Objective Supervised Machine Learning-Based Classification and Inference of Biological Neuronal Networks
Abstract
:1. Introduction
Related Work
2. Methods
2.1. Cortical Networks Characterisation
2.1.1. Cell Characterisation
2.1.2. Train Discretisation and Probability Analysis
2.1.3. Cortical Topologies
2.2. Information Theoretic-Based Network Tomography Based on Spike Delay Estimation
Delay Estimation
2.3. Neuronal Computational Framework
2.3.1. The NEURON Tool
2.3.2. Cell-Data Source
2.3.3. Python Support Library
2.3.4. Overview of Simulation Framework
2.3.5. Simulation Dataset
2.4. Cell Classification
3. Results
3.1. Communication Features
3.1.1. Delay Estimation
3.1.2. Entropy and Mutual Information
3.2. 2-Cell Classification and Model Training
Network Tomography for Cellular Classification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. E-Type and M-Type Confusion Matrix


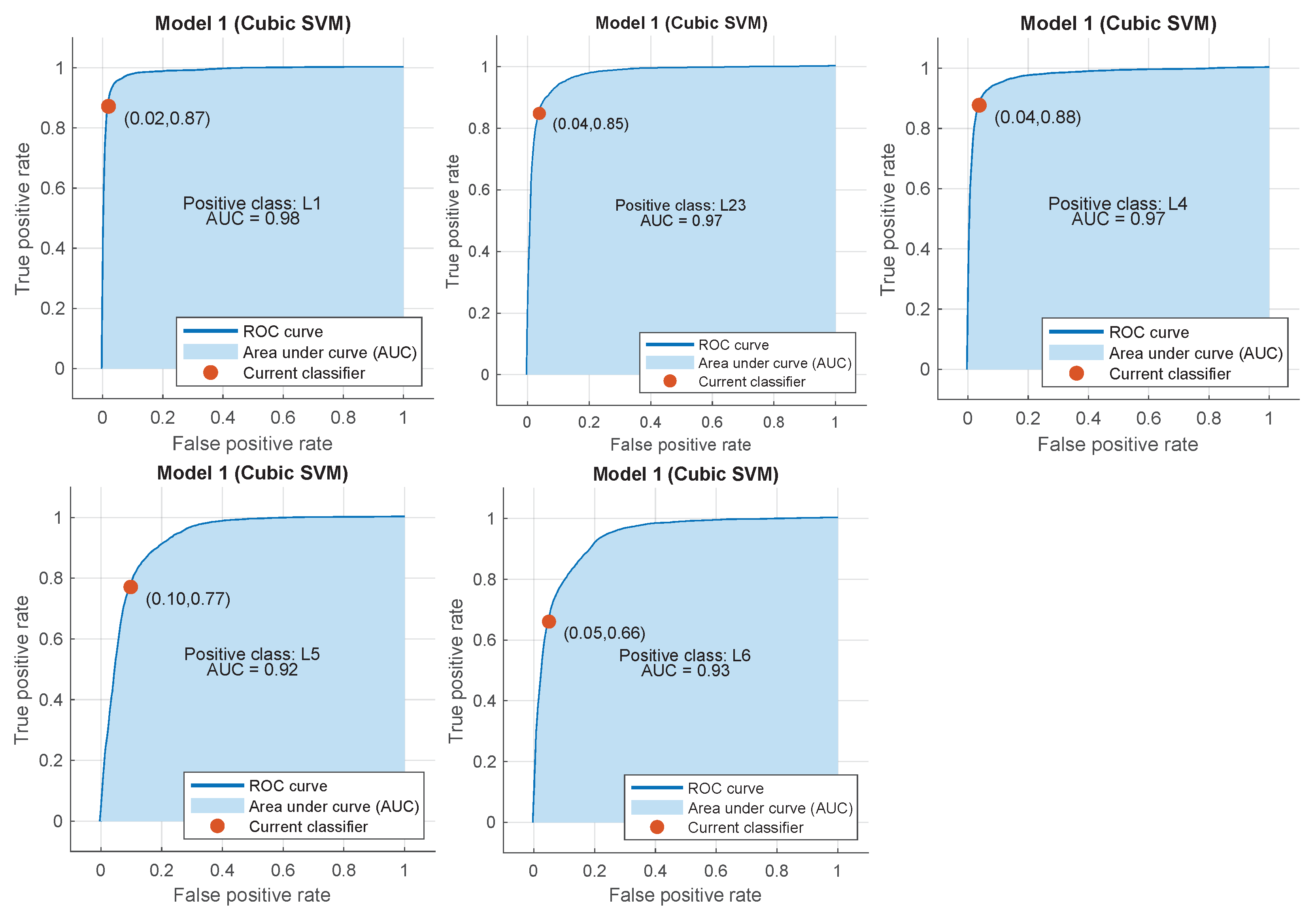
Appendix B. ROC Curves
References
- Markram, H.; Muller, E.; Ramaswamy, S.; Reimann, M.W.; Abdellah, M.; Sanchez, C.A.; Ailamaki, A.; Alonso-Nanclares, L.; Antille, N.; Arsever, S.; et al. Reconstruction and Simulation of Neocortical Microcircuitry. Cell 2015, 163, 456–492. [Google Scholar] [CrossRef] [PubMed]
- Kanari, L.; Ramaswamy, S.; Shi, Y.; Morand, S.; Meystre, J.; Perin, R.; Abdellah, M.; Wang, Y.; Hess, K.; Markram, H. Objective morphological classification of neocortical pyramidal cells. Cereb. Cortex 2019, 29, 1719–1735. [Google Scholar] [CrossRef] [PubMed]
- Vasques, X.; Vanel, L.; Villette, G.; Cif, L. Morphological neuron classification using machine learning. Front. Neuroanat. 2016, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.T.; Siljak, H.; Ekky, A.; Marchetti, N. A Topology Inference Method of Cortical Neuron Networks Based on Network Tomography and the Internet of Bio-Nano Things. IEEE Netw. Lett. 2019, 1, 142–145. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Wirdatmadja, S.A.; Barros, M.T.; Koucheryavy, Y.; Stachowiak, M.; Jornet, J.M. Wireless communications for optogenetics-based brain stimulation: Present technology and future challenges. IEEE Commun. Mag. 2018, 56, 218–224. [Google Scholar] [CrossRef]
