Methanol Oxidation at Platinum Coated Black Titania Nanotubes and Titanium Felt Electrodes
Abstract
1. Introduction
2. Results
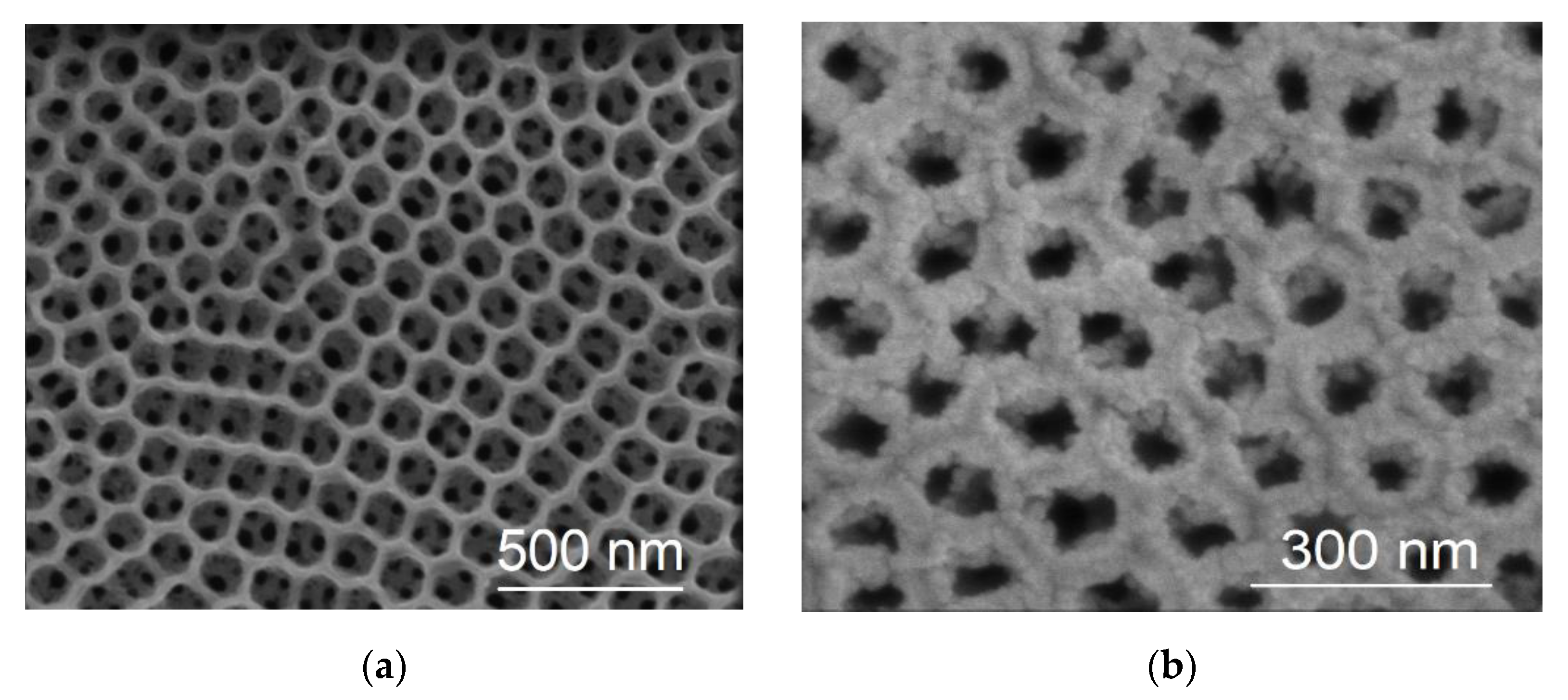
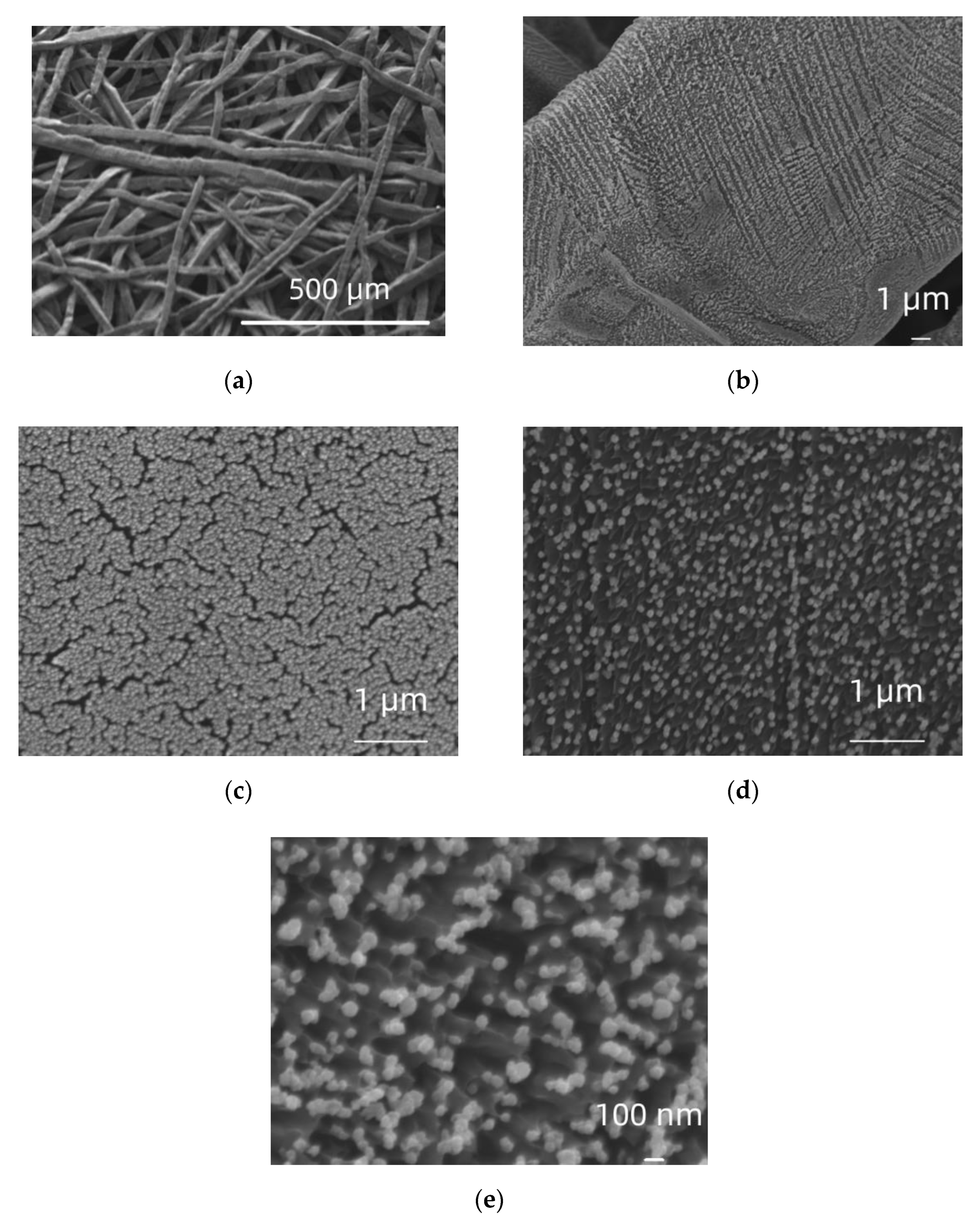
2.1. Microscopic (SEM) and Spectroscopic (EDS, ICP-MS) Characterization
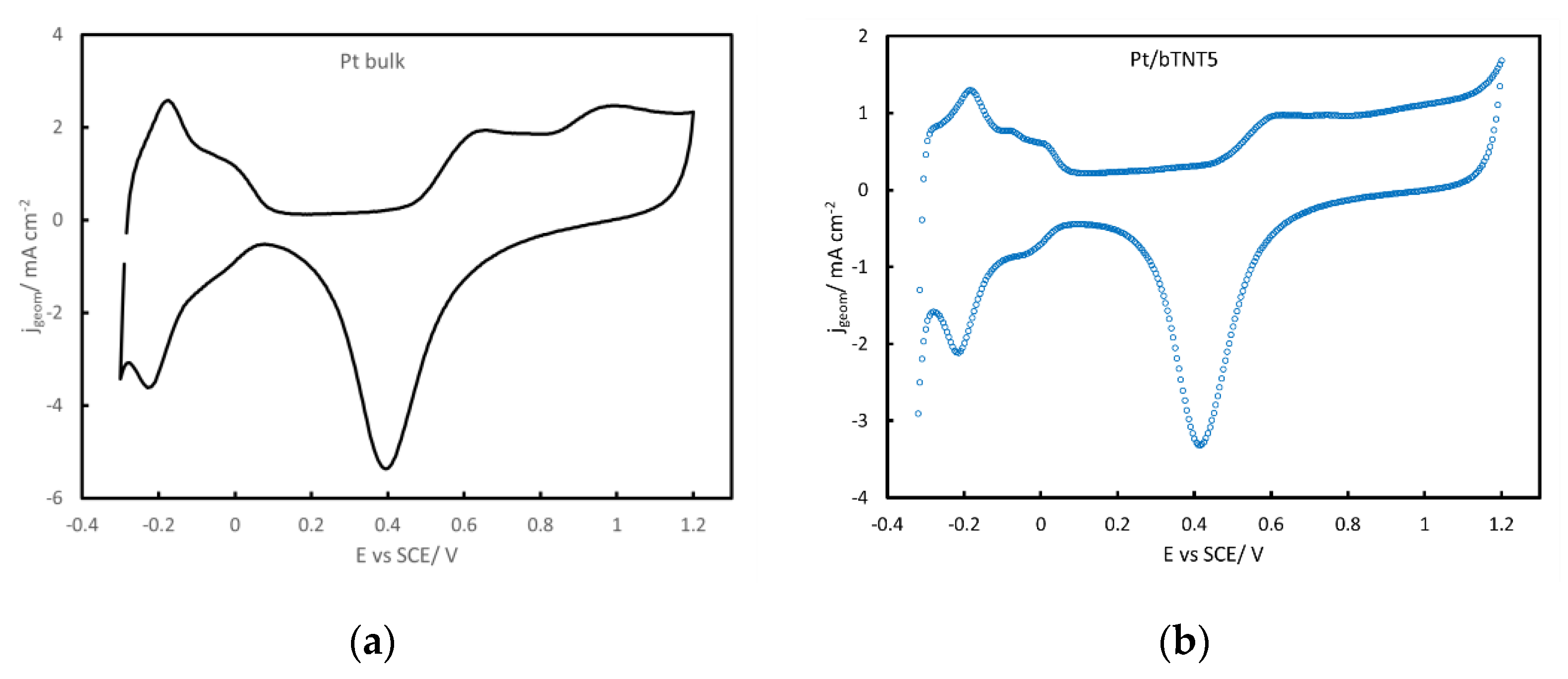
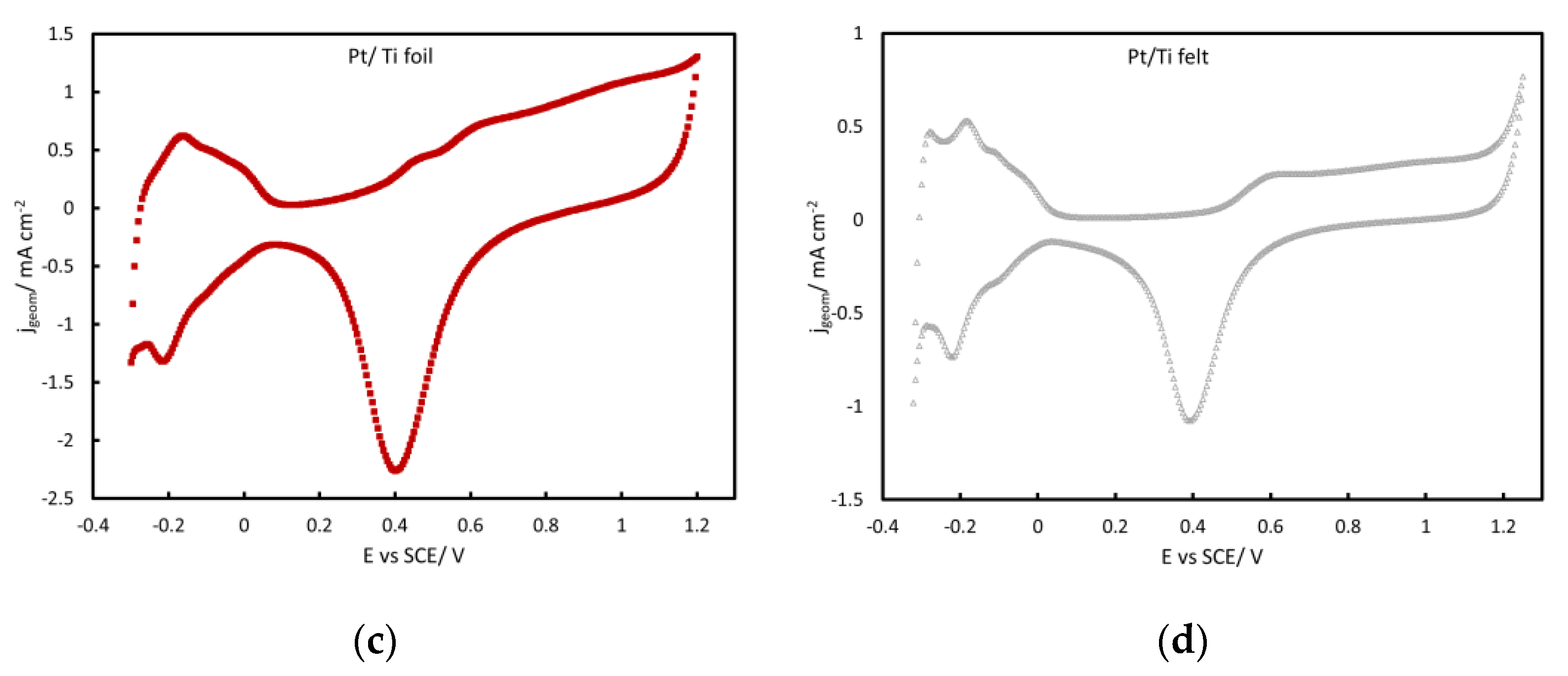
2.2. Surface Electrochemistry in Acid
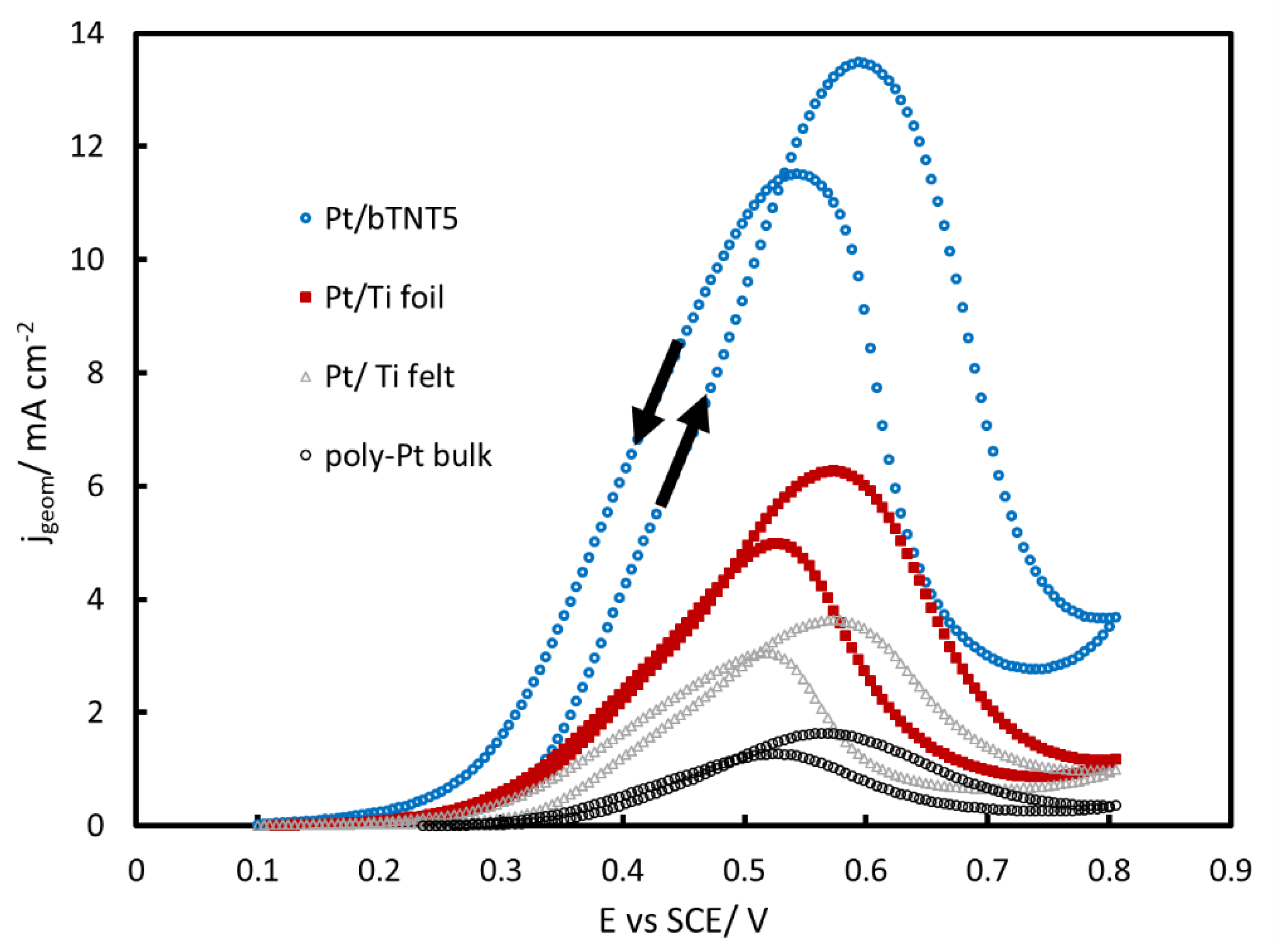
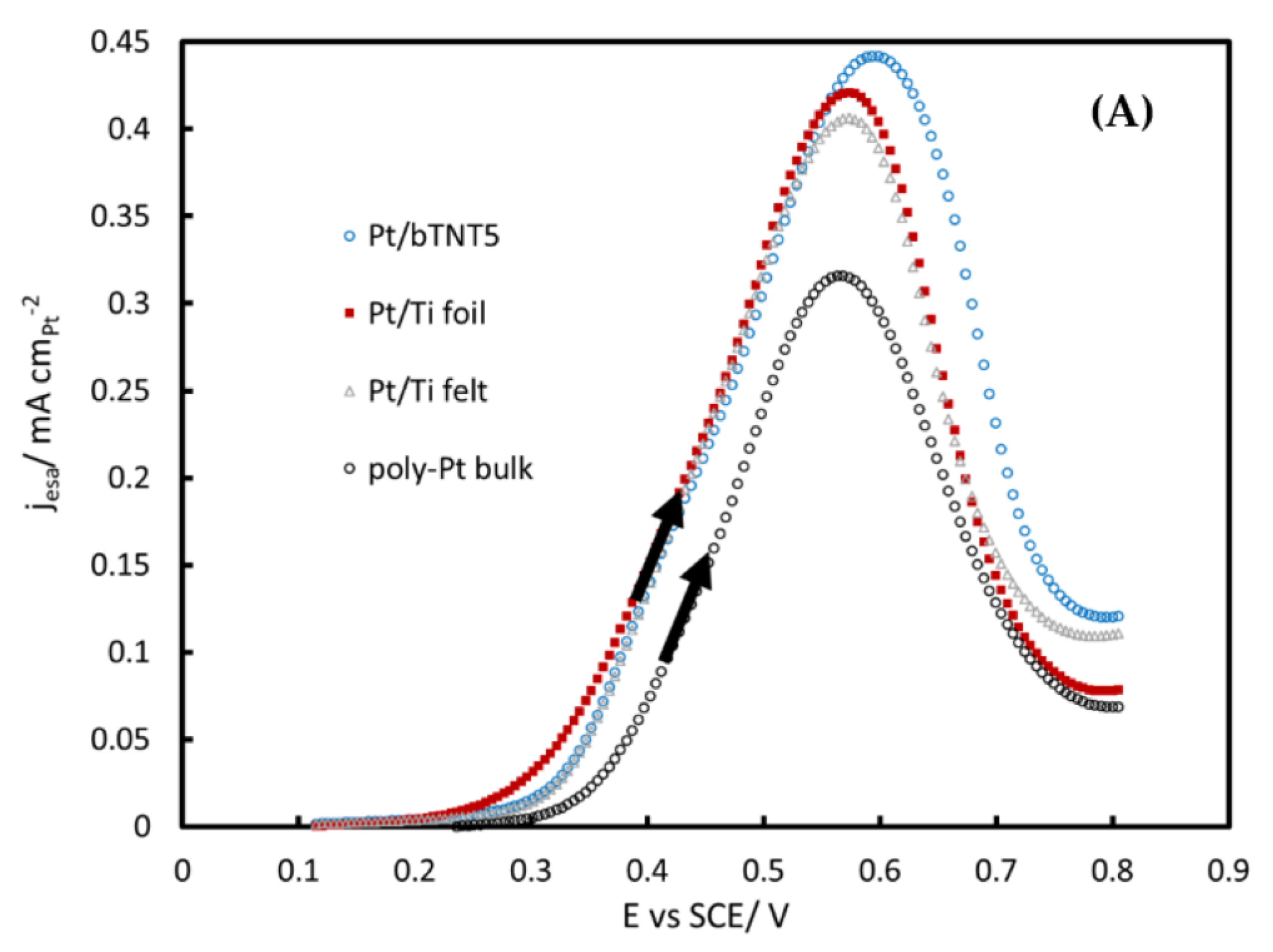
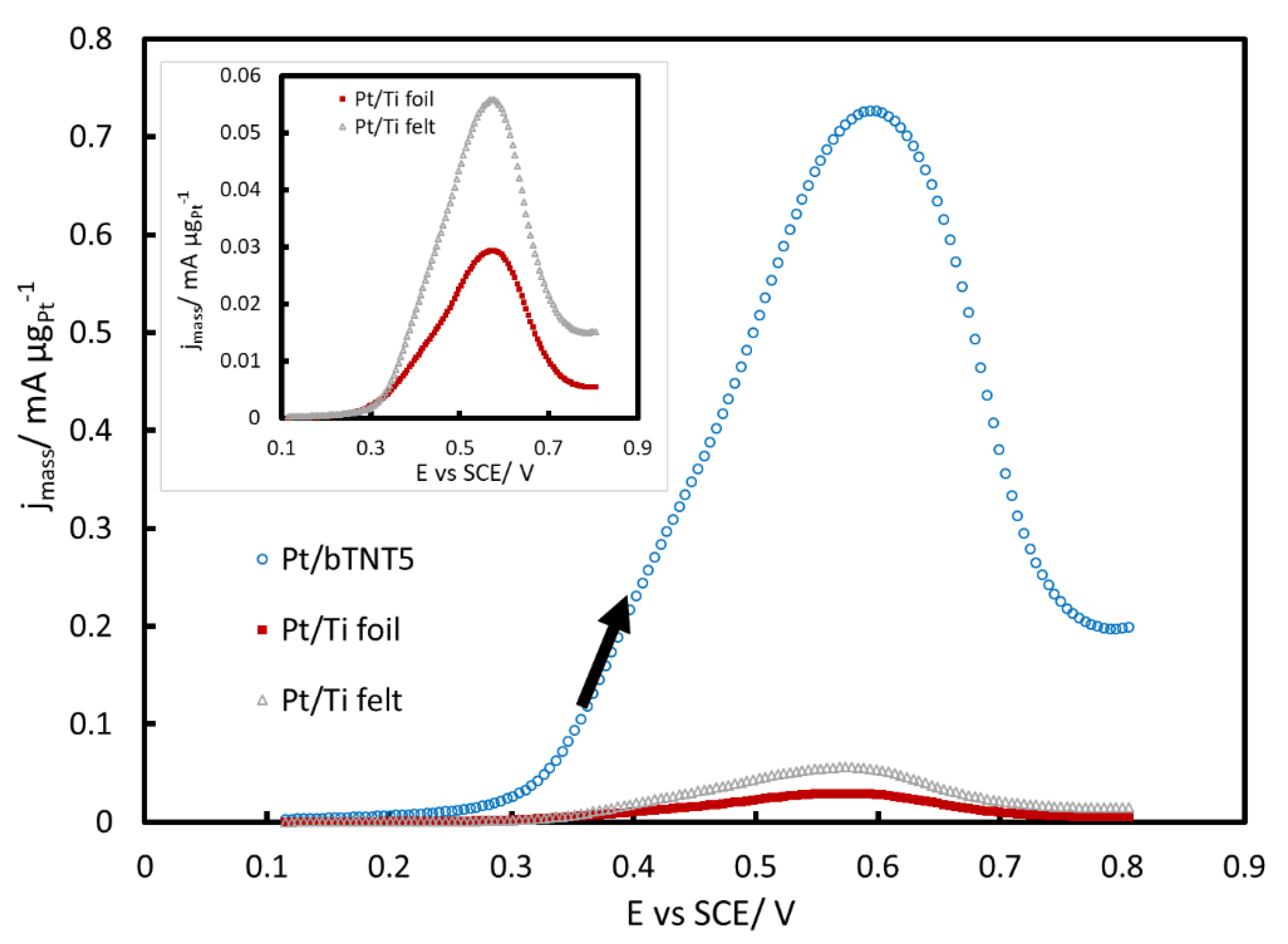
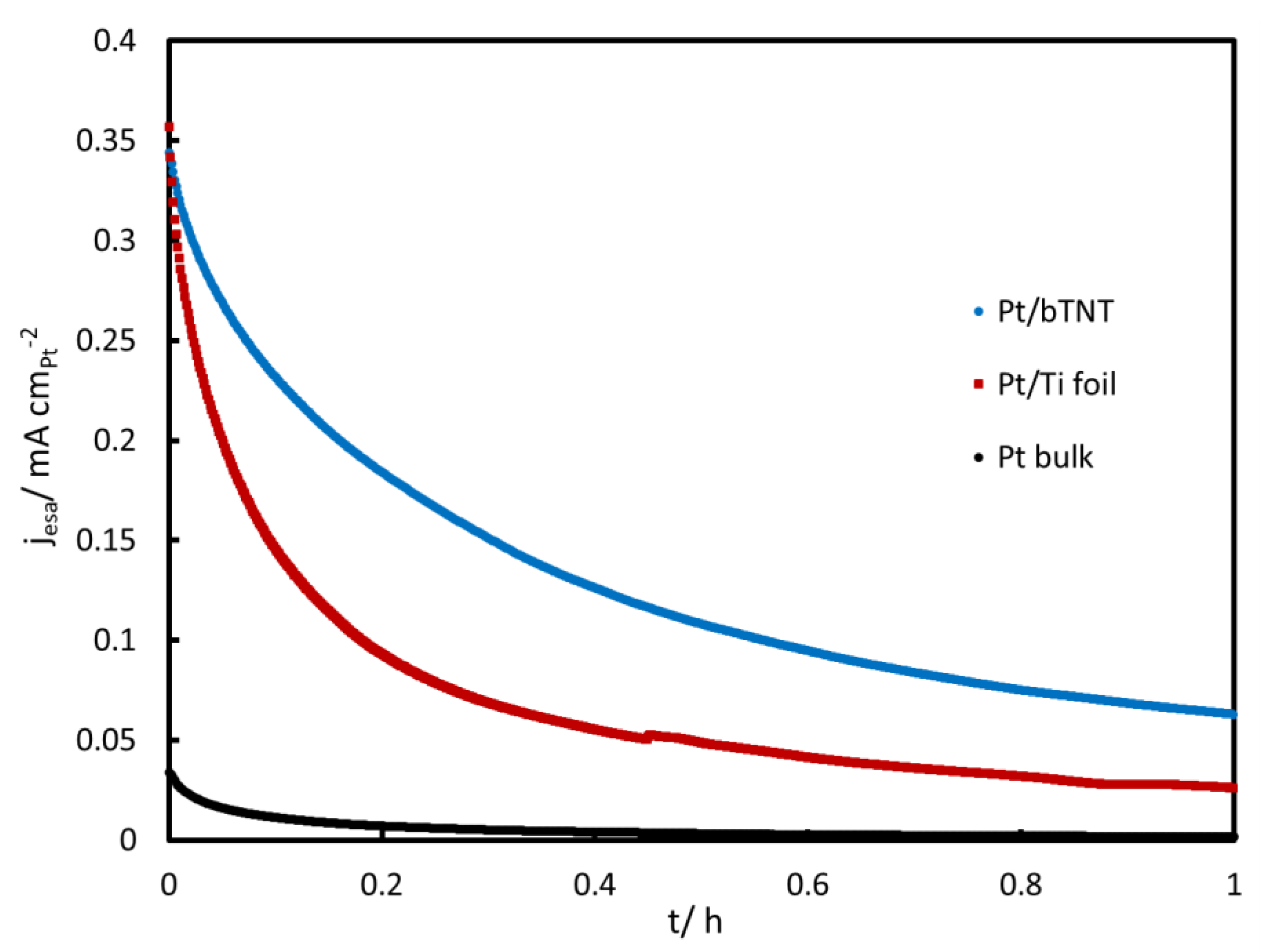
2.3. MOR in Acid
3. Discussion
3.1. Deposit Formation Mechanism and Morphology
3.2. Pt loading and Electroactive Surface Area
3.3. MOR in Acid
- Oxidative chemisorption/dehydrogenationPt + CH3OH → Pt-CH3Oads + H+ + e−Pt-CH3Oads → Pt-CHOads + 2H+ + 2e−
- Poison formationPt-CHOads → Pt-COads + H+ + e−
- Reactive path (where M denotes Pt or second metal or reduced metal oxide)M + H2O → M-OHads + H+ + e−Pt-CHOads + M-OHads → Pt-COOHads + M + H+ + e−Pt-COOHads → Pt + CO2 + H+ + e−
- Poison removalM + H2O → M-OHads + H+ + e−Pt-COads + M-OHads → Pt + M + CO2 + H+ + e−
4. Materials and Methods
4.1. Electrodes Preparation
4.2. Microscopy and Spectroscopy
4.3. Electrochemical Setup
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- She, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining Theory and Experiment in Electrocatalysis: Insights into Materials Design. Science 2017, 355, 146. [Google Scholar] [CrossRef]
- Yuda, A.; Ashok, A.; Kumar, A. A Comprehensive and Critical Review on Recent Progress in Anode Catalyst for Methanol Oxidation Reaction. Catal. Rev. Sci. Eng. 2022, 64, 126–228. [Google Scholar] [CrossRef]
- Siwal, S.S.; Thakur, S.; Zhang, Q.B.; Thakur, V.K. Electrocatalysts for Electrooxidation of Direct Alcohol Fuel Cell: Chemistry and Applications. Mater. Today Chem. 2019, 14, 100182. [Google Scholar] [CrossRef]
- Lamy, C.; Leger, J.M.; Srinivasan, S. Modern Aspects of Electrochemistry; Bockris, J.O., Conway, B.E., Eds.; Plenum Press: New York, NY, USA, 2000; Volume 34, p. 35. [Google Scholar]
- Hamnett, A. Handbook of Fuel Cells: Funtamentals Technology and Applications; Vielstich, W., Lamm, A., Gasteiger, H.A., Eds.; Wiley: Chichester, UK, 2003; Volume 1, p. 305. [Google Scholar]
