Elsholtzia ciliata (Thunb.) Hyland: A Review of Phytochemistry and Pharmacology
Abstract
1. Introduction
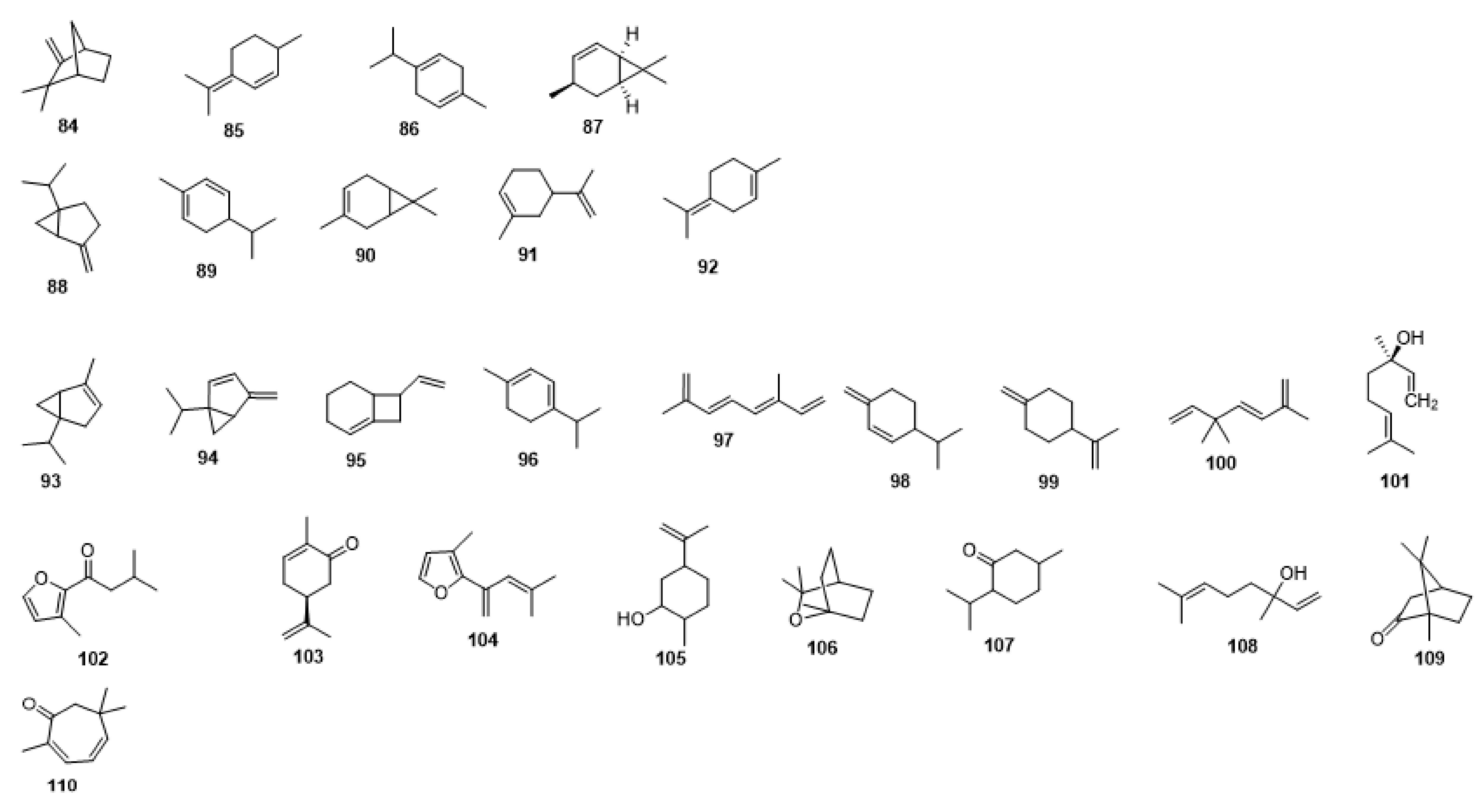
2. Chemical Constituents
3. Pharmacological Activities
3.1. Antioxidant Activity
3.2. Anti-Inflammatory Activity
3.3. Antimicrobial Activity
3.4. Insecticidal Activity
3.5. Antiviral Activity
3.6. Hypolipidemic Activity
3.7. Antitumor Activity
3.8. Immunoregulatory Activity
3.9. Others
| No. | Compound Name | Formula | E. ciliata | JXR | MCM | References |
|---|---|---|---|---|---|---|
| Flavonoids | ||||||
| 1 | vitexin | C21H20O10 | + | - | - | [11] |
| 2 | pedalin | C22H22O12 | + | - | - | [11] |
| 3 | luteolin-7-O-β-D-glucopyranoside | C20H20O11 | + | - | - | [11] |
| 4 | apigenin-5-O-β-D-glucopyranoside | C20H20O10 | + | - | - | [11] |
| 5 | apigenin-7-O-β-D-glucopyranoside | C20H20O10 | + | - | - | [11] |
| 6 | chrysoeriol-7-O-β-D-glucopyranoside | C21H22O11 | + | - | - | [11] |
| 7 | 7,3′-dimethoxyluteolin-6-O-β-D-glucopyranoside | C21H24O12 | + | - | - | [11] |
| 8 | luteolin | C15H10O6 | + | + | - | [11,18] |
| 9 | 5,6,4′-trihydroxy-7,3′-dimethoxyflavone | C17H14O7 | + | - | - | [9] |
| 10 | 5-hydroxy-6,7-dimethoxyflavone | C17H14O5 | + | - | - | [9] |
| 11 | 5-hydroxy-7,8-dimethoxyflavone | C17H14O5 | + | - | - | [9] |
| 12 | negletein | C16H12O5 | - | - | + | [50] |
| 13 | acacetin-7-O-[β-D-glucopyranosyl(1″″→2″)-4‴-O-acetyl-α-L-rhamnopyranosyl(1‴→6″)]-β-D-glucopyranoside | C36H44O20 | + | - | - | [51] |
| 14 | acacetin-7-O-[6″″-O-acetyl-β-D-glucopyranosyl(1″″→2″)-α-L-rhamnopyranosyl(1‴→6″)]-β-D-glucopyranoside | C36H44O20 | + | - | - | [51] |
| 15 | acacetin-7-O-[6″″-O-acetyl-β-D-glucopyranosyl(1″″→2″)-3‴-O-acetyl-α-L-rhamnopyranosyl(1‴→6″)]-β-D-glucopyranoside | C38H46O21 | + | - | - | [51] |
| 16 | acacetin-7-O-[6″″-O-acetyl-β-D-glucopyranosyl(1″″→2″)-4‴-O-acetyl-α-L-rhamnopyranosyl(1‴→6″)]-β-D-glucopyranoside | C38H46O21 | + | - | - | [51] |
| 17 | acacetin-7-O-[3″″,6″″-di-Oacetyl-β-D-glucopyranosyl(1″″→2″)-4‴-O-acetyl-α-L-rhamnopyranosyl(1‴→6″)]-β-D-glucopyranoside | C40H45O25 | + | - | - | [51] |
| 18 | 7-O-β-D-glucopyranosyl-(1 → 2)[α-L-rhamnopyranosyl(1 → 6)]-β-D-glucopyranoside | C34H42O19 | + | - | - | [49] |
| 19 | linarin | C28H32O14 | + | - | - | [51] |
| 20 | acacetin-7-O-[4‴-O-acetyl-α-L-rhamnopyranosyl(1‴→6″)]-β-D-glucopyranoside | C30H34O15 | + | - | - | [51] |
| 21 | apigenin | C15H10O5 | + | - | + | [50,51] |
| 22 | apigetrin | C21H20O10 | + | - | - | [51] |
| 23 | diosmetin | C16H12O6 | + | - | - | [51] |
| 24 | 5-hydroxy-6,7-dimethoxyflavone | C17H14O5 | + | - | - | [51] |
| 25 | 5-hydroxy-7,8-dimethoxyflavone | C17H14O5 | + | - | - | [51] |
| 26 | 6-hydroxy-5,7,8-trimethoxyflavone | C18H16O6 | + | - | - | [51] |
| 27 | butin | C16H14O4 | + | - | - | [51] |
| 28 | isookanin | C16H14O5 | + | - | - | [51] |
| 29 | sulfuretin | C15H10O5 | + | - | - | [51] |
| 30 | 3,2′,4′-trihy-droxy-4-methoxychalcone | C16H14O5 | + | - | - | [51] |
| 31 | 3,2′,4′-trihy4′-O-β-D-glucopyranosyl-3,2′-dihydroxy-4-methoxychalcone | C21H24O10 | + | - | - | [51] |
| 32 | okanin-4-methoxy-4′-O-β-D-glucopyranoside | C21H24O11 | + | - | - | [51] |
| 33 | neoisoliquiritin | C20H22O9 | + | - | - | [51] |
| 34 | kumatakenin | C17H14O6 | + | - | - | [52] |
| 35 | isoorientin-2′′-O-rhamnoside | C27H30O15 | - | + | - | [18] |
| 36 | isovitexin-2′′-O-rhamnoside | C27H30O14 | - | + | - | [18] |
| 37 | quercetin-3-O-rutinoside | C27H30O16 | - | + | - | [18] |
| 38 | quercetin-3-O-glucoside | C21H20O12 | - | + | - | [18] |
| 39 | orientin-2′′-O-rhamnoside | C27H30O15 | - | + | - | [18] |
| 40 | vitexin-2′′-O-rhamnoside | C27H30O14 | - | + | - | [18] |
| 41 | orientin | C21H20O11 | - | + | - | [18] |
| 42 | isoorientin | C21H20O11 | - | + | - | [18] |
| 43 | swertisin | C22H22O10 | - | + | - | [18] |
| 44 | peonidin-3-O-glucoside | C22H22O11 | - | + | - | [18] |
| 45 | chrysoeriol | C16H12O6 | - | + | - | [50] |
| 46 | quercetin | C15H10O7 | - | + | - | [50] |
| 47 | kaempferol | C15H10O6 | - | + | - | [53] |
| 48 | catechin | C15H14O6 | + | - | - | [54] |
| Phenylpropanoids | ||||||
| 49 | caffeic acid | C9H8O4 | + | + | - | [9,18] |
| 50 | (E)-p-coumaric acid | C9H8O3 | + | - | - | [9] |
| 51 | osmundacetone | C10H10O3 | + | - | - | [9] |
| 52 | 4-(E)-caffeoyl-L-threonic acid | C17H16O12 | + | - | - | [9] |
| 53 | 4-O-(E)-p-coumaroyl-L-threonic acid | C13H14O7 | + | - | - | [9] |
