Determination of Apoptotic Mechanism of Action of Tetrabromobisphenol A and Tetrabromobisphenol S in Human Peripheral Blood Mononuclear Cells: A Comparative Study
Abstract
1. Introduction
2. Results
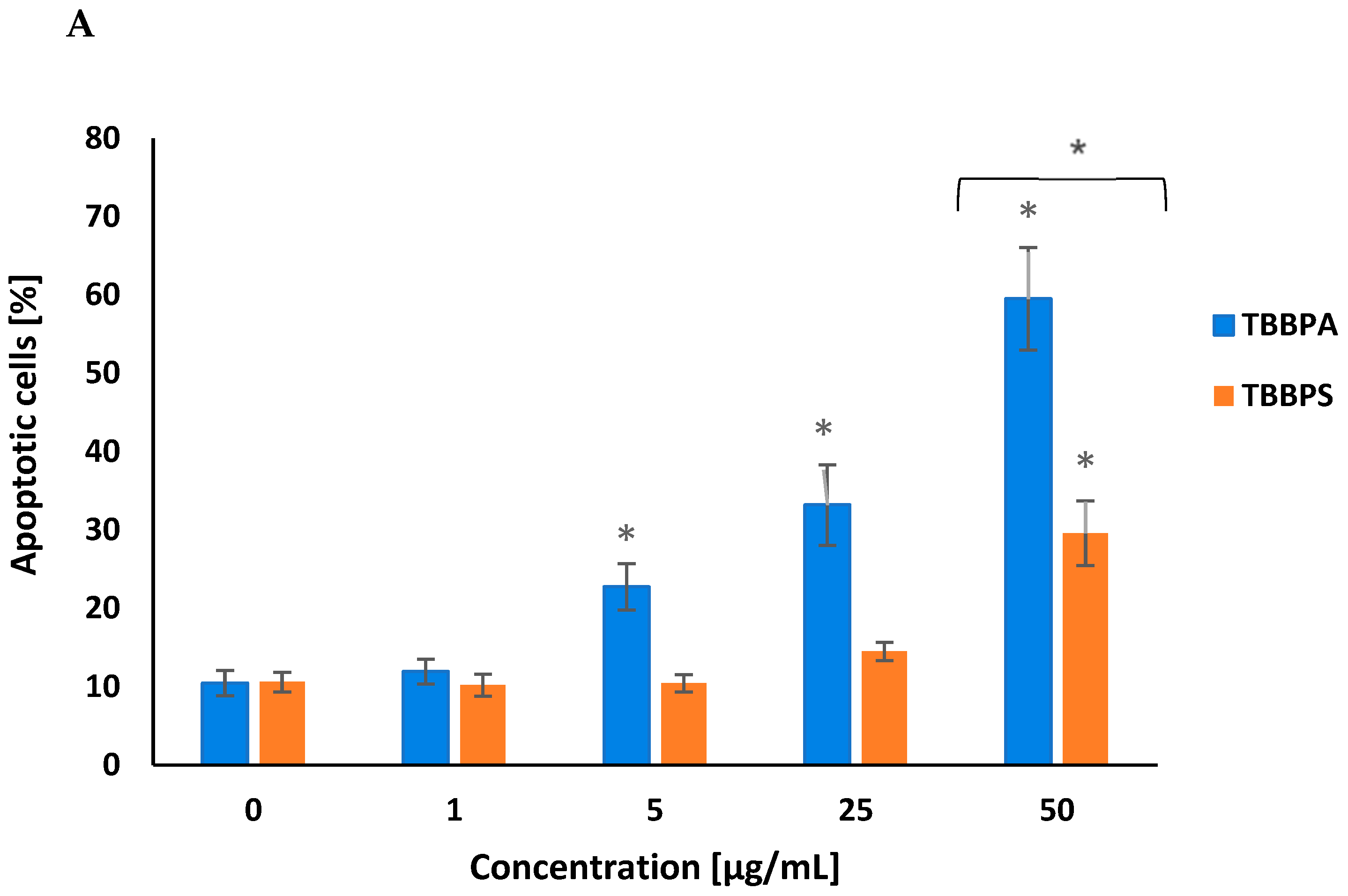
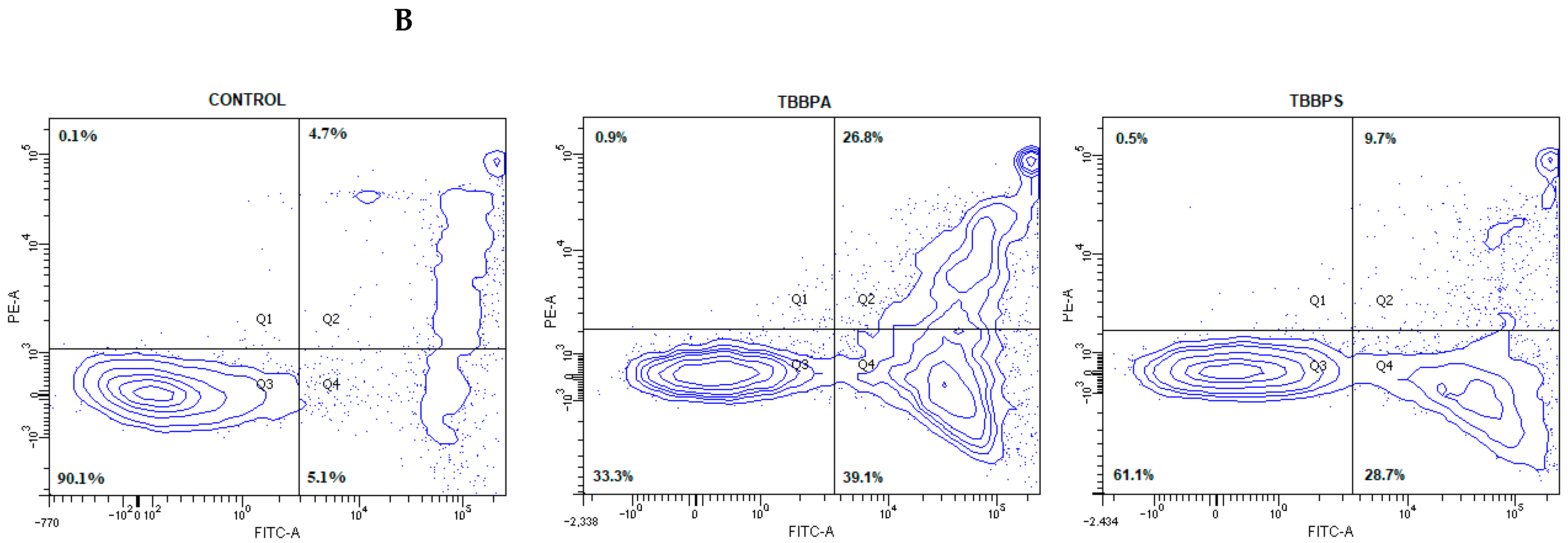
2.1. Quantitative Analysis of Apoptosis
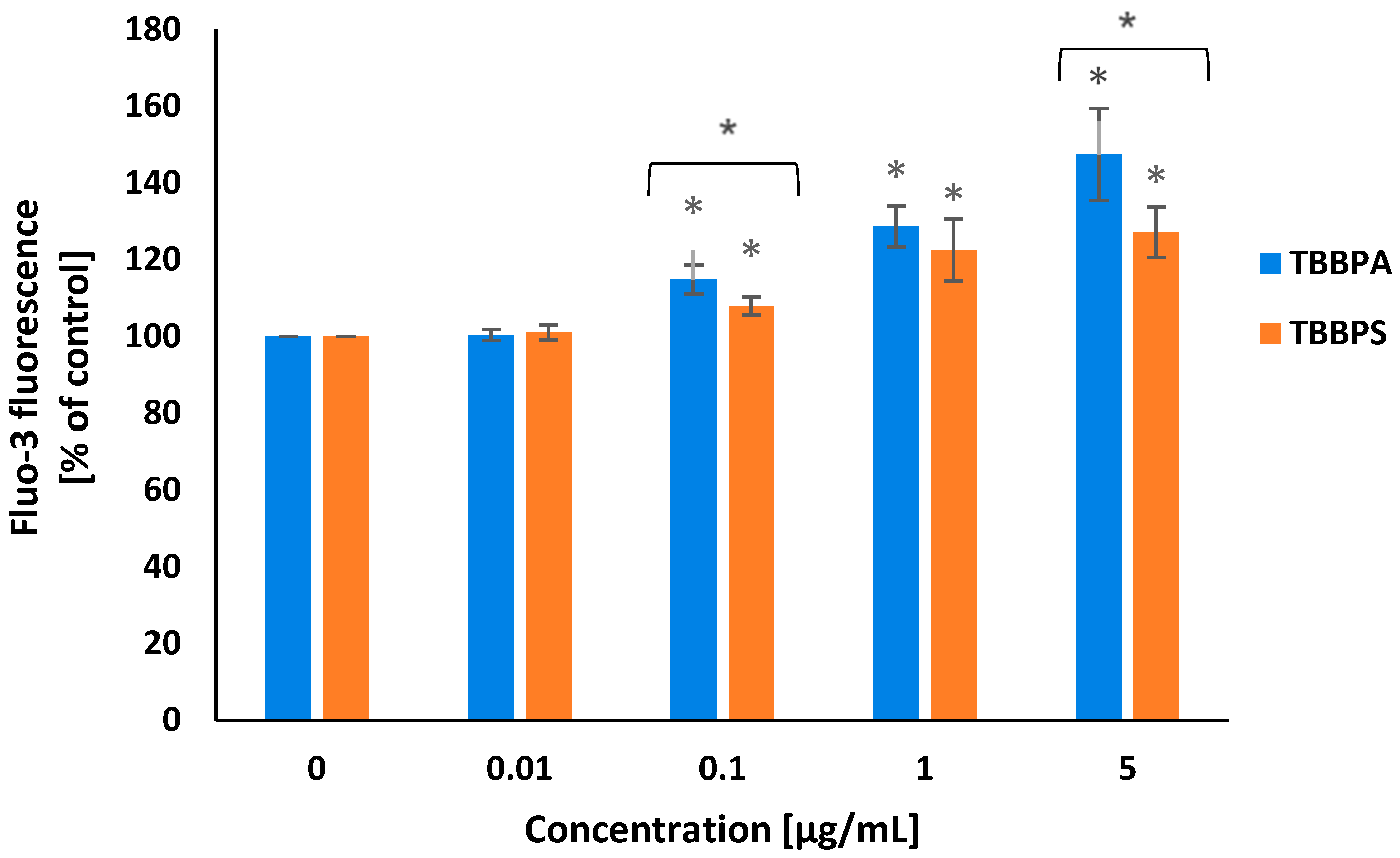
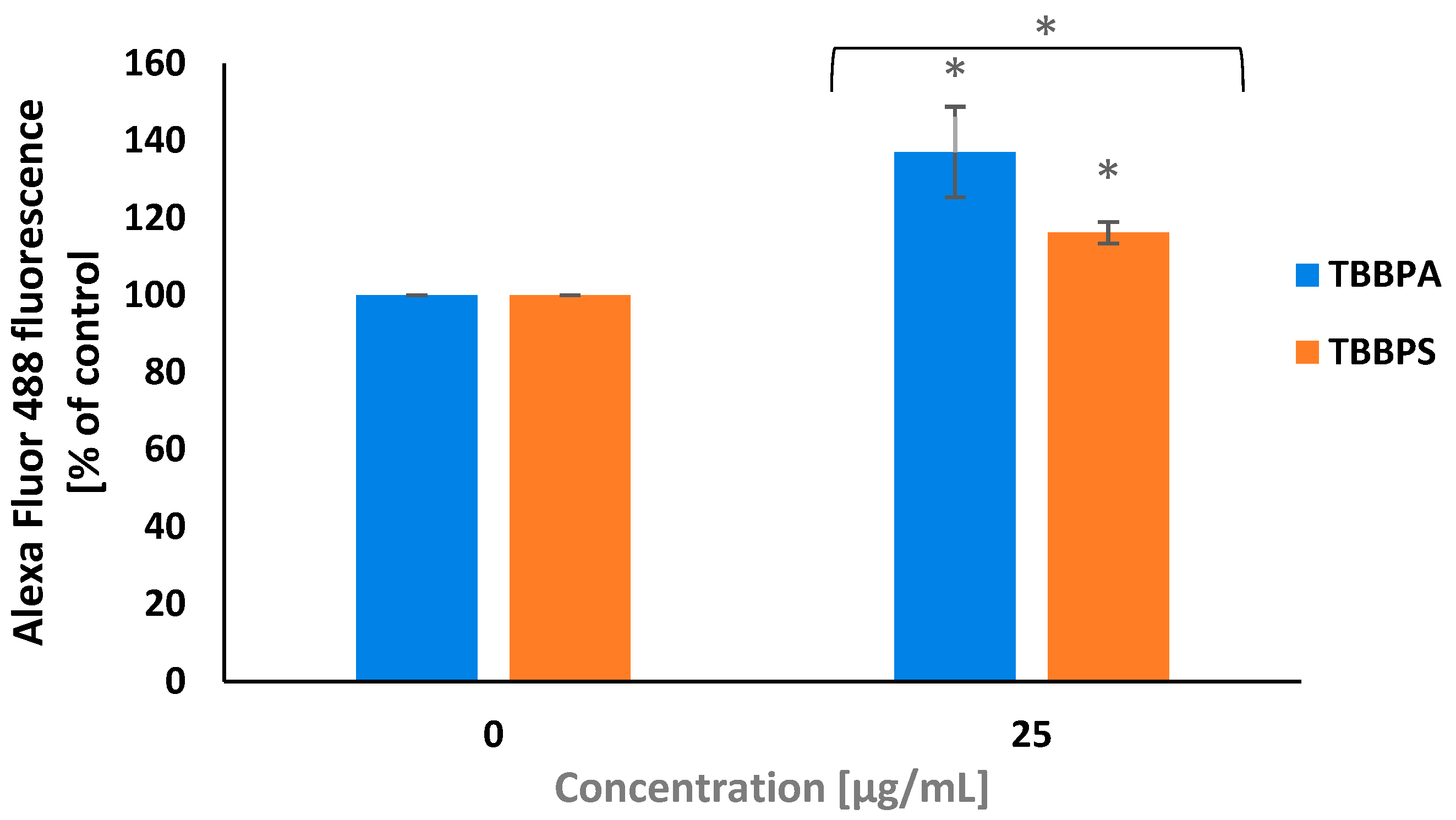
2.2. Cytosolic Calcium Ions Level
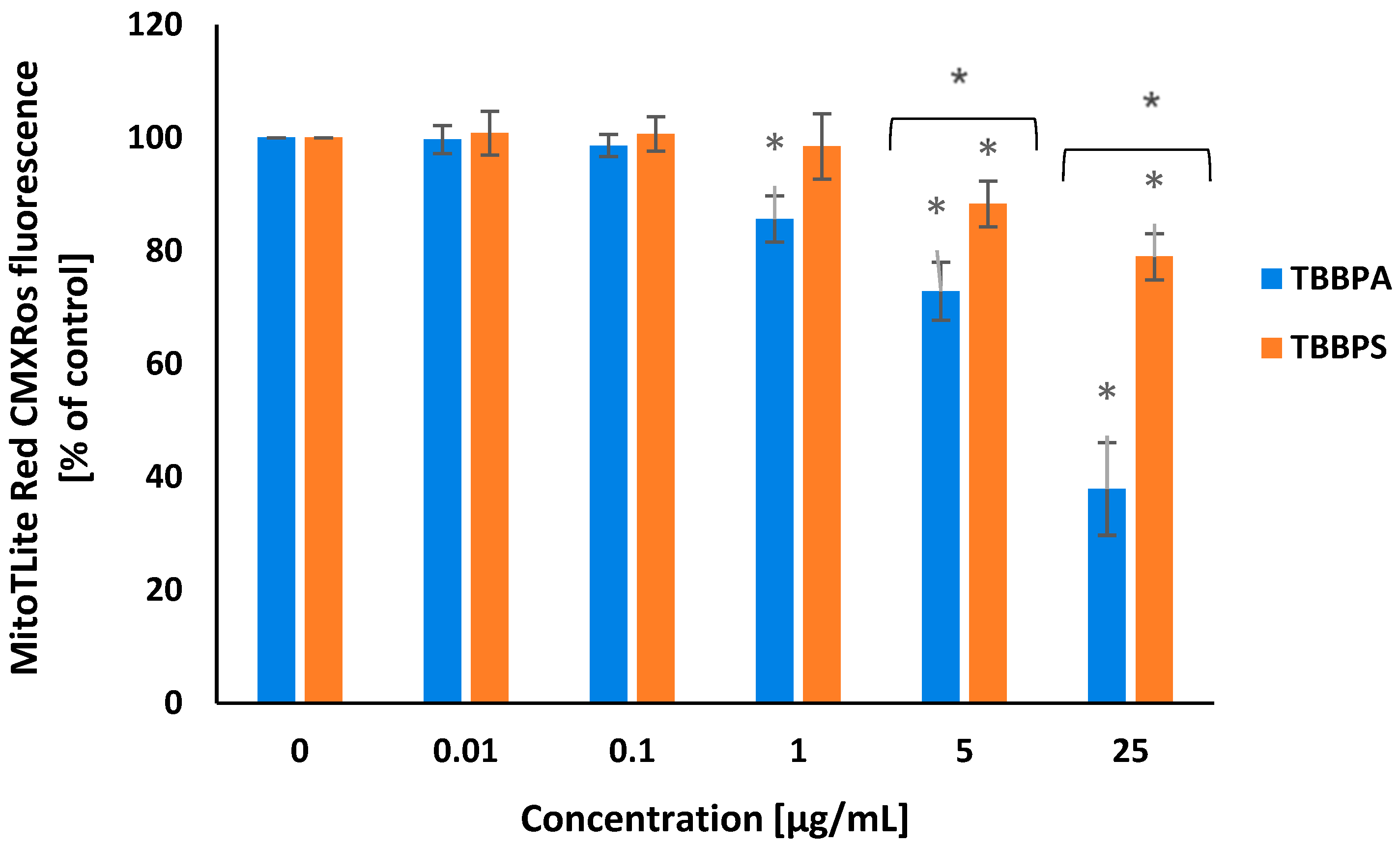
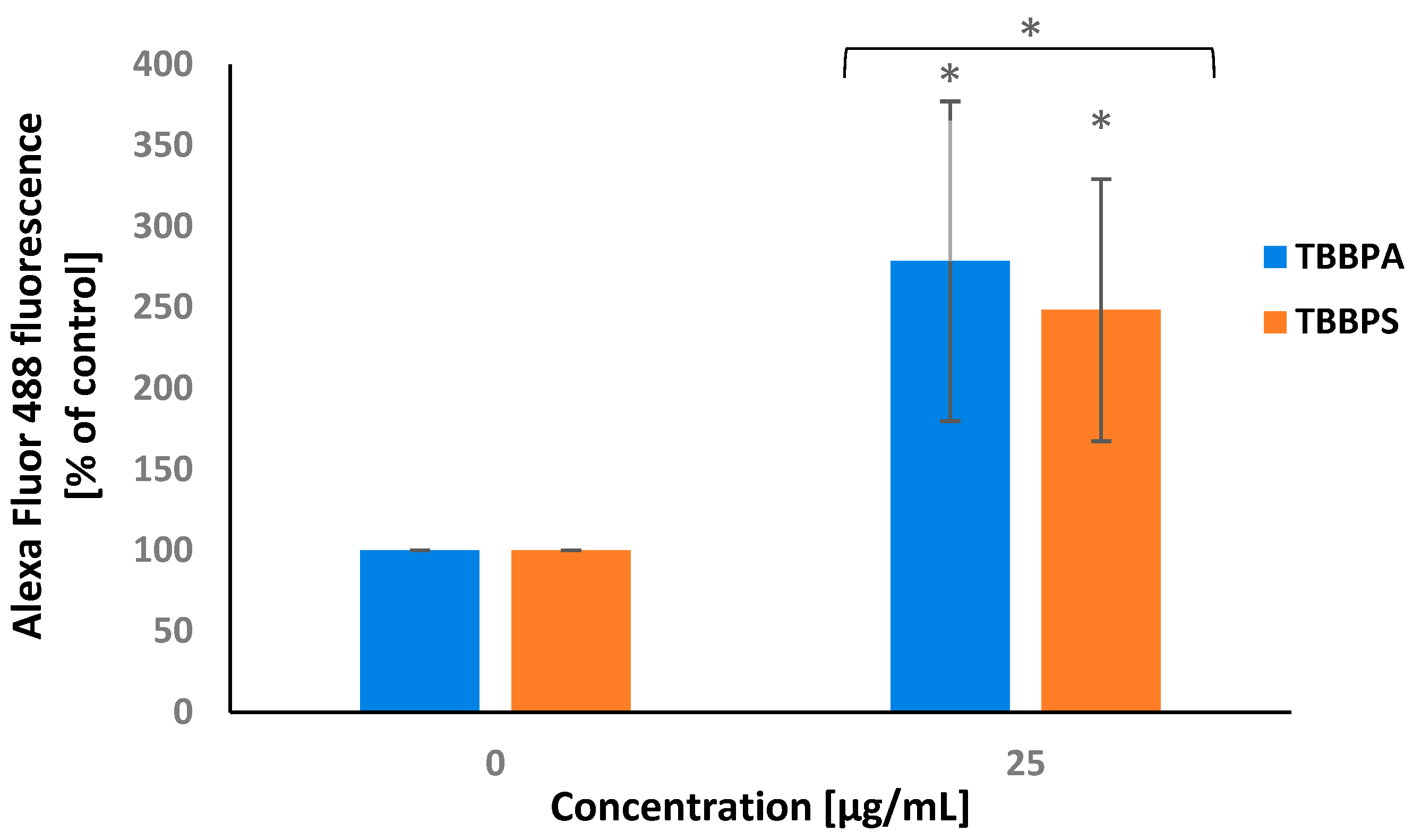
2.3. Transmembrane Mitochondrial Potential
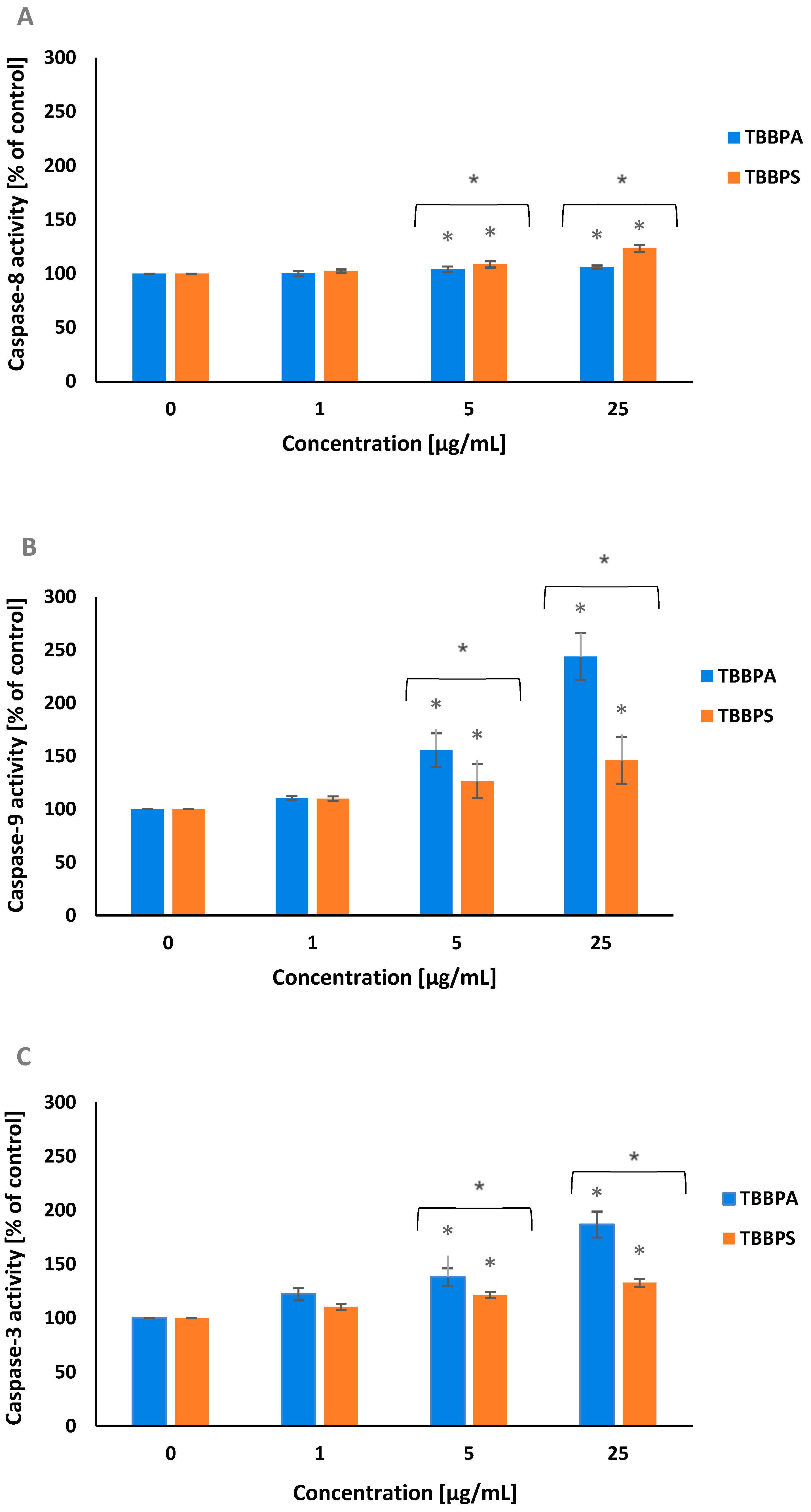
2.4. Caspase-8, -9 and -3 Activation
2.5. PARP-1 Cleavage
2.6. APO-BrdU TUNEL Assay
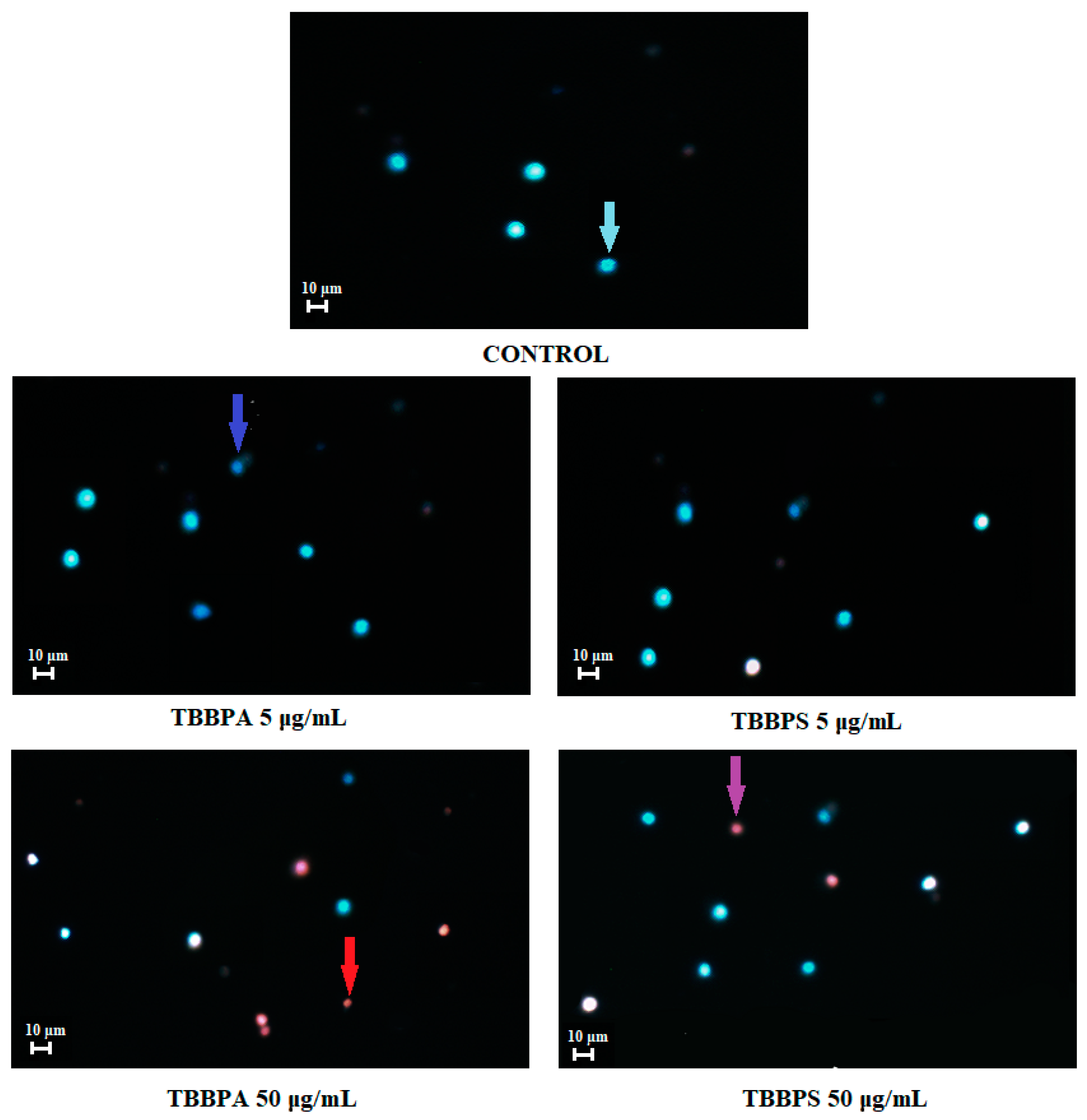
2.7. Apoptosis Detection by Fluorescence Microscopy
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals
5.2. Methods
5.2.1. Cell Isolation and Treatment
5.2.2. Quantitative Determination of Apoptosis (Annexin V-FITC/PI Staining)
5.2.3. Cytosolic Calcium Ion Level
5.2.4. Mitochondrial Transmembrane Potential
5.2.5. Caspase-3, -8, -9 Activation
5.2.6. PARP-1 Cleavage
5.2.7. APO-BrdU TUNEL Assay
5.2.8. Apoptosis Determination by Fluorescence Microscopy
5.2.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, A.; Shi, J.; Shen, Z.; Lin, Y.; Qu, G.; Zhao, Z.; Jiang, G. Identification of unknown brominated bisphenol S congeners in contaminated soils as the transformation products of tetrabromobisphenol S derivatives. Environ. Sci. Technol. 2018, 52, 10480–10489. [Google Scholar] [CrossRef] [PubMed]
- Michałowicz, J.; Włuka, A.; Bukowska, B. A review of environmental occurrence, toxic effects and transformation of man-made bromophenols. Sci. Total Environ. 2022, 811, 152289. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Luo, X.-J.; Zeng, Y.-H.; Mai, B.-X. Determination of tetrabromobisphenol-A/S and their eight derivatives in abiotic (soil/dust) samples using ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromat. A 2021, 1647, 462152. [Google Scholar] [CrossRef]
- Kacew, S.; Hayes, A. Absence of neurotoxicity and lack of neurobehavioral consequences due to exposure to tetrabromobisphenol A (TBBPA) exposure in humans, animals and zebrafish. Arch. Toxicol. 2019, 94, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, W.; Sánchez, F.; Zaiat, M. What drives tetrabromobisphenol A degradation in biotreatment systems? Rev. Environ. Sci. Bio/Technol. 2021, 20, 729–750. [Google Scholar] [CrossRef]
