Fast Detection of Cadmium in Chocolate by Solid Sampling Electrothermal Vaporization Atomic Absorption Spectrometry and Its Application on Dietary Exposure Risk Assessment
Abstract
:1. Introduction
2. Results and Discussion
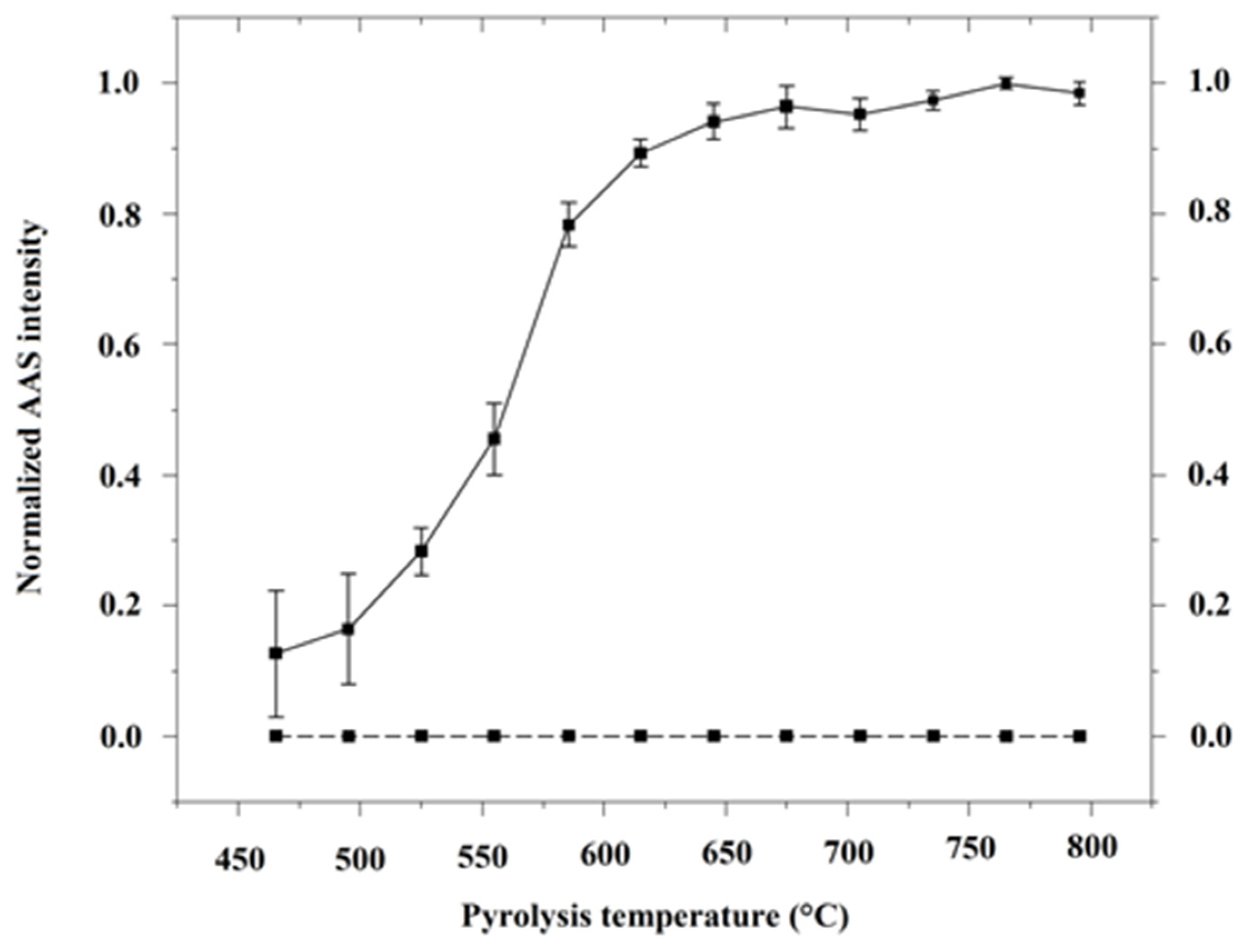
