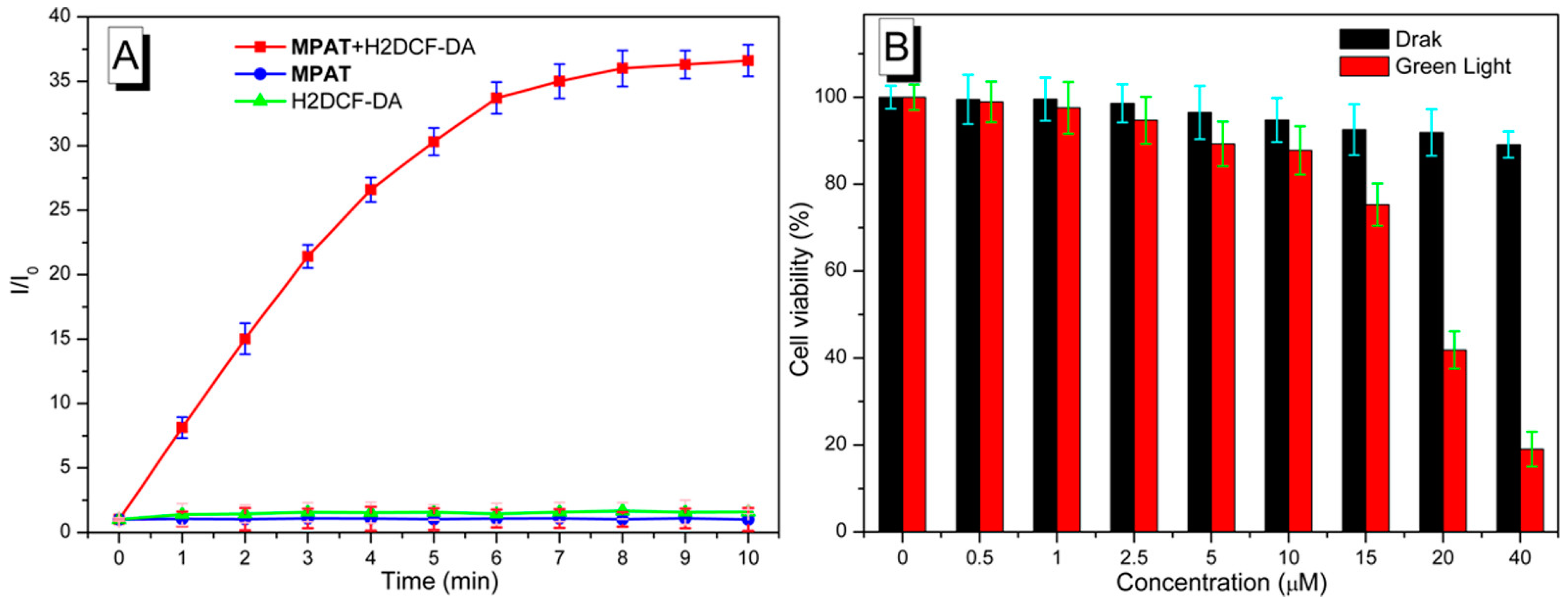
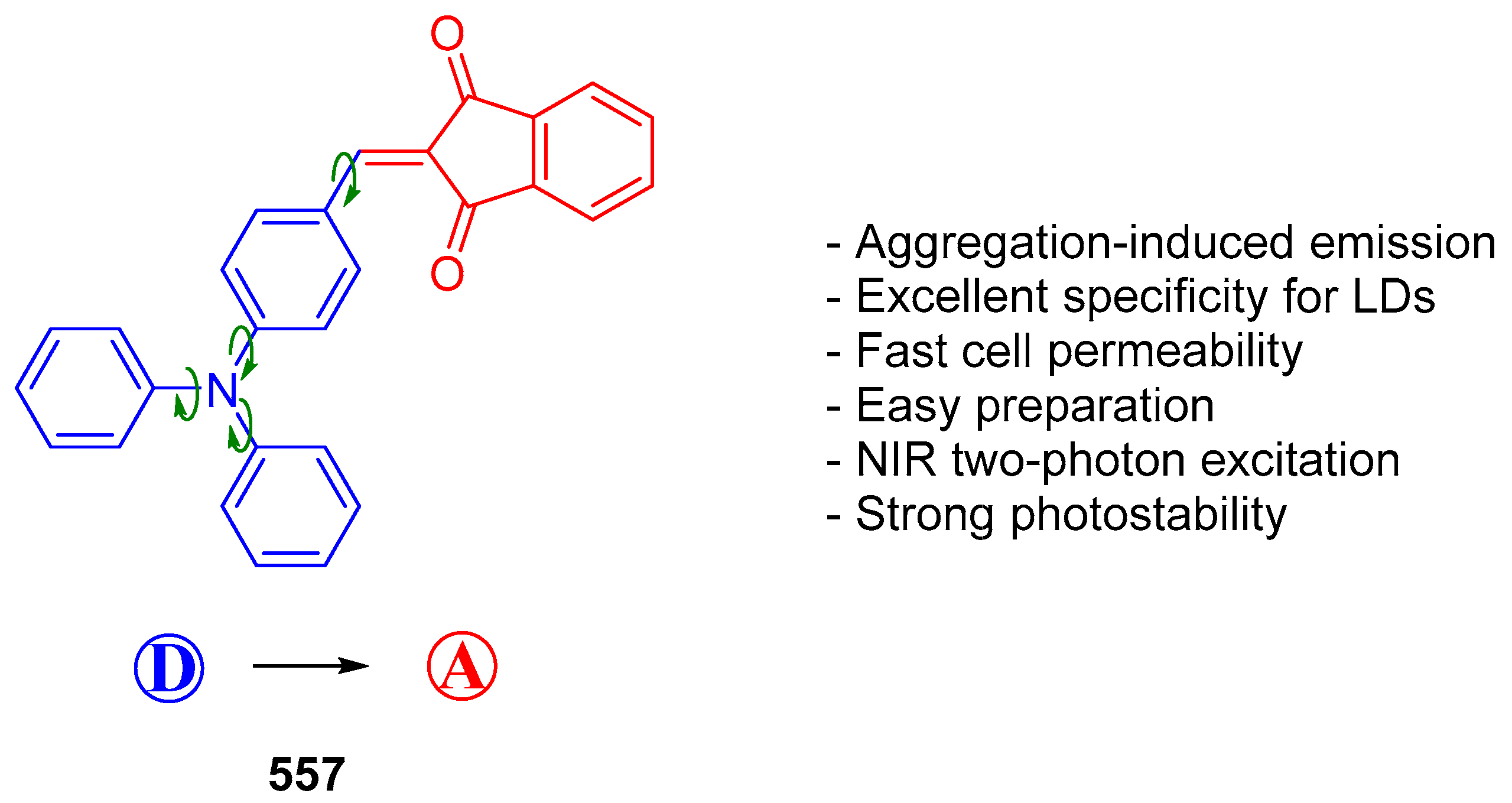

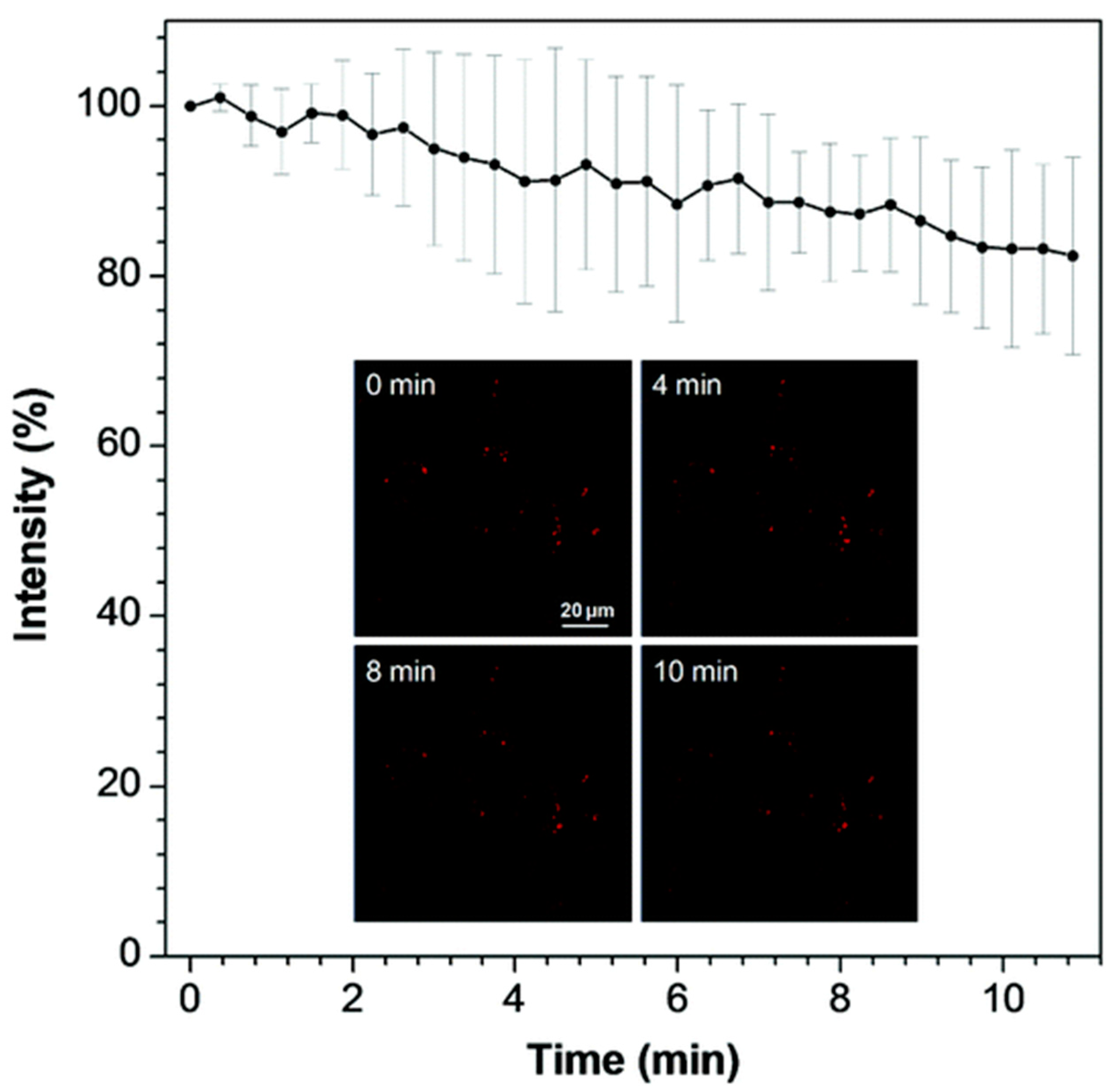
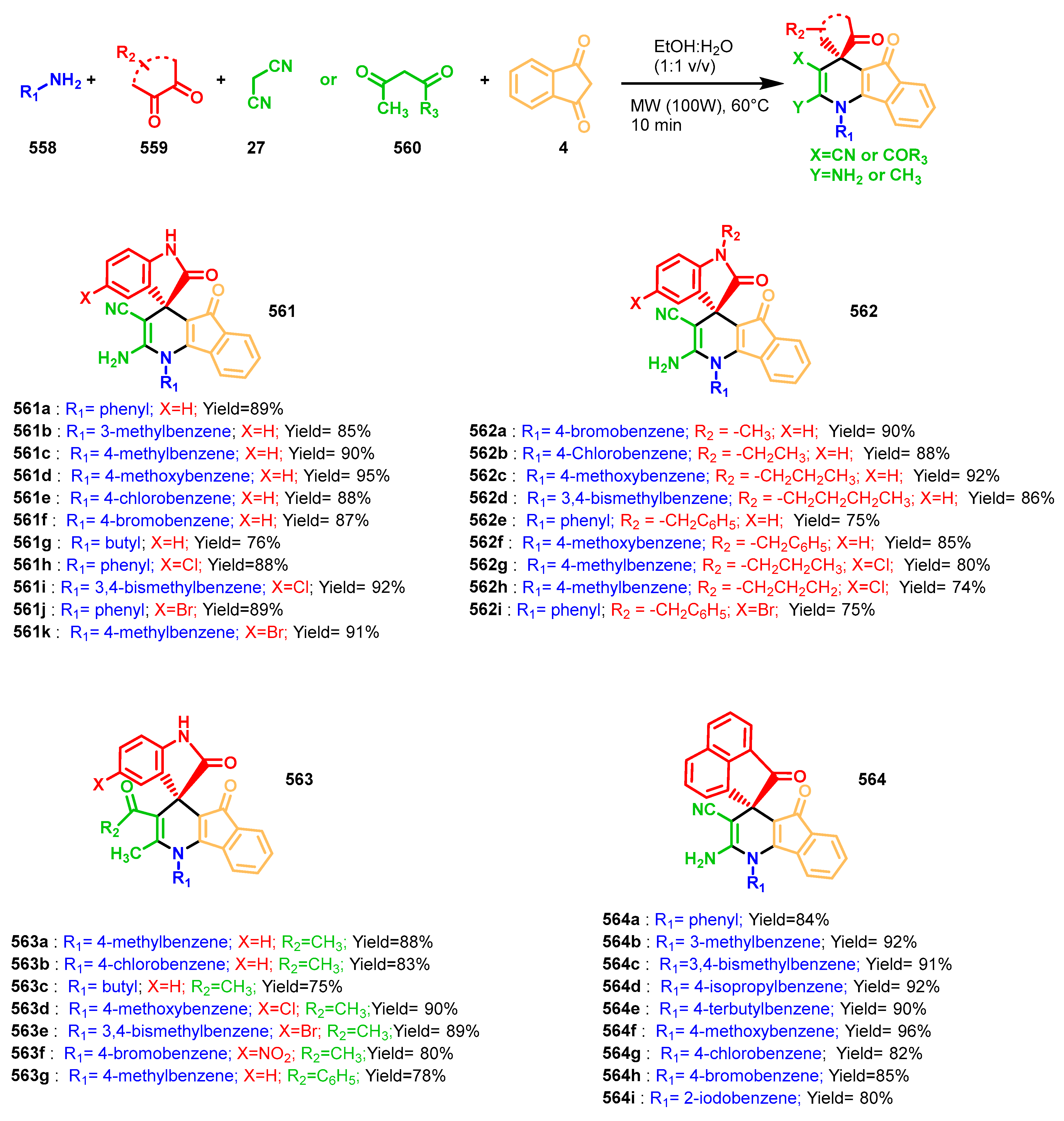
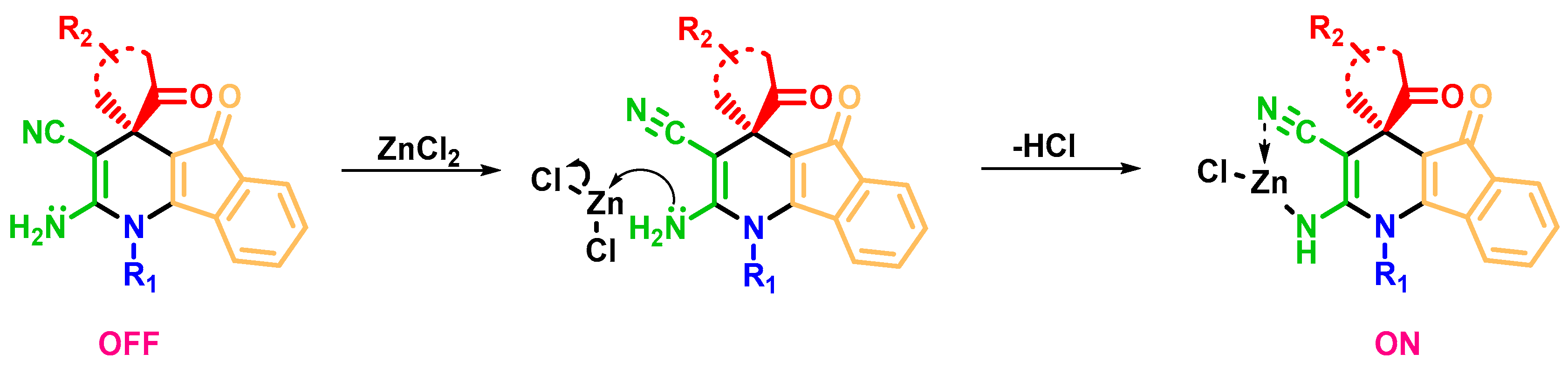
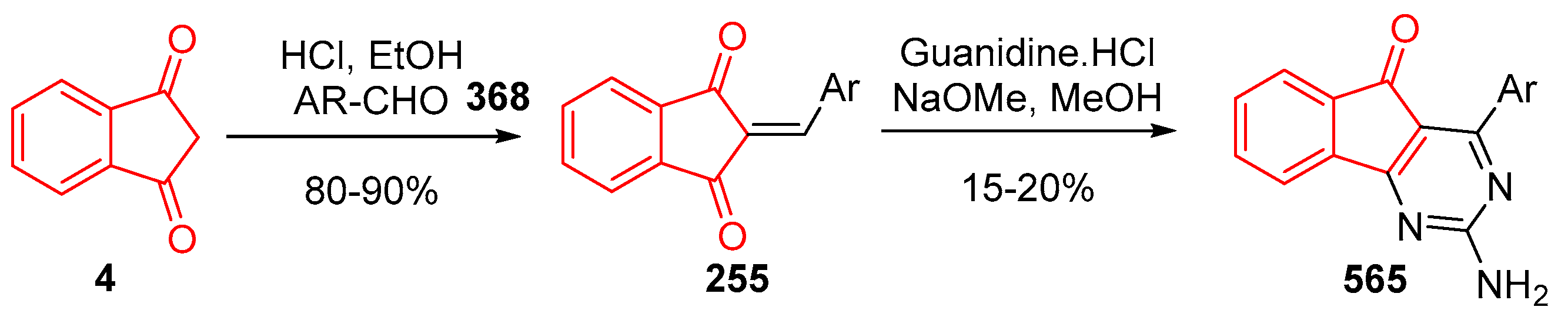


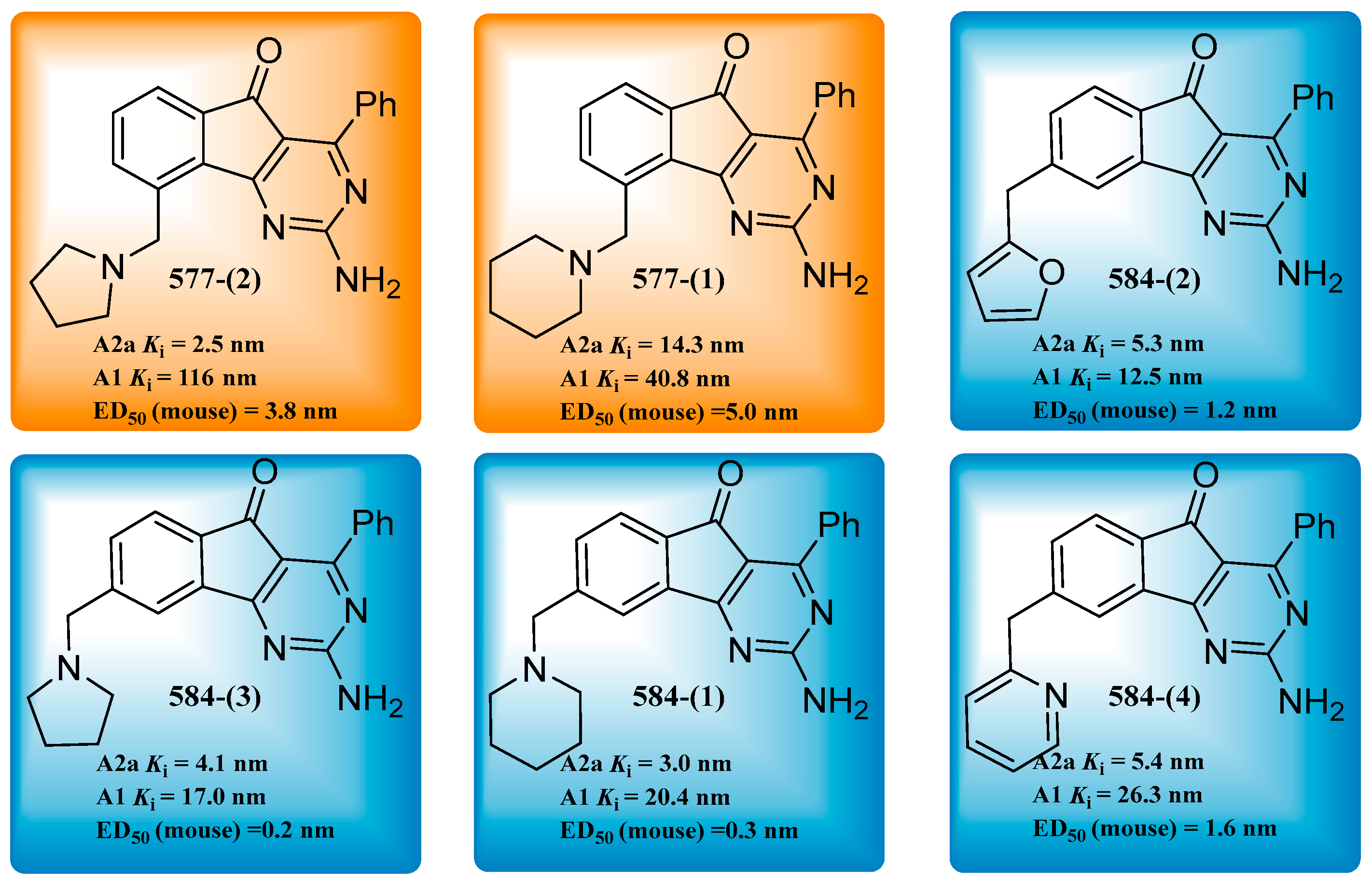

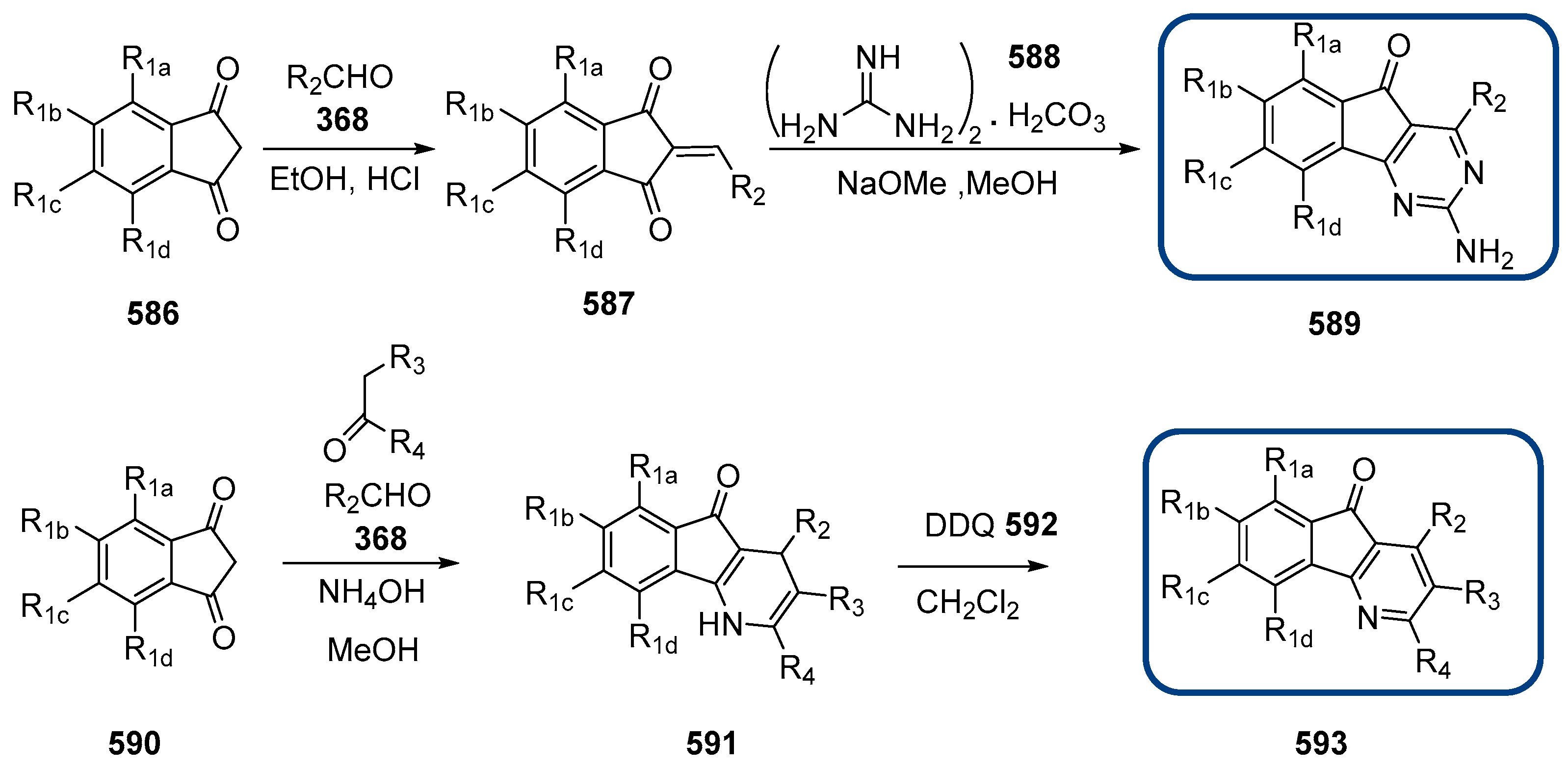

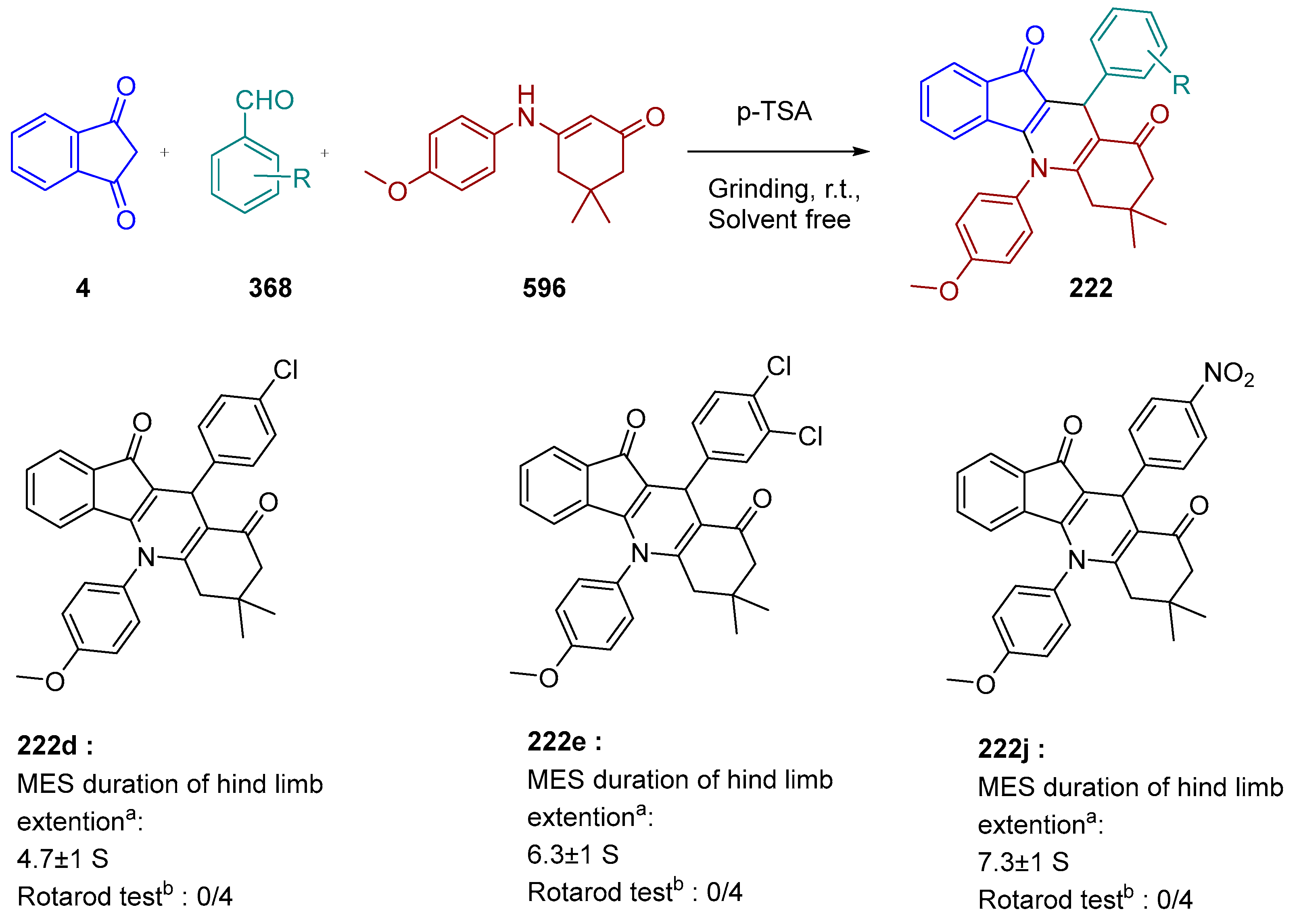
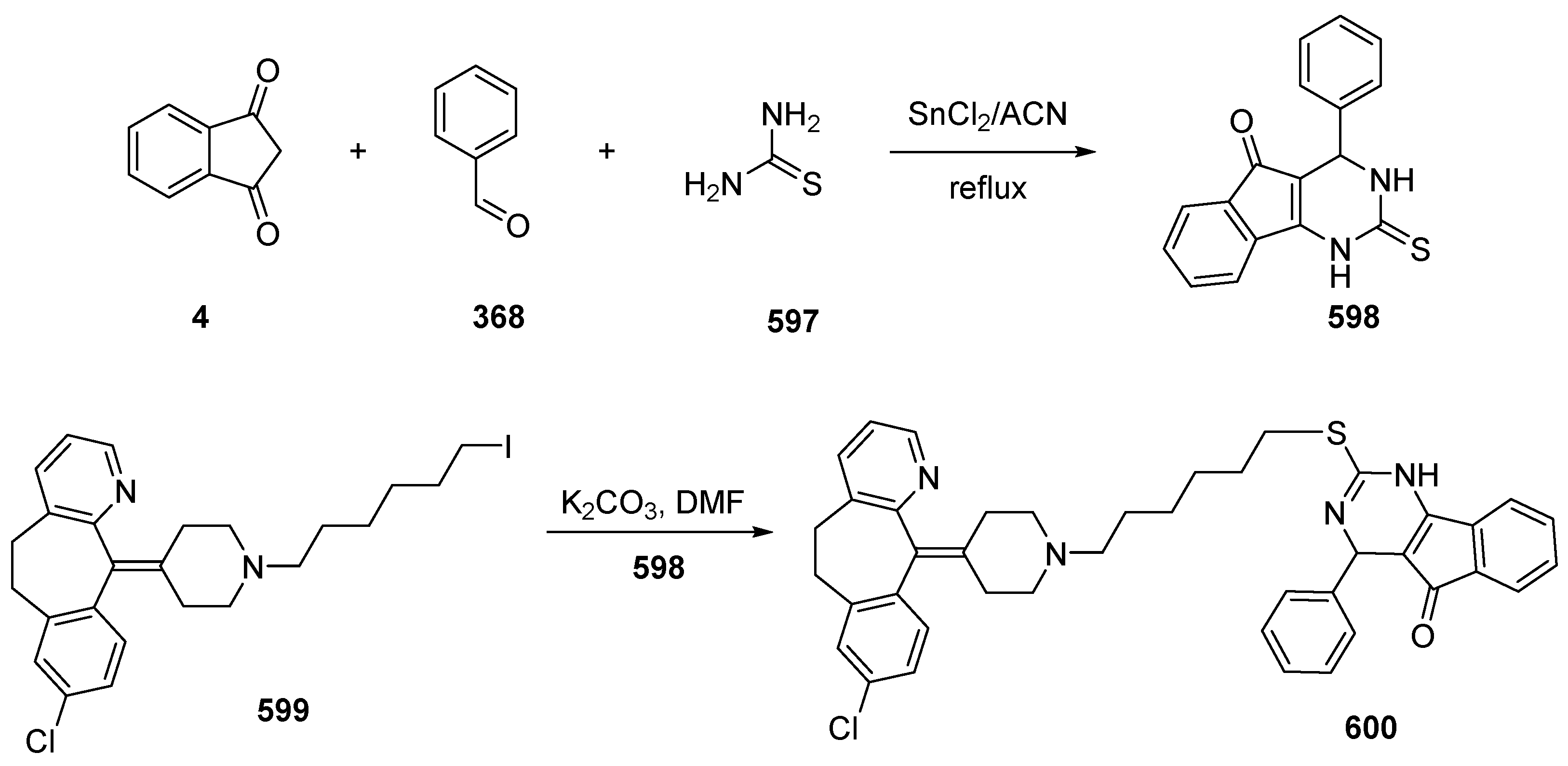
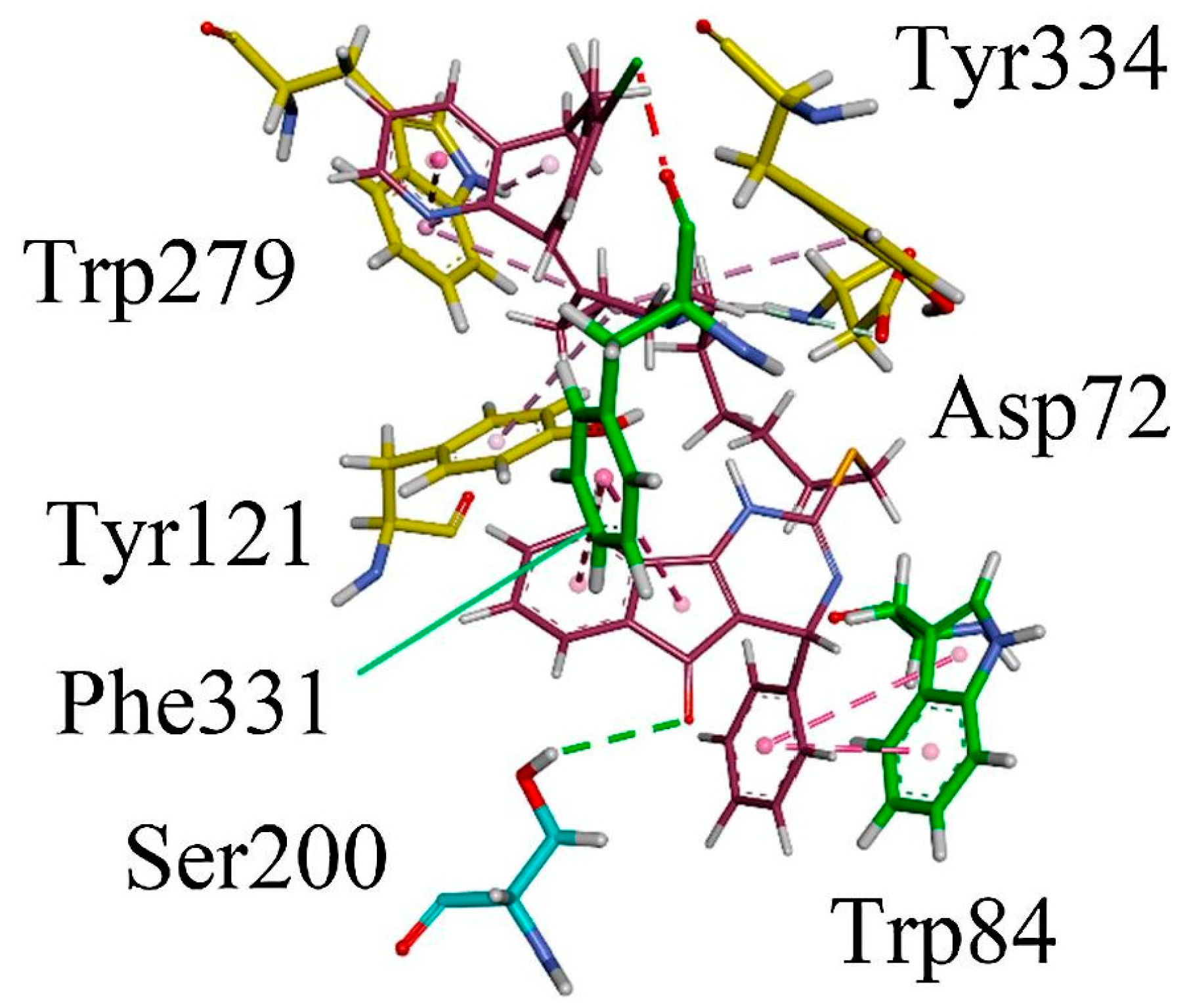
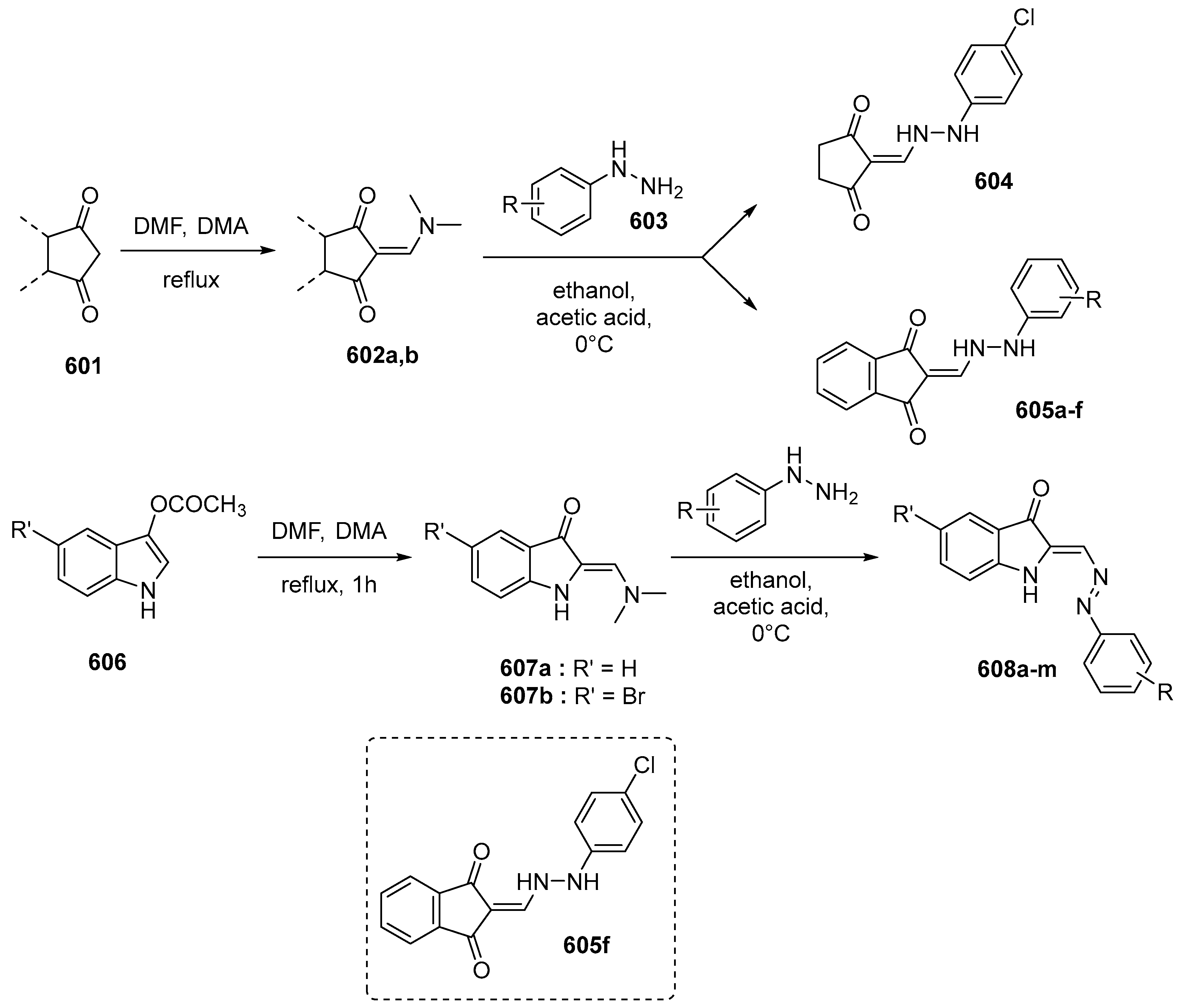

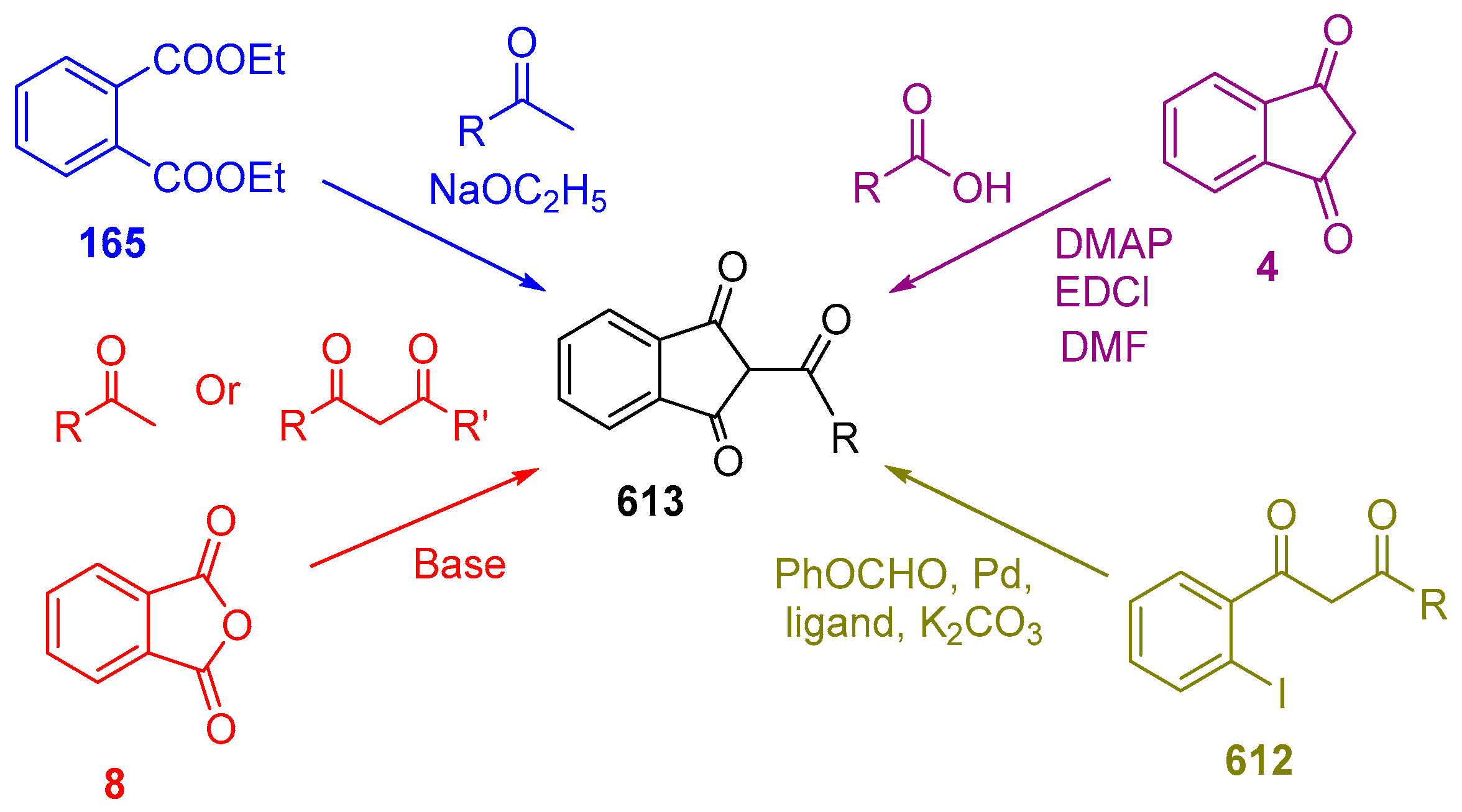
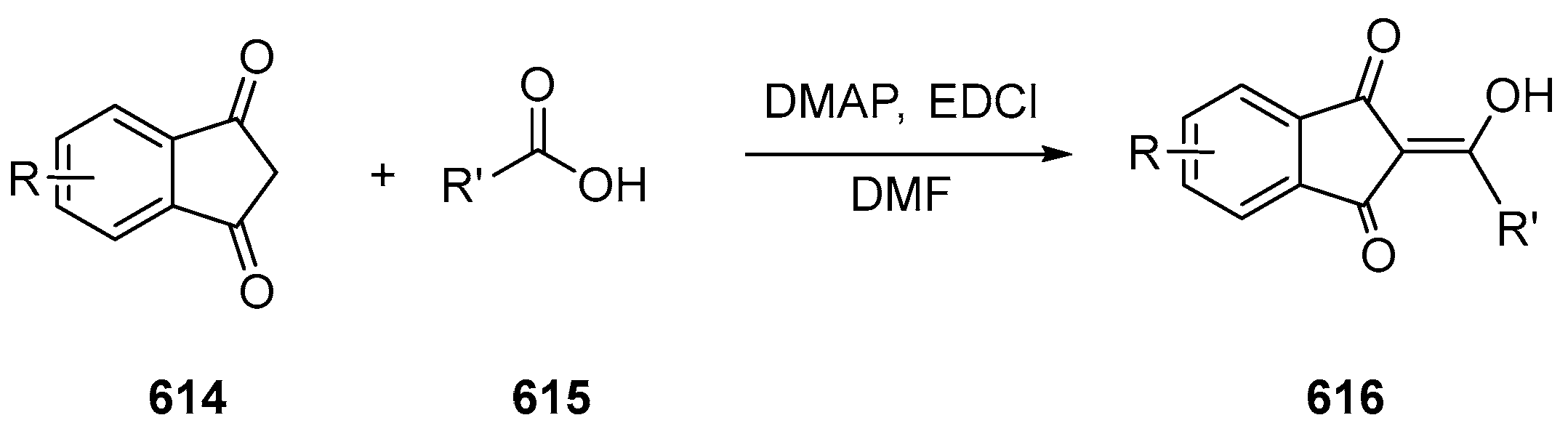
| compounds | 95 | 96 | 97 | 98 | 99 | 100 | 101 | 102 | 103 | 104 |
| reaction yields | 88 | 84 | 88 | 74 | 94 | 89 | 92 | 85 | 84 | 88 |
| compounds | 105 | 106 | 107 | 108 | 109 | 110 | 111 | 112 | 113 | 114 |
| reaction yields | 74 | 85 | 75 | 82 | 92 | 87 | 81 | 78 | 89 | 85 |
| Ar1 | Ar2 | Yield a | dr b |
|---|---|---|---|
| C6H5 | C6H5 | 98 | >20:1 |
| 4-FC6H4 | C6H5 | 75 | >20:1 |
| 4-ClC6H4 | C6H5 | 85 | >20:1 |
| 4-BrC6H4 | C6H5 | 84 | >20:1 |
| 4-CF3C6H4 | C6H5 | 92 | 4:1 |
| 4-MeC6H4 | C6H5 | 83 | >20:1 |
| 3-BrC6H4 | C6H5 | 65 | >20:1 |
| 3-NO2C4H4 | C6H5 | 71 | >20:1 |
| 3-MeC6H4 | C6H5 | 93 | >20:1 |
| 3-MeOC6H4 | C6H5 | 82 | 7:1 |
| 2-C4H3S | C6H5 | 70 | >20:1 |
| R1 | R2 | t (h) | Yield (%) a |
|---|---|---|---|
| 5-Br | H | 18 | 69 |
| H | H | 18 | 62 |
| 5-Cl | H | 12 | 70 |
| 5-NO2 | H | 30 | 58 |
| 5-OMe | H | 18 | 59 |
| 4-OMe | H | 24 | 71 |
| 3-OMe | H | 18 | 79 |
| 5-F | H | 11 | 46 b |
| 5-Br | 5-Cl | 18 | 75 |
| 5-Br | 5-Br | 12 | 72 |
| 5-Br | 5-NO2 | 18 | 63 |
| 5-Br | 5-OMe | 24 | 72 |
| 5-Br | 4-OMe | 18 | 61 |
| 5-Br | 3-OMe | 48 | 48 |
| R1 | R2 | t (h) | Yield (%) a |
|---|---|---|---|
| 5-Br | H | 4 | 79 |
| H | H | 5 | 65 |
| 5-Cl | H | 4 | 71 |
| 5-NO2 | H | 4 | 50 |
| 5-OMe | H | 24 | 26 |
| 4-OMe | H | 15 | 10 |
| 3-OMe | H | 15 | 47 |
| 5-F | H | 5 | 53 |
| 5-Br | 5-Cl | 6 | 75 |
| 5-Br | 5-Br | 4 | 79 |
| 5-Br | 5-NO2 | 48 | 56 |
| 5-Br | 5-OMe | 5 | 70 |
| 5-Br | 4-OMe | 8 | 76 |
| 5-Br | 3-OMe | 4 | 69 |
| R1 | Ar1 | Ar2 | Yield (%) a | dr b |
|---|---|---|---|---|
| H | C6H5 | C6H5 | 98 | 100:0:0:0 |
| H | 4-BrC6H4 | C6H5 | 70 | 100:0:0:0 |
| H | 4-OMeC6H4 | C6H5 | 32 | 96:4:0:0 |
| H | 4-NO2C6H4 | C6H5 | 73 | 100:0:0:0 |
| H | 4-ClC6H4 | C6H5 | 68 | 100:0:0:0 |
| H | 4-PhC6H4 | C6H5 | 84 | 100:0:0:0 |
| H | C10H7 | C6H5 | 72 | 100:0:0:0 |
| H | C4H3O | C6H5 | 61 | 100:0:0:0 |
| H | C4H3S | C6H5 | 56 | 100:0:0:0 |
| F | C6H5 | C6H5 | 40 | 50:50:0:0 |
| NO2 | C6H5 | C6H5 | 46 | 60:40:0:0 |