- Xiong, J.K.; An, T.C.; Zhang, C.S.; Li, G.Y. Pollution profiles and risk assessment of PBDEs and phenolic brominated flame retardants in water environments within a typical electronic waste dismantling region. Environ. Geochem. Health 2015, 37, 457–473. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.; Yan, S.; Zghang, W.; Li, Y.; Han, D. A review of status of tetrabromobisphenol A (TBBPA) in China. Chemosphere 2016, 148, 8–20. [Google Scholar] [CrossRef]
- Rothenbacher, K.; Pecquet, A. Summary of historical terrestrial toxicity data for the brominated flame retardant tetrabromobisphenol A (TBBPA): Effects on soil microorganisms, earthworms, and seedling emergence. Environ. Sci. Pollut. Res. 2018, 25, 17268–17277. [Google Scholar] [CrossRef]
- Abafe, O.A.; Martincigh, B.S. Determination and human exposure assessment of polybrominated diphenyl ethers and tetrabromobisphenol A in indoor dust in South Africa. Environ. Sci. Pollut. Res. 2016, 23, 7038–7049. [Google Scholar] [CrossRef]
- Xie, Z.; Ebinghaus, R.; Lohmann, R.; Heemken, O.; Caba, A.; Püttmann, W. Trace determination of the flame retardant tetrabromobisphenol A in the atmosphere by gas chromatography–mass spectrometry. Anal. Chim. Acta 2007, 584, 333–342. [Google Scholar] [CrossRef]
- Abdallah, M.A.E.; Harrad, S.; Covaci, A. Hexabromocyclododecanes and tetrabromobisphenol-A in indoor air and dust in Birmingham, UK: Implications for human exposure. Environ. Sci. Technol. 2008, 42, 6855–6861. [Google Scholar] [CrossRef] [PubMed]
- Cariou, R.; Antignac, J.P.; Zalko, D.; Berrebi, A.; Cravedi, J.P.; Maume, D.; Marchand, P.; Montenau, F.; Riu, A.; Andre, F.; et al. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: Occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere 2008, 73, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Harada, K.H.; Hitomi, T.; Kobayashi, H.; Koizumi, A.; Haraguchi, K. Temporal trend and age-dependent serum concentration of phenolic organohalogen contaminants in Japanese men during 1989–2010. Environ. Pollut. 2014, 185, 228–233. [Google Scholar] [CrossRef]
- Lankova, D.; Lacina, O.; Pulkrabova, J.; Majslova, J. The determination of perfluoroalkyl substances, brominated flame retardants and their metabolites in human breast milk and infant formula. Talanta 2013, 117, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Ito, Y.; Yamaguchi, M.; Mitumori, K.; Koizumi, M.; Hasegawa, R.; Kamata, E.; Ema, M. Unexpected nephrotoxicity induced by tetrabromobisphenol A in newborn rats. Toxicol. Lett. 2004, 150, 145–155. [Google Scholar] [CrossRef]
