Evaluation of the Anti-Cancer Potential of Rosa damascena Mill. Callus Extracts against the Human Colorectal Adenocarcinoma Cell Line
Abstract
:1. Introduction
2. Results
2.1. R. damascena Callus Fresh, Dry and Crude Weight (g)
2.2. GC–MS Analysis of R. damascena Callus
2.2.1. Control Treatment
2.2.2. Citric Acid Treatment
2.2.3. L-Ascorbic Acid Treatment
2.3. In Vitro Anti-Cancer Study (Cell Line Studies)
2.3.1. Cellular Cytotoxicity Assay
2.3.2. Clonogenic Assay
2.3.3. Ki-67 Flow Cytometry Proliferation Assay
2.3.4. Migration Assay (Scratch Assay)
3. Discussion
4. Materials and Methods
4.1. Callus Initiation of R. damascena
4.1.1. Explant Sterilization
4.1.2. R. damascena Callus Generation in MS Medium Culture
4.2. R. damascena Elicitation Secondary Metabolites
4.2.1. Experiment Design and Setting
4.2.2. Callus Crude Extraction
4.2.3. The GC–MS Analysis
4.3. In Vitro Anti-Cancer Study (Cell Line Studies)
4.3.1. Cellular Cytotoxicity Assay
4.3.2. Colony-Forming Assay
4.3.3. Ki-67 Flow Cytometry Proliferation Assay
4.3.4. Migration Assay (Scratch Assay)
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Labianca, R.; Beretta, G.D.; Kildani, B.; Milesi, L.; Merlin, F.; Mosconi, S.; Pessi, M.A.; Prochilo, T.; Quadri, A.; Gatta, G.; et al. Colon cancer. Crit. Rev. Oncol. Hematol. 2010, 74, 106–133. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef] [PubMed]
- Yuhara, H.; Steinmaus, C.; Cohen, S.E.; Corley, D.A.; Tei, Y.; Buffler, P.A. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am. J. Gastroenterol. 2011, 106, 1911. [Google Scholar] [CrossRef]
- Banerjee, A.; Pathak, S.; Subramanium, V.D.; Dharanivasan, G.; Murugesan, R.; Verma, R.S. Strategies for targeted drug delivery in treatment of colon cancer: Current trends and future perspectives. Drug Discov. Today 2017, 22, 1224–1232. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 4, 523–530. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010. [Google Scholar]
- Olivoto, T.; Nardino, M.; Carvalho, I.R.; Follmann, D.N.; Szareski, V.J.; Ferrari, M.; de Pelegrin, A.J.; de Souza, V.Q. Plant secondary metabolites and its dynamical systems of induction in response to environmental factors: A review. Afr. J. Agr. Res. 2017, 12, 71–84. [Google Scholar]
- Kittakoop, P.; Mahidol, C.; Ruchirawat, S. Alkaloids as important scaffolds in therapeutic drugs for the treatments of cancer, tuberculosis, and smoking cessation. Curr. Top. Med. Chem. 2014, 14, 239–252. [Google Scholar] [CrossRef]
