The Chemical Profiling and Anticancer Potential of Functional Polysaccharides from Flos Sophorae Immaturus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
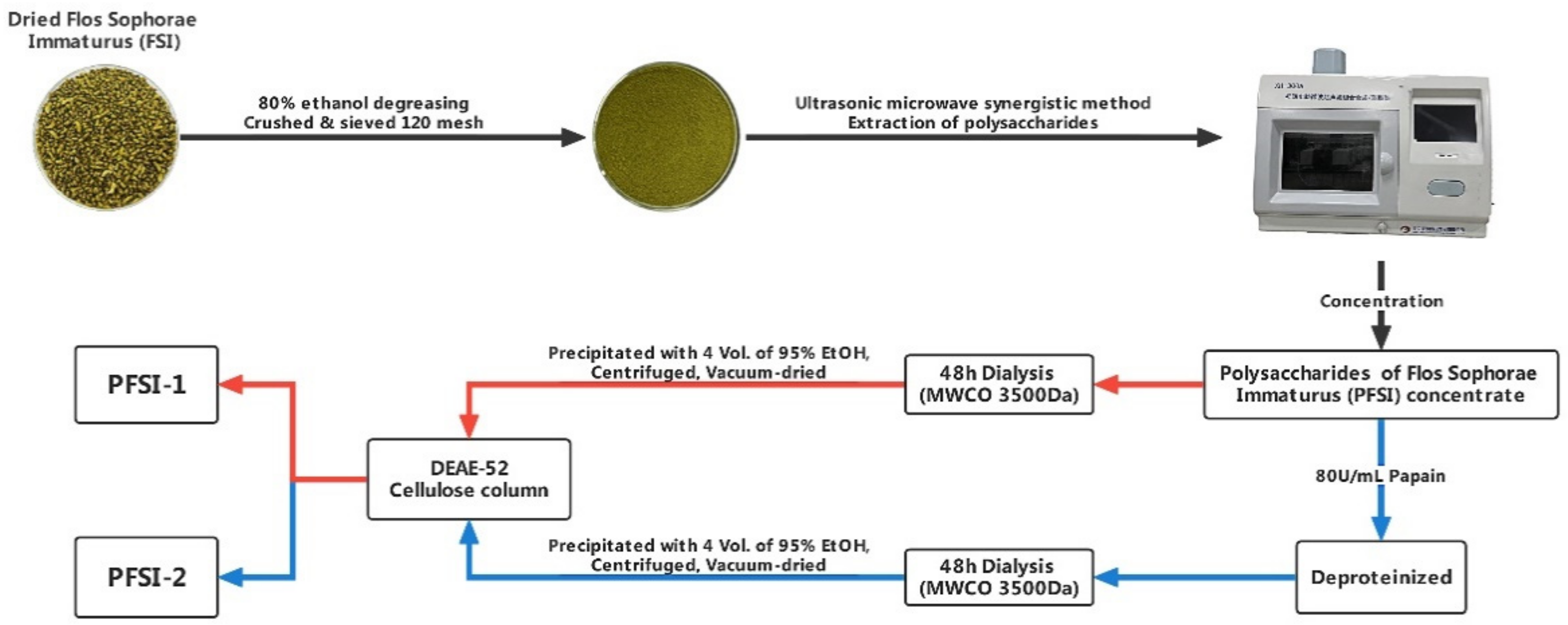
2.2.1. Extraction, Separation, and Purification of Polysaccharides
2.2.2. Infrared Spectral Analysis
2.2.3. Monosaccharide Composition Determination
2.2.4. Scanning Electron Microscope (SEM) Analysis
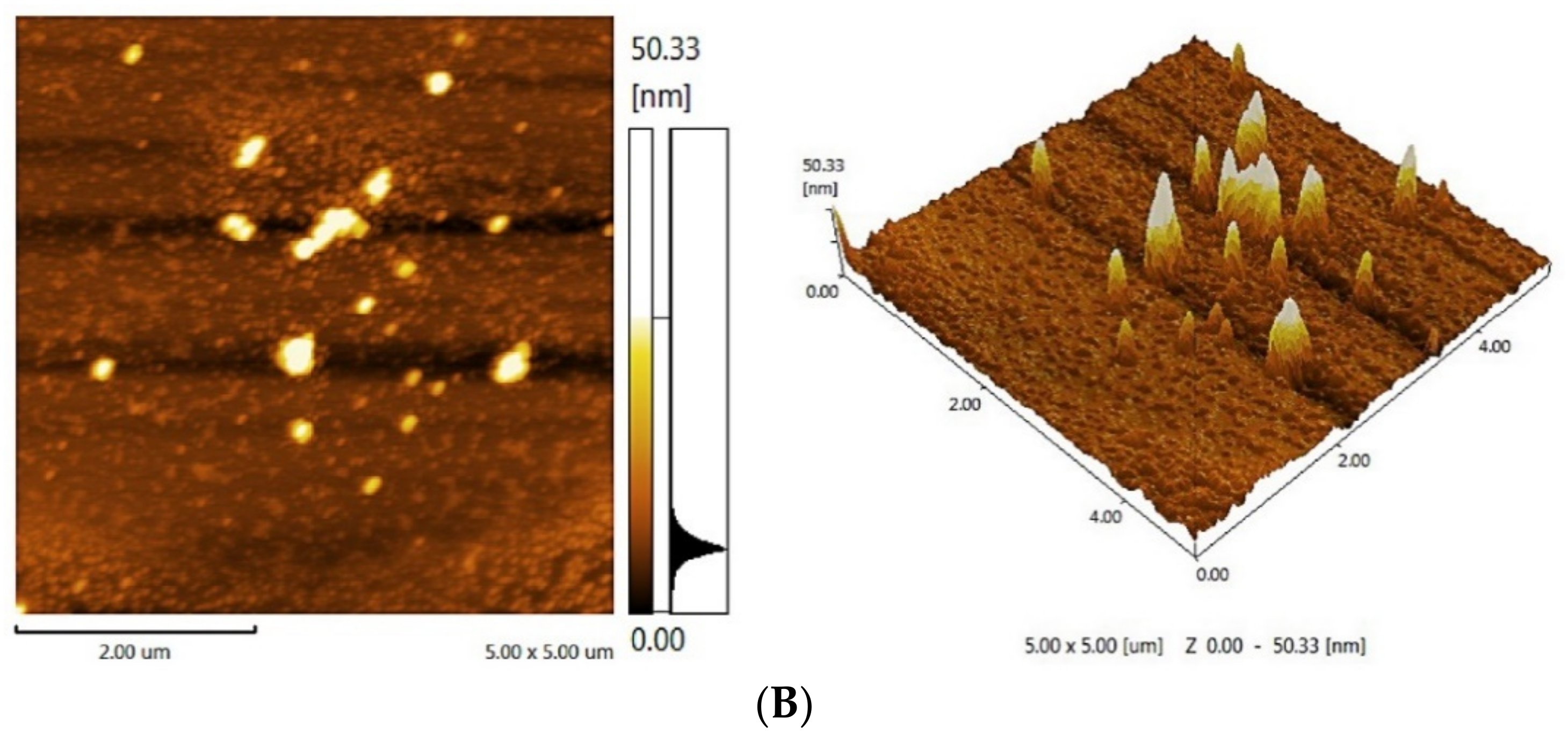
2.2.5. Atomic Force Microscopy (AFM) Analysis
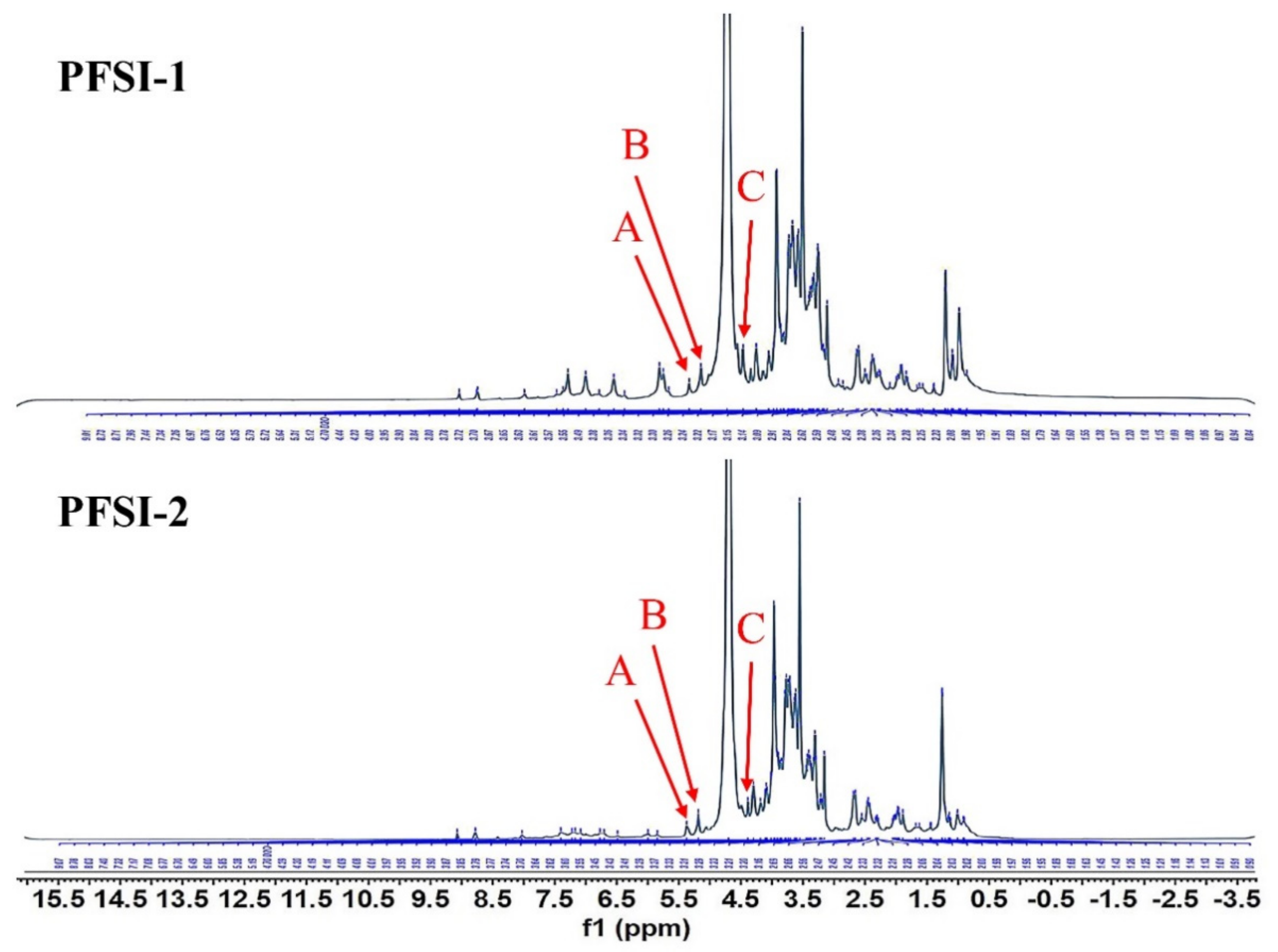
2.2.6. Nuclear Magnetic Resonance Spectroscopy (NMR) Analysis
2.2.7. Cell Viability Assay
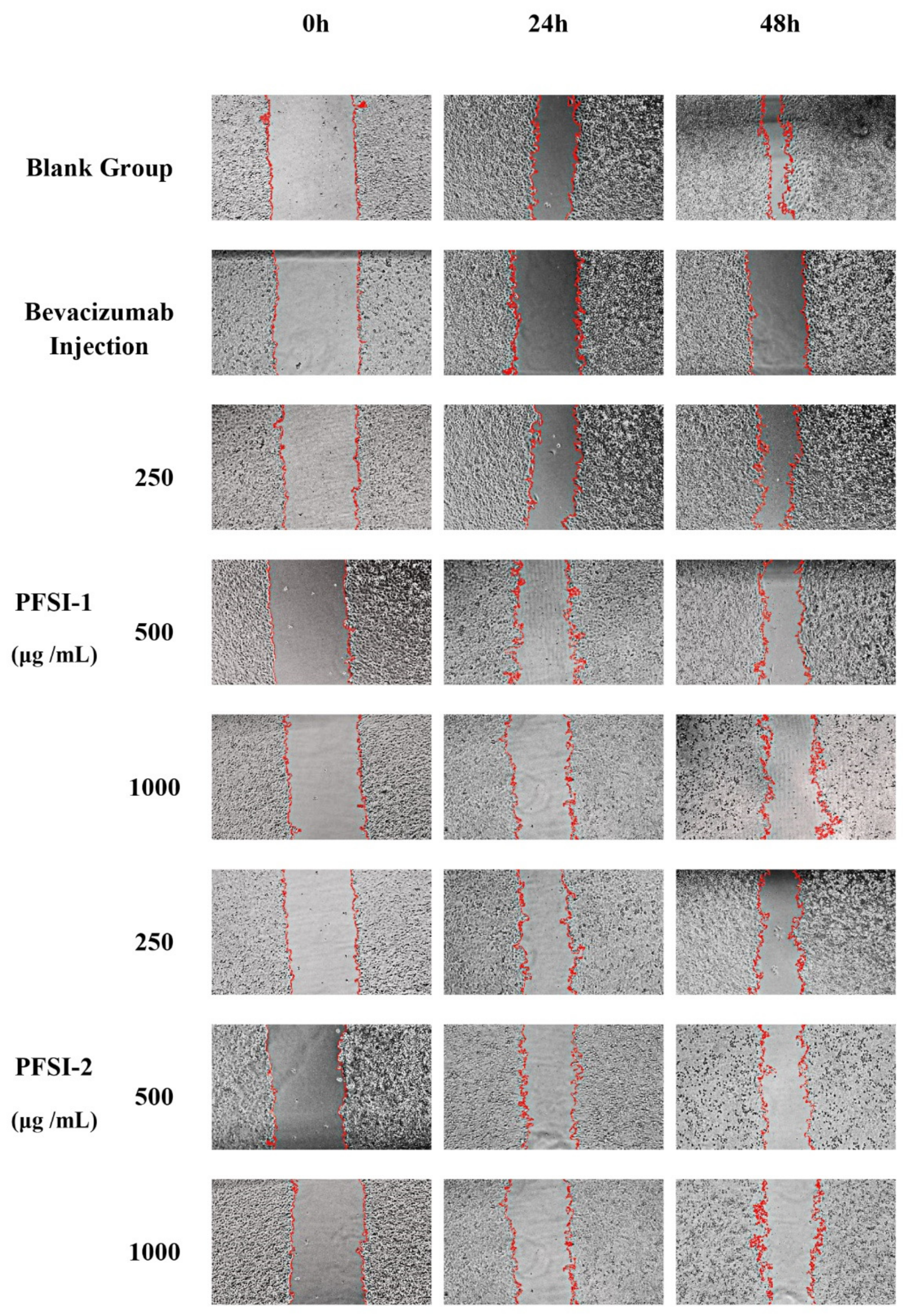
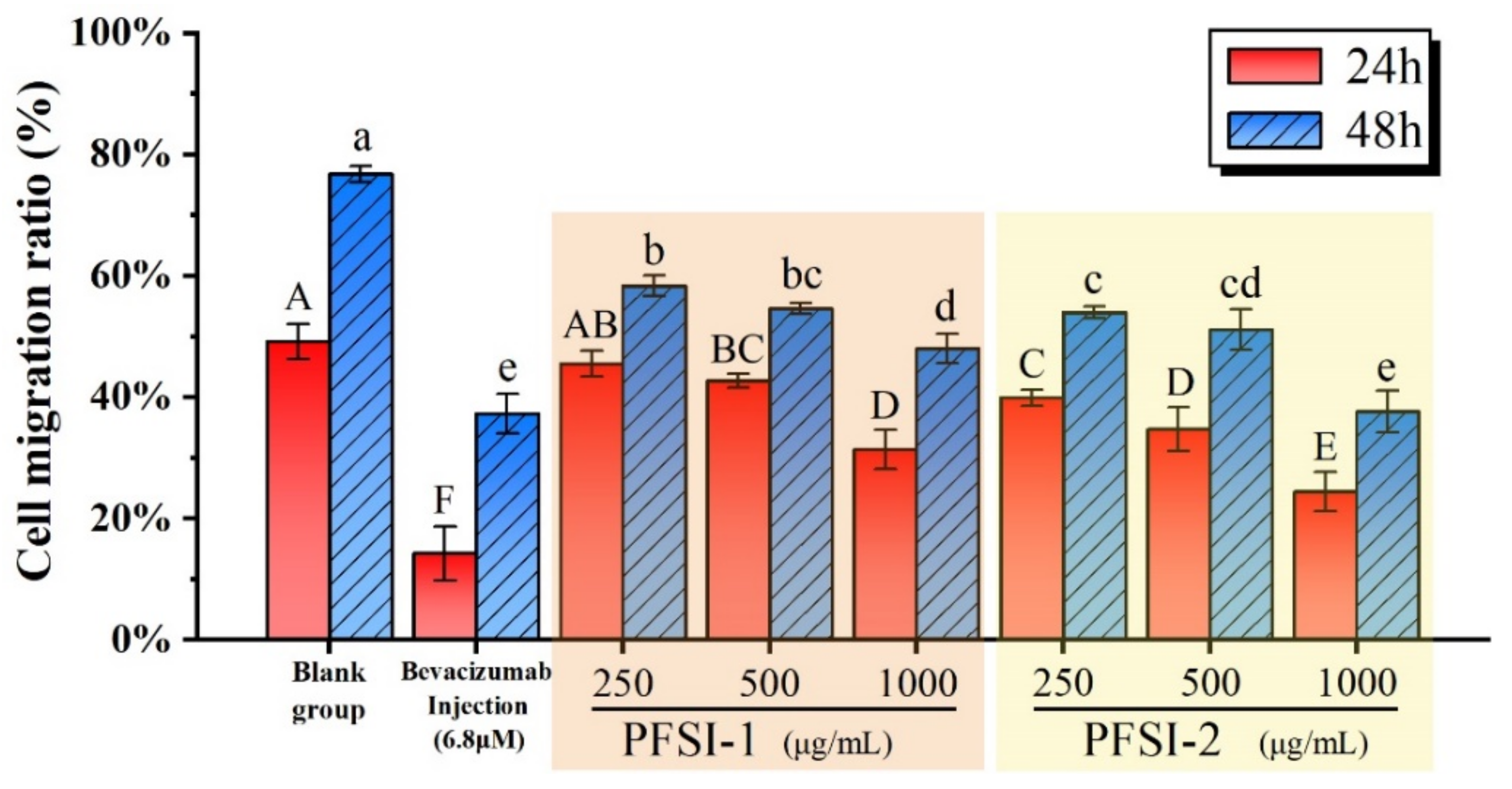
2.2.8. Cell Scratch Wound Healing Assay In Vitro
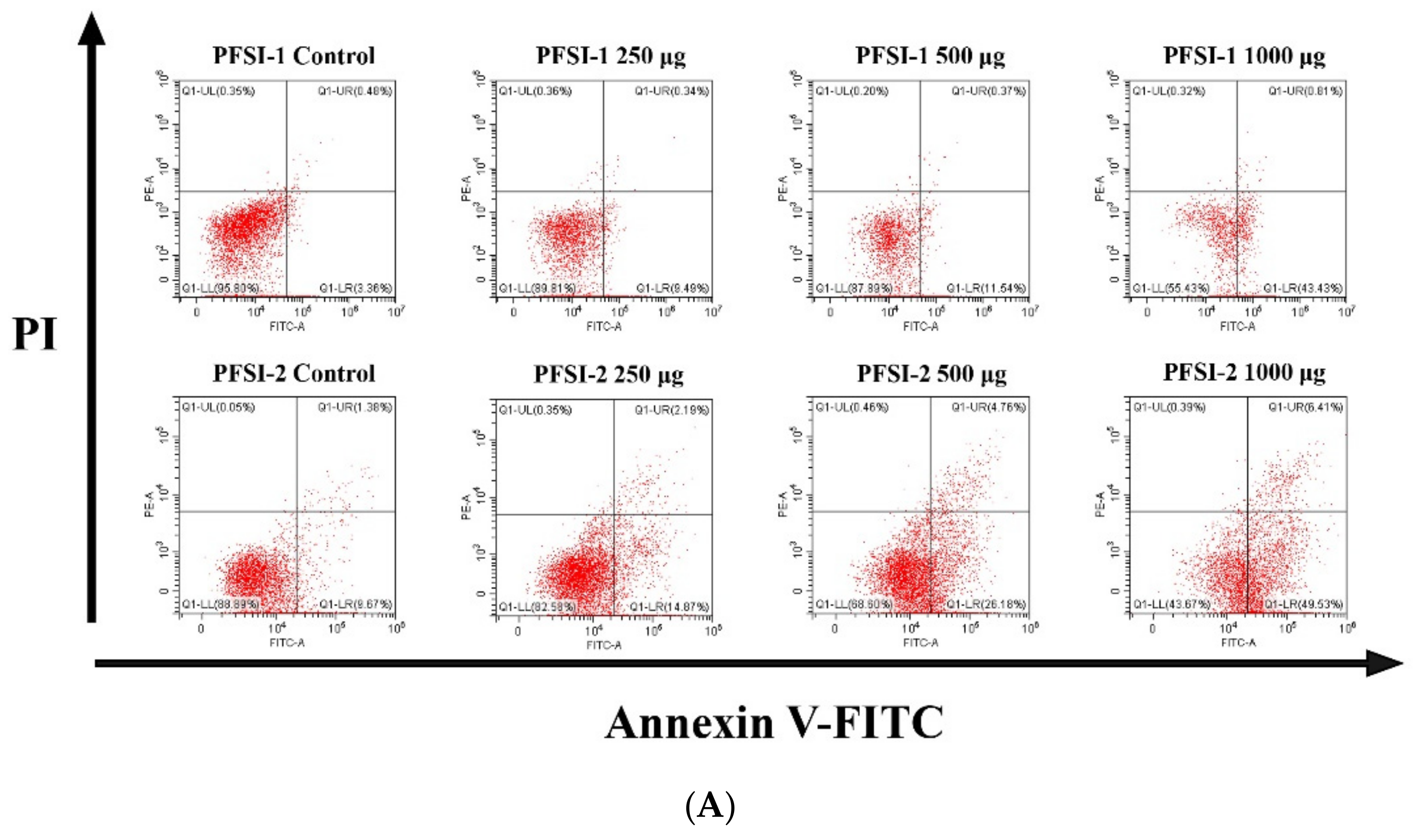
2.2.9. Determination of Apoptosis
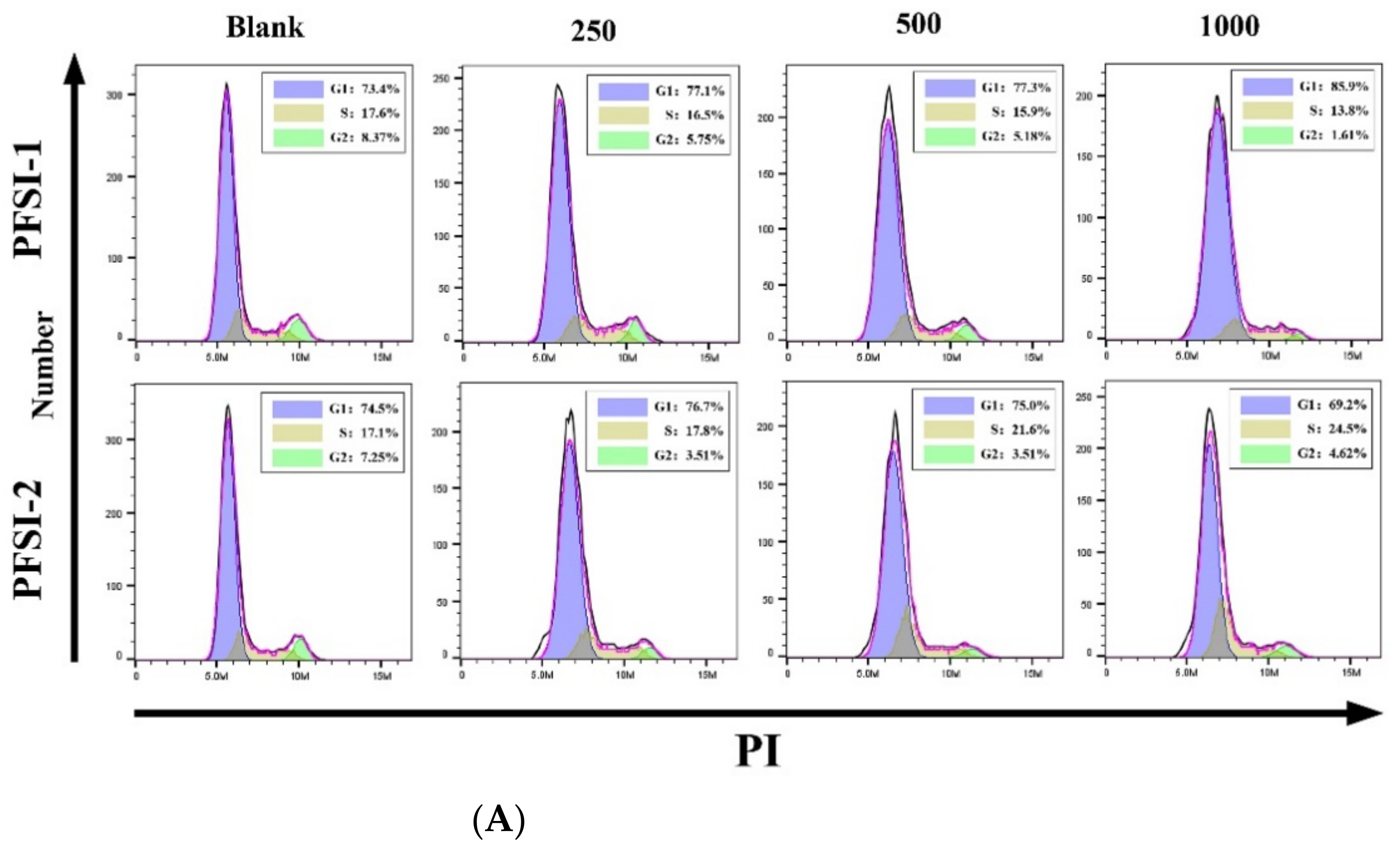
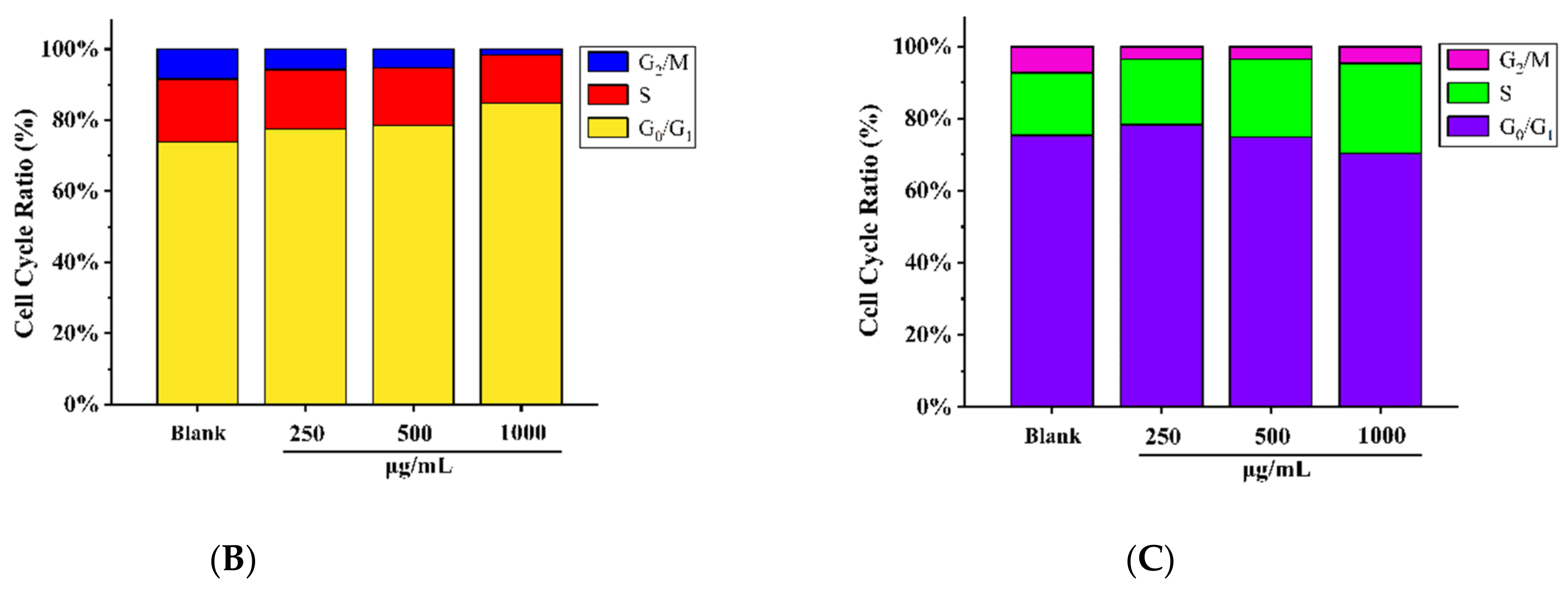
2.2.10. Investigation of Cell Cycle
2.2.11. Statistical Analysis
3. Results
3.1. Monosaccharide Composition of Analysis
3.2. IR of Analysis
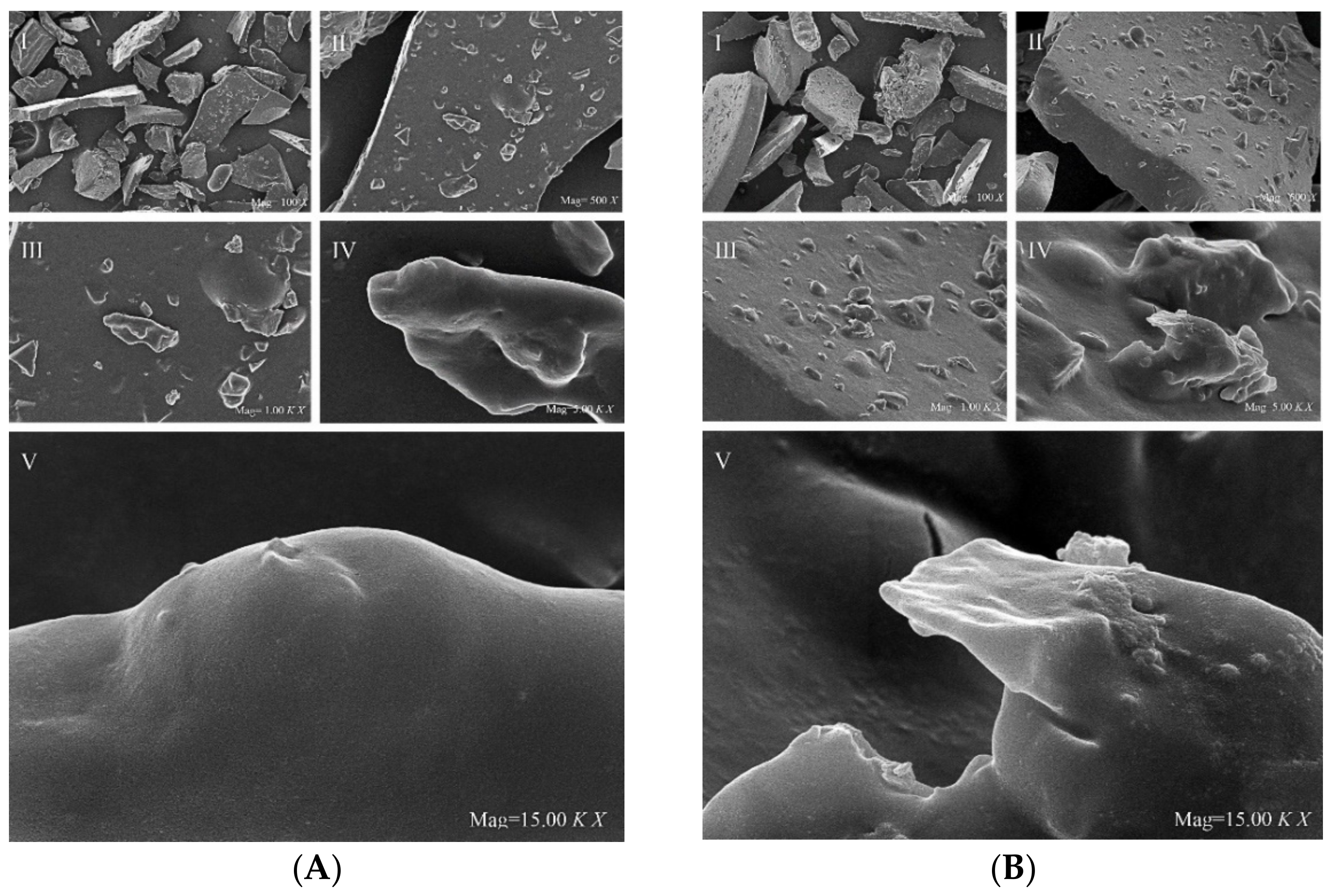
3.3. SEM of Analysis
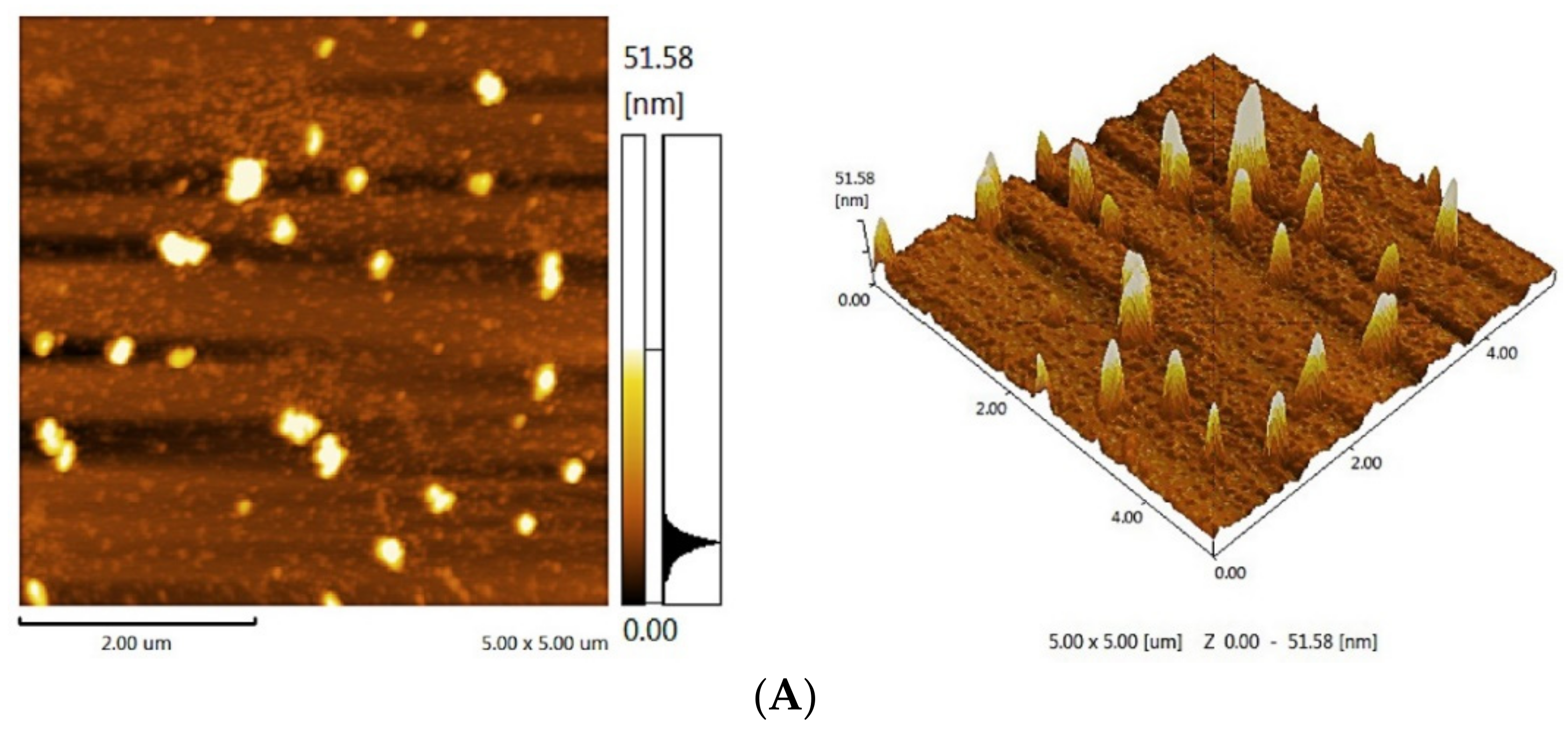
3.4. AFM of Analysis
3.5. NMR of Analysis
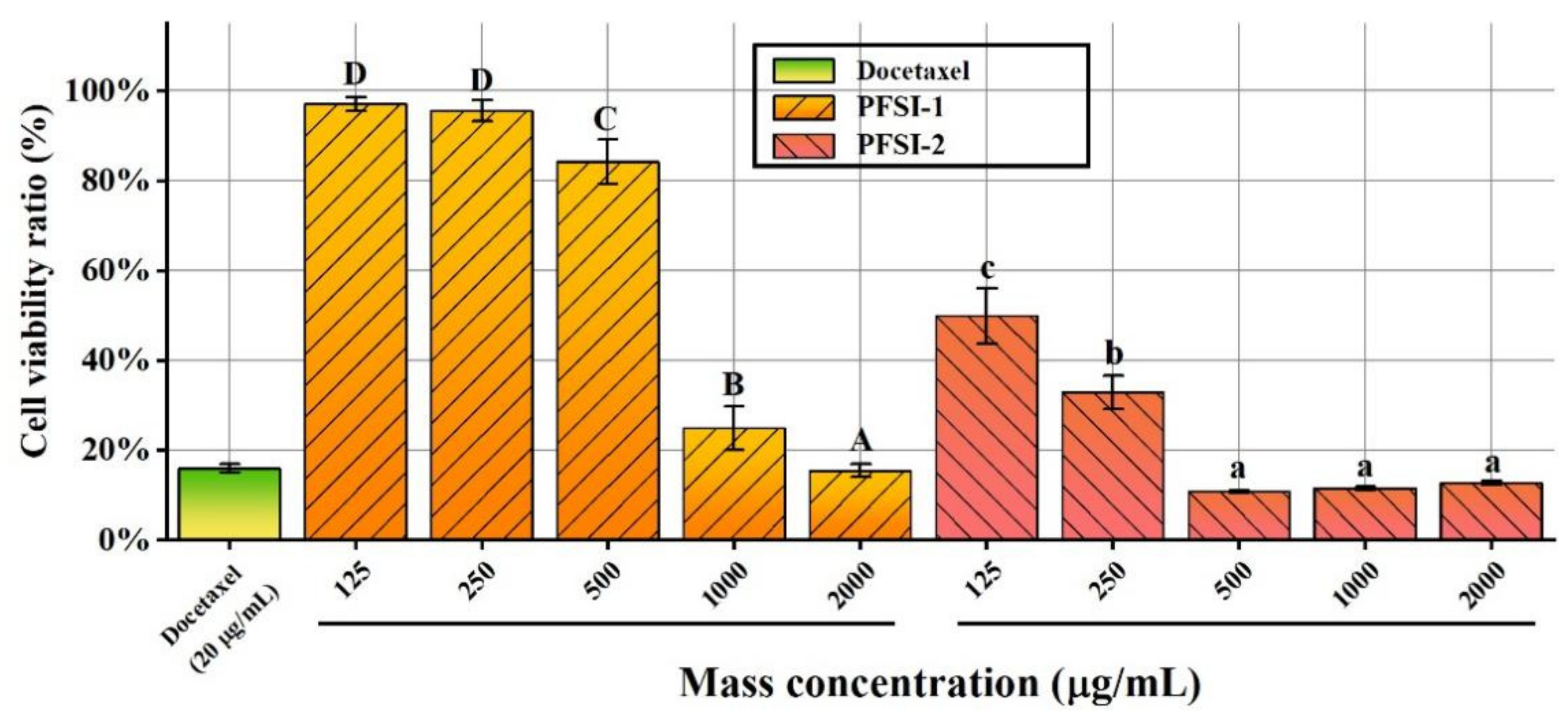
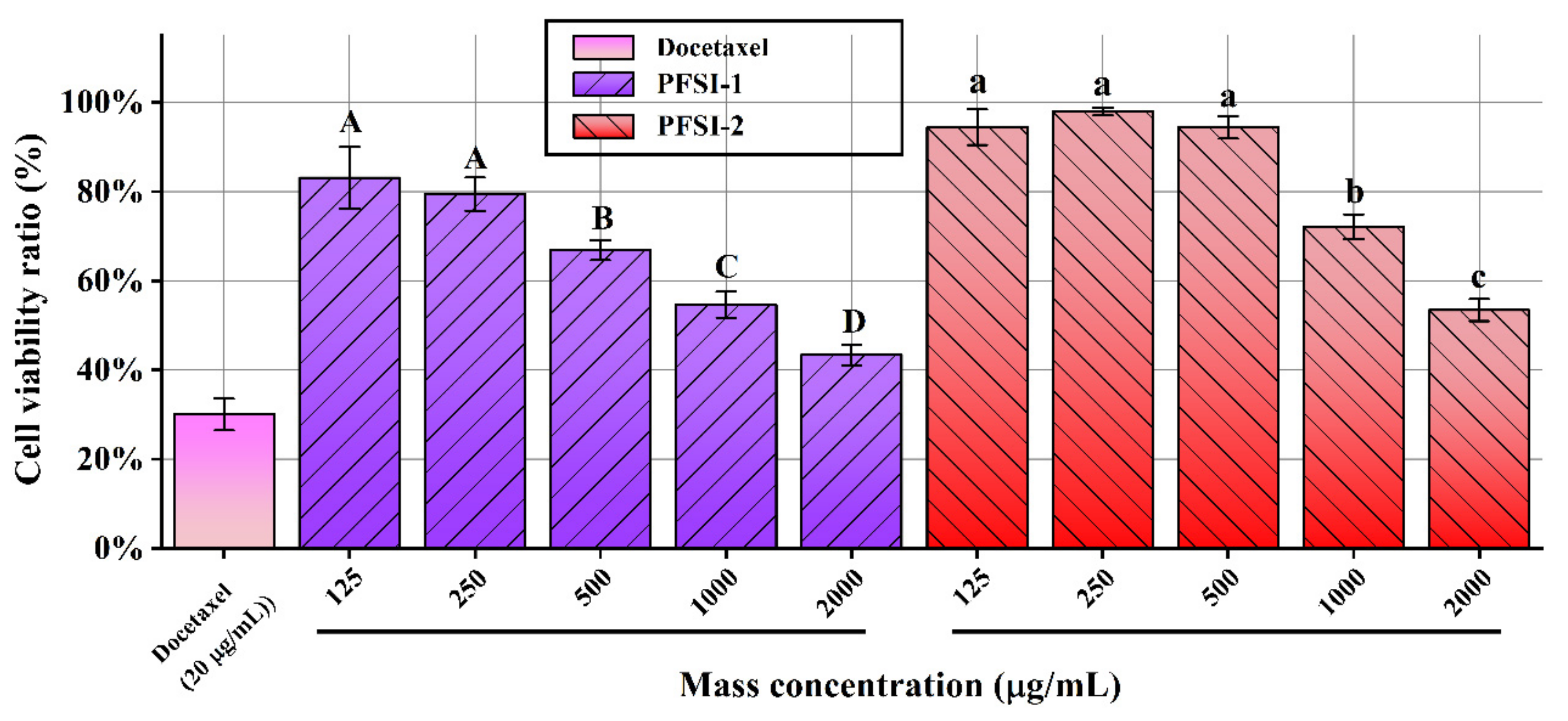
3.6. Cell Viability Assay Analysis
3.6.1. Proliferation Inhibition of SMMC 7721 Cells
3.6.2. Proliferation Inhibition of HL-7702 Cells
3.7. Cell Scratch Wound Healing Assay In Vitro Analysis
