Synthesis and Characterization of Silver Nanoparticles from Rhizophora apiculata and Studies on Their Wound Healing, Antioxidant, Anti-Inflammatory, and Cytotoxic Activity
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis
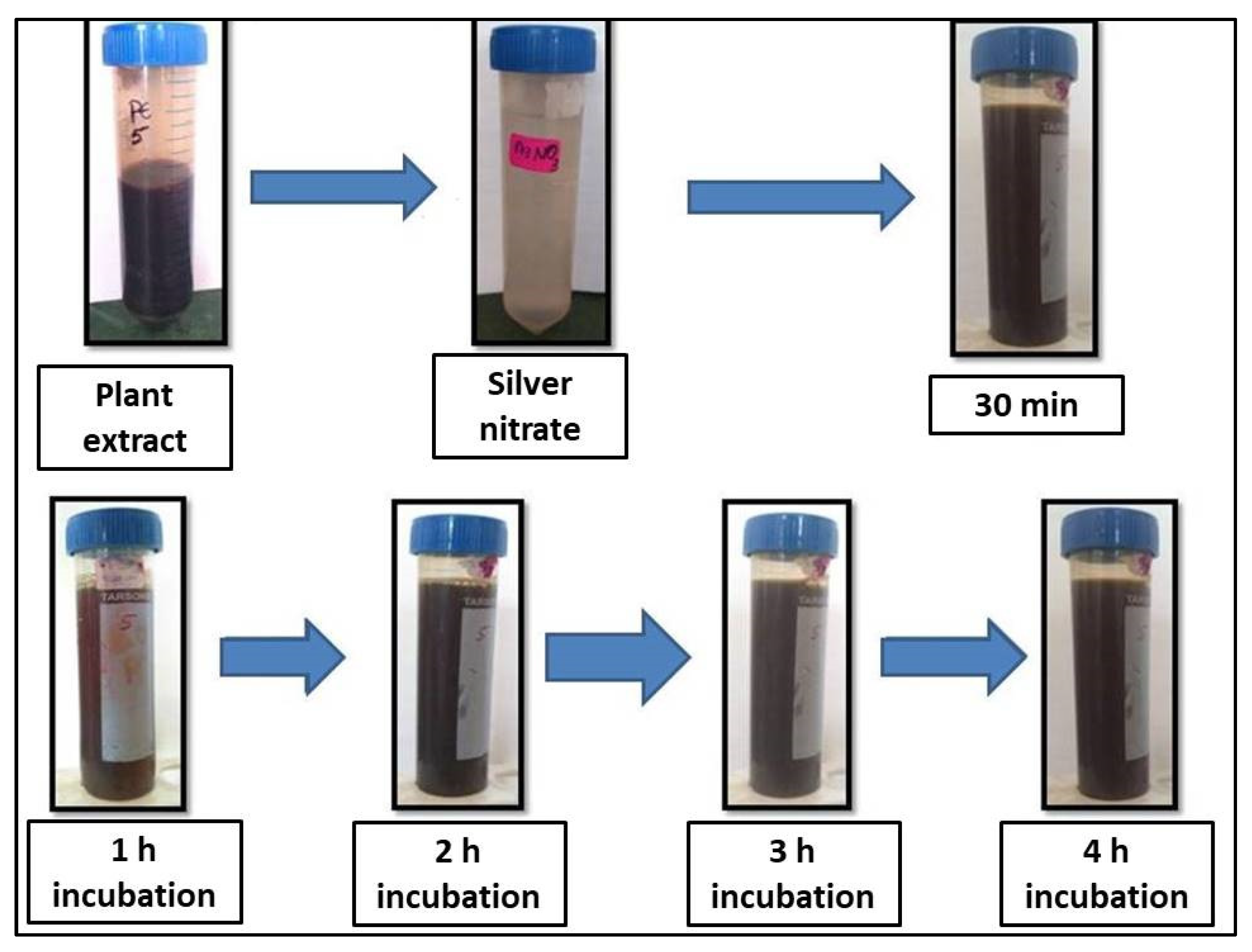
2.2. Visual Observation and UV-VIS Characterization
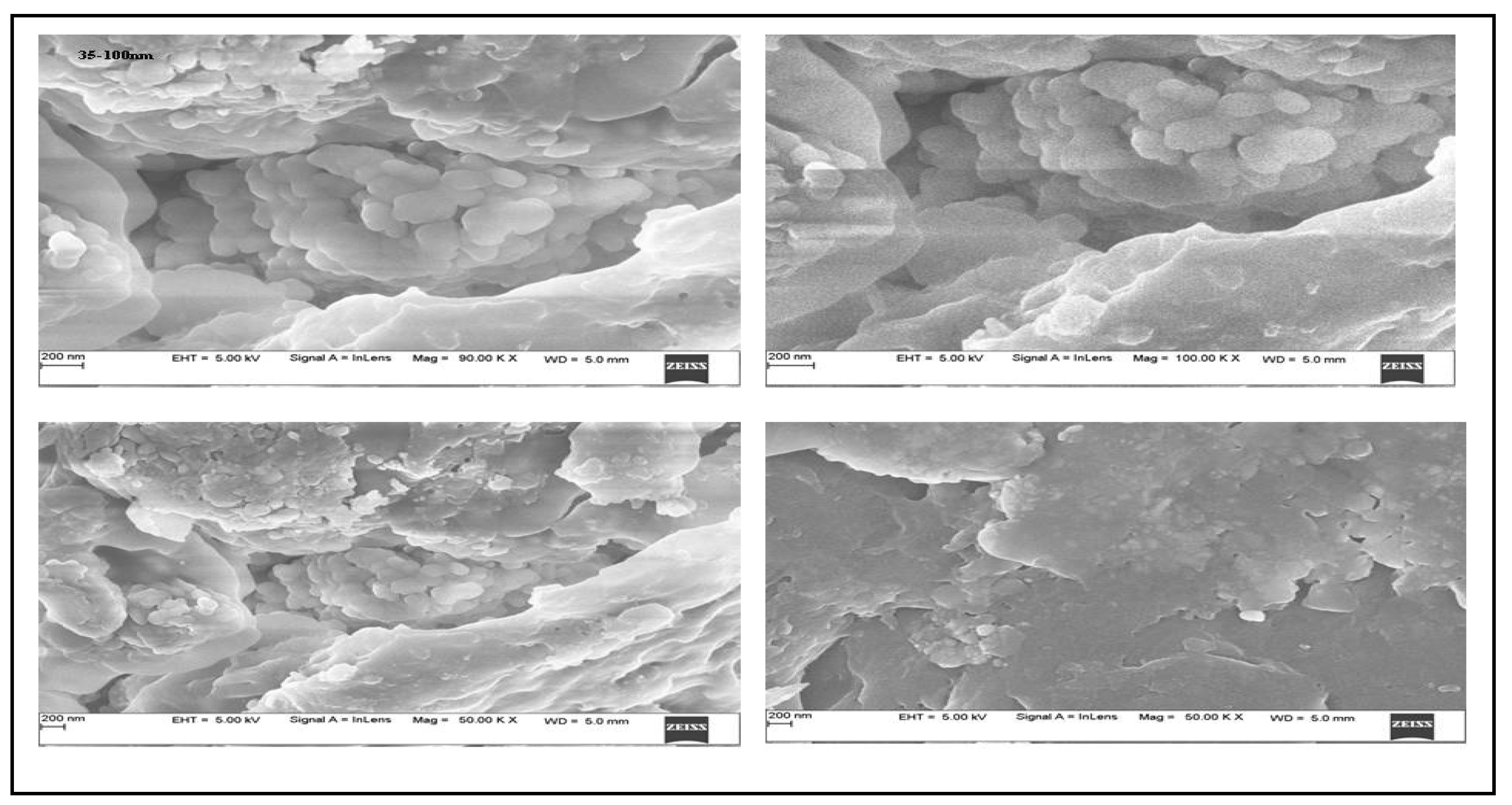
2.3. SEM and EDX Studies
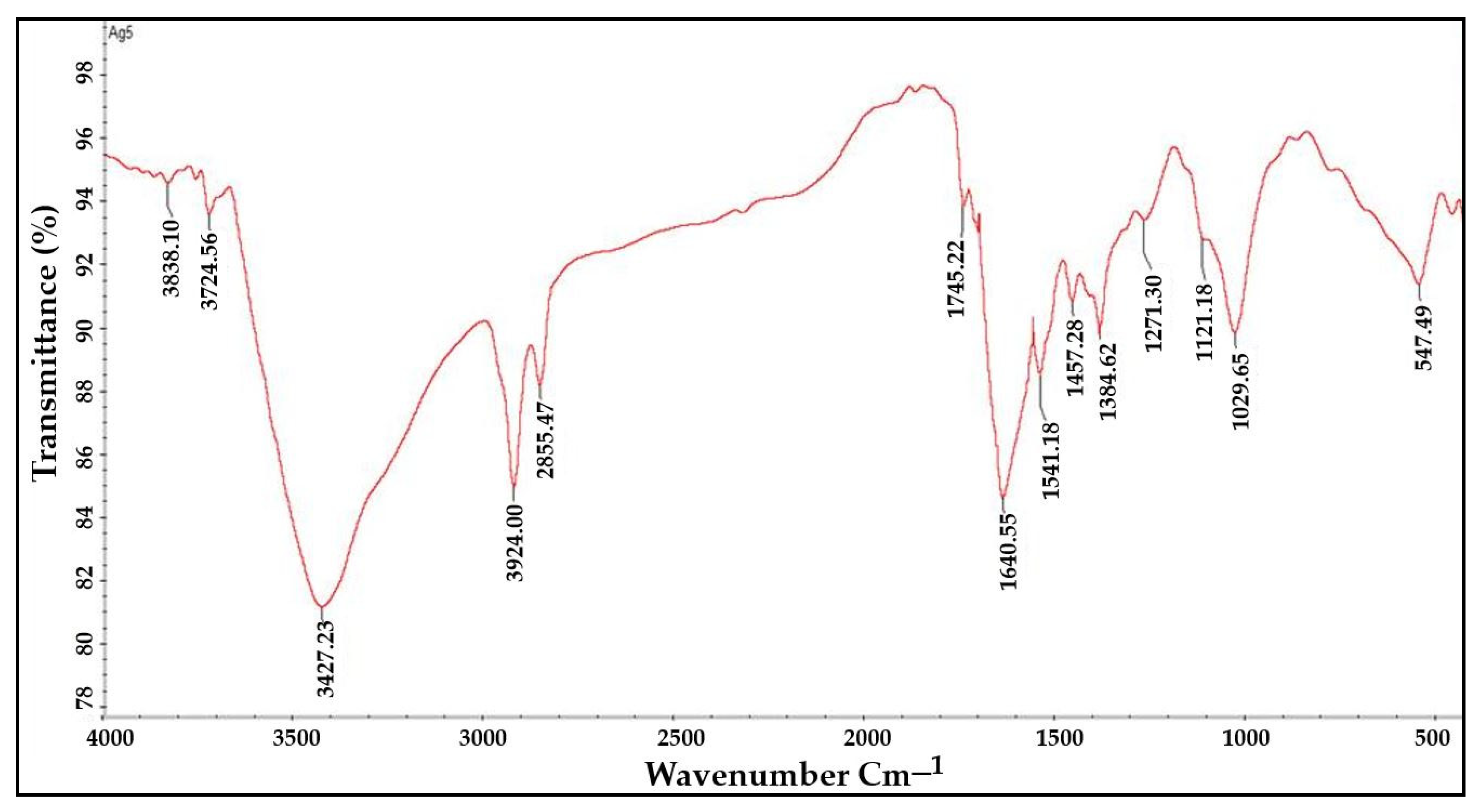
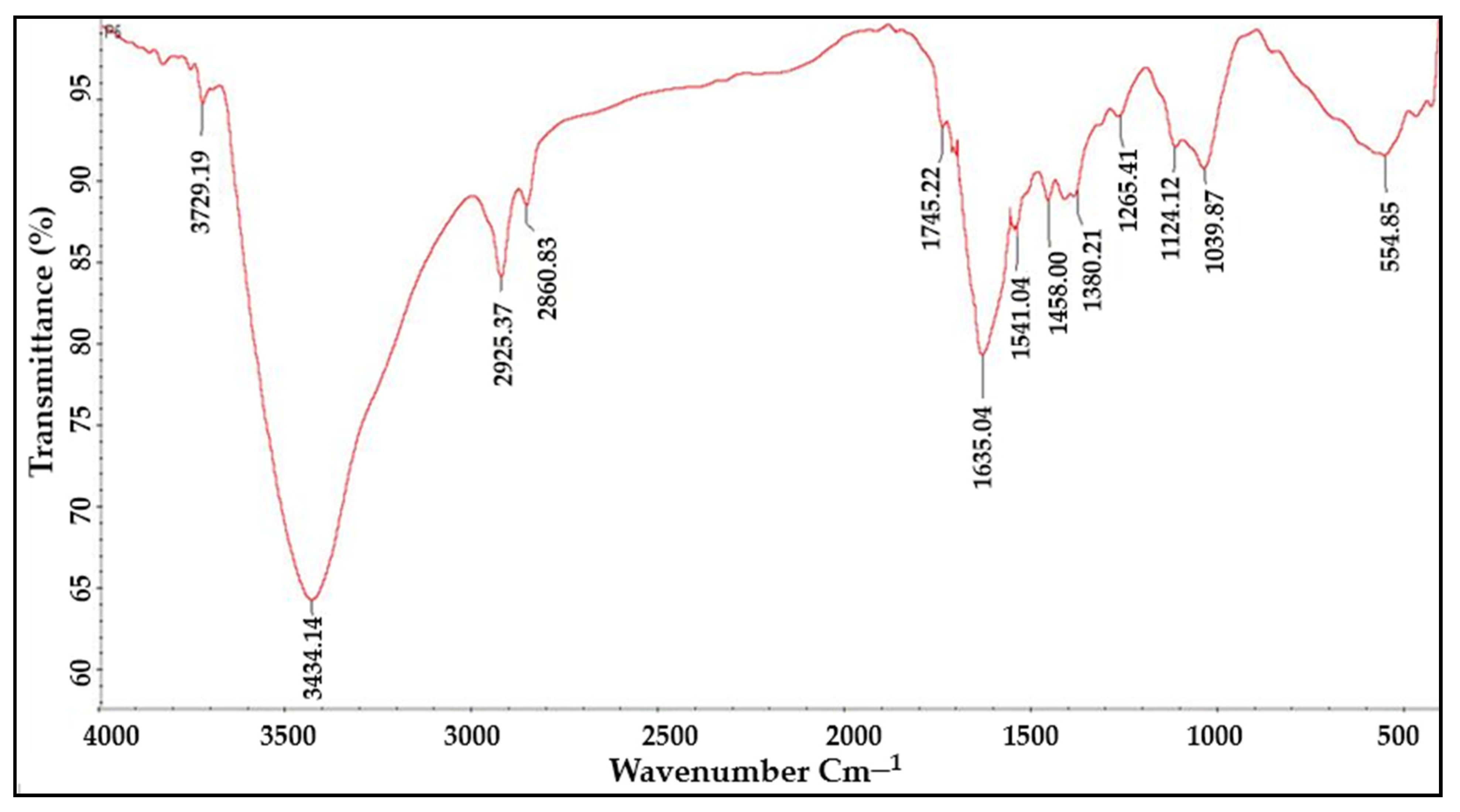
2.4. FTIR Analysis
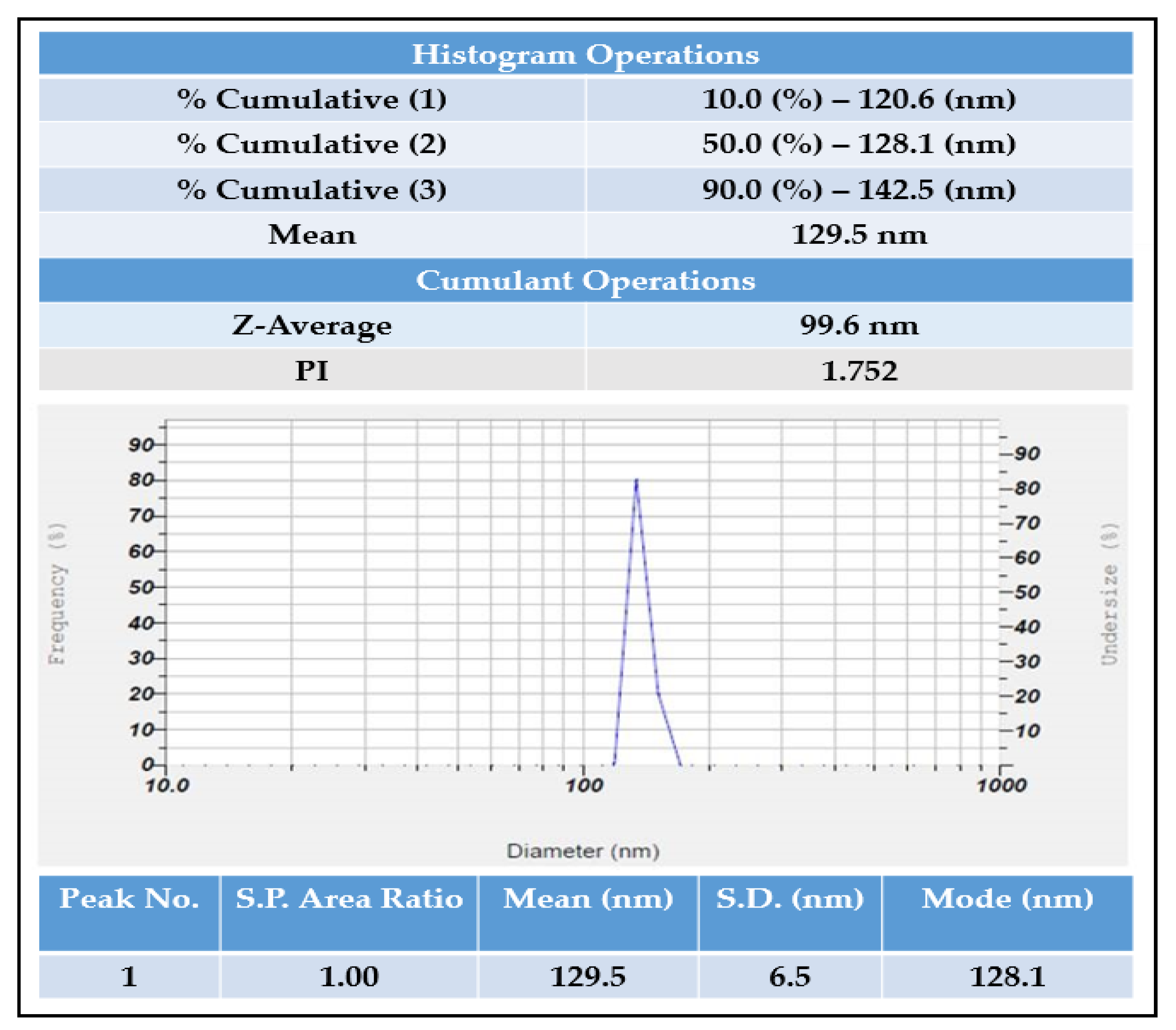
2.5. Particle Size Analyzer and Zeta Potential
XRD Analysis
2.6. In Vitro Antioxidant Activity
2.6.1. FRAP Assay
2.6.2. H2O2 Assay
2.6.3. DPPH Assay
2.6.4. PM Assay
2.6.5. In Vitro Anti-Inflammatory Assay
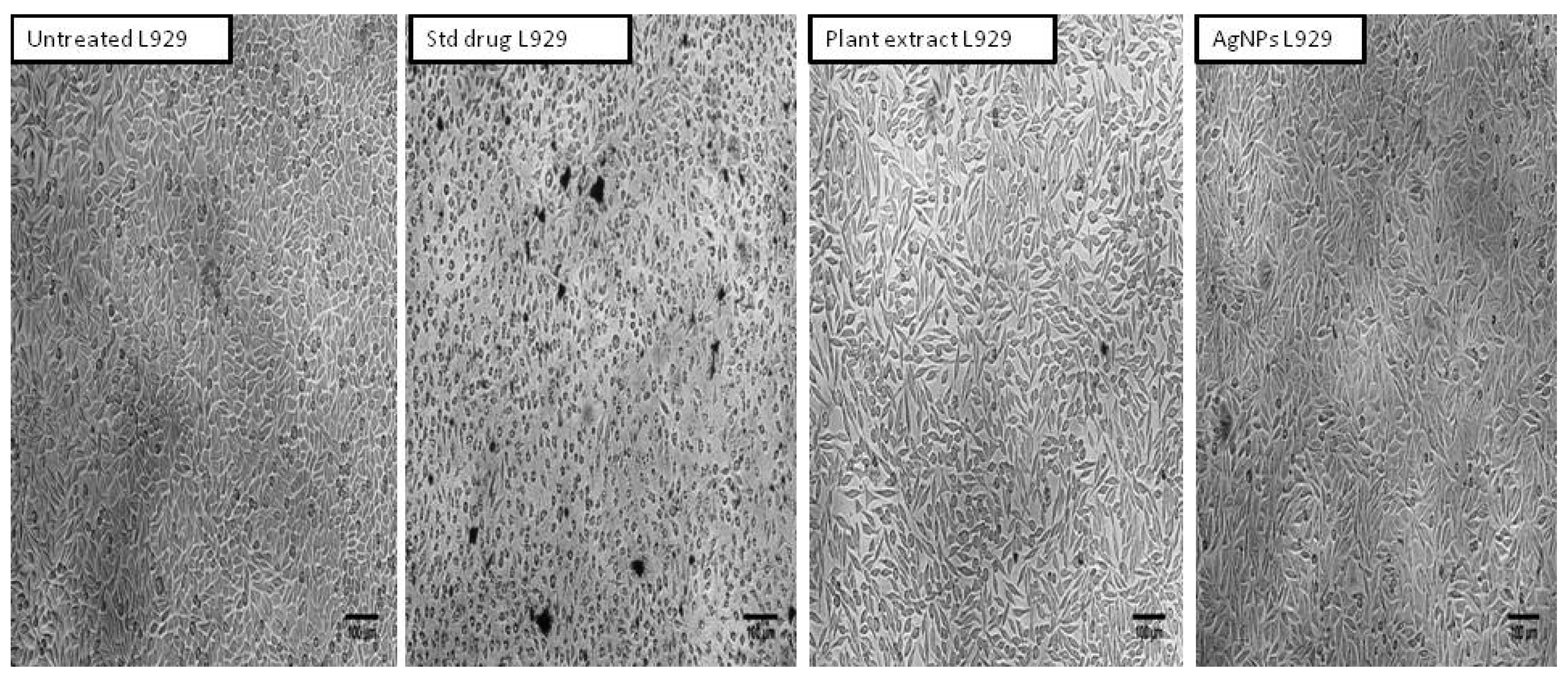
2.7. Cytotoxicity Activity of Aqueous Leaf Extract of R. Apiculata and Its Synthesized AgNPs against Non-Cancerous Fibroblast L929 Cell Line
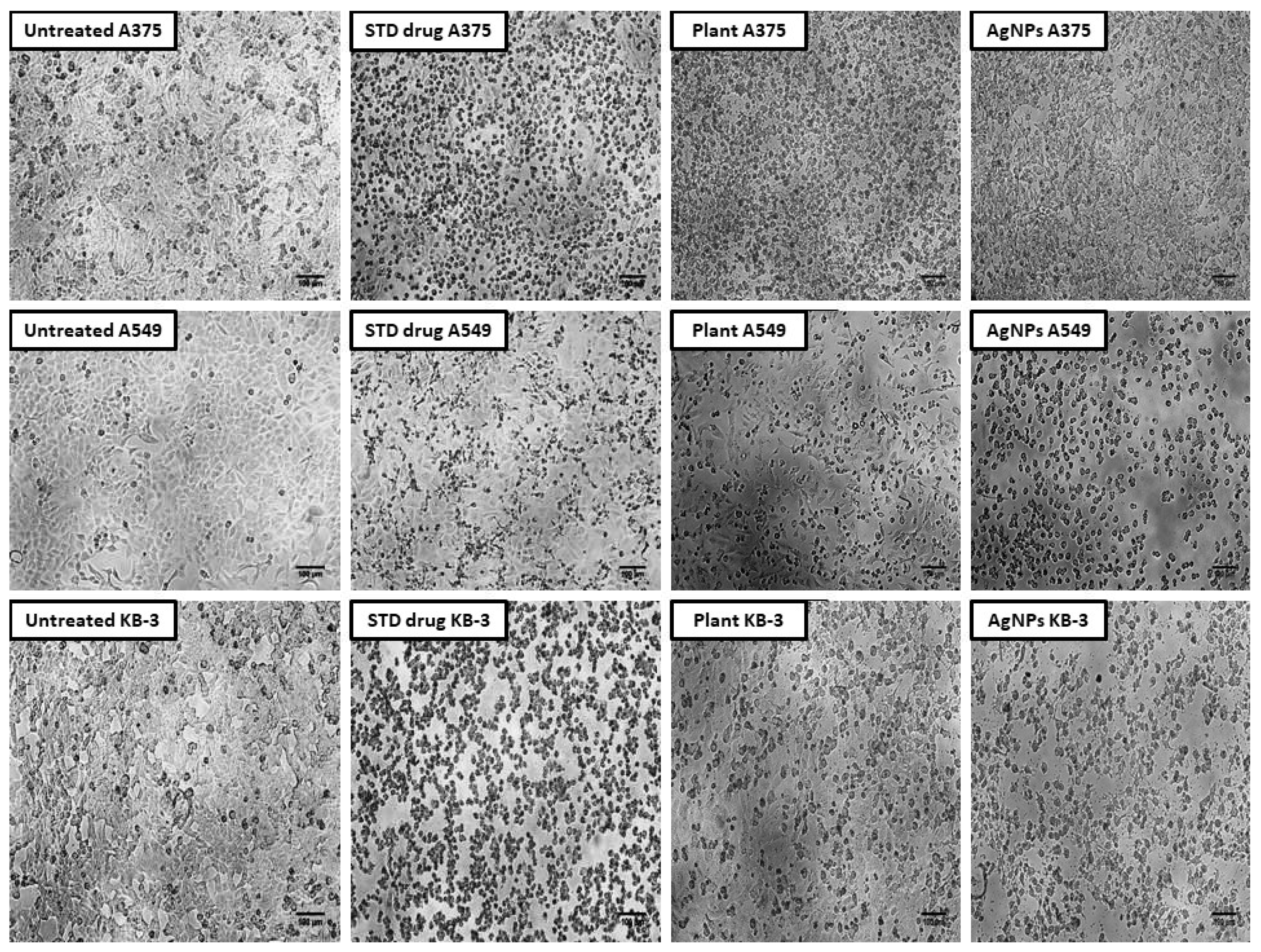
2.8. Cytotoxic Activity of Aqueous Leaf Extract of R. apiculata and Its Synthesized AgNPs against A375 (Skin Cancer), A549 (Lung Cancer), and KB-3 (Oral Cancer)
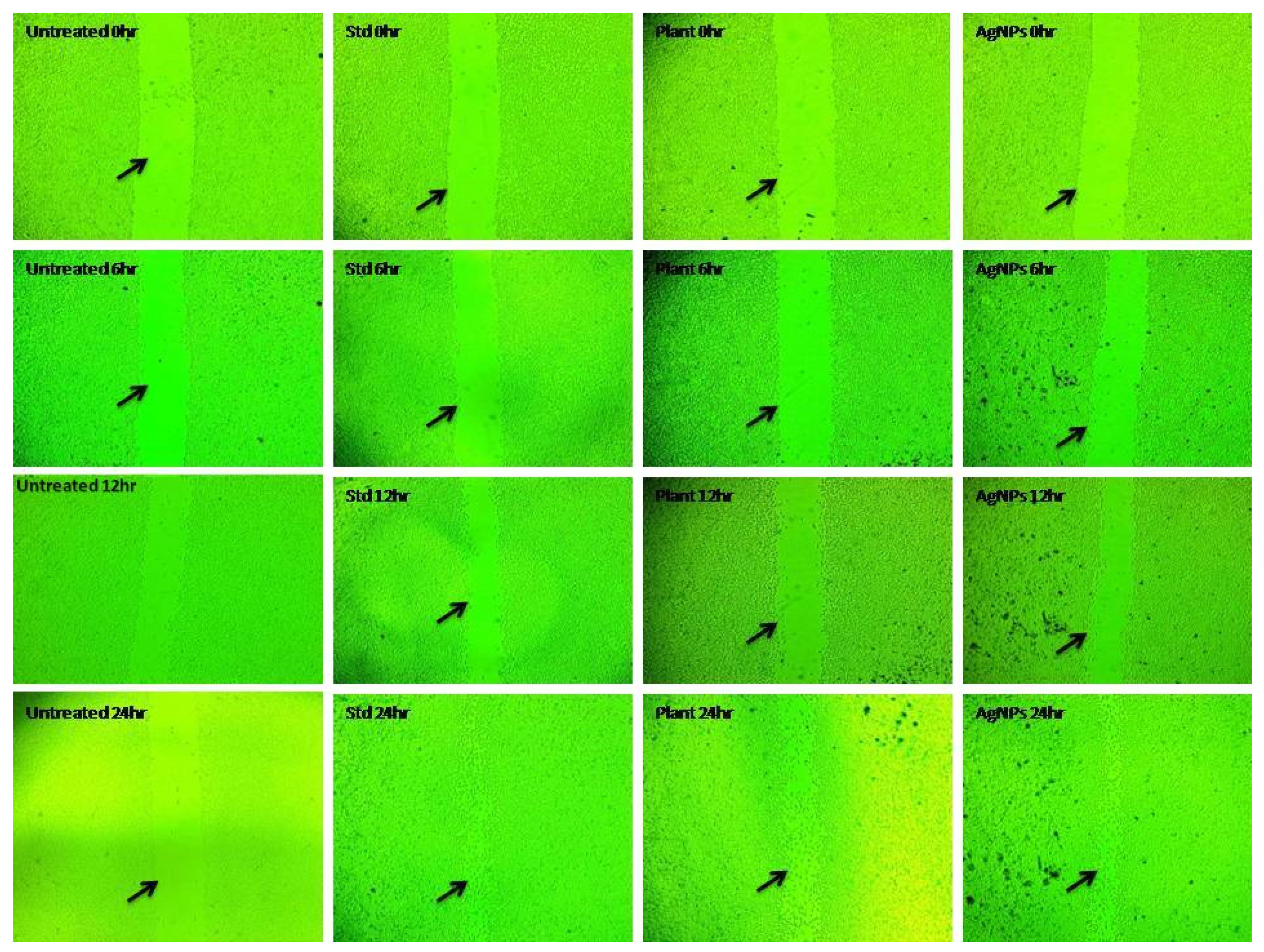
2.9. In Vitro Wound Healing Activity of Aqueous Leaf Extract of R. apiculata and Its Synthesized AgNPs
3. Discussion
4. Materials and Methods
4.1. Collection of Plant Material
4.2. Preparation of Plant Extract
4.3. Qualitative Analysis of Metabolites
4.4. Estimation of Total Phenolic Content
4.5. Estimation of Flavonoids Content
4.6. Synthesis of Silver Nanoparticles
4.7. Characterization of Newly Synthesized AgNPs
4.7.1. U.V.–Visible Spectroscopy-Based Analysis
4.7.2. FTIR-Based Analysis
4.7.3. Scanning-Electron-Microscopy-Based Analysis
4.7.4. Energy Dispersive X-ray
4.7.5. Zeta Potential Observations of N.P.s
4.7.6. Particle Size Analyzer
4.7.7. X-ray Diffraction (XRD) Analysis
4.8. In Vitro Measurement of Antioxidant Activity
4.8.1. Ferric Ion-Reducing Antioxidant Power Assay (FRAP)
4.8.2. Hydrogen Peroxide Scavenging Assay
4.8.3. DPPH Free-Radical-Scavenging Ability Assay
4.8.4. Phosphomolybdenum (PM) Assay
4.9. Evaluation of In Vitro Anti-Inflammatory Activity
4.10. Determination of the Cytotoxic Activity of Aqueous Leaf Extract of R. apiculata and Its Synthesized Silver Nanoparticles Using MTT Assay
4.11. In Vitro Wound Healing Study by Using Scratch Assay Test
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and Their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef]
- Muddapur, U.M.; Alshehri, S.; Ghoneim, M.M.; Mahnashi, M.H.; Alshahrani, M.A.; Khan, A.A.; Iqubal, S.M.S.; Bahafi, A.; More, S.S.; Shaikh, I.A.; et al. Plant-Based Synthesis of Gold Nanoparticles and Theranostic Applications: A Review. Molecules 2022, 27, 1391. [Google Scholar] [CrossRef]
