Nanolipogel Loaded with Tea Tree Oil for the Management of Burn: GC-MS Analysis, In Vitro and In Vivo Evaluation
Abstract
1. Introduction
2. Results and Discussion
2.1. GC-MS Analysis
2.2. Rational for the Selection of the SLN Components
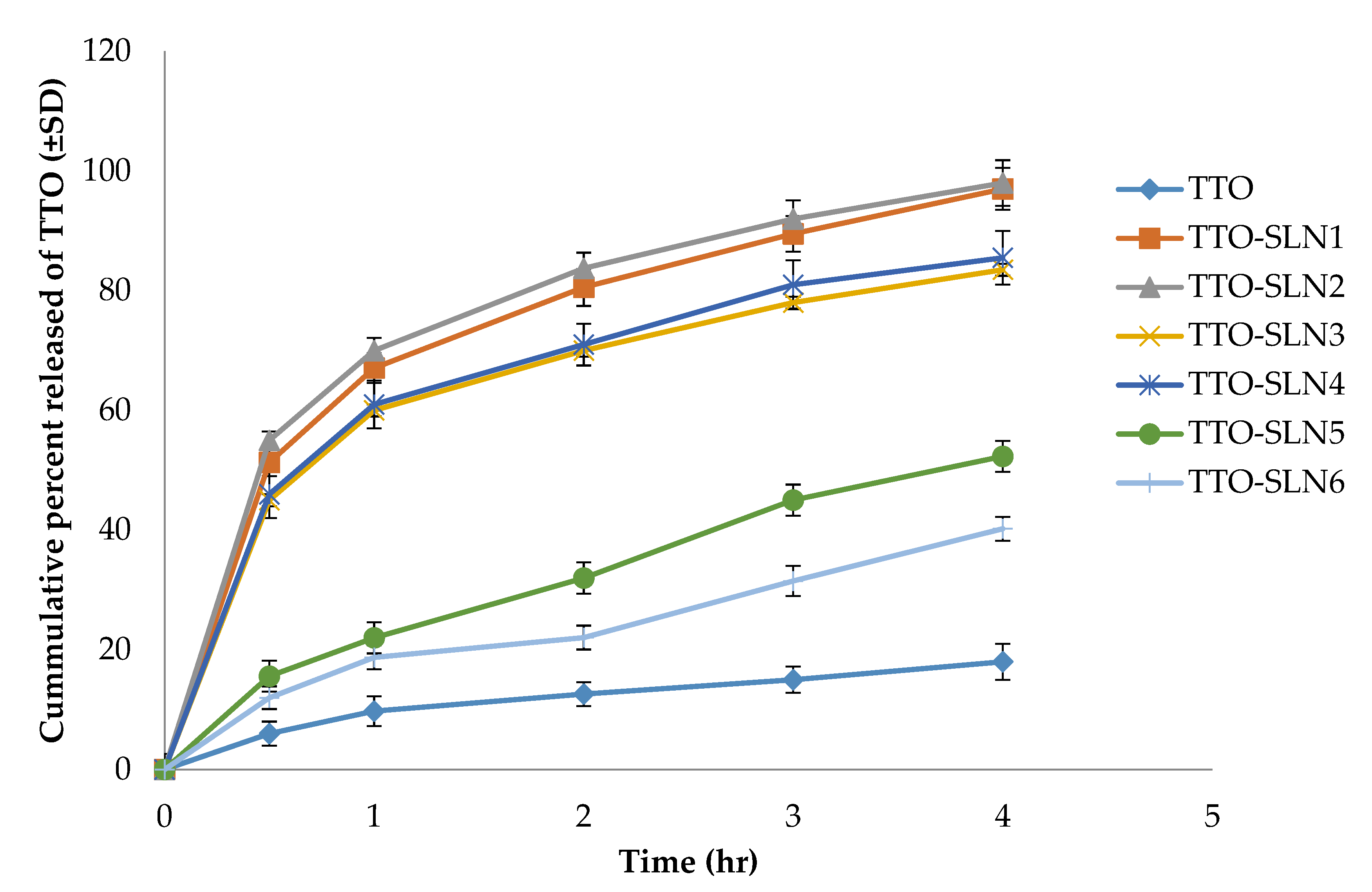
2.3. Physicochemical Properties of Prepared TTO-Loaded SLN
2.3.1. Entrapment Efficiency and Loading Capacity
2.3.2. Particle Size Analysis (Mean Particle Size, Polydispersity Index, and Zeta Potential)
2.3.3. In Vitro Release Studies
2.4. Characterization of Optimized TTO-SLN
2.4.1. Transmission Electron Microscopy (TEM)
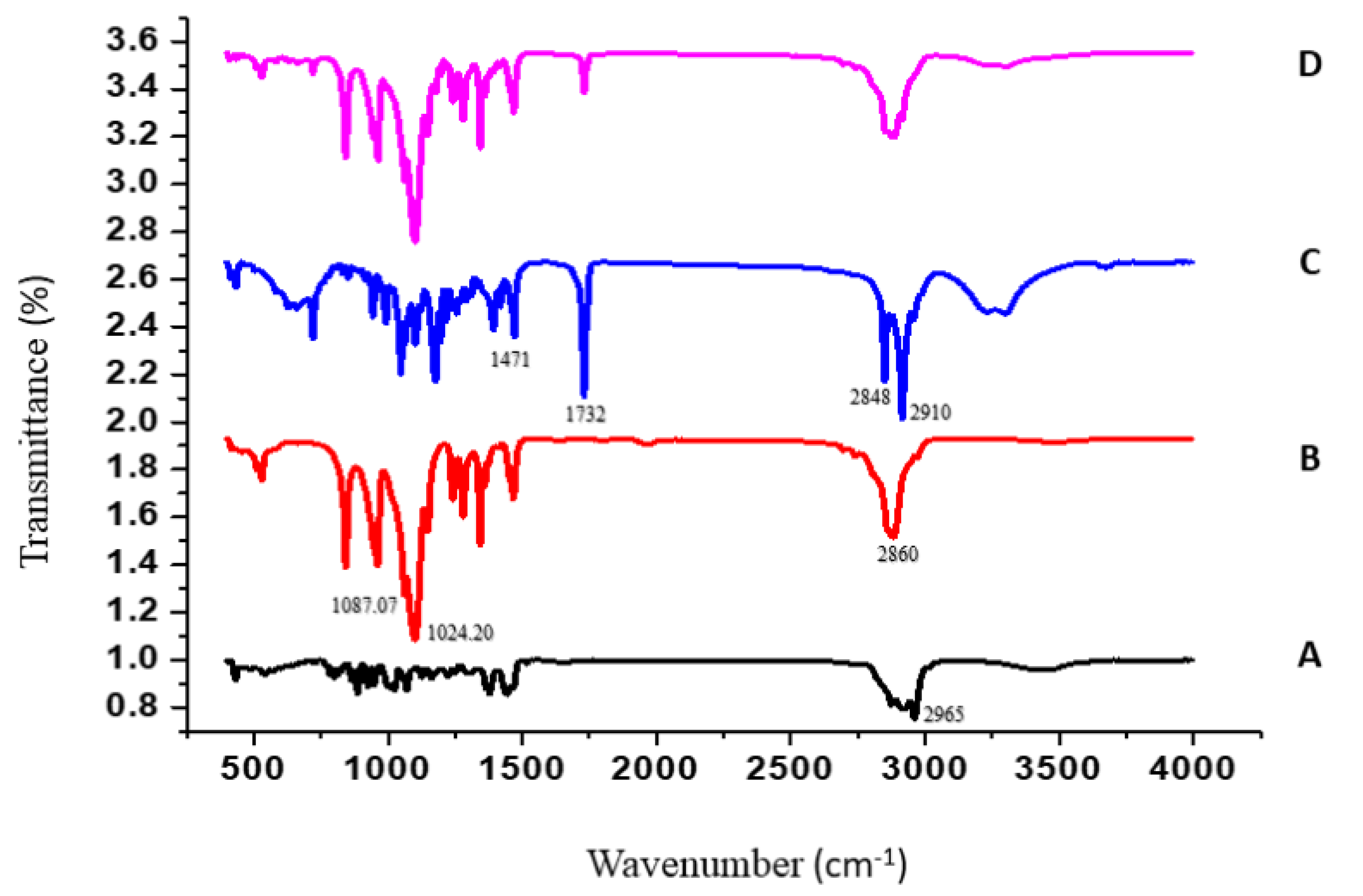
2.4.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5. Evaluation of TTO-Loaded Nanolipogel
2.5.1. Determination of Gel pH
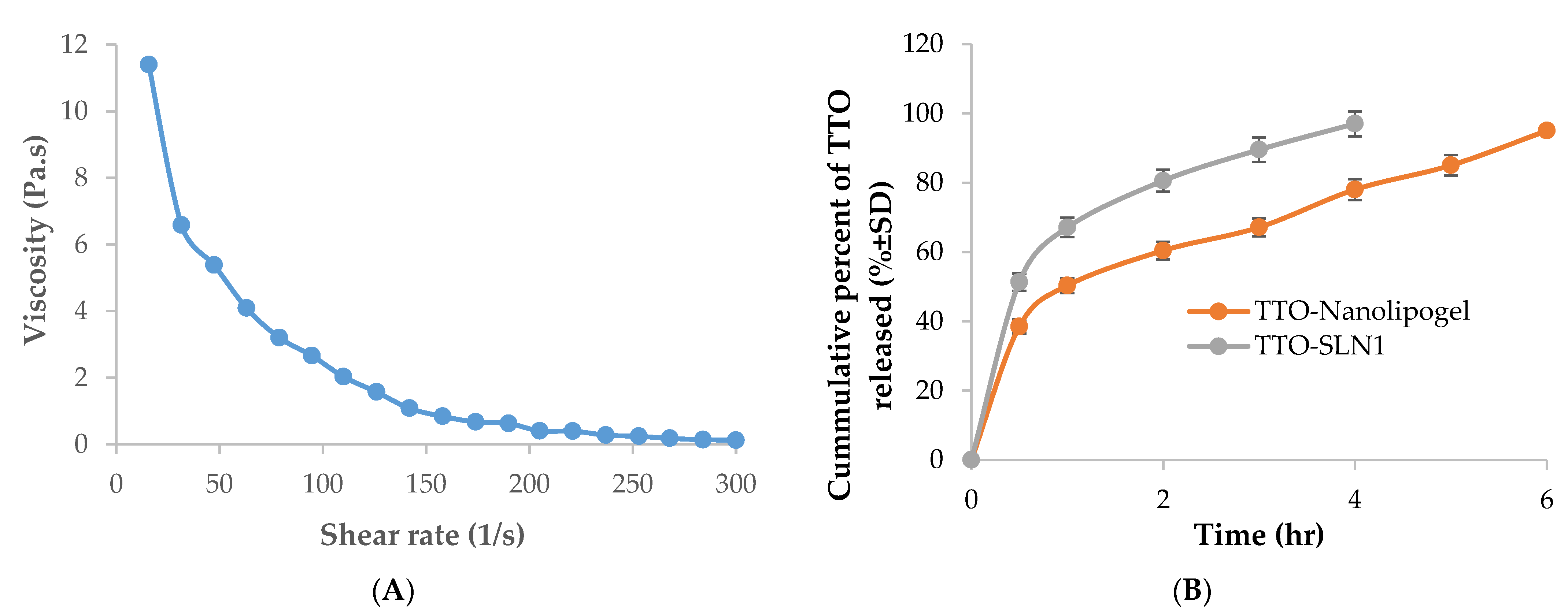
2.5.2. Rheological Properties
2.5.3. In Vitro Release Studies for TTO-Nanolipogel
2.6. In Vivo Studies
2.6.1. Skin Irritation Test
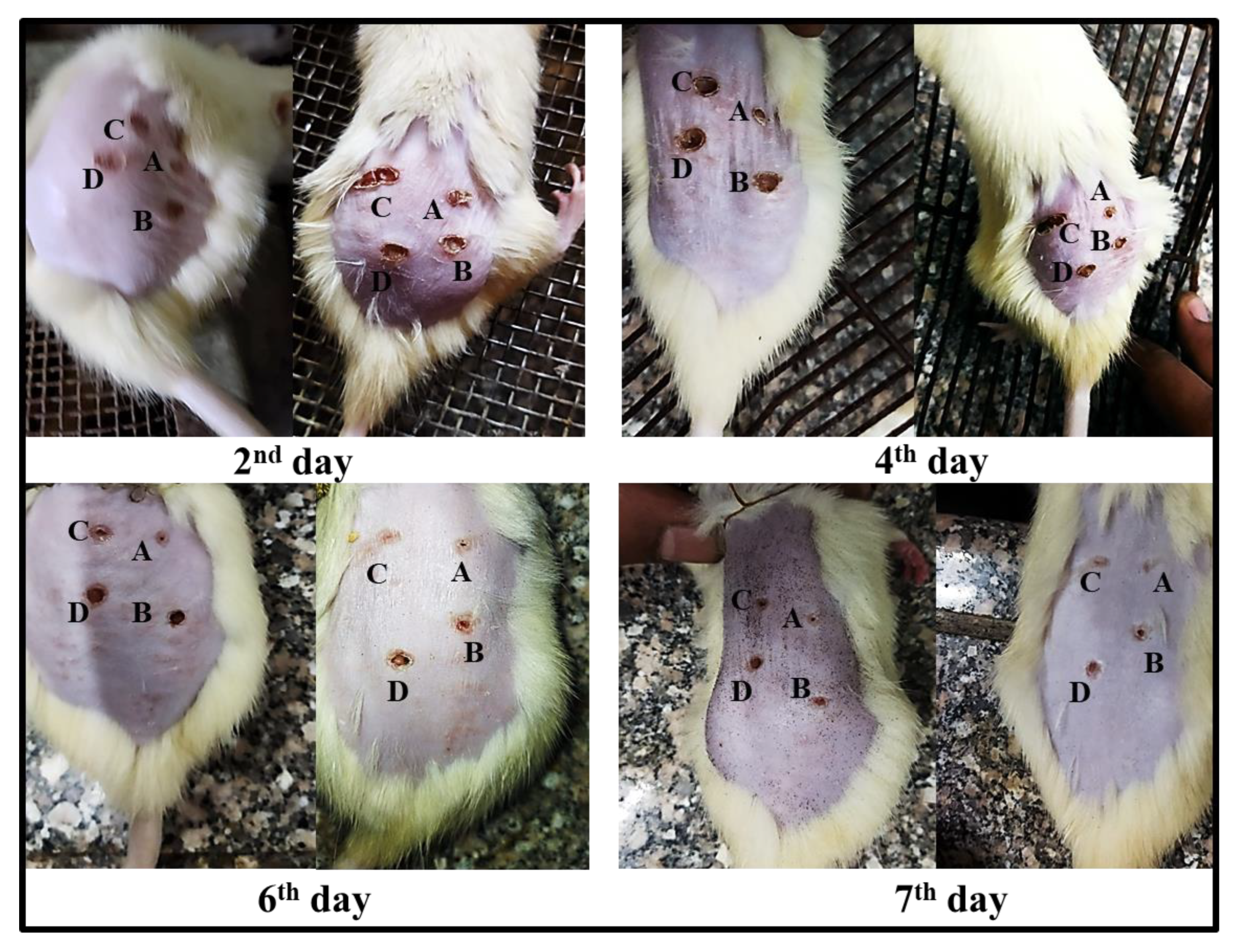
2.6.2. Burn Healing
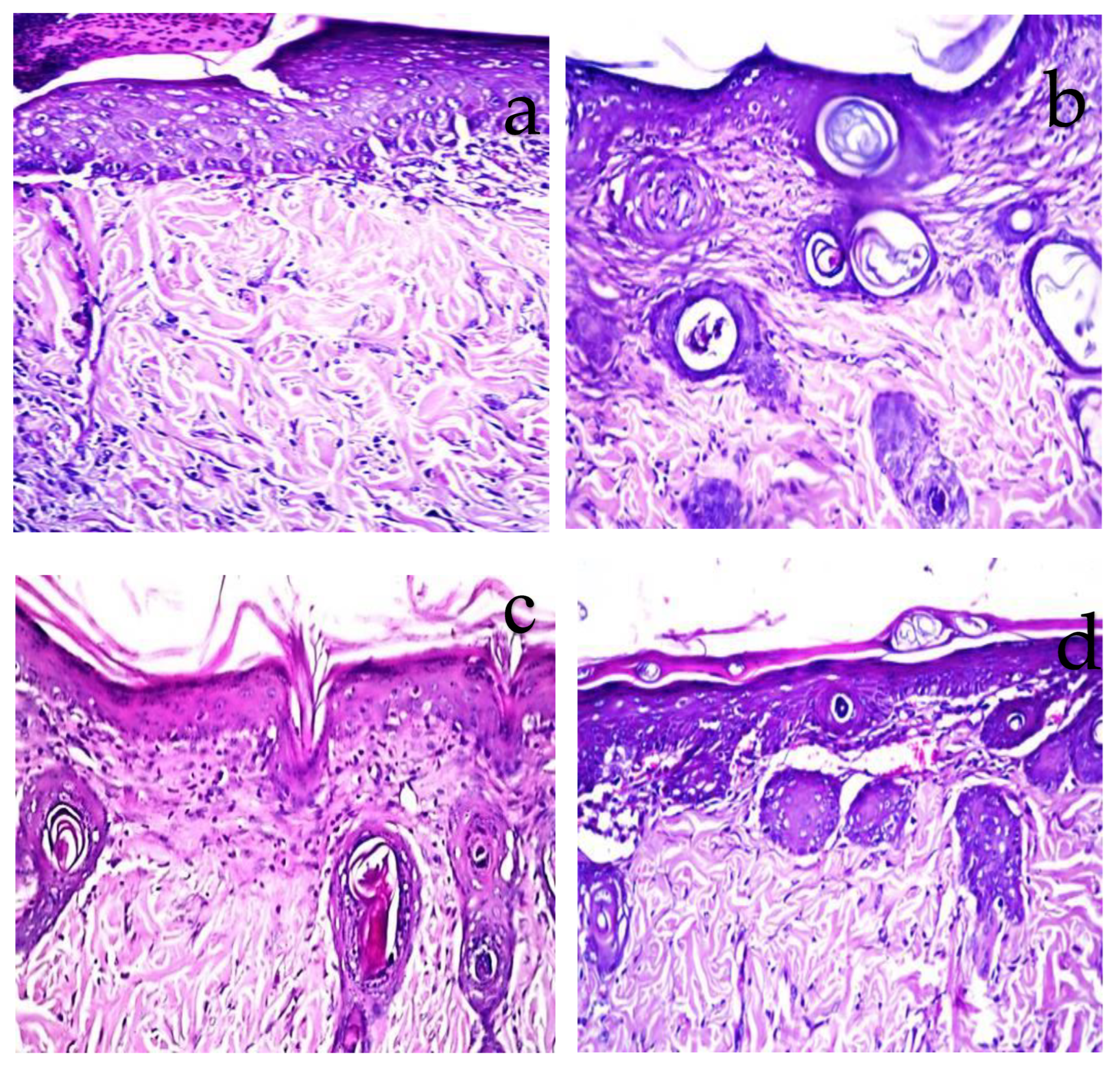
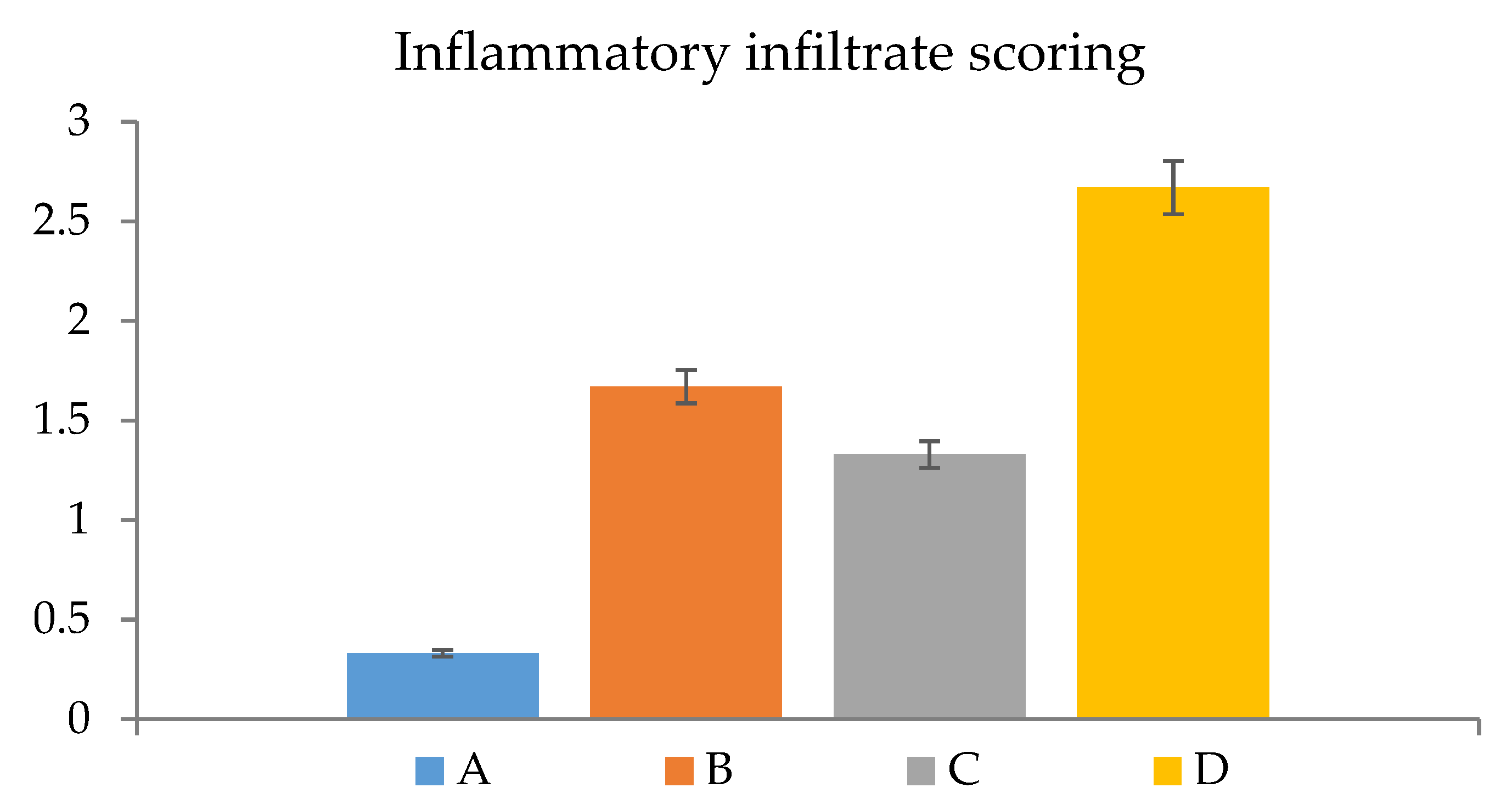
2.7. Histopathological Observations
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. GC-MS Analysis
3.2.2. Preparation of Oil-Loaded Solid Lipid Nanoparticles (SLNs)
3.2.3. Encapsulation Efficiency and Loading Capacity
3.2.4. Examination of Physicochemical Properties of Nanoparticles: Particle Size, Zeta Potential, and Polydispersity Index
3.2.5. In Vitro Release Study
3.2.6. Characterization of Optimized TTO-SLN
Transmission Electron Microscopy
Fourier-Transform Infrared Spectroscopy (FTIR)
Preparation of TTO-Loaded Nanolipogel
3.2.7. Characterization of TTO-Loaded Nanolipogel
Determination of pH
Rheological Study
In Vitro Release Study
3.3. In Vivo Studies
3.3.1. Animals
3.3.2. Skin Irritation Test
3.3.3. Induction of Burn Wounds
3.4. Histopathological Evaluation
3.5. Assessment of Inflammation
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sharaf, S.M.; Al-Mofty, S.E.-D.; El-Sayed, E.-S.M.; Omar, A.; Dena, A.S.A.; El-Sherbiny, I.M. Deacetylated cellulose acetate nanofibrous dressing loaded with chitosan/propolis nanoparticles for the effective treatment of burn wounds. Int. J. Biol. Macromol. 2021, 193, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel asymmetric wettable agnps/chitosan wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Hydrogel dressings for the treatment of burn wounds: An up-to-date overview. Materials 2020, 13, 2853. [Google Scholar] [CrossRef]
- Namviriyachote, N.; Manosittisak, K.; Ritthidej, G.C. Physico-mechanical characterization of polyurethane foam dressings containing natural polyols. Asian J. Pharm. Sci. 2016, 11, 114–115. [Google Scholar] [CrossRef]
- Jarrahi, M. An experimental study of the effects of Matricaria chamomilla extract on cutaneous burn wound healing in albino rats. Nat. Prod. Res. 2008, 22, 422–427. [Google Scholar] [CrossRef]
- Khalil, E.A.; Abou-Zekry, S.S.; Sami, D.G.; Abdellatif, A. Natural products as wound healing agents. In Wound Healing Research: Current Trends and Future Directions; Kumar, P., Kothari, V., Eds.; Springer: Singapore, 2021; pp. 77–94. [Google Scholar]
- Essa, M.M.; Vijayan, R.K.; Castellano-Gonzalez, G.; Memon, M.A.; Braidy, N.; Guillemin, G.J. Neuroprotective effect of natural products against alzheimer’s disease. Neurochem. Res. 2012, 37, 1829–1842. [Google Scholar] [CrossRef]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef]
- Duncan, B.; Li, X.; Landis, R.F.; Kim, S.T.; Gupta, A.; Wang, L.-S.; Ramanathan, R.; Tang, R.; Boerth, J.A.; Rotello, V.M. Nanoparticle-stabilized capsules for the treatment of bacterial biofilms. ACS Nano 2015, 9, 7775–7782. [Google Scholar] [CrossRef]
