Responses of Adult Hypera rumicis L. to Synthetic Plant Volatile Blends
Abstract
:1. Introduction
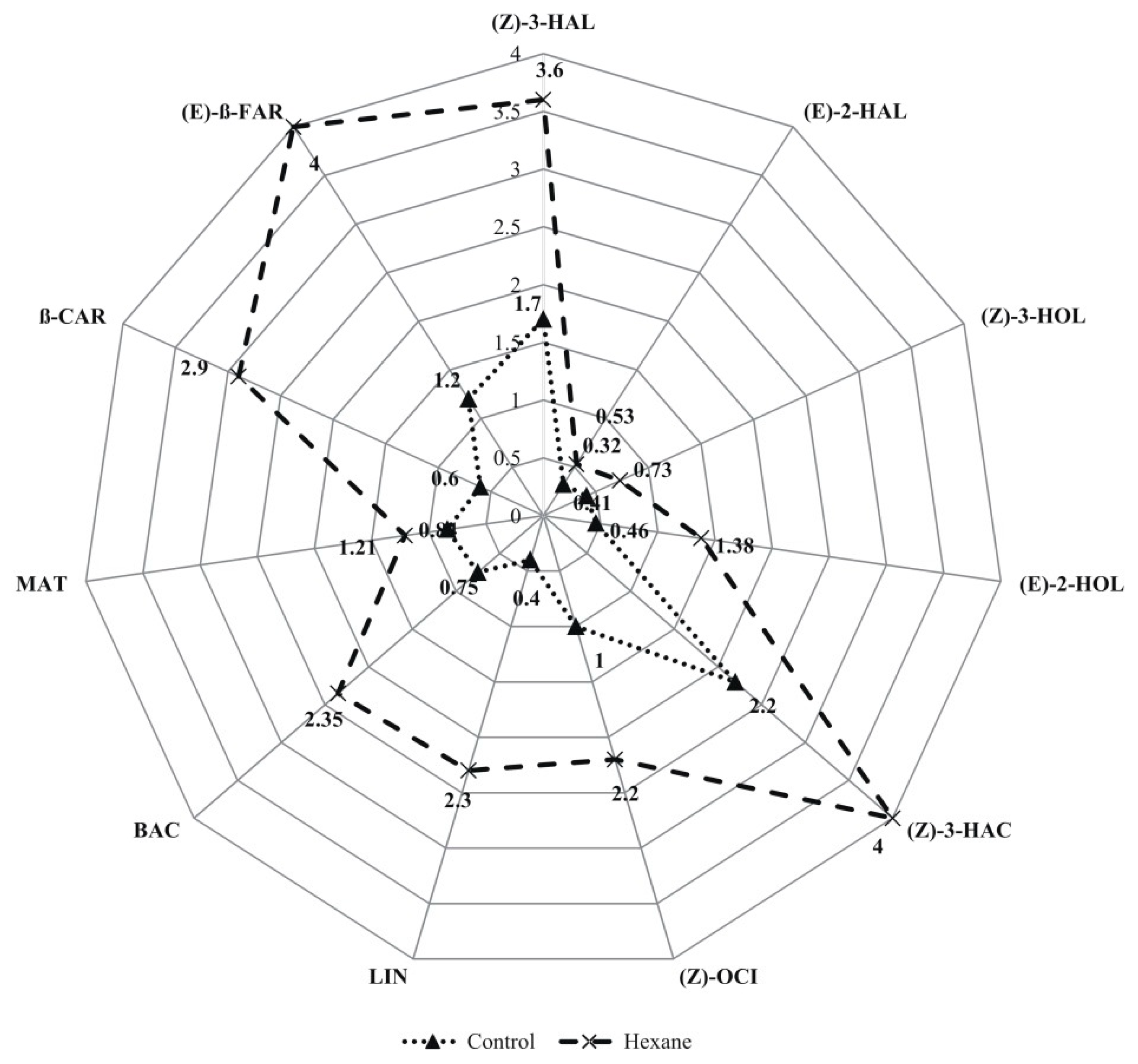
2. Results
3. Discussion
4. Materials and Methods
4.1. Plants
4.2. Application of VOC Blends
4.3. Volatile Collection System
4.4. Analytical Methods
4.5. Insects
4.6. Synthetic Chemicals
4.7. Y-Tube Olfactometer
4.8. Release and Calibration of Odors
4.9. Statistical Analyses for VOC Collection
4.10. Data Analyses for Y-Tube Olfactometer
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, Y.; Wang, Y.M.; Guo, H.; Yang, F.Y. Cultivated rice enhances an insect herbivore-parasitoid interaction compared with a wild grass due to its thinner stems. Biol. Control 2021, 163, 104737. [Google Scholar] [CrossRef]
- Degregorio, R.E.; Ashley, R.A.; Adams, R.G.; Streams, F.A.; Schaefer, C.W. Biocontrol potential of Hypera rumicis (L.) (Coleoptera: Curculionidae) on curly dock (Rumex crispus L.). J. Sustain. Agric. 2010, 2, 7–24. [Google Scholar] [CrossRef]
- Clarke, J.H.; Wynn, S.C.; Twining, S.E. Impact of changing pesticide availability. Asp. Appl. Biol. 2011, 106, 263–267. [Google Scholar]
- Opit, G.P.; Phillips, T.W.; Aikins, M.J.; Hasan, M.M. Phosphine resistance in Tribolium castaneum and Rhyzopertha dominica from stored wheat in oklahoma. J. Econ. Entomol. 2012, 105, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Skevas, T.; Swinton, S.M.; Meehan, T.D.; Kim, T.N.; Gratton, C.; Egbendewe-Mondzozo, A. Integrating agricultural pest biocontrol into forecasts of energy biomass production. Ecol. Econ. 2014, 106, 195–203. [Google Scholar] [CrossRef]
- Gonzalez, J.O.W.; Gutierrez, M.M.; Murray, A.P.; Ferrero, A.A. Composition and biological activity of essential oils from Labiatae against Nezara viridula (Hemiptera: Pentatomidae) soybean pest. Pest. Manag. Sci. 2011, 67, 948–955. [Google Scholar] [CrossRef]
- Reyes, M.; Franck, P.; Charmillot, P.J.; Ioriatti, C.; Olivares, J.; Pasqualini, E.; Sauphanor, B. Diversity of insecticide resistance mechanisms and spectrum in european populations of the codling moth, cydia pomonella. Pest. Manag. Sci. 2007, 63, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Wenda-Piesik, A.; Piesik, D.; Buszewski, B. Do mated Tribolium confusum adults respond to blends of odors? Pol. J. Environ. Stud. 2017, 26, 447–452. [Google Scholar] [CrossRef]
