Characterization of Hyaluronidase 4 Involved in the Catabolism of Chondroitin Sulfate
Abstract
1. Introduction
2. Results
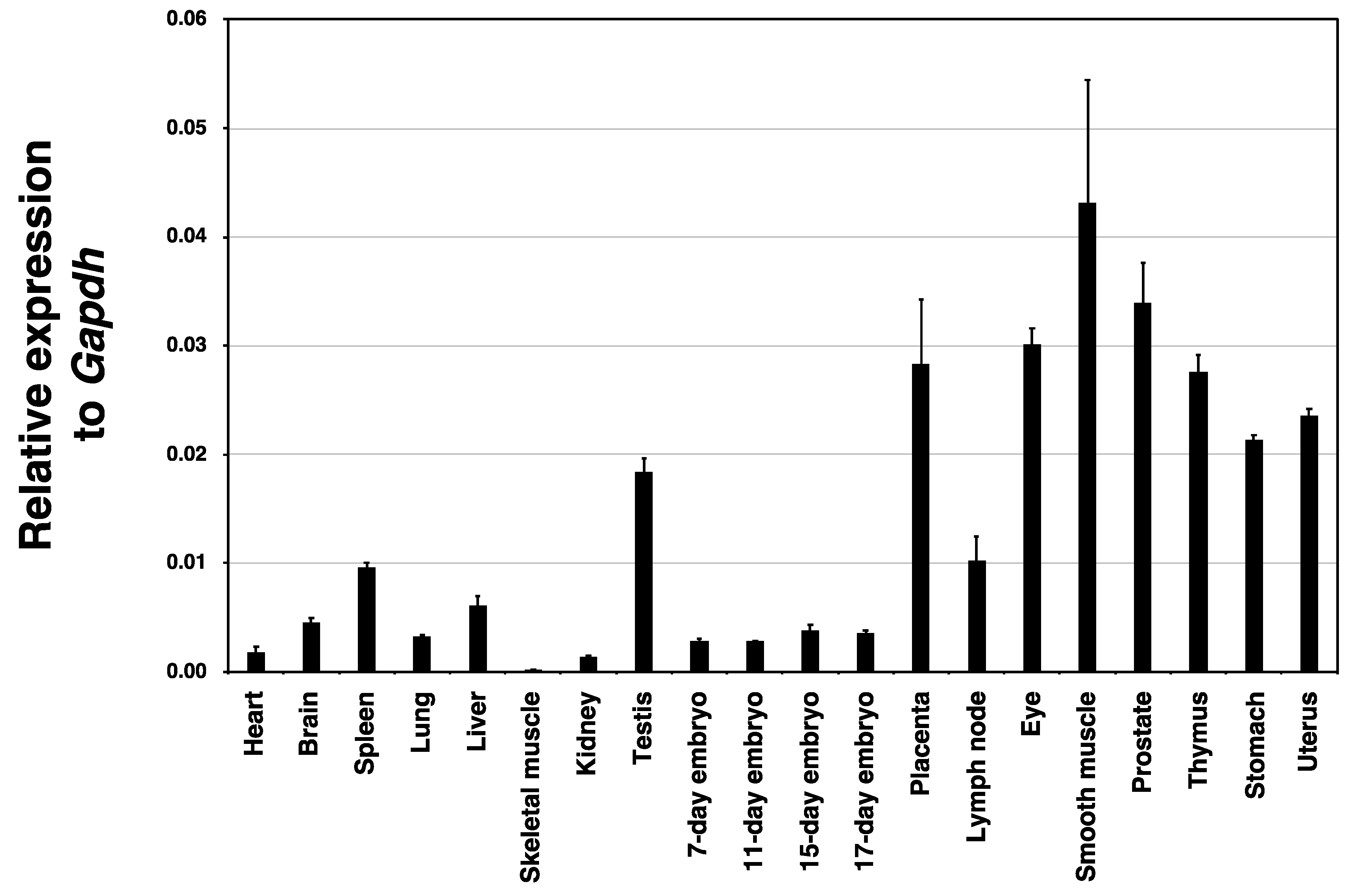
2.1. Expression Analysis of Hyal4 mRNA in Various Tissues
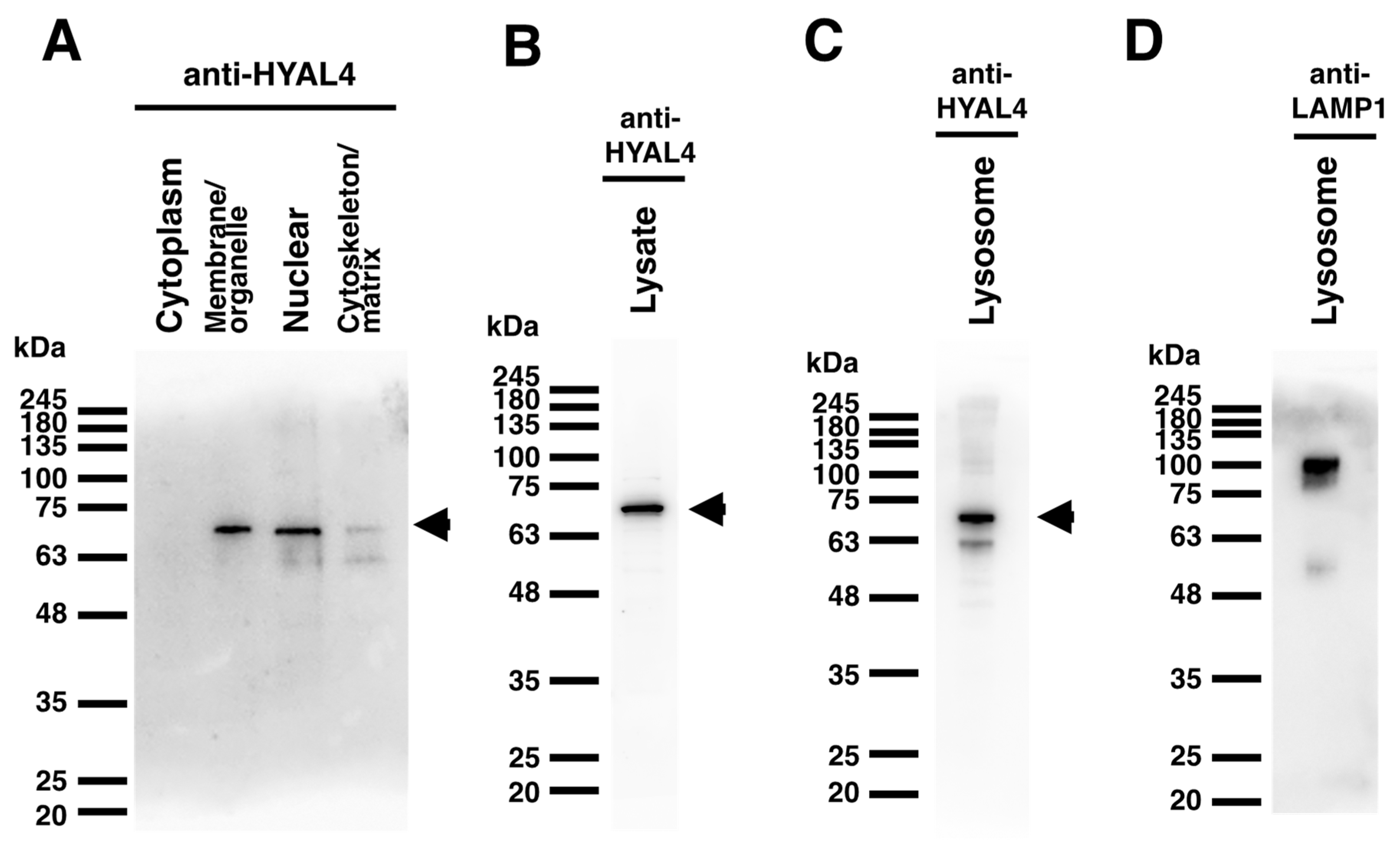
2.2. Expression Analysis of Mouse HYAL4 Protein in Various Tissues
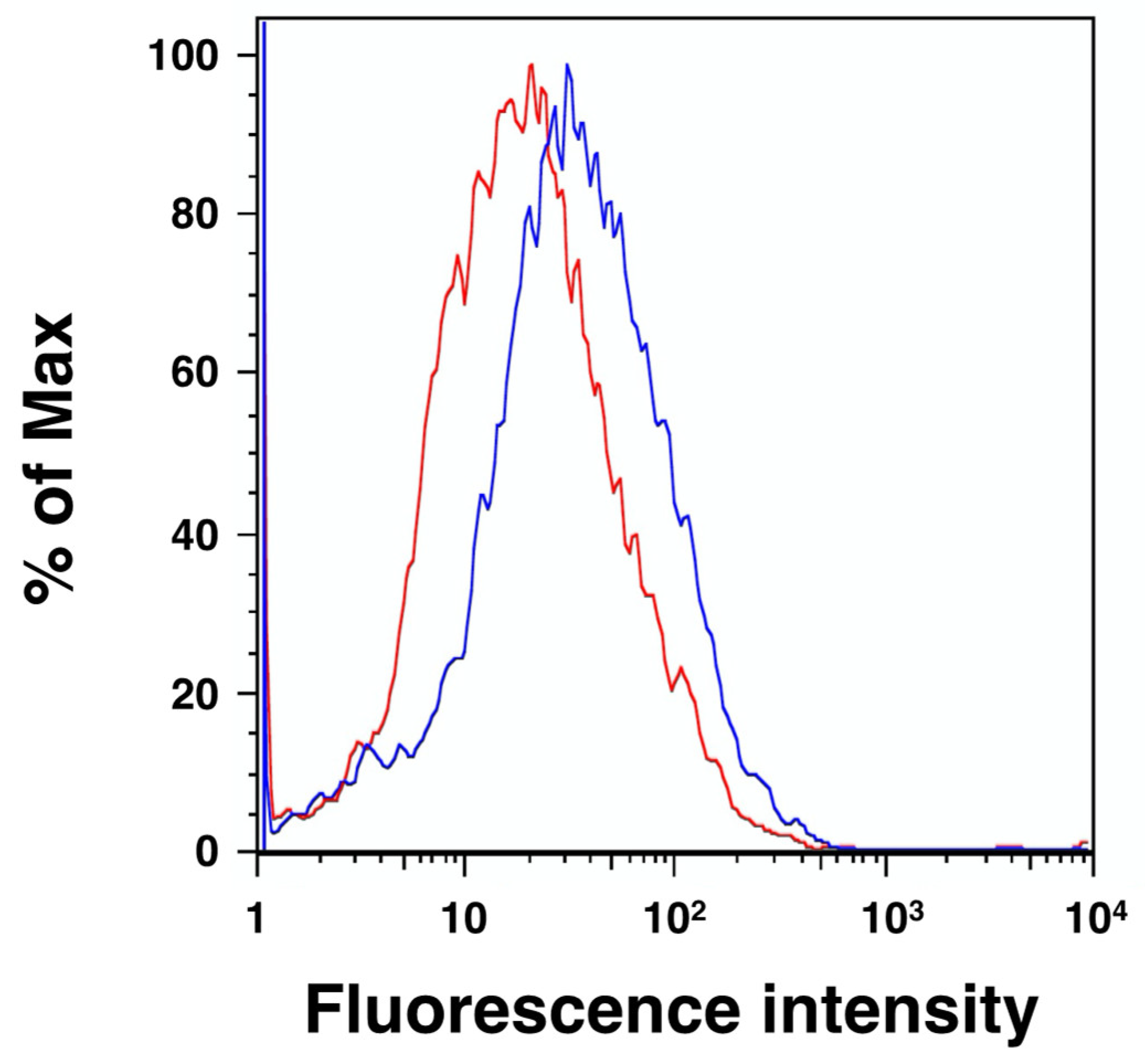
2.3. Cellular Localization of Mouse HYAL4 Protein
2.4. Effects of Overexpression of Mouse HYAL4 on CS Metabolism of CHO Cells
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics for Animal Experiments
4.2. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.3. Semiquantitative and Real-Time RT-PCR
4.4. Western Blotting
4.5. Flow Cytometry Analysis
4.6. Preparation of Membrane and Lysosome Fractions
4.7. Overexpression of Mouse HYAL4 in CHO Cells
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duran-Reynals, F. A study of the generalization of vaccine virus from enhanced skin lesions. J. Exp. Med. 1929, 50, 327–340. [Google Scholar] [CrossRef]
- Meyer, K.; Rapport, M.M. The hydrolysis of chondroitin sulfate by testicular hyaluronidase. Arch. Biochem. 1950, 27, 287–293. [Google Scholar]
- Prabhakar, V.; Sasisekharan, R. The biosynthesis and catabolism of galactosaminoglycans. Adv. Pharmacol. 2006, 53, 69–115. [Google Scholar] [CrossRef]
- Mizumoto, S.; Yamada, S.; Sugahara, K. Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr. Opin. Struct. Biol. 2015, 34, 35–42. [Google Scholar] [CrossRef]
- Mikami, T.; Kitagawa, H. Sulfated glycosaminoglycans: Their distinct roles in stem cell. Glycoconj. J. 2017, 34, 725–735. [Google Scholar] [CrossRef]
- Mizumoto, S.; Yamada, S. An overview of in vivo functions of chondroitin sulfate and dermatan sulfate revealed by their deficient mice. Front. Cell Dev. Biol. 2021, 9, 764781. [Google Scholar] [CrossRef]