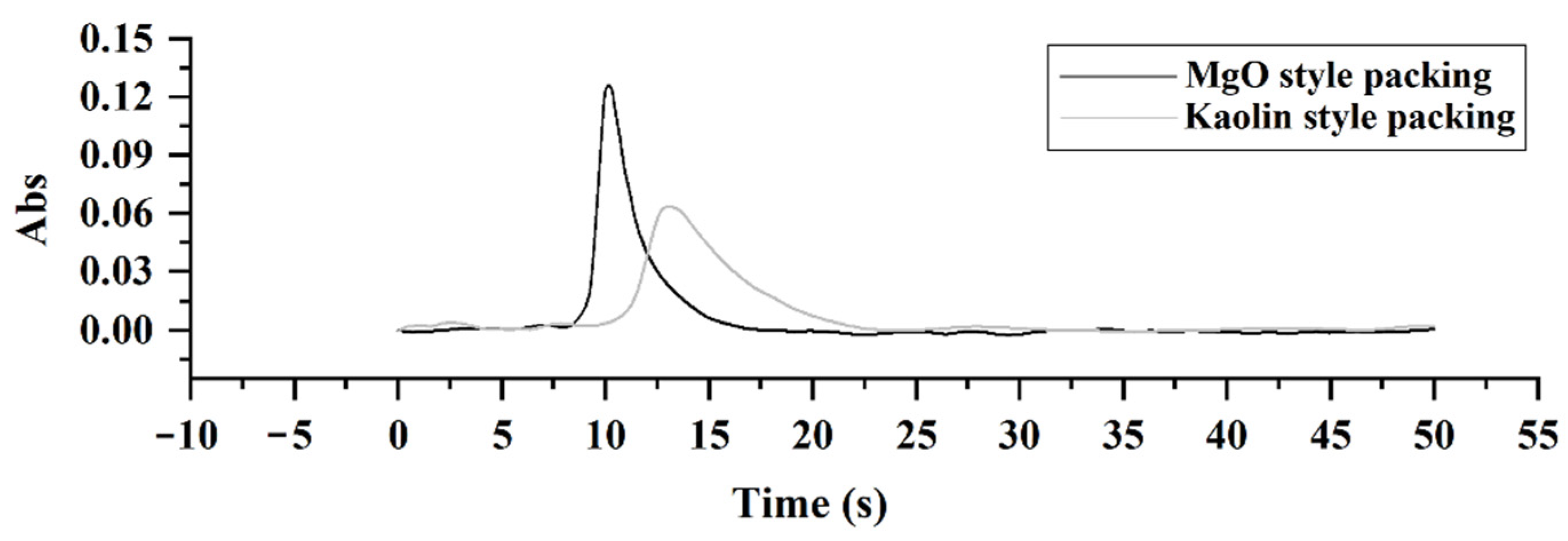
2.1. Modification of the Pyrolysis Filler
2.2. Sample Dehydration and Cd Pre-Vaporization
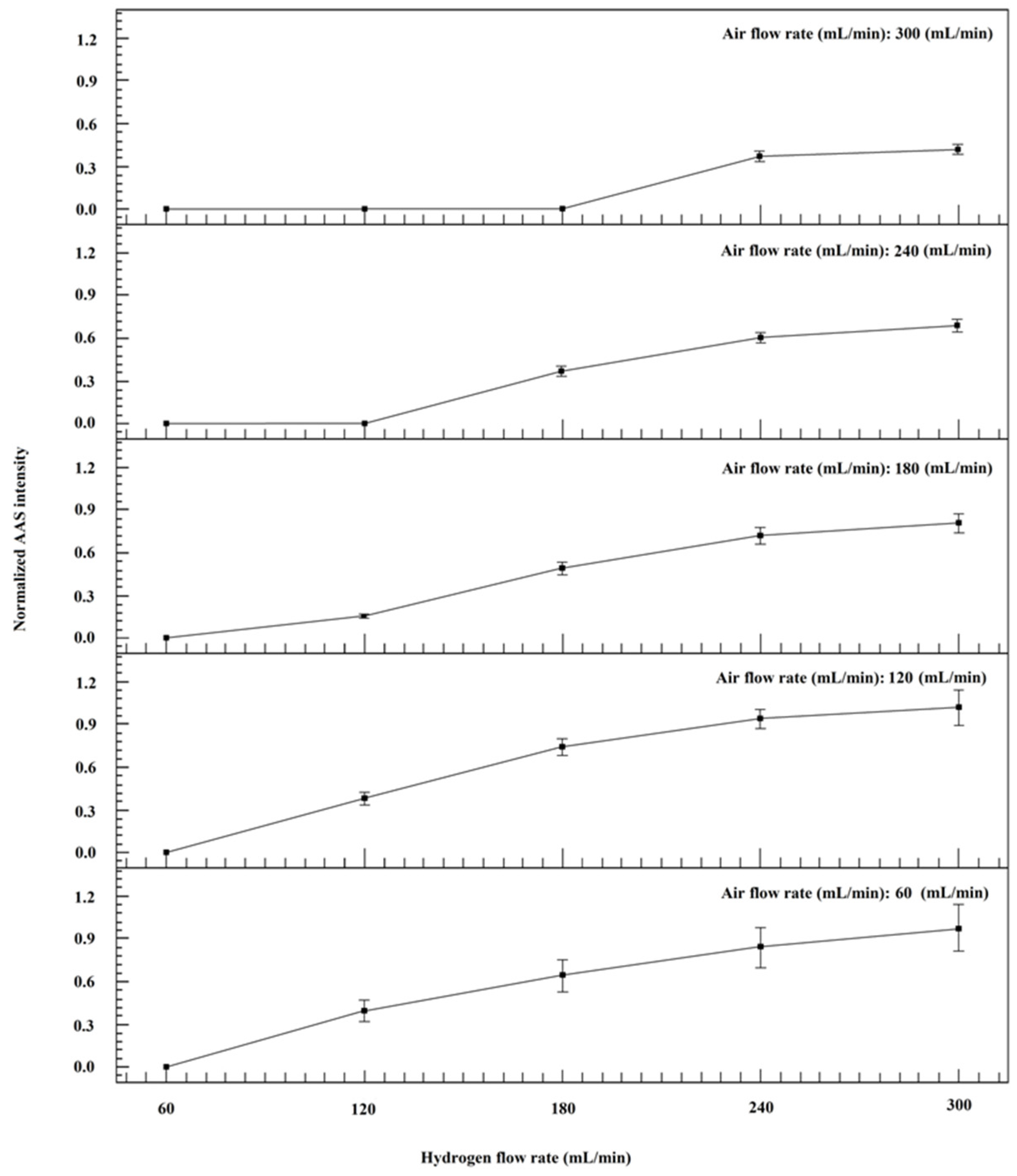
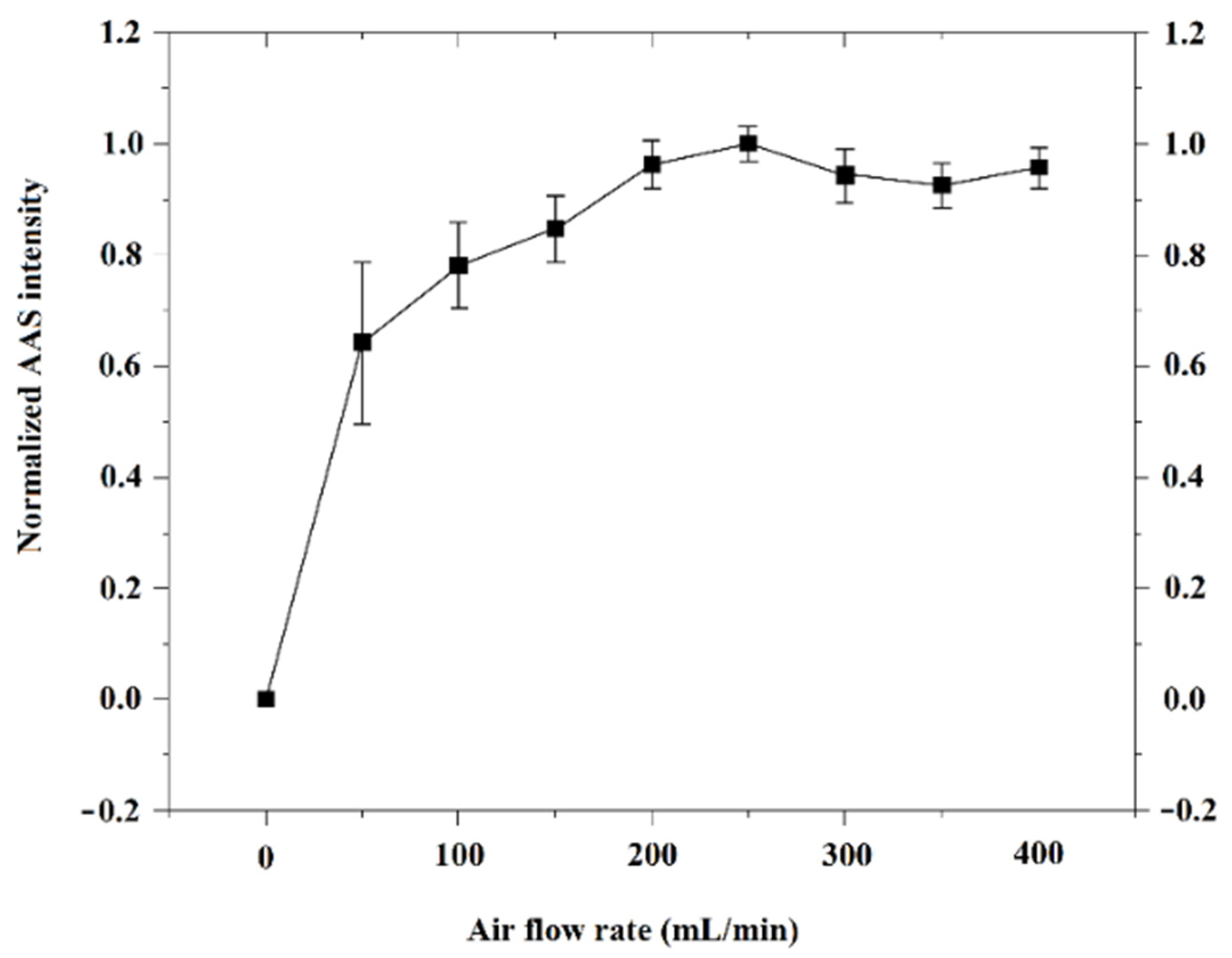
2.3. Gas Atmosphere and Condition
