Experimental and Theoretical Studies on Optical Properties of Tetra(Imidazole) of Palladium (II) Phthalocyanine
Abstract
:1. Introduction
2. Experimental and Theoretical Studies
2.1. Synthesis Method and Deposition of Thin Films
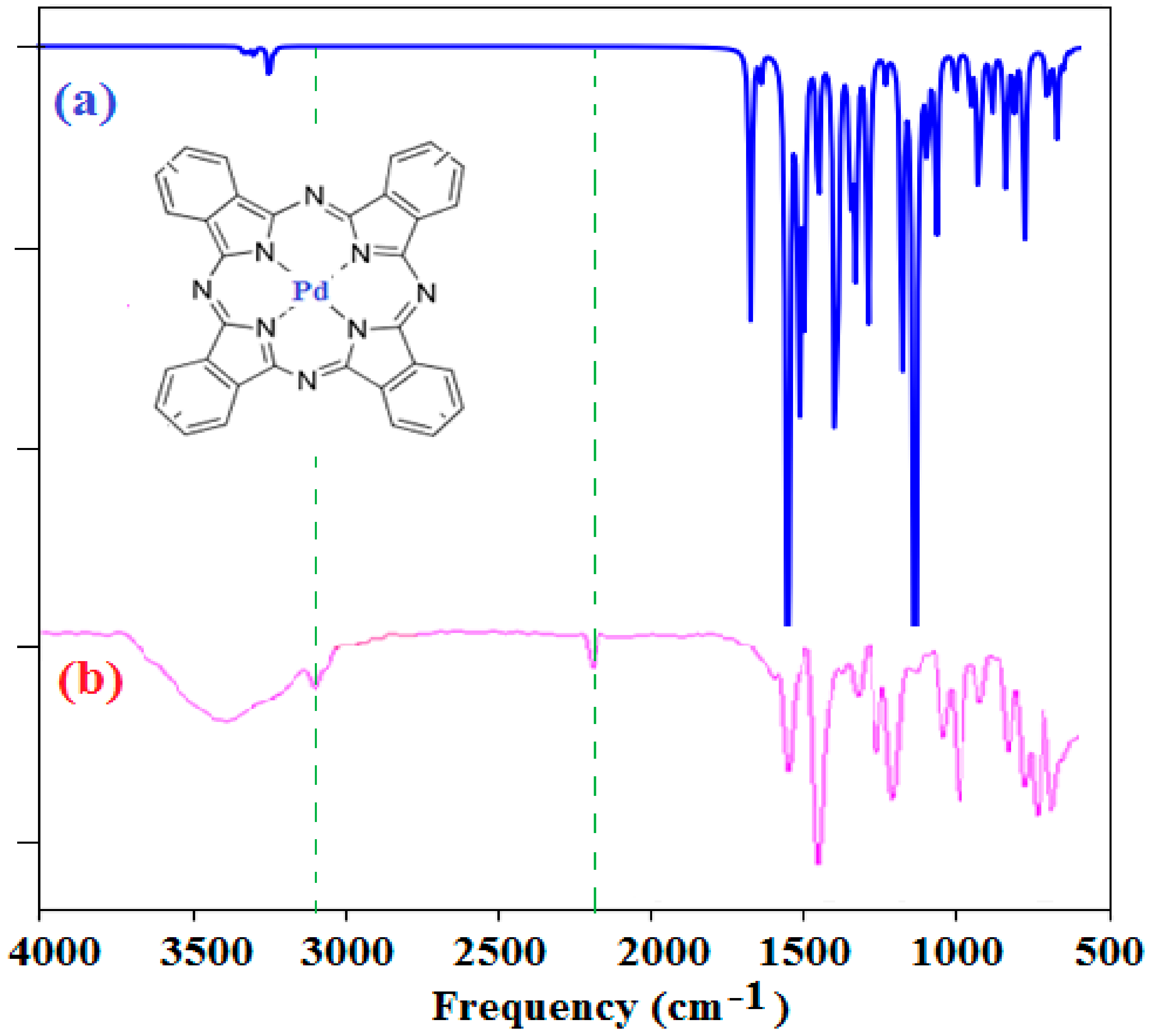
2.2. Characterization
2.3. Theoretical Calculations
3. Results and Discussion
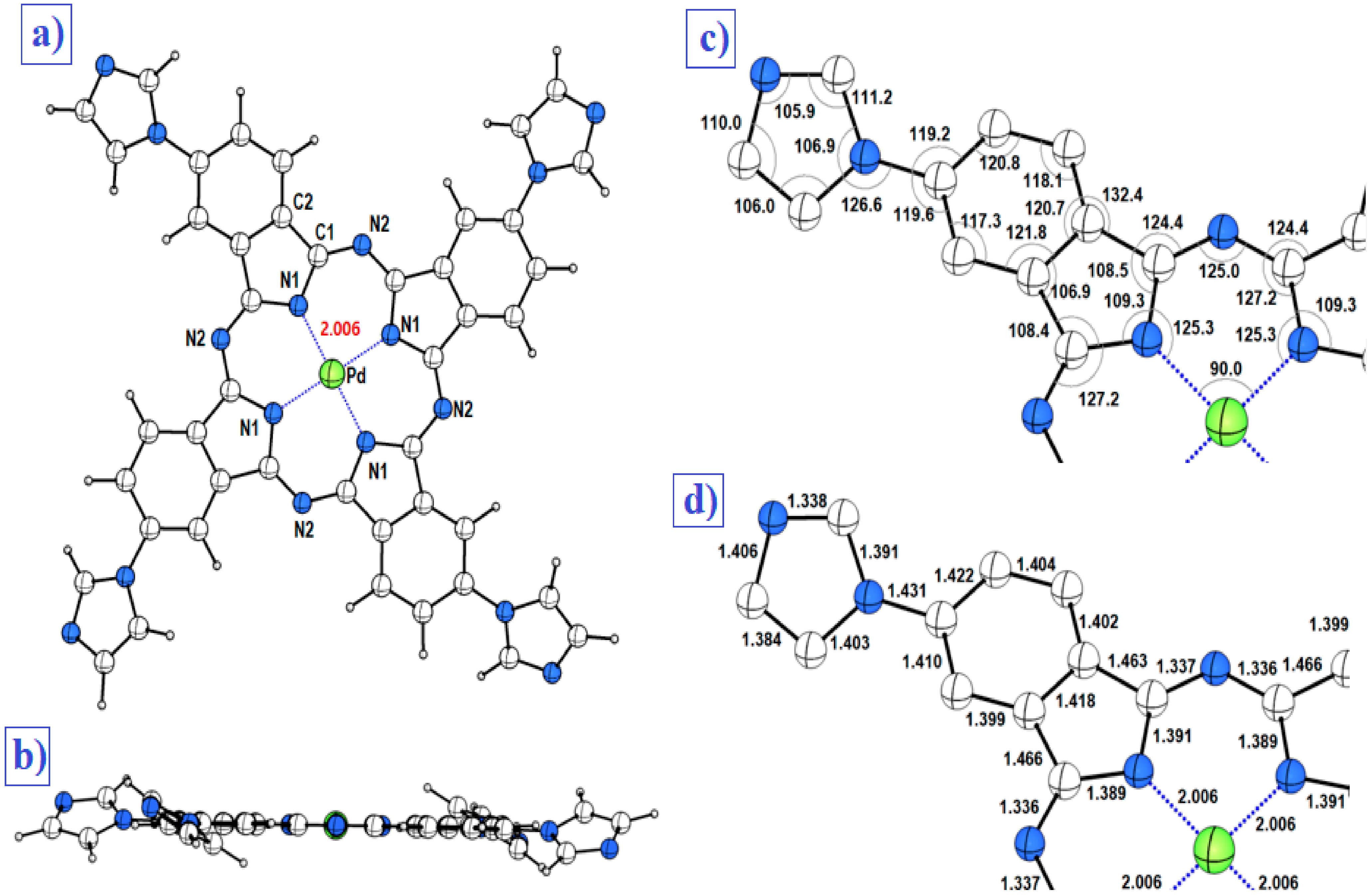
3.1. The Optimized Molecular Structure
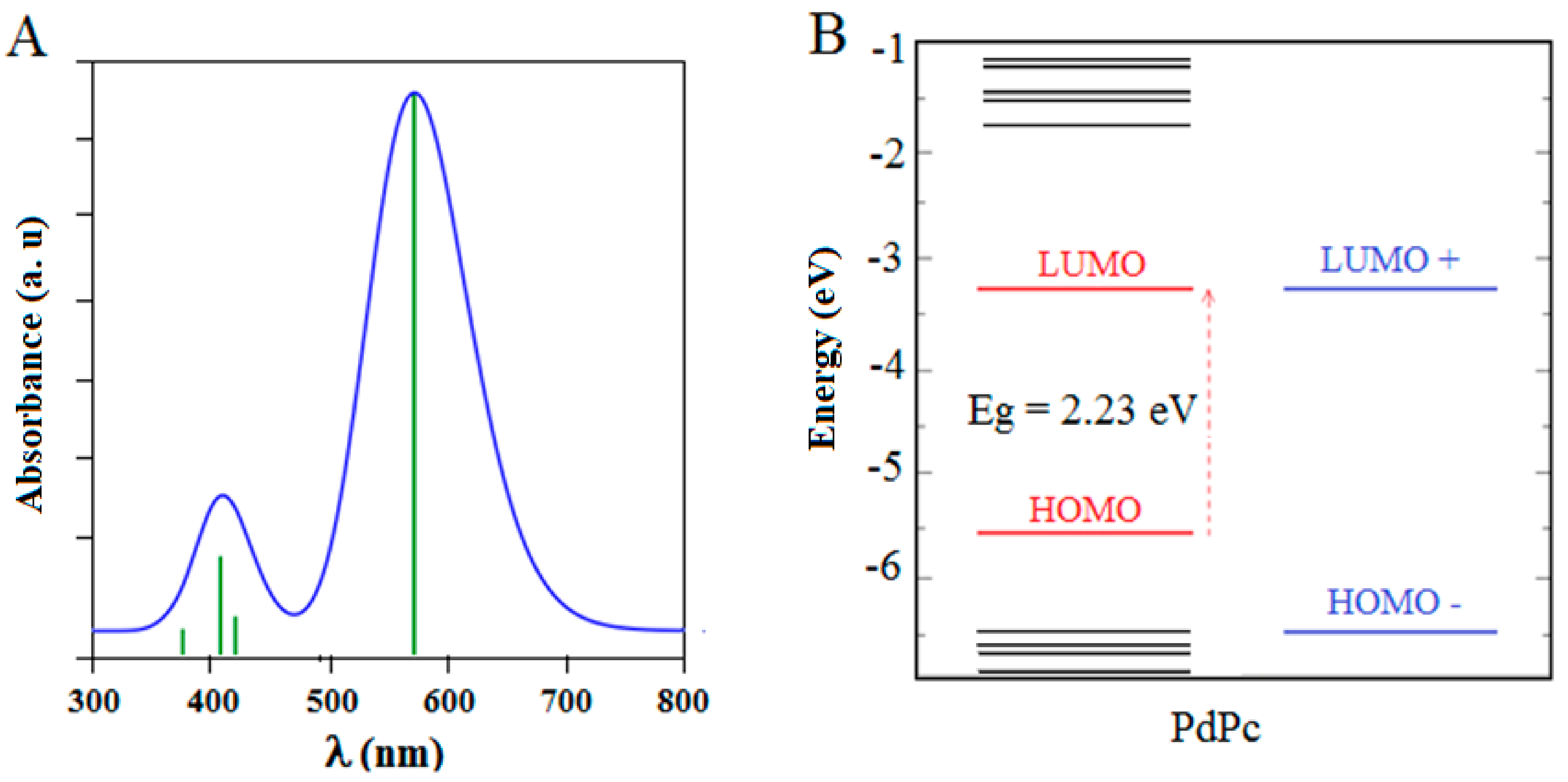
3.2. The Frontier Molecular Orbitals (FMOs)
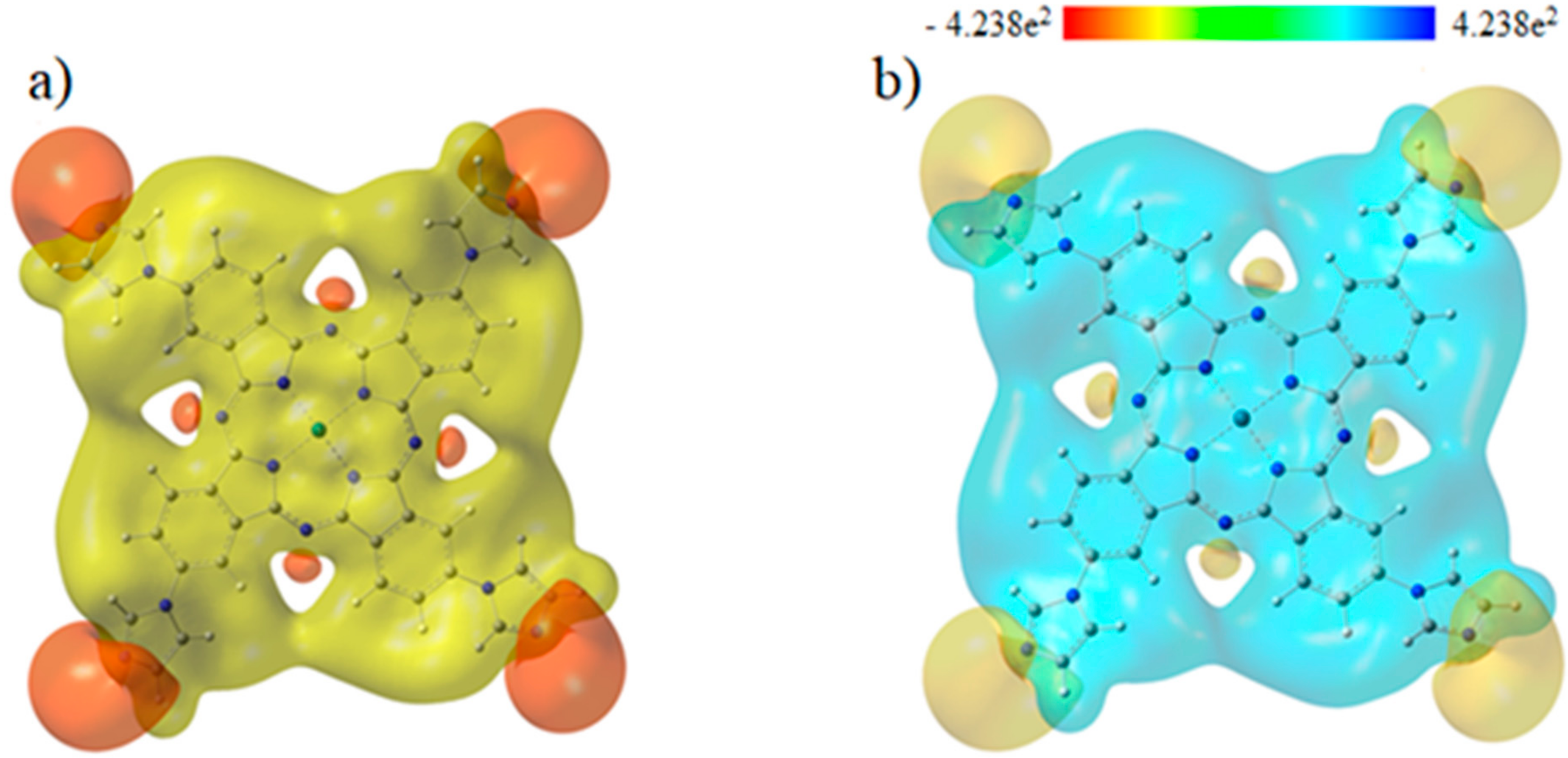
3.3. Molecular Electrostatic Potentials
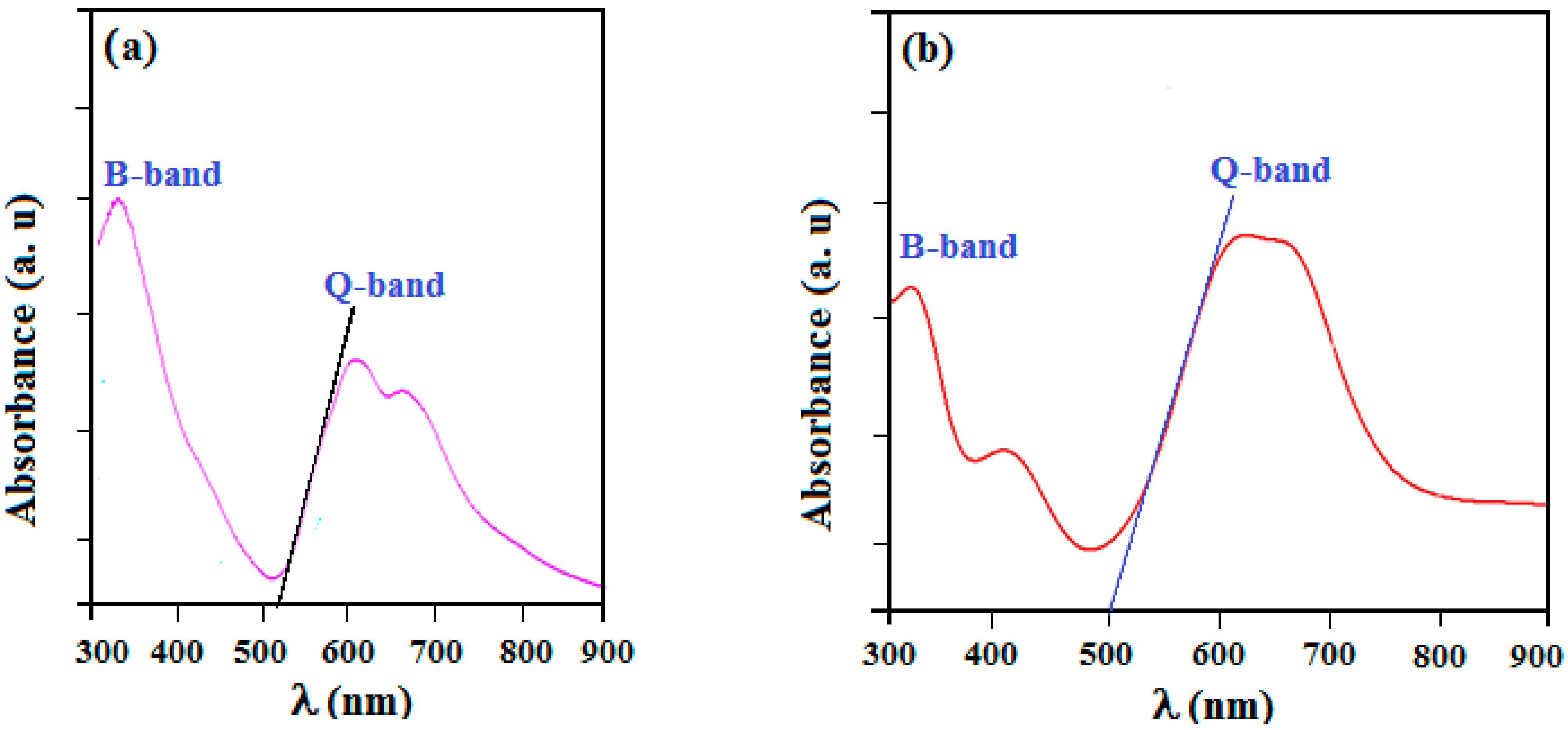
3.4. Optical Absorption Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yin, P.; Shi, Z.; Sun, L.; Xie, P.; Dastan, D.; Sun, K.; Fan, R. Improved breakdown strengths and energy storage properties of polyimide composites: The effect of internal interfaces of C/SiO2 hybrid nanoparticles. Polym. Compos. 2021, 42, 3000–3010. [Google Scholar]
- Altaf, F.; Ahmed, S.; Dastan, D.; Batool, R.; Rehman, Z.U.; Shi, Z.; Hameed, M.U.; Bocchetta, P.; Jacob, K. Novel sepiolite reinforced emerging composite polymer electrolyte membranes for high-performance direct methanol fuel cells. Mater. Today Chem. 2022, 24, 100843. [Google Scholar] [CrossRef]
- Ashraf, I.; Ahmad, S.; Dastan, D.; Wang, C.; Garmestani, H.; Iqbal, M. Fabrication of ionic liquid based D-Ti3C2/MoO3 hybrid electrode system for efficient energy storage applications. Electrochim. Acta 2022, 429, 141036. [Google Scholar]
- Zhang, W.; Zhu, X.; Liang, L.; Yin, P.; Xie, P.; Dastan, D.; Sun, K.; Fan, R.; Shi, Z. Significantly enhanced dielectric permittivity and low loss in epoxy composites incorporating 3d W-WO3/BaTiO3 foams. J. Mater. Sci. 2021, 56, 4254–4265. [Google Scholar]
- Alsulamei, A.; Timoumi, A. Tailoring the physical and optical properties of Sn-doped In2S3 thin films obtained using VTE technique. Opt. Mater. X 2022, 15, 100176. [Google Scholar]
- Ashraf, I.; Ahmad, S.; Nazir, F.; Dastan, D.; Shi, Z.; Garmestani, H.; Iqbal, M. Hydrothermal synthesis and water splitting application of d-Ti3C2 MXene/V2O5 hybrid nanostructures as an efficient bifunctional catalyst. Int. J. Hydrog. Energy 2022, 47, 27383–27396. [Google Scholar]
- Fathinezhad, M.; AbbasiTarighat, M.; Dastan, D. Chemometrics heavy metal content clusters using electrochemical data of modified carbon paste electrode. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100307. [Google Scholar]
- Alamoudi, E.; Timoumi, A. The synthesis and the effect of Cu on optoelectronic qualities of β-In2S3 as a window layer for CIGS thin film solar cells. Results Phys. 2022, 40, 105858. [Google Scholar] [CrossRef]