3.8. Determination of Apoptosis Analysis
3.9. Determination of Cell Cycle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, W.Y.; Wang, L.R.; Xu, C.J.; Wang, Q.; Zhu, S.S.; Chen, Q.C. Research on the hypoglycemic activity of Sophora japonica L. extract. Shanghai J. Tradit. Chin. Med. 2017, 51, 93–97. [Google Scholar] [CrossRef]
- Li, X.; Li, J. Research progress on the extraction process of rutin from Sophora japonica rice. Mod. Agric. Sci. Technol. 2022, 3, 214–216. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C.; Zhu, F.Y.; Jiamei, Y.L.; Sun, Y.; Wang, Z.Y. Purification of flavonoids from Flos Sophorae Immaturns and their antioxidant activity in vitro. Appl. Chem. Ind. 2021, 50, 2182–2186. [Google Scholar] [CrossRef]
- Wang, R.; Wu, C.R.; Li, B.; Luo, Q.; Feng, Y.; Qin, B. Study on Effects and Mechanism of Sophorae Flos Extract on Exercise Performance of Subacute Aging Model Mice. Tradit. Chin. Drug Res. Clin. Pharmacol. 2021, 32, 1752–1756. [Google Scholar] [CrossRef]
- Zu, X.N.; Wang, J.H.; Yu, W.X. Study on extraction and antibacterial activity of rutin in locust. J. Harbin Univ. Commer. 2018, 34, 648–652. [Google Scholar] [CrossRef]
- Jin, N.; Mao, L.; Zhu, Y.; Zhao, H.; Yu, J.; Zhao, R.; Chen, J.; Tong, J.; Fang, T. Experimental study on the inhibitory effects of quercetin and Flos Sophorae immaturus extract on Lewis lung cancer in mice. J. Nanjing Univ. Tradit. Chin. Med. 2005, 2, 108–110. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, L.; Wang, L.; Li, J. Flos Sophorae immaturus: Phytochemistry, bioactivities, and its potential applications. Food Rev. Int. 2021, 1–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Zhao, C.; Gong, X.; Zhou, X. Research progress on anti-hepatocellular carcinoma mechanism of active ingredients of traditional Chinese medicine. China J. Chin. Mater. Med. 2020, 45, 3395–3406. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, B.; Zhang, F.; Santhanam, R.K.; Wang, X.; Lu, J. Extraction, Structural Characterization, and Anti-Hepatocellular Carcinoma Activity of Polysaccharides from Panax ginseng Meyer. Front. Oncol. 2021, 11, 785455. [Google Scholar] [CrossRef]
- Li, A.; Shuai, X.; Jia, Z.; Li, H.; Liang, X.; Su, D.; Guo, W. Ganoderma lucidum polysaccharide extract inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation and function by inducing microRNA-125b. J. Transl. Med. 2015, 13, 100. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Shi, S.; Wang, H.; Li, N.; Su, J.; Chou, G.; Wang, S. A Homogeneous Polysaccharide from Fructus Schisandra chinensis (Turz.) Baill Induces Mitochondrial Apoptosis through the Hsp90/AKT Signalling Pathway in HepG2 Cells. Int. J. Mol. Sci. 2016, 17, 1015. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Xia, W.; Wei, J.; Ding, X. Therapeutic Effect of Astragalus Polysaccharides on Hepatocellular Carcinoma H22-Bearing Mice. Dose Response 2017, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, J.; Feng, Y.; Weng, L.; Zhao, G.; Xiang, J.; Zhang, M.; Xing, D. Targeting of growth factors in the treatment of hepatocellular carcinoma: The potentials of polysaccharides (Review). Oncol. Lett. 2017, 13, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Xiang, Y.; Lin, S.; Xin, D.; Liu, X.; Weng, L.; Chen, T.; Zhang, M. Anticancer effects of deproteinized asparagus polysaccharide on hepatocellular carcinoma in vitro and in vivo. Tumor Biol. 2014, 35, 3517–3524. [Google Scholar] [CrossRef]
- Ren, F.; Li, J.; Yuan, X.; Wang, Y.; Wu, K.; Kang, L.; Luo, Y.; Zhang, H.; Yuan, Z. Dandelion polysaccharides exert anticancer effect on Hepatocellular carcinoma by inhibiting PI3K/AKT/mTOR pathway and enhancing immune response. J. Funct. Foods 2019, 55, 263–274. [Google Scholar] [CrossRef]
- Zong, S.; Li, J.; Yang, L.; Huang, Q.; Ye, Z.; Hou, G.; Ye, M. Synergistic antitumor effect of polysaccharide from Lachnum sp. in combination with cyclophosphamide in hepatocellular carcinoma. Carbohydr. Polym. 2018, 196, 33–46. [Google Scholar] [CrossRef]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer. 2020, 1873, 188314. [Google Scholar] [CrossRef]
- Xu, X.J.; Cai, Y.X.; Mao, Y.; Xu, F.; Liu, X.X.; He, J.H. Study on Anti-tumor Effect of Pseudobulbus Cremastrae Seu Pleiones Polysaccharide on Mice with H22 Hepatocellular Carcinoma. Food Res. Dev. 2015, 36, 23–25. [Google Scholar] [CrossRef]