- Choi, E.; Suh, K.; Rhee, S.; Oh, S.; Kim, S.; Pak, Y.; Choe, W.; Ha, J.; Chon, S. Exposure to tetrabromobisphenol A induces cellular dysfunction in osteoblastic MC3T3-E1 cells. J. Environ. Sci. Health Part A 2017, 52, 561–570. [Google Scholar] [CrossRef]
- Al-Mousa, F.; Michelangel, F.; Vavvas, D. Some commonly used brominated flame retardants cause Ca2+-ATPase inhibition, beta-amyloid peptide release and apoptosis in SH-SY5Y neuronal cells. PLoS ONE 2012, 7, e33059. [Google Scholar] [CrossRef]
- Koike, E.; Yanagisawa, R.; Takano, H. Brominated flame retardants, hexabromocyclododecane and tetrabromobisphenol A, affect proinflammatory protein expression in human bronchial epithelial cells via disruption of intracellular signalling. Toxicol. In Vitro 2016, 32, 212–219. [Google Scholar] [CrossRef]
- Sanders, J.; Coulter, S.; Knudsen, G.; Dunnick, J.; Kissling, G.; Birnbaum, L. Disruption of estrogen homeostasis as a mechanism for uterine toxicity in Wistar Han rats treated with tetrabromobisphenol A. Toxicol. Appl. Pharmacol. 2016, 298, 31–39. [Google Scholar] [CrossRef]
- Yasmin, S.; Whalen, M. Flame retardants, hexabromocyclododecane (HCBD) and tetrabromobisphenol a (TBBPA), alter secretion of tumor necrosis factor alpha (TNFα) from human immune cells. Arch. Toxicol. 2018, 92, 1483–1494. [Google Scholar] [CrossRef]
- Sharma, P.; Chadha, P.; Saini, H. Tetrabromobisphenol A induced oxidative stress and genotoxicity in fish Channa punctatus. Drug Chem. Toxicol. 2019, 42, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Włuka, A.; Woźniak, A.; Woźniak, E.; Michałowicz, J. Tetrabromobisphenol A, terabromobisphenol S and other bromophenolic flame retardants cause cytotoxic effects and induce oxidative stress in human peripheral blood mononuclear cells (in vitro study). Chemosphere 2020, 261, 127705. [Google Scholar] [CrossRef] [PubMed]
- Barańska, A.; Woźniak, A.; Mokra, K.; Michałowicz, J. Genotoxic mechanism of action of TBBPA, TBBPS and selected bromophenols in human peripheral blood mononuclear cells. Front. Immunol. 2022, 13, 869741. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, Y.; Lu, J.; Lu, J.; Zhou, L.; Chovelo, J.M.; Ji, Y. Aqueous photodecomposition of the emerging brominated flame retardant tetrabromobisphenol S (TBBPS). Environ. Pollut. 2021, 271, 116406. [Google Scholar] [CrossRef]