- Cele, T. Preparation of Nanoparticles. In Engineered Nanomaterials—Health and Safety; IntechOpen: London, UK, 2020; ISBN 9781838804114. [Google Scholar]
- Adlim, A. Preparations and Application of Metal Nanoparticles. Indones. J. Chem. 2010, 6, 1–10. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Li, X.; Xu, H.; Chen, Z.-S.; Chen, G. Biosynthesis of Nanoparticles by Microorganisms and Their Applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Huang, Y.; Parsons, J.G.; Zhao, L.; Lopez-Moreno, L.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Plant-Based Green Synthesis of Metallic Nanoparticles: Scientific Curiosity or a Realistic Alternative to Chemical Synthesis? Nanotechnol. Environ. Eng. 2016, 1, 4. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A Review on Green Synthesis of Silver Nanoparticles and Their Applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Al-Rajhi, A.M.H.; Al Abboud, M.A.; Alawlaqi, M.M.; Ganash Magdah, A.; Helmy, E.A.M.; Mabrouk, A.S. Recent Advances in Green Synthesis of Silver Nanoparticles and Their Applications: About Future Directions. A Review. Bionanoscience 2018, 8, 5–16. [Google Scholar] [CrossRef]
- Raj, S.; Trivedi, R.; Soni, V. Biogenic Synthesis of Silver Nanoparticles, Characterization and Their Applications—A Review. Surfaces 2021, 5, 3. [Google Scholar] [CrossRef]
- Rahim, A.A.; Rocca, E.; Steinmetz, J.; Jain Kassim, M.; Sani Ibrahim, M.; Osman, H. Antioxidant Activities of Mangrove Rhizophora Apiculata Bark Extracts. Food Chem. 2008, 107, 200–207. [Google Scholar] [CrossRef]
- Baishya, S.; Banik, S.K.; Choudhury, M.D.; Das Talukdar, D.; Das Talukdar, A. Therapeutic Potentials of Littoral Vegetation: An Antifungal Perspective. In Biotechnological Utilization of Mangrove Resources; Elsevier: Berlin/Heidelberg, Germany, 2020; pp. 275–292. ISBN 9780128195321. [Google Scholar]
- Mitra, S.; Naskar, N.; Chaudhuri, P. A Review on Potential Bioactive Phytochemicals for Novel Therapeutic Applications with Special Emphasis on Mangrove Species. Phytomed. Plus 2021, 1, 100107. [Google Scholar] [CrossRef]
- Antony, J.J.; Sivalingam, P.; Siva, D.; Kamalakkannan, S.; Anbarasu, K.; Sukirtha, R.; Krishnan, M.; Achiraman, S. Comparative Evaluation of Antibacterial Activity of Silver Nanoparticles Synthesized Using Rhizophora Apiculata and Glucose. Colloids Surf. B Biointerfaces 2011, 88, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jacob, J.A.; Jiang, Z.; Xu, S.; Sun, K.; Zhong, Z.; Varadharaju, N.; Shanmugam, A. Hepatoprotective Effect of Silver Nanoparticles Synthesized Using Aqueous Leaf Extract of Rhizophora Apiculata. Int. J. Nanomed. 2019, 14, 3517–3524. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wang, Q.; Dai, T.; Shao, J.; Wu, X.; Jiang, Z.; Jacob, J.A.; Jiang, C. Identification of Possible Reductants in the Aqueous Leaf Extract of Mangrove Plant Rhizophora Apiculata for the Fabrication and Cytotoxicity of Silver Nanoparticles against Human Osteosarcoma MG-63 Cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111252. [Google Scholar] [CrossRef]
