Fluorine-Nitrogen-Codoped Carbon Dots as Fluorescent Switch Probes for Selective Fe(III) and Ascorbic Acid Sensing in Living Cells
Abstract
:1. Introduction
2. Experiment Section
2.1. Materials
2.2. Instruments
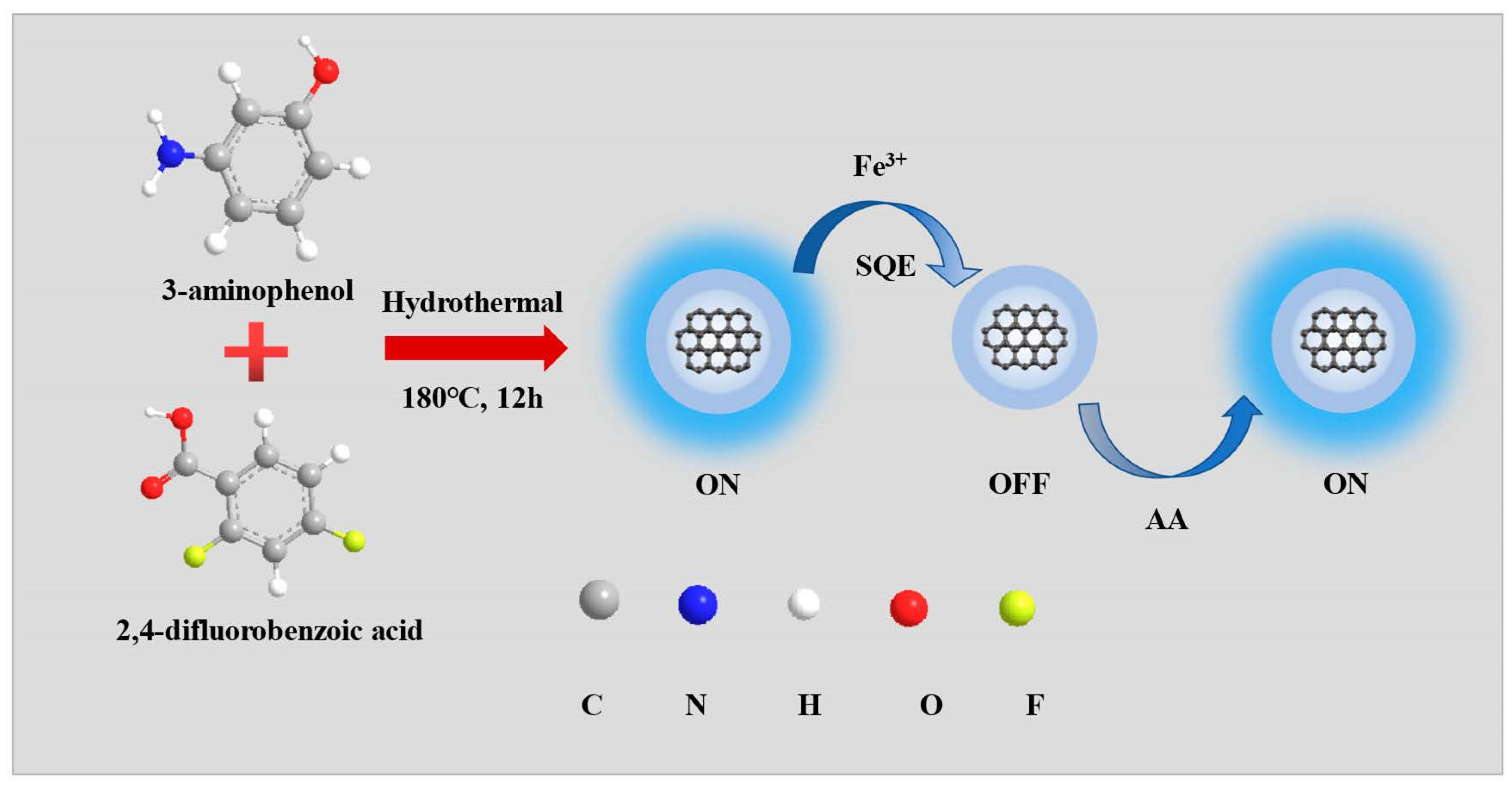
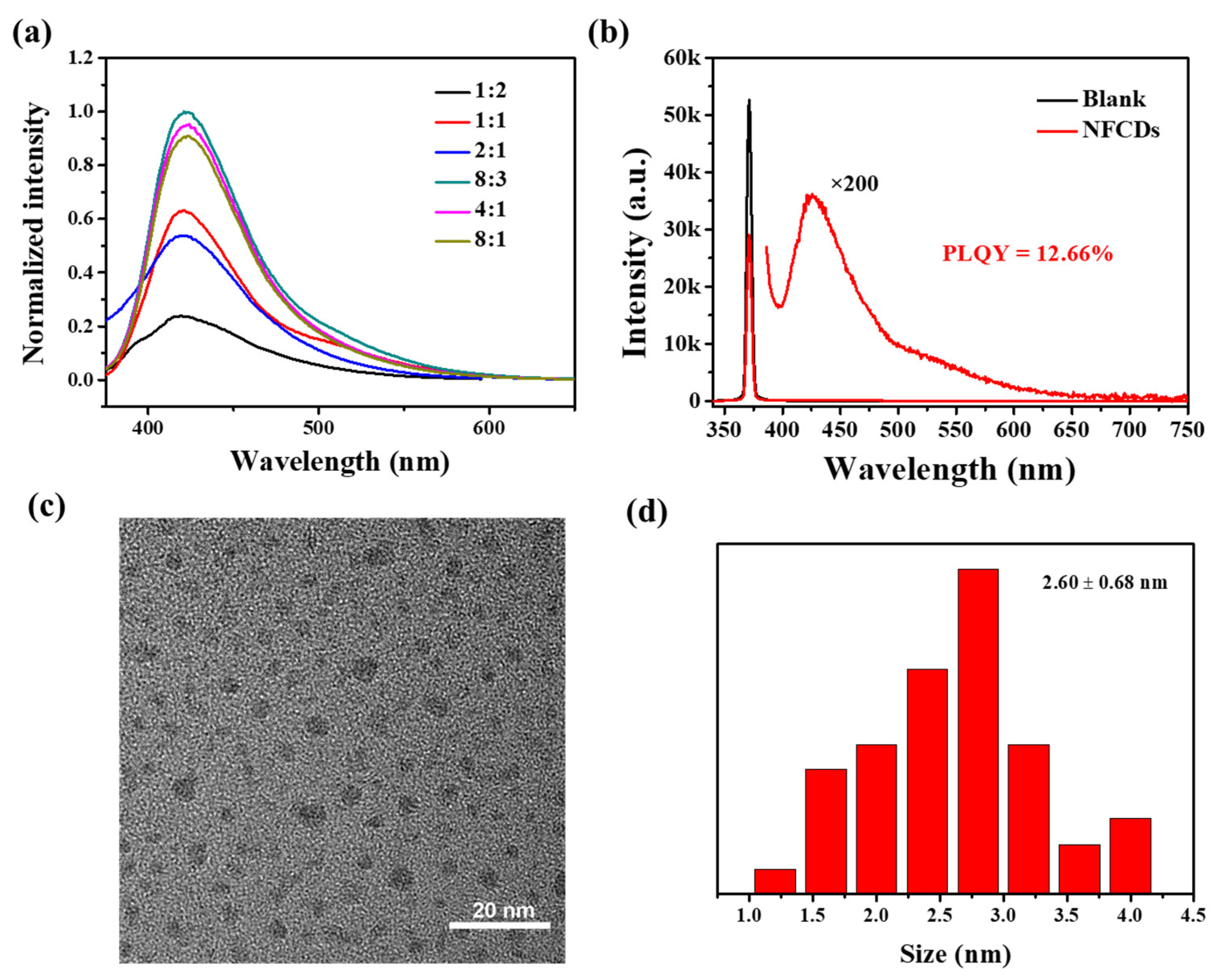
2.3. Synthesis of NFCDs and Measurement of Fluorescence Quantum Yield of NFCDs
2.4. Quantitative Detection of Fe3+ and AA
2.5. Cell Imaging and Cell Viability
3. Results and Discussion
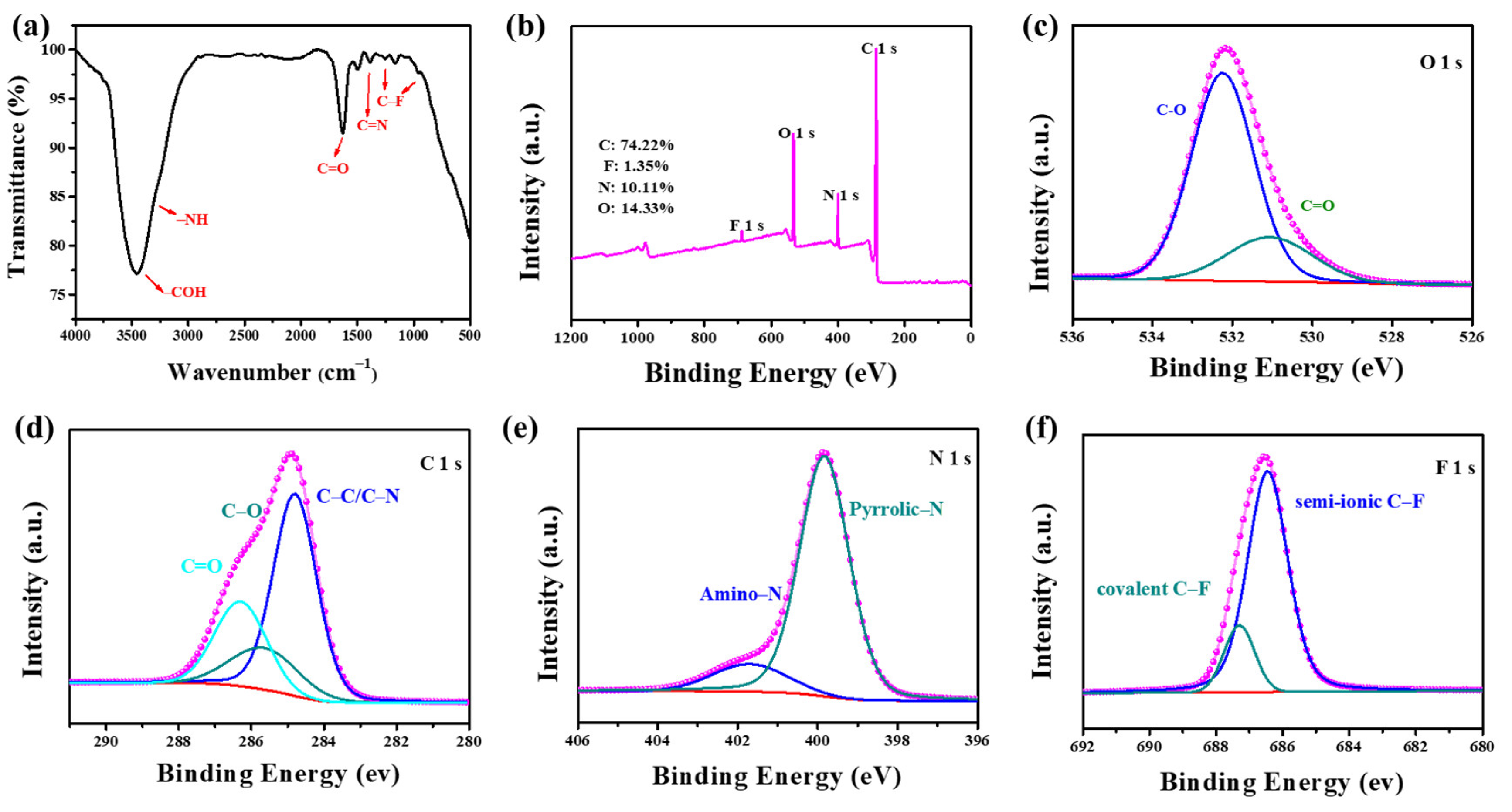
3.1. Characterization of Nanoprobe NFCDs
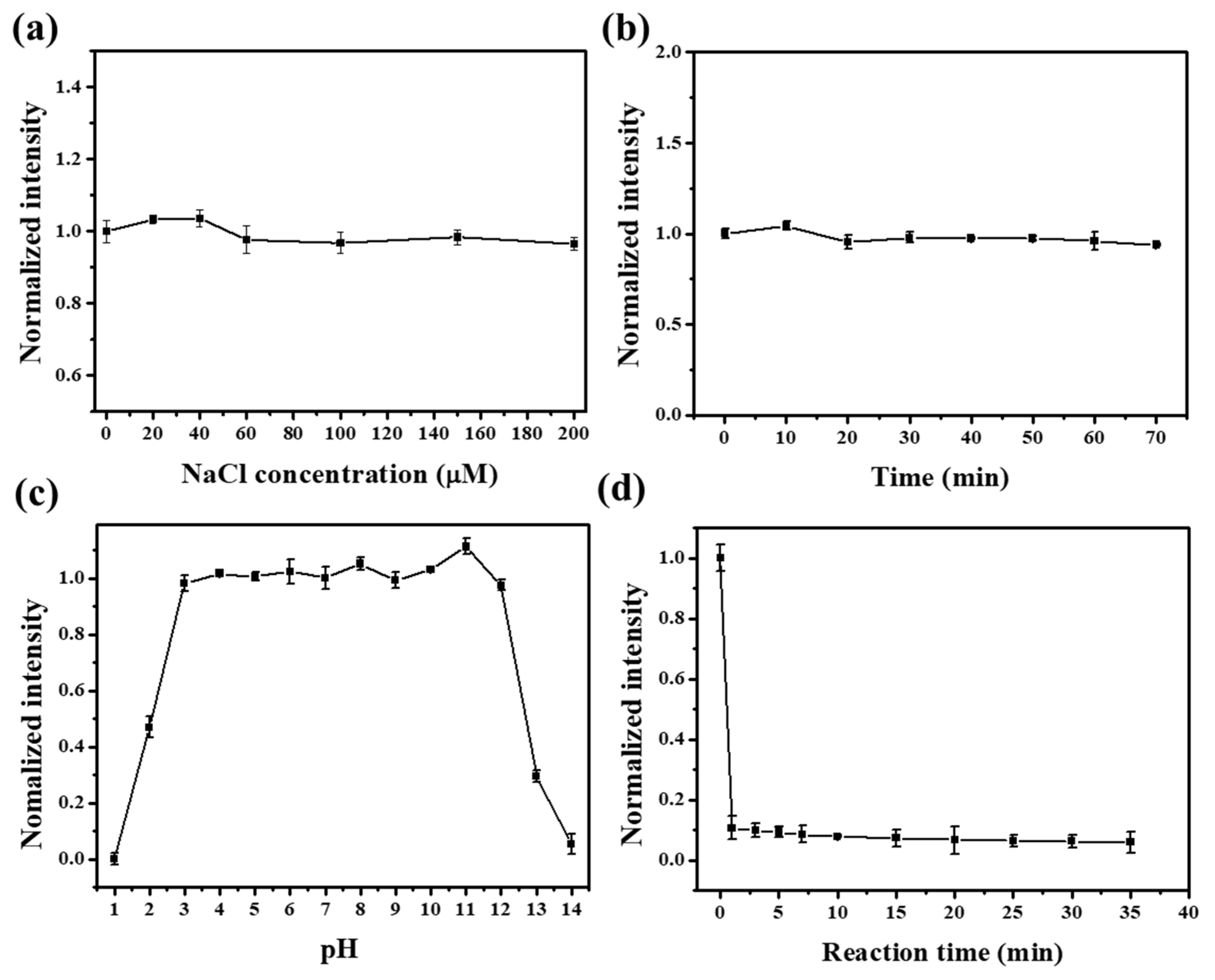
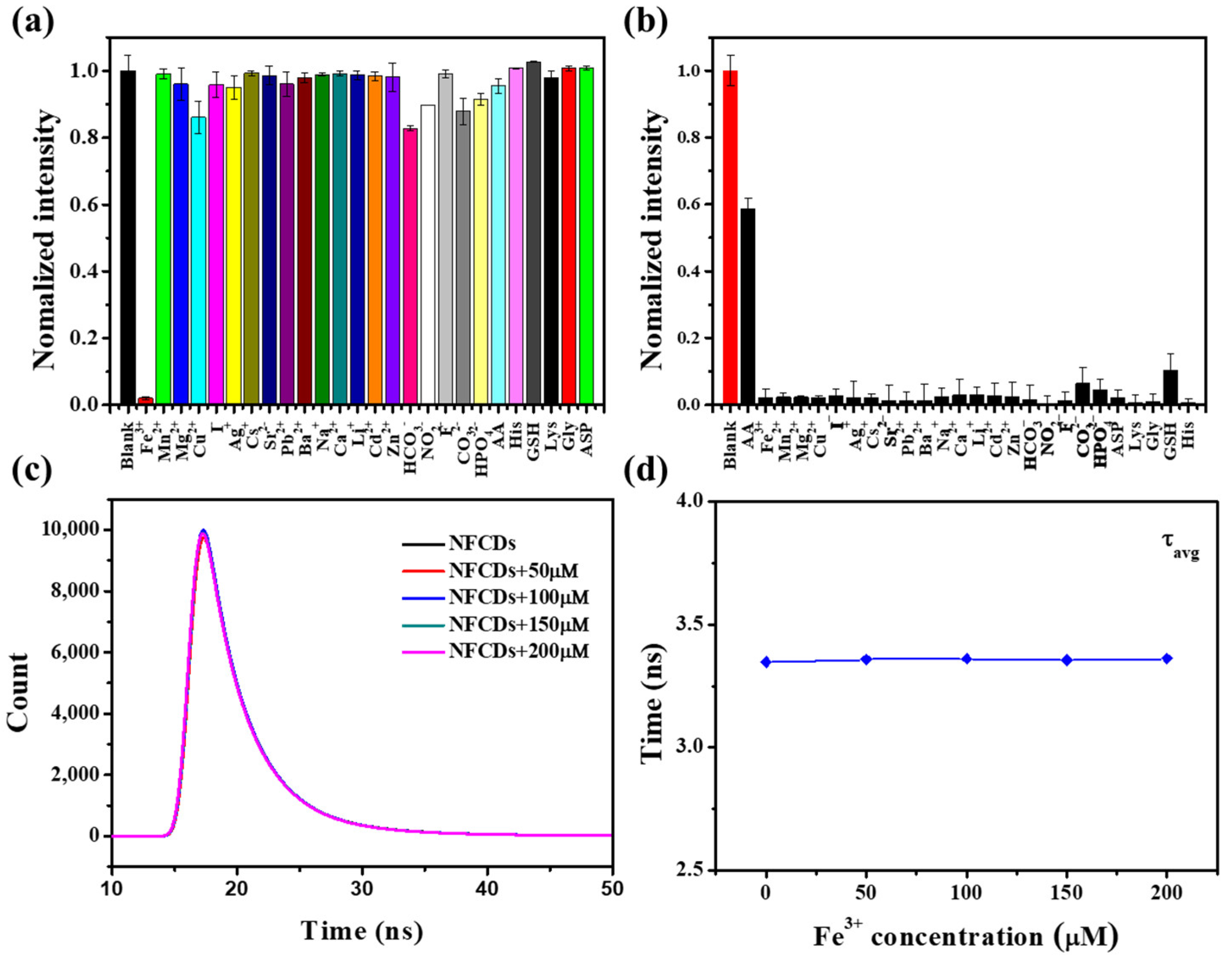
3.2. Selectivity toward Fe3+ and AA
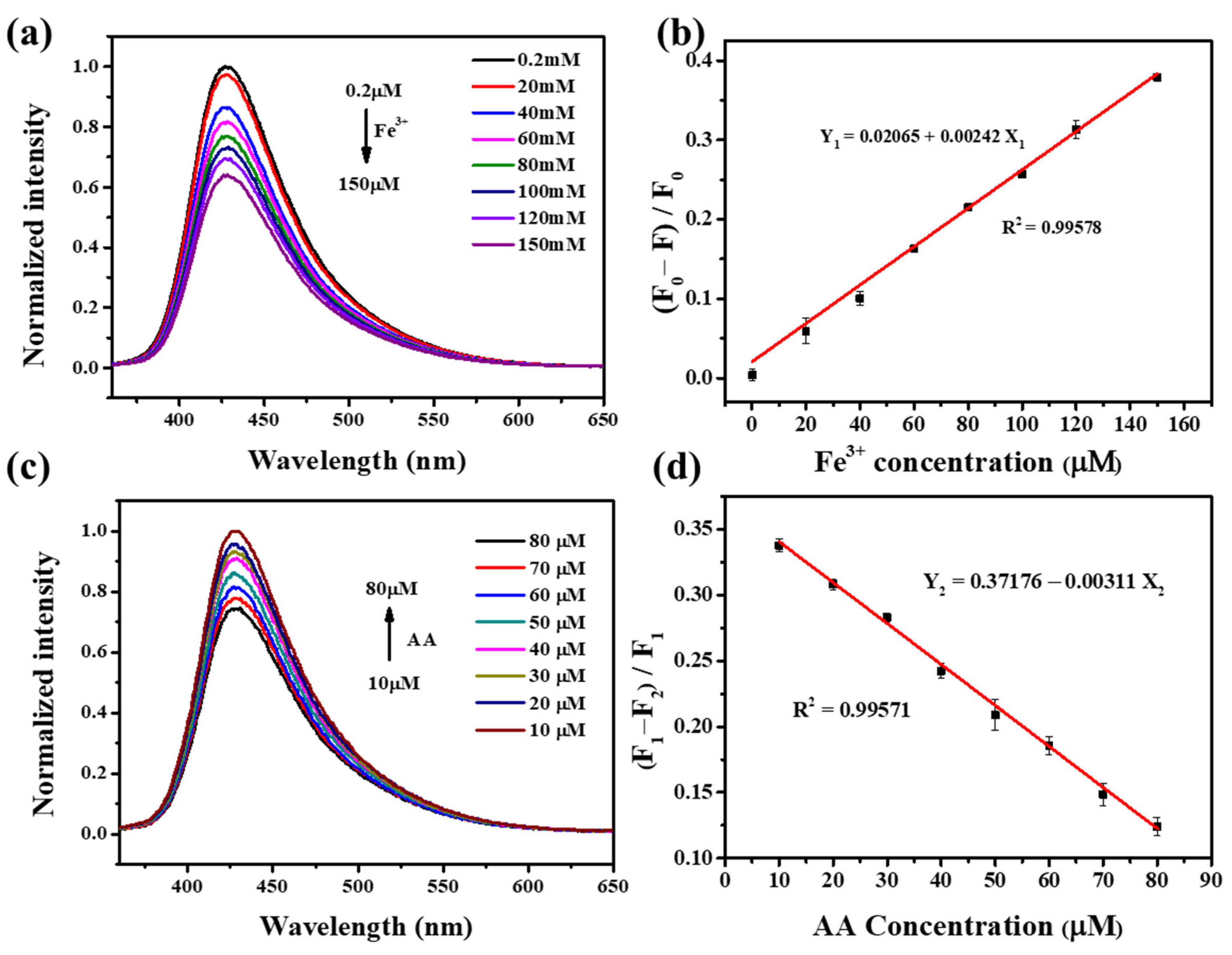
3.3. Detection Range and Limit of Fe3+ and AA for the NFCDs-Based Probe
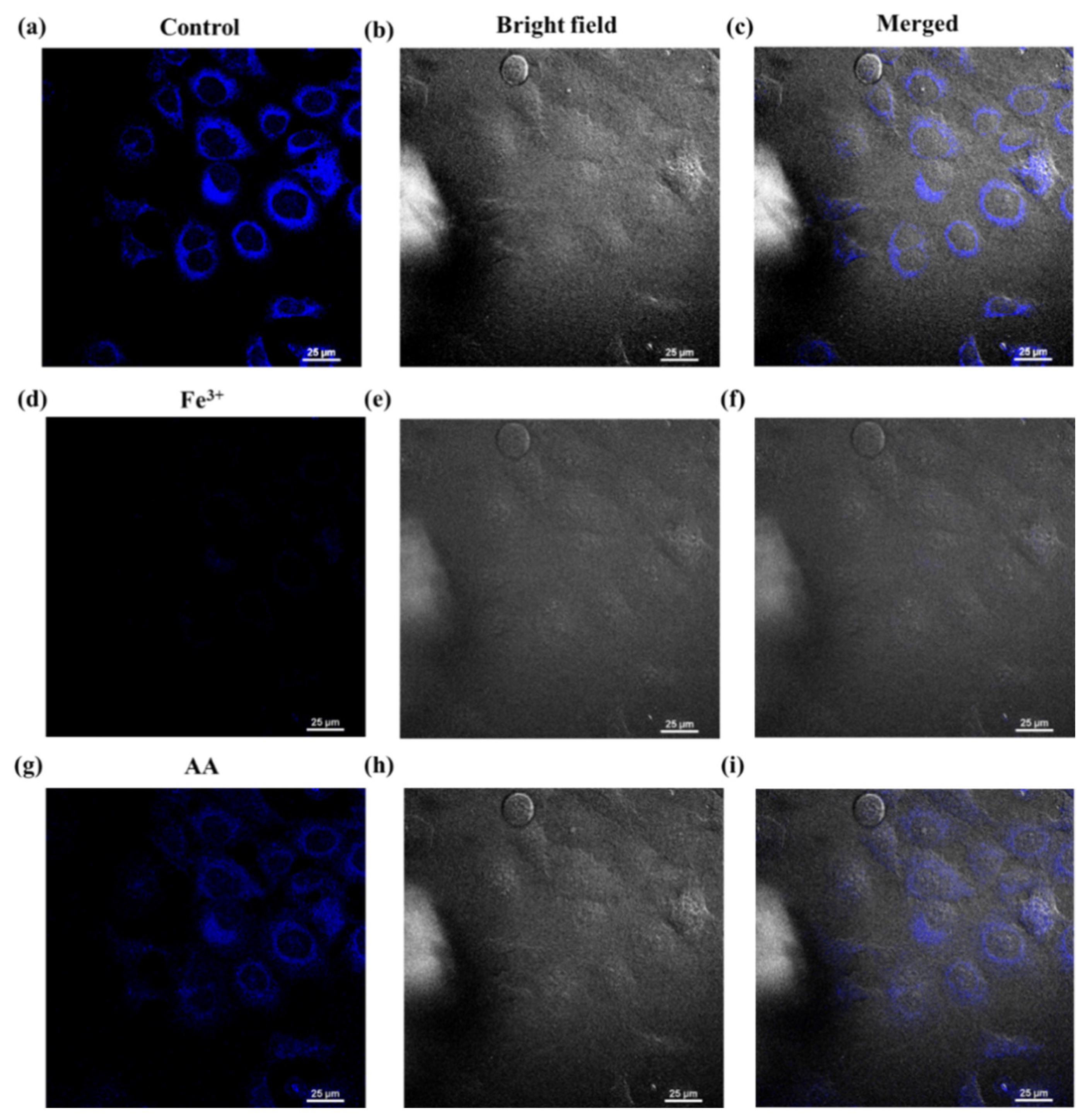
3.4. Cell Imaging of NFCDs in Response to Fe3+ and AA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Xuan, W.; Ruiyi, L.; Saiying, F.; Zaijun, L.; Guangli, W.; Zhiguo, G.; Junkang, L. D-penicillamine-functionalized graphene quantum dots for fluorescent detection of Fe3+ in iron supplement oral liquids. Sens. Actuators B Chem. 2017, 243, 211–220. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Oxygen Species and the Central Nervous System. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Fonseca, V.A.; Alam, M.G.; Shah, S.V. The Role of Iron in Diabetes and Its Complications. Diabetes Care 2007, 30, 1926–1933. [Google Scholar] [CrossRef]
