Effect of Germination on the Avenanthramide Content of Oats and Their in Vitro Antisensitivity Activities
Abstract
1. Introduction
2. Results
2.1. Optimization Methods
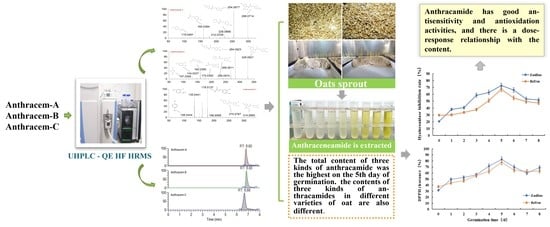
2.2. Variation Pattern of the AVN Content of the Germinated Oats and the Effect of Variety on the Content
2.3. In Vitro Antisensitivity and Antioxidant Activities of the Germinated Oats
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Instruments and Equipment
4.3. Establishment of the Anthranilamide Assay
4.3.1. Preparation of the AVN Standard
4.3.2. Method Establishment
4.4. Germination Treatment of the Oats
4.5. Extraction of Anthranilamide
4.6. In Vitro Determination of the Anti-Allergy
4.7. Measurement of the DPPH Free-Radical-Scavenging Rate
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Koecher, K.; Hansen, L.; Ferruzzi, M.G. Phenolic recovery and bioaccessibility from milled and finished whole grain oat products. Food Funct. 2016, 7, 3370–3381. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Villaluenga, C.; Peñas, E. Health benefits of oat: Current evidence and molecular mechanisms. Curr. Opin. Food Sci. 2017, 14, 26–31. [Google Scholar] [CrossRef]
- Wu, W.; Tang, Y.; Yang, J.; Idehen, E.; Sang, S. Avenanthramide Aglycones and Glucosides in Oat Bran: Chemical Profile, Levels in Commercial Oat Products, and Cytotoxicity to Human Colon Cancer Cells. J. Agric. Food Chem. 2018, 66, 8005–8014. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.W. Oat phenolics: Avenanthramides, novel substi-tuted N-cinnamoylanthranilate alkaloids from oat groats and hulls. J. Agric. Food Chem. 1989, 37, 60–66. [Google Scholar] [CrossRef]
- Boz, H. Phenolic amides (avenanthramides) in oats—A review. Czech J. Food Sci. 2016, 33, 399–404. [Google Scholar] [CrossRef]
- Schendel, R.R. Phenol Content in Sprouted Grains; Sprouted Grain University of Kentucky: Lexington, KY, USA, 2019; pp. 247–315. [Google Scholar] [CrossRef]
- Skoglund, M.; Peterson, D.M.; Andersson, R.; Nilsson, J.; Dimberg, L.H. Avenanthramide content and related enzyme activities in oats as affected by steeping and germination. J. Cereal Sci. 2008, 48, 294–303. [Google Scholar] [CrossRef]
- De Bruijn, W.J.C.; Van Dinteren, S.; Gruppen, H.; Vincken, J.P. Mass spectrometric characterisation of avenanthramides and enhancing their production by germination of oat (Avena sativa). Food Chem. 2019, 277, 682–690. [Google Scholar] [CrossRef]
- Chen, C.; Milbury, P.E.; Collins, F.W.; Blumberg, J.B. Avenanthramides are bioavailable and have antioxidant activity in humans after acute consumption of an enriched mixture from oats. J. Nutr. 2007, 137, 1375–1382. [Google Scholar] [CrossRef]
- Jastrebova, J.; Skoglund, M.; Nilsson, J.; Dimberg, L. Selective and sensitive LC-MS determination of avenanthramides in oats. Chromatographia 2006, 63, 419–423. [Google Scholar] [CrossRef]
