1. Introduction
Antibiotic resistance in bacterial strains is taking root throughout the population and poses a serious public health challenge, which greatly demands pursuing new antibiotics or investigating new antimicrobial compounds [
1]. During the last few decades, much work has been documented, including the research on the production of new antibiotics from diverse strains of microorganisms and plants [
2,
3,
4,
5]. These microbial species and their population size depend on environmental factors such as soil texture, nutrient availability, moisture, and flora-covered soil [
6]. Bacteria produce and use antibiotics in their natural habitats as protective substances to destroy the invasion of other bacterial species. The function of these antibiotics is not only protective but they also play a vital role as signalling molecules to communicate among the cells in the bacterial population [
7,
8].
It is presumed from the past that natural products play a vital role in antibiotic discovery and their development [
9]. There is a dire need to explore novel antimicrobial compounds with significant potential to kill or control a wide range of microorganisms. Antibiotics are one of the essential pillars of present-time medications. Still, unfortunately, the commonly used antibiotics lose their efficacy against several pathogenic strains and there is demand to replace old antibiotics with new ones [
10,
11]. Microorganisms, having the ability to produce bioactive secondary metabolites, show distinct structures and biological activities. Some species of soil microflora also produce these bioactive metabolites, which are used as antibiotics [
6]. Several other significant types of research have also been reported to isolate bacteria from the soil with novel antimicrobial agents [
12,
13].
Public health professionals recently had great difficulty dealing with multidrug-resistant bacterial pathogens; therefore, MDR infections are more dangerous than non-resistant bacterial pathogens. Particularly, the frequency of resistance developed in bacterial pathogens acts as a secondary infection in various life-threatening conditions such as cancer, surgical procedures, transplantation, etc., and influences the treatment impact of modern medications [
14,
15]. As the development of MDR strains is quite rapid, it is obvious that very limited therapeutic agents are available to treat these pathogens effectively [
16,
17]. This study focused on isolating potential bacterial species from the soil and preparing a crude extract from isolated bacterial strains to assess antimicrobial activities against most common human pathogens. In addition, GC-MS was performed to determine bioactive compounds from the crude extract. This study will facilitate the development of novel antibiotics against MDR bacterial strains.
2. Materials and Methods
2.1. Sample Collection and Processing
While conducting the present study, n = 10 samples were collected from different regions of Khyber Pakhtunkhwa, including Haripur, Swat, and Charsadda. Approximately 4 gm of each soil sample was collected in a sterile polythene bag using a sterile spatula. The collected samples were immediately transferred to the laboratory in ice baskets for bacteriological investigation. The pH and moisture content of each sample was also determined. Samples were processed and inoculated using suitable culture media, including nutrient agar, GYM medium, and Tryptic Soy broth with 5% yeast extract for obtaining bacterial isolates. Before inoculation, each soil sample was serially diluted in sterile normal saline and homogeneously mixed by vertexing. The inoculum (100 µL from each dilution) was spread on culture media and was allowed to incubate overnight at 37 °C. After incubation, the fresh culture was subjected to morphological identification. Slightly raised, flat, white, and cream-colored colonies were selected for further identification. Gram staining and different biochemical tests were performed for further identification. The biochemical tests included indole, methyl red, Voges–Proskauer, citrate utilization, catalase, oxidase, cellulose test, triple sugar iron, nitrate reduction, gelatin, and casein hydrolysis test.
2.2. Secondary Metabolites Production
The sterilized nutrient broth (150 mL) was inoculated with fresh bacterial culture in the flask and was incubated at 34 °C for seven days at 150 rpm in a shaking incubator. The cultivated bacterial strains secreted the secondary metabolites in the surrounding culture medium. After incubation, the crude broth was filtered using filter paper and was extracted with ethyl acetate using a separate funnel. The extracts were allowed to dry in a rotary evaporator. They were then stored for further processing at 4 °C.
2.3. Testing Bacterial Strains
Different testing bacterial strains of American-type collection centre (ATCC) culture were used as marker strains in the current study, including Acinetobacter baumannii (ATCC 19606), Methicillin-resistant Staphylococcus aureus (BAA-1683), Klebsiella pneumoniae (ATCC 13883), Pseudomonas aeruginosa (BAA-2108), Staphylococcus aureus (ATCC 292013), Escherichia coli (ATCC25922), Salmonella typhimurium (ATCC 14028).
