Protective Effects of Orange Sweet Pepper Juices Prepared by High-Speed Blender and Low-Speed Masticating Juicer against UVB-induced Skin Damage in SKH-1 Hairless Mice
Abstract
:1. Introduction
2. Results and Discussion
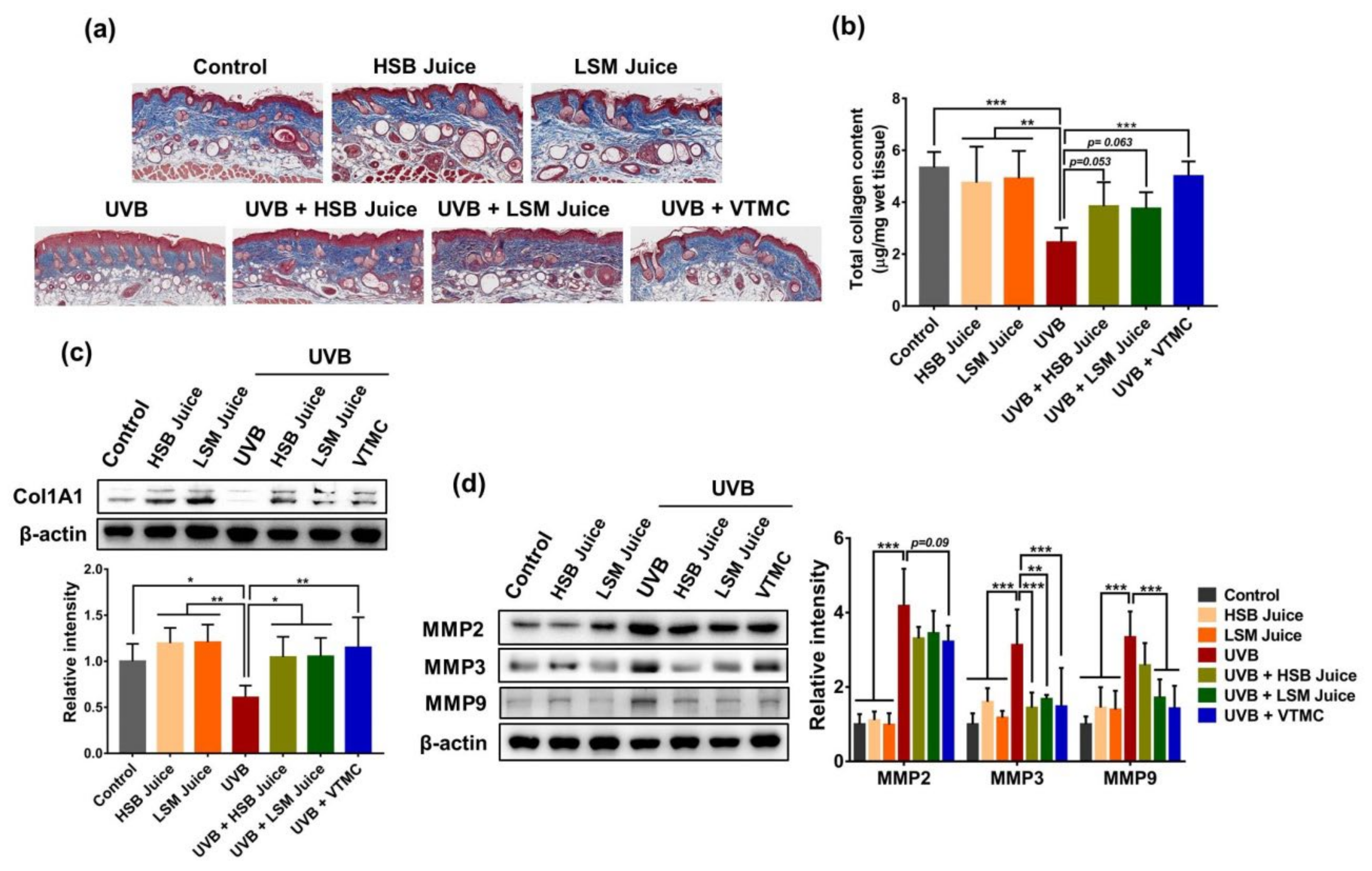
2.1. HSB and LSM Sweet Pepper Juices Inhibit UVB-Induced Photoaging in SKH-1 Hairless Mice
2.2. HSB and LSM Sweet Pepper Juices Attenuate Inflammatory Response in UVB-Irradiated SKH-1 Hairless Mice
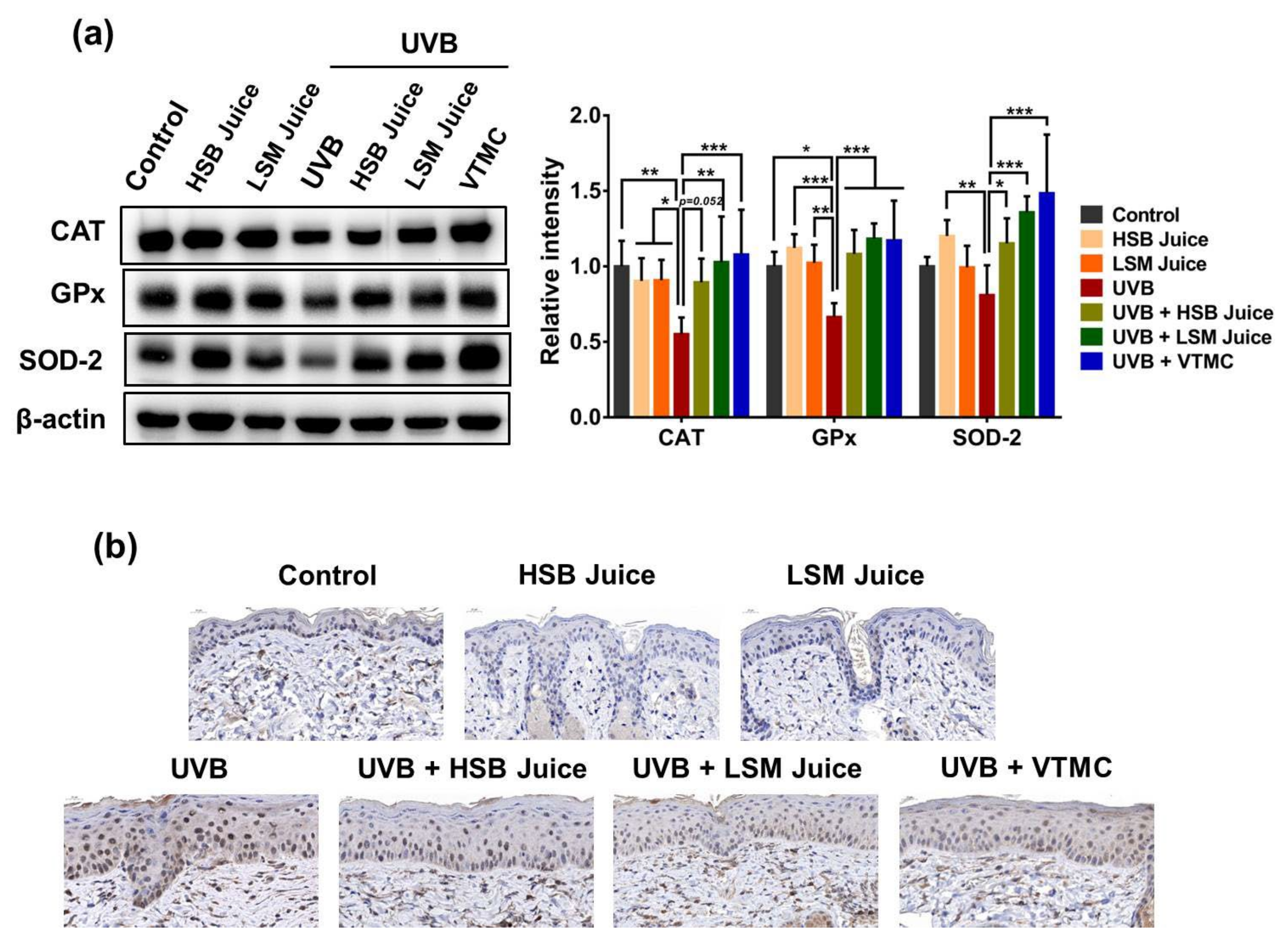
2.3. HSB and LSM Sweet Pepper Juices Enhance Antioxidant Defense Systems in UVB-Irradiated SKH-1 Hairless Mice
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Orange Sweet Pepper Juice
4.3. Animal Experimental Design and UVB Irradiation
4.4. Histological and Immunohistochemical Analyses
4.5. Measurement of Total Collagen Content
4.6. Western Blot Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, R.; Wei, Y.; Zhang, J.; Cai, M.; Lu, L.; Fang, L.; Qin, X.; Gu, R. Protection effects of rice protein hydrolysate on UVB-irradiated photodamage in Hartley guinea pigs skin and human skin fibroblasts. J. Funct. Foods 2021, 82, 104504. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Choi, S.-I.; Han, H.-S.; Kim, J.-M.; Park, G.; Jang, Y.-P.; Shin, Y.-K.; Ahn, H.-S.; Lee, S.-H.; Lee, K.-T. Eisenia bicyclis Extract Repairs UVB-Induced Skin Photoaging In Vitro and In Vivo: Photoprotective Effects. Mar. Drugs 2021, 19, 693. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, S.; Chen, J.; Li, C.; Zhou, C.; Hong, P.; Sun, S.; Qian, Z.-J. Trehalose against UVB-induced skin photoaging by suppressing MMP expression and enhancing procollagen I synthesis in HaCaT cells. J. Funct. Foods 2020, 74, 104198. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of Premature Skin Aging Induced by Ultraviolet Light. N. Engl. J. Med. 1997, 337, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Fam, V.W.; Charoenwoodhipong, P.; Sivamani, R.K.; Holt, R.R.; Keen, C.L.; Hackman, R.M. Plant-Based Foods for Skin Health: A Narrative Review. J. Acad. Nutr. Diet. 2022, 122, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; An, C.G.; Park, J.-S.; Lim, Y.P.; Kim, S. Carotenoid profiling from 27 types of paprika (Capsicum annuum L.) with different colors, shapes, and cultivation methods. Food Chem. 2016, 201, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Hong, H.T.; Hayward, A.; Harper, S.; Mitter, N.; O’Hare, T.J. Carotenoid Profiling of Orange-Coloured Capsicums: In Search of High-Zeaxanthin Varieties for Eye Health. Proceedings 2021, 70, 84. [Google Scholar] [CrossRef]