- Ramalingam, V.; Rajaram, R. Enhanced Antimicrobial, Antioxidant and Anticancer Activity of Rhizophora Apiculata: An Experimental Report. 3 Biotech 2018, 8, 200. [Google Scholar] [CrossRef]
- Alshabi, A.M.; Shaikh, I.A.; Asdaq, S.M.B. The Antiepileptic Potential of Vateria Indica Linn in Experimental Animal Models: Effect on Brain GABA Levels and Molecular Mechanisms. Saudi J. Biol. Sci. 2022, 29, 3600–3609. [Google Scholar] [CrossRef]
- Pandiarajan, J.; Krishnan, M. Properties, synthesis and toxicity of silver nanoparticles. Environ. Chem. Lett. 2017, 15, 387–397. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Daphne, J.; Francis, A.; Mohanty, R.; Ojha, N.; Das, N. Green synthesis of antibacterial silver nanoparticles using yeast isolates and its characterization. Res. J. Pharm. Technol. 2018, 11, 83–92. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef]
- Rashid, T.M.; Nayef, U.M.; Jabir, M.S.; Mutlak, F.A.-H. Synthesis and Characterization of Au:ZnO (Core:Shell) Nanoparticles via Laser Ablation. Optik 2021, 244, 167569. [Google Scholar] [CrossRef]
- Lotfi-Attari, J.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Alipour, S.; Farajzadeh, R.; Javidfar, S.; Zarghami, N. Co-delivery of curcumin and chrysin by polymeric nanoparticles inhibit synergistically growth and hTERT gene expression in human colorectal cancer cells. Nutr. Cancer 2017, 69, 1290–1299. [Google Scholar] [CrossRef]
- Firoozi, M.; Rezapour-Jahani, S.; Shahvegharasl, Z.; Anarjan, N. Ginger essential oil nanoemulsions: Preparation and physicochemical characterization and antibacterial activities evaluation. J. Food Process Eng. 2020, 43, e13434. [Google Scholar] [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Rajkumar, V.; Guha, G.; Kumar, R.A.; Lazar, M. Evaluation of antioxidant activities of Bergenia ciliata rhizome. Rec. Nat. Prod. 2010, 4, 38. [Google Scholar]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and AAnalysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Chung, J.H.; Seo, J.Y.; Choi, H.R.; Lee, M.K.; Youn, C.S.; Rhie, G.E.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J. Investig. Dermatol. 2001, 117, 1218–1224. [Google Scholar] [CrossRef]
- Roth, S.H. Coming to terms with nonsteroidal anti-inflammatory drug gastropathy. Drugs 2012, 72, 873–879. [Google Scholar] [CrossRef]