- Wei, S.; Shi, Z.; Wei, W.; Wang, H.; Dastan, D.; Huang, M.; Shi, J.; Chen, S. Facile preparation of ultralight porous carbon hollow nanoboxes for electromagnetic wave absorption. Ceram. Int. 2021, 47, 28014–28020. [Google Scholar]
- Law, K.Y. Organic photoconductive materials: Recent trends and developments. Chem. Rev. 1993, 93, 449. [Google Scholar] [CrossRef]
- Anthopoulos, T.D.; Shafai, T.S. Influence of oxygen doping on the electrical and photovoltaic properties of Schottky type solar cells based on α-nickel phthalocyanine. Thin Solid Film. 2003, 441, 207–213. [Google Scholar]
- Yin, X.; Zhou, W.; Dastan, D.; Li, J.; Tan, X.; Liu, Y.; Gao, X.; Ma, X. Selectivity sensing response of ZnO-xCo3O4 based sensor to CO against CH4. Mater. Sci. Semicond. Processing 2022, 149, 106883. [Google Scholar] [CrossRef]
- Schueppel, R.; Timmreck, R.; ALllinger, N.; Mueller, T.; Furno, M.; Uhrich, C.; Leo, K.; Riede, M. Controlled current matching in small molecule organic tandem solar cells using doped spacer layers. J. Appl. Phys. 2010, 107, 044503. [Google Scholar]
- Yin, X.; Li, J.; Wang, Q.; Dastan, D.; Shi, Z.; Alharbi, N.; Garmestani, H.; Tan, X.; Liu, Y.; Ma, X. Opposite Sensing Response of Heterojunction Gas Sensors Based on SnO2-Cr2O3 Nanocomposites to H2 against CO and Its Selectivity Mechanism. Langmuir 2021, 37, 13548–13558. [Google Scholar]
- De la Torre, G.; Vazquez, P.; Agullo-Lopez, F.; Torres, T. Phthalocyanines and related compounds:organic targets for nonlinear optical applications. J. Mater. Chem. 1998, 8, 1671–1683. [Google Scholar]
- Böttger, B.; Schindewolf, U.; Avila, J.L.; Rodrigues-Amaro, R. Catalytic electrodeposition of silver on glassy carbon electrodes modified with films of cobalt phthalocyanine. J. Electroanal. Chem. 1997, 432, 139–144. [Google Scholar]
- Neghabi, M.; Zadsar, M.; Ghorashi, S.M.B. Investigation of structural and optoelectronic properties of annealed nickel phthalocyanine thin films. Mater. Sci. Semicond. Process. 2014, 17, 13–20. [Google Scholar]
- Cho, S.W.; Piper, L.F.J.; DeMasi, A.; Preston, A.R.H.; Smith, K.E.; Chauhan, K.V.; Sullivan, P.; Hatton, R.A.; Jones, T.S. Soft X-ray Spectroscopy of C60/Copper Phthalocyanine/MoO3 Interfaces: Role of Reduced MoO3 on Energetic Band Alignment and Improved Performance. J. Phys. Chem. C 2010, 114, 18252–18257. [Google Scholar] [CrossRef]
- El-Nahass, M.M.; Abd-El-Rahman, K.F.; Darwish, A.A.A. Fourier-transform infrared and UV-vis spectroscopes of nickel phthalocyanine thin films. Mater. Chem. Phys. 2005, 92, 185–189. [Google Scholar]
- Yin, X.; Wu, S.; Dastan, D.; Nie, S.; Liu, Y.; Li, Z.; Zhou, Y.; Li, J.; Faik, A.; Shan, K.; et al. Sensing Selectivity of SnO2-Mn3O4 Nanocomposite Sensors for the Detection of H2 and CO Gases. Surf. Interfaces 2021, 25, 101190. [Google Scholar]