2.4. Interference Study
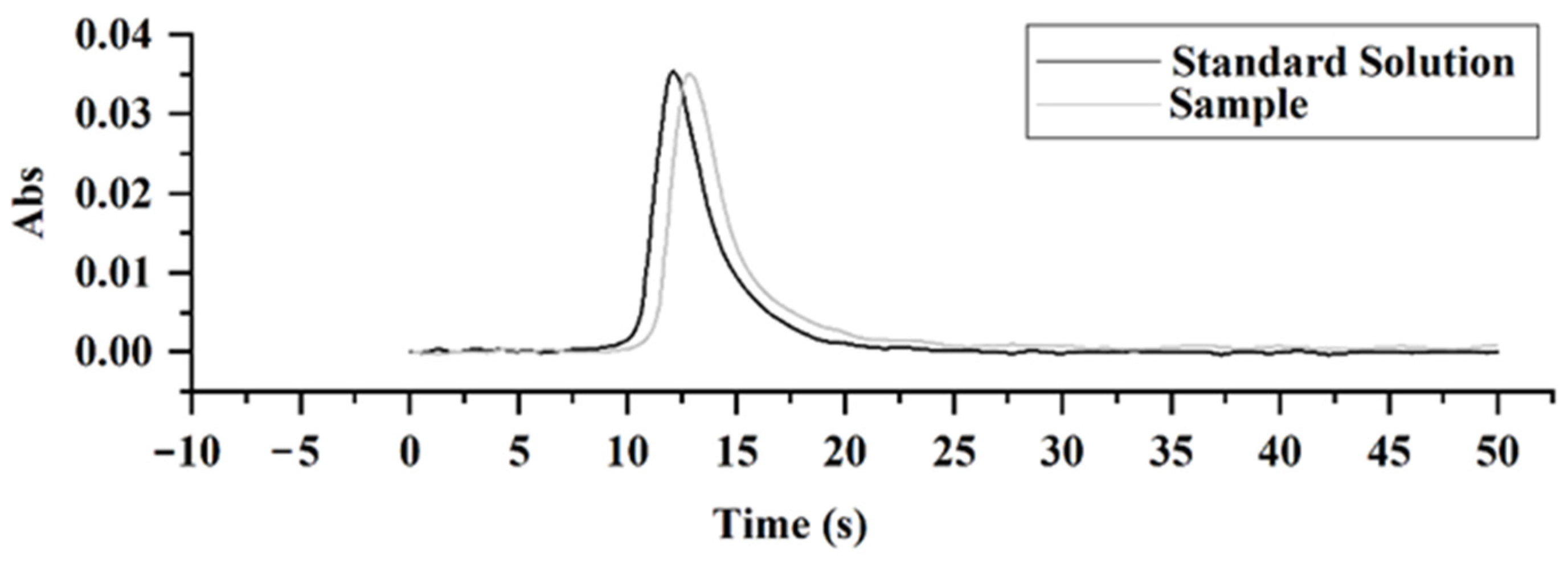
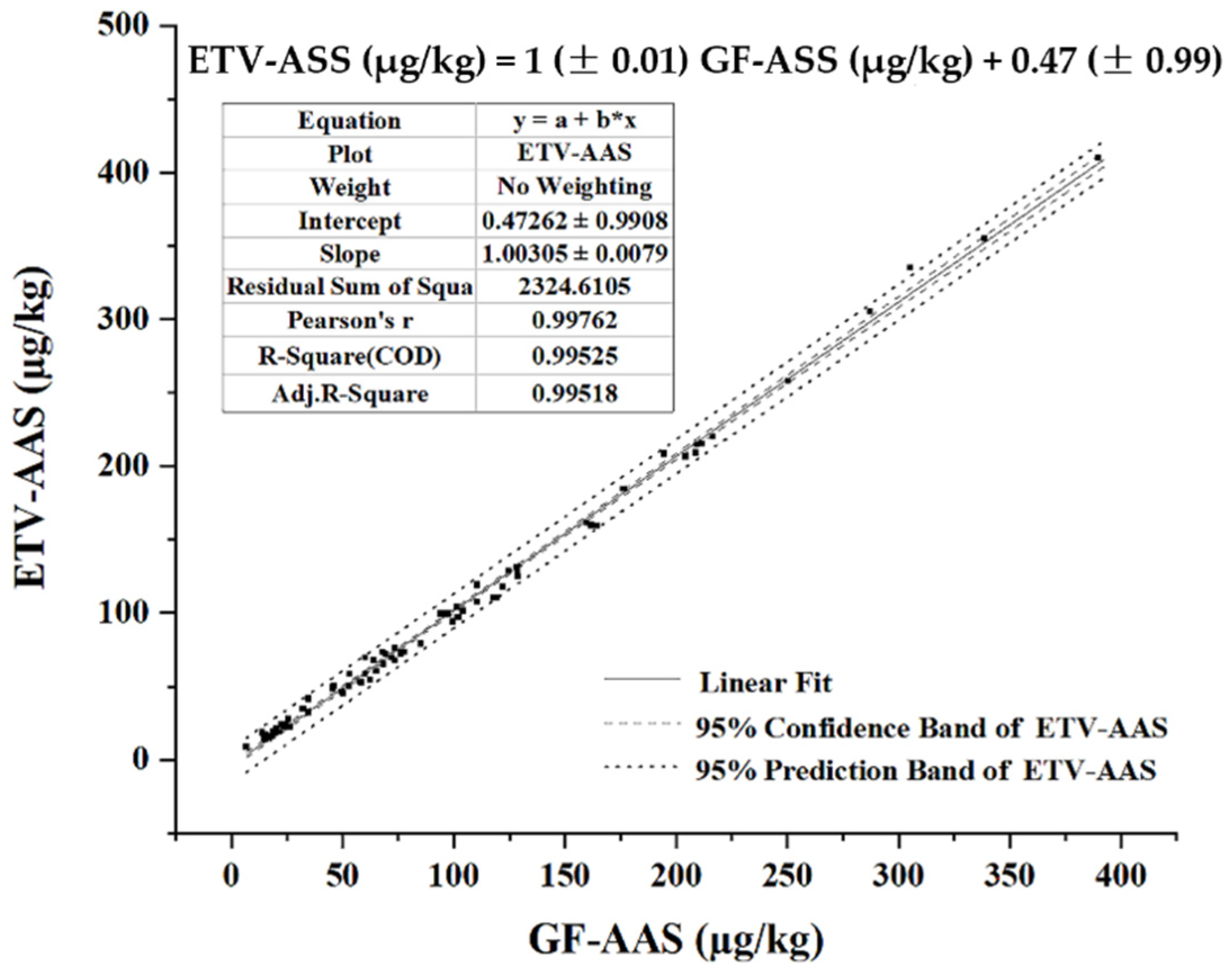
2.5. Analytical Performance
2.6. Cadmium Contamination of Chocolate in China Market and Its Dietary Exposure Risk Assessment
3. Materials and Methods
3.1. Instrumentation
3.2. Chemicals and Materials
3.3. Analytical Procedures of the SS-ETV-AAS Method
3.4. Risk Assessment of Cd through Chocolate Consumption
3.5. Target Hazard Quotient
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Statista Research Department. Chocolate Confectionery Market Size Worldwide 2016–2026. Statista. 29 June 2022. Available online: https://www.statista.com/forecasts/983554/global-chocolate-confectionery-market-size#statisticContainer (accessed on 19 July 2022).
