Programed Thermoresponsive Polymers with Cleavage-Induced Phase Transition
Abstract
:1. Introduction
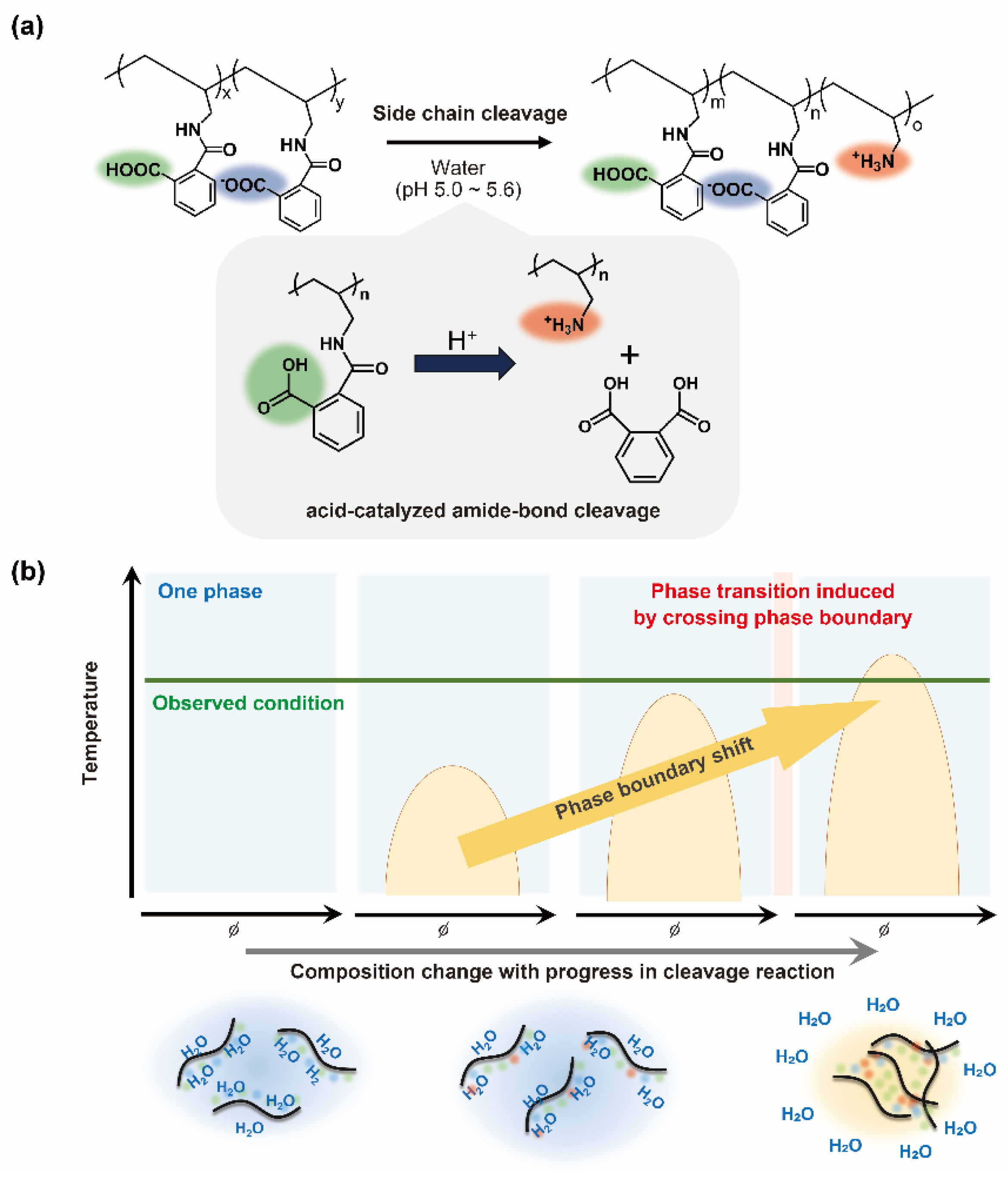
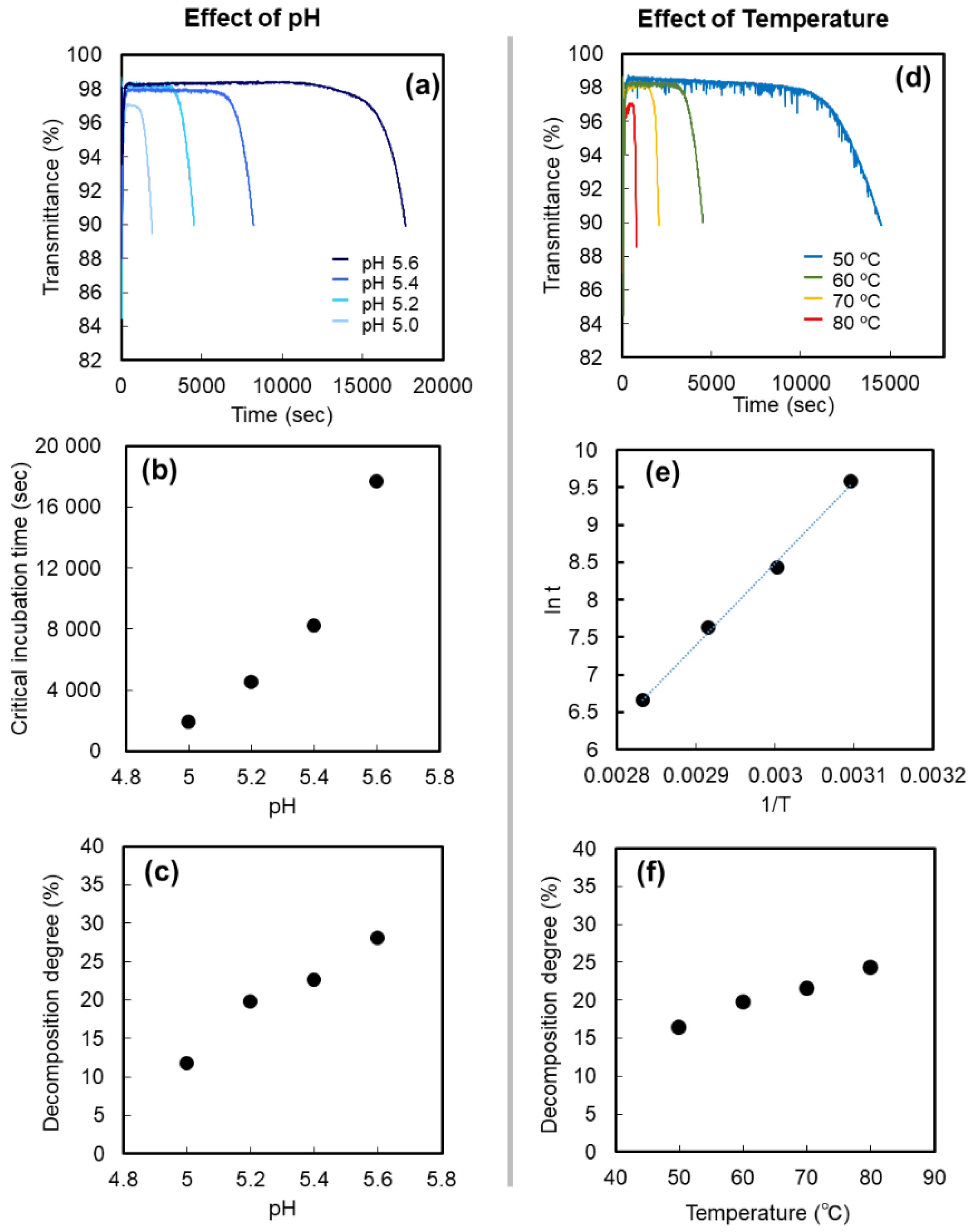
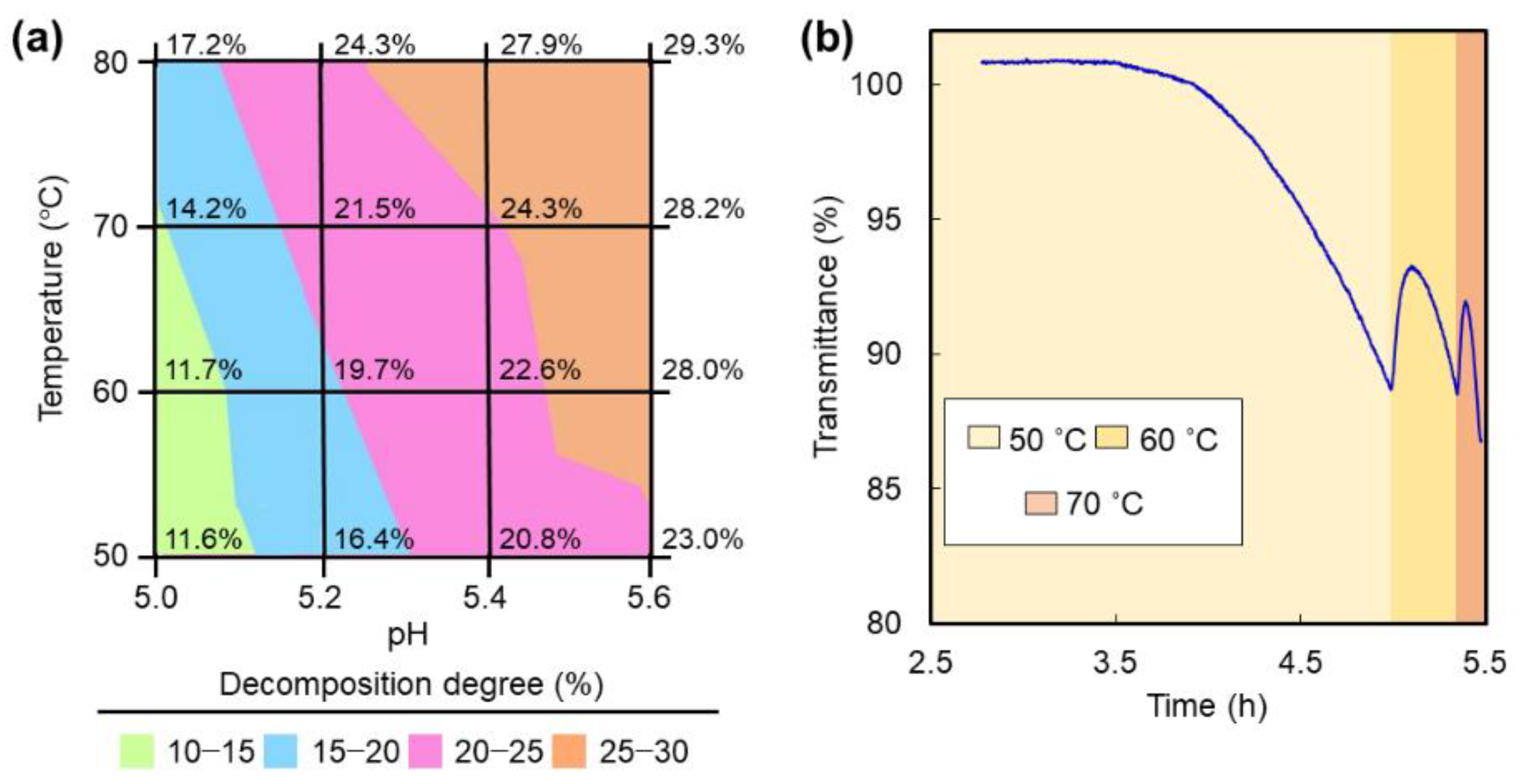
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Acid-Base Titration
3.3. Transmittance Measurements
3.4. Determination of Cleavage Degree of PAA-Pht
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, C.; Ma, Z.; Zhu, X.X. Rational design of thermoresponsive polymers in aqueous solutions: A thermodynamics map. Prog. Polym. Sci. 2019, 90, 269–291. [Google Scholar] [CrossRef]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Bedrov, D.J. Roles of enthalpy, entropy, and hydrogen bonding in the lower critical solution temperature behavior of poly (ethylene oxide)/water solutions. J. Phys. Chem. B 2003, 107, 3095–3097. [Google Scholar] [CrossRef]
- Barker, J.A.; Fock, W. Theory of upper and lower critical solution temperatures. Discuss. Faraday Soc. 1953, 15, 188–195. [Google Scholar] [CrossRef]
- Trzebicka, B.; Szweda, R.; Kosowski, D.; Szweda, D.; Otulakowski, Ł.; Haladjova, E.; Dworak, A. Thermoresponsive polymer-peptide/protein conjugates. Prog. Polym. Sci. 2017, 68, 35–76. [Google Scholar] [CrossRef]
- Vanparijs, N.; Nuhn, L.; De Geest, B.G. Transiently thermoresponsive polymers and their applications in biomedicine. Chem. Soc. Rev. 2017, 46, 1193–1239. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T.J. Thermoresponsive polymers and their biomedical application in tissue engineering—A review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef]