- DeFelipe, J.; López-Cruz, P.L.; Benavides-Piccione, R.; Bielza, C.; Larrañaga, P.; Anderson, S.; Burkhalter, A.; Cauli, B.; Fairén, A.; Feldmeyer, D.; et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat. Rev. Neurosci. 2013, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Deitcher, Y.; Eyal, G.; Kanari, L.; Verhoog, M.B.; Atenekeng Kahou, G.A.; Mansvelder, H.D.; De Kock, C.P.; Segev, I. Comprehensive morpho-electrotonic analysis shows 2 distinct classes of L2 and L3 pyramidal neurons in human temporal cortex. Cereb. Cortex 2017, 27, 5398–5414. [Google Scholar] [CrossRef]
- Barros, M.T. Ca2+-signaling-based molecular communication systems: Design and future research directions. Nano Commun. Netw. 2017, 11, 103–113. [Google Scholar] [CrossRef]
- Moioli, R.C.; Nardelli, P.H.; Barros, M.T.; Saad, W.; Hekmatmanesh, A.; Silva, P.E.G.; de Sena, A.S.; Dzaferagic, M.; Siljak, H.; Van Leekwijck, W.; et al. Neurosciences and wireless networks: The potential of brain-type communications and their applications. IEEE Commun. Surv. Tutor. 2021, 23, 1599–1621. [Google Scholar] [CrossRef]
- Gal, E.; London, M.; Globerson, A.; Ramaswamy, S.; Reimann, M.W.; Muller, E.; Markram, H.; Segev, I. Rich cell-type-specific network topology in neocortical microcircuitry. Nat. Neurosci. 2017, 20, 1004. [Google Scholar] [CrossRef]
- Yang, R.; Sala, F.; Bogdan, P. Hidden network generating rules from partially observed complex networks. Commun. Phys. 2021, 4, 199. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, H.; Bogdan, P. Deciphering the generating rules and functionalities of complex networks. Sci. Rep. 2021, 11, 22964. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Xiao, X.; Balaban, V.; Kandel, M.E.; Lee, Y.J.; Popescu, G.; Bogdan, P. Network science characteristics of brain-derived neuronal cultures deciphered from quantitative phase imaging data. Sci. Rep. 2020, 10, 15078. [Google Scholar] [CrossRef] [PubMed]
- Akan, O.B.; Ramezani, H.; Khan, T.; Abbasi, N.A.; Kuscu, M. Fundamentals of molecular information and communication science. Proc. IEEE 2016, 105, 306–318. [Google Scholar] [CrossRef]
- Ramezani, H.; Akan, O.B. Impacts of spike shape variations on synaptic communication. IEEE Trans. Nanobiosci. 2018, 17, 260–271. [Google Scholar] [CrossRef]
- Balevi, E.; Akan, O.B. A physical channel model for nanoscale neuro-spike communications. IEEE Trans. Commun. 2013, 61, 1178–1187. [Google Scholar] [CrossRef]
- Barros, M.T. Capacity of the hierarchical multi-layered cortical microcircuit communication channel. In Proceedings of the 5th ACM International Conference on Nanoscale Computing and Communication, Reykjavik, Iceland, 5–7 September 2018; ACM: New York, NY, USA, 2018; p. 7. [Google Scholar]