2.4. Antibacterial Activity
The antibacterial activity of isolated strains was determined against pathogenic bacteria following the well-diffusion method. The crude ethyl acetates extract dimethyl sulfoxide (DMSO) (5 mg/mL) was poured into a labelled well of MHA plate and pre-inoculated with pathogenic marker strain. The culture plates were incubated for 24 h at 37 °C to observe the antibacterial activity of each bacterial isolate by the previously described method [
18]. Ciprofloxacin solution was used as a positive control with the concentration by dissolving 1 gm of ciprofloxacin per ml of DMSO, while pure DMSO was used as a negative control.
Furthermore, the broth microdilution method determined each potent bacterial extract’s minimum inhibitory concentration (MIC) [
19]. Each extract’s minimum inhibitory concentration (MIC) was carried out in triplicate to measure the effective concentration against each tested pathogen. After the test application, the microtiter plate was incubated, the optical density of each isolate was incubated overnight, and each isolate’s optical density was measured at a wavelength of 600 nm using a microtiter plate reader. The minimum extract concentration able to inhibit the growth of marker/test bacteria was considered the MIC of that isolate.
2.5. Antifungal Activity
Using the agar dilution method, the antifungal activity of isolated bacterial isolates was checked against the two-marker fungal pathogenic strains, including
Aspergillus niger and
Aspergillus flavus. Seven-day-old, pathogenic marker strains of fungi were inoculated in labelled tubes containing defined crude bacterial extract 3 mg/mL. They were allowed to incubate for seven days at 27 °C in a cool incubator [
20]. The growth was examined carefully and was calculated using the following formula:
where Igc is the linear growth of the control sample while Igt is the linear growth of test sample.
2.6. Gas Chromatography-Mass Spectrum Analysis of Metabolites
Using Thermo Scientific GC Focus Series DSQ, bacterial secondary metabolites were analyzed by GC-MS analysis. Helium gas was used as a carrier gas at a constant flow rate of 1 mL per minute with an infection volume of 1 µL. The temperature of the injector and the hot oven was maintained at 250 °C and 110 °C, respectively, with the increase rate of 10 °C per minute up to 200 °C, then 5 °C per minute up to 280 °C and closing after 9 min at the temperature 280 °C. Peaks of varied compounds were eluted from the GC column, and their retention time was recorded. Data were matched with mass spectra of the compounds, and the database searched for similar compounds with the same molecular mass and retention time. Previously studied natural compounds reported for bioactivities were also studied, and the activities of bacterial extracts with their components in the current research were comparably correlated.
2.7. Molecular Characterization of Bacterial Isolates
Molecular characterization of isolated bacterial strains was based on 16S rRNA conserved gene sequences using universal bacterial primers. The targeted gene sequence was amplified using the conventional PCR method, and the size of the amplified fragments was confirmed by running the final product by 1% gel electrophoresis. The amplified samples and appropriate sequencing fragments were sent for sequencing, and the retrieved nucleotide sequences were phylogenetically studied using MEGA software (MEGA-11). Bacterial isolates were further verified/classified at the species level by BLAST search using GenBank NCBI (National Center for Biotechnology Information). To permit public access to these probiotic strains, 16S rRNA gene sequences were submitted to the GenBank database (
www.ncbi.nlm.nih.gov/projects/genome/clone/) with accession numbers MT256113, MT255013, and MT256091.
3. Results
3.1. Isolation and Identification
A total of n = 10 strains of bacteria were isolated and identified based on colonial morphology, microscopy, biochemical characteristics, and sugar fermentation. Among all, Gram-positive, rod-shaped, mycelial, and spore-forming bacterial strains were selected for further confirmatory tests. The molecular analysis further validated the bacterial strains (A6S7, A1S6, A1S10) as Brevibacillus formosus A6S7, Bacillus Subtilis A1S6, Paenibacillus dendritiformis A1S10.
3.2. Morphological and Biochemical Characterization
The morphology of each colony by different bacterial isolates showed regular, irregular, slightly raised, flat, white, and cream-colored colonies. By motility test, bacterial isolates were motile and possessed terminal and subterminal spores (
Table 1).
The biochemical characterizations of bacterial isolates are mentioned in (
Table 2) and verified these isolates as
Bacillus subtilis,
Brevibacillus formosus, and
Paenibacillus dendritiformis.
3.3. Carbohydrate Fermentations
The isolates showed a color change from pink to yellow, indicating that the production of fermented sugars such as glucose, sucrose, lactose, arabinose, starch, glycogen, maltose, and mannitol of a gas bubble in the Durham’s tube (
Table 3).