- Letcher, R.; Chu, S. High-sensitivity method for determination of tetrabromobisphenol-S and tetrabromobisphenol-A derivative flame retardants in Great Lakes Herring Gull eggs by liquid chromatography−atmospheric pressure photoionization−tandem mass spectrometry. Environ. Sci. Technol. 2010, 44, 8615–8621. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Lu, J.; Ji, Y. Formation of brominated disinfection by-products and bromate in cobalt catalyzed peroxymonosulfate oxidation of phenol. Water Res. 2015, 84, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Liu, A.; Hu, A.; Liu, S.; Shi, J.; Jiang, G. Recent advances in the analysis of TBBPA/TBBPS, TBBPA/TBBPS derivatives and their transformation products. TrAC Trends Anal. Chem. 2016, 83, 14–24. [Google Scholar] [CrossRef]
- Wang, X.L.; Luan, J.; Lu, Q.L.; Lin, H.G.; Xu, C. Three new metal–organic complexes derived from N,N′-bis(3-pyridinecarboxamide)-1,2-ethane and polycarboxylate ligands: Synthesis, fluorescent and electrochemical properties. J. Organometal. Chem. 2012, 719, 1–7. [Google Scholar] [CrossRef]
- Song, S.; Song, M.; Zeng, L.; Wang, T.; Liu, R.; Ruan, T.; Jiang, G. Occurrence and profiles of bisphenol analogues in municipal sewage sludge in China. Environ. Pollut. 2014, 186, 14–19. [Google Scholar] [CrossRef]
- Yang, B.; Ying, G.G.; Chen, Z.F.; Zhao, J.L.; Peng, F.Q.; Chen, X.W. Ferrate(VI) oxidation of tetrabromobisphenol A in comparison with bisphenol A. Water Res. 2014, 62, 211–219. [Google Scholar] [CrossRef]
- Malysheva, S.; Goscinny, S.; Malarvannan, G.; Poma, G.; Andjelkovic, M. Development and validation of a quantitative UHPLC-MS/MS method for selected brominated flame retardants in food. Food Addit. Contam. Part A 2018, 35, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhuang, T.; Shi, W.; Liang, Y.; Liao, C.; Song, M.; Jiang, G. Serum concentration of bisphenol analogues in pregnant women in China. Sci. Total Environ. 2020, 707, 136100. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Dong, X.; Feng, W.; Mao, G.; Chen, Y.; Oiu, X.; Chen, K.; Xu, H. Tetrabromobisphenol S alters the circadian rhythm network in the early life stages of zebrafish. Sci. Total Environ. 2022, 806, 150543. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Liang, S.; Yin, N.; Hu, B.; Faiola, F. Toxicogenomic analyses of the effects of BDE-47/209, TBBPA/S and TCBPA on early neural development with a human embryonic stem cell in vitro differentiation system. Toxicol. Appl. Pharmacol. 2019, 379, 114685. [Google Scholar] [CrossRef] [PubMed]