- Park, S.-Y.; Choi, S.R.; Lim, S.-H.; Yeo, Y.; Kweon, S.J.; Bae, Y.-S.; Kim, K.W.; Im, K.-H.; Ahn, S.K.; Ha, S.-H.; et al. Identification and quantification of carotenoids in paprika fruits and cabbage, kale, and lettuce leaves. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 355–358. [Google Scholar] [CrossRef]
- Guzman, I.; Hamby, S.; Romero, J.; Bosland, P.W.; O’Connell, M.A. Variability of carotenoid biosynthesis in orange colored Capsicum spp. Plant Sci. 2010, 179, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Xu, Z.; Wu, C.-T.; Janes, M.; Prinyawiwatkul, W.; No, H.K. Antioxidant Activities of Different Colored Sweet Bell Peppers (Capsicum annuum L.). J. Food Sci. 2007, 72, S98–S102. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ortega, M.; Ortiz-Moreno, A.; Hernández-Navarro, M.D.; Chamorro-Cevallos, G.; Dorantes-Alvarez, L.; Necoechea-Mondragón, H. Antioxidant, Antinociceptive, and Anti-Inflammatory Effects of Carotenoids Extracted from Dried Pepper (Capsicum annuum L.). J. Biomed. Biotechnol. 2012, 2012, 524019. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T.; Mochida, K.; Kozuka, M.; Ito, Y.; Fujiwara, Y.; Hashimoto, K.; Enjo, F.; Ogata, M.; Nobukuni, Y.; Tokuda, H.; et al. Cancer chemopreventive activity of carotenoids in the fruits of red paprika Capsicum annuum L. Cancer Lett. 2001, 172, 103–109. [Google Scholar] [CrossRef]
- Aizawa, K.; Inakuma, T. Dietary capsanthin, the main carotenoid in paprika (Capsicum annuum), alters plasma high-density lipoprotein-cholesterol levels and hepatic gene expression in rats. Br. J. Nutr. 2009, 102, 1760–1766. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Kim, E.-S.; Lee, Y.-E. Anti-inflammatory effects of paprika fruit and leaf through heme oxygenase-1 induction in RAW264. 7 macrophages. J. Korean Soc. Food Sci. Nutr. 2020, 49, 578–585. [Google Scholar] [CrossRef]
- Kim, H.-R.; Kim, S.; Lee, S.-W.; Sin, H.-S.; Kim, S.-Y. Protective Effects of Fermented Paprika (Capsicum annuum L.) on Sodium Iodate-Induced Retinal Damage. Nutrients 2020, 13, 25. [Google Scholar] [CrossRef]