| H | C6H5 | 4-ClC6H4 | 69 | 100:0:0:0 |
| H | C6H5 | 4-NO2C6H4 | 74 | 100:0:0:0 |
| H | C6H5 | 3,5-(CF3)2C6H3 | 31 | 100:0:0:0 |
| R | Yield (%) a |
|---|---|
| C6H5 | 94 |
| 4-FC6H4 | 93 |
| 2-ClC6H4 | 88 |
| 4-ClC6H4 | 89 |
| 2-BrC6H4 | 91 |
| 4-BrC6H4 | 92 |
| 4-CNC6H4 | 90 |
| 4-NO2C6H4 | 91 |
| 4-MeC6H4 | 95 |
| 4-OMeC6H4 | 96 |
| 3,4,5-Me3C6H2 | 91 |
| 2-C10H7 | 92 |
| 2-C4H3O | 88 |
| 2-C4H3S | 91 |
| C6H13 | 65 |
| R1 | R2 | R3 | t (d) | Yield (%) | ee a |
|---|---|---|---|---|---|
| C6H5 | H | C6H5 | 1 | 89 | 96 |
| 4-NO2C6H4 | H | C6H5 | 1 | 84 | 92 |
| 4-CNC6H4 | H | C6H5 | 2 | 90 | 94 |
| 4-ClC6H4 | H | C6H5 | 2 | 88 | 94 |
| 4-BrC6H4 | H | C6H5 | 1 | 85 | 93 |
| 4-MeC6H4 | H | C6H5 | 3 | 92 | 93 |
| 4-MeOC6H4 | H | C6H5 | 4 | 84 | 91 |
| 4-HOC6H4 | H | C6H5 | 7 | 70 | 69 |
| 2-MeOC6H4 | H | C6H5 | 7 | 93 | 88 |
| 2-HOC6H4 | H | C6H5 | 5 | 42 | 25 |
| 2-BrC6H4 | H | C6H5 | 2 | 88 | 91 |
| C4H3O | H | C6H5 | 4 | 73 | 89 |
| C4H3S | H | C6H5 | 5 | 76 | 88 |
| C5H4N | H | C6H5 | 1 | 82 | 77 |
| C6H5 | H | 4-ClC6H4 | 2 | 90 | 95 |
| C6H5 | H | CH3 | 3 | 85 | 93 |
| C6H5 | 4-Cl | C6H5 | 1 | 92 | 91 |
| C6H5 | 4-Br | C6H5 | 2 | 82 | 95 |
| C6H5 | 4-MeO | C6H5 | 1 | 86 | 92 |
| C6H5 | 2,4-Cl2 | C6H5 | 2 | 87 | 90 |
| C6H5 | 2-MeO | C6H5 | 2 | 95 | 93 |
| R1 | R2 | Yield (%) a | ee (%) b |
|---|---|---|---|
| 4-NO2C6H4 | 4-Cl | 52 | 88 |
| 4-NO2C6H4 | 4-Br | 51 | 92 |
| 4-NO2C6H4 | 4-CN | 65 | 91 |
| 4-NO2C6H4 | 4-CF3 | 50 | 91 |
| 4-NO2C6H4 | 2-Cl | 68 | 97 |
| 4-NO2C6H4 | 2-Br | 50 | 97 |
| 4-NO2C6H4 | 3-NO2 | 75 | 87 |
| 4-NO2C6H4 | 3-Cl | 60 | 91 |
| 4-NO2C6H4 | 3-Br | 51 | 90 |
| 4-NO2C6H4 | 2,4-Cl2 | 62 | 96 |
| 4-NO2C6H4 | 2,3-Cl2 | 67 | 98 |
| 4-NO2C6H4 | 3,4-Cl2 | 54 | 92 |
| 4-NO2C6H4 | 2,6-Cl2 | 30 | 92 |
| 4-NO2C6H4 | 4-F, 3-Br | 50 | 90 |
| 4-NO2C6H4 | 4-CH3 | Trace | - |
| C6H5 | 4-NO2 | 75 c | 66 |
| 3-BrC6H4 | 4-NO2 | 64 c | 67 |
| 4-C6H4 | 4-NO2 | 75 c | 65 |
| C6H11 | 4-NO2 | 68 c | 67 |
| R | Yield (%) | er | dr |
|---|---|---|---|
| C6H5 | 97 | 92:8 | 3:1 |
| 4-MeC6H4 | 98 | 75:25 | 3:1 |
| 4-BrC6H4 | 90 | 77:23 | 3:1 |
| 4-NO2C6H4 | 98 | 80:20 | 3:1 |
| 4-CF3C6H4 | 93 | 87:13 | 3:1 |
| C10H7 | 99 | 84:16 | 10:1 |
| 3-ClC6H4 | 90 | 72:28 | 3:1 |
| 3-MeOC6H4 | 90 | 83:17 | 3:1 |
| C5H4N | 81 | 75:25 | 3:1 |
| C4H3O | 59 | 97:3 | 4:1 |
| CH2C6H5 | 91 | 74:26 | 3:1 |
| R1 | R2 | Yield (%) a | dr b | ee c |
|---|---|---|---|---|
| C6H5 | C6H5 | 99 | 53:47 | 99.98 |
| 3-MeC6H4 | C6H5 | 99 | 52:48 | 99.95 |
| 4-MeC6H4 | C6H5 | 97 | 51:49 | 96.95 |
| 2-MeOC6H4 | C6H5 | 82 | 54:46 | 99.95 |
