PLGA-PVA-PEG Single Emulsion Method as a Candidate for Aminolevulinic Acid (5-ALA) Encapsulation: Laboratory Scaling Up and Stability Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Particle Development
2.3. System Characterization
2.3.1. Physical Properties
2.3.2. Ultraviolet-Visible (UV-VIS) Spectroscopy
2.3.3. Nanoparticle Tracking Analysis (NTA)
2.3.4. Dynamic Light Scattering (DLS) and Zeta Potential
2.3.5. Contact Angle and Surface Energy
2.3.6. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.7. Proton Nuclear Magnetic Resonance (1H-NMR)
2.4. System Stability
2.4.1. Forced Stability of 5–ALA
2.4.2. Forced Stability of Nanoemulsions (3000 rpm at 56 °C)
2.4.3. Physicochemical Stability under Storage Conditions
2.5. Data Analysis
3. Results and Discussion
3.1. Particle Development
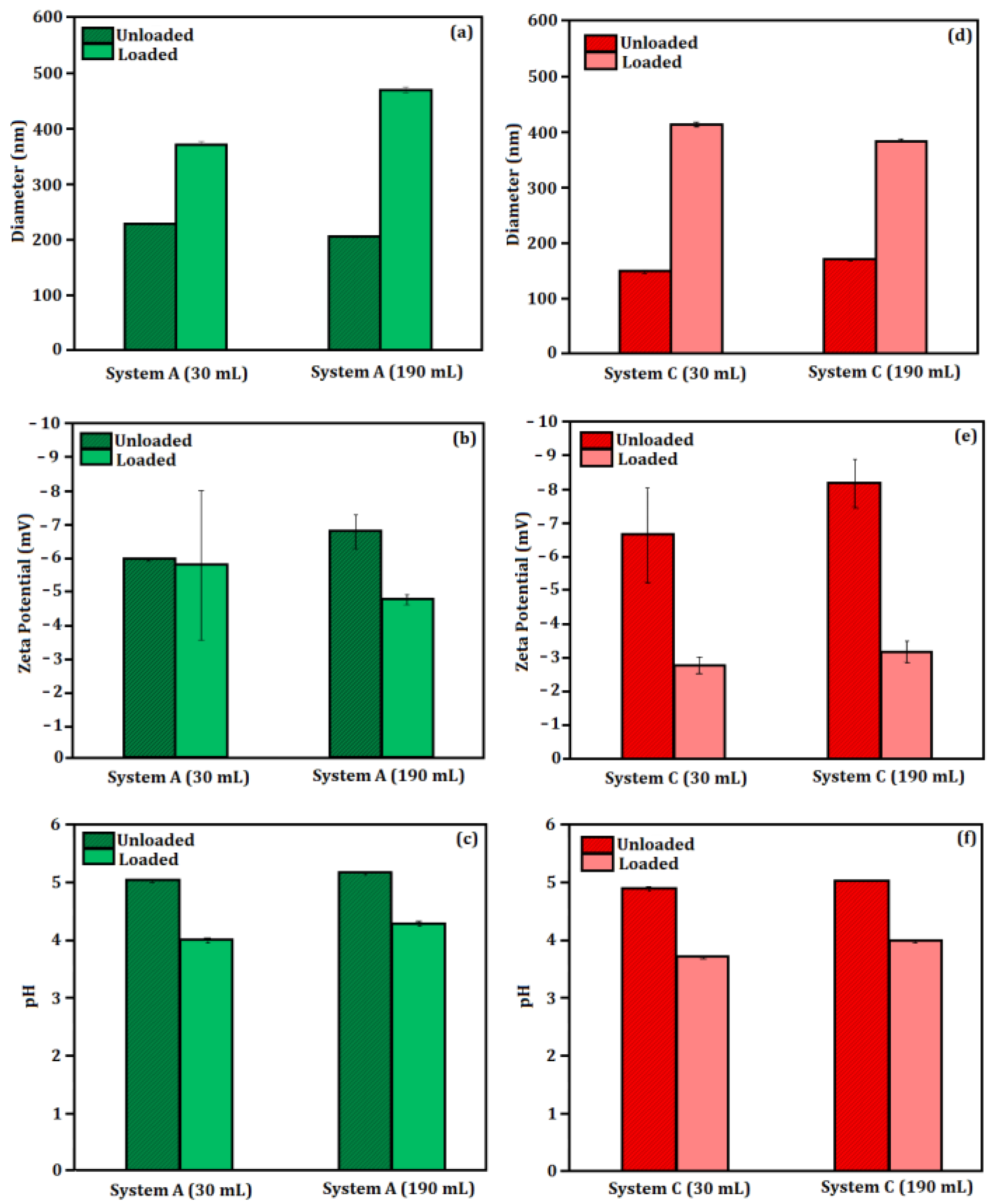
Laboratory Scaling Up
3.2. Characterization