- Costa, M.F.; Durço, A.O.; Rabelo, T.K.; Barreto, R.d.S.; Guimarães, A.G. Effects of carvacrol, thymol and essential oils containing such monoterpenes on wound healing: A systematic review. J. Pharm. Pharmacol. 2018, 71, 141–155. [Google Scholar] [CrossRef]
- Sherry, E.; Boeck, H.; Warnke, P.H. Topical application of a new formulation of eucalyptus oil phytochemical clears methicillin-resistant staphylococcus aureus infection. Am. J. Infect. Control 2001, 29, 346. [Google Scholar] [CrossRef] [PubMed]
- Pazyar, N.; Yaghoobi, R.; Bagherani, N.; Kazerouni, A. A review of applications of tea tree oil in dermatology. Int. J. Dermatol. 2013, 52, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.C.; De Lima, J.A.; Da Silva, C.R.; Benvegnú, D.; Ferreira, J.; Burger, M.E.; Beck, R.; Rolim, C.M.B.; Rocha, M.I.U.M.; da Veiga, M.L.; et al. Hydrogels containing nanocapsules and nanoemulsions of tea tree oil provide antiedematogenic effect and improved skin wound healing. J. Nanosci. Nanotechnol. 2015, 15, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.; Loughlin, R.; Gilpin, D.; Kearney, P.; Tunney, M. In vitro activity of tea-tree oil against clinical skin isolates of meticillin-resistant and -sensitive Staphylococcus aureus and coagulase-negative staphylococci growing planktonically and as biofilms. J. Med. Microbiol. 2006, 55, 1375–1380. [Google Scholar] [CrossRef]
- Hammer, K.A. Treatment of acne with tea tree oil (melaleuca) products: A review of efficacy, tolerability and potential modes of action. Int. J. Antimicrob. Agents 2015, 45, 106–110. [Google Scholar] [CrossRef]
- Lee, C.-J.; Chen, L.-W.; Chen, L.-G.; Chang, T.-L.; Huang, C.-W.; Huang, M.-C.; Wang, C.-C. Correlations of the components of tea tree oil with its antibacterial effects and skin irritation. J. Food Drug Anal. 2013, 21, 169–176. [Google Scholar] [CrossRef]
- Raman, A.; Weir, U.; Bloomfield, S. Antimicrobial effects of tea-tree oil and its major components on Staphylococcus aureus, staph. Epidermidis and propionibacterium acnes. Lett. Appl. Microbiol. 1995, 21, 242–245. [Google Scholar] [CrossRef]
- Hart, P.; Brand, C.; Carson, C.; Riley, T.; Prager, R.; Finlay-Jones, J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Agents Actions 2000, 49, 619–626. [Google Scholar] [CrossRef]
- Yu, D.; Wang, J.; Shao, X.; Xu, F.; Wang, H. Antifungal modes of action of tea tree oil and its two characteristic components against Botrytis cinerea. J. Appl. Microbiol. 2015, 119, 1253–1262. [Google Scholar] [CrossRef]
- Pandit, J.; Aqil, M.; Sultana, Y. Nanoencapsulation technology to control release and enhance bioactivity of essential oils. In Encapsulations; Elsevier: Amsterdam, The Netherlands, 2016; pp. 597–640. [Google Scholar]
- Maryam, I.; Huzaifa, U.; Hindatu, H.; Zubaida, S. Nanoencapsulation of essential oils with enhanced antimicrobial activity: A new way of combating antimicrobial resistance. J. Pharmacogn. Phytochem. 2015, 4, 165. [Google Scholar]
- Kamel, R.; Salama, A.; Shaffie, N.M.; Salah, N.M. Cerebral effect of optimized allium sativum oil-loaded chitosan nanorods: GC-MS analysis and in vitro/in vivo evaluation. Food Funct. 2020, 11, 5357–5376. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.; Elmotasem, H.; Abdelsalam, E.; Salama, A. Lepidium sativum seed oil 3D nano-oleogel for the management of diabetic wounds: GC/MS analysis, in-vitro and in-vivo studies. J. Drug Deliv. Sci. Technol. 2021, 63, 102504. [Google Scholar] [CrossRef]