- Klyamer, D.; Sukhikh, A.; Nikolaeva, N.; Morozova, N.; Basova, T. Vanadyl Phthalocyanine Films and Their Hybrid Structures with Pd Nanoparticles: Structure and Sensing Properties. Sensors 2020, 20, 1893. [Google Scholar] [CrossRef]
- Zhou, W.; Dastan, D.; Yin, X.; Nie, S.; Wu, S.; Wang, Q.; Li, J. Optimization of gas sensing properties of n-SnO2/p-x CuO sensors for homogenous gases and the sensing mechanism. J. Mater. Sci. Mater. Electron. 2020, 31, 18412–18426. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A.; Saini, R.; Bedi, R.K.; Kumar, S.; Debnath, A.K.; Aswal, D.K. Reversible and fast responding ppb level Cl2 sensor based on noncovalent modified carbon nanotubes with Hexadecafluorinated copper phthalocyanine. Sens. Actuators B Chem. 2018, 255, 87–99. [Google Scholar] [CrossRef]
- Shan, K.; Yi, Z.; Yin, X.T.; Dastan, D.; Altaf, F.; Garmestani, H.; Alamgir, F.M. Mixed conductivity evaluation and sensing characteristics of limiting current oxygen sensors. Surf. Interfaces 2020, 21, 100762. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, L.; Su, X.; Zhou, F.; Duan, G. Interfacial self-assembly of CoPc thin films with their high sensing use as NO2 sensors. Mater. Chem. Phys. 2019, 234, 94–101. [Google Scholar] [CrossRef]
- Xia, S.; Shi, Z.; Sun, L.; Sun, S.; Dastan, D.; Fan, R. Suppressing the loss and enhancing the breakdown strengths of high-k materials via constructing layered structure. Mater. Lett. 2022, 312, 131654. [Google Scholar]
- Ambily, S.; Menon, C.S. The effect of growth parameters on the electrical, optical and structural properties of copper phthalocyanine thin films. Thin Solid Film. 1999, 347, 284–288. [Google Scholar]
- Janczak, J. Temperature dependence on recrystallisation of the magnesium phthalocyanine (MgPc) in triethylamine. Polyhedron 2010, 29, 941–949. [Google Scholar] [CrossRef]
- Kim, I.; Haverinen, H.M.; Wang, Z.; Madakuni, S.; Li, J.; Jabbour, G.E. Effect of molecular packing on interfacial recombination of organic solar cells based on palladium phthalocyanine and perylene derivatives. Appl. Phys. Lett. 2009, 95, 023305. [Google Scholar] [CrossRef]
- Jarosz, G. On small signal capacitance spectra of organic diode formed by ITO-palladium phthalocyanine-Al sandwich system. Thin Solid Film. 2010, 518, 4015–4018. [Google Scholar]
- Gould, R.D. Structure and electrical conduction properties of phthalocyanine thin films. Coord. Chem. Rev. 1996, 156, 237–274. [Google Scholar] [CrossRef]
- De Haan, A.; Debliquy, M.; Decroly, A. Influence of atmospheric pollutants on the conductance of phthalocyanine films. Sens. Actuators B Chem. 1999, 57, 69–74. [Google Scholar] [CrossRef]