- Galaris, D.; Skiada, V.; Barbouti, A. Redox signaling and cancer: The role of “labile” iron. Cancer Lett. 2008, 266, 21–29. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Pires, A.S.; Marques, C.R.; Encarnação, J.C.; Abrantes, A.M.; Mamede, A.C.; Laranjo, M.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Botelho, M.F. Ascorbic acid and colon cancer: An oxidative stimulus to cell death depending on cell profile. Eur. J. Cell Biol. 2016, 95, 208–218. [Google Scholar] [CrossRef]
- Fiorani, M.; Azzolini, C.; Guidarelli, A.; Cerioni, L.; Cantoni, O. A novel biological role of dehydroascorbic acid: Inhibition of Na+-dependent transport of ascorbic acid. Pharmacol. Res. 2014, 84, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, Á.; Takayama, K.; Tuñón-Molina, A.; Seyran, M.; Hassan, S.S.; Pal Choudhury, P.; Uversky, V.N.; Lundstrom, K.; Adadi, P.; Palù, G.; et al. Carbon-Based Nanomaterials: Promising Antiviral Agents to Combat COVID-19 in the Microbial-Resistant Era. ACS Nano 2021, 15, 8069–8086. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.; Ghosh, S.; Mishra, Y.K.; Tripathi, K.M. Prospects of nano-carbons as emerging catalysts for enzyme-mimetic applications. Mater. Adv. 2022, 3, 3101–3122. [Google Scholar] [CrossRef]