- Sugahara, K.; Mikami, T. Chondroitin/dermatan sulfate in the central nervous system. Curr. Opin. Struct. Biol. 2007, 17, 536–545. [Google Scholar] [CrossRef]
- Mikami, T.; Yasunaga, D.; Kitagawa, H. Contactin-1 is a functional receptor for neuroregulatory chondroitin sulfate-E. J. Biol. Chem. 2009, 284, 4494–4499. [Google Scholar] [CrossRef]
- Shida, M.; Mikami, T.; Tamura, J.I.; Kitagawa, H. Chondroitin sulfate-D promotes neurite outgrowth by acting as an extracellular ligand for neuronal integrin αVβ3. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1319–1331. [Google Scholar] [CrossRef]
- Stevens, R.L.; Fox, C.C.; Lichtenstein, L.M.; Austen, K.F. Identification of chondroitin sulfate E proteoglycans and heparin proteoglycans in the secretory granules of human lung mast cells. Proc. Natl. Acad. Sci. USA 1988, 85, 2284–2287. [Google Scholar] [CrossRef]
- Iida, J.; Meijne, A.M.; Knutson, J.R.; Furcht, L.T.; McCarthy, J.B. Cell surface chondroitin sulfate proteoglycans in tumor cell adhesion, motility and invasion. Semin. Cancer Biol. 1996, 7, 155–162. [Google Scholar] [CrossRef]
- Yamada, S.; Sugahara, K. Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Curr. Drug Discov. Technol. 2008, 5, 289–301. [Google Scholar] [CrossRef]
- Galindo, L.T.; Mundim, M.; Pinto, A.S.; Chiarantin, G.; Almeida, M.; Lamers, M.L.; Horwitz, A.R.; Santos, M.F.; Porcionatto, M. Chondroitin sulfate impairs neural stem cell migration through ROCK activation. Mol. Neurobiol. 2018, 55, 3185–3195. [Google Scholar] [CrossRef]
- Silbert, J.E.; Sugumaran, G. Biosynthesis of chondroitin/dermatan sulfate. IUBMB Life 2002, 54, 177–186. [Google Scholar] [CrossRef]