- Thuphairo, K.; Sornchan, P.; Suttisansanee, U. Bioactive Compounds, Antioxidant Activity and Inhibition of Key Enzymes Relevant to Alzheimer’s Disease from Sweet Pepper (Capsicum annuum) Extracts. Prev. Nutr. Food Sci. 2019, 24, 327–337. [Google Scholar] [CrossRef]
- Nishino, A.; Sugimoto, K.; Sambe, H.; Ichihara, T.; Takaha, T.; Kuriki, T. Effects of Dietary Paprika Xanthophylls on Ultraviolet Light-Induced Skin Damage: A Double-Blind Placebo-Controlled Study. J. Oleo Sci. 2018, 67, 863–869. [Google Scholar] [CrossRef]
- Yatsuhashi, H.; Takumi, H.; Terada, Y.; Kuriki, T. Effects of Oral Supplementation with Paprika Xanthophylls on Human Skin Moisture. J. Oleo Sci. 2022, 71, 735–745. [Google Scholar] [CrossRef]
- Agarwal, S.; Fulgoni Iii, V.L.; Welland, D. Intake of 100% Fruit Juice Is Associated with Improved Diet Quality of Adults: NHANES 2013-2016 Analysis. Nutrients 2019, 11, 2513. [Google Scholar] [CrossRef] [PubMed]
- Khaksar, G.; Assatarakul, K.; Sirikantaramas, S. Effect of cold-pressed and normal centrifugal juicing on quality attributes of fresh juices: Do cold-pressed juices harbor a superior nutritional quality and antioxidant capacity? Heliyon 2019, 5, e01917. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Jun, J.-G.; Park, S.-Y.; Choi, M.-J.; Park, E.; Kim, J.-I.; Kim, M.-J. Antioxidant activities of fresh grape juices prepared using various household processing methods. Food Sci. Biotechnol. 2017, 26, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Gouws, C.A.; Georgouopoulou, E.; Mellor, D.D.; Naumovski, N. The Effect of Juicing Methods on the Phytochemical and Antioxidant Characteristics of the Purple Prickly Pear (Opuntia ficus indica)—Preliminary Findings on Juice and Pomace. Beverages 2019, 5, 28. [Google Scholar] [CrossRef]
- Baswan, S.M.; Klosner, A.E.; Weir, C.; Salter-Venzon, D.; Gellenbeck, K.W.; Leverett, J.; Krutmann, J. Role of ingestible carotenoids in skin protection: A review of clinical evidence. Photodermatol. Photoimmunol. Photomed. 2021, 37, 490–504. [Google Scholar] [CrossRef]
- Lee, J.; Jiang, S.; Levine, N.; Watson, R.R. Carotenoid supplementation reduces erythema in human skin after simulated solar radiation exposure. Proc. Soc. Exp. Biol. Med. 2000, 223, 170–174. [Google Scholar] [CrossRef]
- Heinrich, U.; Gärtner, C.; Wiebusch, M.; Eichler, O.; Sies, H.; Tronnier, H.; Stahl, W. Supplementation with β-Carotene or a Similar Amount of Mixed Carotenoids Protects Humans from UV-Induced Erythema. J. Nutr. 2003, 133, 98–101. [Google Scholar] [CrossRef]