- Kobayashi, J.; Yamato, M.; Okano, T.J. On-off affinity binding modulation on thermoresponsive polymer-grafted surfaces for capture and release of proteins and cells. J. Biomater. Sci. Polym. Ed. 2017, 28, 939–957. [Google Scholar] [CrossRef]
- Nagase, K.; Okano, T.J. Thermoresponsive-polymer-based materials for temperature-modulated bioanalysis and bioseparations. J. Mater. Chem. B 2016, 4, 6381–6397. [Google Scholar] [CrossRef]
- Agbim, K.A.; Schaefer, L.A. Investigation of thermoresponsive microgel polymer swelling theory. Polym. Rev. 2020, 60, 648–670. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: From fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Kikuchi, A.; Okano, T. Intelligent thermoresponsive polymeric stationary phases for aqueous chromatography of biological compounds. Prog. Polym. Sci. 2002, 27, 1165–1193. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Ultrathin poly (N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir 2004, 20, 5506–5511. [Google Scholar] [CrossRef]
- Tan, H.; Ramirez, C.M.; Miljkovic, N.; Li, H.; Rubin, J.P.; Marra, K.G. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials 2009, 30, 6844–6853. [Google Scholar] [CrossRef] [PubMed]
- Calejo, M.T.; Sande, S.A.; Nyström, B. Thermoresponsive polymers as gene and drug delivery vectors: Architecture and mechanism of action. Expert Opin. Drug Deliv. 2013, 10, 1669–1686. [Google Scholar] [CrossRef]