- Qi, C.-X.; Xu, Y.-B.; Li, H.; Chen, X.-B.; Xu, L.; Liu, B. A highly sensitive and selective turn-off fluorescence sensor for Fe3+ detection based on a terbium metal-organic framework. J. Solid State Chem. 2021, 294, 121835. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Kong, R.-M.; Xia, L.; Qu, F. Metal-organic framework as a multi-component sensor for detection of Fe3+, ascorbic acid and acid phosphatase. Chin. Chem. Lett. 2021, 32, 198–202. [Google Scholar] [CrossRef]
- Šafranko, S.; Stanković, A.; Hajra, S.; Kim, H.-J.; Strelec, I.; Dutour-Sikirić, M.; Weber, I.; Bosnar, M.H.; Grbčić, P.; Pavelić, S.K.; et al. Preparation of Multifunctional N-Doped Carbon Quantum Dots from Citrus clementina Peel: Investigating Targeted Pharmacological Activities and the Potential Application for Fe3+ Sensing. Pharmaceuticals 2021, 14, 857. [Google Scholar] [CrossRef]
- Apak, R. Current Issues in Antioxidant Measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Teng, X.; Lu, C. Carbonate interlayered hydrotalcites-enhanced peroxynitrous acid chemiluminescence for high selectivity sensing of ascorbic acid. Analyst 2012, 137, 1876–1881. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, Y.; Ye, J. Determination of Phenolic Compounds and Ascorbic Acid in Different Fractions of Tomato by Capillary Electrophoresis with Electrochemical Detection. J. Agric. Food Chem. 2008, 56, 1838–1844. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Huang, T.; Huang, L.; Sun, Z.; Jiang, Y.; Yao, T.; Wei, S. Red-emitting Au7 nanoclusters with fluorescence sensitivity to Fe2+ ions. J. Mater. Chem. C 2017, 5, 4448–4454. [Google Scholar] [CrossRef]
- Tai, Y.-T.; Simon, T.; Chu, Y.-Y.; Ko, F.-H. One-pot synthesis of copper nanoconjugate materials as luminescent sensor for Fe3+ and I− detection in human urine sample. Sens. Bio-Sens. Res. 2020, 27, 100319. [Google Scholar] [CrossRef]
- Ungor, D.; Csapó, E.; Kismárton, B.; Juhász, Á.; Dékány, I. Nucleotide-directed syntheses of gold nanohybrid systems with structure-dependent optical features: Selective fluorescence sensing of Fe3+ ions. Colloids Surf. B Biointerfaces 2017, 155, 135–141. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, S.; Fang, W.; Fan, Y. Synthesis of N-doped graphene quantum dots from bulk N-doped carbon nanofiber film for fluorescence detection of Fe3+ and ascorbic acid. Fuller. Nanotub. Carbon Nanostruct. 2021, 29, 218–226. [Google Scholar] [CrossRef]
- Shi, X.; Meng, H.; Sun, Y.; Qu, L.; Lin, Y.; Li, Z.; Du, D. Far-Red to Near-Infrared Carbon Dots: Preparation and Applications in biotechnology. Small 2019, 15, 1901507. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing Graphene Quantum Dots and Carbon Dots: Properties, Syntheses, and Biological Applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Huang, H.; Liu, Y.; Kang, Z. Carbon Dots: A Small Conundrum. Trends Chem. 2019, 1, 235–246. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, H.; Yu, N.; Sun, Z.; Chen, L. Conjugate area-controlled synthesis of multiple-color carbon dots and application in sensors and optoelectronic devices. Sens. Actuators B Chem. 2021, 329, 129263. [Google Scholar] [CrossRef]
- Guo, J.; Ye, S.; Li, H.; Song, J.; Qu, J. Novel fluorescence probe based on bright emitted carbon dots for ClO-detection in real water samples and living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 240, 118592. [Google Scholar] [CrossRef]
- Long, P.; Feng, Y.; Cao, C.; Li, Y.; Han, J.; Li, S.; Peng, C.; Li, Z.; Feng, W. Self-Protective Room-Temperature Phosphorescence of Fluorine and Nitrogen Codoped Carbon Dots. Adv. Funct. Mater. 2018, 28, 1800791. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, H.; Lu, S.; Geng, T.; Xiao, G.; Zou, B.; Bi, H. Photoactivated Fluorescence Enhancement in F, N-Doped Carbon Dots with Piezochromic Behavior. Angew Chem. Int Ed Engl. 2020, 59, 9986–9991. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, K.; Hua, X.; Wang, W.; Li, J.; Zhang, S.; Chen, S. Pyridinic-N Protected Synthesis of 3D Nitrogen-Doped Porous Carbon with Increased Mesoporous Defects for Oxygen Reduction. Small 2019, 15, 1805325. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, A.Y.; Xie, X.F.; Wang, X.Y.; Wang, D.; Wang, P.; Li, H.J.; Yang, J.H.; Li, Y. Doping effect and fluorescence quenching mechanism of N-doped graphene quantum dots in the detection of dopamine. Talanta 2019, 196, 563–571. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Liu, M.; Liu, Y. Selective detection of Fe3+ ions based on fluorescence MXene quantum dots via a mechanism integrating electron transfer and inner filter effect. Nanoscale 2020, 12, 1826–1832. [Google Scholar] [CrossRef]
- Starzak, K.; Matwijczuk, A.; Creaven, B.; Matwijczuk, A.; Wybraniec, S.; Karcz, D. Fluorescence Quenching-Based Mechanism for Determination of Hypochlorite by Coumarin-Derived Sensors. Int. J. Mol. Sci. 2019, 20, 281. [Google Scholar] [CrossRef] [Green Version]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Raveendran, V.; Suresh Babu, A.R.; Renuka, N.K. Mint leaf derived carbon dots for dual analyte detection of Fe(iii) and ascorbic acid. RSC Adv. 2019, 9, 12070–12077. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Man, H.; Dong, L.; Huang, J.; Wang, X. Preparation of highly crystalline nitrogen-doped carbon dots and their application in sequential fluorescent detection of Fe3+ and ascorbic acid. Food Chem. 2020, 326, 126935. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.; Zhang, M.; Wang, Y.; Zhang, B.; Mei, X. Tiopronin protected gold-silver bimetallic nanoclusters for sequential detection of Fe3+ and ascorbic acid in serum. Microchem. J. 2022, 174, 107048. [Google Scholar] [CrossRef]