- Takemura, M.; Tanaka, R.; Misawa, N. Pathway engineering for the production of β-amyrin and cycloartenol in Escherichia coli, a method to biosynthesize plant-derived triterpene skeletons in E. coli. Appl. Microbiol. Biotechnol. 2017, 101, 6615–6625. [Google Scholar] [CrossRef]
- Lamarti, A.; Badoc, A.; Deffieux, G.; Carde, J.P. Biogenesis of monoterpenes: The isoprenic chain. Bull. Soc. Pharm. Bordx. 1994, 133, 79–99. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Soriano, I.R.; Riley, I.T.; Potter, M.J.; Bowers, W.S. Phytoecdysteroids: A novel defense against plant-parasitic nematodes. J. Chem. Ecol. 2004, 30, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- Veitch, G.E.; Boyer, A.; Ley, S.V. The azadirachtin story. Angew. Chem. Int. Ed. Engl. 2008, 47, 9402–9429. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A.; Weber, M.G. On the study of plant defence and herbivory using comparative approaches: How important are secondary plant compounds. Ecol. Lett. 2015, 18, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J. Plant Growth Regul. 2012, 32, 216–232. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Gómez-Romero, M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Advances in the analysis of phenolic compounds in products derived from bees. J. Pharm. Biomed. Anal. 2006, 41, 1220–1234. [Google Scholar] [CrossRef]
- Ali, M.B.; Hahn, E.J.; Paek, K.Y. Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules 2007, 12, 607–621. [Google Scholar] [CrossRef]
- Homoki, J.R.; Nemes, A.; Fazekas, E.; Gyémánt, G.; Balogh, P.; Gál, F.; Al-Asri, J.; Mortier, J.; Wolber, G.; Babinszky, L.; et al. Anthocyanin composition, antioxidant efficiency, and α-amylase inhibitor activity of different hungarian sour cherry varieties (Prunus cerasus L.). Food Chem. 2016, 194, 222–229. [Google Scholar] [CrossRef]
- Salawu, S.O.; Alao, O.F.; Faloye, O.F.; Akindahunsi, A.A.; Boligon, A.A.; Athayde, M.L. Antioxidant potential of phenolic-rich two varieties of Nigerian local rice and their anti-cholinesterase activities after in vitro digestion. Nutr. Food Sci. 2016, 46, 171–189. [Google Scholar] [CrossRef]
- Ben Mrid, R.; Bouargalne, Y.; El Omari, R.; Nhiri, M. New insights into the therapeutic effects of phenolic acids from sorghum seeds. J. Rep. Pharma. Sci. 2019, 8, 91–101. [Google Scholar]
- Darwish, H.; Abdelmigid, H.; Albogami, S.; Alotaibi, S.; Noureldeen, A.; Alnefaie, A. Induction of biosynthetic genes related to rosmarinic acid in plant callus culture and antiproliferative activity against breast cancer cell line. Pak. J. Biol. Sci. 2020, 23, 1025–1036. [Google Scholar]