- Lasmi, M.; Mahtout, S.; Rabilloud, F. The effect of palladium and platinum doping on the structure, stability and optical properties of germanium clusters: DFT study of PdGen and PtGen (n = 1–20) clusters. Comput. Theor. Chem. 2020, 1181, 112830. [Google Scholar] [CrossRef]
- Azam, S.; Goumri-Said, S.; Khan, S.; Kanoun, M. Electronic, optical and thermoelectric properties of new metal-rich homological selenides with palladium–indium: Density functional theory and Boltzmann transport model. J. Phys. Chem. Solids 2020, 138, 109229. [Google Scholar] [CrossRef]
- Brahim, H. DFT/TD-DFT investigation on the UV-vis absorption and phosphorescence spectra of platinum (II) and palladium (II) complexeswith Schiff-base ligands. J. Lumin. 2019, 210, 96–103. [Google Scholar] [CrossRef]
- Timoumi, A.; Al Turkestani, M.K.; Alamri, S.N.; Alamri, H.; Ouerfelli, J.; Jamoussi, B. Synthesis and characterization of thin films of palladium (II) phthalocyanine and its derivatives using the thermal evaporation technique. Mater. Sci. Mater. Electron. 2017, 28, 7480–7488. [Google Scholar] [CrossRef]
- Timoumi, A.; Bouguila, N.; Koaib, J.; Al Turkestani, M.K.; Jamoussi, B. Study of electrical and dielectric properties of palladium phthalocyanine (PdPc) in pellet form. Mater. Res. Express 2019, 6, 055103. [Google Scholar] [CrossRef]
- Melville, O.A.; Lessard, B.H.; Bender, T.P. Phthalocyanine-Based Organic Thin-Film Transistors: A Review of Recent Advances. ACS Appl. Mater. Interfaces 2015, 7, 13105–13118. [Google Scholar] [CrossRef] [PubMed]
- Juan, A.; Tejada, J.; Lopez-Varo, P.; Chaure, N.B.; Chambrier, I.; Cammidge, A.N.; Cook, M.J.; Jafari-Fini, A.; Ray, A.K. Organic thin film transistors using a liquid crystalline palladium phthalocyanine as active layer. J. Appl. Phys. 2018, 123, 115501. [Google Scholar]
- Zheng, X.; Wang, Y.; Hu, J.; Yang, G.; Guo, Z.; Xia, J.; Xu, Z.; Fang, G. Octamethyl-substituted Pd(ii) phthalocyanine with long carrier lifetime as a dopant-free hole selective material for performance enhancement of perovskite solar cells. J. Mater. Chem. A 2017, 246, 24416–24424. [Google Scholar] [CrossRef]
- Karl, N.; Kraft, K.H.; Marktanner, J.; Munch, M.; Schatz, F.; Stehle, R.; Uhde, H.M.; Vac, J. Fast electronic transport in organic molecular solids? Sci. Technol. A 1999, 17, 2318–2328. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, L.; Hou, W.; Guo, H.; Zhang, H. Dependence of morphology, substrate and thickness of iron phthalocyanine thin films on the photocatalytic degradation of rhodamine B dye. Chem. Pap. 2018, 72, 2327–2337. [Google Scholar] [CrossRef]
- Chakane, S.; Datir, A.; Koinkar, P. Spin coated unsubstituted copper phthalocyanine thin films for nitrogen dioxide sensors. Mod. Phys. Lett. B 2015, 29, 1540032. [Google Scholar] [CrossRef]