3.4. Antibacterial Potency of Crude Extracts of Bacterial Isolates
The in-vitro antibacterial potency of crude extracts of bacterial isolates against the MDR strains of both Gram-positive and Gram-negative bacteria was obtained either by the absence or presence of inhibition zones (IZ). The crude extract exhibited an inhibitory effect against the 12 MDR strains with variable diameters of inhibition zones ranging from 7 mm to 28 mm. The bacterial extracts exhibited antibacterial potency against almost all Gram-positive and Gram-negative tested strains of MDR bacteria, but with few exceptions. The best activity was shown by
Paenibacillus dendritiformis (A1S10) against
S. aureus with an IZ of 28.22 mm in diameter, followed by
P. aeruginosa,
K. pneumoniae, and
Acinetobacter baumannii was 28 mm, 27 mm and 21 mm respectively. Similarly,
Brevibacillus formosus (A6S7) inhibited the growth of MRSA,
K. pneumoniae P. aeruginosa,
S. aureus, and
S. typhi with the zone of inhibition of 24 mm, 22 mm, 22 mm, 21 mm, and 24 mm, respectively.
Bacillus subtilis (A1S6) inhibited the growth of
E. coli,
S. typhi,
K. pneumoniae, MRSA, and
S. aureus with the zone of inhibition of 28 mm, 28 mm, 27 mm, 23 mm, and 22 mm, respectively (
Figure 1). All the bacterial strains exhibited the best activity against
E. coli,
S. aureus,
K. pneumoniae,
Acinetobacter baumannii,
P. aeruginosa. In contrast, the least activity was observed against
P. aeruginosa and MRSA. The MIC of (A1S10) against
S. typhi,
S. aureus, and
E. coli was 0.412, 1.25, and 0.312 mg/mL, respectively. Ethyl acetate extract of bacterial isolates exhibited MIC values of 0.312, 0.422, 1.25 mg/mL, and 2.5 mg/mL against
S. typhi,
S. aureus, and
E. coli, respectively.
3.5. Detection of MIC of Crude Bacterial Extracts
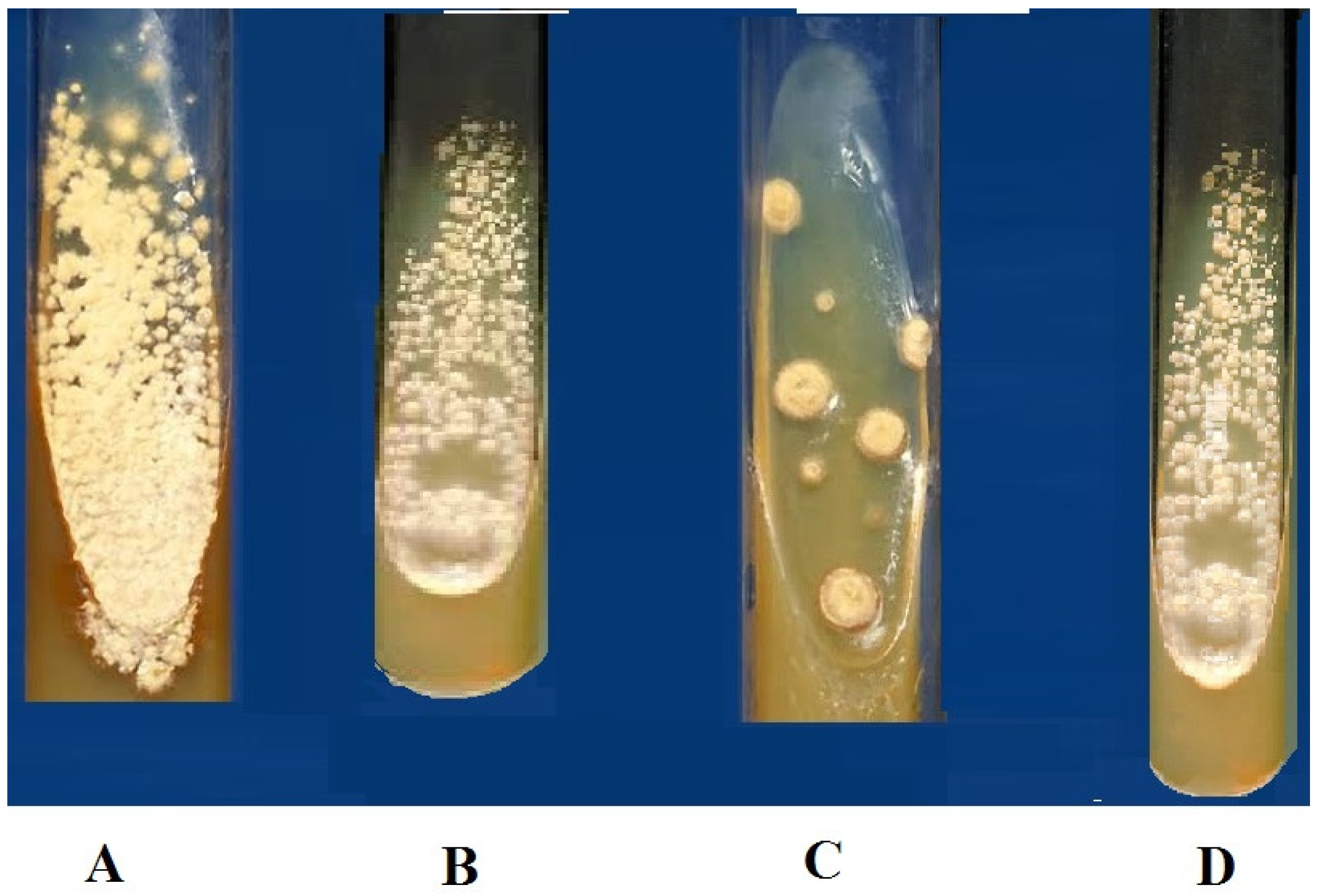
The bacterial extracts were able to inhibit the growth of tested MDR strains isolated from hospital patients. The least concentration of the methanolic extracts that could stop the growth of pathogenic bacteria was 100 mg/mL for all three bacterial isolates (
Figure 2).
3.6. Antifungal Potency of Bacterial Extracts
Ethyl acetate extract of bacterial species was analyzed to observe its antifungal potency against the pathogenic strains of fungi using the tube dilution method. Bacterial extracts were tested against two pathogenic fungal species;
Aspergillus flavus and
Aspergillus niger (
Table 4,
Figure 3).
3.7. GC-MS Analysis
The results of GC-MS evaluation indicated the presence of several compounds in crude extracts of bacterial species. The most important and highest components exhibited in the crude extract analyzed with the help of GC-MS are explained in
Table 5,
Table 6 and
Table 7, describing where compounds observed in this study were previously identified. These compounds showed resemblance with the natural products of plant and bacterial origin. The GC–MS data analysis revealed that most were derivatives of volatile compounds such as alkaloids, esters, ethers, and phenolic compounds.