- Mikami, T.; Kitagawa, H. Biosynthesis and function of chondroitin sulfate. Biochim. Biophys. Acta 2013, 1830, 4719–4733. [Google Scholar] [CrossRef]
- Csoka, A.B.; Frost, G.I.; Stern, R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001, 20, 499–508. [Google Scholar] [CrossRef]
- Yamada, S. Catabolism of chondroitin sulfate. Cell. Mol. Biol. Lett. 2015, 20, 196–212. [Google Scholar] [CrossRef]
- Honda, T.; Kaneiwa, T.; Mizumoto, S.; Sugahara, K.; Yamada, S. Hyaluronidases have strong hydrolytic activity toward chondroitin 4-sulfate comparable to that for hyaluronan. Biomolecules 2012, 2, 549–563. [Google Scholar] [CrossRef]
- Kinoshita, A.; Yamada, S.; Haslam, S.M.; Morris, H.R.; Dell, A.; Sugahara, K. Novel tetrasaccharides isolated from squid cartilage chondroitin sulfate E contain unusual sulfated disaccharide units GlcA(3-O-sulfate)beta1-3GalNAc(6-O-sulfate) or GlcA(3-O-sulfate)beta1-3GalNAc. J. Biol. Chem. 1997, 272, 19656–19665. [Google Scholar] [CrossRef]
- Kaneiwa, T.; Mizumoto, S.; Sugahara, K.; Yamada, S. Identification of human hyaluronidase-4 as a novel chondroitin sulfate hydrolase that preferentially cleaves the galactosaminidic linkage in the trisulfated tetrasaccharide sequence. Glycobiology 2010, 20, 300–309. [Google Scholar] [CrossRef]
- Kaneiwa, T.; Miyazaki, A.; Kogawa, R.; Mizumoto, S.; Sugahara, K.; Yamada, S. Identification of amino acid residues required for the substrate specificity of human and mouse chondroitin sulfate hydrolase (conventional hyaluronidase-4). J. Biol. Chem. 2012, 287, 42119–42128. [Google Scholar] [CrossRef] [PubMed]