- Al-Raqa, S.Y.; Solieman, A.S.; Joraid, A.A.; Alamri, S.N.; Moussa, Z.; Aljuhani, A. Preparation and optical properties of novel symmetrical hexadecachlorinatedphthalocyaninato zinc (II) spin coated thin films. Polyhedron 2008, 27, 1256–1261. [Google Scholar] [CrossRef]
- Critchley, S.M.; Willis, M.R. Deposition of thin phthalocyanine films by spin coating. Int. J. Electron. 1994, 76, 809–814. [Google Scholar] [CrossRef]
- Jafari, M.J.; Azim-Araghi, M.E.; Barhemat, S. Effect of chemical environments on palladium phthalocyanine thin film sensors for humidity analysis. J. Mater. Sci. 2012, 47, 1992–1999. [Google Scholar] [CrossRef]
- Mphuthi, N.G.; Adekunle, A.S.; Fayemi, O.E.; Olasunkanmi, L.O.; Ebenso, E.E. Phthalocyanine Doped Metal Oxide Nanoparticles on Multiwalled Carbon Nanotubes Platform for the detection of Dopamine. Sci. Rep. 2017, 7, 1–23. [Google Scholar] [CrossRef]
- Silva, J.; Sekhar, K.; Negrea, R.; Ghica, C.; Dastan, D.; Gomes, M. Ferroelectric properties of ZrO2 films deposited on ITO-coated glass. Ceram. Int. 2022, 48, 6131–6137. [Google Scholar] [CrossRef]
- Chu, Q.; Sun, Z.; Liu, Y.; Cui, H.; Cheng, B.; Dastan, D.; Moon, K.; Yang, G.; Wong, C. Difluorobenzylamine Treatment of Organolead Halide Perovskite Boosting High Efficiency and Stable Photovoltaic Cells. ACS Appl. Mater. Interfaces 2022, 14, 11388–11397. [Google Scholar] [CrossRef]
- Lokesh, K.S.; Adriaens, A. Synthesis and characterization of tetra-substituted palladium phthalocyanine complexes. Dye. Pigment. 2013, 96, 269–277. [Google Scholar] [CrossRef]
- Mohammed, M.; Al-Mousoi, A.; Singh, S.; Younis, U.; Kumar, A.; Dastan, D.; Ravi, G. Ionic Liquid Passivator for Mesoporous Titanium Dioxide Electron Transport Layer to Enhance Efficiency and Stability of Hole Conductor-Free Perovskite Solar Cells. Energy Fuels 2022. [Google Scholar] [CrossRef]
- Mohammad Beigia, S.; Mesgari, F.; Hossein, M.; Dastan, D.; Xu, G. Electrochemiluminescence Sensors based on Lanthanide Nanomaterials as Modifiers. Curr. Anal. Chem. 2022, 18, 53–62. [Google Scholar] [CrossRef]
- Borker, P.; Salker, A.V.J. Synthesis, characterization and photocatalytic studies of some metal phthalocyanines. Chem. Tech. 2006, 13, 341. [Google Scholar]
- Seoudi, R.; El-Bahy, G.S.; El Sayed, Z.A. Ultraviolet and visible spectroscopic studies of phthalocyanine and its complexes thin films. Opt. Mater. 2016, 29, 304–312. [Google Scholar] [CrossRef]
- Sumimoto, M.; Honda, T.; Kawashima, Y.; Hori, K.; Fujimoto, H. Theoretical and experimental investigation on the electronic properties of the shuttlecock shaped and the double-decker structured metal phthalocyanines, MPc and M(Pc)2 (M = Sn and Pb). Dalton Trans. 2012, 23, 7141–7150. [Google Scholar] [CrossRef]