GC-MS analysis revealed propanoic acid, oxalic acid, phenol, and 1,3,5-Trioxaneas the major metabolites present in the extracts of Bacillus species. Other compounds viz.9-octadecenoic acid (z)-, 2-hydroxyl-1,3-propanedyl ester, cholestan-3-ol,2methylene (3a,5a), stearic acid, chondrilla sterol, octadeccenoic acid, hexadecanoic acid, cyclobutane, and dasycarpidan were also detected possessing antibacterial, antifungal activity, and antioxidant activities.
3.8. Molecular Characterization
Three (n = 3) bacterial isolates were obtained with increased antimicrobial activities from variable samples. Phylogenetic analysis of the 16S rRNA gene sequences indicated that all the three candidate bacterial isolates, A6S7, A1S10, and A1S6, belong to three different bacterial genera, i.e.,
Brevibacillus, Paenibacillus, and
Bacillus, respectively (
Figure 4) as they cluster with the above-mentioned bacterial
spp. in the phylogenetic tree.
The top hit sequence similarity determined that these bacterial isolates presumably belong to
Brevibacillus formosus (99%),
Bacillus Subtilis (99%), and
Paenibacillus dendritiformis (99%), respectively (
Table 8). The presumably described taxonomy was validated by tree topology and high bootstrap values obtained after phylogenetic analysis.
4. Discussion
Soil is a complex and diverse habitat with extensive microbial communities and composition, thus providing highly versatile metabolic biosynthesis and a source of diverse secondary metabolites. Nearly 500 antibiotics are found each year, in which 60% of antibiotics are obtained from soil microbes. Recent analyses have shown that soil screening for antimicrobial activities has been carried out in many parts of the world. A teaspoon of soil contains a hundred million to one billion bacteria active in each acre of the soil. Soil microflora was interesting to investigate, and microbes are considered tiny antimicrobial factories that produce biologically active secondary metabolites. The extreme microbial diversity, abundance and structure also correlate with their diverse metabolic activities, which result in producing countless metabolites with numerous activities, including antimicrobial, anti-parasitic, anti-cancerous and anti-pesticidal activities, etc. The current study aimed to investigate selected soil microbial communities for their potential to produce antimicrobial activities. Twenty different bacterial isolates were found after several steps of isolation, identification on various general purpose, and selected bacterial growth media along with biochemical investigations. In recent years, several microorganisms that can produce antibiotics grown in suitable cultures have increased the probability of developing novel antibiotics to encounter/control untreated infectious diseases. Various studies confirmed soil microflora produce antimicrobial compounds having pharmaceutical and biotechnological applications [
21,
22].