- Ishihara, A.; Kojima, K.; Fujita, T.; Yamamoto, Y.; Nakajima, H. New series of avenanthramides in oat seed. J. Agric. Chem. Soc. Jpn. 2014, 78, 1975–1983. [Google Scholar] [CrossRef]
- Xie, Z.; Mui, T.; Sintara, M.; Ou, B.; Johnson, J.; Chu, Y.; O’Shea, M.; Kasturi, P.; Chen, Y. Rapid quantitation of avenanthramides in oat-containing products by high-performance liquid chromatographycoupled with triple quadrupole mass spectrometry (HPLC-TQMS). Food Chem. 2016, 224, 280–288. [Google Scholar] [CrossRef]
- Wise, M.L. Avenanthramides: Chemistry and Biosynthesis; John Wiley & Sons, Ltd.: Madison, WI, USA, 2014; pp. 195–226. [Google Scholar] [CrossRef]
- Chu, Y.F.; Wise, M.L.; Gulvady, A.A.; Chang, T.; Kendra, D.F.; van Klinken, B.J.-W.; Shi, Y.; O’Shea, M. In vitro antioxidant capacity and anti-inflammatory activity of seven common oats. Food Chem. 2013, 139, 426–431. [Google Scholar] [CrossRef]
- Dimberg, L.H.; Sunnerheim, K.; Sundberg, B.; Walsh, K. Stability of Oat Avenanthramides. Cereal Chem. J. 2001, 78, 278–281. [Google Scholar] [CrossRef]
- Rao, H.; Chen, C.; Tian, Y.; Li, Y.; Gao, Y.; Tao, S.; Xue, W. Germination results in reduced allergenicity of peanut by degradation of allergens and resveratrol enrichment. Innov. Food Sci. Emerg. Technol. 2018, 50, 188–195. [Google Scholar] [CrossRef]
- Aguilera, Y.; Herrera, T.; Liébana, R.; Rebollo-Hernanz, M.; Sanchez-Puelles, C.; Martín-Cabrejas, M.A. Impact of Melatonin Enrichment during Germination of Legumes on Bioactive Compounds and Antioxidant Activity. J. Agric. Food Chem. 2015, 63, 7967–7974. [Google Scholar] [CrossRef]
- Collins, F.W.; Burrows, V.D. Method for Increasing Concentration of Avenanthramides in Oats. U.S. Patent US13259447, 4 July 2012. [Google Scholar]
- Liu, L.; Zubik, L.; Collins, F.W.; Marko, M.; Meydani, M. The anti-atherogenic potential of oat phenolic compounds. Atherosclerosis 2004, 175, 39–49. [Google Scholar] [CrossRef]
- Sur, R.; Nigam, A.; Grote, D.; Liebel, F.; Southall, M.D. Avenanthramides, polyphenols from oats, exhibit anti-inflammatory andanti-itch activity. Arch. Dermatol. Res. 2008, 300, 569–574. [Google Scholar] [CrossRef]
- Azuma, H.; Banno, K.; Yoshimura, T. Pharmacological propertiesof N-(3′,4′-dimethoxycinnamoyl)anthranilic acid (N-5), a new anti-atopic agent. Br. J. Pharmacol. 1976, 58, 483–488. [Google Scholar] [CrossRef]
- Dhakal, H.; Yang, E.J.; Lee, S.; Kim, M.J.; Baek, M.C.; Lee, B.; Park, P.H.; Kwon, T.K.; Khang, D.; Song, K.S. Avenanthramide C from germinated oats exhibits anti-allergic inflammatory effects in mast cells. Sci. Rep. 2019, 9, 6884. [Google Scholar] [CrossRef]
- Zhang, L. Study on Optimization of High-Pressure Microfluidization and Mixed Fermentation Processes and Biological Activity of Solanum nigrum L. Master’s Thesis, Beijing Forestry University, Beijing, China, 2020. [Google Scholar] [CrossRef]