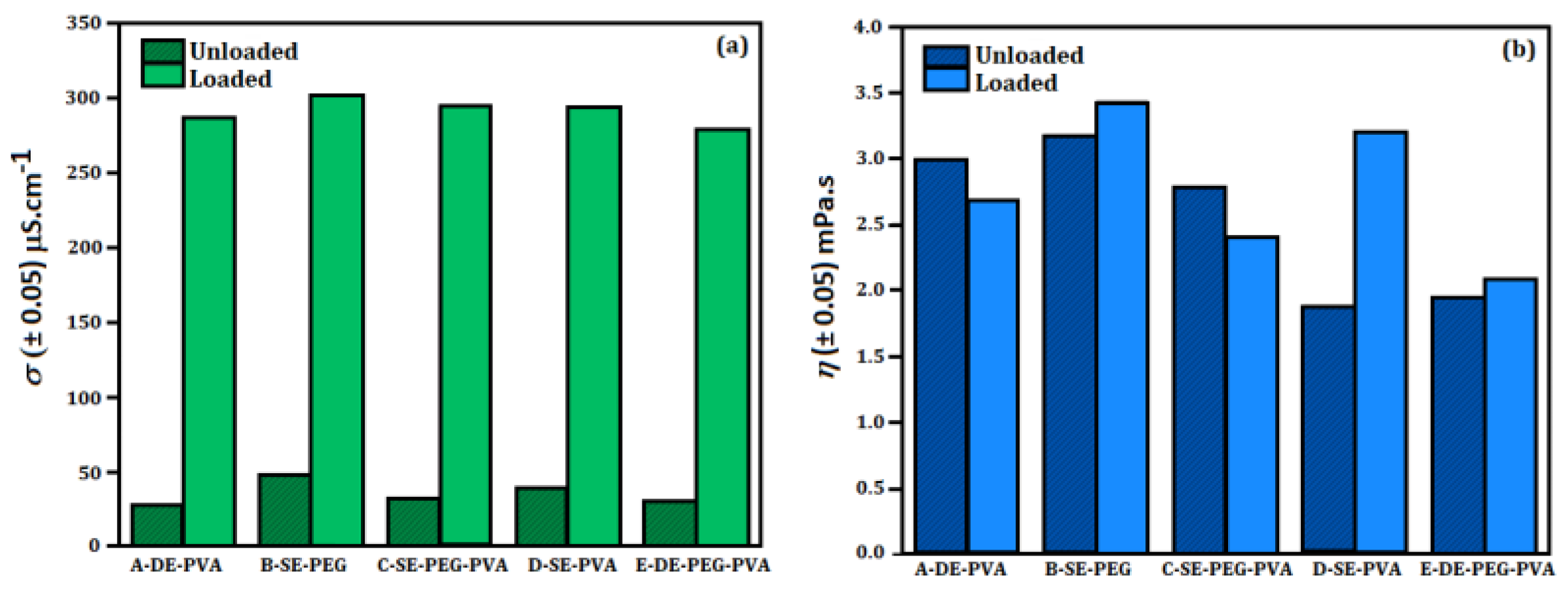
3.2.1. Organoleptic Properties, Electrical Conductivity, Relative Density and Dynamic Viscosity
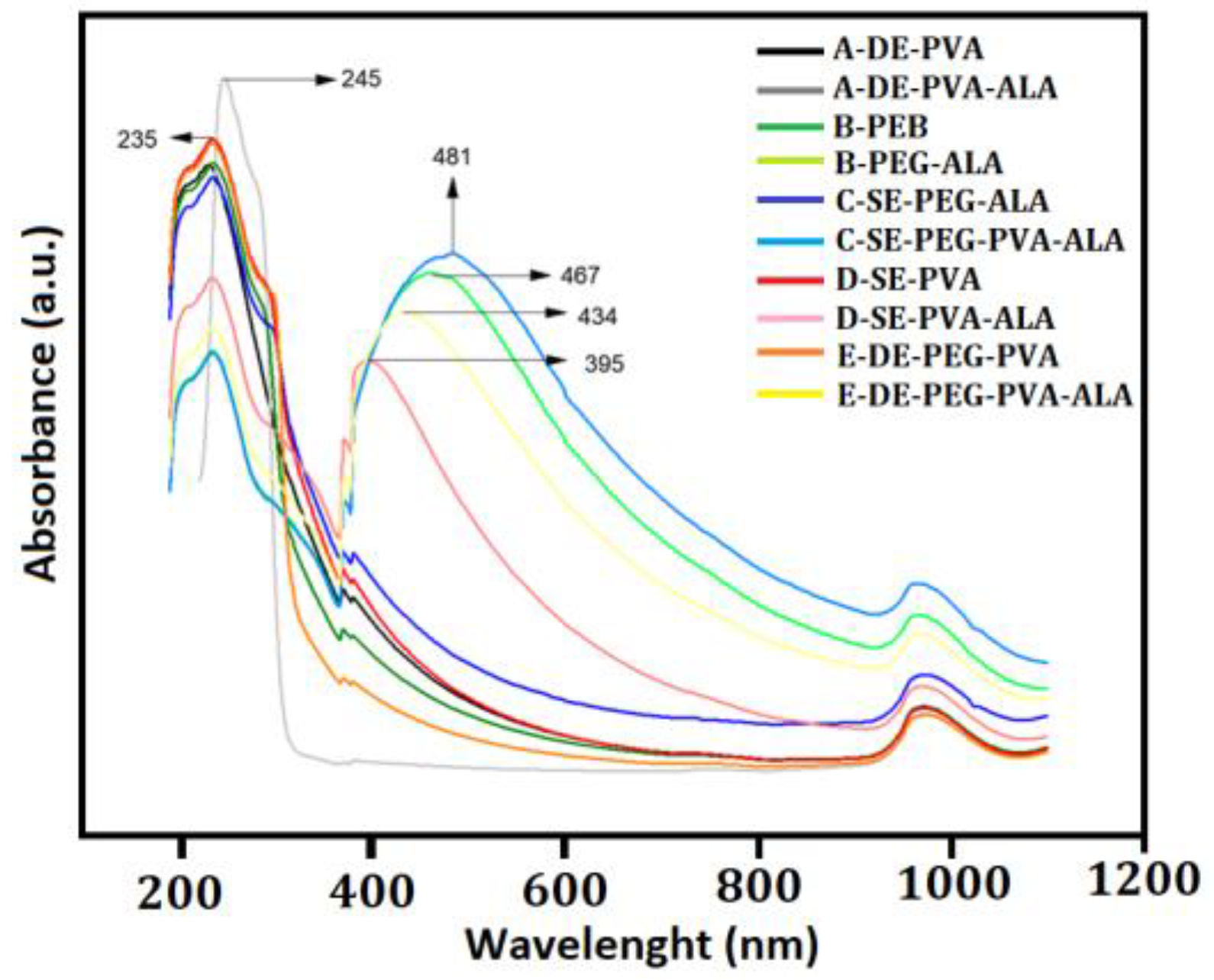
3.2.2. UV-VIS Spectroscopy
3.2.3. Nanoparticle Tracking Analysis (NTA)
3.2.4. Contact Angle and Surface Energy
3.2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
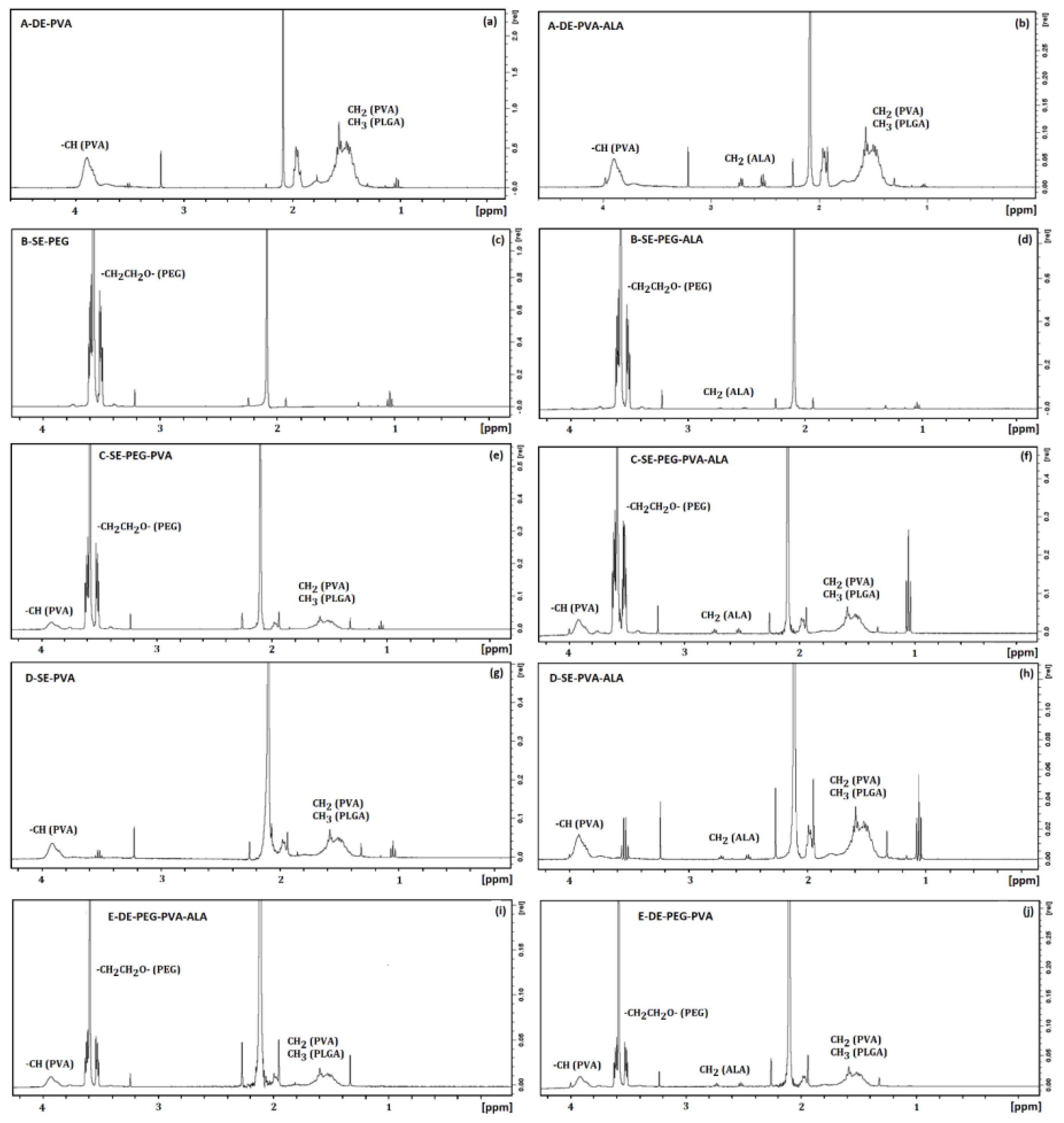
3.2.6. H-RMN Analysis
3.2.7. Forced Stability of 5-ALA
