Recombinant Thaumatin-Like Protein (rTLP) and Chitinase (rCHI) from Vitis vinifera as Models for Wine Haze Formation
Abstract
:1. Introduction
2. Results
2.1. Validation of Transformants
2.2. Heterologous Expression of rTLP and rCHI
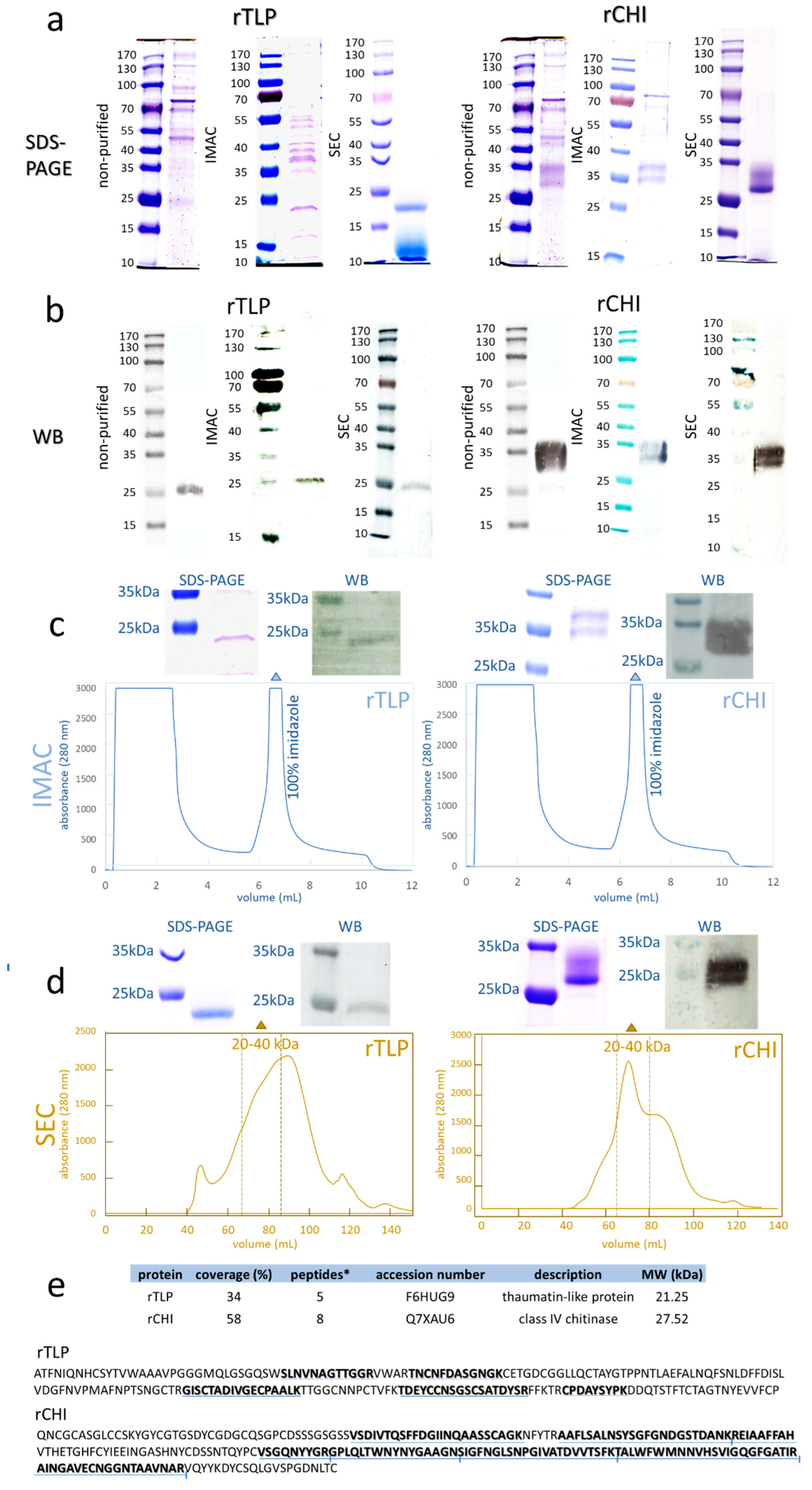
2.3. Purification of rTLP and rCHI and Characterization by MS-Based Bottom-Up Proteomics
2.4. TLP and CHI: Recombinant Versus Native Proteins
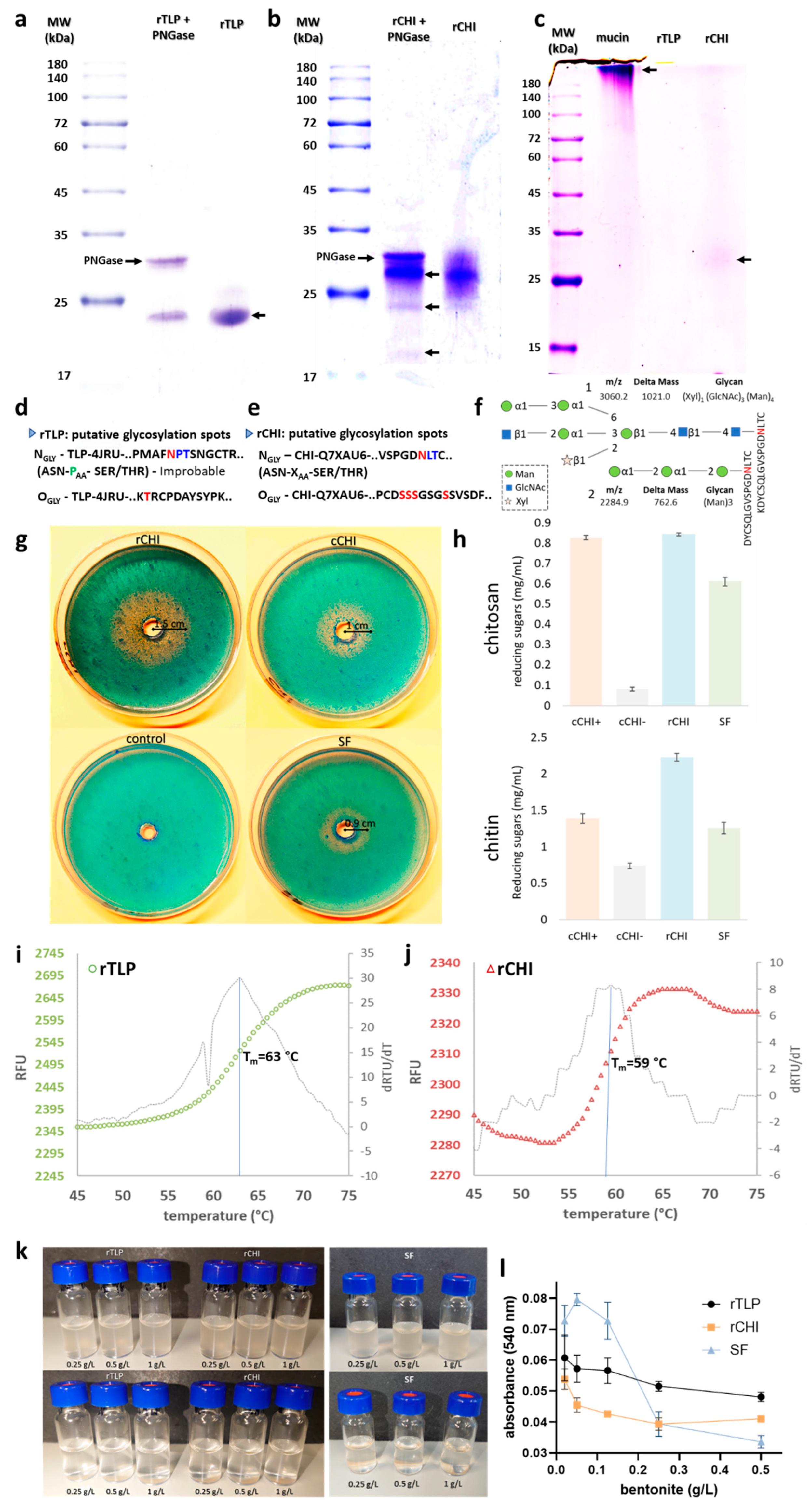
2.4.1. Glycosylation Analysis
2.4.2. Chitinolytic Activity
2.4.3. Thermostability of rTLP and rCHI
2.4.4. Adsorption of the Proteins to Bentonite (Bentonite Fining)
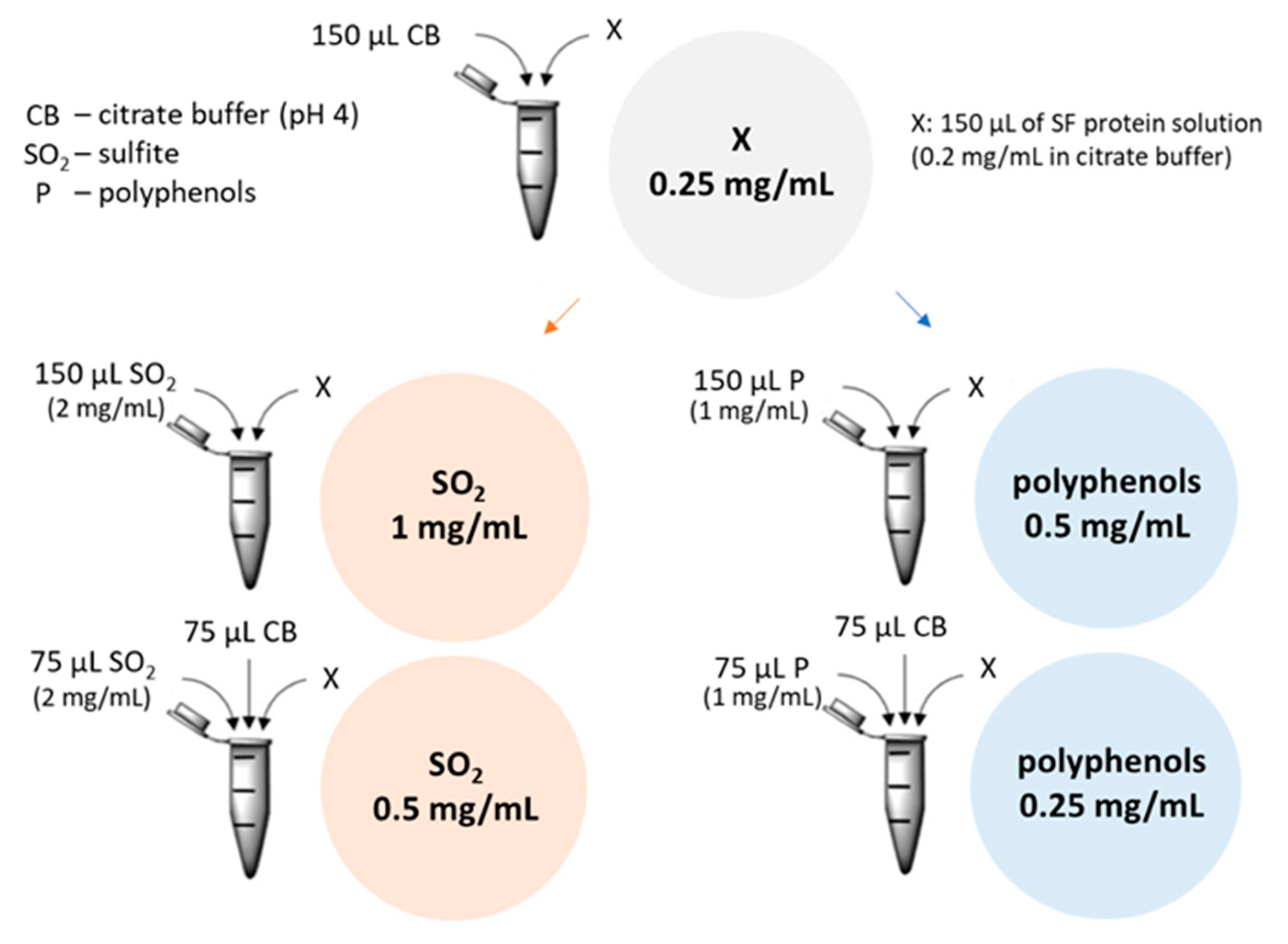
2.4.5. Influence of Polyphenols and Sulfite Ions on the Haze Potential of rTLP and rCHI
3. Discussion
3.1. Molecular Characterization and Comparison with Native Proteins
3.2. Influence of Polyphenols and Sulfite Ion on the Haze Potential of TLP and CHI
3.3. Heterologous rTLP and rCHI as Haze-Forming Protein Models for Research and Applications
4. Materials and Methods
4.1. Plasmid Amplification and Isolation
4.2. Transformation into K. phaffii, Selection of Transformed Cells and Phenotype Determination
4.3. Recombinant Expression, Purification and Identification of rTLP and rCHI
4.3.1. Recombinant Expression
4.3.2. Protein Purification
4.3.3. MS-Based Proteomics Analysis
4.4. Protein Glycosylation
4.5. Chitinolytic Activity
4.6. Analysis of Protein Thermostability by Differential Scanning Fluorimetry (DSF)
4.7. Bentonite Fining
4.8. Influence of Haze-Inducing Agents on Protein Aggregation
4.8.1. Haze Test
4.8.2. Extraction and Analysis of Polyphenols and Monosaccharides from Grape (V. vinifera) Juices
4.8.3. Aggregation Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gazzola, D.; van Sluyter, S.C.; Curioni, A.; Waters, E.J.; Marangon, M. Roles of Proteins, Polysaccharides, and Phenolics in Haze Formation in White Wine via Reconstitution Experiments. J. Agric. Food Chem. 2012, 60, 10666–10673. [Google Scholar] [CrossRef] [PubMed]