| 3-MeOC6H4 | C6H5 | 84 | 53:47 | 99.98 |
| 4-MeOC6H4 | C6H5 | 93 | 54:46 | 96.89 |
| 2-FC6H4 | C6H5 | 96 | 53:47 | 93.92 |
| 3-FC6H4 | C6H5 | 86 | 56:44 | 97.94 |
| 4-FC6H4 | C6H5 | 96 | 52:48 | 94.92 |
| 3-ClC6H4 | C6H5 | 94 | 52:48 | 93.95 |
| 4-ClC6H4 | C6H5 | 76 | 51:49 | 96.95 |
| 3-BrC6H4 | C6H5 | 77 | 52:48 | 93.89 |
| 4-BrC6H4 | C6H5 | 68 | 50:50 | 95.95 |
| 4-PhC6H4 | C6H5 | 71 | 53:47 | 93.95 |
| C6H5 | 3-MeC6H4 | 94 | 56:44 | 96.97 |
| C6H5 | 2-MeOC6H4 | 73 | 64:36 | 84.88 |
| C6H5 | 3-MeOC6H4 | 99 | 62:38 | 96.96 |
| C6H5 | 4-MeOC6H4 | 93 | 59:41 | 98.97 |
| C6H5 | 3,4-(MeO)2C6H4 | 79 | 81:19 | 99.95 |
| C6H5 | 2-FC6H4 | 89 | 68:32 | 91.90 |
| C6H5 | 3-FC6H4 | 91 | 68:32 | 96.90 |
| C6H5 | 4-FC6H4 | 89 | 53:47 | 94.92 |
| C6H5 | 3-CF3C6H4 | 90 | 73:27 | 93.91 |
| C6H5 | 4-CF3C6H4 | 99 | 59:41 | 97.81 |
| C6H5 | 2-C4H3O | 97 | 63:37 | 99.99 |
| C6H5 | 4-Br-2-C4H2S | 99 | 76:24 | 99.96 |
| R. | Yield (%) a | dr b | ee c |
|---|---|---|---|
| C6H5 | 80 | 5:1 | 97:95 |
| 4-FC6H4 | 87 | 5:1 | 96:95 |
| 4-BrC6H4 | 84 | 4.4:1 | 92:80 |
| 2-CF3C6H4 | 75 | 14:1 | 99:55 |
| 2-FC6H4 | 82 | 6.4:1 | 96:89 |
| 4-MeOC6H4 | 92 | 5.5:1 | 98:90 |
| 2-F-6-ClC6H3 | 85 | 5.3:1 | 97:90 |
| 2-MeOC6H4 | 90 | 2.6:1 | 97:97 |
| 4-MeC6H4 | 81 | 5:1 | 96:88 |
| 2-BrC6H4 | 79 | 4.1:1 | 92:84 |
| 4-ClC6H4 | 90 | 5.3:1 | 92:68 |
| 1-C10H7 | 83 | 2.3:1 | 97:97 |
| 2-C10H7 | 81 | 5.7:1 | 98:80 |
| C4H3S | 80 | 6:1 | 97:87 |
| C4H3O | 89 | 5.7:1 | 96:78 |
| C3H4 | 86 | 1.7:1 | 99:99 |
| C6H11 | 88 | 1.2:1 | 99:99 |
| R1 | R2 | Yield % a | ee % b |
|---|---|---|---|
| H | CH2C6H5 | 95 | 78 |
| 5-F | CH2C6H5 | 99 | 64 |
| 5-Cl | CH2C6H5 | 92 | 78 |
| 5-I | CH2C6H5 | 98 | 69 |
| 5-Me | CH2C6H5 | 99 | 82 |
| 6-Me | CH2C6H5 | 78 | 59 |
| 6-MeO | CH2C6H5 | 96 | 77 |
| 6-Cl | CH2C6H5 | 96 | 65 |
| 7-CF3 | CH2C6H5 | 52 | 68 |
| 5,7-Me2 | CH2C6H5 | 80 | 48 |
| 5,7-Cl2 | CH2C6H5 | 39 | 75 |
| H | CH2OMe | 65 | 77 |
| H | Me | 81 | 84 |
| R1 | R2 | Yield (%) a | dr b |
|---|---|---|---|
| C10H7 | C6H5 | 75 | 3:1 |
| 3,4-(OMe)2C6H4 | C6H5 | 93 | 1:4.2 |
| 4-BrC6H4 | C6H5 | 80 | 3.5:1 |
| 3-ClC6H4 | C6H5 | 86 | 10.0:1 |
| C4H9 | C6H5 | 84 | 9.5:1 |
| C6H5 | 4-NO2C6H4 | 81 | 4.8:1 |
| C6H5 | 4-OMeC6H4 | 77 | 1:1.2 |
| C6H5 | 3-MeC6H4 | 79 | 1.6:1 |
| C6H5 | 4-ClC6H4CH2 | 50 | 1:9.2 |
| Ar1 | Ar2 | Yield (%) a |
|---|---|---|
| C6H5 | C6H5 | 95 |
| 2-BrC6H5 | C6H5 | 92 |
| 2-NO2C6H5 | C6H5 | 78 |
| 4-FC6H4 | C6H5 | 85 |
| 4-CF3C6H4 | C6H5 | 88 |
| 4-MeC6H4 | C6H5 | 81 |
| 4-MeOC6H4 | C6H5 | 61 |
| 3-BrC6H4 | C6H5 | 95 |
| C10H7 | C6H5 | 51 |