- Veletić, M.; Floor, P.A.; Babić, Z.; Balasingham, I. Peer-to-peer communication in neuronal nano-network. IEEE Trans. Commun. 2016, 64, 1153–1166. [Google Scholar] [CrossRef]
- Strong, S.P.; Koberle, R.; van Steveninck, R.R.D.R.; Bialek, W. Entropy and information in neural spike trains. Phys. Rev. Lett. 1998, 80, 197. [Google Scholar] [CrossRef]
- Glaser, J.I.; Benjamin, A.S.; Farhoodi, R.; Kording, K.P. The roles of supervised machine learning in systems neuroscience. Prog. Neurobiol. 2019, 175, 126–137. [Google Scholar] [CrossRef]
- Herfurth, T.; Tchumatchenko, T. Quantifying encoding redundancy induced by rate correlations in Poisson neurons. Phys. Rev. E 2019, 99, 042402. [Google Scholar] [CrossRef]
- Chichilnisky, E. A simple white noise analysis of neuronal light responses. Netw. Comput. Neural Syst. 2001, 12, 199–213. [Google Scholar] [CrossRef]
- Smith, J.O. Techniques for Digital Filter Design and System Identification with Application to the Violin. Ph.D. Thesis, Stanford University, Stanford, CA, USA, 1983. [Google Scholar]
- Ljung, L.; Söderström, T. Theory and Practice of Recursive Identification; MIT Press: Cambridge, MA, USA, 1983. [Google Scholar]
- Zbili, M.; Debanne, D. Past and future of analog-digital modulation of synaptic transmission. Front. Cell. Neurosci. 2019, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.S.; Bullmore, E.T. Small-world brain networks revisited. Neuroscientist 2017, 23, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.; Hero, A.; Nowak, R.; Yu, B. Internet tomography. IEEE Signal Process. Mag. 2002, 19, 47–65. [Google Scholar] [CrossRef]
- Chen, A.; Cao, J.; Bu, T. Network Tomography: Identifiability and Fourier Domain Estimation. IEEE Trans. Signal Process. 2010, 58, 6029–6039. [Google Scholar] [CrossRef]
- Hines, M.L.; Carnevale, N.T. The NEURON simulation environment. Neural Comput. 1997, 9, 1179–1209. [Google Scholar] [CrossRef]
- Project, Blue Brain NMC Portal. 2016. Available online: https://bbp.epfl.ch/nmc-portal/welcome.html (accessed on 21 July 2022).
- Ramaswamy, S.; Courcol, J.D.; Abdellah, M.; Adaszewski, S.R.; Antille, N.; Arsever, S.; Atenekeng, G.; Bilgili, A.; Brukau, Y.; Chalimourda, A.; et al. The neocortical microcircuit collaboration portal: A resource for rat somatosensory cortex. Front. Neural Circuits 2015, 9, 44. [Google Scholar] [CrossRef]
- Mullen, P. Neurpy Library. 2019. Available online: https://zenodo.org/record/4009922#.YxHBUHZBxPY (accessed on 21 July 2022).