- Blomqvist, P.; Feltelius, N.; Ekbom, A.; Klareskog, L. Rheumatoid arthritis in Sweden. Drug prescriptions, costs, and adverse drug reactions. J. Reumatol. 2000, 27, 1171–1177. [Google Scholar]
- Ishiyama, M.; Tominaga, H.; Shiga, M.; Sasamoto, K.; Ohkura, Y.; Ueno, K. A combined assay of cell vability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol. Pharm. Bull. 1996, 19, 1518–1520. [Google Scholar] [CrossRef]
- Rufen, L.; Zhen, C.; Na, R.; Yixuan, W.; Yujia, W.; Fengye, Y. Biosynthesis of silver oxide nanoparticles and their photocatalytic and antimicrobial activity evaluation for wound healing applications in nursing care. J. Photochem. Photobiol. B 2019, 199, 111593. [Google Scholar]
- Suganya, S.; Ishwarya, S.; Jayakumar, S.; Govindarajan, M.; Alharbi, N.S.; Kadaikanna, S.; Khaled, J.M.; Al-Anbir, M.N.; Vaseeharan, B. New insecticides and antimicrobials derived from Sargassum wightii and Halimeda gracillis seaweeds: Toxicity against mosquito vectors and antibiofilm activity against microbial pathogens. S. Afr. J. Bot. 2019, 125, 466–480. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Malaikozhundan, B.; Saravanakumar, S.; Duran-Lara, E.F.; Wang, M.H.; Vaseeharan, B. Garlic clove extract assisted silver nanoparticle- Antibacterial, antibiofilm, antihelminthic, anti-inflammatory, anticancer and ecotoxicity assessment. J. Photochem. Photobiol. B Biol. 2019, 198, 111558. [Google Scholar] [CrossRef] [PubMed]
- Anna, R.F.; Małgorzata, K.L.; Victor, S.; Silvia, I.; Mnuel, A.; Agnieszka, K.; Grazyna, S. Development of noncytotoxic silver–chitosan nanocomposites for efficient control of biofilm forming microbes. RSC Adv. 2017, 7, 52398. [Google Scholar]
- Greinert, R.; de Vries, E.; Erdmann, F.; Espina, C.; Auvinen, A.; Kesminiene, A.; Schüz, J. European Code against Cancer 4th edition: Ultraviolet radiation and cancer. Cancer Epidemiol. 2015, 39, S75–S83. [Google Scholar] [CrossRef]
- Zhao, B.; He, Y.-Y. Recent advances in the prevention and treatment of skin cancer using photodynamic therapy. Expert Rev. Anticancer. Ther. 2010, 10, 1797–1809. [Google Scholar] [CrossRef]
- Zhao, Q.-H.; Zhang, Y.; Liu, Y.; Wang, H.-L.; Shen, Y.-Y.; Yang, W.-J.; Wen, L.-P. Anticancer effect of realgar nanoparticles on mouse melanoma skin cancer in vivo via transdermal drug delivery. Med. Oncol. 2009, 27, 203–212. [Google Scholar] [CrossRef]
- Malvin, S.S.; Sujin, J.; Selvarajan, E.; Thangavel, M.; Arivazhagan, P. Biosynthasized silver nanoparticle using Bacillus amyloliquefaciens: Application of cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol. J. Photochem. Photobiol. B Biol. 2020, 202, 111642. [Google Scholar]