| C4H3O | C6H5 | 56 |
| C4H3S | C6H5 | 54 |
| C3H7 | C6H5 | 21 |
| C6H5 | 2-BrC6H4 | 82 |
| C6H5 | 4-MeC6H4 | 85 |
| C6H5 | 4-MeOC6H4 | 86 |
| C6H5 | 4-NO2C6H4 | 86 |
| 4-FC6H4 | 3-BrC6H4 | 93 |
| 3-BrC6H4 | 4-ClC6H4 | 84 |
| 4-BrC6H4 | 4-MeC6H4 | 87 |
| 3-NO2C6H4 | 4-MeOC6H4 | 84 |
| 2-ClC6H4 | 4-MeOC6H4 | 92 |
| C6H5 | C4H3S | 77 |
| Ar1 | R | Ar2 | Yield (%) a | ee (%) b |
|---|---|---|---|---|
| C6H5 | Me | 4-BrC6H4 | 56 | −87 |
| C6H5 | Et | 4-BrC6H4 | 60 | −74 |
| 2,4-Cl2C6H3 | Et | 4-BrC6H4 | 85 | −70 |
| 2-MeC6H4 | Me | 4-BrC6H4 | 79 | −73 |
| 1-Br-2-C10H6 | Me | 4-BrC6H4 | 89 | −81 |
| 1-Br-2-C10H6 | Et | 3-NO2C6H4 | 73 | −81 |
| 2,4-F2C6H3 | Et | 3-NO2C6H4 | 89 | −76 |
| 3-MeC6H4 | Me | 3-NO2C6H4 | 75 | −76 |
| 2-MeC6H4 | Et | 3-NO2C6H4 | 76 | −72 |
| 4-ClC6H5 | Et | 3-NO2C6H4 | 55 | 64 |
| 4-BrC6H4 | Et | 3-NO2C6H4 | 76 | −77 |
| 2-ClC6H4 | Et | 2-NO2C6H4 | 59 | −79 |
| 4-NO2C6H4 | Me | C6H5 | 90 | −74 |
| 4-ClC6H4 | Me | 2-C4H3O | 65 | −78 |
| Ar. | R | Yield (%) a |
|---|---|---|
| 4-OMeC6H4 | CO2Me | 81 |
| 4-MeC6H4 | CO2Me | 88 |
| C6H5 | CO2Me | 92 |
| 3-ClC6H4 | CO2Me | 92 |
| 3-NO2C6H4 | CO2Me | 70 |
| 4-ClC6H4 | CO2Me | 73 |
| 4-OMeC6H4 | CO2Et | 56 |
| 4-MeC6H4 | CO2Et | 72 |
| C6H5 | CO2Et | 90 |
| 3-FC6H4 | CO2Et | 68 |
| 3-ClC6H4 | CO2Et | 70 |
| 3-NO2C6H4 | CO2Et | 65 |
| 4-ClC6H4 | CO2Et | 78 |
| 4-BrC6H4 | CO2Et | 90 |
| 4-NO2C6H4 | CO2Et | 75 |
| C6H5 | CO2tBu | 86 |
| 2-ClC6H4 | CO2tBu | 55 |
| 3-ClC6H4 | CO2tBu | 60 |
| 3-FC6H4 | CO2tBu | 63 |
| 3-NO2C6H4 | CO2tBu | 54 |
| 4-BtC6H4 | CO2tBu | 62 |
| 4-NO2C6H4 | CO2tBu | 63 |
| C6H5 | CN | 73 |
| 4-MeC6H4 | CN | 48 |
| 3-ClC6H4 | CN | 37 |
| 4-ClC6H4 | CN | 55 |
| 4-BrC6H4 | CN | 57 |
| Ar. | Yield (%) a |
|---|---|
| 4-MeC6H4 | 70 |
| 3-OMeC6H4 | 68 |
| 2-ClC6H4 | 92 |
| 2-BrC6H4 | 81 |
| 3-FC6H4 | 73 |
| 3-ClC6H4 | 89 |
| 4-ClC6H4 | 56 |
| 4-BrC6H4 | 72 |
| R1 | R2 | Yield (%) a |
|---|---|---|
| CH2Br | CH2Br | 72 |
| CH2Cl | H | 80 |
| CH2Cl | CH2Cl | 79 |
| R | t (h) | Yield (%) a | ee (%) b |
|---|---|---|---|
| C6H5 | 12 | 87 | 97 |
| 2-FC6H4 | 14 | 99 | 98 |
| 3-FC6H4 | 48 | 62 | 97 |
| 4-FC6H4 | 10 | 94 | 97 |
| 2-ClC6H4 | 5 | 62 | 98 |
| 3-ClC6H4 | 36 | 94 | 98 |
| 4-ClC6H4 | 24 | 99 | 98 |
| 2,4-Cl2C6H3 | 4 | 90 | 98 |
| 2-MeC6H4 | 5 | 99 | 98 |
| 3-MeC6H4 | 12 | 99 | 96 |
| 4- MeC6H4 | 12 | 83 | 95 |
| 4-EtC6H4 | 12 | 64 | 97 |
| 2-MeOC6H4 | 11 | 99 | 97 |
| 3-MeOC6H4 | 14 | 99 | 99 |
| 4- MeOC6H4 | 14 | 99 | 94 |
| 1-C10H6 | 20 | 99 | 99 |
| 2-C4H3S | 24 | n.r. | - |
| R1 | R2 | Yield (%) | dr cis:trans |
|---|---|---|---|