- Tao, L.; Huang, J.; Dastan, D.; Wang, T.; Li, J.; Yin, X.; Wang, Q. New insight into absorption characteristics of CO2 on the surface of calcite, dolomite, and magnesite. Appl. Surf. Sci. 2021, 540, 148320. [Google Scholar] [CrossRef]
- Toader, M.; Hietschold, M. Tuning the energy level alignment at the SnPc/Ag (111) interface using an STM tip. J. Phys. Chem. C 2011, 115, 3099–3105. [Google Scholar] [CrossRef]
- Tao, L.; Huang, J.; Dastan, D.; Li, J.; Yin, X.; Wang, Q. Flue Gas Separation at Organic-Inorganic Interface under Geological Conditions. Surf. Interfaces 2011, 27, 101462. [Google Scholar] [CrossRef]
- Sumimoto, M.; Honda, T.; Kawashima, Y.; Horia, K.; Fujimoto, H. Significance of dimer models describing physical properties in a triclinic solid of tin(ii) phthalocyanine. RSC Adv. 2012, 33, 12798–12803. [Google Scholar] [CrossRef]
- Ţălu, Ş.; Kulesza, S.; Bramowicz, M.; Stępień, K.; Dastan, D. Analysis of the Surface Microtexture of Sputtered Indium Tin Oxide Thin Films. Arch. Metall. Mater. 2021, 66, 443. [Google Scholar]
- Timoumi, A.; Albetran, H.M.; Alamri, H.R.; Alamri, S.N.; Low, I.M. Impact of annealing temperature on structural, morphological and optical properties of GO-TiO2 thin films prepared by spin coating technique. Superlattices Microstruct. 2020, 139, 106423. [Google Scholar] [CrossRef]
- Timoumi, A.; Bouzouita, H.; Kanzari, M.; Rezig, B. Fabrication and characterization of In2S3 thin films deposited by thermal evaporation technique. Thin Solid Film. 2005, 480, 124–128. [Google Scholar] [CrossRef]
- Timoumi, A.; Bouzouita, H.; Rezig, B. Optical constants of Na–In2S3 thin films prepared by vacuum thermal evaporation technique. Thin Solid Film. 2011, 519, 7615–7619. [Google Scholar] [CrossRef]
- Dastan, D.; Shan, K.; Jafari, A.; Gity, F.; Yin, X.; Shi, Z.; Alharbi, N.; Reshi, B.; Fu, W.; Ţălu, Ş.; et al. Influence of nitrogen concentration on electrical, mechanical, and structural properties of tantalum nitride thin films prepared via DC magnetron sputtering. Appl. Phys. A 2022, 128, 400. [Google Scholar] [CrossRef]
- Yin, X.; Huang, H.; Xie, J.; Dastan, D.; Li, J.; Liu, Y.; Tan, X.; Gao, X.; Shah, W.; Ma, X. High-performance visible-light active Sr-doped porous LaFeO3 semiconductor prepared via sol-gel method. Green Chem. Lett. Rev. 2022, 15, 546–556. [Google Scholar] [CrossRef]
- Frisch, M.J. Gaussian 09, Revision D. 2016; 1. [Google Scholar]
- Liu, L.; Sheng, Y.Y.; Liu, M.; Dienwiebel, M.; Zhang, Z.; Dastan, D. Formation of the third bodies of steel sliding against brass under lubricated conditions. Tribol. Int. 2019, 140, 105727. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Fuentealba, P.; Preuss, H.; Stoll, H.; Von Szentpály, L. A proper account of core-polarization with pseudopotentials: Single valence-electron alkali compounds. Chem. Phys. Lett. 1982, 89, 418–422. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; John Wiley & Sons, Ltd.: London, UK, 1982; Volume 879. [Google Scholar]