- Rajivgandhi, G.; Muneeswaran, T.; Maruthupandy, M.; Ramakritinan, C.; Saravanan, K.; Ravikumar, V.; Manoharan, N. Antibacterial and anticancer potential of marine endophytic actinomycetes Streptomyces coeruleorubidus GRG 4 (KY457708) compound against colistin resistant uropathogens and A549 lung cancer cells. Microb. Pathog. 2018, 125, 325–335. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Lao, X.Q.; Ho, K.-F.; Goggins, W.B.; Tse, S.L.A. Incidence and mortality of lung cancer: Global trends and association with socioeconomic status. Sci. Rep. 2017, 7, 14300. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Brocatti, L.K.; Rustenbach, S.J.; Schäfer, I.; Herberger, K. Cost-ofillness of leg ulcers in the community. Int Wound J. 2014, 11, 283–292. [Google Scholar] [CrossRef]
- Moore, D. Hypochlorites: A review of the evidence. J. Wound Care 1992, 1, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Toppo, F.S.; Pawar, R.S. Novel drug delivery strategies and approaches for wound healing managements. J. Crit. Rev. 2015, 2, 12–20. [Google Scholar]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef]
- Veeraraghavan, V.P.; Periadurai, N.D.; Karunakaran, T.; Hussain, S.; Surapaneni, K.M.; Jiao, X. Green Synthesis of Silver Nanoparticles from Aqueous Extract of Scutellaria Barbata and Coating on the Cotton Fabric for Antimicrobial Applications and Wound Healing Activity in Fibroblast Cells (L929). Saudi J. Biol. Sci. 2021, 28, 3633–3640. [Google Scholar] [CrossRef]
- Nazem, A.; Mansoori, G.A. Nanotechnology solutions for Alzheimer’s disease: Advances in research tools, diagnostic methods and therapeutic agents. J. Alzheimer’s Dis. 2008, 13, 199–223. [Google Scholar] [CrossRef]
- Sanpui, P.; Chattopadhyay, A.; Ghosh, S.S. Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. Appl. Mater. Interfaces 2011, 3, 218–228. [Google Scholar] [CrossRef]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied. Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef]
- Haider, A.; Kang, I.K. Preparation of silver nanoparticles and their industrial and biomedical applications: A comprehensive review. Adv. Mater. Sci. Eng. 2015, 165, 257. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [PubMed]
- Ontong, J.C.; Paosen, S.; Shankar, S.; Voravuthikunchai, S.P. Eco-friendly synthesis of silver nanoparticles using Senna alata bark extract and its antimicrobial mechanism through enhancement of bacterial membrane degradation. J. Microbiol. Methods 2019, 165, 105692. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, O.; Puiu, R.A.; Bîrcă, A.C.; Burdușel, A.-C.; Grumezescu, A.M. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials 2020, 10, 2318. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Arif, R.; Uddin, R. A Review on Recent Developments in the Biosynthesis of Silver Nanoparticles and Its Biomedical Applications. Med. Devices Sens. 2021, 4, e10158. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Shaikh, I.A.; Muddapur, U.M.; Bagewadi, Z.K.; Chiniwal, S.; Ghoneim, M.M.; Mahnashi, M.H.; Alsaikhan, F.; Yaraguppi, D.; Niyonzima, F.N.; More, S.S.; et al. Characterization of Bioactive Compounds from Acacia concinna and Citrus limon, Silver Nanoparticles’ Production by A. concinna Extract, and Their Biological Properties. Molecules 2022, 27, 2715. [Google Scholar] [CrossRef]
- Kumar, R.; Roopan, S.M.; Prabhakarn, A.; Khanna, V.G.; Chakroborty, S. Agricultural waste Annona squamosa peel extract: Biosynthesis of silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 173–176. [Google Scholar] [CrossRef]
- Deepti, K.; Umadevi, P.; Vijayalakshmi, G. Antimicrobial activity and phytochemical analysis of Morinda tinctoria Roxb. leaf extracts. Asian Pac. J. Trop. Biomed. 2012, 2, S1440–S1442. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Kannan, R.R.R.; Stirk, W.A.; Van Staden, J. Synthesis of silver nanoparticles using the seaweed Codium capitatum P.C. Silva (Chlorophyceae). S. Afr. J. Bot. 2013, 86, 1–4. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J. Biol. Sci. 2017, 24, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.J.; Im, S.H.; Li, Z.Y.; McLellan, J.; Siekkinen, A.; Xia, Y. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J. Phys. Chem. B 2006, 110, 15666–15675. [Google Scholar] [CrossRef]
- Bahjat, H.H.; Ismail, R.A.; Sulaiman, G.M.; Jabir, M.S. Magnetic Field-Assisted Laser Ablation of Titanium Dioxide Nanoparticles in Water for Antibacterial Applications. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3649–3656. [Google Scholar] [CrossRef]
- Jabir, M.S.S.; Nayef, U.M.M.; Abdulkadhim, W.K.; Taqi, Z.J.J.; Sulaiman, G.M.; Sahib, U.I.I.; Al-Shammari, A.M.; Wu, Y.-J.; El-Shazly, M.; Su, C.-C. Fe3O4 Nanoparticles Capped with PEG Induce Apoptosis in Breast Cancer AMJ13 Cells via Mitochondrial Damage and Reduction of NF-ΚB Translocation. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1241–1259. [Google Scholar] [CrossRef]
- Meléndrez, M.F.; Cárdenas, G.; Arbiol, J. Synthesis and characterization of gallium colloidal nanoparticles. J. Colloid Interface Sci. 2010, 346, 279–287. [Google Scholar] [CrossRef]
- Oyaizu, M. Antioxidative activities of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Ruiz, J.C.R. Free Radical Scavenging Activity. PLoS ONE 2018, 1. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Jangle, S.N. Evaluation of in-vitro anti-inflammatory activity of herbal preparation, a combination of four medicinal plants. Int. J. Basic Appl. Med. Sci. 2012, 2, 109–116. [Google Scholar]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Fronza, M.; Heinzmann, B.; Hamburger, M.; Laufer, S.; Merfort, I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J. Ethnopharmacol. 2009, 126, 463–467. [Google Scholar] [CrossRef] [PubMed]