- Liu, S.J.; Zhou, W.Q.; Wang, J.Q.; Chen, B.; He, G.S.; Jia, Y.N. Association between Mobile Phone Addiction Index and Sugar-Sweetened Food Intake in Medical College Students Stratified by Sex from Shanghai, China. Nutrients 2021, 13, 2256. [Google Scholar] [CrossRef] [PubMed]
- Ewusi, A.; Sunkari, E.D.; Seidu, J.; Coffie-Anum, E. Hydrogeochemical characteristics, sources and human health risk assessment of heavy metal dispersion in the mine pit water-surface water-groundwater system in the largest manganese mine in Ghana. Environ. Technol. Innov. 2022, 26, 102312. [Google Scholar] [CrossRef]
- Chamba-Eras, I.; Griffith, D.M.; Kalinhoff, C.; Ramirez, J.; Gazquez, M.J. Native Hyperaccumulator Plants with Differential Phytoremediation Potential in an Artisanal Gold Mine of the Ecuadorian Amazon. Plants 2022, 11, 1186. [Google Scholar] [CrossRef] [PubMed]
- Soumahoro, N.S.; Kouassi, N.L.B.; Yao, K.M.; Kwa-Koffi, E.K.; Kouassi, A.M.; Trokourey, A. Impact of municipal solid waste dumpsites on trace metal contamination levels in the surrounding area: A case study in West Africa, Abidjan, Cote d’Ivoire. Environ. Sci. Pollut. Res. 2021, 28, 30425–30435. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.X.; Liu, G.; Zhang, K.L.; Tan, Y.; Zou, H.; Yuan, Y.; Gu, J.H.; Song, R.L.; Zhu, J.Q.; Liu, Z.P. Cadmium exposure induces rat proximal tubular cells injury via p62-dependent Nrf2 nucleus translocation mediated activation of AMPK/AKT/mTOR pathway. Ecotoxicol. Environ. Saf. 2021, 214, 112058. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Lee, S.J. Association of Blood Heavy Metal Levels and Renal Function in Korean Adults. Int. J. Environ. Res. Public Health 2022, 19, 6646. [Google Scholar] [CrossRef] [PubMed]
- Pouillot, R.; Santillana, F.S.; Van, D.J.M. Modeling the risk of low bone mass and osteoporosis as a function of urinary cadmium in U.S adults aged 50–79 years. Environ. Res. 2022, 212, 113315. [Google Scholar] [CrossRef] [PubMed]
- Tarhonska, K.; Lesicka, M.; Janasik, B.; Roszak, J.; Reszka, E.; Braun, M.; Kolacinska-Wow, A.; Jablonska, E. Cadmium and breast cancer-Current state and research gaps in the underlying mechanisms. Toxicol. Lett. 2022, 361, 29–42. [Google Scholar] [PubMed]
- Hu, W.R.; Xia, M.Z.; Zhang, C.; Song, B.D.; Xia, Z.M.; Guo, C.Y.; Cui, Y.Y.; Jiang, W.Y.; Zhang, S.C.; Xu, D.X.; et al. Chronic cadmium exposure induces epithelial mesenchymal transition in prostate cancer cells through a TGF-beta-independent, endoplasmic reticulum stress induced pathway. Toxicol. Lett. 2022, 353, 107–117. [Google Scholar] [CrossRef]
- Dai, S.D.; Wang, S.; Qin, Y.N.; Zhu, J.C. Multiomics Landscape Uncovers the Molecular Mechanism of the Malignant Evolution of Lung Adenocarcinoma Cells to Chronic Low Dose Cadmium Exposure. Front. Oncol. 2021, 11, 654687. [Google Scholar] [PubMed]
- Mortoglou, M.; Djordjevic, A.B.; Djordjevic, V.; Collins, H.; York, L.; Mani, K.; Valle, E.; Wallace, D.; Uysal-Onganer, P. Role of microRNAs in response to cadmium chloride in pancreatic ductal adenocarcinoma. Arch. Toxicol. 2022, 96, 467–485. [Google Scholar] [CrossRef] [PubMed]
- The Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants; WHO Technical Report Series No. 960; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- The Joint FAO/WHO Committee on Food Additives. The Joint FAO/WHO Committee on Food Additives (JECFA) Work on Cadmium in Chocolates and Cocoa-Derived Products. CCCF14 Webinars; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Peixoto, R.R.A.; Oliverira, A.; Cadore, S. Risk assessment of cadmium and chromium from chocolate powder. Food Addit. Contam. Part B Surveill 2018, 11, 256–263. [Google Scholar] [CrossRef]
- Xiao, N.C.; Wang, F.P.; Tang, L.B.; Zhu, L.L.; Song, B.; Chen, T.B. Recommended risk screening values for Cd in high geological background area of Guangxi, China. Environ. Monit. Assess. 2022, 194, 202. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Du, P.; Luo, H.L.; Wu, H.; Zhang, Y.H.; Chen, J.; Wu, M.H.; Xu, G.; Gao, H.F. Soil contamination with cadmium and potential risk around various mines in China during 2000–2020. J. Environ. Manag. 2022, 310, 114509. [Google Scholar] [CrossRef]
- Huang, L.J.; Huang, W.; Shen, R.J.; Shuai, Q. Chitosan/thiol functionalized metal-organic framework composite for the simultaneous determination of lead and cadmium ions in food samples. Food Chem. 2020, 330, 127212. [Google Scholar] [CrossRef] [PubMed]