- Esposito, E.; Pecorelli, A.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Carducci, F.; Cervellati, F.; Nastruzzi, C.; Cortesi, R.; Valacchi, G. Production and characterization of nanoparticle based hyaluronate gel containing retinyl palmitate for wound healing. Curr. Drug Deliv. 2018, 15, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, D.A.A.; Kamel, R.; Fadel, M. PEGylated lipid nanocarrier for enhancing photodynamic therapy of skin carcinoma using curcumin: In-vitro/in-vivo studies and histopathological examination. Sci. Rep. 2020, 10, 10435. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.; Mostafa, D.M. Rutin nanostructured lipid cosmeceutical preparation with sun protective potential. J. Photochem. Photobiol. B Biol. 2015, 153, 59–66. [Google Scholar] [CrossRef]
- Jindal, S.; Kumar, A.; Goyal, K.; Awasthi, R.; Kulkarni, G.T. Lipid nanocarriers for dermal delivery of lutein. In Nanomedicine for Bioactives; Springer: Singapore, 2020; pp. 341–366. [Google Scholar]
- Montenegro, L. Lipid-based nanoparticles as carriers for dermal delivery of antioxidants. Curr. Drug Metab. 2017, 18, 469–480. [Google Scholar] [CrossRef]
- Kamel, R.; El-Wakil, N.A.; Dufresne, A.; Elkasabgy, N.A. Nanocellulose: From an agricultural waste to a valuable pharmaceutical ingredient. Int. J. Biol. Macromol. 2020, 163, 1579–1590. [Google Scholar] [CrossRef]
- Kamel, R.; El-Wakil, N.A.; Abdelkhalek, A.A.; Elkasabgy, N.A. Nanofibrillated cellulose/cyclodextrin based 3D scaffolds loaded with raloxifene hydrochloride for bone regeneration. Int. J. Biol. Macromol. 2020, 156, 704–716. [Google Scholar] [CrossRef]
- Kamel, R.; Mabrouk, M.; El-Sayed, S.A.; Beherei, H.H.; Abouzeid, R.E.; El-Fadl, M.T.A.; Mahmoud, A.A.; Maged, A. Nanofibrillated cellulose/glucosamine 3D aerogel implants loaded with rosuvastatin and bioactive ceramic for dental socket preservation. Int. J. Pharm. 2022, 616, 121549. [Google Scholar] [CrossRef]
- Meftahi, A.; Samyn, P.; Geravand, S.A.; Khajavi, R.; Alibkhshi, S.; Bechelany, M.; Barhoum, A. Nanocelluloses as skin biocompatible materials for skincare, cosmetics, and healthcare: Formulations, regulations, and emerging applications. Carbohydr. Polym. 2021, 278, 118956. [Google Scholar] [CrossRef]
- Farag, M.A.; Kabbash, E.M.; Mediani, A.; Döll, S.; Esatbeyoglu, T.; Afifi, S.M. Comparative metabolite fingerprinting of four different cinnamon species analyzed via UPLC–MS and GC–MS and chemometric tools. Molecules 2022, 27, 2935. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.; Khattab, A.; Shamma, S.; Afifi, S. Profiling of primary metabolites and volatile determinants in mahlab cherry (Prunus mahaleb L.) seeds in the context of its different varieties and roasting as analyzed using chemometric tools. Foods 2021, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, J.; Avula, B.; Wang, Y.-H.; Chittiboyina, A.G.; Parcher, J.F.; Khan, I.A. Quality evaluation of terpinen-4-ol-type australian tea tree oils and commercial products: An integrated approach using conventional and chiral GC/MS combined with chemometrics. J. Agric. Food Chem. 2015, 63, 2674–2682. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lyu, S.; Chen, D.; Lin, Y.; Chen, J.; Chen, G.; Ye, N. Volatiles emitted at different flowering stages of jasminum sambac and expression of genes related to α-farnesene biosynthesis. Molecules 2017, 22, 546. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, L.; Liao, Y.; Li, J.; Tang, J.; Yang, Z. Formation of α-farnesene in tea (camellia sinensis) leaves induced by herbivore-derived wounding and its effect on neighboring tea plants. Int. J. Mol. Sci. 2019, 20, 4151. [Google Scholar] [CrossRef]
- Wan Salleh, W.M.N.H.; Kammil, M.F.; Ahmad, F.; Sirat, H.M. Antioxidant and anti-inflammatory activities of essential oil and extracts of Piper miniatum. Nat. Prod. Commun. 2015, 10, 1934578X1501001151. [Google Scholar] [CrossRef]
- Islam, R.; Rahman, M.S.; Rahman, S.M. GC-MS analysis and antibacterial activity of cuscuta reflexa against bacterial pathogens. Asian Pac. J. Trop. Dis. 2015, 5, 399–403. [Google Scholar] [CrossRef]