| Tests | Water Extract |
|---|---|
| Alkaloids | − |
| Flavonoids | + |
| Glycosides | + |
| Phenols | + |
| Saponins | + |
| Tannins | − |
| Terpenoids | + |
| Steroids | − |
| Sl.No | Concentration (µL/mL) | Optical Density at 700 nm | ||
|---|---|---|---|---|
| Std Ascorbic Acid | R. apiculata Extract | AgNPs | ||
| 1 | 100 | 0.46 ± 0.003 | 0.44 ± 0.003 | 0.60 ± 0.036 |
| 2 | 200 | 0.75 ± 0.004 | 0.48 ± 0.003 | 0.80 ± 0.036 |
| 3 | 300 | 0.99 ± 0.004 | 0.53 ± 0.005 | 1.03 ± 0.054 |
| 4 | 400 | 1.13 ± 0.004 | 0.63 ± 0.003 | 1.51 ± 0.017 |
| 5 | 500 | 1.46 ± 0.003 | 0.86 ± 0.025 | 1.81 ± 0.025 * |
| Sl. No | Concentration (µg/mL) | Samples | Percentage (%) Inhibition |
|---|---|---|---|
| 1 | 100 | Standard | 74.46 ± 0.13 * |
| 2 | 100 | R. apiculata extract | 63.58 ± 0.44 * |
| 3 | 100 | AgNPs | 74.98 ± 0.31 * |
| Sl.no | Concentration (µg/mL) | Percentage Inhibition | ||
|---|---|---|---|---|
| Std Ascorbic Acid | R. apiculata Extract | AgNPs | ||
| 1 | 10 | 67.78 ± 0.17 | 43.56 ± 0.70 | 51.04 ± 1.42 |
| 2 | 20 | 73.65 ± 0.23 | 46.27 ± 0.23 | 55.34 ± 0.98 |
| 3 | 30 | 78.20 ± 0.30 | 63.64 ± 0.35 | 63.48 ± 1.33 |
| 4 | 40 | 80.80 ± 0.35 | 72.9833 ± 0.66 | 66.39 ± 0.70 |
| 5 | 50 | 83.91 ± 0.35 | 77.36 ± 0.35 | 76.74 ± 0.76 * |
| Sl.no | Concentration (µg/mL) | Optical Density at 695 nm | ||
|---|---|---|---|---|
| Std Ascorbic Acid | R. apiculata Extract | AgNPs | ||
| 1 | 100 | 0.27 ± 0.004 | 0.52 ± 0.002 | 0.71 ± 0.039 |
| 2 | 200 | 0.50 ± 0.004 | 0.79 ± 0.013 | 0.95 ± 0.008 |
| 3 | 300 | 0.68 ± 0.004 | 0.92 ± 0.011 | 1.12 ± 0.044 |
| 4 | 400 | 0.90 ± 0.003 | 1.07 ± 0.001 | 1.37 ± 0.042 |
| 5 | 500 | 1.13 ± 0.007 | 1.27 ± 0.004 | 1.44 ± 0.004 * |
| Sl. No. | Concentration (µg/mL) | Treatment | % Inhibition |
|---|---|---|---|
| 1 | 100 | Standard | 94.24 ± 1.90 * |
| 2 | 500 | AgNPs | 71.65 ± 0.88 * |
| 3 | 500 | R. apiculata extract | 54.34 ± 3.26 * |
| Samples | Concentration in µg/mL | Percentage of Cell Viability | IC50 in µg/mL |
|---|---|---|---|
| R. apiculata extract | 10 | 97.44 ± 0.001 | 47.47 |
| 20 | 84.03 ± 0.005 | ||
| 30 | 73.55 ± 0.001 | ||
| 40 | 58.65 ± 0.001 | ||
| 50 | 46.81 ± 0.002 | ||
| AgNPs | 10 | 98.57 ± 0.005 | 105.50 |
| 20 | 92.47 ± 0.001 | ||
| 30 | 90.22 ± 0.001 | ||
| 40 | 83.69 ± 0.005 | ||
| 50 | 77.50 ± 0.005 |
| Samples | Concentration in µg/mL | Percentage of Cell Viability for A375 | Percentage of Cell Viability for A549 | Percentage of Cell Viability for KB-3-1 |
|---|---|---|---|---|
| R. apiculata extract | 10 | 99.72 ± 0.001 | 99.07 ± 0.003 | 92.20 ± 0.005 |