- Zhao, B.J.; Guo, Y.B. Progress in the extraction and purification of polysaccharides from edible mushrooms and their biological activities. China Biotechnol. 2022, 42, 146–159. [Google Scholar] [CrossRef]
- Kucukler, S.; Benzer, F.; Yildirim, S.; Gur, C.; Kandemir, F.M.; Bengu, A.S.; Ayna, A.; Caglayan, C.; Dortbudak, M.B. Protective effects of chrysin against oxidative stress and inflammation induced by lead acetate in rat kidneys: A biochemical and histopathological approach. Biol. Trace Elem. Res. 2020, 199, 1501–1514. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Ma, L.J.; Hu, L.; Li, J. Inhibition effects and mechanisms of sophora flower bud abstract on tumor in S180 tumor-bearing mice. Pharmacol. Clin. Chin. Mater. Med. 2014, 30, 100–102. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, Y.; Wu, Y.; Liu, H.; Liu, Q.; Kang, Z.; Wu, M.; Yin, H.; Duan, J. A homogeneous polysaccharide from Lycium barbarum: Structural characterizations, anti-obesity effects and impacts on gut microbiota. Int. J. Biol. Macromol. 2021, 183, 2074–2087. [Google Scholar] [CrossRef]
- Katja, S.; Nataša, U.; Darja, K.; Samo, K. Simple method for the determination of polysaccharides in herbal syrup. J. Carbohydr. Chem. 2018, 37, 7–8. [Google Scholar] [CrossRef]
- Cai, W.T. The comparision of MTT and CCK-8 by detecting cytotoxicity of antiviral active components in traditional Chinese medicine. J. Hubei Univ. Nat. Sci. 2017, 39, 305–310. [Google Scholar] [CrossRef]