- Chagas, R.; Laia, C.A.T.; Ferreira, R.B.; Ferreira, L.M. Sulfur Dioxide Induced Aggregation of Wine Thaumatin-like Proteins: Role of Disulfide Bonds. Food Chem. 2018, 259, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Batista, L.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R.; Ferreira, R.B. Protein Haze Formation in Wines Revisited. The Stabilising Effect of Organic Acids. Food Chem. 2010, 122, 1067–1075. [Google Scholar] [CrossRef]
- Claus, H.; Mojsov, K. Enzymes for Wine Fermentation: Current and Perspective Applications. Fermentation 2018, 4, 52. [Google Scholar] [CrossRef]
- Tchouakeu Betnga, P.F.; Longo, E.; Poggesi, S.; Boselli, E. Effects of Transport Conditions on the Stability and Sensory Quality of Wines. OENO One 2021, 55, 197–208. [Google Scholar] [CrossRef]
- Marangon, M.; Sauvage, F.-X.; Waters, E.J.; Vernhet, A. Effects of Ionic Strength and Sulfate upon Thermal Aggregation of Grape Chitinases and Thaumatin-like Proteins in a Model System. J. Agric. Food Chem. 2011, 59, 2652–2662. [Google Scholar] [CrossRef]
- Dufrechou, M.; Vernhet, A.; Roblin, P.; Sauvage, F.X.; Poncet-Legrand, C. White Wine Proteins: How Does the pH Affect Their Conformation at Room Temperature? Langmuir 2013, 29, 10475–10482. [Google Scholar] [CrossRef]
- Marangon, M.; Vincenzi, S.; Lucchetta, M.; Curioni, A. Heating and Reduction Affect the Reaction with Tannins of Wine Protein Fractions Differing in Hydrophobicity. Anal. Chim. Acta 2010, 660, 110–118. [Google Scholar] [CrossRef]
- Albuquerque, W.; Seidel, L.; Zorn, H.; Will, F.; Gand, M. Haze Formation and the Challenges for Peptidases in Wine Protein Fining. J. Agric. Food Chem. 2021, 69, 14402–14414. [Google Scholar] [CrossRef]
- van Sluyter, S.C.; McRae, J.M.; Falconer, R.J.; Smith, P.A.; Bacic, A.; Waters, E.J.; Marangon, M. Wine Protein Haze: Mechanisms of Formation and Advances in Prevention. J. Agric. Food Chem. 2015, 63, 4020–4030. [Google Scholar] [CrossRef] [Green Version]