- Kavitha, E.; Sandaraganesan, N.; Sebastian, S. Molecular structure, vibrational spectroscopic and HOMO, LUMO studies of 4-nitroaniline by density functional method. Indian J. Pure Appl. Phys. 2010, 48, 20. [Google Scholar]
- Zainuri, D.A.; Razak, I.A.; Arshad, S. Crystal structures, DFT studies and UV–visible absorption spectra of two anthracenyl chalcone derivatives. Acta Cryst. E 2018, 74, 1491–1496. [Google Scholar] [CrossRef]
- Lisi, S.; Gargiani, P.; Scardamaglia, M.; Brookes, N.B.; Sessi, V.; Mariani, C.; Betti, M.G. Graphene-Induced Magnetic Anisotropy of a Two-Dimensional Iron Phthalocyanine Network. J. Phys. Chem. Lett. 2015, 6, 1690–1695. [Google Scholar] [CrossRef]
- Bufon, C.C.B.; Vervacke, C.; Thurmer, D.J.; Fronk, M.; Salvan, G.; Lindner, S.; Knupfer, M.; Zahn, D.R.T.; Schmidt, O.G. Determination of the Charge Transport Mechanisms in Ultrathin Copper Phthalocyanine Vertical Heterojunctions. J. Phys. Chem. C 2014, 118, 7272–7279. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis; Cengage Learning: Boston, MA, USA, 2017; Volume 335. [Google Scholar]
- Davidson, A.T. The effect of the meal atom on the absorption spectra of phthalocyanine films. J. Chem. Phys. 1982, 77, 168–172. [Google Scholar] [CrossRef]
- Martínez-Bourget, D.; Rocha, E.; Labra-Vázquez, P.; Santillan, R.; Ortiz-López, B.; Ortiz-Navarrete, V.; Maraval, V.; Chauvin, R.; Farfán, N. BODIPY-Ethynylestradiol molecular rotors as fluorescent viscosity probes in endoplasmic reticulum. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 283, 121704. [Google Scholar] [CrossRef]
- Haghnegahdar, N.; AbbasiTarighat, M.; Dastan, D. Curcumin-functionalized nanocomposite AgNPs/SDS/MWCNTs for electrocatalytic simultaneous determination of dopamine, uric acid, and guanine in co-existence of ascorbic acid by glassy carbon electrode. J. Mater. Sci. Mater. Electron. 2021, 32, 5602–5613. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, Z.; Zhang, J.; Zhang, K.; Lei, L.; Dastan, D.; Dong, B. Greatly Enhanced Dielectric Charge Storage Capabilities of Layered Polymer Composites Incorporated with low loading fractions of Ultrathin Amorphous Iron Phosphate Nanosheets. J. Mater. Chem. C 2021, 9, 10414–10424. [Google Scholar] [CrossRef]
- Kartha, M.; Reshi, B.; Walke, P.; Dastan, D. Morphological Study of Thin Films: Simulation and Experimental Insights using Horizontal Visibility Graph. Ceram. Int. 2022, 48, 5066–5074. [Google Scholar] [CrossRef]