- Mullen, P. Research Release Code. 2019. Available online: https://zenodo.org/record/4009917#.YxHBjXZBxPY (accessed on 21 July 2022).
- Ofer, N.; Shefi, O.; Yaari, G. Axonal tree morphology and signal propagation dynamics improve interneuron classification. bioRxiv 2019, 414615. [Google Scholar] [CrossRef]
- Barros, M.; Dey, S. Feed-forward and feedback control in astrocytes for Ca2+-based molecular communications nanonetworks. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018, 17, 1174–1186. [Google Scholar] [CrossRef]
- Barros, M.T.; Silva, W.; Regis, C.D.M. The multi-scale impact of the Alzheimer’s disease on the topology diversity of astrocytes molecular communications nanonetworks. IEEE Access 2018, 6, 78904–78917. [Google Scholar] [CrossRef]











| Classifier | Decision Tree | Random Forest | SVM | NN |
|---|---|---|---|---|
| Accuracy | 67.30% | 53.26% | 80.60% | 81.31% |
| FactorImprovement | 3.365 | 2.663 | 4.03 | 4.0655 |
| Classifier | Decision Tree | Random Forest | SVM | NN |
|---|---|---|---|---|
| Accuracy | 52.10% | 52.93% | 74.20% | 69.62% |
| FactorImprovement | 13.025 | 13.2325 | 18.55 | 17.405 |
| Classifier | Decision Tree | Random Forest | SVM | NN |
|---|---|---|---|---|
| Accuracy | 54.90% | 49.06% | 64.70% | 60.59% |
| FactorImprovement | 7.685846283 | 6.868262635 | 9.057818844 | 8.482430351 |
| Predicted Class | True Pos. | False Neg. | ||||||
|---|---|---|---|---|---|---|---|---|
| L1 | L2/3 | L4 | L5 | L6 | ||||
| True Class | L1 | 87.1% | 9.3% | 0.7% | 2.4% | 0.5% | 87.1% | 12.9% |
| L2/3 | 5.8% | 84.9% | 7.9% | 0.5% | 0.9% | 84.9% | 15.1% | |
| L4 | 0.3% | 5.9% | 87.7% | 4.4% | 1.7% | 87.7% | 12.3% | |
| L5 | 0.9% | 0.2% | 5.2% | 77.7% | 15.9% | 77.7% | 22.3% | |
| L6 | 0.6% | 0.2% | 1.0% | 32.5% | 65.7% | 65.7% | 34.3% | |
| Prediction | Layer | m-Type | e-Type | Whole-Cell |
|---|---|---|---|---|
| # classes | 5 | 25 | 14 | 1750 |
| Equiv. random guess accuracy | 20.0% | 4.0% | 7.143% | 0.0571% |
| Classifieraccuracy | 61.82% | 56.34% | 64.62% | 36.23% |
| FactorImprovement | 3.091 | 14.085 | 9.05 | 634.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, M.T.; Siljak, H.; Mullen, P.; Papadias, C.; Hyttinen, J.; Marchetti, N. Objective Supervised Machine Learning-Based Classification and Inference of Biological Neuronal Networks. Molecules 2022, 27, 6256. https://doi.org/10.3390/molecules27196256
Barros MT, Siljak H, Mullen P, Papadias C, Hyttinen J, Marchetti N. Objective Supervised Machine Learning-Based Classification and Inference of Biological Neuronal Networks. Molecules. 2022; 27(19):6256. https://doi.org/10.3390/molecules27196256
Chicago/Turabian StyleBarros, Michael Taynnan, Harun Siljak, Peter Mullen, Constantinos Papadias, Jari Hyttinen, and Nicola Marchetti. 2022. "Objective Supervised Machine Learning-Based Classification and Inference of Biological Neuronal Networks" Molecules 27, no. 19: 6256. https://doi.org/10.3390/molecules27196256