3.2.8. Forced Stability of Nanoemulsions (3000 rpm and 56 °C)
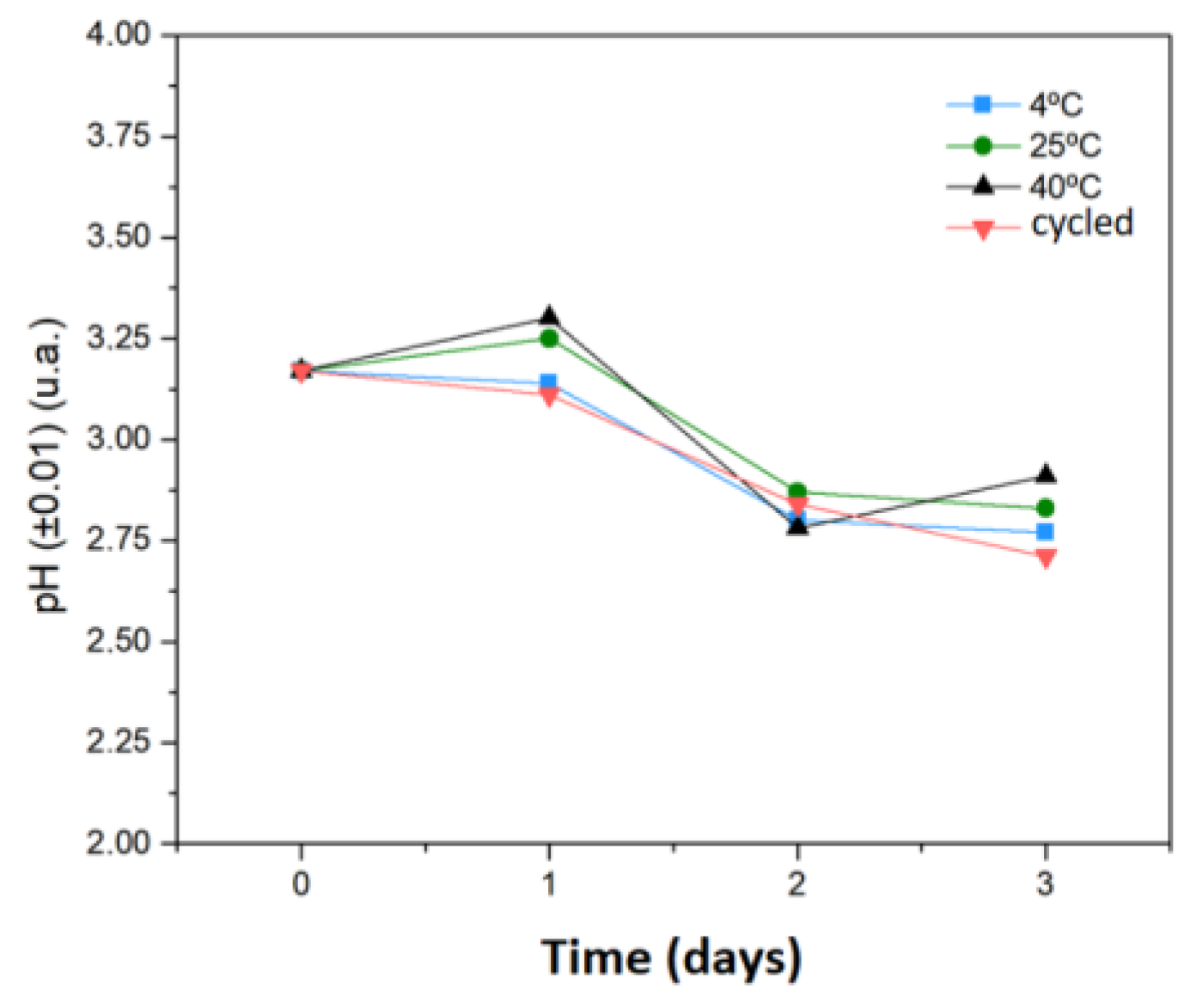
3.2.9. Influence of Storage Temperature on Physicochemical Stability
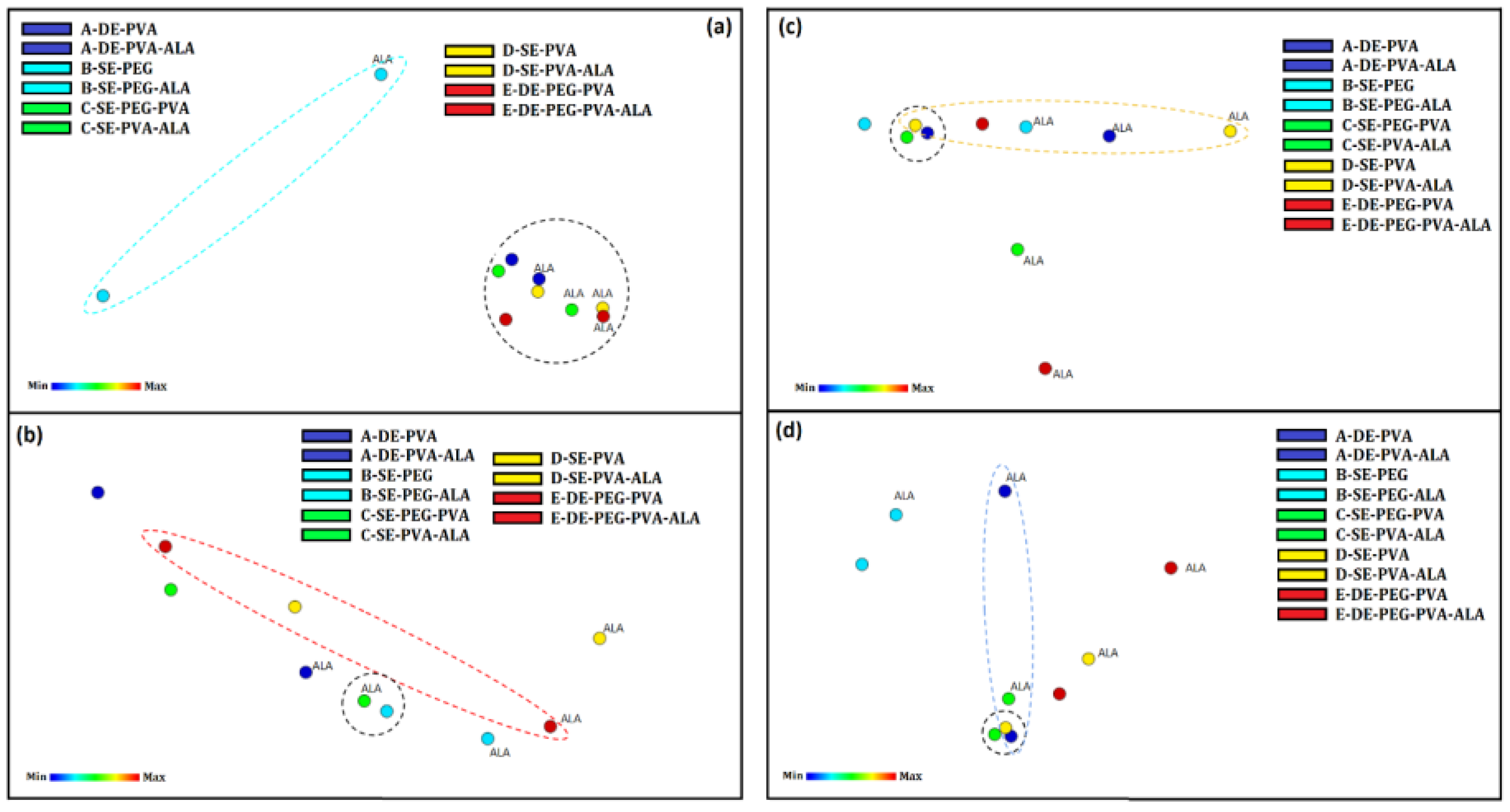
3.2.10. IDMAP Tool Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- INCA—Instituto Nacional de Câncer Tipos de Câncer—Câncer de Pele. Available online: https://www.inca.gov.br/assuntos/cancer-de-pele (accessed on 15 August 2022).