- Ong, B.C.; Kamarudin, S.K.; Basri, S. Direct Liquid Fuel Cells: A Review. Int. J. Hydrogen Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, X.; Sun, H.; Wang, S.; Sun, G. Recent Advances in Multi-Scale Design and Construction of Materials for Direct Methanol Fuel Cells. Nano Energy 2019, 65, 104048. [Google Scholar] [CrossRef]
- Alias, M.S.; Kamarudin, S.K.; Zainoodin, A.M.; Masdar, M.S. Active Direct Methanol Fuel Cell: An Overview. Int. J. Hydrogen Energy 2020, 45, 19620–19641. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K.; Bahru, R.; Osman, S.H.; Md Ishak, N.A.I. Progress and Challenges: Review for Direct Liquid Fuel Cell. Int. J. Energy Res. 2021, 45, 6644–6688. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Shen, J.; Hu, Z. Micro Direct Methanol Fuel Cell: Functional Components, Supplies Management, Packaging Technology and Application. Int. J. Energy Res. 2017, 41, 613–627. [Google Scholar] [CrossRef]
- Dillon, R.; Srinivasan, S.; Aricò, A.S.; Antonucci, V. International Activities in DMFC R&D: Status of Technologies and Potential Applications. J. Power Sources 2004, 127, 112–126. [Google Scholar] [CrossRef]
- Aricò, A.S.; Srinivasan, S.; Antonucci, V. DMFCs: From Fundamental Aspects to Technology Development. Fuel Cells 2001, 1, 133–161. [Google Scholar] [CrossRef]
- Take, T.; Tsurutani, K.; Umeda, M. Hydrogen Production by Methanol-Water Solution Electrolysis. J. Power Sources 2007, 164, 9–16. [Google Scholar] [CrossRef]
- Uhm, S.; Jeon, H.; Kim, T.J.; Lee, J. Clean Hydrogen Production from Methanol-Water Solutions via Power-Saved Electrolytic Reforming Process. J. Power Sources 2012, 198, 218–222. [Google Scholar] [CrossRef]
- Chen, L.; Shi, J. Chemical-Assisted Hydrogen Electrocatalytic Evolution Reaction (CAHER). J. Mater. Chem. A 2018, 6, 13538–13548. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, M.; Wang, M.; Ren, T.; Ren, K.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Methanol Electroreforming Coupled to Green Hydrogen Production over Bifunctional NiIr-Based Metal-Organic Framework Nanosheet Arrays. Appl. Catal. B Environ. 2022, 300, 120753. [Google Scholar] [CrossRef]
- Hasa, B.; Vakros, J.; Katsaounis, A.D. Effect of TiO2 on Pt-Ru-Based Anodes for Methanol Electroreforming. Appl. Catal. B Environ. 2018, 237, 811–816. [Google Scholar] [CrossRef]