- Falconer, R.J.; Marangon, M.; van Sluyter, S.C.; Neilson, K.A.; Chan, C.; Waters, E.J. Thermal Stability of Thaumatin-like Protein, Chitinase, and Invertse Isolated from Sauvignon Blanc and Semillon Juice and Their Role in Haze Formation in Wine. J. Agric. Food Chem. 2010, 58, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Siebert, K.J.; Carrasco, A.; Lynn, P.Y. Formation of Protein−Polyphenol Haze in Beverages. J. Agric. Food Chem. 1996, 44, 1997–2005. [Google Scholar] [CrossRef]
- Dufrechou, M.; Poncet-Legrand, C.; Sauvage, F.X.; Vernhet, A. Stability of White Wine Proteins: Combined Effect of pH, Ionic Strength, and Temperature on Their Aggregation. J. Agric. Food Chem. 2012, 60, 1308–1319. [Google Scholar] [CrossRef]
- Kosińska-Cagnazzo, A.; Heeger, A.; Udrisard, I.; Mathieu, M.; Bach, B.; Andlauer, W. Phenolic Compounds of Grape Stems and Their Capacity to Precipitate Proteins from Model Wine. J. Food Sci. Technol. 2020, 57, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Salazar, F.N.; Marangon, M.; Labbé, M.; Lira, E.; Rodríguez-Bencomo, J.J.; López, F. Comparative Study of Sodium Bentonite and Sodium-Activated Bentonite Fining During White Wine Fermentation: Its Effect on Protein Content, Protein Stability, Lees Volume, and Volatile Compounds. Eur. Food Res. Technol. 2017, 243, 2043–2054. [Google Scholar] [CrossRef]
- Hsu, J.C.; Heatherbell, D.A.; Riesling, W. Heat-Unstable Proteins in Wine. I. Characterization and Removal by Bentonite Fining and Heat Treatment. Am. J. Enol. Vitic. 1987, 38, 11–16. [Google Scholar]
- Pocock, K.F.; Waters, E.J. Protein Haze in Bottled White Wines: How Well Do Stability Tests and Bentonite Fining Trials Predict Haze Formation during Storage and Transport? Aust. J. Grape Wine Res. 2006, 12, 212–220. [Google Scholar] [CrossRef]
- Marangon, M.; van Sluyter, S.C.; Neilson, K.A.; Chan, C.; Haynes, P.A.; Waters, E.J.; Falconer, R.J. Roles of Grape Thaumatin-like Protein and Chitinase in White Wine Haze Formation. J. Agric. Food Chem. 2011, 59, 733–740. [Google Scholar] [CrossRef]
- Pocock, K.F.; Alexander, G.M.; Hayasaka, Y.; Jones, P.R.; Waters, E.J. Sulfate—A Candidate for the Missing Essential Factor That Is Required for the Formation of Protein Haze in White Wine. J. Agric. Food Chem. 2007, 55, 1799–1807. [Google Scholar] [CrossRef]