- Farag, M.A.; Fathi, D.; Shamma, S.; Shawkat, M.S.A.; Shalabi, S.M.; El Seedi, H.R.; Afifi, S.M. Comparative metabolome classification of desert truffles Terfezia claveryi and Terfezia boudieri via its aroma and nutrients profile. LWT 2021, 142, 111046. [Google Scholar] [CrossRef]
- de Fátima Souto Maior, L.; Maciel, P.P.; Ferreira, V.Y.N.; de Lima Gouveia Dantas, C.; de Lima, J.M.; Castellano, L.R.C.; Batista, A.U.D.; Bonan, P.R.F. Antifungal activity and shore a hardness of a tissue conditioner incorporated with terpinen-4-ol and cinnamaldehyde. Clin. Oral Investig. 2019, 23, 2837–2848. [Google Scholar] [CrossRef]
- Dos Santos, E.; Radai, J.A.S.; Nascimento, K.F.D.; Formagio, A.S.N.; Balsalobre, N.D.M.; Ziff, E.B.; Konkiewitz, E.C.; Kassuya, C.A.L. Contribution of spathulenol to the anti-nociceptive effects of psidium guineense. Nutr. Neurosci. 2020, 25, 812–822. [Google Scholar] [CrossRef]
- Alitonou, G.A.; Sessou, P.; Tchobo, P.F.; Noudogbessi, J.P.; Avlessi, F.; Yehouenou, B.; Menut, C.; Villeneuve, P.; Sohounhloue, D.C.K. Chemical composition and biological activities of essential oils of Chenopodium ambrosioides L. collected in two areas of Benin. Int. J. Biosci. 2012, 2, 58–66. [Google Scholar]
- Sales, A.; Felipe, L.D.O.; Bicas, J.L. Production, properties, and applications of α-terpineol. Food Bioprocess Technol. 2020, 13, 1261–1279. [Google Scholar] [CrossRef]
- Hosseini, F.; Adlgostar, A.; Sharifnia, F. Antibacterial activity of pistacia atlantica extracts on streptococcus mutans biofilm. Int Res. J. Biol. Sci. 2013, 2, 1–7. [Google Scholar]
- Adhikari, U.; Goliaei, A.; Tsereteli, L.; Berkowitz, M.L. Properties of poloxamer molecules and poloxamer micelles dissolved in water and next to lipid bilayers: Results from computer simulations. J. Phys. Chem. B 2016, 120, 5823–5830. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Kamel, R.; El Sayed, N. Dermal anti-oxidant, anti-inflammatory and anti-aging effects of compritol ATO-based resveratrol colloidal carriers prepared using mixed surfactants. Int. J. Pharm. 2018, 541, 37–47. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Üner, M.; Yener, G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int. J. Nanomed. 2007, 2, 289–300. [Google Scholar]
- He, J.; Huang, S.; Sun, X.; Han, L.; Chang, C.; Zhang, W.; Zhong, Q. Carvacrol loaded solid lipid nanoparticles of propylene glycol monopalmitate and glyceryl monostearate: Preparation, characterization, and synergistic antimicrobial activity. NanoMater. 2019, 9, 1162. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Kaul, V.; Waghule, T.; Gorantla, S.; Sharma, S.; Roy, A.; Dubey, S.K.; Singhvi, G. Curcumin loaded nanostructured lipid carriers for enhanced skin retained topical delivery: Optimization, scale-up, in-vitro characterization and assessment of ex-vivo skin deposition. Eur. J. Pharm. Sci. 2020, 152, 105438. [Google Scholar] [CrossRef]
- Rarokar, N.R.; Menghani, S.S.; Kerzare, D.R.; Khedekar, P.B.; Bharne, A.P.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Sreeharsha, N.; Asdaq, S.M.B. Preparation of terbinafin-encapsulated solid lipid nanoparticles containing antifungal carbopol(®) hydrogel with improved efficacy: In vitro, ex vivo and in vivo study. Pharmaceutics 2022, 14, 1393. [Google Scholar] [CrossRef]
- Kang, J.H.; Yoo, K.H.; Park, H.Y.; Hyun, S.M.; Han, S.D.; Kim, D.W.; Park, C.W. Preparation and in vivo evaluation of a lidocaine self-nanoemulsifying ointment with glycerol monostearate for local delivery. Pharmaceutics 2021, 13, 1468. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Fang, X.; Zhou, Y.; Wang, J.; Guo, F.; Li, F.; Peng, X. Development and optimization of solid lipid nanoparticle formulation for ophthalmic delivery of chloramphenicol using a Box-Behnken design. Int. J. Nanomed. 2011, 6, 683–692. [Google Scholar]