- Song, X.; Zhu, J.-L.; Wen, Y.; Zhao, F.; Zhang, Z.-X.; Li, J.J. Thermoresponsive supramolecular micellar drug delivery system based on star-linear pseudo-block polymer consisting of β-cyclodextrin-poly (N-isopropylacrylamide) and adamantyl-poly (ethylene glycol). J. Colloid Interface Sci. 2017, 490, 372–379. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. Polymers with upper critical solution temperature in aqueous solution: Unexpected properties from known building blocks. ACS Macro Lett. 2013, 2, 597–600. [Google Scholar] [CrossRef]
- Lutz, J.F.; Akdemir, Ö.; Hoth, A.J. Point by point comparison of two thermosensitive polymers exhibiting a similar LCST: Is the age of poly (NIPAM) over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef]
- Xia, Y.; Yin, X.; Burke, N.A.D.; Stöver, H.D.H. Thermal response of narrow-disperse poly (N-isopropylacrylamide) prepared by atom transfer radical polymerization. Macromolecules 2005, 38, 5937–5943. [Google Scholar] [CrossRef]
- Schild, H.G. Poly (N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Thijs, H.M.L.; Jochems, M.J.H.C.; Van Lankvelt, B.M.; Fijten, M.W.M.; Schubert, U.S. Tuning the LCST of poly (2-oxazoline) s by varying composition and molecular weight: Alternatives to poly (N-isopropylacrylamide)? Chem. Comm. 2008, 44, 5758–5760. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kataoka, K. Comprehensive and accurate control of thermosensitivity of poly (2-alkyl-2-oxazoline) s via well-defined gradient or random copolymerization. Macromolecules 2007, 40, 3599–3609. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Chen, X.D.; Luo, W.A.; Yang, J.; Zhang, M.Q.; Zhu, F.M. Study of phase separation of poly (vinyl methyl ether) aqueous solutions with Rayleigh scattering technique. Macromolecules 2009, 42, 1720–1725. [Google Scholar] [CrossRef]