- Csóka, A.B.; Scherer, S.W.; Stern, R. Expression analysis of six paralogous human hyaluronidase genes clustered on chromosomes 3p21 and 7q31. Genomics 1999, 60, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, B.L.; Mizumoto, S.; Lord, M.S.; O’Grady, R.L.; Kuchel, R.P.; Yamada, S.; Whitelock, J.M. Hyaluronidase-4 is produced by mast cells and can cleave serglycin chondroitin sulfate chains into lower molecular weight forms. J. Biol. Chem. 2019, 294, 11458–11472. [Google Scholar] [CrossRef]
- Maciej-Hulme, M.L. New Insights into Human Hyaluronidase 4/Chondroitin Sulphate Hydrolase. Front. Cell Dev. Biol. 2021, 9, 767924. [Google Scholar] [CrossRef] [PubMed]
- Mollinedo, F.; Gajate, C. Lipid rafts as major platforms for signaling regulation in cancer. Adv. Biol. Regul. 2015, 57, 130–146. [Google Scholar] [CrossRef]
- Levental, I.; Levental, K.R.; Heberle, F.A. Lipid Rafts: Controversies Resolved, Mysteries Remain. Trends Cell Biol. 2020, 30, 341–353. [Google Scholar] [CrossRef]
- Pantazaka, E.; Papadimitriou, E. Chondroitin sulfate-cell membrane effectors as regulators of growth factor-mediated vascular and cancer cell migration. Biochim. Biophys. Acta 2014, 1840, 2643–2650. [Google Scholar] [CrossRef]
- Wang, B.; Jia, J.; Zhang, X.; Zcharia, E.; Vlodavsky, I.; Pejler, G.; Li, J.P. Heparanase affects secretory granule homeostasis of murine mast cells through degrading heparin. J. Allergy Clin. Immunol. 2011, 128, 1310–1317. [Google Scholar] [CrossRef]
- Higashi, N.; Waki, M.; Sue, M.; Kogane, Y.; Shida, H.; Tsunekawa, N.; Hasan, A.; Sato, T.; Kitahara, A.; Kasaoka, T.; et al. Heparanase-mediated cleavage of macromolecular heparin accelerates release of granular components of mast cells from extracellular matrices. Biochem. J. 2014, 458, 291–299. [Google Scholar] [CrossRef]