- Pallardy, M.; Biola, A.; Lebrec, H.; Breard, J. Assessment of apoptosis in xenobiotic-induced immunotoxicity. Methods 1999, 19, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Hartig, M.; Dzhagalov, I.; Draper, D.; He, Y.W. The role of apoptosis in the development and function of T lymphocytes. Cell Res. 2005, 15, 749–769. [Google Scholar] [CrossRef] [PubMed]
- Karuma, M.M.; Tan, N.Y.; Bennett, M.R. Death receptors and their ligands in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1694–1702. [Google Scholar]
- LaRosa, F.D.; Orange, S.J. Lymphocytes. J. Allergy Clin. Immunol. 2008, 121, 364–369. [Google Scholar] [CrossRef]
- Weinberg, A.; Jesser, R.; Edelstein, C.; Billy, J.; Wohl, D. Excess apoptosis of mononuclear cells contributes to the depressed cytomegalovirus-specific immunity in HIV-infected patients on HAART. Virology 2004, 330, 313–321. [Google Scholar] [CrossRef]
- Ratomski, K.; Skotnicka, B.; Kasprzycka, E.; Żelazowska-Rutkowska, B.; Wysocka, J.; Anisimowicz, S. Evaluation of percentage of the CD19+ CD5+ lymphocytes in hypertrophied adenoids at children with otitis media with effusion. Otolaryngol. Pol. 2007, 61, 962–966. [Google Scholar] [CrossRef]
- Zhou, C.-X.; Zhou, D.-H.; Liu, G.-X.; Suo, X.; Zhu, X.-Q. Transcriptomic analysis of porcine PBMCs infected with Toxoplasma gondii RH strain. Acta Tropica. 2016, 154, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Halit, S.; Salameh, P. Exposure to toxins during pregnancy and childhood and asthma in children: A pilot study. J. Epidemiol. Global Health 2017, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Kemppainen, E.; Orešič, M. Perspectives on Systems Modeling of Human Peripheral Blood Mononuclear Cells. Front. Mol. Biosci. 2017, 4, 96. [Google Scholar] [CrossRef] [PubMed]
- Feiterio, J.; Mariana, M.; Cairrao, E. Health toxicity effects of brominated flame retardants: From environmental to human exposure. Environ. Pollut. 2021, 285, 117475. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.M.; Coulter, S.J.; Knudsen, G.A.; Sanders, J.M.; Birnbaum, L.S. Gene expression changes in immune response pathways following oral administration of tetrabromobisphenol A (TBBPA) in female Wistar Han rats. Toxicol. Lett. 2017, 272, 68–74. [Google Scholar] [CrossRef]