- Szigeti, Z.; Paradi, I. On the communication of plants—What happens above the ground? Bot. Közlemények 2020, 107, 19–32. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L. Specificity and context-dependency of plant-plant communication in response to insect herbivory. Curr. Opin. Insect. Sci. 2019, 32, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.F.; Zhang, K.D.; Wu, Z.W.; Xu, J.M.; Erb, M. Plant volatiles as regulators of plant defense and herbivore immunity: Molecular mechanisms and unanswered questions. Curr. Opin. Insect. Sci. 2021, 44, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.Q.; Tian, L.B.; Han, X.; Yang, Y. Research advances in allelopathy of volatile organic compounds (VOCs) of plants. Horticulturae 2021, 7, 278. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The role of volatiles in plant communication. Plant. J. 2019, 100, 892–907. [Google Scholar] [CrossRef] [PubMed]
- Lev-Yadun, S. Avoiding rather than resisting herbivore attacks is often the first line of plant defence. Biol J. Linn. Soc. 2021, 134, 775–802. [Google Scholar] [CrossRef]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2018, 220, 666–683. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell. Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.M. Social networking in crop plants: Wired and wireless cross-plant communications. Plant Cell. Environ. 2021, 44, 1095–1110. [Google Scholar] [CrossRef]
- Clavijo McCormick, A.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef]
- Mithofer, A.; Wanner, G.; Boland, W. Effects of feeding Spodoptera littoralis on lima bean leaves. Ii. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiol. 2005, 137, 1160–1168. [Google Scholar] [CrossRef]
- Piesik, D.; Panka, D.; Jeske, M.; Wenda-Piesik, A.; Delaney, K.J.; Weaver, D.K. Volatile induction of infected and neighbouring uninfected plants potentially influence attraction/repellence of a cereal herbivore. J. Appl. Entomol. 2013, 137, 296–309. [Google Scholar] [CrossRef]
- Yoneya, K.; Kugimiya, S.; Takabayashi, J. Can herbivore-induced plant volatiles inform predatory insect about the most suitable stage of its prey? Physiol. Entomol. 2009, 34, 379–386. [Google Scholar] [CrossRef]
- Bertea, C.M.; Casacci, L.P.; Bonelli, S.; Zampollo, A.; Barbero, F. Chemical, physiological and molecular responses of host plants to lepidopteran egg-laying. Front. Plant Sci. 2019, 10, 1768. [Google Scholar] [CrossRef] [PubMed]
- Poelman, E.H.; Zheng, S.J.; Zhang, Z.; Heemskerk, N.M.; Cortesero, A.M.; Dicke, M. Parasitoid-specific induction of plant responses to parasitized herbivores affects colonization by subsequent herbivores. Proc. Natl. Acad. Sci. USA 2011, 108, 19647–19652. [Google Scholar] [CrossRef] [PubMed]
- Pickett, J.A.; Ardottir, G.I.; Birkett, M.A.; Bruce, T.J.A.; Chamberlain, K.; Khan, Z.R.; Midega, C.A.O.; Smart, L.E.; Woodcock, C.M. Aspects of insect chemical ecology: Exploitation of reception and detection as tools for deception of pests and beneficial insects. Physiol. Entomol. 2012, 37, 2–9. [Google Scholar] [CrossRef]