| C6H5 | 4-NO2C6H4 | 97 | ≥99:1 |
| C6H5 | 4-MeOC6H4 | 71 | 6:1 |
| C6H5 | 4-HOC6H4 | ≥99 | ≥99:1 |
| C6H5 | 4-ClC6H4 | ≥99 | ≥99:1 |
| C6H5 | 2-NO2C6H4 | 80 | ≥99:1 |
| C6H5 | 4-CNC6H4 | ≥99 | ≥99:1 |
| C6H5 | 4-CO2MeC6H4 | ≥99 | ≥99:1 |
| C6H5 | C10H7 | ≥99 | ≥99:1 |
| C6H5 | C4H3O | ≥99 | 10:1 |
| C6H5 | C4H3S | 57 | 13:1 |
| C6H5 | C4H4N | 30 | ≥99:1 |
| C6H5 | C2H2C6H5 | 74 | ≥99:1 |
| C6H5 | C6H5 | 93 | ≥99:1 |
| C10H7 | C10H7 | 95 | ≥99:1 |
| C4H3S | C4H3S | 93 | ≥99:1 |
| C4H3O | C4H3O | 60 | ≥99:1 |
| 4-MeOC6H4 | 4-MeOC6H4 | 95 | ≥99:1 |
| C7H5O2 | C7H5O2 | 98 | ≥99:1 |
| 4-Me2NC6H4 | 4-Me2NC6H4 | 90 | ≥99:1 |
| 4-HOC6H4 | 4-HOC6H4 | 95 | ≥99:1 |
| 4-ClC6H4 | 4-ClC6H4 | 98 | ≥99:1 |
| 4-NO2C6H4 | 4-NO2C6H4 | 98 | ≥99:1 |
| 4-CNC6H4 | 4-CNC6H4 | 85 | ≥99:1 |
| 4-CO2MeC6H4 | 4-CO2MeC6H4 | 83 | ≥99:1 |
| Ar | Yield (%) b | ee (%) c | dr cis:trans |
|---|---|---|---|
| C6H5 | 86 | 99 | 94.8:5.2 |
| 4-FC6H4 | 81 | 99 | 95.3:4.7 |
| 4-MeOC6H4 | 80 | 98 | 95.6:4.4 |
| 3-BrC6H4 | 78 | 97 | 91.6:8.4 |
| 3-NO2C6H4 a | 85 | 91 | 84.4:15.6 |
| 3-MeC6H4 | 83 | 98 | 95.0:5.0 |
| 2-C4H3O | 78 | 97 | 92.1:7.9 |
| R | Time (h) | Yield (%) a | dr cis:trans b | ee c |
|---|---|---|---|---|
| C6H5 | 3 | 96 | 9:1 | 68 |
| C10H7 | 3 | 93 | 8:1 | 65 |
| 4-NO2C6H4 | 2 | 99 | 7.5:1 | 56 |
| 4-FC6H4 | 1 | 96 | 9:1 | 66 |
| 4-BrC6H4 | 5 | 97 | 1.3:1 | 68 |
| 2-ClC6H4 | 2 | 93 | 1.5:1 | 72 |
| 4-MeC6H4 | 2.5 | 98 | 9:1 | 71 |
| 3-MeOC6H4 | 3 | 88 | 9:1 | 60 |
| 3,4-(OCH2O)C6H3 | 4.5 | 96 | 9:1 | 74 |
| 3-C4H3S | 24 | 98 | 9:1 | 72 |
| Ar | Yield (%) a | rr b |
|---|---|---|
| 4-MeC6H4 | 78 | ≥95:5 |
| 4-MeOC6H4 | 83 | ≥95:5 |
| 2-C10H7 | 74 | ≥95:5 |
| 4-ClC6H4 | 79 | ≥95:5 |
| 4-BrC6H4 | 80 | ≥95:5 |
| Ar | X | Yield (%) |
|---|---|---|
| 4-BrC6H4 | NH | 78 |
| 4-MeOC6H4 | NH | 80 |
| 4-ClC6H4 | S | 76 |
| 4-MeOC6H4 | S | 85 |
| R1 | R2 | R3 | t (h) | Yield (%) a |
|---|---|---|---|---|
| C6H5 | H | C6H5 | 15 | 90 |
| C6H5 | H | 2-BrC6H4 | 10 | 93 |
| C6H5 | H | 3-BrC6H4 | 10 | 96 |
| C6H5 | H | 4-BrC6H4 | 10 | 90 |
| C6H5 | H | 2-MeC6H4 | 10 | 91 |
| C6H5 | H | 3-MeC6H4 | 10 | 95 |
| C6H5 | H | 4-MeC6H4 | 10 | 94 |
| C6H5 | H | 4-ClC6H4 | 12 | 88 |
| C6H5 | H | 4-FC6H4 | 12 | 87 |
| C6H5 | H | 4-MeOC6H4 | 3 | 90 b |
| C6H5 | H | 4-NO2C6H4 | 24 | 73 |
| C6H5 | H | 2,4-Cl2C6H3 | 12 | 62 |
| C6H5 | H | 2-C4H3O | 48 | 87 |
| C6H5 | H | 2-C4H3S | 53 | 84 |
| C6H5 | H | 1-C10H7 | 20 | 96 |
| C6H5 | H | C2H4C6H4 | 35 | 89 |
| C6H5 | Me | 4-BrC6H4 | 18 | 95 |
| C6H5 | Di-Me | 4-BrC6H4 | 18 | 97 |
| 4-FC6H4 | H | 4-BrC6H4 | 21 | 83 |