- Garmyn, M.; Ribaya-Mercado, J.D.; Russel, R.M.; Bhawan, J.; Gilchrest, B.A. Effect of beta-carotene supplementation on the human sunburn reaction. Exp. Dermatol. 1995, 4, 104–111. [Google Scholar] [CrossRef]
- Baswan, S.M.; Marini, A.; Klosner, A.E.; Jaenicke, T.; Leverett, J.; Murray, M.; Gellenbeck, K.W.; Krutmann, J. Orally administered mixed carotenoids protect human skin against ultraviolet A-induced skin pigmentation: A double-blind, placebo-controlled, randomized clinical trial. Photodermatol. Photoimmunol. Photomed. 2020, 36, 219–225. [Google Scholar] [CrossRef]
- Obana, A.; Gohto, Y.; Gellermann, W.; Ermakov, I.V.; Sasano, H.; Seto, T.; Bernstein, P.S. Skin Carotenoid Index in a large Japanese population sample. Sci. Rep. 2019, 9, 9318. [Google Scholar] [CrossRef] [Green Version]
- Ribaya-Mercado, J.D.; Garmyn, M.; Gilchrest, B.A.; Russell, R.M. Skin Lycopene Is Destroyed Preferentially over β-Carotene during Ultraviolet Irradiation in Humans. J. Nutr. 1995, 125, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K.; Obermueller-Jevic, U.C. UV Light, Beta-carotene and Human Skin—Beneficial and Potentially Harmful Effects. Arch. Biochem. Biophys. 2001, 389, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Misawa, E.; Tanaka, M.; Saito, M.; Nabeshima, K.; Yao, R.; Yamauchi, K.; Abe, F.; Yamamoto, Y.; Furukawa, F. Protective effects of Aloe sterols against UVB-induced photoaging in hairless mice. Photodermatol. Photoimmunol. Photomed. 2017, 33, 101–111. [Google Scholar] [CrossRef] [PubMed]
- El-Abaseri, T.B.; Putta, S.; Hansen, L.A. Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis 2006, 27, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, J.H.; Nam, K.T.; Lee, S.H. Molecular events associated with apoptosis and proliferation induced by ultraviolet-B radiation in the skin of hairless mice. J. Dermatol. Sci. 2003, 32, 171–179. [Google Scholar] [CrossRef]
- Ouhtit, A.; Muller, H.K.; Davis, D.W.; Ullrich, S.E.; McConkey, D.; Ananthaswamy, H.N. Temporal events in skin injury and the early adaptive responses in ultraviolet-irradiated mouse skin. Am. J. Pathol. 2000, 156, 201–207. [Google Scholar] [CrossRef]