- Tamiru, A.; Bruce, T.J.; Midega, C.A.; Woodcock, C.M.; Birkett, M.A.; Pickett, J.A.; Khan, Z.R. Oviposition induced volatile emissions from african smallholder farmers’ maize varieties. J. Chem. Ecol. 2012, 38, 231–234. [Google Scholar] [CrossRef]
- Rosenkranz, M.; Chen, Y.Y.; Zhu, P.Y.; Vlot, A.C. Volatile terpenes-mediators of plant-to-plant communication. Plant J. 2021, 108, 617–631. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; von Dahl, C.C.; Preston, C.A. Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef]
- Ayelo, P.M.; Pirk, C.W.W.; Yusuf, A.A.; Chailleux, A.; Mohamed, S.A.; Deletre, E. Exploring the kairomone-based foraging behaviour of natural enemies to enhance biological control: A review. Front. Ecol. Evol. 2021, 9, 641974. [Google Scholar] [CrossRef]
- Guo, H.; Wang, C.Z. The ethological significance and olfactory detection of herbivore-induced plant volatiles in interactions of plants, herbivorous insects, and parasitoids. Arthropod-Plant Interact. 2019, 13, 161–179. [Google Scholar] [CrossRef]
- Piesik, D.; Wenda-Piesik, A.; Kotwica, K.; Lyszczarz, A.; Delaney, K.J. Gastrophysa polygoni herbivory on Rumex confertus: Single leaf VOC induction and dose dependent herbivore attraction/repellence to individual compounds. J. Plant Physiol. 2011, 168, 2134–2138. [Google Scholar] [CrossRef]
- Piesik, D.; Wenda-Piesik, A.; Krasinska, A.; Wrzesinska, D.; Delaney, K.J. Volatile organic compounds released by Rumex confertus following Hypera rumicis herbivory and weevil responses to volatiles. J. Appl. Entomol. 2016, 140, 308–316. [Google Scholar] [CrossRef]
- Skoczek, A.; Piesik, D.; Wenda-Piesik, A.; Buszewski, B.; Bocianowski, J.; Wawrzyniak, M. Volatile organic compounds released by maize following herbivory or insect extract application and communication between plants. J. Appl. Entomol. 2017, 141, 630–643. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Matsui, K.; Takabayashi, J. Chemical and molecular ecology of herbivore-induced plant volatiles: Proximate factors and their ultimate functions. Plant Cell. Physiol. 2009, 50, 911–923. [Google Scholar] [CrossRef]
- Farre-Armengol, G.; Filella, I.; Llusia, J.; Penuelas, J. Floral volatile organic compounds: Between attraction and deterrence of visitors under global change. Perspect. Plant Ecol. 2013, 15, 56–67. [Google Scholar] [CrossRef]
- Gantner, M.; Najda, A. Essential oils from buds and leaves of two hazelnut (Corylus L.) cultivars with different resistance to filbert big bud mite (Phytoptus avellanae Nal.) and filbert aphid (Myzocallis coryli Goetze). Arthropod-Plant Interact. 2013, 7, 659–666. [Google Scholar] [CrossRef]