| 4-MeC6H4 | Me | 4-BrC6H4 | 21 | 84 |
| n-Bu | H | 4-BrC6H4 | 22 | NR |
| R1 | R2 | R3 | t (h) | Yield (%) |
|---|---|---|---|---|
| C6H5 | H | C6H5 | 15 | 90 |
| C6H5 | H | 2-BrC6H4 | 10 | 93 |
| C6H5 | H | 3-BrC6H4 | 10 | 96 |
| C6H5 | H | 4-BrC6H4 | 10 | 90 |
| C6H5 | H | 2-MeC6H4 | 10 | 91 |
| C6H5 | H | 3-MeC6H4 | 10 | 95 |
| C6H5 | H | 4-MeC6H4 | 10 | 94 |
| C6H5 | H | 4-ClC6H4 | 12 | 88 |
| C6H5 | H | 4-FC6H4 | 12 | 87 |
| C6H5 | H | 4-MeOC6H4 | 3 | 90 |
| C6H5 | H | 4-NO2C6H4 | 24 | 73 |
| C6H5 | H | 2,4-Cl2C6H3 | 12 | 62 |
| C6H5 | H | 2-C4H3O | 48 | 87 |
| C6H5 | H | 2-C4H3S | 53 | 84 |
| C6H5 | H | 1-C10H7 | 20 | 96 |
| C6H5 | H | C2H4C6H4 | 35 | 89 |
| C6H5 | Me | 4-BrC6H4 | 18 | 95 |
| C6H5 | Di-Me | 4-BrC6H4 | 18 | 97 |
| 4-FC6H4 | H | 4-BrC6H4 | 21 | 83 |
| 4-MeC6H4 | Me | 4-BrC6H4 | 21 | 84 |
| n-Bu | H | 4-BrC6H4 | 22 | NR |
| Ar | R1 | R2 | Yield % | dr |
|---|---|---|---|---|
| C6H5 | C6H5 | H | 85 | 4:1 |
| C6H5 | 4-FC6H4 | H | 81 | 4:1 |
| C6H5 | 4-OMeC6H4 | H | 69 | 4:1 |
| C6H5 | 4-NO2C6H4 | H | 51 | 4:1 |
| 4-OMeC6H4 | C6H5 | H | 60 | 4:1 |
| 4-OMeC6H4 | 4-FC6H4 | H | 48 | 4:1 |
| 4-OMeC6H4 | 4-OMeC6H4 | H | 51 | 4:1 |
| 4-OMeC6H4 | 4-NO2C6H4 | H | 40 | 4:1 |
| C6H5 | Me | H | 72 | 3:2 |
| 4-OMeC6H4 | Me | H | 60 | 3:2 |
| C6H5 | H | Me | 60 a | - b |
| C6H5 | Me | Me | 69 | - |
| R1 a | R2 | R3 | Time (h) | Yield % b |
|---|---|---|---|---|
| - | C6H5 | C6H5 | 24 | 82 |
| 5-OH | C6H5 | C6H5 | 24 | 82 |
| 5-OMe | C6H5 | C6H5 | 36 | 77 |
| 4-OMe | C6H5 | C6H5 | 27 | 83 |
| 5-Br | C6H5 | C6H5 | 24 | 82 |
| - | 4-OMeC6H4 | C6H5 | 75.5 | 72 |
| - | 4-BrC6H4 | C6H5 | 96 | 72 |
| - | 4-ClC6H4 | C6H5 | 24.5 | 67 |
| - | 3-ClC6H4 | C6H5 | 24 | 72 |
| - | 2-ClC6H4 | C6H5 | 24 | 72 |
| - | C6H5 | 4-BrC6H4 | 24.5 | 76c |
| - | C6H5 | 3-BrC6H4 | 24.5 | 76 |
| - | C6H5 | 4-ClC6H4 | 27 | 82 |
| - | C6H5 | 3-ClC6H4 | 24.5 | 73 |
| - | C6H5 | 2-ClC6H4 | 24.5 | 77 c |
| - | C6H5 | 4-OMeC6H4 | 48 | 84 |
| - | C6H5 | C4H3S | 24 | 71 |
| - | C6H5 | C3H7 | 24 | 58 c |
| R | t (h) | Yield (%) a |
|---|---|---|
| C6H5 | 10 | 67 |
| 2-ClC6H4 | 10 | 69 |
| 4-ClC6H4 | 8 | 72 |
| 4-FC6H4 | 9 | 64 |
| 3-NO2C6H4 | 10 | 62 |
| 4-MeC6H4 | 10 | 52 |
| 4-MeOC6H4 | 9 | 62 |
| 3,5-(MeO)2C6H3 | 9 | 58 |
| 2-HOC6H4 | 10 | 63 |
| 4-HOC6H4 | 9 | 55 |
| 2-HO-3-MeOC6H4 | 8 | 57 |
| 3-MeO-4-HOC6H4 | 8 | 60 |
| 4-NMe2C6H4 | 10 | 52 |
| R1 | R2 | Yield Product 1 (%) | Yield Product 2 (%) |
|---|---|---|---|
| C6H5 | C6H5 | 30 | 31 |
| 4-NMe2C6H4 | 4-NMe2C6H4 | 28 | 32 |
| 4-OMeC6H4 | 4-OMeC6H4 | 32 | 36 |
| 4-NO2C6H4 | 4-NO2C6H4 | 20 | 22 |