- Caravaca, A.; De Lucas-Consuegra, A.; Calcerrada, A.B.; Lobato, J.; Valverde, J.L.; Dorado, F. From Biomass to Pure Hydrogen: Electrochemical Reforming of Bio-Ethanol in a PEM Electrolyser. Appl. Catal. B Environ. 2013, 134–135, 302–309. [Google Scholar] [CrossRef]
- Chen, Y.X.; Lavacchi, A.; Miller, H.A.; Bevilacqua, M.; Filippi, J.; Innocenti, M.; Marchionni, A.; Oberhauser, W.; Wang, L.; Vizza, F. Nanotechnology Makes Biomass Electrolysis More Energy Efficient than Water Electrolysis. Nat. Commun. 2014, 5, 4036. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Tsampas, M.N.; Fredriksson, H.O.A.; Gracia, J.M.; Niemantsverdriet, J.W. Hydrogen from Electrochemical Reforming of C1–C3 Alcohols Using Proton Conducting Membranes. Int. J. Hydrogen Energy 2017, 42, 10762–10774. [Google Scholar] [CrossRef]
- Araujo, R.B.; Martín-Yerga, D.; dos Santos, E.C.; Cornell, A.; Pettersson, L.G.M. Elucidating the Role of Ni to Enhance the Methanol Oxidation Reaction on Pd Electrocatalysts. Electrochim. Acta 2020, 360, 136954. [Google Scholar] [CrossRef]
- Mekazni, D.S.; Arán-Ais, R.M.; Ferre-Vilaplana, A.; Herrero, E. Why Methanol Electro-Oxidation on Platinum in Water Takes Place Only in the Presence of Adsorbed OH. ACS Catal. 2022, 12, 1965–1970. [Google Scholar] [CrossRef]
- Ferrin, P.; Mavrikakis, M. Structure Sensitivity of Methanol Electrooxidation on Transition Metals. J. Am. Chem. Soc. 2009, 131, 14381–14389. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.C.S.; Lebedeva, N.P.; Housmans, T.H.M.; Koper, M.T.M. Mechanisms of Carbon Monoxide and Methanol Oxidation at Single-Crystal Electrodes. Top. Catal. 2007, 46, 320–333. [Google Scholar] [CrossRef]
- Lamy, C.; Lima, A.; LeRhun, V.; Delime, F.; Coutanceau, C.; Léger, J.M. Recent Advances in the Development of Direct Alcohol Fuel Cells (DAFC). J. Power Sources 2002, 105, 283–296. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Singh, G.; Kim, K.S. Recent Progress in the Development of Anode and Cathode Catalysts for Direct Methanol Fuel Cells. Nano Energy 2013, 2, 553–578. [Google Scholar] [CrossRef]
- Das, S.; Dutta, K.; Shul, Y.G.; Kundu, P.P. Progress in Developments of Inorganic Nanocatalysts for Application in Direct Methanol Fuel Cells. Crit. Rev. Solid State Mater. Sci. 2015, 40, 316–357. [Google Scholar] [CrossRef]
- Liu, H.; Song, C.; Zhang, L.; Zhang, J.; Wang, H.; Wilkinson, D.P. A Review of Anode Catalysis in the Direct Methanol Fuel Cell. J. Power Sources 2006, 155, 95–110. [Google Scholar] [CrossRef]