- Xiang, A.; Ling, C.; Zhang, W.; Chen, H. Effects of Rhizopus Nigricans Exopolysaccharide on Proliferation, Apoptosis, and Migration of Breast Cancer MCF-7 Cells and Akt Signaling Pathway. Int. J. Polym. Sci. 2021, 2021, 5621984. [Google Scholar] [CrossRef]
- Hu, N.; Gao, Z.; Cao, P.; Song, H.; Hu, J.; Qiu, Z.; Chang, C.; Zheng, G.; Shan, X.; Meng, Y. Uniform and disperse selenium nanoparticles stabilized by inulin fructans from Codonopsis pilosula and their anti-hepatoma activities. Int. J. Biol. Macromol. 2022, 203, 105–115. [Google Scholar] [CrossRef]
- Wang, L.; Mao, Y.; Zeng, X.; Liu, N.; Niu, C.; Li, X.; Ma, B.; Guo, L.; Yang, X. Structure and Bioactivities of a Novel Polysaccharide Extracted from Dendrobium huoshanense by Subcritical Water. Front. Nutr. 2022, 9, 877871. [Google Scholar] [CrossRef]
- Ren, Y.; Bai, Y.; Zhang, Z.; Cai, W.; Del Rio Flores, A. The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef]
- Li, J.; Qi, H.; Xia, H.; Xu, H. Comparison of Deproteinization Methods for Onion Crude Polysaccharide. J. Henan Agric. Sci. 2015, 44, 164–168. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, R.; Chen, J.; Luo, Z.G. Structural characterization, anticancer, hypoglycemia and immune activities of polysaccharides from Russula virescens. Int. J. Biol. Macromol. 