| 54 | (7E,9E)-3-hydroxyavenalumic acid-3-O-[6′-O-(E)-caffeoyl]-β-D-glucopyranoside | C26H26O12 | + | - | - | [51] |
| 55 | gnaphaliin C | C25H24O11 | + | - | - | [51] |
| 56 | 3,5-di-O-caffeoylquinic acid | C25H24O12 | + | - | - | [51] |
| 57 | everlastoside L | C25H26O11 | + | - | - | [51] |
| 58 | 1-(3′,4′-dihydroxycinnamoyl)cyclopentane2,3-diol | C14H16O6 | + | - | - | [51] |
| 59 | ethyl caffeate | C11H12O4 | + | - | - | [51] |
| 60 | (Z)-p-coumaric acid | C9H8O3 | + | - | - | [51] |
| 61 | p-hydroxybenzaldehyde | C7H6O2 | + | - | - | [51] |
| 62 | p-hydroxybenzoic acid | C7H6O3 | + | + | - | [18,51] |
| 63 | 3,4-dihydroxybenzoic acid | C7H6O4 | + | - | - | [51] |
| 64 | vanillic acid | C8H8O4 | + | - | - | [51] |
| 65 | rosmarinic acid | C18H16O8 | + | + | - | [18,51] |
| 66 | estra-1,3,5(10)-trien-17-β-ol | C18H24O | + | - | - | [55] |
| 67 | 5-caffeoylquinic acid | C16H17O9 | - | + | - | [18] |
| 68 | 4-caffeoylquinic acid | C16H17O9 | - | + | - | [18] |
| 69 | Danshensu | C9H10O5 | - | + | - | [18] |
| 70 | (+)lyoniresinol | C24H38O8 | - | + | - | [53] |
| 71 | (-)-5-methoxyisolariciresinol | C22H30O7 | - | + | - | [53] |
| 72 | pinoresinol | C21H26O6 | - | + | - | [53] |
| 73 | isoeucommin A | C27H34O12 | - | + | - | [53] |
| 74 | episyringaresinol-4-O-β-D-glucopyranoside | C28H36O13 | - | + | - | [53] |
| 75 | methyl-3-(3′,4′dihydroxyphenyl) lactate | C10H12O5 | - | + | - | [56] |
| 76 | (s)-pencedanol-7-O-β-D-glucopyranoside | C20H26O10 | - | + | - | [56] |
| 77 | stearyl ferulate | C28H46O4 | + | - | - | [54] |
| Terpenoids | ||||||
| (1) Monoterpene | ||||||
| 78 | α-Pinene | C10H16 | + | + | + | [5,26,57] |
| 79 | β-Pinene | C10H16 | + | - | + | [5,57] |
| 80 | myrcene | C10H16 | + | - | + | [5,57] |
| 81 | β-Phellandrene | C10H16 | + | - | - | [5] |
| 82 | limonene | C10H16 | + | - | + | [5,57] |
| 83 | β-Ocimene | C10H16 | + | - | + | [5,57] |
| 84 | camphene | C10H16 | + | - | + | [57,58] |
| 85 | isoterpinolene | C10H16 | + | - | - | [58] |
| 86 | γ-terpinene | C10H16 | + | + | - | [26,58] |
| 87 | 4-carene | C10H16 | + | - | - | [6] |
| 88 | sabinene | C10H16 | + | - | + | [57,59] |
| 89 | α-phellandrene | C10H16 | - | + | + | [26,57] |
| 90 | 3-carene | C10H16 | - | + | + | [26,57] |
| 91 | sylvestrene | C10H16 | - | + | - | [26] |
| 92 | terpinolene | C10H16 | - | + | + | [26,57] |
| 93 | α-thujene | C10H16 | - | - | + | [57] |
| 94 | bicyclo [3.1.0]hex-2-ene, 4-methylene-1-(1-methylethyl)- | C10H14 | - | - | + | [57] |
| 95 | bicyclo[4.2.0]oct-1-ene,7-exo-ethenyl- | C10H14 | - | - | + | [57] |
| 96 | α-terpinene | C10H16 | - | - | + | [57] |
| 97 | 2,6-dimethyl-1,3,5,7-octatetraene | C10H14 | - | - | + | [57] |
| 98 | p-mentha-1(7),2-diene | C10H16 | + | - | - | [60] |
| 99 | cyclohexane, 1-methylene-4-(1-methylethenyl)- | C10H16 | + | - | - | [61] |
| 100 | artemisia triene | C10H16 | - | - | + | [28] |
| (2) Oxygenated monoterpene | ||||||
| 101 | linalool | C10H18O | + | - | + | [5,57] |
| 102 | elsholtzia ketone | C10H14O2 | + | - | - | [5] |
| 103 | carvone | C10H14O | + | - | - | [5] |
| 104 | dehydroelsholtzia ketone | C10H12O2 | + | - | - | [5] |
| 105 | neodihydrocarveol | C10H18O | + | - | - | [58] |
| 106 | eucalyptol | C10H18O | + | + | - | [33,58] |
| 107 | menthone | C10H18O | + | - | - | [58] |
| 108 | linalool | C10H18O | + | - | - | [58] |
| 109 | camphor | C10H16O | + | - | - | [58] |
| 110 | eucarvone | C10H14O | + | - | - | [58] |
| 111 | perillene | C10H14O | + | - | - | [58] |
| 112 | jasmone | C10H14O | + | - | - | [58] |
| 113 | dehydroelsholtzia ketone | C10H12O2 | + | - | - | [58] |
| 114 | eucalyptol | C10H18O | + | + | - | [33,58] |
| 115 | (−)-1R-8-hydroxy-p-menth-4-en-3-one | C10H16O2 | + | - | - | [43] |
| 116 | fenchol | C10H18O | + | - | - | [6] |
| 117 | lavandulol | C10H18O | + | - | - | [6] |
| 118 | α-terpineol | C10H18O | + | - | - | [6] |
| 119 | 1,6-dihydrocarveol | C10H18O | + | - | - | [6] |
| 120 | cis-carveol | C10H16O | + | - | - | [6] |
| 121 | terpinen-4-ol | C10H18O | + | + | - | [33,59] |
| 122 | nerol | C10H18O | + | - | - | [59] |
| 123 | neral | C10H16O | + | - | - | [59] |
| 124 | geraniol | C10H18O | + | - | - | [59] |
| 125 | borneol | C10H18O | - | + | - | [33] |
| 126 | trans-4-thujanol | C10H18O | - | + | - | [26] |
| 127 | sabinol | C10H16O | - | + | - | [26] |
| 128 | thymoquinone | C10H12O2 | - | - | + | [57] |
| 129 | umbellulon | C10H14O | - | - | + | [27] |