- Gupta, R.; Brunak, S. Prediction of Glycosylation across the Human Proteome and the Correlation to Protein Function. Pac. Symp. Biocomput. 2002, 322, 310–322. [Google Scholar] [CrossRef]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.-B.G.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L.; et al. Precision Mapping of the Human O-GalNAc Glycoproteome through SimpleCell Technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasprzewska, A. Plant Chitinases-Regulation and Function. Cell Mol. Biol. Lett. 2003, 8, 809–824. [Google Scholar] [PubMed]
- Sharma, A.; Sharma, H.; Upadhyay, S.K. Thaumatin-like Proteins: Molecular Characterization, Evolutionary Analysis and Expression Profiling in Major Cereal Crops. Plant Sci. 2020, 290, 110317. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, G.; Antonacci, D.; Larsen, M.R. Glycoproteomic Profile in Wine: A ‘Sweet’ Molecular Renaissance. J. Proteome Res. 2010, 9, 6148–6159. [Google Scholar] [CrossRef]
- Cereghino, J.L.; Cregg, J.M. Heterologous Protein Expression in the Methylotrophic Yeast Pichia pastoris. FEMS Microbiol. Rev. 2000, 24, 45–66. [Google Scholar] [CrossRef]
- Landim, P.G.C.; Correia, T.O.; Silva, F.D.A.; Nepomuceno, D.R.; Costa, H.P.S.; Pereira, H.M.; Lobo, M.D.P.; Moreno, F.B.M.B.; Brandão-Neto, J.; Medeiros, S.C.; et al. Production in Pichia pastoris, Antifungal Activity and Crystal Structure of a Class I Chitinase from Cowpea (Vigna Unguiculata): Insights into Sugar Binding Mode and Hydrolytic Action. Biochimie 2017, 135, 89–103. [Google Scholar] [CrossRef]
- Orlando, M.; Buchholz, P.C.F.; Lotti, M.; Pleiss, J. The GH19 Engineering Database: Sequence Diversity, Substrate Scope, and Evolution in Glycoside Hydrolase Family 19. PLoS ONE 2021, 16, e0256817. [Google Scholar] [CrossRef]
- Boivin, S.; Kozak, S.; Meijers, R. Optimization of Protein Purification and Characterization Using Thermofluor Screens. Protein Expr. Purif. 2013, 91, 192–206. [Google Scholar] [CrossRef]