- Ramli, Z.A.C.; Kamarudin, S.K. Platinum-Based Catalysts on Various Carbon Supports and Conducting Polymers for Direct Methanol Fuel Cell Applications: A Review. Nanoscale Res. Lett. 2018, 13, 410. [Google Scholar] [CrossRef]
- Ehelebe, K.; Schmitt, N.; Sievers, G.; Jensen, A.W.; Hrnjić, A.; Jiménez, P.C.; Kaiser, P.; Geuß, M.; Ku, Y.P.; Jovanovič, P.; et al. Benchmarking Fuel Cell Electrocatalysts Using Gas Diffusion Electrodes: Inter-Lab Comparison and Best Practices. ACS Energy Lett. 2022, 7, 816–826. [Google Scholar] [CrossRef]
- Yu, E.H.; Scott, K.; Reeve, R.W.; Yang, L.; Allen, R.G. Characterisation of Platinised Ti Mesh Electrodes Using Electrochemical Methods: Methanol Oxidation in Sodium Hydroxide Solutions. Electrochim. Acta 2004, 49, 2443–2452. [Google Scholar] [CrossRef]
- Cheng, T.T.; Gyenge, E.L. Direct Methanol Fuel Cells with Reticulated Vitreous Carbon, Uncompressed Graphite Felt and Ti Mesh Anodes. J. Appl. Electrochem. 2008, 38, 51–62. [Google Scholar] [CrossRef]
- Chen, C.S.; Pan, F.M. Electrocatalytic Activity of Pt Nanoparticles Deposited on Porous TiO2 Supports toward Methanol Oxidation. Appl. Catal. B Environ. 2009, 91, 663–669. [Google Scholar] [CrossRef]
- Georgieva, J.; Valova, E.; Mintsouli, I.; Sotiropoulos, S.; Tatchev, D.; Armyanov, S.; Hubin, A.; Dille, J.; Hoell, A.; Raghuwanshi, V.; et al. Pt(Ni) Electrocatalysts for Methanol Oxidation Prepared by Galvanic Replacement on TiO2 and TiO2-C Powder Supports. J. Electroanal. Chem. 2015, 754, 65–74. [Google Scholar] [CrossRef]
- Dimitrova, N.; Georgieva, J.; Sotiropoulos, S.; Boiadjieva-Scherzer, T.Z.; Valova, E.; Armyanov, S.; Steenhaut, O.; Hubin, A.; Karashanova, D. Pt(Cu) Catalyst on TiO2 Powder Support Prepared by Photodeposition-Galvanic Replacement Method. J. Electroanal. Chem. 2018, 823, 624–632. [Google Scholar] [CrossRef]
- Papaderakis, A.; Spyridou, O.; Karanasios, N.; Touni, A.; Banti, A.; Dimitrova, N.; Armyanov, S.; Valova, E.; Georgieva, J.; Sotiropoulos, S. The Effect of Carbon Cotent on Methanol Oxidation and Photo-Oxidation at Pt-TiO2-C Electrodes. Catalysts 2020, 10, 248. [Google Scholar] [CrossRef]
- Yang, L.; Xiao, Y.; Zeng, G.; Luo, S.; Kuang, S.; Cai, Q. Fabrication and Characterization of Pt/C—TiO2 Nanotube Arrays as Anode Materials for Methanol Electrocatalytic Oxidation. Energy Fuels 2009, 23, 3134–3138. [Google Scholar] [CrossRef]