- Kakegawa, H.; Matsumoto, H.; Satoh, T. Inhibitory Effects of Hydrangenol Derivatives on the Activation of Hyaluronidase and their Antiallergic Activities. Planta Medica 1988, 54, 385–389. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Teng, J.W.; Huang, L.; Wei, B.Y.; Ning, X.; Feng, Z.C. Study on antioxidant and anti-allergic effects of ground elm extract. Shi Zhen Guo Yi Guo Yao 2009, 20, 1958–1960. [Google Scholar]
- Žilić, S.; Basić, Z.; Hadži-Tašković Šukalović, V.; Maksimović, V.; Janković, M.; Filipović, M. Can the sprouting process applied to wheat improve the contents of vitamins and phenolic compounds and antioxidant capacity of the flour? Int. J. Food Sci. Technol. 2014, 49, 1040–1047. [Google Scholar] [CrossRef]
- Harmuth-Hoene, A.E.; Bognar, A.E.; Kornemann, U.; Diehl, J.F. Der Einfluss der Keimung auf den Nährwert von Weizen, Mungbohnen und Kichererbsen. Z. Fur Lebensm. Unters. Und Forsch. 1987, 185, 386–393. [Google Scholar] [CrossRef]
- Krapf, J.; Kandzia, F.; Brühan, J.; Walther, G.; Flöter, E. Sprouting of oats: A new approach to quantify compositional changes. Cereal Chem. 2019, 96, 994–1003. [Google Scholar] [CrossRef]
- Tian, B.; Xie, B.; Shi, J.; Wu, J.; Cai, Y.; Xu, T.; Deng, Q. Physicochemical changes of oat seeds during germination. Food Chem. 2010, 119, 1195–1200. [Google Scholar] [CrossRef]
- Xu, J.; Tian, C.; Hu, Q.P.; Luo, J.Y.; Wang, X.D.; Tian, X. Dynamic changes in phenolic compounds and antioxidant activity in oats (Avena nuda L.) during steeping and germination. J. Agric. Food Chem. 2009, 57, 10392–10398. [Google Scholar] [CrossRef]
- Damazo-Lima, M.; Rosas-Pérez, G.; Reynoso-Camacho, R.; Pérez-Ramírez, I.F.; Rocha-Guzmán, N.E.; de Los Ríos, E.A.; Ramos-Gomez, M. Chemopreventive Effect of the Germinated Oat and its Phenolic-AVA Extract in Azoxymethane/Dextran Sulfate Sodium (AOM/DSS) Model of Colon Carcinogenesis in Mice. Foods 2020, 9, 169. [Google Scholar] [CrossRef]
- Fagerlund, A.; Sunnerheim, K.; Dimberg, L.H. Radical-scavenging and antioxidant activity of avenanthramides. Food Chem. 2009, 113, 550–556. [Google Scholar] [CrossRef]




| Name | Molecular Formula | CAS Number | Molecular Structure | Precursor Ion (m/z) | Collision Energy (CE) | Retention Time (min) | Fragment Ion (m/z) |
|---|---|---|---|---|---|---|---|
| Avenanthramide A | C16H13NO5 | 108605-70-5 |  | 298.0721 | 30/40 | 6.8 | 254.0817/160.0394 |
| Avenanthramide B | C17H15NO6 | 108605-69-2 |  | 328.0821 | 30/40 | 6.83 | 284.0923/268.0611 |
| Avenanthramide C | C16H13NO6 | 116764-15-9 |  | 314.0665 | 20/30 | 6.66 | 178.0137/135.0441 |
| Time (min) | Flow (mL/min) | B (%) | Injection Volume (µL) |
|---|---|---|---|
| 0 | 0.45 | 2 | 5 |
| 3.5 | 0.45 | 2 | 5 |
| 7.0 | 0.45 | 98 | 5 |
| 7.5 | 0.45 | 98 | 5 |
| 8.0 | 0.45 | 2 | 5 |