- Veeresham, C.; Chitti, P. Therapeutic Agents from Tissue Cultures of Medicinal Plants. College of Pharmaceutical Sciences, Kakatiya University, Warangal, Andhra Pradesh, India Natural Products Chemistry and Research. Nat. Prod. Chem. Res. 2013, 1, 4. [Google Scholar]
- Basim, E.; Basim, H. Antibacterial activity of Rosa damascene essential oil. Fitoterapia 2003, 74, 394–396. [Google Scholar] [CrossRef]
- Ozkan, G.; Sagdic, O.; Baydar, N.G.; Baydar, H. Antioxidant and antibacterial activities of Rosa damascena flower extracts. Food Sci. Technol. Int. 2004, 10, 277–281. [Google Scholar] [CrossRef]
- Achuthan, C.R.; Babu, B.H.; Padikkala, J. Antioxidant and hepatoprotective effects of Rosa damascene. J. Padikkala Pharm. Biol. 2003, 41, 357–361. [Google Scholar] [CrossRef]
- Mahmood, N.; Piacente, S.; Pizza, C.; Burke, A.; Khan, A.L.; Hay, A.J. The anti-HIV activity and mechanisms of action of pure com-pounds isolated from Rosa damascena. Biochem. Biophys. Res. Commun. 1996, 229, 73–79. [Google Scholar] [CrossRef]
- Jabbarzadeh, Z.; Khosh-Khui, M. Factors affecting tissue culture of Damask rose (Rosa damascena Mill.). Sci. Hortic. 2005, 105, 475–482. [Google Scholar] [CrossRef]
- Rady, M.M.; Sadak, M.S.; El-Lethy, S.R.; Abdelhamid, E.M.; Abdelhamid, M.T. Exogenous α-tocopherolhasa beneficial effect on Glycine max (L.) plants irrigated with diluted sea water. J. Hortic. Sci. Biotechnol. 2015, 90, 195–202. [Google Scholar]
- Orabi, S.A.; Abdelhamid, M.T. Protective role of α-tocopherol on two Vicia faba cultivars against seawater-induced lipid peroxidation by enhancing capacity of anti-oxidative system. J. Saudi Soc. Agric. Sci. 2016, 15, 145–154. [Google Scholar] [CrossRef]
- Beltaji, M.S. Exogenous ascorbic acid (vitamin C) induced anabolic changes for salt tolerance in chickpea (Cicer arietinum L.) plants. Afr. J. Plant Sci. 2008, 2, 118–123. [Google Scholar]
- Zechmann, B. Subcellular distribution of ascorbate in plants. Plant Signal. Behav. 2011, 6, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Gallie, D.R. L-Ascorbic acid, a multifunctional molecule supporting plant growth and development. Scientifica 2013, 795964. [Google Scholar] [CrossRef]
- Smirnoff, N.; Wheeler, G.L. Ascorbic acid in plants: Biosynthesis and function. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 291–314. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, T.; Kakalova, M.; Stefanof, L.; Petkova, M.; Stoyanova, A.; Damyanova, S.; Desyk, M. Chemical composition of essential oil from Rosa damascena mill., growing in new region of Bulgaria. Ukr. Food J. 2016, 5, 492–498. [Google Scholar] [CrossRef]