- Moon, J.-M.; Park, S.-H.; Jhee, K.-H.; Yang, S.-A. Protection against UVB-Induced Wrinkle Formation in SKH-1 Hairless Mice: Efficacy of Tricin Isolated from Enzyme-Treated Zizania latifolia Extract. Molecules 2018, 23, 2254. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lamichhane, S.; Ju, J.-H.; Yun, J. Protective Effect of Octylmethoxycinnamate against UV-Induced Photoaging in Hairless Mouse via the Regulation of Matrix Metalloproteinases. Int. J. Mol. Sci. 2018, 19, 1836. [Google Scholar] [CrossRef]
- Zhang, J.-A.; Yin, Z.; Ma, L.-W.; Yin, Z.-Q.; Hu, Y.-Y.; Xu, Y.; Wu, D.; Permatasari, F.; Luo, D.; Zhou, B.-R. The Protective Effect of Baicalin against UVB Irradiation Induced Photoaging: An In Vitro and In Vivo Study. PLoS ONE 2014, 9, e99703. [Google Scholar] [CrossRef]
- Darawsha, A.; Trachtenberg, A.; Levy, J.; Sharoni, Y. The Protective Effect of Carotenoids, Polyphenols, and Estradiol on Dermal Fibroblasts under Oxidative Stress. Antioxidants 2021, 10, 2023. [Google Scholar] [CrossRef]
- Yum, H.-W.; Park, J.; Park, H.-J.; Shin, J.W.; Cho, Y.-Y.; Kim, S.-J.; Kang, J.X.; Surh, Y.-J. Endogenous ω-3 Fatty Acid Production by fat-1 Transgene and Topically Applied Docosahexaenoic Acid Protect against UVB-induced Mouse Skin Carcinogenesis. Sci. Rep. 2017, 7, 11658. [Google Scholar] [CrossRef]
- Tripp, C.S.; Blomme, E.A.G.; Chinn, K.S.; Hardy, M.M.; LaCelle, P.; Pentland, A.P. Epidermal COX-2 Induction Following Ultraviolet Irradiation: Suggested Mechanism for the Role of COX-2 Inhibition in Photoprotection. J. Investig. Dermatol. 2003, 121, 853–861. [Google Scholar] [CrossRef]
- Rundhaug, J.E.; Mikulec, C.; Pavone, A.; Fischer, S.M. A role for cyclooxygenase-2 in ultraviolet light-induced skin carcinogenesis. Mol. Carcinog. 2007, 46, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K.; Surh, Y.-J. Breaking the relay in deregulated cellular signal transduction as a rationale for chemoprevention with anti-inflammatory phytochemicals. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 591, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.M.; Pavone, A.; Mikulec, C.; Langenbach, R.; Rundhaug, J.E. Cyclooxygenase-2 expression is critical for chronic UV-induced murine skin carcinogenesis. Mol. Carcinog. 2007, 46, 363–371. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Koki, A.T.; Zweifel, B.S.; Kusewitt, D.F.; Rubal, P.A.; Oberyszyn, T.M. Inhibition of cutaneous ultraviolet light B–mediated inflammation and tumor formation with topical celecoxib treatment. Mol. Carcinog. 