- Higashi, N.; Waki, M.; Sudo, Y.; Suzuki, S.; Oku, T.; Tsuiji, M.; Tsuji, T.; Miyagishi, M.; Takahashi, K.; Nakajima, M.; et al. Incorporation, intracellular trafficking and processing of extracellular heparanase by mast cells: Involvement of syndecan-4-dependent pathway. Biochem. Biophys. Res. Commun. 2018, 503, 3235–3241. [Google Scholar] [CrossRef]
- Levy-Adam, F.; Ilan, N.; Vlodavsky, I. Tumorigenic and adhesive properties of heparanase. Semin. Cancer Biol. 2010, 20, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Barash, U.; Cohen-Kaplan, V.; Dowek, I.; Sanderson, R.D.; Ilan, N.; Vlodavsky, I. Proteoglycans in health and disease: New concepts for heparanase function in tumor progression and metastasis. FEBS J. 2010, 277, 3890–3903. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwar, V.B.; Morera, D.S.; Hasanali, S.L.; Yates, T.J.; Hupe, M.C.; Knapp, J.; Lokeshwar, S.D.; Wang, J.; Hennig, M.J.; Baskar, R.; et al. A novel splice variant of HYAL-4 drives malignant transformation and predicts outcome in patients with bladder cancer. Clin. Cancer Res. 2020, 26, 3455–3467. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.; Shirodkar, S.P.; Escudero, D.O.; Ekwenna, O.O.; Yates, T.J.; Ayyathurai, R.; Garcia-Roig, M.; Gahan, J.C.; Manoharan, M.; Bird, V.G.; et al. Molecular characterization of kidney cancer: Association of hyaluronic acid family with histological subtypes and metastasis. Cancer 2012, 118, 2394–2402. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, D.; Wang, L.; Yu, J.; Du, D.; Chen, Y.; Gao, P.; Wang, D.-M.; Yu, J.; Zhang, F.; et al. Distinct genomic aberrations between low-grade and high-grade gliomas of Chinese patients. PLoS ONE 2013, 8, e57168. [Google Scholar] [CrossRef] [PubMed]