- Kaplan, I. Attracting carnivorous arthropods with plant volatiles: The future of biocontrol or playing with fire? Biol. Control 2012, 60, 77–89. [Google Scholar] [CrossRef]
- Niinemets, U.; Kannaste, A.; Copolovici, L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front. Plant Sci. 2013, 4, 262. [Google Scholar] [CrossRef]
- Trowbridge, A.M.; Stoy, P.C. Bvoc mediated plant–herbivore interactions. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson, R.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 21–46. [Google Scholar]
- Cavers, P.B.; Harper, J.L. Biological flora of the British Isles, Rumex obtusifolius L. and Rumex crispus L. Ecology 1964, 52, 737–766. [Google Scholar] [CrossRef]
- Koner, A.; Das, S.; Karmakar, A.; Barik, A. Attraction of the biocontrol agent, Galerucella placida towards volatile blends of two polygonaceae weeds, rumex dentatus and polygonum glabrum. J. Chem. Ecol. 2022, 48, 165–178. [Google Scholar] [CrossRef]
- Koner, A.; Das, S.; Mobarak, S.H.; Barik, A. Short-rang.ge attraction and oviposition stimulant of a biocontrol agent, Galerucella placida Baly (Coleoptera: Chrysomelidae) toward weed leaf surface waxes. Bull. Entomol. Res. 2022, 112, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Koner, A.; Debnath, R.; Barik, A. Age-stage, two-sex life table and food utilization efficiencies of Galerucella placida Baly (Coleoptera: Chrysomelidae) on two Polygonaceae weeds. J. Asia-Pac. Entomol. 2019, 22, 1136–1144. [Google Scholar] [CrossRef]
- Bengtsson, J.M.; Gonzalez, F.; Cattaneo, A.M.; Montagne, N.; Walker, W.B.; Bengtsson, M.; Anfora, G.; Ignell, R.; Jacquin-Joly, E.; Witzgall, P. A predicted sex pheromone receptor of codling moth Cydia pomonella detects the plant volatile pear ester. Front. Ecol. Evol. 2014, 2, 33. [Google Scholar] [CrossRef]
- Delaney, K.J.; Wawrzyniak, M.; Lemanczyk, G.; Wrzesinska, D.; Piesik, D. Synthetic Cis-jasmone exposure induces wheat and barley volatiles that repel the pest cereal leaf beetle, Oulema melanopus L. J. Chem. Ecol. 2013, 39, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.; Bengtsson, J.M.; Walker, W.B.; Sousa, M.F.R.; Cattaneo, A.M.; Montagne, N.; de Fouchier, A.; Anfora, G.; Jacquin-Joly, E.; Witzgall, P.; et al. A conserved odorant receptor detects the same 1-indanone analogs in a tortricid and a noctuid moth. Front. Ecol. Evol. 2015, 3, 131. [Google Scholar] [CrossRef]
- Witzgall, P.; Proffit, M.; Rozpedowska, E.; Becher, P.G.; Andreadis, S.; Coracini, M.; Lindblom, T.U.T.; Ream, L.J.; Hagman, A.; Bengtsson, M.; et al. “This is not an apple”-yeast mutualism in codling moth. J. Chem. Ecol. 2012, 38, 949–957. [Google Scholar] [CrossRef]