- Cato, A.; Celada, L.; Kibakaya, E.; Simmons, N.; Whalen, M. Brominated flame retardants, tetrabromobisphenol A and hexabromocyclododecane, activate mitogen-activated protein kinases (MAPKs) in human natural killer cells. Cell. Biol. Toxicol. 2014, 30, 345–360. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Rogalska, A.; Gajek, A.; Szwed, M.; Joźwiak, Z.; Marczak, A. The role of reactive oxygen species in WP 631-induced death of human ovarian cancer cells: A comparison with the effect of doxorubicin. Toxicol. Vitr. 2011, 25, 1712–1720. [Google Scholar] [CrossRef]
- Rutkowska, J. Zjawisko apoptozy w chorobach alergicznych. Now. Lek. 2007, 76, 48–54. [Google Scholar]
- Mokra, K.; Kocia, M.; Michałowicz, J. Bisphenol A and its analogs exhibit different apoptotic potential in peripheral blood mononuclear cells (in vitro study). Food Chem. Toxicol. 2015, 84, 79–88. [Google Scholar] [CrossRef]
- Cho, J.-H.; Lee, S.; Jeon, H.; Kim, A.; Lee, W.; Lee, Y.; Yang, S.; Yun, J.; Jung, Y.-S.; Lee, J. Tetrabromobisphenol A-induced apoptosis in neural stem cells through oxidative stress and mitochondrial dysfunction. Neurotox. Res. 2020, 38, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Chen, C.; An, J.; Shang, Y.; Li, H.; Xa, H.; Yu, J.; Wang, C.; Liu, Y.; et al. Regulation of TBBPA-induced oxidative stress on mitochondrial apoptosis in L02 cells through the Nrf2 signaling pathway. Chemosphere 2019, 226, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.; Choi, E.; Rhee, S.; Oh, S.; Kim, S.; Pak, Y.; Choe, W.; Ha, J.; Chon, S. Tetrabromobisphenol A induces cellular damages in pancreatic β-cells in vitro. J. Environ. Sci. Health Part A 2017, 52, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hu, J.; Li, G.; An, T. Low concentration Tetrabromobisphenol A (TBBPA) elevating overall metabolism by inducing activation of the Ras signalling pathway. J. Hazard. Mat. 2021, 416, 125797. [Google Scholar] [CrossRef]
- Wu, S.; Ji, G.; Liu, J.; Zhang, S.; Gong, Y.; Shi, L. TBBPA induces developmental toxicity, oxidative stress, and apoptosis in embryos and Zebrafish Larvae (Danio rerio). Environ. Toxicol. 2016, 31, 1241–1249. [Google Scholar] [CrossRef]
- Hajnoczky, G.; Davies, E.; Madesh, M. Calcium signalling and apoptosis. Biochem. Biophys. Res. Commun. 2003, 304, 445–454. [Google Scholar] [CrossRef]