- Eilers, M.; Patel, A.B.; Liu, W.; Smith, S.O. Comparison of Helix Interactions in Membrane and Soluble α-Bundle Proteins. Biophys. J. 2002, 82, 2720–2736. [Google Scholar] [CrossRef]
- Alexandrov, A.I.; Mileni, M.; Chien, E.Y.T.; Hanson, M.A.; Stevens, R.C. Microscale Fluorescent Thermal Stability Assay for Membrane Proteins. Structure 2008, 16, 351–359. [Google Scholar] [CrossRef]
- van der Plank, I.; van Loey, A.; Hendrickx, M.E.G. Changes in Sulfhydryl Content of Egg White Proteins Due to Heat and Pressure Treatment IESEL. J. Agric. Food Chem. 2005, 53, 5726–5733. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, W.; Ghezellou, P.; Li, B.; Spengler, B.; Will, F.; Zorn, H.; Gand, M. Identification of Intact Peptides by Top-down Peptidomics Reveals Cleavage Spots in Thermolabile Wine Proteins. Food Chem. 2021, 363, 130437. [Google Scholar] [CrossRef]
- Marangon, M.; van Sluyter, S.C.; Robinson, E.M.C.; Muhlack, R.A.; Holt, H.E.; Haynes, P.A.; Godden, P.W.; Smith, P.A.; Waters, E.J. Degradation of White Wine Haze Proteins by Aspergillopepsin I and II during Juice Flash Pasteurization. Food Chem. 2012, 135, 1157–1165. [Google Scholar] [CrossRef]
- Pocock, K.F.; Salazar, F.N.; Waters, E.J. The Effect of Bentonite Fining at Different Stages of White Winemaking on Protein Stability. Aust. J. Grape Wine Res. 2011, 17, 280–284. [Google Scholar] [CrossRef]
- Chagas, R.; Monteiro, S.; Boavida Ferreira, R. Assessment of Potential Effects of Common Fining Agents Used for White Wine Protein Stabilization. Am. J. Enol. Vitic. 2012, 63, 574–578. [Google Scholar] [CrossRef]
- Nolan, C.M.; Reyes, C.D.; Debord, J.D.; García, A.J.; Lyon, L.A. Phase Transition Behavior, Protein Adsorption, and Cell Adhesion Resistance of Poly(Ethylene Glycol) Cross-Linked Microgel Particles. Biomacromolecules 2005, 6, 2032–2039. [Google Scholar] [CrossRef]
- Sommer, S.; Tondini, F. Sustainable Replacement Strategies for Bentonite in Wine Using Alternative Protein Fining Agents. Sustainability 2021, 13, 1860. [Google Scholar] [CrossRef]