- Kamel, R.; Abbas, H.; Fayez, A. Diosmin/essential oil combination for dermal photo-protection using a lipoid colloidal carrier. J. Photochem. Photobiol. B Biol. 2017, 170, 49–57. [Google Scholar] [CrossRef] [PubMed]
- AbouSamra, M.M.; Basha, M.; Awad, G.E.; Mansy, S.S. A promising nystatin nanocapsular hydrogel as an antifungal polymeric carrier for the treatment of topical candidiasis. J. Drug Deliv. Sci. Technol. 2019, 49, 365–374. [Google Scholar] [CrossRef]
- Vijayan, V.; Rao, D.S.; Jayachandran, E.; Anburaj, J. Preparation and characterization of anti diabetic drug loaded solid lipid nanoparticles. JITPS 2010, 8, 324. [Google Scholar]
- El-Housiny, S.; Eldeen, M.A.S.; El-Attar, Y.A.; Salem, H.A.; Attia, D.; Bendas, E.; El-Nabarawi, M.A. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: Formulation and clinical study. Drug Deliv. 2017, 25, 78–90. [Google Scholar] [CrossRef]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Aqil, M.; Shafiq, S. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS PharmSciTech 2007, 8, 191–199. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions a particulate drug formulations in therapy: Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Kumar, P.P.; Gayatri, P.; Sunil, R.; Jaganmohan, S.; Rao, Y.M. Atorvastatin Loaded Solidlipid Nanoparticles: Formulation, Optimization, and in vitro characterization. IOSR J. Pharm. 2012, 2, 23–32. [Google Scholar] [CrossRef]
- zur Mühlen, A.; Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) for controlled drug delivery–Drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. [Google Scholar] [CrossRef]
- Yang, X.; Dogan, I.; Pannala, V.R.; Kootala, S.; Hilborn, J.; Ossipov, D. A hyaluronic acid–camptothecin nanoprodrug with cytosolic mode of activation for targeting cancer. Polym. Chem. 2013, 4, 4621–4630. [Google Scholar] [CrossRef]
- Dubes, A.; Parrot-Lopez, H.; Abdelwahed, W.; Degobert, G.; Fessi, H.; Shahgaldian, P.; Coleman, A.W. Scanning electron microscopy and atomic force microscopy imaging of solid lipid nanoparticles derived from amphiphilic cyclodextrins. Eur. J. Pharm. Biopharm. 2003, 55, 279–282. [Google Scholar] [CrossRef]
- Lin, G.; Chen, H.; Zhou, H.; Zhou, X.; Xu, H. Preparation of tea tree oil/poly(styrene-butyl methacrylate) microspheres with sustained release and anti-bacterial properties. Materials 2018, 11, 710. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhang, C.; Zheng, Y.; Mei, L.; Tang, L.; Song, C.; Sun, H.; Huang, L. The effect of poloxamer 188 on nanoparticle morphology, size, cancer cell uptake, and cytotoxicity. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 170–178. [Google Scholar] [CrossRef]
- Girotra, P.; Singh, S.K.; Kumar, G. Development of zolmitriptan loaded PLGA/poloxamer nanoparticles for migraine using quality by design approach. Int. J. Biol. Macromol. 2016, 85, 92–101. [Google Scholar] [CrossRef]
- Kraisit, P.; Yonemochi, E.; Furuishi, T.; Mahadlek, J.; Limmatvapirat, S. Chitosan film containing antifungal agent-loaded SLNs for the treatment of candidiasis using a Box-Behnken design. Carbohydr. Polym. 2022, 283, 119178. [Google Scholar] [CrossRef]
- Plasencia, I.; Norlén, L.; Bagatolli, L. Direct visualization of lipid domains in human skin stratum corneum’s lipid membranes: Effect of pH and temperature. Biophys. J. 2007, 93, 3142–3155. [Google Scholar] [CrossRef]
- Bhowmik, M.; Bain, M.K.; Ghosh, L.K.; Chattopadhyay, D. Effect of salts on gelation and drug release profiles of methylcellulose-based ophthalmic thermo-reversible in situ gels. Pharm. Dev. Technol. 2010, 16, 385–391. [Google Scholar] [CrossRef]
- Crawford, R.J.; Edler, K.J.; Lindhoud, S.; Scott, J.L.; Unali, G. Formation of shear thinning gels from partially oxidised cellulose nanofibrils. Green Chem. 2011, 14, 300–303. [Google Scholar] [CrossRef]