- De Souza, R.J.S.A.P.; Mattedi, A.P.; Corrêa, M.P.; Rezende, M.L.; Ferreira, A.C.A. Estimativa do custo do tratamento do câncer de pele tipo não-melanoma no estado de São Paulo—Brasil. An. Bras. Dermatol. 2011, 86, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of third generation photosensitizers used in anticancer photodynamic therapy: Review. Photodiagn. Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef] [PubMed]
- Hamdoon, Z.; Jerjes, W.; Rashed, D.; Kawas, S.; Sattar, A.A.; Samsudin, R.; Hopper, C. In vivo optical coherence tomography-guided photodynamic therapy for skin pre-cancer and cancer. Photodiagn. Photodyn. Ther. 2021, 36, 102520. [Google Scholar] [CrossRef] [PubMed]
- Sharman, W.M.; Allen, C.M.; Van Lier, J.E. Photodynamic therapeutics: Basic principles and clinical applications. Drug Discov. Today 1999, 4, 507–517. [Google Scholar] [CrossRef]
- Kennedy, J.C.; Pottier, R.H.; Pross, D.C. Photodynamic therapy with endogenous protoporphyrin IX: Basic principles and present clinical experience. J. Photochem. Photobiol. B Biol. 1990, 6, 143–148. [Google Scholar] [CrossRef]
- Elfsson, B.; Wallin, I.; Eksborg, S.; Rudaeus, K.; Ros, A.M.; Ehrsson, H. Stability of 5-aminolevulinic acid in aqueous solution. Eur. J. Pharm. Sci. 1999, 7, 87–91. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photossensitizersfot photodynamic therapy. Biochem. J. 2017, 473, 347–364. [Google Scholar] [CrossRef]
- Dianzani, C.; Zara, G.P.; Maina, G.; Pettazzoni, P.; Pizzimenti, S.; Rossi, F.; Gigliotti, C.L.; Ciamporcero, E.S.; Daga, M.; Barrera, G. Drug delivery nanoparticles in skin cancers. BioMed Res. Int. 2014, 2014, 895986. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Issadore, D.; Mitchell, M.J. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials 2021, 274, 120826. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, G.; Liu, C.; Fu, J.; Hu, D.; Rong, J.; Yang, X. Recent progress in the preparation, chemical interactions and applications of biocompatible polysaccharide-protein nanogel carriers. Food Res. Int. 2021, 147, 110564. [Google Scholar] [CrossRef]
- Cortés-Morales, E.A.; Mendez-Montealvo, G.; Velazquez, G. Interactions of the molecular assembly of polysaccharide-protein systems as encapsulation materials. A review. Adv. Colloid Interface Sci. 2021, 295, 102398. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Pereira, M.C. PLGA Based drug carrier and pharmaceutical applications: The most recent advances. Pharmaceutics 2020, 12, 903. [Google Scholar] [CrossRef]
- Wang, Y. FDA’s Regulatory Science Program for Generic PLA/PLGA Based Drug Products. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/188841-FDA-s-Regulatory-Science-Program-for-Generic-PLA-PLGA-Based-Drug-Products/ (accessed on 15 August 2022).
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Roca, M.F. Biofrontera Bioscience GmbH. US Patent US 2009/0186126 A1, 30 December 2009. [Google Scholar]
- Cerize, N.N.P.; de Oliveira, A.M.; Ré, M.I.; Tedesco, A.C. Nanocarreadores Coloidais para Tivos Hidrofílicos e Processo de Produção. Patent WO2011156880A1, 22 December 2011. [Google Scholar]
- Torres, B.B.M. Filmes Finos do Ácido Poli 3-tiofeno Acético. Master’s Thesis, Instituto de Física e Química de São Carlos da Universidade de São Paulo, São Carlos, Brazil, 2011; 108p. [Google Scholar]
- ANVISA. Guia de Estabilidade de Produtos Cosméticos, 1st ed.; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2004; Volume 1, ISBN 8588233150. [Google Scholar]
- Duvall, M.N.; Knight, K. FDA Regulation of Nanotechnology; Food Drug Administration: Silver Spring, MD, USA, 2011. [Google Scholar]
- Paulovich, F.V.; Moraes, M.L.; Maki, R.M.; Ferreira, M.; Oliveira, O.N., Jr.; de Oliveira, M.C.F. Information visualization techniques for sensing and biosensing. Analyst 2011, 136, 1344–1350. [Google Scholar] [CrossRef]
- Cancino, J.; Marangoni, V.S.; Zucolotto, V. Nanotechnology in medicine: Concepts and concerns. Quim. Nova 2014, 37, 521–526. [Google Scholar] [CrossRef]
- Shi, L.; Wang, X.; Zhao, F.; Luan, H.; Tu, Q.; Huang, Z.; Wang, H.H.; Wang, H.H. In vitro evaluation of 5-aminolevulinic acid (ALA) loaded PLGA nanoparticles. Int. J. Nanomed. 2013, 8, 2669–2676. [Google Scholar] [CrossRef]
- Mishra, P.; Nayak, B.; Dey, R.K. PEGylation in anti-cancer therapy: An overview. Asian J. Pharm. Sci. 2016, 11, 337–348. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Nimesh, S. Poly(D,L-lactide-co-glycolide)-based nanoparticles. In Gene Therapy: Potential Applications of Nanotechnology; Woodhead Publishing: Sawston, UK, 2013; pp. 309–329. ISBN 9781908818645. [Google Scholar]
- Grama, C.N.; Venkatpurwar, V.P.; Lamprou, D.A.; Ravi Kumar, M.N.V. Towards scale-up and regulatory shelf-stability testing of curcumin encapsulated polyester nanoparticles. Drug Deliv. Transl. Res. 2013, 3, 286–293. [Google Scholar] [CrossRef]