- Marangon, M.; van Sluyter, S.C.; Waters, E.J.; Menz, R.I. Structure of Haze Forming Proteins in White Wines: Vitis vinifera Thaumatin-like Proteins. PLoS ONE 2014, 9, e113757. [Google Scholar] [CrossRef]
- Esteruelas, M.; Poinsaut, P.; Sieczkowski, N.; Manteau, S.; Fort, M.F.; Canals, J.M.; Zamora, F. Comparison of Methods for Estimating Protein Stability in White Wines. Am. J. Enol. Vitic. 2009, 60, 302–311. [Google Scholar]
- Darias-Martín, J.J.; Andrés-Lacueva, C.; Díaz-Romero, C.; Lamuela-Raventós, R.M. Phenolic Profile in Varietal White Wines Made in the Canary Islands. Eur. Food Res. Technol. 2008, 226, 871–876. [Google Scholar] [CrossRef]
- di Gaspero, M.; Ruzza, P.; Hussain, R.; Honisch, C.; Biondi, B.; Siligardi, G.; Marangon, M.; Curioni, A.; Vincenzi, S. The Secondary Structure of a Major Wine Protein Is Modified upon Interaction with Polyphenols. Molecules 2020, 25, 1646. [Google Scholar] [CrossRef] [PubMed]
- Toledo, L.N.; Salazar, F.N.; Aquino, A.J.A. A Theoretical Approach for Understanding the Haze Phenomenon in Bottled White Wines at Molecular Level. S. Afr. J. Enol. Vitic. 2017, 38, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Yu, D.; Sun, J.; Liu, X.; Jiang, L.; Guo, H.; Ren, F. Interaction of Plant Phenols with Food Macronutrients: Characterisation and Nutritional–Physiological Consequences. Nutr. Res. Rev. 2014, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mierczynska-Vasilev, A.; Boyer, P.; Vasilev, K.; Smith, P.A. A Novel Technology for the Rapid, Selective, Magnetic Removal of Pathogenesis-Related Proteins from Wines. Food Chem. 2017, 232, 508–514. [Google Scholar] [CrossRef]
- Cosme, F.; Fernandes, C.; Ribeiro, T.; Filipe-Ribeiro, L.; Nunes, F.M. White Wine Protein Instability: Mechanism, Quality Control and Technological Alternatives for Wine Stabilisation—An Overview. Beverages 2020, 6, 19. [Google Scholar] [CrossRef]