Three bacterial isolates with antibacterial activity were found positive for fermentation of sugars such as glucose, arabinose, sucrose, starch, rhamnose, glycerol, glycogen, lactose, fructose, raffinose, maltose, and mannitol. All these bacterial isolates were also positive for indole, methyl red, Voges–Proskauer, citrate utilization, catalase, oxidase, cellulose test, triple sugar iron, nitrate reduction, and amino acid utilization tests (
Table 1 and
Table 2). A study by Tariq, Sudha [
23] reported the same results of biochemical analysis and sugar fermentation. Isolated bacterial strains were partially characterized based on different sugars fermentation and biochemical tests. These results showed similarities with previous literature [
24].
Our results showed that
Bacillus subtilis,
Paenibacillus, and
Brevibacillus inhibit the growth of multidrug-resistant bacterial strains, which is a contiguous finding previously reported [
25,
26]. It was also noticed that the crude extract of all three bacterial isolates showed prominent antifungal activities against chosen fungal species (
Figure 3 and
Table 4). Previous studies determined that the inhibitory effect of
Bacillus is due to a change in pH in the growth medium or due to the production of volatile compounds. Several other studies reveal that
Bacillus produces polypeptide antibacterial compounds such as bacitracin, polymyxin, gramicidin S., and tyrothricin. These compounds are effective against a wide range of Gram-positive and Gram-negative bacteria [
27].
A previous study revealed that three different bacterial genera were isolated and investigated against MDRS [
28]. Another study also found almost similar results [
29]. Some of the studies reported that
Brevibacillus ssp. [
25,
30] showed robust antimicrobial activity against pathogenic bacteria and fungi. A similar study was conducted on the activity of
Brevibacillus against MDRS [
28]. The GC-MS studies of the bacterial extracts revealed compounds presence (
Table 5,
Table 6 and
Table 7). Mostly these compounds were structurally similar to natural products of plant and bacterial origin and were derivatives of volatile compounds such as esters, alkaloids, ethers and phenolics, etc. The most prominent metabolites of the exact used in this study include propanoic acid, oxalic acid, phenol and 1,3,5-Trioxaneas, possessing antibacterial antifungal activity and antioxidant activities.
Similar observations were reported in several cases; for example, Pecilocin Bb produced by the
Brevibacillus from soil [
26,
31] is a bacteriocin-like inhibitory substance (BLIS) that is stable within the pH range of 1.0–9.0, resistant to heat (100 °C for 30 min), as well as detergents and organic solvents. Kim, Bae [
32] demonstrated the broad-spectrum antimicrobial activity of
Paneibacillus. The study also found a variety of secondary metabolites, which were analyzed by GC-MS, including chondrillasterol, stigmasterol, benzedicarboxylic acid, and octadeconoic acid. A similar study was conducted by [
33] with the same compounds isolated from plants showing antimicrobial activity against MRSA and
E. coli. Another similar study was conducted by Phuong, Han [
34] on marine
Bacillus subtilis strain HD16b producing benz dicarboxylic acid and octadecanoic acid.
Molecular investigation revealed the taxonomy of three different isolated belonging to three different genera, including
Bacillus,
Paenibacillus, and other
Brevibacillus. Based on phylogenetic analysis and top hit sequence similarity results, and supported by a high bootstrap value, it was provisionally assumed that the three most active candidates of bacterial isolates A6S7, A1S10, and A1S6 belong to
Brevibacillus formosus (99%),
Bacillus subtilis (99%), and
Paenibacillus dendritiformis (99%), respectively. It was reported by Amin, Rakhisi [
35] that the
Bacillus spp. is very common in soil. For their successful colonization in various environmental habitats, their highly resistant endospores help them. On the other hand, the isolation strategy applied favors the spore-formers. Identification of three different bacterial strains and their anti microbial activity would help to facilitate microbial screening in the future and the isolation of active metabolites against MDRS.