- Duarte, D.S.; Nascimento, J.A.d.A.; de Britto, D. Scale-up in the synthesis of nanoparticles for encapsulation of agroindustrial active principles. Ciênc. Agrotec. 2019, 43, e02381. [Google Scholar] [CrossRef]
- Ye, Z.; Squillante, E. The development and scale-up of biodegradable polymeric nanoparticles loaded with ibuprofen. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 422, 75–80. [Google Scholar] [CrossRef]
- Vauthier, C.; Bouchemal, K. Methods for the Preparation and Manufacture of Polymeric Nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef]
- Parhizkar, M.; Reardon, P.J.T.; Knowles, J.C.; Browning, R.J.; Stride, E.; Barbara, P.R.; Harker, A.H.; Edirisinghe, M. Electrohydrodynamic encapsulation of cisplatin in poly (lactic-co-glycolic acid) nanoparticles for controlled drug delivery. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1919–1929. [Google Scholar] [CrossRef]
- Rahman, S.M.; Mohd Said, S.B.; Subramanian, B.; Long, B.D.; Kareem, M.A.; Soin, N. Synthesis and Characterization of Polymer Electrolyte Using Deep Eutectic Solvents and Electrospun Poly(vinyl alcohol) Membrane. Ind. Eng. Chem. Res. 2016, 55, 8341–8348. [Google Scholar] [CrossRef]
- Wiedmann, T.S.; Naqwi, A. Pharmaceutical salts: Theory, use in solid dosage forms and in situ preparation in an aerosol. Asian J. Pharm. Sci. 2016, 11, 722–734. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahmad, J.; Hägg, M.B. Fabrication and characterization of polymer blends consisting of cationic polyallylamine and anionic polyvinyl alcohol. Ionics 2014, 20, 957–967. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Sadasivuni, K.K.; Ponnamma, D.; Deshmukh, R.R.; Pasha, S.K.K.; AlMaadeed, M.A.A.; Chidambaram, K. Graphene oxide reinforced polyvinyl alcohol/polyethylene glycol blend composites as high-performance dielectric material. J. Polym. Res. 2016, 23, 159. [Google Scholar] [CrossRef]
- Gupta, R.; Dwadasi, B.S.; Rai, B.; Mitragotri, S. Effect of Chemical Permeation Enhancers on Skin Permeability: In silico screening using Molecular Dynamics simulations. Sci. Rep. 2019, 9, 1456. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim. Biophys. Acta—Biomembr. 2009, 1788, 2362–2373. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, C. Tuning the size of poly(lactic-co-glycolic acid) (PLGA) nanoparticles fabricated by nanoprecipitation. Biotechnol. J. 2018, 13, 1700203. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Deshmukh, R.R.; Bhagat, P.R.; Pasha, S.K.K.; Bhagat, A.; Shirbhate, R.; Telare, F.; Lakhani, C. Influence of K2CrO4 Doping on the Structural, Optical and Dielectric Properties of Polyvinyl Alcohol/K2CrO4 Composite Films. Polym.—Plast. Technol. Eng. 2016, 55, 231–241. [Google Scholar] [CrossRef]
- Mallakpour, S.; Nezamzadeh Ezhieh, A. Preparation and characterization of chitosan-poly(vinyl alcohol) nanocomposite films embedded with functionalized multi-walled carbon nanotube. Carbohydr. Polym. 2017, 166, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, M.A.; Nia Asli, M.A.; Oveisi, M.; Babaei, R.; Qasemi, K.; Khandan, S. An organic-inorganic hybrid based on an Anderson-type polyoxometalate immobilized on PVA as a reusable and efficient nanocatalyst for oxidative desulphurization of gasoline. RSC Adv. 2016, 6, 53069–53079. [Google Scholar] [CrossRef]
- Slepička, P.; Elashnikov, R.; Ulbrich, P.; Staszek, M.; Kolská, Z.; Švorčík, V. Stabilization of sputtered gold and silver nanoparticles in PEG colloid solutions. J. Nanopart. Res. 2015, 17, 11. [Google Scholar] [CrossRef]
- Basu, T.; Pal, B.; Singh, S. Fabrication of core–shell PLGA/PLA–pNIPAM nanocomposites for improved entrapment and release kinetics of antihypertensive drugs. Particuology 2018, 40, 169–176. [Google Scholar] [CrossRef]
- Basu, T.; Pal, B.; Singh, S. Synthesis and Characterization of Ramipril Embedded Nanospheres of Biodegradable Poly-D,L-Lactide-co-Glycolide and Their Kinetic Release Study. Adv. Sci. Eng. Med. 2016, 8, 444–449. [Google Scholar] [CrossRef]
- Yi, S.; Yang, F.; Jie, C.; Zhang, G. A novel strategy to the formulation of carmustine and bioactive nanoparticles co-loaded PLGA biocomposite spheres for targeting drug delivery to glioma treatment and nursing care. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3438–3447. [Google Scholar] [CrossRef] [PubMed]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Malvern Instruments Limited. Comparison of Statistical Measures Reported by NTA and DLS Techniques. Available online: https://www.malvernpanalytical.com/en/learn/knowledge-center/technical-notes/tn160115comparingstatisticalmeasuresntadls (accessed on 15 August 2022).