2003, 38, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Rundhaug, J.E.; Fischer, S.M. Cyclo-oxygenase-2 Plays a Critical Role in UV-induced Skin Carcinogenesis. Photochem. Photobiol. 2008, 84, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Buckman, S.Y.; Gresham, A.; Hale, P.; Hruza, G.; Anast, J.; Masferrer, J.; Pentland, A.P. COX-2 expression is induced by UVB exposure in human skin: Implications for the development of skin cancer. Carcinogenesis 1998, 19, 723–729. [Google Scholar] [CrossRef]
- Bermudez, Y.; Stratton, S.P.; Curiel-Lewandrowski, C.; Warneke, J.; Hu, C.; Bowden, G.T.; Dickinson, S.E.; Dong, Z.; Bode, A.M.; Saboda, K.; et al. Activation of the PI3K/Akt/mTOR and MAPK Signaling Pathways in Response to Acute Solar-Simulated Light Exposure of Human Skin. Cancer Prev. Res. 2015, 8, 720–728. [Google Scholar] [CrossRef]
- Ha, S.J.; Lee, J.; Kim, H.; Song, K.-M.; Lee, N.H.; Kim, Y.E.; Lee, H.; Kim, Y.H.; Jung, S.K. Preventive effect of Rhus javanica extract on UVB-induced skin inflammation and photoaging. J. Funct. Foods 2016, 27, 589–599. [Google Scholar] [CrossRef]
- Martinez, R.M.; Fattori, V.; Saito, P.; Melo, C.B.P.; Borghi, S.M.; Pinto, I.C.; Bussmann, A.J.C.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; et al. Lipoxin A4 inhibits UV radiation-induced skin inflammation and oxidative stress in mice. J. Dermatol. Sci. 2018, 91, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Naringenin Inhibits UVB Irradiation-Induced Inflammation and Oxidative Stress in the Skin of Hairless Mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.R.; Mitrani, R.; Werth, V.P. Effect of TNFα blockade on UVB-induced inflammatory cell migration and collagen loss in mice. J. Photochem. Photobiol. B Biol. 2020, 213, 112072. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Truong, V.-L.; Jeong, W.-S. Cellular Defensive Mechanisms of Tea Polyphenols: Structure-Activity Relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Kundu, J.K.; Shin, J.-W.; Na, H.-K.; Surh, Y.-J. Docosahexaenoic Acid Inhibits UVB-Induced Activation of NF-κB and Expression of COX-2 and NOX-4 in HR-1 Hairless Mouse Skin by Blocking MSK1 Signaling. PLoS ONE 2011, 6, e28065. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Spandau, D.F. UVB activation of NF-κB in normal human keratinocytes occurs via a unique mechanism. Arch. Dermatol. Res. 2007, 299, 93–101. [Google Scholar] [CrossRef]