| 4-C6H5C6H4 | 4-C6H5C6H4 | 32 | 28 |
| C4H3S | C4H3S | 42 | 41 |
| C4H3O | C4H3O | 36 | 36 |
| 3-NO2C4H2O | 3-NO2C4H2O | 39 | 31 |
| 3-MeC4H2O | 3-MeC4H2O | 30 | 33 |
| R1 | R2 | Time (min) | Yield (%) |
|---|---|---|---|
| 4-ClC6H4 | 4-FC6H4 | 45 | 85 |
| 4-ClC6H4 | 4-MeC6H4 | 50 | 80 |
| 4-BrC6H4 | 4-FC6H4 | 50 | 83 |
| 4-FC6H4 | 4-MeC6H4 | 40 | 82 |
| 4-BrC6H4 | 4-MeC6H4 | 50 | 81 |
| 4-FC6H4 | 4-FC6H4 | 45 | 84 |
| 4-ClC6H4 | 4-NO2C6H4 | 50 | 82 |
| 4-BrC6H4 | 4-NO2C6H4 | 45 | 86 |
| 4-MeC6H4 | 4-NO2C6H4 | 40 | 79 |
| 4-NO2C6H4 | 4-OMeC6H4 | 45 | 84 |
| 4-FC6H4 | 4-NO2C6H4 | 40 | 82 |
| 4-(CF3)C6H4 | 4-NO2C6H4 | 40 | 87 |
| 4-(CF3)C6H4 | 7-ClC9H5N | 50 | 80 |
| 4-(CF3)C6H4 | 4-FC6H4 | 45 | 85 |
| 4-MeC6H4 | 7-ClC9H5N | 50 | 74 |
| C4H3O | C4H9 | 50 | 80 |
| (CH2O2)C6H3 | 4-FC6H4 | 50 | 86 |
| C4H9 | 7-ClC9H5N | 60 | 78 |
| C4H9 | 4-(Ome)C6H4 | 65 | 74 |
| Ar | Yield (%) a |
|---|---|
| 4-CH2OHC6H4 | 81 |
| 4-CHOC6H4 | 80 |
| 4-OMeC6H4 | 93 |
| 3,5-(OMe)2C6H4 | 40 |
| 4-CF3C6H4 | 81 |
| 4-OHC6H4 | 70 |
| 4-COOHC6H4 | 54 |
| R1 | R2 | Yield (%) a |
|---|---|---|
| - | - | 84 |
| 4-Me | - | 80 |
| 4-OMe | - | 73 |
| 2-OMe | - | 70 |
| 3-OMe | - | 78 |
| 4-Cl | - | 92 |
| 4-Br | - | 90 |
| 3-OMe | 4-OMe | 83 |
| 4-Br | 4-OMe | 86 |
| 4-Br | 4-Br | 91 |
| R | Methyle | n | Yield (%) a |
|---|---|---|---|
| C6H5 | Yes | 1 | 82 |
| 4-BrC6H4 | Yes | 1 | 55 |
| 4-CF3C6H4 | Yes | 1 | 59 |
| 4-MeC6H4 | Yes | 1 | 53 |
| 4-NMe2C6H4 | Yes | 1 | 45 |
| 4-ClC6H4 | Yes | 1 | 42 |
| 4-CO2MeC6H4 | Yes | 1 | 69 |
| 4-OMeC6H4 | Yes | 1 | 54 |
| 2-OMeC6H4 | Yes | 1 | 72 |
| 3-OMeC6H4 | Yes | 1 | 54 |
| 3-CNC6H4 | Yes | 1 | 48 |
| 2,4,5-C6H2 | Yes | 1 | 50 |
| C4H3O | Yes | 1 | 58 |
| C4H3S | Yes | 1 | 48 |
| C10H7 | Yes | 1 | 74 |
| 3-CNC6H4 | No | 1 | 63 b |
| C4H3S | No | 1 | 46 b |
| 4-OMeC6H4 | No | 2 | 58 |
| C6H5 | Yes | 2 | 50 |
| R1 | R2 | Yield (%) a |
|---|---|---|
| 4-FC6H4 | C6H5 | 90 |
| 4-BrC6H4 | C6H5 | 61 |
| 4-CF3C6H4 | C6H5 | 84 |
| 4-MeC6H4 | C6H5 | 73 |
| 4-OMeC6H4 | C6H5 | 82 |
| 3-MeC6H4 | C6H5 | 81 |
| 3-OMeC6H4 | C6H5 | 66 |
| 3-ClC6H4 | C6H5 | 69 |
| 2-BrC6H4 | C6H5 | 85 |
| C10H7 | C6H5 | 71 |
| C4H3O | C6H5 | 64 |
| C4H3S | C6H5 | 66 |
| C6H5 | 4-FC6H4 | 74 |
| C6H5 | 4-ClC6H4 | 87 |
| C6H5 | 4-BrC6H4 | 91 |
| C6H5 | 4-NO2C6H4 | 75 |
| C6H5 | 4-MeC6H4 | 76 |
| C6H5 | 4-OMeC6H4 | 60 |
| C6H5 | 3-ClC6H4 | 89 |
| C6H5 | 3-MeC6H4 | 78 |
| C6H5 | 3-OMeC6H4 | 74 |
| C6H5 | 2-BrC6H4 | 68 |
| C6H5 | 2-MeC6H4 | 70 |
| 4-BrC6H4 | 4-MeC6H4 | 58 |
| 4-FC6H4 | 3-BrC6H4 | 62 |
| 3-BrC6H4 | 4-ClC6H4 | 71 |