| 130 | furan,3-methyl-2-(3-methyl-2-buten-1-yl)- | C10H14O | + | - | - | [24] |
| 131 | verbenol | C10H16O | - | - | + | [28] |
| 132 | β-dehydro-elsholtzione | C10H12O2 | + | - | - | [22] |
| 133 | rose furan epoxide | C10H14O2 | + | - | - | [65] |
| 134 | 1-octen-3-yl acetate | C10H18O2 | + | - | - | [24] |
| 135 | neryl formate | C11H18O2 | + | - | - | [59] |
| 136 | geranyl formate | C11H18O2 | + | - | - | [59] |
| 137 | neryl acetate | C12H20O2 | + | - | - | [59] |
| 138 | citronellal | C10H18O | + | - | - | [6] |
| 139 | isobornyl formate | C11H18O2 | - | - | + | [57] |
| (3) Sesquiterpene | ||||||
| 140 | cubebene | C15H24 | + | - | - | [5] |
| 141 | β-bourbonene | C15H24 | + | - | - | [5] |
| 142 | β-caryophyllene | C15H24 | + | + | + | [5,26,57] |
| 143 | α-caryophyllene | C15H24 | + | + | - | [5,33] |
| 144 | α-farnesene | C15H24 | + | + | + | [5,26,57] |
| 145 | β-bourbonene | C15H24 | + | - | - | [58] |
| 146 | β-gurjunene | C15H24 | + | - | - | [58] |
| 147 | π-cubebene | C15H24 | + | - | - | [58] |
| 148 | α-bergamotene | C15H28 | + | + | - | [26,58] |
| 149 | humulene | C15H24 | + | - | - | [58] |
| 150 | π-sesquiphellandrene | C15H24 | + | - | - | [58] |
| 151 | germacrene D | C15H24 | + | + | - | [26,58] |
| 152 | π-bisabolene | C15H24 | + | - | - | [58] |
| 153 | γ-elemene | C15H24 | + | - | - | [58] |
| 154 | (Z, E)-α-farnesene | C15H24 | + | - | - | [58] |
| 155 | caryophyllene | C15H24 | + | + | - | [33,58] |
| 156 | π-muurolene | C15H24 | + | - | - | [58] |
| 157 | π-cadinene | C15H24 | + | - | - | [58] |
| 158 | α-farnesene | C15H24 | + | - | - | [43] |
| 159 | ledene | C15H24 | + | - | - | [43] |
| 160 | α-cubebene | C15H24 | + | - | - | [43] |
| 161 | γ-cadinene | C15H24 | + | - | - | [43] |
| 162 | δ-cadinene | C15H24 | + | - | - | [43] |
| 163 | β-elemene | C15H24 | + | - | - | [6] |
| 164 | aromadendrene | C15H24 | + | - | - | [6] |
| 165 | β-bisabolene | C15H24 | + | - | - | [59] |
| 166 | trans-α-bergamotene | C15H24 | - | + | - | [33] |
| 167 | γ-muurolene | C15H24 | - | + | - | [26] |
| 168 | eudesma-3,7(11)-diene | C15H24 | - | + | - | [26] |
| 169 | longifolene | C15H24 | - | - | + | [57] |
| 170 | trans-α-bergamotene | C15H24 | - | - | + | [57] |
| 171 | trans-β-bergamotene | C15H24 | - | - | + | [57] |
| 172 | (Z,E)-α-farnesene | C15H24 | - | - | + | [57] |
| 173 | β-himachalene | C15H24 | - | - | + | [57] |
| 174 | γ-cadinene | C15H24 | - | - | + | [57] |
| 175 | guaiene | C15H24 | - | - | + | [64] |
| 176 | α-zingiberene | C15H24 | - | - | + | [64] |
| 177 | α-muurolene | C15H24 | - | - | + | [64] |
| 178 | β-selinene | C15H24 | + | - | - | [65] |
| (4) Oxygenated sesquiterpene | ||||||
| 179 | (-)-humulene epoxide II | C15H24O | + | - | - | [5] |
| 180 | nerolidol | C15H26O | + | - | - | [58] |
| 181 | caryophyllene oxide | C15H24O | + | + | - | [33,58] |
| 182 | spathulenol | C15H24O | + | - | - | [6] |
| 183 | humulene-1,2-epoxide | C15H24O | - | + | - | [26] |
| 184 | cedrol | C15H26O | - | - | + | [57] |
| 185 | cedrenol | C15H24O | - | - | + | [64] |
| 186 | levomenol | C15H26O | - | - | + | [64] |
| 187 | germacrone | C15H22O | + | - | - | [22] |
| (5) Oxygenated diterpene | ||||||
| 188 | 2,3-dimethyl-5-(2,6,10trimethylundecyl) furan | C20H36O | + | - | - | [43] |
| 189 | phytol | C20H40O | - | - | + | [28] |
| (6) Triterpene | ||||||
| 190 | forrestin A | C30H42O11 | - | + | - | [18] |
| 191 | betulinic acid | C30H48O3 | - | + | - | [50] |
| 192 | oleanolic acid | C30H48O3 | - | + | - | [50] |
| 193 | ursolic acid | C30H48O3 | - | + | - | [50] |
| Alkaloids | ||||||
| 194 | N-trans-feruloyloctopamine | C18H19NO5 | + | - | - | [51] |
| 195 | 5-methyl-furan-2-carboxylic acid (1H-[1,2,4]triazol3-yl) -amide | C8H8N4O2 | + | - | - | [51] |
| 196 | furane-2-carboxaldehyde, 5 (nitrophenoxymethyl) | C12H9NO5 | + | - | - | [51] |
| 197 | uridine | C9H12N2O6 | - | + | - | [18] |
| 198 | carbamult | C12H17NO2 | - | + | - | [26] |
| 199 | phenol,O-amino- | C6H7NO | + | - | - | [61] |
| 200 | adenosin | C10H13N5O4 | - | + | - | [67] |
| 201 | prunasin | C14H17NO6 | - | + | - | [68] |
| 202 | sambunigrin | C13H17NO7 | - | + | - | [68] |
| Others | ||||||
| (1) Aliphatic | ||||||
| 203 | pentacosane | C25H52 | + | - | - | [55] |
| 204 | heptacosane | C27H56 | + | - | - | [55] |
| 205 | octacosane | C28H58 | + | - | - | [55] |
| 206 | tetratriacontane | C34H70 | + | - | - | [55] |