- Adhami, V.M.; Afaq, F.; Ahmad, N. Suppression of ultraviolet B exposure-mediated activation of NF-κB in normal human keratinocytes by resveratrol. Neoplasia 2003, 5, 74–82. [Google Scholar] [CrossRef]
- Chang, E.-J.; Kundu, J.K.; Liu, L.; Shin, J.-W.; Surh, Y.-J. Ultraviolet B radiation activates NF-κB and induces iNOS expression in HR-1 hairless mouse skin: Role of IκB kinase-β. Mol. Carcinog. 2011, 50, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Wang, Y.; Liu, N.; Yang, M.; Hu, Y.; Li, X.; Fu, Y.; Luo, M.; Sun, J.; Yang, X. Potential skin protective effects after UVB irradiation afforded by an antioxidant peptide from Odorrana andersonii. Biomed. Pharmacother. 2019, 120, 109535. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, C.; Wang, Q.; Gao, R.; Cai, X.; Wang, S.; Zhang, Y. The protective effect of collagen peptides from bigeye tuna (Thunnus obesus) skin and bone to attenuate UVB-induced photoaging via MAPK and TGF-β signaling pathways. J. Funct. Foods 2022, 93, 105101. [Google Scholar] [CrossRef]
- Filip, A.; Daicoviciu, D.; Clichici, S.; Bolfa, P.; Catoi, C.; Baldea, I.; Bolojan, L.; Olteanu, D.; Muresan, A.; Postescu, I.D. The effects of grape seeds polyphenols on SKH-1 mice skin irradiated with multiple doses of UV-B. J. Photochem. Photobiol. B Biol. 2011, 105, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Kang, Y.-H. Anti-inflammatory and antioxidant activities of red pepper (Capsicum annuum L.) stalk extracts: Comparison of pericarp and placenta extracts. J. Funct. Foods 2013, 5, 1724–1731. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, D.W.; Park, C.W.; Kim, B.; Sim, H.; Kim, H.S.; Lee, T.-K.; Lee, J.-C.; Yang, G.E.; Her, Y.; et al. Laminarin Attenuates Ultraviolet-Induced Skin Damage by Reducing Superoxide Anion Levels and Increasing Endogenous Antioxidants in the Dorsal Skin of Mice. Mar. Drugs 2020, 18, 345. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.; Bae, G.Y.; Cho, K.; Park, S.S.; Suh, H.J.; Hong, K.-B. An Anthocyanin-Enriched Extract from Vaccinium uliginosum Improves Signs of Skin Aging in UVB-Induced Photodamage. Antioxidants 2020, 9, 844. [Google Scholar] [CrossRef]
- Kwak, C.S.; Yang, J.; Shin, C.-Y.; Chung, J.H. Topical or oral treatment of peach flower extract attenuates UV-induced epidermal thickening, matrix metalloproteinase-13 expression and pro-inflammatory cytokine production in hairless mice skin. Nutr. Res. Pract. 2018, 12, 29–40. [Google Scholar] [CrossRef]