- Kaufman, R.J.; Scheuner, D.; Schröder, M. The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 2002, 3, 411–421. [Google Scholar] [CrossRef]
- Kroemer, G.; Reed, J.C. Mitochondrial control of cell death. Nat. Med. 2000, 6, 513–519. [Google Scholar] [CrossRef]
- Ogunbayo, O.; Lai, P.; Conolly, T.; Michelangeli, A. Tetrabromobisphenol A (TBBPA), induces cell death in TM4 Sertoli cells by modulating Ca2+ transport proteins and causing dysregulation of Ca2+ homeostasis. Toxicol. Vitr. 2008, 22, 943–952. [Google Scholar] [CrossRef]
- Łabędzka, K.; Grzanka, A.; Izdebska, M. Mitochondrium a śmierć komórki. Post. Hig. Med. Dośw. 2006, 60, 439–446. [Google Scholar]
- Hongmei, Z. Extrinsic and intrinsic apoptosis signal pathway review. In Apoptosis and Medicine; IntechOpen: London, UK, 2012; Volume 16, pp. 1–22. [Google Scholar]
- Stępień, A.; Izdebska, M.; Grzanka, A. Rodzaje śmierci komórki. Post. Hig. Med. Dośw. 2007, 61, 420–428. [Google Scholar]
- Pihán, P.; Carreras-Sureda, A.; Hetz, C. BCL-2 family: Integrating stress responses at the ER to control cell demise. Cell Death Different. 2017, 24, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.; Wójtowicz, A. TBBPA causes neurotoxic and the apoptotic responses in cultured mouse hippocampal neurons in vitro. Pharmacol. Rep. 2015, 68, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, M.; Qi, M.; Zhong, L.; Qiu, L. Effects of novel brominated flame retardant TBBPA on human airway epithelial cell (A549) in vitro and proteome profiling. Environ. Toxicol. 2018, 33, 1245–1253. [Google Scholar] [CrossRef]
- Jarosiewicz, M.; Michałowicz, J.; Bukowska, B. In vitro assessment of eryptotic potential of tetrabromobisphenol A and other bromophenolic flame retardants. Chemosphere 2019, 215, 404–412. [Google Scholar] [CrossRef]
- Mashimo, M.; Onishi, M.; Uno, A.; Tanimichi, A.; Nobeyama, A.; Mori, M.; Yamada, S.; Negi, S.; Bu, X.; Kato, J.; et al. The 89-kDa PARP1 cleavage fragment serves as a cytoplasmic PAR carrier to induce AIF-mediated apoptosis. J. Biol. Chem. 2021, 296, 100046. [Google Scholar] [CrossRef]
- Chmielewski, M.; Linke, K.; Zabel, M. Metody wykrywania zjawiska apoptozy w komórkach wątrobowych in situ. Now. Lek. 2008, 77, 223–226. [Google Scholar]
- Park, C.; Se-Jin, K.; Lee, W.K.; Moon, S.K.; Kwak, S.; Choe, S.K.; Park, R. Tetrabromobisphenol-A induces apoptotic death of auditory cells and hearing loss. Biochem. Biophys. Res. Commun. 2016, 478, 1667–1673. [Google Scholar] [CrossRef]