- Abbasi, S.; Dastan, D.; Ţălu, Ş.; Tahir, M.; Elias, M.; Tao, L.; Li, Z. Evaluation of the dependence of methyl orange organic pollutant removal rate on the amount of titanium dioxide nanoparticles in MWCNTs-TiO2 photocatalyst using statistical methods and Duncan’s multiple range test. Int. J. Environ. An. Chem. 2022, 1–15. [Google Scholar] [CrossRef]
- Timoumi, A.; Zayoud, W.; Sharma, A.; Kraini, M.; Bouguila, N.; Hakamy, A.; Revaprasadu, N.; Alaya, S. Impact of thermal annealing inducing oxidation processon the crystalline powder of In2S3. J. Mater. Sci. Mater. Electron. 2020, 31, 13636–13645. [Google Scholar] [CrossRef]
- Liang, L.; Shi, Z.; Tan, X.; Sun, S.; Chen, M.; Dastan, D.; Dong, B.; Cao, L. Largely Improved Breakdown Strength and Discharge Efficiency of Layer-Structured Nanocomposites by Filling with a Small Loading Fraction of 2D Zirconium Phosphate Nanosheets. Adv. Mater. Interfaces 2022, 2101646. [Google Scholar] [CrossRef]
- Asadzadeh, M.; Tajabadi, F.; Dastan, D.; Sangpour, P.; Shi, Z.; Taghavinia, N. Facile deposition of porous fluorine doped tin oxide by Dr. Blade method for capacitive applications. Ceram. Int. 2021, 47, 5487–5494. [Google Scholar] [CrossRef]
- El-Nhass, M.M.; Solman, H.S.; Metwally, H.S.; Farid, A.M.; Farag, A.A.M.; El Shazly, A.A. Optical properties of evaporated iron phthalocyanine (FePc) thin films. J. Opti. 2001, 30, 121–129. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.W.; Lee, H.H.; Lee, B.; Kim, J.-J. Initial growth mode, nanostructure, and molecular stacking of a ZnPc:C60 bulk heterojunction. Adv. Funct. Mater. 2012, 22, 4244–4248. [Google Scholar] [CrossRef]





| Q-Band | ||
|---|---|---|
| Material | Wavelength (nm) | Eg (eV) |
| PdPc(Im)4 in DMSO solution | 515 | 2.41 |
| PdPc(Im)4-thin films | 500 | 2.48 |
| PdPc(Im)4-DFT | 490 | 2.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timoumi, A.; Dastan, D.; Jamoussi, B.; Essalah, K.; Alsalmi, O.H.; Bouguila, N.; Abassi, H.; Chakroun, R.; Shi, Z.; Ţălu, Ş. Experimental and Theoretical Studies on Optical Properties of Tetra(Imidazole) of Palladium (II) Phthalocyanine. Molecules 2022, 27, 6151. https://doi.org/10.3390/molecules27196151
Timoumi A, Dastan D, Jamoussi B, Essalah K, Alsalmi OH, Bouguila N, Abassi H, Chakroun R, Shi Z, Ţălu Ş. Experimental and Theoretical Studies on Optical Properties of Tetra(Imidazole) of Palladium (II) Phthalocyanine. Molecules. 2022; 27(19):6151. https://doi.org/10.3390/molecules27196151
Chicago/Turabian StyleTimoumi, Abdelmajid, Davoud Dastan, Bassem Jamoussi, Khaled Essalah, Omar Hammed Alsalmi, Noureddine Bouguila, Henda Abassi, Radhouane Chakroun, Zhicheng Shi, and Ştefan Ţălu. 2022. "Experimental and Theoretical Studies on Optical Properties of Tetra(Imidazole) of Palladium (II) Phthalocyanine" Molecules 27, no. 19: 6151. https://doi.org/10.3390/molecules27196151