- Melo, C.C. Nanopartículas de Quitosana como Veículo para Entrega de Oligodeoxiribonucleotídeos Antisense. Master’s Thesis, Instituto de Física de São Carlos da Universidade de São Paulo, São Carlos, Brazil, 2018. [Google Scholar]
- Zhang, L.; Webster, T.J. Effects of chemically modified nanostructured PLGA on functioning of lung and breast cancer cells. Int. J. Nanomed. 2013, 8, 1907–1919. [Google Scholar] [CrossRef]
- Gyulai, G.; Kiss, É. Interaction of poly(lactic-co-glycolic acid) nanoparticles at fluid interfaces. J. Colloid Interface Sci. 2017, 500, 9–19. [Google Scholar] [CrossRef]
- Azarbayjani, A.F.; Lin, H.; Yap, C.W.; Chan, Y.W.; Chan, S.Y. Surface tension and wettability in transdermal delivery: A study on the in-vitro permeation of haloperidol with cyclodextrin across human epidermis. J. Pharm. Pharmacol. 2010, 62, 770–778. [Google Scholar] [CrossRef]
- Mansur, H.S.; Oréfice, R.L.; Mansur, A.A.P. Characterization of poly(vinyl alcohol)/poly(ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy. Polymer 2004, 45, 7193–7202. [Google Scholar] [CrossRef]
- Mannock, D.A.; Lewis, R.N.A.H.; McMullen, T.P.W.; McElhaney, R.N. The effect of variations in phospholipid and sterol structure on the nature of lipid-sterol interactions in lipid bilayer model membranes. Chem. Phys. Lipids 2010, 163, 403–448. [Google Scholar] [CrossRef]
- Nagle, J.F. Theory of the Main Lipid Bilayer Phase Transition. Annu. Rev. Phys. Chem. 1980, 31, 157–196. [Google Scholar] [CrossRef]
- Barbosa, L.C.A. Espectroscopia no Infravermelho na Caracterização de Compostos Orgânicos; Universidade Federal de Viçosa: Viçosa, Brazil, 2013; 183p. [Google Scholar]
- Dos Reis, E.F.; Campos, F.S.; Lage, A.P.; Leite, R.C.; Heneine, L.G.; Vasconcelos, W.L.; Lobato, Z.I.P.; Mansur, H.S. Synthesis and characterization of poly(vinyl alcohol) hydrogels and hybrids for rMPB70 protein adsorption. Mater. Res. 2006, 9, 185–191. [Google Scholar] [CrossRef]
- Porjazoska, A.; Goracinova, K.; Mladenovska, K.; Glavas, M.; Simonovska, M.; Janjević, E.I.; Cvetkovska, M. Poly(lactide-co-glycolide) microparticles as systems for controlled release of proteins—Preparation and characterization. Acta Pharm. 2004, 54, 215–229. [Google Scholar]
- Fasehee, H.; Dinarvand, R.; Ghavamzadeh, A.; Esfandyari-Manesh, M.; Moradian, H.; Faghihi, S.; Ghaffari, S.H. Delivery of disulfiram into breast cancer cells using folate-receptor-targeted PLGA-PEG nanoparticles: In vitro and in vivo investigations. J. Nanobiotechnol. 2016, 14, 32. [Google Scholar] [CrossRef]
- Chen, J.Y.; Peng, Q.; Jodl, H.J. Infrared spectral comparison of 5-aminolevulinic acid and its hexyl ester. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2003, 59, 2571–2576. [Google Scholar] [CrossRef]
- Moreno, M.M.; Garidel, P.; Suwalsky, M.; Howe, J.; Brandenburg, K. The membrane-activity of Ibuprofen, Diclofenac, and Naproxen: A physico-chemical study with lecithin phospholipids. Biochim. Biophys. Acta—Biomembr. 2009, 1788, 1296–1303. [Google Scholar] [CrossRef]
- Severcan, F.; Sahin, I.; Kazanci, N. Melatonin strongly interacts with zwitterionic model membranes—Evidence from Fourier transform infrared spectroscopy and differential scanning calorimetry. Biochim. Biophys. Acta—Biomembr. 2005, 1668, 215–222. [Google Scholar] [CrossRef]
- Garner, J.; Skidmore, S.; Park, H.; Park, K.; Choi, S.; Wang, Y. A protocol for assay of poly(lactide-co-glycolide) in clinical products. Int. J. Pharm. 2015, 495, 87–92. [Google Scholar] [CrossRef]
- Shapiro, Y.E. 1H NMR self-diffusion study of morphology and structure of polyvinyl alcohol cryogels. J. Colloid Interface Sci. 1999, 212, 453–465. [Google Scholar] [CrossRef]
- Petit, J.M.; Zhu, X.X. 1H and 13C NMR study on local dynamics of poly(vinyl alcohol) in aqueous solutions. Macromolecules 1996, 29, 2075–2081. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, N.; Sun, Y.; Li, X.; Yang, H. Absolute quantification of poly(DL-lactide-co-glycolide) in microspheres using quantitative 1H NMR spectroscopy. J. Pharm. Biomed. Anal. 2017, 146, 273–278. [Google Scholar] [CrossRef]
- Butler, A.R.; George, S. The nonenzymatic cyclic dimerisation of 5-aminolevulinic acid. Tetrahedron 1992, 48, 7879–7886. [Google Scholar] [CrossRef]
- Mello, N.C.; Bonagamba, T.J.; Panepucci, H.; Dahmouche, K.; Judeinstein, P.; Aegerter, M.A. NMR study of ion-conducting organic-inorganic nanocomposites poly(ethylene glycol)—Silica—LiClO4. Macromol. Wash. Am. Chem. Soc. 2000, 33, 1280–1288. [Google Scholar] [CrossRef]
- Austin, D.T.R.; Hills, B.P. Two-dimensional NMR relaxation study of the pore structure in silicone hydrogel contact lenses. Appl. Magn. Reson. 2009, 35, 581–591. [Google Scholar] [CrossRef]
- Clausen, A.E.; Bernkop-schnu, A. In vitro evaluation of the effect of natural-resumos. J. Pharm. Sci. 2000, 89, 1253–1261. [Google Scholar] [CrossRef]
- Cerize, N.N.P. Estudo de Sistemas Nanocarreadores para o Ácido 5-aminolevulínico com Aplicação na Terapia Fotodinâmica. Ph.D. Thesis, Faculdade de Ciências Farmacêuticas de Ribeirão Preto—USP, São Paulo, Brazil, 2012; 174p. [Google Scholar]