2021, 184, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, F. Research Progress on the Anticancer Activities and Mechanisms of Polysaccharides from Ganoderma. Front. Pharmacol. 2022, 13, 891171. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, C.; Georgiev, M.I.; Bajpai, V.K.; Tundis, R.; Simal-Gandara, J.; Lu, X.; Xiao, J.; Tang, X.; Qiao, X. Advances in dietary polysaccharides as anticancer agents: Structure-activity relationship. Trends Food Sci. Technol. 2021, 111, 360–377. [Google Scholar] [CrossRef]
- Zhang, G.F.; Liu, C.; Liu, G.D.; Zhang, R.T. Advances in Antitumor Effects and Structure-activity Relationship of Plant Polysaccharides. Sci. Technol. Food Ind. 2022, 1–21. [Google Scholar] [CrossRef]
- Bian, L.; Chen, H.G.; Zhou, X. Recent Advances in Understanding the Antitumor Activity of Polysaccharides from Plants. Food Sci. 2020, 41, 275–282. [Google Scholar]
- Sajadimajd, S.; Momtaz, S.; Haratipour, P.; El-Senduny, F.F.; Panah, A.I.; Navabi, J.; Zhaleh, S.; Farzaei, M.H.; Rahimi, R. Molecular Mechanisms Underlying Cancer Preventive and Therapeutic Potential of Algal Polysaccharides. Curr. Pharm. Des. 2019, 25, 1210–1235. [Google Scholar] [CrossRef]
- Yarley, O.P.; Kojo, A.B.; Zhou, C.; Yu, X.; Gideon, A.; Kwadwo, H.H.; Richard, O. Reviews on mechanisms of in vitro antioxidant, antibacterial and anticancer activities of water-soluble plant polysaccharides. Int. J. Biol. Macromol. 2021, 183, 2262–2271. [Google Scholar] [CrossRef]
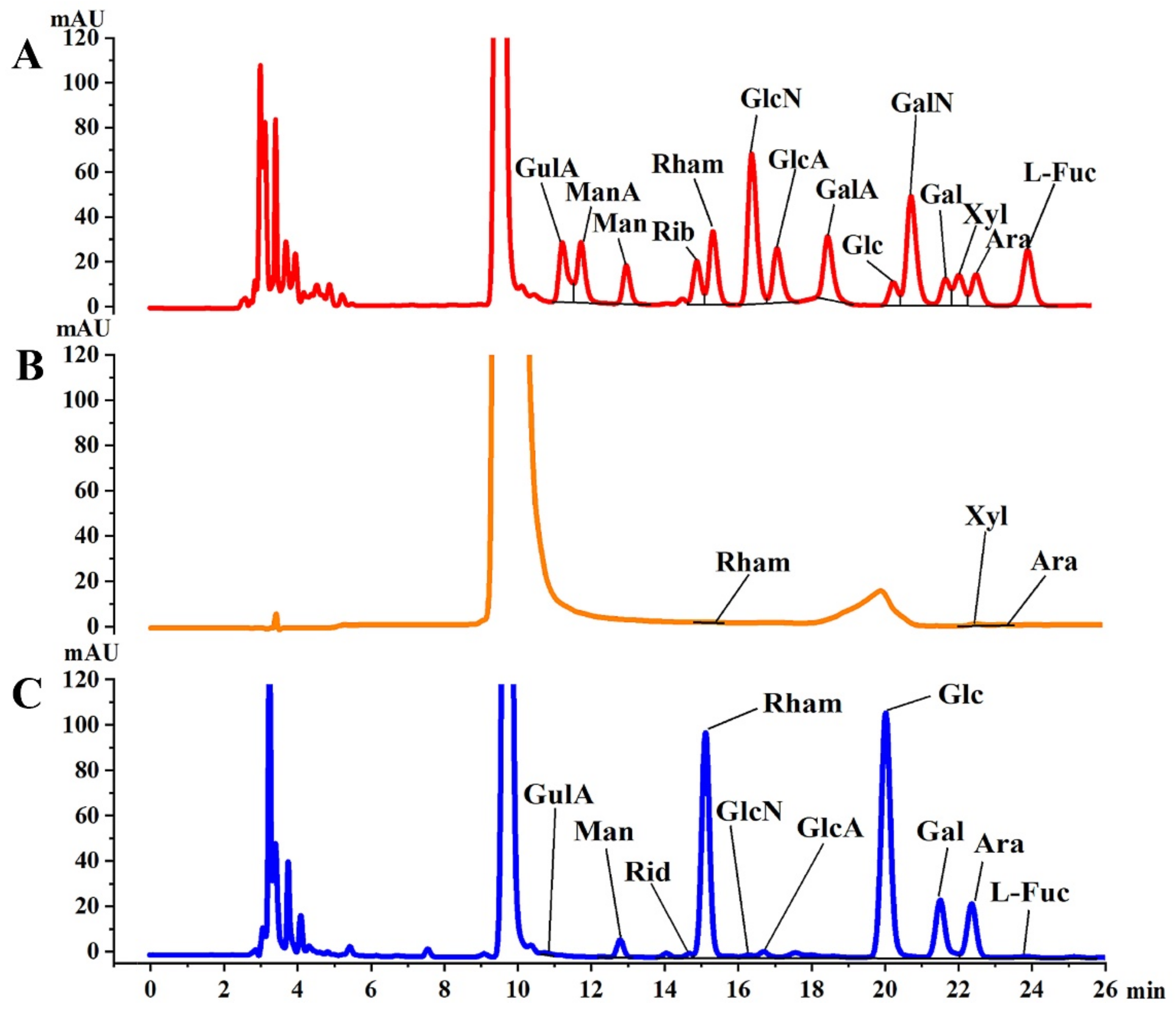