- Kim, E.-J.; Kim, S.-H. Anti-inflammatory effects of low-level laser in burn wound models in rats. Phys. Ther. Rehabil. Sci. 2017, 6, 170–175. [Google Scholar] [CrossRef][Green Version]
- Khedir, S.B.; Bardaa, S.; Chabchoub, N.; Moalla, D.; Sahnoun, Z.; Rebai, T. The healing effect of Pistacia lentiscus fruit oil on laser burn. Pharm. Biol. 2017, 55, 1407–1414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, H.; Chen, J.; Cen, Y. Burn wound healing potential of a polysaccharide from Sanguisorba officinalis L. in mice. Int. J. Biol. Macromol. 2018, 112, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Barzegari, A.A.; Hashemzaei, M.; Majdani, R.; Alihemmati, A.R. Effects of topical treatment of second-degree burn wounds with Lactobacillus acidophilus on the wound healing process in male rats. Pharm. Biomed. Res. 2017, 3, 20–30. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Tijing, L.D.; Lim, J.K. Characterization of the surface biocompatibility of an electrospun nylon 6/CaP nanofiber scaffold using osteoblasts. Chem. Eng. J. 2013, 215, 57–64. [Google Scholar] [CrossRef]
- Afifi, S.M.; El-Mahis, A.; Heiss, A.G.; Farag, M.A. Gas chromatography–Mass spectrometry-based classification of 12 fennel (foeniculum vulgare miller) varieties based on their aroma profiles and estragole levels as analyzed using chemometric tools. ACS Omega 2021, 6, 5775–5785. [Google Scholar] [CrossRef]
- Fathi, M.; Varshosaz, J.; Mohebbi, M.; Shahidi, F. Hesperetin-loaded solid lipid nanoparticles and nanostructure lipid carriers for food fortification: Preparation, characterization, and modeling. Food Bioprocess Technol. 2013, 6, 1464–1475. [Google Scholar] [CrossRef]
- Laein, S.S.; Khanzadi, S.; Hashemi, M.; Gheybi, F.; Azizzadeh, M. Peppermint essential oil-loaded solid lipid nanoparticle in gelatin coating: Characterization and antibacterial activity against foodborne pathogen inoculated on rainbow trout (Oncorhynchus mykiss) fillet during refrigerated storage. J. Food Sci. 2022, 87, 2920–2931. [Google Scholar] [CrossRef]
- Upadhyay, S.U.; Patel, J.K.; Patel, V.A.; Saluja, A.K. Effect of different lipids and surfactants on formulation of solid lipid nanoparticles incorporating tamoxifen citrate. J. Pharm. Bioallied Sci. 2012, 4 (Suppl. 1), S112–S113. [Google Scholar] [CrossRef]
- Bhaskar, K.; Mohan, C.K.; Lingam, M.; Mohan, S.J.; Venkateswarlu, V.; Rao, Y.M.; Anbu, J.; Ravichandran, V. Development of SLN and NLC enriched hydrogels for transdermal delivery of nitrendipine: In vitro and in vivo characteristics. Drug Dev. Ind. Pharm. 2008, 35, 98–113. [Google Scholar] [CrossRef]
- Neves, A.R.; Lúcio, M.; Martins, S.; Lima, J.L.C. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar]
- Kumar, V.V.; Chandrasekar, D.; Ramakrishna, S.; Kishan, V.; Rao, Y.M.; Diwan, P.V. Development and evaluation of nitrendipine loaded solid lipid nanoparticles: Influence of wax and glyceride lipids on plasma pharmacokinetics. Int. J. Pharm. 2007, 335, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Emami, J.; Mohiti, H.; Hamishehkar, H.; Varshosaz, J. Formulation and optimization of solid lipid nanoparticle formulation for pulmonary delivery of budesonide using taguchi and box-behnken design. Res. Pharm. Sci. 2015, 10, 17–33. [Google Scholar] [PubMed]
- Matloub, A.A.; AbouSamra, M.M.; Salama, A.H.; Rizk, M.Z.; Aly, H.F.; Fouad, G.I. Cubic liquid crystalline nanoparticles containing a polysaccharide from Ulva fasciata with potent antihyperlipidaemic activity. Saudi Pharm. J. 2018, 26, 224–231. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Vuddanda, P.R.; Karunanidhi, P.; Singh, S.K. Development and evaluation of solid lipid nanoparticles of raloxifene hydrochloride for enhanced bioavailability. BioMed. Res. Int. 2013, 2013, 584549. [Google Scholar] [CrossRef]