- Sun, L.; Srinivas, A.; Runnebaum, R.C. Understanding the Impact of Key Wine Components on the Use of a Non-Swelling Ion-Exchange Resin for Wine Protein Fining Treatment. Molecules 2021, 26, 3905. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Souza-da Costa, B.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Nanotechnology: Recent Advances in Viticulture and Enology. J. Sci. Food Agric. 2021, 101, 6156–6166. [Google Scholar] [CrossRef]
- Yang, X.; Dai, J.; Wei, X.; Zhong, Y.; Liu, X.; Guo, D.; Wang, L.; Huang, Y.; Zhang, C.; Liu, Y.; et al. Characterization of Recombinant GRIP32 as a Novel Haze Protein for Protein-Polyphenol Haze Models and Prevention of Haze Formation with Polysaccharides in the Models. LWT 2021, 136, 110317. [Google Scholar] [CrossRef]
- Mohammadzadeh, R.; Karbalaei, M.; Soleimanpour, S.; Mosavat, A.; Rezaee, S.A.; Ghazvini, K.; Farsiani, H. Practical Methods for Expression of Recombinant Protein in the Pichia pastoris System. Curr. Protoc. 2021, 1, 1–24. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Ghezellou, P.; Albuquerque, W.; Garikapati, V.; Casewell, N.R.; Kazemi, S.M.; Ghassempour, A.; Spengler, B. Integrating Top-Down and Bottom-Up Mass Spectrometric Strategies for Proteomic Profiling of Iranian Saw-Scaled Viper, Echis Carinatus Sochureki, Venom. J. Proteome Res. 2021, 20, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE Database and Related Tools and Resources in 2019: Improving Support for Quantification Data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed]
- Doerner, K.C.; White, B.A. Detection of Glycoproteins Separated by Nondenaturing Polyacrylamide Gel Electrophoresis Using the Periodic Acid-Schiff Stain. Anal. Biochem. 1990, 187, 147–150. [Google Scholar] [CrossRef]
- Patil, S.; Rohrer, J. An Improved Method for Galactosyl Oligosaccharide Characterization. J. Chromatogr. B 2021, 1184, 122967. [Google Scholar] [CrossRef]
- Trudel, J.; Asselin, A. Detection of Chitin Deacetylase Activity after Polyacrylamide Gel Electrophoresis. Anal. Biochem. 1990, 189, 249–253. [Google Scholar] [CrossRef]
- Zou, X.; Nonogaki, H.; Welbaum, G.E. A Gel Diffusion Assay for Visualization and Quantification of Chitinase Activity. Mol. Biotechnol. 2002, 22, 19–24. [Google Scholar] [CrossRef]