- Bruce, T.J.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects—Finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.L.; Cheng, K.; Wang, Z.W.; Zhang, Q.; Yang, X.T. Use of odor by host-finding insects: The role of real-time odor environment and odor mixing degree. Chemoecology 2021, 31, 149–158. [Google Scholar] [CrossRef]
- Piesik, D.; Weaver, D.K.; Runyon, J.B.; Buteler, M.; Peck, G.E.; Morrill, W.L. Behavioural responses of wheat stem sawflies to wheat volatiles. Agric. For. Entomol. 2008, 10, 245–253. [Google Scholar] [CrossRef]
- Heil, M.; Karban, R. Explaining evolution of plant communication by airborne signals. Trends Ecol. Evol. 2010, 25, 137–144. [Google Scholar] [CrossRef]
- Kessler, A.; Heil, M. The multiple faces of indirect defences and their agents of natural selection. Funct. Ecol. 2011, 25, 348–357. [Google Scholar] [CrossRef]
- Sidorova, D.E.; Plyuta, V.A.; Padiy, D.A.; Kupriyanova, E.V.; Roshina, N.V.; Koksharova, O.A.; Khmel, I.A. The effect of volatile organic compounds on different organisms: Agrobacteria, plants and insects. Microorganisms 2021, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.; Gezan, S.; Bruce, T.; Hardie, J.; Pickett, J. Between plant and diurnal variation in quantities and ratios of volatile compounds emitted by Vicia faba plants. Phytochemistry 2010, 71, 81–89. [Google Scholar] [CrossRef]
- Ali, A.N.; Wright, M.G. Response of Trichogramma papilionis to semiochemicals induced by host oviposition on plants. Biol. Control 2021, 154, 104510. [Google Scholar] [CrossRef]
- Carroll, M.J.; Schmelz, E.A.; Meagher, R.L.; Teal, P.E.A. Attraction of Spodoptera frugiperda larvae to volatiles from herbivore-damaged maize seedlings. J. Chem. Ecol. 2006, 32, 1911–1924. [Google Scholar] [CrossRef] [PubMed]
- Beyaert, I.; Waschke, N.; Scholz, A.; Varama, M.; Reinecke, A.; Hilker, M. Relevance of resource-indicating key volatiles and habitat odour for insect orientation. Anim. Behav. 2010, 79, 1077–1086. [Google Scholar] [CrossRef]
- Davidson-Lowe, E.; Ali, J.G. Herbivore-induced plant volatiles mediate behavioral interactions between a leaf-chewing and a phloem-feeding herbivore. Basic Appl. Ecol. 2021, 53, 39–48. [Google Scholar] [CrossRef]
- Piesik, D.; Kalka, I.; Wenda-Piesik, A.; Bocianowski, J. Apion miniatum Germ. Herbivory on the mossy sorrel, Rumex confertus willd.: Induced plant volatiles and weevil orientation responses. Pol. J. Environ. Stud. 2014, 23, 2149–2156. [Google Scholar] [CrossRef]
- Bougherra, H.H.; Bedini, S.; Flamini, G.; Cosci, F.; Belhamel, K.; Conti, B. Pistacia lentiscus essential oil has repellent effect against three major insect pests of pasta. Ind. Crop Prod. 2015, 63, 249–255. [Google Scholar] [CrossRef]