- Londnio, A.; Morzan, E.; Smichowski, P. Simultaneous on-line preconcentration and determination of toxic elements in rice and rice-based products by SPE-ICP-MS: Multiple response optimization. J. Food Compost. Anal. 2022, 107, 104388. [Google Scholar] [CrossRef]
- de Santana, F.A.; Portugal, L.A.; Serra, A.M.; Ferrer, L.; Cerda, V.; Ferreira, S.L.C. Development of a MSFIA system for sequential determination of antimony, arsenic and selenium using hydride generation atomic fluorescence spectrometry. Talanta 2016, 156, 29–33. [Google Scholar] [CrossRef]
- Fiorito, F.; Amoroso, M.G.; Lambiase, S.; Serpe, F.P.; Bruno, T.; Scaramuzzo, A.; Maglio, P.; Fusco, G.; Esposito, M. A relationship between environmental pollutants and enteric viruses in mussels(Mytilus galloprovincialis). Environ. Res. 2019, 169, 156–162. [Google Scholar] [CrossRef]
- Karas, K.; Ziola-Frankowska, A.; Bartoszewicz, M.; Krzysko, G.; Frankowski, M. Investigation of chocolate types on the content of selected metals and non-metals determined by ICP-OES analytical technique. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2021, 38, 293–303. [Google Scholar] [CrossRef]
- Wang, B.; Feng, L.; Mao, X.F.; Liu, J.X.; Yu, C.C.; Ding, L.; Li, S.Q.; Zheng, C.M.; Qian, Y.Z. Direct determination of trace mercury and cadmium in food by sequential electrothermal vaporization atomic fluorescence spectrometry using tungsten and gold coil traps. J. Anal. At. Spectrom. 2018, 33, 1209–1216. [Google Scholar] [CrossRef]
- Li, S.Q.; Hao, C.C.; Xing, P.Z.; Xia, X.L.; Liu, T.P.; Mao, X.F. Rapid Screening Analysis of Methylmercury in Fish Samples Using Stannous Chloride Reduction and Direct Sampling Electrothermal Vaporization Atomic Absorption Spectrometry. At. Spectrosc. 2020, 41, 211–217. [Google Scholar] [CrossRef]
- Medvedev, N.S.; Volzhenin, A.V.; Saprykin, A.I. Multi-elemental Analysis of High-purity Molybdenum by Electrothermal Vaporization-Inductively Coupled Plasma Mass Spectrometry. At. Spectrosc. 2021, 42, 71–78. [Google Scholar] [CrossRef]
- Gomez-Nieto, B.; Motyzhov, V.; Gismera, M.J.; Procopio, J.R.; Sevilla, M.T. Fast-sequential determination of cadmium and copper in milk powder and infant formula by direct solid sampling high-resolution continuum source graphite furnace atomic absorption spectrometry. Microchem. J. 2021, 159, 105335. [Google Scholar] [CrossRef]
- Bustos, D.E.; Toro, J.A.; Briceno, M.; Rivas, R.E. Use of slow atomization ramp in high resolution continuum source graphite furnace atomic absorption spectrometry for the simultaneous determination of Cd and Ni in slurry powdered chocolate samples. Talanta 2022, 247, 123547. [Google Scholar] [CrossRef] [PubMed]
- Xing, P.Z.; Li, X.; Zhang, J.Y.; Feng, L.; Liu, G.Q.; Mao, X.F. Direct Determination on Cadmium in Tea Sample by Electrothermal VaporizationCatalytic Pyrolysis-Atomic Absorption Spect. Food Nutr. China 2021, 27, 24–29. [Google Scholar]
- Xing, P.Z.; Li, X.; Feng, L.; Mao, X.F. Novel solid sampling electrothermal vaporization atomic absorption spectrometry for fast detection of cadmium in grain samples. J. Anal. At. Spectrom. 2021, 36, 285–293. [Google Scholar] [CrossRef]
- Thongsaw, A.; Sananmuang, R.; Udnan, Y.; Ross, G.M.; Chaiyasith, W.C. Speciation of mercury in water and freshwater fish samples using two-step hollow fiber liquid phase microextraction with electrothermal atomic absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2019, 152, 102–108. [Google Scholar] [CrossRef]
- Maddela, N.R.; Kakarla, D.; Megharaj, M. Cocoa-laden cadmium threatens human health and cacao economy: A critical view. Sci. Total Environ. 2020, 720, 137645. [Google Scholar] [CrossRef]
- Caprioli, G.; Fiorini, D.; Maggi, F.; Nicoletti, M.; Ricciutelli, M.; Toniolo, C.; Prosper, B.; Vittori, S.; Sagratini, G. Nutritional composition, bioactive compounds and volatile profile of cocoa beans from different regions of Cameroon. Int. J. Food Sci. Nutr. 2016, 67, 422–430. [Google Scholar] [CrossRef]
- Santana, D.M.U.; Roldan, L.M.S.; Florencio, B.P. Factors which favor the exports of cocoa grains from Ecuador. Interciencia 2021, 46, 272–279. [Google Scholar]
- U.S. Environmental Protection Agency. Integrated Risk Information System (IRIS) on Cadmium; National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 1999.