- Domínguez-Villegas, V.; Clares-Naveros, B.; García-López, M.L.; Calpena-Campmany, A.C.; Bustos-Zagal, P.; Garduño-Ramírez, M.L. Development and characterization of two nano-structured systems for topical application of flavanones isolated from Eysenhardtia platycarpa. Colloids Surf. B Biointerfaces 2014, 116, 183–192. [Google Scholar] [CrossRef]
- Draize, J.H. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- Kamel, R.; Abbas, H.A. Self-assembled carbohydrate hydrogels for prolonged pain management. Pharm. Dev. Technol. 2011, 18, 990–1004. [Google Scholar] [CrossRef]
- Abbas, H.; Kamel, R. Potential role of resveratrol-loaded elastic sorbitan monostearate nanovesicles for the prevention of UV-induced skin damage. J. Liposome Res. 2019, 30, 45–53. [Google Scholar] [CrossRef]
- Upadhyay, N.; Kumar, R.; Mandotra, S.; Meena, R.; Siddiqui, M.; Sawhney, R.; Gupta, A. Safety and healing efficacy of Sea buckthorn (Hippophae rhamnoides L.) seed oil on burn wounds in rats. Food Chem. Toxicol. 2009, 47, 1146–1153. [Google Scholar] [CrossRef]
- Pereira, D.D.S.T.; Lima-Ribeiro, M.H.M.; de Pontes-Filho, N.T.; Carneiro-Leão, A.M.D.A.; Correia, M.T.D.S. Development of animal model for studying deep second-degree thermal burns. J. Biomed. Biotechnol. 2012, 2012, 460841. [Google Scholar]
- Ammar, H.O.; Ghorab, M.; El-Nahhas, S.A.; Kamel, R. Evaluation of chemical penetration enhancers for transdermal delivery of aspirin. Asian J. Pharm. Sci. 2007, 2, 96–105. [Google Scholar]
- Banchroft, J.D.; Stevens, A.; Turner, D.R. Theory and Practice of Histological Techniques, 4th ed.; Churchil Livingstone: New York, NY, USA; London, UK; San Francisco, CA, USA; Tokyo, Japan, 1996. [Google Scholar]
- Lin, H.-C.; Lee, H.-S.; Chiueh, T.-S.; Lin, Y.-C.; Cha, T.-L.; Meng, E. Histopathological assessment of inflammation and expression of inflammatory markers in patients with ketamine-induced cystitis. Mol. Med. Rep. 2014, 11, 2421–2428. [Google Scholar] [CrossRef] [PubMed]


| No. | RT (min) | Compounds | KI | Class | Relative % |
|---|---|---|---|---|---|
| 1 | 4.77 | γ-Terpinene | 983 | Monoterpene hydrocarbons | 2.88 ± 0.05 |
| 2 | 5.05 | α-Terpinolene | 1027 | Monoterpene hydrocarbons | 5.27 ± 0.84 |
| 3 | 6.8 | Terpinen-4-ol | 1112 | Alcohol | 4.19 ± 0.42 |
| 4 | 8.27 | Terpinen-4-ol isomer | 1139 | Alcohol | 7.87 ± 0.79 |
| 5 | 8.39 | α-Terpineol | 1148 | Alcohol | 4.43 ± 0.16 |
| 6 | 8.57 | α-Cyclocitral | 1164 | Aldehyde | 0.52 ± 0.04 |
| 7 | 8.74 | 7-Hydroxyterpineol | 1210 | Alcohol | 1.61 ± 0.09 |
| 8 | 9.41 | trans-Ascaridol glycol | 1225 | Alcohol | 4.66 ± 0.43 |
| 9 | 9.58 | p-Mentha-3-en-8-ol | 1232 | Alcohol | 1.17 ± 0.05 |
| 10 | 10.04 | α-Copaene | 1241 | Sesquiterpene hydrocarbons | 1.7 ± 0.07 |
| 11 | 10.1 | 4-Heptenal | 1265 | Aldehyde | 0.63 ± 0.03 |
| 12 | 10.43 | Isoledene | 1317 | Sesquiterpene hydrocarbons | 0.89 ± 0.07 |
| 13 | 10.53 | α-Copaene isomer | 1324 | Sesquiterpene hydrocarbons | 2.22 ± 0.16 |
| 14 | 10.84 | α-Cadinol | 1368 | Alcohol | 0.96 ± 0.31 |
| 15 | 11.17 | α-Cadinol isomer | 1383 | Alcohol | 2.84 ± 0.52 |
| 16 | 11.37 | Caryophyllene | 1462 | Sesquiterpene hydrocarbons | 2.58 ± 0.28 |
| 17 | 11.47 | Caryophyllene isomer | 1478 | Sesquiterpene hydrocarbons | 0.66 ± 0.05 |
| 18 | 11.87 | α-Bergamotene | 1493 | Sesquiterpene hydrocarbons | 5.3 ± 0.18 |
| 19 | 11.98 | Alloaromadendrene | 1529 | Sesquiterpene hydrocarbons | 1.5 ± 0.53 |
| 20 | 12.23 | δ-Cadinene | 1561 | Sesquiterpene hydrocarbons | 3.73 ± 0.59 |
| 21 | 12.43 | δ-Cadinene isomer | 1583 | Sesquiterpene hydrocarbons | 3.88 ± 0.76 |
| 22 | 13.05 | α-Cubebene | 1647 | Sesquiterpene hydrocarbons | 8.15 ± 2.37 |
| 23 | 13.6 | α-Farnesene | 1684 | Sesquiterpene hydrocarbons | 9.43 ± 3.62 |