- Cloyd, R.A. How effective is conservation biological control in regulating insect pest populations in organic crop production systems? Insects 2020, 11, 744. [Google Scholar] [CrossRef]
- Cusumano, A.; Harvey, J.A.; Bourne, M.E.; Poelman, E.H.; J, G.d.B. Exploiting chemical ecology to manage hyperparasitoids in biological control of arthropod pests. Pest. Manag. Sci. 2020, 76, 432–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffke, A.M.; Alborn, H.T.; Dudley, T.L.; Bean, D.W. Using chemical ecology to enhance weed biological control. Insects 2021, 12, 695. [Google Scholar] [CrossRef] [PubMed]
- Qawasmeh, A.; Raman, A.; Wheatley, W. Volatiles in perennial ryegrass infected with strains of endophytic fungus: Impact on african black beetle host selection. J. Appl. Entomol. 2015, 139, 94–104. [Google Scholar] [CrossRef]
- Thoming, G. Behavior matters-future need for insect studies on odor-mediated host plant recognition with the aim of making use of allelochemicals for plant protection. J. Agric. Food Chem. 2021, 69, 10469–10479. [Google Scholar] [CrossRef] [PubMed]
- Piesik, D.; Lemańczyk, G.; Bocianowski, B.; Buszewski, B.; Vidal, S.; Mayhew, C.A. Induction of volatile organic compunds in Triticum aestivum (wheat) plants following. Phytochemistry 2022, 198, 113162. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Mahalanobis, P.C. On the generalized distance in statistics. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 1936, 12, 49–55. [Google Scholar]
- Seidler-Lozykowska, K.; Bocianowski, J. Evaluation of variability of morphological traits of selected caraway (Carum carvi L.) genotypes. Ind. Crop Prod. 2012, 35, 140–145. [Google Scholar] [CrossRef]
- Camussi, A.; Ottaviano, E.; Calinski, T.; Kaczmarek, Z. Genetic distances based on quantitative traits. Genetics 1985, 111, 945–962. [Google Scholar] [CrossRef]
- Bocianowski, J.; Liersch, A. Multidimensional Analysis of Diversity in Genotypes of Winter Oilseed Rape (Brassica napus L.). Agronomy 2022, 12, 633. [Google Scholar] [CrossRef]

| Source of Variation | Concentrations | Residual |
|---|---|---|
| The number of degrees of freedom | 3 | 36 |
| (Z)-3-HAL | 907,362 *** | 3422 |
| (E)-2-HAL | 30,606.3 *** | 231.2 |
| (Z)-3-HOL | 23,262.1 *** | 221.9 |
| (E)-2-HOL | 27,375 *** | 386.9 |
| (Z)-3-HAC | 951,397 *** | 3434 |
| (Z)-OCI | 637,135 *** | 3501 |
| LIN | 365,700 *** | 1280 |
| BAC | 81,395 *** | 1311 |
| MAT | 113,526 *** | 1044 |
| β-CAR | 671,452 *** | 2345 |
| (E)-β-FAR | 1,104,779 *** | 1349 |