| 207 | hexatriacontane | C36H74 | + | - | - | [55] |
| 208 | n-dodecane | C11H24 | - | - | + | [57] |
| 209 | n-tridecane | C13H28 | - | - | + | [57] |
| 210 | n-tetradecane | C14H30 | - | - | + | [57] |
| 211 | n-pentadecane | C15H32 | - | - | + | [57] |
| 212 | n-hexadecane | C16H34 | - | - | + | [57] |
| 213 | heptadecane | C17H36 | - | + | - | [63] |
| 214 | octadecane | C18H38 | - | + | - | [63] |
| 215 | tridecane,4-cyclohexyl | C19H38 | - | + | - | [63] |
| 216 | nonadecane | C19H40 | - | + | - | [63] |
| 217 | eicosane | C20H42 | - | + | - | [63] |
| 218 | heneicosane | C21H44 | - | + | - | [63] |
| 219 | docosane | C22H46 | - | + | - | [63] |
| 220 | tricosane | C23H48 | - | + | - | [63] |
| 221 | tetracosane | C24H50 | - | + | - | [63] |
| 222 | 9-cyclohexyl eicosane | C26H52 | - | + | - | [63] |
| 223 | (R, R)-2,3-butanediol | C4H10O2 | + | - | - | [58] |
| 224 | 3-hexen-1-ol | C6H12O | + | - | - | [58] |
| 225 | 1-hexanol | C6H14O | + | - | - | [58] |
| 226 | 3-octen-1-ol | C8H16O | + | - | - | [58] |
| 227 | 3-octanol | C8H18O | + | - | - | [58] |
| 228 | 3,13-octadecadien-1-ol | C18H34O | - | + | - | [63] |
| 229 | 2-octene-1-ol | C8H16O | + | - | - | [66] |
| 230 | hexanal | C6H12O | + | - | - | [58] |
| 231 | heptana | C7H14O | + | - | - | [58] |
| 232 | octanal | C8H16O | + | - | - | [58] |
| 233 | nonanal | C9H18O | + | - | - | [58] |
| 234 | nonacosan-10-one | C29H58O | + | - | - | [55] |
| 235 | 3-hexanone | C6H12O | + | - | - | [58] |
| 236 | 3-heptanone | C7H14O | + | - | - | [58] |
| 237 | 3-octanone | C8H16O | + | - | - | [58] |
| 238 | irisone | C13H20O | + | - | - | [24] |
| 239 | 2-pentadecanone,6,10,14-trimethyl | C18H36O | - | + | - | [63] |
| 240 | α-linolenic acid | C18H30O2 | + | - | - | [9] |
| 241 | n-hexadecanoic acid | C16H32O2 | + | - | - | [55] |
| 242 | 9,12-octadecadienoic acid | C18H32O2 | + | - | - | [55] |
| 243 | 3-methylpentanoic acid | C6H12O2 | + | - | - | [58] |
| 244 | 2-methylbutanoic acid | C5H10O2 | + | - | - | [58] |
| 245 | galactonic acid | C6H12O7 | - | + | - | [18] |
| 246 | malic acid | C4H6O5 | - | + | - | [18] |
| 247 | citric acid | C6H8O7 | - | + | - | [18] |
| 248 | quinic acid | C7H12O6 | - | + | - | [18] |
| 249 | succinic acid | C4H6O4 | - | + | - | [18] |
| 250 | azelaic acid | C9H16O4 | - | + | - | [18] |
| 251 | 9-hydroxy-10,12-octadecadienoic acid | C18H32O3 | - | + | - | [18] |
| 252 | palmitic acid | C16H32O2 | - | + | - | [18] |
| 253 | oleic acid | C18H34O2 | - | + | - | [18] |
| 254 | tetradecanoic acid | C14H28O2 | - | + | - | [63] |
| 255 | linoleic acid | C18H32O2 | + | [66] | ||
| 256 | hexadecanoic acid, ethyl ester | C18H36O2 | + | - | - | [55] |
| 257 | 9,12,15-octadecatrienoic acid methyl ester | C19H32O2 | + | - | - | [55] |
| 258 | linoleic acid ethyl ester | C20H36O2 | + | - | - | [55] |
| 259 | 9,12,15-octadecatrienoic acidethyl ester | C20H34O2 | + | - | - | [55] |
| 260 | octadecanoic acidethyl ester | C20H40O2 | + | - | - | [55] |
| 261 | linolenic acide, 2-hydroxy-1(hydroxymethyl) ethyl ester | C21H36O4 | + | - | - | [55] |
| 262 | octen-1-ol, acetate | C10H18O2 | + | - | - | [58] |
| 263 | 3-octanol, acetate | C10H20O2 | + | - | - | [58] |
| 264 | 2-propenoic acid, 2-methyl-, ethenyl ester | C6H8O2 | + | - | - | [43] |
| 265 | diglycol laurate | C16H32O4 | - | + | - | [18] |
| 266 | butanoic acid, 2-methylpropyl ester | C8H16O2 | - | - | + | [57] |
| 267 | propanoic acid, 2-methyl-,butyl ester | C8H16O2 | - | - | + | [57] |
| 268 | 2-propenoic acid, 2-methyl-,butyl ester | C8H14O2 | - | - | + | [57] |
| 269 | butanoic acid, butyl ester | C8H16O2 | - | - | + | [57] |
| 270 | propanoic acid, 2-methyl,2-methylpropyl ester | C8H16O2 | - | - | + | [57] |
| 271 | heptadecanoic acid, ethylester | C19H38O2 | - | + | - | [63] |
| 272 | acetic acid, bornyl ester | C12H20O2 | - | - | + | [34] |
| 273 | ethyl linoleate | C20H36O2 | + | - | - | [22] |
| 274 | octenylacetate | C10H18O2 | + | - | - | [65] |
| 275 | 2,5-diethyltetrahydrofuran | C8H16O | + | - | - | [58] |
| 276 | 1,8-cineole | C10H18O | + | - | - | [6] |
| 277 | dihydroartemisinin ethyl ether | C17H28O5 | - | + | - | [18] |
| 278 | pentane, 1-butoxy- | C9H20O | - | - | + | [57] |
| 279 | ethane, 1,1-dibutoxy- | C10H22O2 | - | - | + | [57] |
| 280 | 1,1-dibutoxy-isobutane | C12H26O2 | - | - | + | [57] |
| 281 | butane, 1,1-dibutoxy- | C12H26O2 | - | - | + | [57] |