- Breuil, C.; Saddler, J.N. Comparison of the 3,5-Dinitrosalicylic Acid and Nelson-Somogyi Methods of Assaying for Reducing Sugars and Determining Cellulase Activity. Enzyme Microb. Technol. 1985, 7, 327–332. [Google Scholar] [CrossRef]
- Brandt, S.C.; Ellinger, B.; van Nguyen, T.; Thi, Q.D.; van Nguyen, G.; Baschien, C.; Yurkov, A.; Hahnke, R.L.; Schäfer, W.; Gand, M. A Unique Fungal Strain Collection from Vietnam Characterized for High Performance Degraders of Bioecological Important Biopolymers and Lipids. PLoS ONE 2018, 13, e0202695. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, C.; Zhang, X.; Wei, Y. Cysteine Residue Is Not Essential for CPM Protein Thermal-Stability Assay. Anal. Bioanal. Chem. 2015, 407, 3683–3691. [Google Scholar] [CrossRef]
- Chan, D.S.H.; Kavanagh, M.E.; McLean, K.J.; Munro, A.W.; Matak-Vinković, D.; Coyne, A.G.; Abell, C. Effect of DMSO on Protein Structure and Interactions Assessed by Collision-Induced Dissociation and Unfolding. Anal. Chem. 2017, 89, 9976–9983. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albuquerque, W.; Sturm, P.; Schneider, Q.; Ghezellou, P.; Seidel, L.; Bakonyi, D.; Will, F.; Spengler, B.; Zorn, H.; Gand, M. Recombinant Thaumatin-Like Protein (rTLP) and Chitinase (rCHI) from Vitis vinifera as Models for Wine Haze Formation. Molecules 2022, 27, 6409. https://doi.org/10.3390/molecules27196409
Albuquerque W, Sturm P, Schneider Q, Ghezellou P, Seidel L, Bakonyi D, Will F, Spengler B, Zorn H, Gand M. Recombinant Thaumatin-Like Protein (rTLP) and Chitinase (rCHI) from Vitis vinifera as Models for Wine Haze Formation. Molecules. 2022; 27(19):6409. https://doi.org/10.3390/molecules27196409
Chicago/Turabian StyleAlbuquerque, Wendell, Pia Sturm, Quintus Schneider, Parviz Ghezellou, Leif Seidel, Daniel Bakonyi, Frank Will, Bernhard Spengler, Holger Zorn, and Martin Gand. 2022. "Recombinant Thaumatin-Like Protein (rTLP) and Chitinase (rCHI) from Vitis vinifera as Models for Wine Haze Formation" Molecules 27, no. 19: 6409. https://doi.org/10.3390/molecules27196409