| Monosaccharides | Molar Percentage % | |
|---|---|---|
| PFSI-1 | PFSI-2 | |
| Gulonic acid | - | 0.04 |
| Mannuronic acid | - | - |
| Mannose | - | 2.19 |
| Ribose | - | 0.44 |
| Rhamnose | 8.28 | 16.95 |
| Glucosamine | - | 0.07 |
| Glucuronic acid | - | 0.48 |
| Galacturonic acid | - | - |
| Glucose | - | 58.01 |
| Galactosamine | - | - |
| Galactose | - | 11.62 |
| Xylose | 70.10 | - |
| Arabinose | 21.62 | 10.00 |
| Fucose | - | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, W.; Yang, C.; Zhang, Y.; Liu, Y.; Yang, D. The Chemical Profiling and Anticancer Potential of Functional Polysaccharides from Flos Sophorae Immaturus. Molecules 2022, 27, 5978. https://doi.org/10.3390/molecules27185978
Zhong W, Yang C, Zhang Y, Liu Y, Yang D. The Chemical Profiling and Anticancer Potential of Functional Polysaccharides from Flos Sophorae Immaturus. Molecules. 2022; 27(18):5978. https://doi.org/10.3390/molecules27185978
Chicago/Turabian StyleZhong, Wenting, Chunmiao Yang, Yongze Zhang, Yumeng Liu, and Dongsheng Yang. 2022. "The Chemical Profiling and Anticancer Potential of Functional Polysaccharides from Flos Sophorae Immaturus" Molecules 27, no. 18: 5978. https://doi.org/10.3390/molecules27185978
APA StyleZhong, W., Yang, C., Zhang, Y., Liu, Y., & Yang, D. (2022). The Chemical Profiling and Anticancer Potential of Functional Polysaccharides from Flos Sophorae Immaturus. Molecules, 27(18), 5978. https://doi.org/10.3390/molecules27185978