| Anthracamide Type | Ranger (μg/L) | Linear Equations | Coefficient (R) | LOD (μg/L) | LOQ (μg/L) |
|---|---|---|---|---|---|
| AVN-A | 1–2000 | y = 0.9654x + 5.5375 | 0.9989 | 2.2 | 6.6 |
| AVN-B | 1–2000 | y = 0.9737x − 2.9207 | 0.9968 | 1.7 | 5.1 |
| AVN-C | 1–2000 | y = 0.9713x − 2.5372 | 0.9971 | 3.7 | 11.1 |
| 0.5 μg/L | 1 μg/L | 1.5 μg/L | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | Inter RSD (%) | Inter RSD (%) | Recovery (%) | Inter RSD (%) | Inter RSD (%) | Recovery (%) | Inter RSD (%) | Inter RSD (%) | |
| AVN-A | 109.5 | 4.8 | 5.9 | 112.9 | 8.4 | 8.3 | 113.2 | 5.1 | 10.1 |
| AVN-B | 103.9 | 5.4 | 2.7 | 115.9 | 3.9 | 6.4 | 111.6 | 10.2 | 17.1 |
| AVN-C | 100.4 | 4.7 | 4.2 | 110.2 | 4.3 | 6.6 | 118 | 8.8 | 12.8 |
| Germination Time | Zaohau Varieties | Bayou Varieties | ||||
|---|---|---|---|---|---|---|
| (d) | AVN-A (μg/g) | AVN-B (μg/g) | AVN-C (μg/g) | AVN-A (μg/g) | AVN-B (μg/g) | AVN-C (μg/g) |
| 0 | 2.98 ± 0.24 | 1.63 ± 0.18 | 3.36 ± 0.27 | 7.58 ± 0.45 | 5.41 ± 0.19 | 5.29 ± 0.89 |
| 1 | 36.31 ± 2.49 | 34.09 ± 2.36 | 30.15 ± 1.99 | 18.37 ± 0.88 | 17.79 ± 0.74 | 9.25 ± 0.54 |
| 2 | 41.39 ± 2.94 | 39.36 ± 2.08 | 29.67 ± 1.28 | 19.51 ± 1.64 | 37.12 ± 1.68 | 20.91 ± 1.55 |
| 3 | 38.97 ± 1.51 | 49.22 ± 1.51 | 37.65 ± 2.14 | 34.76 ± 1.78 | 34.45 ± 2.18 | 27.14 ± 1.92 |
| 4 | 43.14 ± 1.69 | 51.53 ± 1.75 | 49.21 ± 2.64 | 35.42 ± 3.81 | 40.08 ± 3.37 | 28.22 ± 1.23 |
| 5 | 49.44 ± 2.12 | 53.46 ± 2.17 | 50.61 ± 1.79 | 39.24 ± 1.94 | 50.46 ± 2.05 | 36.6 ± 3.16 |
| 6 | 43.21 ± 4.11 | 50.35 ± 3.58 | 54.23 ± 3.77 | 39.73 ± 1.50 | 33.13 ± 1.71 | 36.57 ± 2.55 |
| 7 | 38.03 ± 3.04 | 39.58 ± 1.33 | 55.43 ± 2.48 | 36.28 ± 1.07 | 35.40 ± 1.22 | 36.86 ± 1.35 |
| 8 | 39.34 ± 2.80 | 47.26 ± 2.33 | 60.75 ± 2.40 | 33.92 ± 2.82 | 27.76 ± 1.16 | 41.04 ± 2.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Suo, D.; Guan, X.; Wang, S.; Xiao, Z.; Li, Y.; Liu, X.; Fan, X. Effect of Germination on the Avenanthramide Content of Oats and Their in Vitro Antisensitivity Activities. Molecules 2022, 27, 6167. https://doi.org/10.3390/molecules27196167
Feng Y, Suo D, Guan X, Wang S, Xiao Z, Li Y, Liu X, Fan X. Effect of Germination on the Avenanthramide Content of Oats and Their in Vitro Antisensitivity Activities. Molecules. 2022; 27(19):6167. https://doi.org/10.3390/molecules27196167
Chicago/Turabian StyleFeng, Yuchao, Decheng Suo, Xin Guan, Shi Wang, Zhiming Xiao, Yang Li, Xiaolu Liu, and Xia Fan. 2022. "Effect of Germination on the Avenanthramide Content of Oats and Their in Vitro Antisensitivity Activities" Molecules 27, no. 19: 6167. https://doi.org/10.3390/molecules27196167
APA StyleFeng, Y., Suo, D., Guan, X., Wang, S., Xiao, Z., Li, Y., Liu, X., & Fan, X. (2022). Effect of Germination on the Avenanthramide Content of Oats and Their in Vitro Antisensitivity Activities. Molecules, 27(19), 6167. https://doi.org/10.3390/molecules27196167