- Hussein, M.M.; Alva, A.K. Effects of Zinc and Ascorbic Acid Application on the Growth and Photosynthetic Pigments of Millet Plants Grown under Different Salinity. Agric. Sci. 2014, 5, 1253–1260. [Google Scholar] [CrossRef]
- De Pinto, M.C.; Francis, D.; De Gara, D. The redox state of the ascorbate-dehydroascrobate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma 1999, 209, 90–97. [Google Scholar] [CrossRef]
- Joy, R.W.; Patel, K.R.; Thorpe, T.A. Ascorbic acid enhancement of organogenesis in tobacco. Plant Cell Tissue Organ Cult. 1988, 13, 219–228. [Google Scholar] [CrossRef]
- El-Sayed, O.M.; El-Gammal, O.; Salama, A. Effect of ascorbic acid, proline and jasmonic acid foliar spraying on fruit set and yield of Manzanillo olive trees under salt stress. Sci. Hortic. 2014, 176, 32–37. [Google Scholar] [CrossRef]
- Chen, Z.; Gallie, D.R. The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 2004, 16, 1143–1162. [Google Scholar] [CrossRef]
- El-Hifny, I.M.; El-Sayed, M. Response of sweet pepper plant growth and productivity to application of ascorbic acid and biofertilizers under saline conditions. Aust. J. Basic. Appl. Sci. 2011, 5, 1273–1283. [Google Scholar]
- Midan, S.A.; Sorial, M.E. Some antioxidants application in relation to lettuce growth, chemical constituents and yield. Aust. J. Basic Appl. Sci. 2011, 5, 127. [Google Scholar]
- Shalaby, T.A.; El-Ramady, H. Effect of foliar application of bio-stimulants on growth, yield, components, and storability of garlic (Allium sativum L.). Aust. J. Crop Sci. 2014, 8, 271–275. [Google Scholar]
- Fatima, A.; Singh, A.A.; Mukherjee, A.; Agrawal, M.; Agrawal, S.B. Ascorbic acid and thiols as potential biomarkers of ozone tolerance in tropical wheat cultivars. Ecotoxicol. Environ. Saf. 2019, 171, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Desoky, E.M.; Mansour, E.; Yasin, M.A.; El Sobky, E.-S.E.; Rady, M.M. Improvement of drought tolerance in five different cultivars of Vicia faba with foliar application of ascorbic acid or silicon. Span. J. Agric. Res. 2020, 18, 16. [Google Scholar] [CrossRef]
- Mazher, A.A.; Zaghloul, S.M.; Mahmoud, S.A.; Siam, H.S. Stimulatory effect of kinetin, ascorbic acid and glutamic acid ongrowth and chemical constituents of Codiaeum variegatum L. plants. Am. Eurasian. J. Agric. Environ. Sci. 2011, 10, 318–323. [Google Scholar]
- El-Sanatawy, A.M.; Ash-Shormillesy, S.M.; Qabil, N.; Awad, M.F.; Mansour, E. Seed halo-priming improves seedling vigor, grain yield, and water use efficiency of maize under varying irrigation regimes. Water 2021, 13, 2115. [Google Scholar] [CrossRef]