- Maeda, Y. IR spectroscopic study on the hydration and the phase transition of poly (vinyl methyl ether) in water. Langmuir 2001, 17, 1737–1742. [Google Scholar] [CrossRef]
- Becer, C.R.; Hahn, S.; Fijten, M.W.M.; Thijs, H.M.L.; Hoogen-boom, R.; Schubert, U.S. Libraries of methacrylic acid and oligo (ethylene glycol) methacrylate copolymers with LCST behavior. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7138–7147. [Google Scholar] [CrossRef]
- Emoto, J.; Kitayama, Y.; Harada, A. Thermoresponsiveness of Carboxylated Polyallylamines Induced by Divalent Counterions as Ionic Effectors. Macromolecules 2022, 55, 7204–7211. [Google Scholar] [CrossRef]
- Kirby, A.J.; Lancaster, P.W. Structure and efficiency in intramolecular and enzymic catalysis. Catalysis of amide hydrolysis by the carboxy-group of substituted maleamic acids. J. Chem. Soc. Perkin Trans. 1972, 2, 1206–1214. [Google Scholar] [CrossRef]
- Scorsin, L.; Affeldt, R.F.; Oliveira, B.S.; Silveira, E.V.; Ferraz, M.S.; Souza, F.P.S.; Caramori, G.F.; Nome, F.J. Coordination among bond formation/cleavage in a bifunctional-catalyzed fast amide hydrolysis: Evidence for an optimized Intramolecular N-protonation event. J. Org. Chem. 2020, 85, 4663–4671. [Google Scholar] [CrossRef]