- Zatecka, E.; Castillo, J.; Elzeinova, F.; Kubatova, A.; Ded, J.; Peknicova, J.; Oliva, R. The effect of tetrabromobisphenol A on protamine content and DNA integrity in mouse spermatozoa. Andrology 2014, 2, 910–917. [Google Scholar] [CrossRef]
- Lue, J.; Han, L.-W.; Lin, J.-X.; Cao, R. Organic complexes built by halogenated molecules: Unexpected in situ C_N bond formation in metal-free solvothermal conditions. Cryst. Growth Des. 2010, 10, 4217–4220. [Google Scholar]
- Barańska, A.; Sicińska, P.; Michałowicz, J. Apoptosis-inducing potential of selected bromophenolic flame retardants 2,4,6-tribromophenol and pentabromophenol in human peripheral blood mononuclear cells. Molecules 2022, 27, 5056. [Google Scholar] [CrossRef] [PubMed]
- Gralewska, P.; Gajek, A.; Marczak, A.; Rogalska, A. Metformin affects olaparib sensitivity through induction of apoptosis in epithelial ovarian cancer cell lines. Int. J. Mol. Sci. 2021, 22, 10557. [Google Scholar] [CrossRef] [PubMed]
- Watała, C. Biostatystyka—Wykorzystanie Metod Statystycznych w Pracy Badawczej w Naukach Biomedycznych, 2nd ed.; Alfa-Medica Press: Bielsko-Biała, Poland, 2002; pp. 54–68. [Google Scholar]
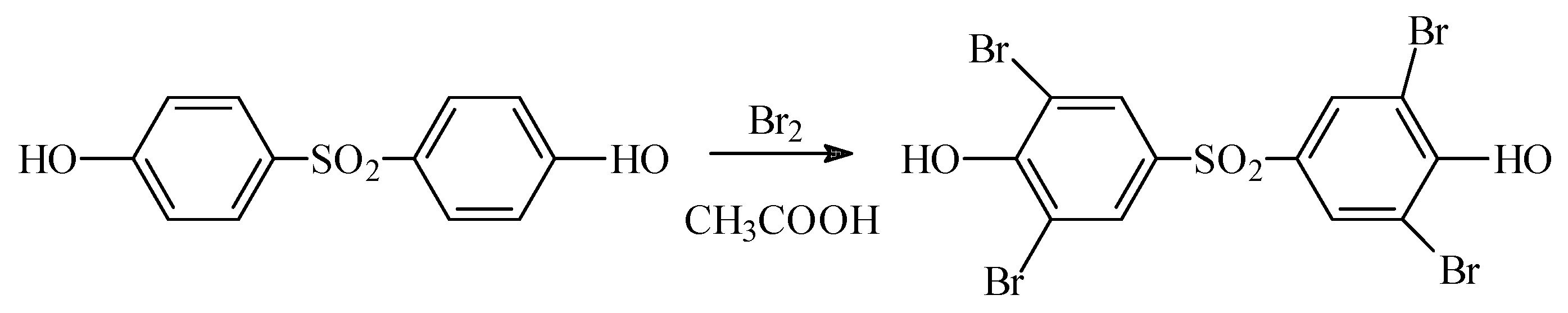
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barańska, A.; Bukowska, B.; Michałowicz, J. Determination of Apoptotic Mechanism of Action of Tetrabromobisphenol A and Tetrabromobisphenol S in Human Peripheral Blood Mononuclear Cells: A Comparative Study. Molecules 2022, 27, 6052. https://doi.org/10.3390/molecules27186052
Barańska A, Bukowska B, Michałowicz J. Determination of Apoptotic Mechanism of Action of Tetrabromobisphenol A and Tetrabromobisphenol S in Human Peripheral Blood Mononuclear Cells: A Comparative Study. Molecules. 2022; 27(18):6052. https://doi.org/10.3390/molecules27186052
Chicago/Turabian StyleBarańska, Anna, Bożena Bukowska, and Jaromir Michałowicz. 2022. "Determination of Apoptotic Mechanism of Action of Tetrabromobisphenol A and Tetrabromobisphenol S in Human Peripheral Blood Mononuclear Cells: A Comparative Study" Molecules 27, no. 18: 6052. https://doi.org/10.3390/molecules27186052
APA StyleBarańska, A., Bukowska, B., & Michałowicz, J. (2022). Determination of Apoptotic Mechanism of Action of Tetrabromobisphenol A and Tetrabromobisphenol S in Human Peripheral Blood Mononuclear Cells: A Comparative Study. Molecules, 27(18), 6052. https://doi.org/10.3390/molecules27186052