| 24 | 13.68 | α-Copaene isomer | 1694 | Sesquiterpene hydrocarbons | 1.33 ± 0.06 |
| 25 | 13.77 | α-Calacorene | 1728 | Aromatics | 0.27 ± 0.09 |
| 26 | 14.06 | 10-Aromadendranol | 1750 | Sesquiterpene hydrocarbons | 1.74 ± 0.16 |
| 27 | 14.18 | Spathulenol | 1765 | Alcohol | 2.25 ± 0.25 |
| 28 | 14.54 | Unknown 1 | 1769 | - | 2.64 ± 0.04 |
| 29 | 14.76 | Guaiol | 1782 | Alcohol | 1.84 ± 0.08 |
| 30 | 14.88 | Isospathulenol | 1798 | Alcohol | 1.79 ± 0.03 |
| 31 | 15.26 | 7-epi-α-Eudesmol | 1801 | Alcohol | 1.71 ± 0.29 |
| 32 | 15.35 | Cubenol | 1816 | Alcohol | 2.06 ± 0.45 |
| 33 | 15.61 | Spathulenol isomer | 1820 | Alcohol | 3.65 ± 0.82 |
| 34 | 15.92 | Epoxyguaiene | 1835 | Sesquiterpene hydrocarbons | 0.45 ± 0.27 |
| 35 | 21.68 | Unknown 2 | 1841 | - | 1.08 ± 0.09 |
| 36 | 23.11 | Unknown 3 | 1867 | - | 0.62 ± 0.37 |
| 37 | 35.02 | Unknown 4 | 1886 | - | 0.75 ± 0.08 |
| 38 | 35.12 | Unknown 5 | 1894 | - | 0.72 ± 0.05 |
| Formulations | Entrapment Efficiency (E.E. ± SD) % | TTO Loading (LC ± SD)% | Particle Size (P.S ± SD) nm | Polydispersity Index (PDI ± SD) | Zeta Potential (Z.P.) mV |
|---|---|---|---|---|---|
| TTO-SLN1 | 96.26 ± 2.3 | 29.81 ± 1.2 | 235.0 ± 20.4 | 0.31 ± 0.01 | −32.0 |
| TTO-SLN2 | 96.73 ± 2.5 | 19.63 ± 1.8 | 226.6 ± 20.6 | 0.34 ± 0.01 | −29.3 |
| TTO-SLN3 | 97.12 ± 3.1 | 21.40 ± 2.1 | 335.6 ± 19.7 | 0.38 ± 0.02 | −25.4 |
| TTO-SLN4 | 97.70 ± 3.0 | 15.23 ± 2.2 | 324.5 ± 33.4 | 0.37 ± 0.01 | −26.5 |
| TTO-SLN5 | 98.50 ± 2.8 | 14.31 ± 2.7 | 426.0 ± 40.7 | 0.44 ± 0.02 | −25.0 |
| TTO-SLN6 | 98.60 ± 3.0 | 10.23 ± 1.3 | 429.8 ± 35.8 | 0.43 ± 0.02 | −26.6 |
| Rat Number | Percentage of Burn Area Contraction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | |||||||||
| Day 4 | Day 6 | Day 7 | Day 4 | Day 6 | Day 7 | Day 4 | Day 6 | Day 7 | Day 4 | Day 6 | Day 7 | |
| 1 | 4.6 | 88.6 | 92.5 | 5.0 | 59.9 | 68.5 | 3.3 | 84.9 | 90.5 | 2.0 | 51.5 | 54.8 |
| 2 | 5.4 | 89.1 | 97.3 | 3.2 | 47.2 | 67.4 | 2.8 | 79.8 | 80.9 | 3.0 | 51.1 | 59.6 |
| 3 | 5.1 | 81.6 | 97.8 | 3.1 | 65.1 | 65.4 | 4.9 | 60.0 | 78.2 | 2.2 | 39.0 | 39.7 |
| 4 | 4.9 | 91.2 | 91.5 | 3.6 | 56.1 | 56.1 | 3.3 | 72.9 | 82.9 | 3.1 | 52.7 | 56.4 |
| 5 | 5.0 | 92.1 | 91.1 | 4.9 | 64.0 | 66.5 | 3.4 | 75.0 | 68.1 | 2.3 | 53.4 | 56.7 |
| Mean | 5.0 | 88.5 | 94.0 | 4.0 | 58.5 | 64.8 | 3.5 | 74.5 | 80.1 | 2.5 | 49.5 | 53.4 |
| SD | 0.3 | 4.1 | 3.2 | 0.9 | 7.2 | 5.0 | 0.8 | 9.3 | 8.1 | 0.5 | 6.0 | 7.9 |
| Formulations | Composition | |
|---|---|---|
| GMS (%w/w) | P188 (%w/w) | |
| TTO-SLN1 | 2 | 5 |
| TTO-SLN2 | 2 | 10 |
| TTO-SLN3 | 4 | 5 |
| TTO-SLN4 | 4 | 10 |
| TTO-SLN5 | 8 | 5 |
| TTO-SLN6 | 8 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamel, R.; Afifi, S.M.; Abdou, A.M.; Esatbeyoglu, T.; AbouSamra, M.M. Nanolipogel Loaded with Tea Tree Oil for the Management of Burn: GC-MS Analysis, In Vitro and In Vivo Evaluation. Molecules 2022, 27, 6143. https://doi.org/10.3390/molecules27196143
Kamel R, Afifi SM, Abdou AM, Esatbeyoglu T, AbouSamra MM. Nanolipogel Loaded with Tea Tree Oil for the Management of Burn: GC-MS Analysis, In Vitro and In Vivo Evaluation. Molecules. 2022; 27(19):6143. https://doi.org/10.3390/molecules27196143
Chicago/Turabian StyleKamel, Rabab, Sherif M. Afifi, Amr M. Abdou, Tuba Esatbeyoglu, and Mona M. AbouSamra. 2022. "Nanolipogel Loaded with Tea Tree Oil for the Management of Burn: GC-MS Analysis, In Vitro and In Vivo Evaluation" Molecules 27, no. 19: 6143. https://doi.org/10.3390/molecules27196143
APA StyleKamel, R., Afifi, S. M., Abdou, A. M., Esatbeyoglu, T., & AbouSamra, M. M. (2022). Nanolipogel Loaded with Tea Tree Oil for the Management of Burn: GC-MS Analysis, In Vitro and In Vivo Evaluation. Molecules, 27(19), 6143. https://doi.org/10.3390/molecules27196143