- Zhu, Y.; Noy, J.M.; Lowe, A.B.; Roth, P.J. The synthesis and aqueous solution properties of sulfobutylbetaine (co) polymers: Comparison of synthetic routes and tuneable upper critical solution temperatures. Polym. Chem. 2015, 6, 5705–5718. [Google Scholar] [CrossRef] [Green Version]
- Maji, T.; Banerjee, S.; Biswas, Y.; Mandal, T.K. Dual-stimuli-responsive l-serine-based zwitterionic UCST-type polymer with tunable thermosensitivity. Macromolecules 2015, 48, 4957–4966. [Google Scholar] [CrossRef]
- Das, E.; Matsumura, K.J. Tunable phase-separation behavior of thermoresponsive polyampholytes through molecular design. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 876–884. [Google Scholar] [CrossRef]
- Matsuda, Y.; Kobayashi, M.; Annaka, M.; Ishihara, K.; Takahara, A. UCST-type cononsolvency behavior of poly (2-methacryloxyethyl phosphorylcholine) in the mixture of water and ethanol. Polym. J. 2008, 40, 479–483. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitayama, Y.; Yazaki, Y.; Emoto, J.; Yuba, E.; Harada, A. Programed Thermoresponsive Polymers with Cleavage-Induced Phase Transition. Molecules 2022, 27, 6082. https://doi.org/10.3390/molecules27186082
Kitayama Y, Yazaki Y, Emoto J, Yuba E, Harada A. Programed Thermoresponsive Polymers with Cleavage-Induced Phase Transition. Molecules. 2022; 27(18):6082. https://doi.org/10.3390/molecules27186082
Chicago/Turabian StyleKitayama, Yukiya, Yasumichi Yazaki, Junya Emoto, Eiji Yuba, and Atsushi Harada. 2022. "Programed Thermoresponsive Polymers with Cleavage-Induced Phase Transition" Molecules 27, no. 18: 6082. https://doi.org/10.3390/molecules27186082