| 282 | 3-methyl-3-oxetanemethanol | C5H10O2 | + | - | - | [43] |
| 283 | neryl-β-D-glucopyranoside | C16H28O6 | + | - | - | [51] |
| 284 | furfural | C5H4O2 | + | - | - | [58] |
| 285 | 2-acetyl-5-methylfuran | C7H8O2 | + | - | - | [58] |
| 286 | geranyl acetate | C12H20O2 | + | - | - | [58] |
| 287 | octen-3-ol | C9H19O | + | - | + | [6,57] |
| 288 | cis-jasmone | C11H16 | + | - | - | [6] |
| 289 | 2,6-octadien-1-ol, 3,7-dimethyl- | C11H20 | - | - | + | [57] |
| 290 | 3,5-dimethylcyclohex-1-ene-4carboxaldehyde | C9H14O | - | + | - | [33] |
| 291 | (6S,9R)-roseoside | C19H30O8 | - | + | - | [67] |
| 292 | 1-hexadecene | C16H32 | - | + | - | [63] |
| 293 | 1-nonene | C9H18 | - | - | + | [34] |
| 294 | ligustilide | C12H14O2 | + | - | - | [22] |
| 295 | cyclohexene, 2-ethenyl-1,3,3-trimethyl | C11H18 | + | - | - | [43] |
| 296 | daucosterol | C35H60O6 | + | - | - | [54] |
| (2) Aromatic | ||||||
| 297 | thymol | C10H14O | + | + | + | [33,57,58] |
| 298 | carvacrol | C10H14O | - | + | + | [33,57] |
| 299 | p-cymene | C10H14 | + | + | + | [6,26,57] |
| 300 | methyl chavicol | C10H12O | + | - | - | [59] |
| 301 | m-cymene | C10H14 | - | - | + | [57] |
| 302 | 3,4-diethylphenol | C10H14O | - | - | + | [57] |
| 303 | p-eugenol | C10H12O2 | - | - | + | [57] |
| 304 | O-cymene | C10H14 | + | - | - | [60] |
| 305 | cis-anethol | C10H12O | - | + | - | [62] |
| 306 | 3,4,5-trimethoxytoluene | C10H14O3 | - | + | - | [62] |
| 307 | cis-asarone | C10H14O2 | - | + | - | [63] |
| 308 | cuminaldehyde | C10H12O | - | - | + | [64] |
| 309 | benzyl-β-D-glucopyranoside | C13H18O6 | + | - | - | [51] |
| 310 | p-xylene | C8H10 | + | - | - | [58] |
| 311 | benzaldehyde | C7H6O | + | - | + | [57,58] |
| 312 | thymol acetate | C12H16O2 | + | + | + | [33,57,58] |
| 313 | acetophenone | C8H8O | + | - | - | [58] |
| 314 | naphthalene | C10H8O | + | - | - | [43] |
| 315 | protocatechuic acid | C7H6O4 | - | + | - | [18] |
| 316 | sodium ferulate | C10H9NaO4 | - | + | - | [18] |
| 317 | 4,5-dimethyl-2-ethylphenol | C10H14O | - | + | - | [33] |
| 318 | thymyl acetate | C12H16O2 | - | + | - | [26] |
| 319 | carvacryl acetate | C12H16O2 | - | + | - | [26] |
| 320 | 4-O-β-D- glucopyranosylbenzyl-4′-hydroxylbenzoate | C20H22O9 | - | - | + | [39] |
| 321 | 4-O-β-D-glucopyranosylbenzyl-3′-hydroxy-4′- methoxybenzoate | C21H24O10 | - | - | + | [39] |
| 322 | amburoside A | C20H22O10 | - | - | + | [39] |
| 323 | 4-[[(2′,5′-Dihydroxybenzoyl)oxy]methyl]phenyl-O-β-D-glucopyranoside | C20H20O10 | - | - | + | [39] |
| 324 | hyprhombin B methyl ester | C26H22O9 | - | - | + | [39] |
| 325 | methyl lithospermate | C28H24O12 | - | - | + | [39] |
| 326 | dimethyl lithospermate | C29H26O12 | - | - | + | [39] |
| 327 | salvianolic acid C methyl ester | C27H23O10 | - | - | + | [39] |
| 328 | sebestenoids C | C36H30O14 | - | - | + | [39] |
| 329 | cucurbitoside D | C25H30O13 | - | - | + | [39] |
| 330 | 2,4-di-tert-butylphenol | C14H22O | - | - | + | [27] |
| 331 | benzyl benzoate | C14H12O2 | + | - | - | [69] |
| 332 | 4-isopropyl-3-methylphenol | C10H14O | + | - | - | [60] |
| 333 | benzenemethanol, 4-(1-methylethyl)- | C10H14O | + | - | - | [61] |
| 334 | methyl eugenol | C11H14O2 | - | + | - | [62] |
| 335 | elemicin | C12H16O3 | - | + | - | [62] |
| 336 | myristicin | C11H12O3 | - | + | - | [62] |
| 337 | methyl thymol ether | C11H16O | - | - | + | [28] |
| 338 | apiole | C12H14O4 | - | - | + | [28] |
| 339 | 4-hydroxy-2,6-dimethoxyphenyl-β-D-glucopyranoside | C14H20O9 | - | + | - | [67] |
| 340 | 4-hydroxy-3,5-dimethoxyphenyl-β-D-glucopyranoside | C14H20O9 | - | + | - | [67] |
| 341 | 3,4,5-dimethoxyphenyl-β-D-glucopyranoside | C15H22O9 | - | + | - | [67] |
| 342 | 3-hydroxyestragole-β-D-glucopyranosides | C16H22O7 | - | + | - | [67] |
| 343 | 4-acetoxy-3-methoxy acetophenone | C11H12O4 | - | + | - | [63] |
| 344 | benzyl-D-glucopyranoside | C13H18O6 | - | + | - | [63] |
| 345 | 2′,4′-dihydroxy-3′methylacetophenone | C9H10O3 | - | - | + | [34] |
| 346 | acetylthymol | C12H16O2 | - | - | + | [64] |
| 347 | carvacrol acetate | C12H16O2 | + | - | - | [66] |
| 348 | bergaptene | C12H8O4 | + | - | - | [66] |
| 349 | 2-butenylbenzene | C10H12 | + | - | - | [22] |
| 350 | α-methyl-benzenepropanol | C10H14O | + | - | - | [22] |
| 351 | cuminic acid | C10H12O | + | - | - | [22] |
| 352 | p-cymen-8-ol | C11H16O | + | - | - | [6] |



















4. Conclusions and Prospects


Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Q.; Lai, F.G.; Yang, S.T. Identification of original plants of Elsholtzia jiangxiang. J. Chin. Med. Mater. 1986, 2, 22–25. [Google Scholar]
- Zhu, G.P.; Shi, J.L. A new variety of Elsholtzia stone, the original plant of Chinese medicinal herb Elsholtzia Jiang. J. Syst. Evol. 1995, 3, 305. [Google Scholar]
- Cao, H.; Zhao, W.L.; Hao, J.D.; Li, Y.H. Research on latin scientific names of medicinal materials in the 2020 edition of Chinese Pharmacopoeia. J. Chin. Med. Mater. 2022, 45, 1002–1007. [Google Scholar]
- Pudziuvelyte, L.; Liaudanskas, M.; Jekabsone, A.; Sadauskiene, I.; Bernatoniene, J. Elsholtzia ciliata (Thunb.) Hyl. extracts from different plant parts: Phenolic composition, antioxidant, and anti-Inflammatory activities. Molecules 2020, 25, 1153. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Y.; Xu, J.; Yang, Y.Y.; Shao, Y.Z.; Zhou, F.; Wang, J.L. Toxicity and synergistic effect of Elsholtzia ciliata essential oil and its main components against the adult and larval stages of tribolium castaneum. Foods 2020, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.P.; Liu, X.C.; Lai, D.W.; Zhou, L.G.; Liu, Z.L. Analysis of the essential oil of Elsholtzia ciliate aerial parts and its insecticidal activities against liposcelis bostrychophila. Helv. Chim. Acta 2016, 99, 90–94. [Google Scholar] [CrossRef]
- Zienkaitė, K.; Liekis, A.; Sadauskienė, I.; Pudžiuvelytė, L.; Bernatonienė, J.; Šimonytė, S. The effect of Elsholtzia Ciliata essential oil on catalase (Cat) activity in mice brain. In Proceedings of the XIII International Conference of the Lithuanian Neuroscience Association “CONSCIOUSNESS” (LNA conference), Kaunas, Lithuania, 26 November 2021. [Google Scholar]
- Trinh, T.A.; Seo, Y.H.; Choi, S.; Lee, J.; Kang, K.S. Protective effect of osmundacetone against neurological cell death caused by oxidative glutamate toxicity. Biomolecules 2021, 11, 328. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Tran, H.; Schwaiger, S.; Stuppner, H.; Marzocco, S. Effect of non-volatile constituents of Elsholtzia ciliata (Thunb.) Hyl. from southern vietnam on reactive oxygen species and nitric oxide release in macrophages. Chem. Biodivers. 2021, 18, e2000577. [Google Scholar] [CrossRef]
- Phong, H.X.; Viet, N.T.; Quyen, N.T.N.; Van Thinh, P.; Trung, N.M.; Ngan, T.T.K. Phytochemical screening, total phenolic, flavonoid contents, and antioxidant activities of four spices commonly used in Vietnamese traditional medicine. Mater. Today Proc. 2021, 56, A1–A5. [Google Scholar] [CrossRef]
- Zhang, Q.; Porto, N.M.; Guilhon, C.C.; Giorno, T.B.S.; Alviano, D.S.; Agra, M.D.; Fernandes, P.D.; Boylan, F. Pharmacognostic study on Elsholtzia ciliata (Thumb.) Hyl: Anatomy, phytochemistry and pharmacological activities. Pharmaceuticals 2021, 14, 1152. [Google Scholar] [CrossRef]
- Li, J.E.; Fan, S.T.; Qiu, Z.H.; Li, C.; Nie, S.P. Total flavonoids content, antioxidant and antimicrobial activities of extracts from Mosla chinensis Maxim. cv. Jiangxiangru. LWT—Food Sci. Technol. 2015, 64, 1022–1027. [Google Scholar] [CrossRef]
- Li, J.E.; Nie, S.P.; Xie, M.Y.; Li, C. Isolation and partial characterization of a neutral polysaccharide from Mosla chinensis Maxim. cv. Jiangxiangru and its antioxidant and immunomodulatory activities. J. Funct. Foods 2014, 6, 410–418. [Google Scholar] [CrossRef]
- Cao, L.; Si, J.Y.; Liu, Y.; Sun, H.; Jin, W.; Li, Z.; Zhao, X.H.; Pan, R.L. Essential oil composition, antimicrobial and antioxidant properties of Mosla chinensis Maxim. Food Chem. 2009, 115, 801–805. [Google Scholar] [CrossRef]
- Liu, X.P.; Jia, J.; Yang, L.; Yang, F.J.; Ge, H.S.; Zhao, C.J.; Zhang, L.; Zu, Y.G. Evaluation of antioxidant activities of aqueous extracts and fractionation of different parts of Elsholtzia ciliata. Molecules 2012, 17, 5430–5441. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.Y.; Gao, H.; Wang, X.T.; Yan, L.; Pang, B. Study on the antipyretic and anti-inflammatory effects of producing area processing integrated Mosla chinensis Maxim. Chin. Herb. Med. 2018, 49, 4737–4742. [Google Scholar]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar] [PubMed]
- Wang, X.; Cheng, K.; Liu, Z.; Sun, Y.; Zhou, L.; Xu, M.; Dai, X.; Xiong, Y.; Zhang, H. Bioactive constituents of Mosla chinensis. cv. Jiangxiangru ameliorate inflammation through MAPK signaling pathways and modify intestinal microbiota in DSS-induced colitis mice. Phytomedicine 2021, 93, 153804. [Google Scholar] [CrossRef]