| 20 | 97.35 ± 0.001 | 92.72 ± 0.001 | 88.59 ± 0.015 | |
| 30 | 91.05 ± 0.001 | 87.36 ± 0.002 | 81.15 ± 0.002 | |
| 40 | 87.75 ± 0.001 | 83.22 ± 0.003 | 75.51 ± 0.005 | |
| 50 | 82.69 ± 0.002 | 73.73 ± 0.002 | 67.17 ± 0.001 | |
| AgNPs | 10 | 86.01 ± 0.005 | 95.56 ± 0.009 | 70.95 ± 0.005 |
| 20 | 65.60 ± 0.002 | 88.58 ± 0.002 | 58.12 ± 0.005 | |
| 30 | 54.49 ± 0.002 | 74.76 ± 0.001 | 43.16 ± 0.010 | |
| 40 | 44.46 ± 0.005 | 67.95± 0.004 | 33.69 ± 0.002 | |
| 50 | 31.84 ± 0.004 | 56.09 ± 0.010 | 22.59 ± 0.022 |
| Sl.No | Test Sample | Duration | Cell Migration in µm |
|---|---|---|---|
| 1 | Untreated | 6 h | 2.96 |
| 12 h | 2.50 | ||
| 24 h | 2.07 | ||
| 2 | Ascorbic acid | 6 h | 25.47 |
| 12 h | 28.03 | ||
| 24 h | 21.74 | ||
| 3 | R. apiculata extract | 6 h | 11.63 |
| 12 h | 14.58 | ||
| 24 h | 18.74 | ||
| 4 | AgNPs | 6 h | 14.43 |
| 12 h | 20.56 | ||
| 24 h | 18.23 |
| Sl.No | Test Sample | Percentage of Wound Closure at 24 h |
|---|---|---|
| 1 | Untreated | 9.13 |
| 2 | Standard drug Ascorbic acid | 96.26 |
| 3 | R. apiculata extract | 75.23 |
| 4 | AgNPs | 82.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsareii, S.A.; Manaa Alamri, A.; AlAsmari, M.Y.; Bawahab, M.A.; Mahnashi, M.H.; Shaikh, I.A.; Shettar, A.K.; Hoskeri, J.H.; Kumbar, V. Synthesis and Characterization of Silver Nanoparticles from Rhizophora apiculata and Studies on Their Wound Healing, Antioxidant, Anti-Inflammatory, and Cytotoxic Activity. Molecules 2022, 27, 6306. https://doi.org/10.3390/molecules27196306
Alsareii SA, Manaa Alamri A, AlAsmari MY, Bawahab MA, Mahnashi MH, Shaikh IA, Shettar AK, Hoskeri JH, Kumbar V. Synthesis and Characterization of Silver Nanoparticles from Rhizophora apiculata and Studies on Their Wound Healing, Antioxidant, Anti-Inflammatory, and Cytotoxic Activity. Molecules. 2022; 27(19):6306. https://doi.org/10.3390/molecules27196306
Chicago/Turabian StyleAlsareii, Saeed Ali, Abdulrahman Manaa Alamri, Mansour Yousef AlAsmari, Mohammed A. Bawahab, Mater H. Mahnashi, Ibrahim Ahmed Shaikh, Arun K. Shettar, Joy H. Hoskeri, and Vijay Kumbar. 2022. "Synthesis and Characterization of Silver Nanoparticles from Rhizophora apiculata and Studies on Their Wound Healing, Antioxidant, Anti-Inflammatory, and Cytotoxic Activity" Molecules 27, no. 19: 6306. https://doi.org/10.3390/molecules27196306
APA StyleAlsareii, S. A., Manaa Alamri, A., AlAsmari, M. Y., Bawahab, M. A., Mahnashi, M. H., Shaikh, I. A., Shettar, A. K., Hoskeri, J. H., & Kumbar, V. (2022). Synthesis and Characterization of Silver Nanoparticles from Rhizophora apiculata and Studies on Their Wound Healing, Antioxidant, Anti-Inflammatory, and Cytotoxic Activity. Molecules, 27(19), 6306. https://doi.org/10.3390/molecules27196306