- Xing, L.; Jia, J.; Wang, Y.; Zhang, B.; Dong, S. Pt Modified TiO2 Nanotubes Electrode: Preparation and Electrocatalytic Application for Methanol Oxidation. Int. J. Hydrogen Energy 2010, 35, 12169–12173. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wei, Z.D.; Gao, B.; Qi, X.Q.; Li, L.; Zhang, Q.; Xia, M.R. The Electrochemical Oxidation of Methanol on a Pt/TNTs/Ti Electrode Enhanced by Illumination. J. Power Sources 2011, 196, 1132–1135. [Google Scholar] [CrossRef]
- Sui, X.L.; Wang, Z.B.; Yang, M.; Huo, L.; Gu, D.M.; Yin, G.P. Investigation on C-TiO2 Nanotubes Composite as Pt Catalyst Support for Methanol Electrooxidation. J. Power Sources 2014, 255, 43–51. [Google Scholar] [CrossRef]
- Cao, H.; Huang, K.; Wu, L.; Hou, G.; Tang, Y.; Zheng, G. Enhanced Catalytic Performance of Pt/TNTs Composite Electrode by Reductive Doping of TNTs. Appl. Surf. Sci. 2016, 364, 257–263. [Google Scholar] [CrossRef]
- Anitha, V.C.; Zazpe, R.; Krbal, M.; Yoo, J.E.; Sopha, H.; Prikryl, J.; Cha, G.; Slang, S.; Schmuki, P.; Macak, J.M. Anodic TiO2 Nanotubes Decorated by Pt Nanoparticles Using ALD: An Efficient Electrocatalyst for Methanol Oxidation. J. Catal. 2018, 365, 86–93. [Google Scholar] [CrossRef]
- Han, J.; Yang, L.; Yang, L.; Jiang, W.; Luo, X.; Luo, S. PtRu Nanoalloys Loaded on Graphene and TiO2 Nanotubes Co-Modified Ti Wire as an Active and Stable Methanol Oxidation Electrocatalyst. Int. J. Hydrogen Energy 2018, 43, 7338–7346. [Google Scholar] [CrossRef]
- Abdel Rahim, M.A.A.; Hassan, H.B. Titanium and Platinum Modified Titanium Electrodes as Catalysts for Methanol Electro-Oxidation. Thin Solid Film. 2009, 517, 3362–3369. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Tang, B.; Lin, N.; Hou, H.; Ma, Y. A Facile Preparation of Novel Pt-Decorated Ti Electrode for Methanol Electro-Oxidation by High-Energy Micro-Arc Cladding Technique. J. Power Sources 2013, 230, 81–88. [Google Scholar] [CrossRef]
- Hassan, H.B. Electrodeposited Pt and Pt-Sn Nanoparticles on Ti as Anodes for Direct Methanol Fuel Cells. J. Fuel Chem. Technol. 2009, 37, 346–354. [Google Scholar] [CrossRef]
- Ting, C.C.; Liu, C.H.; Tai, C.Y.; Hsu, S.C.; Chao, C.S.; Pan, F.M. The Size Effect of Titania-Supported Pt Nanoparticles on the Electrocatalytic Activity towards Methanol Oxidation Reaction Primarily via the Bifunctional Mechanism. J. Power Sources 2015, 280, 166–172. [Google Scholar] [CrossRef]