- Zheng, K.; Wu, S.Z.; Lv, Y.W.; Pang, P.; Deng, L.; Xu, H.C.; Shi, Y.C.; Chen, X.Y. Carvacrol inhibits the excessive immune response induced by influenza virus A via suppressing viral replication and TLR/RLR pattern recognition. J. Ethnopharmacol. 2021, 268, 113555. [Google Scholar] [CrossRef]
- Kim, H.H.; Yoo, J.S.; Lee, H.S.; Kwon, T.K.; Shin, T.Y.; Kim, S.H. Elsholtzia ciliata inhibits mast cell-mediated allergic inflammation: Role of calcium, p38 mitogen-activated protein kinase and nuclear factor-kappa B. Exp. Biol. Med. 2011, 236, 1070–1077. [Google Scholar] [CrossRef]
- Li, Z.M.; Sun, Y.M.; Wang, M.; Peng, L.; Ren, P. Study on antibacterial activity of different polar extracts from Mosla chinensis Maxim. cv. Jiangxiangru. Sci. Technol. Food Ind. 2014, 35, 115–116+120. [Google Scholar]
- Hu, H.B.; Cao, H.; Jian, Y.F.; Zheng, X.D. Study on extraction and antibacterial activity of active components from Elsholtzia chinensis. Grassl. Sci. 2007, 24, 36–39. [Google Scholar]
- Li, Y.Q.; Ren, Y.S.; Wang, L.J.; Ai, J.; Liang, S.; Zhang, T.P.; Liao, M.C.; Li, J. Preparation of nanoemulsion spray from Moslae Herba volatile oil and its antibacterial activity. China J. Chin. Mater. Med. 2021, 46, 4986–4992. [Google Scholar]
- Xiang, P.; Lou, G.Q.; Wang, S.Y.; Chen, Q. Analysis of volatile oils in Elsholtzia ciliata and Elsholtzia cypriani and evaluation of their biological activities. Chin. Tradit. Pat. Med. 2017, 39, 1880–1884. [Google Scholar]
- Li, Z.M.; Wang, M.; Peng, L. Chemical composition analysis of essential oil from Mosla chinensis Maxim. cv. Jiangxiangru and inhibitory activity of the oil and its major constituents on biofilm formation of staphylococcus aureus. Food Sci. 2016, 37, 138–143. [Google Scholar]
- Peng, L.; Xiong, Y.; Wang, M.; Han, M.; Cai, W.; Li, Z. Chemical composition of essential oil in Mosla Chinensis Maxim Cv. Jiangxiangru and its inhibitory effect on Staphylococcus aureus biofilm formation. Open Life Sci. 2018, 13, 1–10. [Google Scholar] [CrossRef]
- Liu, M.T.; Luo, F.Y.; Zeng, J.G. Composition analysis of essential oil of Mosla Chinensis Maxim and its antibacterial and antioxidant activity. Chin. Tradit. Pat. Med. 2020, 42, 3091–3095. [Google Scholar]
- Lin, C.L.; Cai, J.Z.; Lin, G.Y. Chemical constituents study of volatile oils from the Mosla Chinensis Maxim in zhejiang province. Chin. Arch. Tradit. Chin. Med. 2012, 30, 197–198. [Google Scholar]
- Nogueira, J.O.E.; Campolina, G.A.; Batista, L.R.; Alves, E.; Caetano, A.R.S.; Brandão, R.M.; Nelson, D.L.; Cardoso, M.D.G. Mechanism of action of various terpenes and phenylpropanoids against Escherichia coli and Staphylococcus aureus. FEMS Microbiol. Lett. 2021, 368, fnab052. [Google Scholar] [CrossRef]
- Jiang, H.M.; Fang, J.; Lu, X.Y.; Peng, L.S.; Liu, J. Preliminary study on inhibition mechanism of Mosla Chinensis Maxim extract on aspergillus flavus. J. Chin. Inst. Food Sci. Technol. 2007, 7, 47–50. [Google Scholar]
- Qi, W.W.; Shen, Y.T.; Chen, C.Y.; Chen, J.Y.; Wan, C.P. Antifungal mechanisms of the active ingredients carvacrol of Mosla Chinensis ‘Jiangxiangru’ against penicillium digitatum. Mod. Food Sci. Technol. 2018, 34, 65–69+64. [Google Scholar]
- Huang, K.; Zhang, D.; Ren, J.J.; Dong, R.; Wu, H. Screening of the repellent activity of 12 essential oils against adult german cockroach (Dictyoptera: Blattellidae): Preparation of a sustained release repellent agent of binary oil-γ-CD and its repellency in a small container. J. Econ. Entomol. 2020, 113, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.P.; Weng, H.; Li, C.; He, J.; Zhang, X.; Ma, Z.Q. Efficacy of essential oil from Mosla chinensis Maxim. cv. Jiangxiangru and its three main components against insect pests. Ind. Crops Prod. 2020, 147, 112237. [Google Scholar] [CrossRef]
- Chen, F.F.; Peng, Y.H.; Zeng, D.Q.; Zhang, Y.; Qin, Q.H.; Huang, Y. Composition and bioactivity of the essential oils from Mosla chinensis against Aedes albopictus. Chin. J. Vector Biol. Control 2010, 21, 211–214. [Google Scholar]
- Le, T.B.; Beaufay, C.; Nghiem, D.T.; Mingeot-Leclercq, M.P. In vitro anti-leishmanial activity of essential oils extracted from vietnamese plants. Molecules 2017, 22, 1071. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.L.; Cui, Y.; Qin, Y.H.; Ren, Y.X.; Liu, X.; Tao, L.; Dai, X.D. Effect of Mosla chinensis Maximon Trichomonas vaginalis in vitro. J. Dalian Med. Univ. 2009, 31, 282–285. [Google Scholar]