| C4H3O | 4-NO2C6H4 | 71 |
| C6H5 | CO2Me | Nr |
| C6H5 | C6H11 | Nr |
| R1 | R2 | R3 | R4 | Yield (%) a |
|---|---|---|---|---|
| C6H5 | C6H5 | H | H | 74 |
| C6H5 | 4-MeC6H4 | H | H | 82 |
| C6H5 | 4-OMeC6H4 | H | H | 80 |
| C6H5 | 4-IC6H4 | H | H | 64 |
| C6H5 | 4-ClC6H4 | H | H | 83 |
| C6H5 | 4-CF3C6H4 | H | H | 81 |
| C6H5 | 4-NO2C6H4 | H | H | 66 |
| C6H5 | 4-CNC6H4 | H | H | 78 |
| C6H5 | 4-MeSC6H4 | H | H | 81 |
| C6H5 | 3-BrC6H4 | H | H | 85 |
| C6H5 | 3-MeC6H4 | H | H | 89 |
| C6H5 | 2-MeC6H4 | H | H | 78 |
| C6H5 | C10H7 | H | H | 74 |
| C6H5 | C4H9 | H | H | 79 |
| C6H5 | C6H5 | CH3 | CH3 | 50 |
| C6H5 | C6H5 | C2H5 | H | 65 a |
| C6H5 | C3H5 | H | H | 78 |
| C6H5 | CO2Et | H | H | 30 |
| C6H5 | C10H20 | H | 32 a | |
| C6H5 | C8H8 | H | 40 a | |
| 4-OMeC6H4 | C2H5 | CH3 | H | 51 a |
| C6H5 | C5H4N | H | H | 51 |
| C6H5 | C4H3O | H | H | 86 |
| C6H5 | C4H3S | H | H | 56 |
| C6H5 | C9H8N | H | H | 40 |
| C6H5 | C5H6N | H | H | 96 |
| C6H5 | C4H5N2 | H | H | 77 |
| C6H5 | C6H4C3H2SN | H | H | 70 |
| 4-BrC6H4 | C6H3(O2CH2) | H | H | 72 |
| 2-MeC6H4 | C8H5S | H | H | 52 |
| R | Yield (%) a |
|---|---|
| C6H5 | 87 |
| 4-OMeC6H4 | 75 |
| 4-iPrC6H4 | 92 |
| 4-EtC6H4 | 88 |
| 4-MeC6H4 | 88 |
| 4-PhC6H4 | 90 |
| 4-BrC6H4 | 85 |
| 4-ClC6H4 | 77 |
| 3-ClC6H4 | 93 |
| 4-OMeC6H4 | 86 |
| 2-MeC6H4 | 88 |
| 3,4-(OMe)2C6H3 | 65 |
| 3,4,5-(OMe)3C6H2 | 80 |
| C4H4N | 80 |
| C4H3S | 87 |
| C10H7 | 89 |
| Fc | 87 |
| Entry | Zone of Inhibition/mm | ||||||
|---|---|---|---|---|---|---|---|
| Gram-Positive | Gram-Negative | Fungi | |||||
| B. subtilis | S. aureus | P. aeruginosa | E. coli | A. niger | C. albicans | A. fumigatus | |
| 538a | 9 | 10 | 6 | 8 | 8 | 10 | 11 |
| 538b | 15 | 14 | 10 | 11 | 12 | 14 | 17 |
| 538c | 21 | 24 | 12 | 14 | 19 | 17 | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigot, C.; Brunel, D.; Dumur, F. Indane-1,3-Dione: From Synthetic Strategies to Applications. Molecules 2022, 27, 5976. https://doi.org/10.3390/molecules27185976
Pigot C, Brunel D, Dumur F. Indane-1,3-Dione: From Synthetic Strategies to Applications. Molecules. 2022; 27(18):5976. https://doi.org/10.3390/molecules27185976
Chicago/Turabian StylePigot, Corentin, Damien Brunel, and Frédéric Dumur. 2022. "Indane-1,3-Dione: From Synthetic Strategies to Applications" Molecules 27, no. 18: 5976. https://doi.org/10.3390/molecules27185976
APA StylePigot, C., Brunel, D., & Dumur, F. (2022). Indane-1,3-Dione: From Synthetic Strategies to Applications. Molecules, 27(18), 5976. https://doi.org/10.3390/molecules27185976