- Rafique, N.; Raza, S.H.; Qasim, M.; Iqbal, N. Pre-sowing application of ascorbic acid and salicylic acid to seed of pumpkin and seedling response to salt. Pak. J. Bot. 2011, 43, 2677–2682. [Google Scholar]
- Sajid, Z.A.; Aftab, F. Amelioration of salinity tolerance in Solanum tuberosum L. by exogenous application of ascorbic acid. In Vitro Cell. Dev. Biol. Plant. 2009, 45, 540–549. [Google Scholar] [CrossRef]
- Albogami, S.; Darwish, H. A novel therapeutic effect Callus from Rosa damascena that suppresses Human T-cell Activation: Medicine from Cultured Cells. Res. J. Biotechnol. 2017, 12, 20–24. [Google Scholar]
- Etman, S.M.; Abdallah, O.Y.; Mehanna, R.A.; Elnaggar, Y.R. Lactoferrin/Hyaluronic acid double-coated lignosulfonate nanoparticles of quinacrine as a controlled release biodegradable nanomedicine targeting pancreatic cancer. Int. J. Pharm. 2020, 30, 119097. [Google Scholar] [CrossRef] [PubMed]
- El-Habashy, S.E.; Eltaher, H.M.; Gaballah, A.; Eiman, I.Z.; Mehanna, R.A.; El-Kamel, A.H. Hybrid bioactive hydroxyapatite/polycaprolactone nanoparticles for enhanced osteogenesis. Mater. Sci. Eng. C 2021, 119, 111599. [Google Scholar] [CrossRef] [PubMed]
- Elsehly, W.M.; Mourad, G.M.; Mehanna, R.A.; Kholief, M.A.; El-Nikhely, N.A.; Awaad, A.K.; Attia, M.H. The potential implications of estrogenic and antioxidant-dependent activities of high doses of methyl paraben on MCF7 breast cancer cells. J. Biochem. Mol. Toxicol. 2022, 36, e23012. [Google Scholar] [CrossRef] [PubMed]
- Cory, G. Scratch-Wound Assay. In Cell Migration. Methods in Molecular Biology; Wells, C., Parsons, M., Eds.; Humana Press: London, UK, 2011; Volume 769. [Google Scholar]
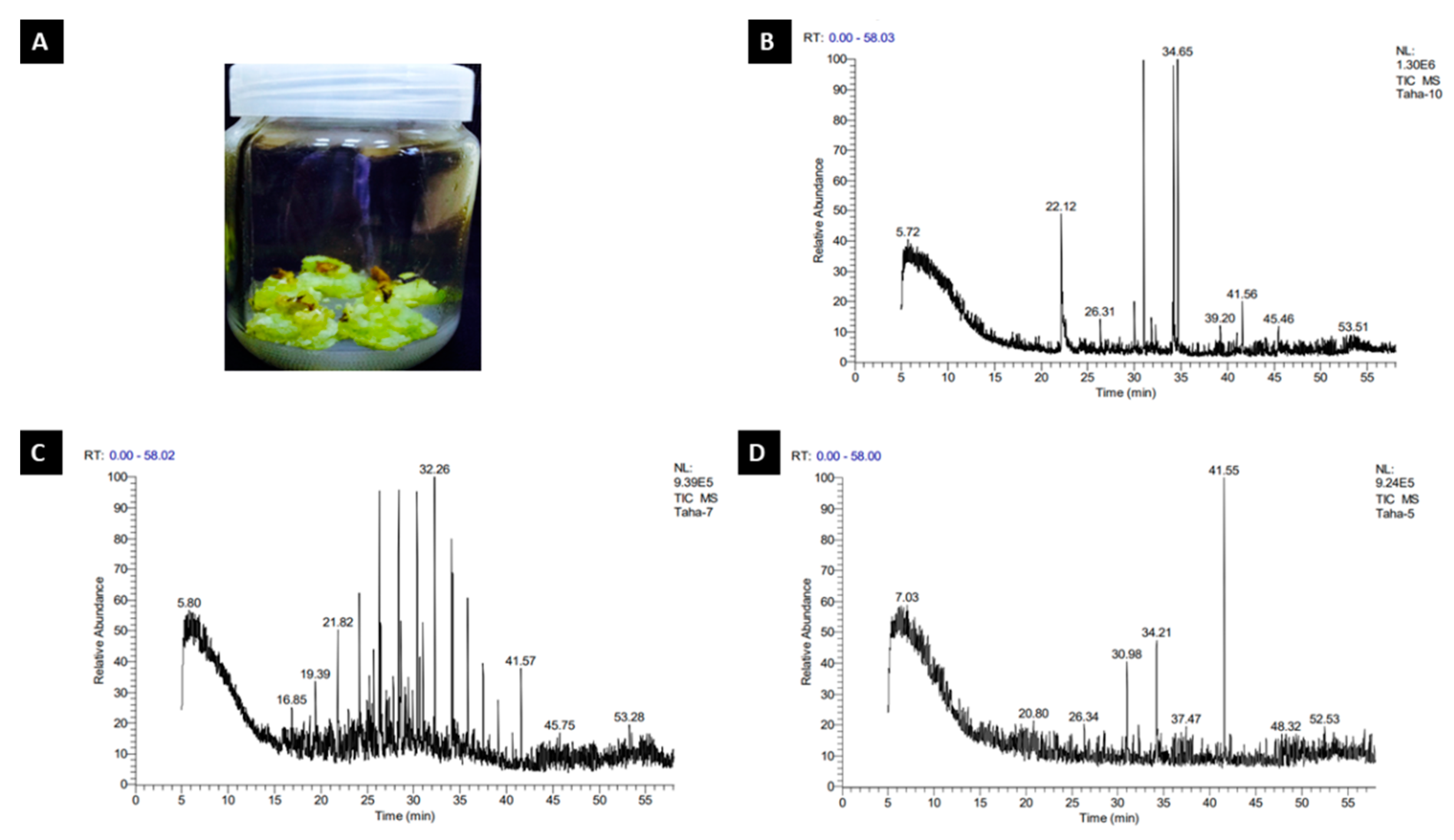



| Treatments | 1 Fresh Weight (g) | Dry Weight (g) | Crude Weight (g) |
|---|---|---|---|
| Control | 2 2.166 ab | 0.214 b | 0.038 a |
| Citric acid 0.5 g/L | 1.267 b | 0.168 b | 0.022 a |
| L-ascorbic acid 0.5 g/L | 2.878 a | 0.306 a | 0.032 a |
| L.S.D at 5% | 1.019 | 0.075 | 2922.8 |
| Peak | R. Time | Area | Area % | Name |
|---|---|---|---|---|
| 1 | 5.72 | 2561 | 1.04 | (2,3-Dihydro-5,10,15,20- tetraphenyl [2-(2)H1]prop hyinato) copper(II) |
| 2 | 22.12 | 2251 | 9.15 | Benzonitrile (CAS) |
| 3 | 26.31 | 3938 | 1.60 | 1-Pentanol, 2,2-dimethyl-(CAS) |
| 4 | 34.65 | 3385 | 13.75 | Octadecanoic acid, methyl ester (CAS) |
| 5 | 39.20 | 3807 | 1.55 | 4-Octanol, propanoate |
| 6 | 41.56 | 7511 | 3.05 | 1,2-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester (CAS) |
| 7 | 45.46 | 4280 | 1.74 | 2,6-Nonadien-1-ol |