| No. of Females | No. of Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Name of mixed compounds | Rep. | ng·min−1 | + (4) | – (5) | χ2 (1) | + (4) | – (5) | χ2 (1) |
| control | 0 | 12 | 8 | 0.45 ns | 7 | 13 | 1.25 ns | |
| (Z)-3-HAL | ||||||||
| + (E)-2-HAL | 1 | 1 | 15 | 5 | 4.05* (a) (3) | 8 | 12 | 0.45 ns |
| + (Z)-3-HOL | 2 | 5 | 16 | 4 | 6.05* (a) (3) | 11 | 9 | 0.05 ns |
| + (E)-2-HOL | 3 | 25 | 15 | 5 | 4.05* (a) (3) | 8 | 12 | 0.45 ns |
| + (Z)-3-HAC | 4 | 125 | 3 | 17 | 8.45** (r) (2) | 4 | 16 | 6.05* (r) (2) |
| control | 0 | 10 | 10 | 0.05 ns | 7 | 13 | 1.25 ns | |
| (Z)-OCI | ||||||||
| + LIN | 1 | 1 | 11 | 9 | 0.05 ns | 6 | 14 | 2.45 ns |
| + BAC | 2 | 5 | 3 | 17 | 8.45** (r) (2) | 9 | 11 | 0.05 ns |
| + MAT | 3 | 25 | 4 | 16 | 6.05* (r) (2) | 5 | 15 | 4.05* (r) (2) |
| + β-CAR | 4 | 125 | 3 | 17 | 8.45** (r) (2) | 3 | 17 | 8.45** (r) (2) |
| + (E)-β-FAR | ||||||||
| No. of Females | No. of Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Rep. | ng·min−1 (7) | + (5) | – (6) | χ2 (1) | + (5) | – (6) | χ2 (1) | |
| (B1) | (M) | (B1) | (F) | |||||
| 1 | 1 | 10 | 10 | 0.05 ns | 8 | 12 | 0.45 ns | |
| Blend/H. rumicis | 2 | 5 | 5 | 15 | 4.05* (f/a/m) (3) | 5 | 15 | 4.05* (m/a/f) (4) |
| 3 | 25 | 4 | 16 | 6.05* (f/a/m) (3) | 3 | 17 | 8.45** (m/a/f) (4) | |
| No. of Females | No. of Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Rep. | ng·min−1 (7) | + (5) | – (6) | χ2 (1) | + (5) | – (6) | χ2 (1) | |
| (B1) | (M) | (B1) | (F) | |||||
| 1 | 1 | 14 | 6 | 2.45 ns | 11 | 9 | 0.05 ns | |
| Blend/H. rumicis | 2 | 5 | 17 | 3 | 8.45** (f/a) (2) | 5 | 15 | 4.05* (m/a/f) (4) |
| 3 | 25 | 18 | 2 | 11.25*** (f/a) (2) | 4 | 16 | 6.05* (m/a/f) (4) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piesik, D.; Bocianowski, J.; Kotwica, K.; Lemańczyk, G.; Piesik, M.; Ruzsanyi, V.; Mayhew, C.A. Responses of Adult Hypera rumicis L. to Synthetic Plant Volatile Blends. Molecules 2022, 27, 6290. https://doi.org/10.3390/molecules27196290
Piesik D, Bocianowski J, Kotwica K, Lemańczyk G, Piesik M, Ruzsanyi V, Mayhew CA. Responses of Adult Hypera rumicis L. to Synthetic Plant Volatile Blends. Molecules. 2022; 27(19):6290. https://doi.org/10.3390/molecules27196290
Chicago/Turabian StylePiesik, Dariusz, Jan Bocianowski, Karol Kotwica, Grzegorz Lemańczyk, Magdalena Piesik, Veronika Ruzsanyi, and Chris A. Mayhew. 2022. "Responses of Adult Hypera rumicis L. to Synthetic Plant Volatile Blends" Molecules 27, no. 19: 6290. https://doi.org/10.3390/molecules27196290
APA StylePiesik, D., Bocianowski, J., Kotwica, K., Lemańczyk, G., Piesik, M., Ruzsanyi, V., & Mayhew, C. A. (2022). Responses of Adult Hypera rumicis L. to Synthetic Plant Volatile Blends. Molecules, 27(19), 6290. https://doi.org/10.3390/molecules27196290