- Bandi, R.; Devulapalli, N.P.; Dadigala, R.; Gangapuram, B.R.; Guttena, V. Facile Conversion of Toxic Cigarette Butts to N, S-Codoped Carbon Dots and Their Application in Fluorescent Film, Security Ink, Bioimaging, Sensing and Logic Gate Operation. ACS Omega 2018, 3, 13454–13466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, C.; Shao, X.; Guan, R.; Hu, Y.; Zhang, K.; Liu, W.; Hong, M.; Yue, Q. Dual-emission ratio fluorescence for selective and sensitive detection of ferric ions and ascorbic acid based on one-pot synthesis of glutathione protected gold nanoclusters. RSC Adv. 2021, 11, 17283–17290. [Google Scholar] [CrossRef]
- Li, H.; Ye, S.; Guo, J.; Wang, H.; Yan, W.; Song, J.; Qu, J. Biocompatible carbon dots with low-saturation-intensity and high-photobleaching-resistance for STED nanoscopy imaging of the nucleolus and tunneling nanotubes in living cells. Nano Res. 2019, 12, 3075–3084. [Google Scholar] [CrossRef]

| Types of Fluorescent Probes | Detection Range of Fe3+ (μM) | Detection Range of AA (μM) | Reference |
|---|---|---|---|
| Carbon dot | 0–0.38 | 0–0.78 | [32] |
| Metal-organic framework | 5–60 | 1–20 | [11] |
| Carbon dot | 0–145 | 0–150 | [33] |