| 8 | 53.51 | 2360 | 0.96 | (5,10,15,20-Tetraphenyl [2-(2)H1]prophyrinato)zinx(II) |
| Peak | R. Time | Area | Area % | Name |
|---|---|---|---|---|
| 1 | 5.80 | 3614 | 0.95 | Dichloroacetaldehyde |
| 2 | 16.85 | 4391 | 1.16 | Dodecane, 5,8-diethyl-(CAS) |
| 3 | 19.39 | 7554 | 2.00 | Tetradecane (CAS) |
| 4 | 21.82 | 1355 | 3.58 | Tetratetracontane (CAS) |
| 5 | 32.26 | 2202 | 5.82 | Octadecane, 2-methyl- |
| 6 | 41.57 | 1018 | 2.69 | 1,2-Benzenedicarboxylic acid, diisooctyl ester (CAS) |
| 7 | 45.75 | 2866 | 0.76 | 3,3′,5,5′-Tetrabromo-2-nitro-2′-propylsulfonylbiphenyl |
| 8 | 53.28 | 2764 | 0.73 | 5á-Pregnan-20-one, 3à,11á,17,21-tetrakis(trim ethylsiloxy)-, O-methyloxime |
| Peak | R. Time | Area | Area % | Name |
|---|---|---|---|---|
| 1 | 7.03 | 2904 | 1.79 | 11-[(t-Butyldimethylsilyl)oxy]-6,9a-dimethyl-6-(m ethoxycarbonyl)-(perhydro )napthaleno[a]benzofulvene |
| 2 | 20.80 | 1980 | 1.22 | Mixture of: 5,6-Dihydro-6-methy l-2H-pyran-2-one and 5-methoxy-3-pente ne-2-ol |
| 3 | 26.34 | 3210 | 1.98 | Nonane, 1-chloro-(CAS) |
| 4 | 30.98 | 9453 | 5.82 | Hexadecanoic acid, methyl ester (CAS) |
| 5 | 34.21 | 1391 | 8.57 | 7-Octadecenoic acid, methyl ester (CAS) |
| 6 | 37.47 | 1777 | 1.09 | (2,3-Dihydro-5,10,15,20-tetraphenyl [2-(2)H1]prophyrinato)copper(II) |
| 7 | 41.55 | 2884 | 17.75 | 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester |
| 8 | 48.32 | 1799 | 1.11 | 6-[2′-(4″-Phenyl)ethyl]-1,2,3-triphenyl-9H-tribenzo[a,c,e]cycloheptatriene |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, H.; Alharthi, S.; Mehanna, R.A.; Ibrahim, S.S.; Fawzy, M.A.; Alotaibi, S.S.; Albogami, S.M.; Albogami, B.; Hassan, S.H.A.; Noureldeen, A. Evaluation of the Anti-Cancer Potential of Rosa damascena Mill. Callus Extracts against the Human Colorectal Adenocarcinoma Cell Line. Molecules 2022, 27, 6241. https://doi.org/10.3390/molecules27196241
Darwish H, Alharthi S, Mehanna RA, Ibrahim SS, Fawzy MA, Alotaibi SS, Albogami SM, Albogami B, Hassan SHA, Noureldeen A. Evaluation of the Anti-Cancer Potential of Rosa damascena Mill. Callus Extracts against the Human Colorectal Adenocarcinoma Cell Line. Molecules. 2022; 27(19):6241. https://doi.org/10.3390/molecules27196241
Chicago/Turabian StyleDarwish, Hadeer, Sarah Alharthi, Radwa A. Mehanna, Samar S. Ibrahim, Mustafa A. Fawzy, Saqer S. Alotaibi, Sarah M. Albogami, Bander Albogami, Sedky H. A. Hassan, and Ahmed Noureldeen. 2022. "Evaluation of the Anti-Cancer Potential of Rosa damascena Mill. Callus Extracts against the Human Colorectal Adenocarcinoma Cell Line" Molecules 27, no. 19: 6241. https://doi.org/10.3390/molecules27196241