- Hasanali, S.L.; Morera, D.S.; Racine, R.R.; Hennig, M.; Ghosh, S.; Lopez, L.E.; Hupe, M.C.; Escudero, D.O.; Wang, J.; Zhu, H.; et al. HYAL4-V1/Chondroitinase (Chase) drives gemcitabine resistance and predicts chemotherapy failure in patients with bladder cancer. Clin. Cancer Res. 2021, 27, 4410–4421. [Google Scholar] [CrossRef]
- Deepa, S.S.; Yamada, S.; Zako, M.; Goldberger, O.; Sugahara, K. Chondroitin sulfate chains on syndecan-1 and syndecan-4 from normal murine mammary gland epithelial cells are structurally and functionally distinct and cooperate with heparan sulfate chains to bind growth factors. A novel function to control binding of midkine, pleiotrophin, and basic fibroblast growth factor. J. Biol. Chem. 2004, 279, 37368–37376. [Google Scholar] [CrossRef]


| Disaccharide | Mock 1 | Hyal41 |
|---|---|---|
| ΔHexA-GalNAc 2 | ND 3 | ND |
| ΔHexA-GalNAc(4-O-sulfate) | 224.4 | 77.2 |
| ΔHexA-GalNAc(6-O-sulfate) | 2.3 | 1.1 |
| ΔHexA(2-O-sulfate)-GalNAc(6-O-sulfate) | ND | ND |
| ΔHexA-GalNAc(4,6-O-disulfate) | ND | ND |
| Total | 226.7 | 78.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, S.; Mizumoto, S. Characterization of Hyaluronidase 4 Involved in the Catabolism of Chondroitin Sulfate. Molecules 2022, 27, 6103. https://doi.org/10.3390/molecules27186103
Yamada S, Mizumoto S. Characterization of Hyaluronidase 4 Involved in the Catabolism of Chondroitin Sulfate. Molecules. 2022; 27(18):6103. https://doi.org/10.3390/molecules27186103
Chicago/Turabian StyleYamada, Shuhei, and Shuji Mizumoto. 2022. "Characterization of Hyaluronidase 4 Involved in the Catabolism of Chondroitin Sulfate" Molecules 27, no. 18: 6103. https://doi.org/10.3390/molecules27186103
APA StyleYamada, S., & Mizumoto, S. (2022). Characterization of Hyaluronidase 4 Involved in the Catabolism of Chondroitin Sulfate. Molecules, 27(18), 6103. https://doi.org/10.3390/molecules27186103