- Novo, M.; Hüttmann, G.; Diddens, H. Chemical instability of 5-aminolevulinic acid used in the fluorescence diagnosis of bladder tumours. J. Photochem. Photobiol. B Biol. 1996, 34, 143–148. [Google Scholar] [CrossRef]
- Schaffazick, S.R.; Guterres, S.S.; Freitas, L.d.L.; Pohlmann, A.R. Caracterização e estabilidade físico-química de sistemas poliméricos nanoparticulados para administração de fármacos. Quim. Nova 2003, 26, 726–737. [Google Scholar] [CrossRef]
- Moraes, C.M.; de Paula, E.; Rosa, A.H.; Fraceto, L.F. Physicochemical stability of poly(lactide-co-glycolide) nanocapsules containing the local anesthetic bupivacaine. J. Braz. Chem. Soc. 2010, 21, 995–1000. [Google Scholar] [CrossRef]
- De, S.; Robinson, D.H. Particle size and temperature effect on the physical stability of PLGA nanospheres and microspheres containing bodipy. AAPS PharmSciTech 2004, 5, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, R.; Sepehri, N.; Manouchehri, S.; Rouhani, H.; Atyabi, F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shen, H.; Zhu, X.; Yao, H.; Wang, W. Preparation and photochromic properties of PEG-400 assisted WO3–TiO2–ZnO composite films. Ceram. Int. 2015, 41, 14008–14012. [Google Scholar] [CrossRef]
- Soares, A.C. Filmes Nanoestruturados Apliacados em Biossensores para Detecção Precoce de Câncer de Pâncreas. Ph.D. Thesis, Escola de Engenharia de São Carlos da Universidade de São Paulo, São Carlos, Brazil, 2016. [Google Scholar]


| Probe Liquid | γtotal | γdispersive | γpolar | γacid | γbasic |
|---|---|---|---|---|---|
| water | 72.80 | 21.08 | 51.72 | 25.50 | 25.50 |
| formamide | 58.00 | 39.00 | 19.00 | 2.28 | 39.60 |
| ethylene glycol | 48.00 | 29.00 | 19.00 | 3.00 | 30.10 |
| diiodomethane | 50.80 | 48.50 | 2.300 | 0.00 | 0.00 |
| Emulsion Method | Sample | Internal Aqueous Phase | External Aqueous Phase | Observation |
|---|---|---|---|---|
| (A) Double | A–DE–PVA | water | PVA | Literature and method review Control of Synthesis E, Comparison to System D |
| A–DE–PVA–ALA | 5-ALA | PVA | ||
| (B) Simple | B–SE–PEG | - | PEG | Method review Control of Synthesis C and E |
| B–SE–PEG–ALA | - | PEG–ALA | ||
| (C) Simple | C–SE–PEG–PVA | - | PVA–PEG | Innovation Comparison to System E |
| C–SE–PEG–PVA–ALA | - | PVA–PEG–ALA | ||
| (D) Simple | D–SE–PVA | - | PVA | Method review Modified synthesis from (A) Comparison to System A System E control. |
| D–SE–PVA–ALA | - | PVA–ALA | ||
| (E) Double | E–DE–PEG–PVA | PVA | PEG | Innovation Comparison to System C. |
| E–DE-PEG–PVA–ALA | PVA–ALA | PEG |
| System | Average Size NTA (nm) | Mode (nm) | Standard Error (nm) | Average Size (DLS) (nm) | Particle mL−1 |
|---|---|---|---|---|---|
| A–DE–PVA | (225.7 ± 0.7) | (218.3 ± 12.8) | (33.5 ± 1.2) | (226.4 ± 70.2) | 1.99 × 1012 ± 3.74 × 1010 |
| A–DE–PVA–ALA | (192.7 ± 6,5) | (195.1 ± 7.9) | (24.7 ± 4.7) | (195.9 ± 61.2) | 1.79 × 1011 ± 1.27 × 1010 |
| C–SE–PEG–PVA | (153.9 ± 1.3) | (149.0 ± 2.7) | (24.8 ± 3.4) | (151.6 ± 49.0) | 1.53 × 1014 ± 5.62 × 1012 |
| C–SE–PEG–PVA–ALA | (334.4 ± 4.0) | (316.3 ± 8.8) | (70.4 ± 3.8) | (377.4 ± 62.6) | 1.85 × 1013 ± 7.92 × 1011 |
| D–SE–PVA | (142.6 ± 0.9) | (137.9 ± 1.9) | (22.5 ± 1.3) | (181.1 ± 51.2) | 2.36 × 1015 ± 5.69 × 1013 |
| D–SE–PVA–ALA | (445.7 ± 18.5) | (341.9 ± 28.5) | (149.9 ± 7.4) | (702.1 ± 118.9) | 7.97 × 1011 ± 6.10 × 1010 |
| E–DE–PEG–PVA | (173.2 ± 0.8) | (165.3 ± 3.5) | (44.2 ± 0.6) | (199.4 ± 60.8) | 3.50 × 1014 ± 7.4 × 1012 |
| E–DE–PEG–PVA–ALA | (164.5 ± 4.2) | (153.8 ± 1.9) | (23.3 ± 3.3) | (274.6 ± 117.5) | 9.23 × 1011 ± 6.12 × 1010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, G.R.; dos Santos, A.L.; Soares, A.C.; dos Santos, M.C.; dos Santos, S.C.; Ţălu, Ş.; Rodrigues de Lima, V.; Bagnato, V.S.; Sanches, E.A.; Inada, N.M. PLGA-PVA-PEG Single Emulsion Method as a Candidate for Aminolevulinic Acid (5-ALA) Encapsulation: Laboratory Scaling Up and Stability Evaluation. Molecules 2022, 27, 6029. https://doi.org/10.3390/molecules27186029
da Silva GR, dos Santos AL, Soares AC, dos Santos MC, dos Santos SC, Ţălu Ş, Rodrigues de Lima V, Bagnato VS, Sanches EA, Inada NM. PLGA-PVA-PEG Single Emulsion Method as a Candidate for Aminolevulinic Acid (5-ALA) Encapsulation: Laboratory Scaling Up and Stability Evaluation. Molecules. 2022; 27(18):6029. https://doi.org/10.3390/molecules27186029
Chicago/Turabian Styleda Silva, Geisiane Rosa, Amanda Luizetto dos Santos, Andrey Coatrini Soares, Marinalva Cardoso dos Santos, Sandra Cruz dos Santos, Ştefan Ţălu, Vânia Rodrigues de Lima, Vanderlei Salvador Bagnato, Edgar Aparecido Sanches, and Natalia Mayumi Inada. 2022. "PLGA-PVA-PEG Single Emulsion Method as a Candidate for Aminolevulinic Acid (5-ALA) Encapsulation: Laboratory Scaling Up and Stability Evaluation" Molecules 27, no. 18: 6029. https://doi.org/10.3390/molecules27186029
APA Styleda Silva, G. R., dos Santos, A. L., Soares, A. C., dos Santos, M. C., dos Santos, S. C., Ţălu, Ş., Rodrigues de Lima, V., Bagnato, V. S., Sanches, E. A., & Inada, N. M. (2022). PLGA-PVA-PEG Single Emulsion Method as a Candidate for Aminolevulinic Acid (5-ALA) Encapsulation: Laboratory Scaling Up and Stability Evaluation. Molecules, 27(18), 6029. https://doi.org/10.3390/molecules27186029