- Abraham, B.G.; Chetty, R. Influence of Electrodeposition Techniques and Parameters towards the Deposition of Pt Electrocatalysts for Methanol Oxidation. J. Appl. Electrochem. 2021, 51, 503–520. [Google Scholar] [CrossRef]
- Lamy, C. Electrocatalytic Oxidation of Low Weight Oxygenated Organic Compounds: A Review on Their Use as a Chemical Source to Produce Either Electricity in a Direct Oxidation Fuel Cell or Clean Hydrogen in an Electrolysis Cell. J. Electroanal. Chem. 2020, 875, 114426. [Google Scholar] [CrossRef]
- Touni, A.; Liu, X.; Kang, X.; Carvalho, P.A.; Diplas, S.; Both, K.G.; Sotiropoulos, S.; Chatzitakis, A. Galvanic Deposition of Pt Nanoparticles on Black TiO2 Nanotubes for Hydrogen Evolving Cathodes. ChemSusChem 2021, 14, 4993–5003. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Di Palma, V.; Zafeiropoulos, G.; Penchev, H.; Verheijen, M.A.; Creatore, M.; Ublekov, F.; Sinigersky, V.; Bik, W.M.A.; Fredriksson, H.O.A.; et al. Overpotential Analysis of Alkaline and Acidic Alcohol Electrolysers and Optimized Membrane-Electrode Assemblies. Int. J. Hydrogen Energy 2019, 44, 10163–10173. [Google Scholar] [CrossRef]
- Kokkinidis, G.; Stoychev, D.; Lazarov, V.; Papoutsis, A.; Milchev, A. Electroless Deposition of Pt on Ti. Part II. Catalytic Activity for Oxygen Reduction. J. Electroanal. Chem. 2001, 511, 20–30. [Google Scholar] [CrossRef]
- Kokkinidis, G.; Papoutsis, A.; Stoychev, D.; Milchev, A. Electroless Deposition of Pt on Ti—Catalytic Activity for the Hydrogen Evolution Reaction. J. Electroanal. Chem. 2000, 486, 48–55. [Google Scholar] [CrossRef]
- Touni, A.; Papaderakis, A.; Karfaridis, D.; Banti, A.; Mintsouli, I.; Lambropoulou, D.; Sotiropoulos, S. Oxygen Evolution at IrO2-Modified Ti Anodes Prepared by a Simple Galvanic Deposition Method. J. Electroanal. Chem. 2019, 855, 113485. [Google Scholar] [CrossRef]
- Trasatti, S.; Petrii, O.A. Real Surface Area Measurements in Electrochemistry. J. Electroanal. Chem. 1992, 327, 353–376. [Google Scholar] [CrossRef]
- Chung, D.Y.; Lee, K.J.; Sung, Y.E. Methanol Electro-Oxidation on the Pt Surface: Revisiting the Cyclic Voltammetry Interpretation. J. Phys. Chem. C 2016, 120, 9028–9035. [Google Scholar] [CrossRef]