| Countries | Producers | Minimum Value (µg/kg) | Maximum Value (µg/kg) | Average Value (µg/kg) | Standard Deviation (µg/kg) | Median Value (µg/kg) | Total Average (µg/kg) |
|---|---|---|---|---|---|---|---|
| CHN | 7 | 52.8 ± 1.5 | 209.3 ± 3.1 | 135.4 | 62.1 | 128.6 | 100.4 |
| USA | 9 | 67.85 ± 0.9 | 253.2 ± 2.4 | 149.4 | 69.8 | 110.4 | |
| ITA | 16 | 15.1 ± 0.7 | 102.4 ± 1.3 | 44.2 | 28.4 | 40.1 | |
| GER | 13 | 6.7 ± 0.5 | 121.8 ± 2.2 | 48.6 | 36.9 | 32.3 | |
| JPA | 10 | 45.4 ± 1.4 | 146.2 ± 2.4 | 95.1 | 37.1 | 89.6 | |
| CHE | 5 | 34.6 ± 1.8 | 110.5 ± 0.8 | 69.9 | 31.7 | 60.2 | |
| FRA | 5 | 18.7 ± 0.9 | 99.5 ± 2.8 | 58.9 | 32.4 | 65.2 | |
| RUS | 9 | 94.5 ± 2.7 | 392.4 ± 4.2 | 225.1 | 109.7 | 159.8 | |
| MAS | 4 | 45.4 ± 0.6 | 173.4 ± 2.5 | 132.7 | 75.4 | 136.8 |
| Countries | Average Cd (µg/kg) | THQ10(g/d) | THQ50(g/d) | THQ100(g/d) |
|---|---|---|---|---|
| CHN | 135.4 | 0.027 | 0.136 | 0.272 |
| USA | 149.3 | 0.030 | 0.150 | 0.300 |
| ITA | 45.4 | 0.009 | 0.046 | 0.091 |
| GER | 50.5 | 0.010 | 0.051 | 0.101 |
| JPA | 95.1 | 0.019 | 0.095 | 0.191 |
| CHE | 69.8 | 0.014 | 0.070 | 0.140 |
| FRA | 58.9 | 0.012 | 0.059 | 0.118 |
| RUS | 225.1 | 0.038 | 0.188 | 0.375 |
| MAS | 132.7 | 0.022 | 0.111 | 0.221 |
| Program | Time (s) | Temperature (°C) | Carrier Gas | Flow Rate (mL/min) |
|---|---|---|---|---|
| Dehydration | 25 | 435–464 | Air | 350 |
| Pre-vaporization | 60 | 465–765 | ||
| Complete vaporization and detection | 50 | 765–767 | N2/H2 | Air: 120 H2: 240 |
| Clean | 20 | 767 | ||
| 15 | 767–435 | Air | 500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Li, X.; Lan, G.; Wang, Z.; Feng, L.; Mao, X. Fast Detection of Cadmium in Chocolate by Solid Sampling Electrothermal Vaporization Atomic Absorption Spectrometry and Its Application on Dietary Exposure Risk Assessment. Molecules 2022, 27, 6197. https://doi.org/10.3390/molecules27196197
Jia H, Li X, Lan G, Wang Z, Feng L, Mao X. Fast Detection of Cadmium in Chocolate by Solid Sampling Electrothermal Vaporization Atomic Absorption Spectrometry and Its Application on Dietary Exposure Risk Assessment. Molecules. 2022; 27(19):6197. https://doi.org/10.3390/molecules27196197
Chicago/Turabian StyleJia, Hongyu, Xue Li, Guanyu Lan, Zhaohui Wang, Li Feng, and Xuefei Mao. 2022. "Fast Detection of Cadmium in Chocolate by Solid Sampling Electrothermal Vaporization Atomic Absorption Spectrometry and Its Application on Dietary Exposure Risk Assessment" Molecules 27, no. 19: 6197. https://doi.org/10.3390/molecules27196197
APA StyleJia, H., Li, X., Lan, G., Wang, Z., Feng, L., & Mao, X. (2022). Fast Detection of Cadmium in Chocolate by Solid Sampling Electrothermal Vaporization Atomic Absorption Spectrometry and Its Application on Dietary Exposure Risk Assessment. Molecules, 27(19), 6197. https://doi.org/10.3390/molecules27196197