| nanoclusters | 0.5–80 | 0.2–80 | [34] |
| Carbon dot | 0–100 | 0–100 | [35] |
| Carbon dot | 0.75–125 | 0.25–30 | [36] |
| Carbon dot | 0.2–150 | 10–80 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, S.; Zhang, M.; Guo, J.; Yu, X.; Song, J.; Zeng, P.; Qu, J.; Chen, Y.; Li, H. Fluorine-Nitrogen-Codoped Carbon Dots as Fluorescent Switch Probes for Selective Fe(III) and Ascorbic Acid Sensing in Living Cells. Molecules 2022, 27, 6158. https://doi.org/10.3390/molecules27196158
Ye S, Zhang M, Guo J, Yu X, Song J, Zeng P, Qu J, Chen Y, Li H. Fluorine-Nitrogen-Codoped Carbon Dots as Fluorescent Switch Probes for Selective Fe(III) and Ascorbic Acid Sensing in Living Cells. Molecules. 2022; 27(19):6158. https://doi.org/10.3390/molecules27196158
Chicago/Turabian StyleYe, Shuai, Mingming Zhang, Jiaqing Guo, Xiantong Yu, Jun Song, Pengju Zeng, Junle Qu, Yue Chen, and Hao Li. 2022. "Fluorine-Nitrogen-Codoped Carbon Dots as Fluorescent Switch Probes for Selective Fe(III) and Ascorbic Acid Sensing in Living Cells" Molecules 27, no. 19: 6158. https://doi.org/10.3390/molecules27196158
APA StyleYe, S., Zhang, M., Guo, J., Yu, X., Song, J., Zeng, P., Qu, J., Chen, Y., & Li, H. (2022). Fluorine-Nitrogen-Codoped Carbon Dots as Fluorescent Switch Probes for Selective Fe(III) and Ascorbic Acid Sensing in Living Cells. Molecules, 27(19), 6158. https://doi.org/10.3390/molecules27196158