- Pletcher, D.; Solis, V. The Effect of Experimental Parameters on the Rate and Mechanism of Oxidation of Methanol at a Platinum Anode in Aqueous Acid. Electrochim. Acta 1982, 27, 775–782. [Google Scholar] [CrossRef]
- Hou, G.; Parrondo, J.; Ramani, V.; Prakash, J. Kinetic and Mechanistic Investigation of Methanol Oxidation on a Smooth Polycrystalline Pt Surface. J. Electrochem. Soc. 2014, 161, F252–F258. [Google Scholar] [CrossRef]
- Gojković, S.L.; Vidaković, T.R. Methanol Oxidation on an Ink Type Electrode Using Pt Supported on High Area Carbons. Electrochim. Acta 2001, 47, 633–642. [Google Scholar] [CrossRef]
- Christensen, P.A.; Hamnett, A.; Troughton, G.L. The Role of Morphology in the Methanol Electro-Oxidation Reaction. J. Electroanal. Chem. 1993, 362, 207–218. [Google Scholar] [CrossRef]
- Bagotzky, V.S.; Vassilyev, Y.B. Mechanism of Electro-Oxidation of Methanol on the Paltinum Electrode. Electrochim. Acta 1967, 12, 1323–1343. [Google Scholar] [CrossRef]
- Hayden, B.E.; Malevich, D.V.; Pletcher, D. Platinum Catalysed Nanoporous Titanium Dioxide Electrodes in H2SO4 Solutions. Electrochem. Commun. 2001, 3, 395–399. [Google Scholar] [CrossRef]
- Momeni, M.M. Evaluation of the Performance of Pt-MWCNTs Nanocomposites Electrodeposited on Titanium for Methanol Electro-Oxidation. Port. Electrochim. Acta 2015, 33, 331–341. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, Z.; Huang, P.; Zhang, X.; Liu, Y.M. Pt/TiO2-C with Hetero Interfaces as Enhanced Catalyst for Methanol Electrooxidation. Electrochim. Acta 2013, 105, 157–161. [Google Scholar] [CrossRef]
- Song, Y.Y.; Gao, Z.D.; Schmuki, P. Highly Uniform Pt Nanoparticle Decoration on TiO2 Nanotube Arrays: A Refreshable Platform for Methanol Electrooxidation. Electrochem. Commun. 2011, 13, 290–293. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.B.; Ding, Y.; Yin, G.P. Investigation of the Pt-Ni-Pb/C Ternary Alloy Catalysts for Methanol Electrooxidation. Electrochem. Commun. 2008, 10, 443–446. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Y.W.; Jin, Z.; Chen, C.; Li, H.; Cai, W. Bin Alternative Aqueous Phase Synthesis of a Ptru/c Electrocatalyst for Direct Methanol Fuel Cells. Catalysts 2021, 11, 925. [Google Scholar] [CrossRef]
- Liu, X.; Carvalho, P.; Getz, M.N.; Norby, T.; Chatzitakis, A. Black Anatase TiO2 Nanotubes with Tunable Orientation for High Performance Supercapacitors. J. Phys. Chem. C 2019, 123, 21931–21940. [Google Scholar] [CrossRef]