- Yin, Y.; Li, W.; Son, Y.-O.; Sun, L.; Lu, J.; Kim, D.; Wang, X.; Yao, H.; Wang, L.; Pratheeshkumar, P.; et al. Quercitrin protects skin from UVB-induced oxidative damage. Toxicol. Appl. Pharmacol. 2013, 269, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, S.; Funakoshi, T.; Sato, Y.; Saito, N.; Ohsawa, H.; Kurita, K.; Nagata, K.; Yoshida, M.; Ishigami, A. Protective effect of pre- and post-vitamin C treatments on UVB-irradiation-induced skin damage. Sci. Rep. 2018, 8, 16199. [Google Scholar] [CrossRef] [PubMed]
- McArdle, F.; Rhodes, L.E.; Parslew, R.; Jack, C.I.A.; Friedmann, P.S.; Jackson, M.J. UVR-induced oxidative stress in human skin in vivo: Effects of oral vitamin C supplementation. Free Radic. Biol. Med. 2002, 33, 1355–1362. [Google Scholar] [CrossRef]
- Han, J.-H.; Bang, J.S.; Choi, Y.J.; Choung, S.-Y. Oral administration of oyster (Crassostrea gigas) hydrolysates protects against wrinkle formation by regulating the MAPK pathway in UVB-irradiated hairless mice. Photochem. Photobiol. Sci. 2019, 18, 1436–1446. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Kim, O.-K.; Lee, J.; Lee, M.-J.; Kang, N.; Hwang, J.-K. Protective effect of the standardized green tea seed extract on UVB-induced skin photoaging in hairless mice. Nutr. Res. Pract. 2014, 8, 398–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truong, V.-L.; Rarison, R.H.G.; Jeong, W.-S. Protective Effects of Orange Sweet Pepper Juices Prepared by High-Speed Blender and Low-Speed Masticating Juicer against UVB-induced Skin Damage in SKH-1 Hairless Mice. Molecules 2022, 27, 6394. https://doi.org/10.3390/molecules27196394
Truong V-L, Rarison RHG, Jeong W-S. Protective Effects of Orange Sweet Pepper Juices Prepared by High-Speed Blender and Low-Speed Masticating Juicer against UVB-induced Skin Damage in SKH-1 Hairless Mice. Molecules. 2022; 27(19):6394. https://doi.org/10.3390/molecules27196394
Chicago/Turabian StyleTruong, Van-Long, Razanamanana. H. G. Rarison, and Woo-Sik Jeong. 2022. "Protective Effects of Orange Sweet Pepper Juices Prepared by High-Speed Blender and Low-Speed Masticating Juicer against UVB-induced Skin Damage in SKH-1 Hairless Mice" Molecules 27, no. 19: 6394. https://doi.org/10.3390/molecules27196394