- Zafeiropoulos, G.; Stoll, T.; Dogan, I.; Mamlouk, M.; van de Sanden, M.C.M.; Tsampas, M.N. Porous Titania Photoelectrodes Built on a Ti-Web of Microfibers for Polymeric Electrolyte Membrane Photoelectrochemical (PEM-PEC) Cell Applications. Sol. Energy Mater. Sol. Cells 2018, 180, 184–195. [Google Scholar] [CrossRef]


| Reference | Electrode | jm/mAmg−1 | jesa/mAcm−2Pt |
|---|---|---|---|
| Hayden 2001 [62] | Pt/TiO2 | 1.41 | 0.14 |
| Momeni 2015 [63] | (Pt + MWCNT)/Ti | 34.23 | |
| Fan 2013 [64] | Pt/TiO2 + C | 31.62 | 0.35 |
| Song 2011 [65] | Pt/TNT | 3.49 | |
| Yang 2009 [37] | Pt/TNT + C | 13.12 | |
| Wang 2011 [39] | Pt/TNT/Ti | 8.62 | |
| Sui 2014 [40] | Pt/TNT + C | 117.00 | |
| Han 2018 [43] | PtRu/graphene-TiO2 | 67.08 | |
| Hassan 2009 [46] | Pt/Ti | 6.96 | 0.12 |
| Abraham 2021 [48] | Pt/Ti | 18.92 | |
| Chen 2008 [66] | Pt/C (E-Tek) | 7.50 | |
| Sui 2014 [40] | Pt/C (Vulcan XC-72) | 117.00 | |
| Wang 2021 [67] | Pt/C (JM) | 395.28 | 0.29 |
| this work | Pt/bTNT | 726.00 | 0.44 |
| this work | Pt/Ti felt | 57.00 | 0.41 |
| this work | Pt/Ti | 29.00 | 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touni, A.; Liu, X.; Kang, X.; Papoulia, C.; Pavlidou, E.; Lambropoulou, D.; Tsampas, M.N.; Chatzitakis, A.; Sotiropoulos, S. Methanol Oxidation at Platinum Coated Black Titania Nanotubes and Titanium Felt Electrodes. Molecules 2022, 27, 6382. https://doi.org/10.3390/molecules27196382
Touni A, Liu X, Kang X, Papoulia C, Pavlidou E, Lambropoulou D, Tsampas MN, Chatzitakis A, Sotiropoulos S. Methanol Oxidation at Platinum Coated Black Titania Nanotubes and Titanium Felt Electrodes. Molecules. 2022; 27(19):6382. https://doi.org/10.3390/molecules27196382
Chicago/Turabian StyleTouni, Aikaterini, Xin Liu, Xiaolan Kang, Chrysanthi Papoulia, Eleni Pavlidou, Dimitra Lambropoulou, Mihalis N. Tsampas, Athanasios Chatzitakis, and Sotiris Sotiropoulos. 2022. "Methanol Oxidation at Platinum Coated Black Titania Nanotubes and Titanium Felt Electrodes" Molecules 27, no. 19: 6382. https://doi.org/10.3390/molecules27196382
APA StyleTouni, A., Liu, X., Kang, X., Papoulia, C., Pavlidou, E., Lambropoulou, D., Tsampas, M. N., Chatzitakis, A., & Sotiropoulos, S. (2022). Methanol Oxidation at Platinum Coated Black Titania Nanotubes and Titanium Felt Electrodes. Molecules, 27(19), 6382. https://doi.org/10.3390/molecules27196382