- Zhang, X.X.; Wu, Q.F.; Yan, Y.L.; Zhang, F.L. Inhibitory effects and related molecular mechanisms of total flavonoids in Mosla chinensis Maxim against H1N1 influenza virus. Inflamm. Res. 2018, 67, 179–189. [Google Scholar] [CrossRef]
- Li, Y.; Fang, S.G. Effects of elsholtzia chinensis ethanol extract on avian infectious bronchitis virus proliferation. J. Yangtze Univ. (Nat. Sci. Ed.) 2019, 16, 75–80, 78. [Google Scholar]
- Du, J.C.; Yang, L.Y.; Shao, L.; Yu, F.; Li, R.T.; Zhong, J.D. Study on phenolic acid compounds from acrial parts of Mosla chinensis and its anti-influenza activity, J. Chin. Med. Mater. 2021, 44, 2594–2599. [Google Scholar]
- Xu, J.L.; Jiang, W.E. Effect of water extract of Mosla chinensis Maxim against influenza virus. Zhejiang J. Tradit. Chin. Med. 2013, 48, 273–274. [Google Scholar]
- Lynn, Y.; Jim, H.; Towers, G. Isolation of the anthropogenic compound fluoranthene in a screening of Chinese medicinal plants for antiviral compounds. Planta Med. 1995, 61, 187–188. [Google Scholar]
- Sung, Y.Y.; Yoon, T.; Yang, W.K.; Kim, S.J.; Kim, H.K. Article inhibitory effects of Elsholtzia ciliata extract on fat accumulation in high-fat diet-induced obese mice. Appl. Biol. Chem. 2011, 54, 388–394. [Google Scholar]
- Pudziuvelyte, L.; Stankevicius, M.; Maruska, A.; Petrikaite, V.; Ragazinskiene, O.; Draksiene, G.; Bernatoniene, J. Chemical composition and anticancer activity of Elsholtzia ciliata essential oils and extracts prepared by different methods. Ind. Crops Prod. 2017, 107, 90–96. [Google Scholar] [CrossRef]
- Sun, D.Y.; Wang, X.Y.; Wang, X.T.; Yan, L.; Liu, X.F.; Pang, B.; Gao, H. Comparison of integration processing technology of origin and traditional cutting processing technology of Moslae Herba for lung-Yang deficiency rats. China J. Chin. Mater. Med. 2018, 43, 2537–2542. [Google Scholar]
- Li, J.E.; Cui, S.W.; Nie, S.P.; Xie, M.Y. Structure and biological activities of a pectic polysaccharide from Mosla chinensis Maxim. cv. Jiangxiangru. Carbohydr. Polym. 2014, 105, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Li, J.E.; Nie, S.P.; Xie, M.Y.; Huang, D.F.; Wang, Y.T.; Li, C. Chemical composition and antioxidant activities in immumosuppressed mice of polysaccharides isolated from Mosla chinensis Maxim cv. Jiangxiangru. Int. Immunopharmacol. 2013, 17, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Z.M.; Peng, L. α-Glucosidase Inhibition of Mosla Chinensis Maxim.cv. Jiangxiangru extracts. J. Nanchang Vocat. Tech. Techers’ Coll. 2015, 6, 40–45. [Google Scholar]
- Macianskiene, R.; Pudziuvelyte, L.; Bernatoniene, J.; Almanaityte, M.; Navalinskas, A.; Andriule, R.; Jurevicius, J. Antiarrhythmic properties of Elsholtzia ciliata essential oil on electrical activity of the isolated rabbit heart and preferential inhibition of sodium conductance. Biomolecules 2020, 10, 948. [Google Scholar] [CrossRef]
- Nugroho, A.; Park, J.H.; Choi, J.S.; Park, K.S.; Hong, J.P.; Park, H.J. Structure determination and quantification of a new flavone glycoside with anti-acetylcholinesterase activity from the herbs of Elsholtzia ciliata. Nat. Prod. Res. 2019, 33, 814–821. [Google Scholar] [CrossRef]
- Shu, R.G.; Hu, H.W.; Zhang, P.Z.; Ge, F. Triterpenes and flavonoids from Mosla chinensis. Chem. Nat. Compd. 2012, 48, 706–707. [Google Scholar] [CrossRef]
- Seo, Y.H.; Trinh, T.A.; Ryu, S.M.; Kim, H.S.; Choi, G.; Moon, B.C.; Shim, S.H.; Jang, D.S.; Lee, D.; Kang, K.S.; et al. Chemical constituents from the aerial parts of Elsholtzia ciliata and their protective activities on glutamate-induced HT22 cell death. J. Nat. Prod. 2020, 83, 3149–3155. [Google Scholar] [CrossRef]
- Kim, T.W.; Kim, Y.J.; Seo, C.S.; Kim, H.T.; Park, S.R.; Lee, M.Y.; Jung, J.Y. Elsholtzia ciliata (Thunb.) Hylander attenuates renal inflammation and interstitial fibrosis via regulation of TGF-beta and Smad3 expression on unilateral ureteral obstruction rat model. Phytomedicine 2016, 23, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Pan, J.P.; Zhou, M. Analysis of ethyl acetate constituents of Mosla chinensis Maxim cv. jiangxiangru and their inhibitory activity of α-glucosidase. China Food Saf. Mag. 2021, 25, 65–68. [Google Scholar]
- Zheng, X.D.; Hu, H.B. Chemical constituents of Elsholtzia ciliata (Thunb.) Hyland. Chem. Res. 2006, 17, 85–87. [Google Scholar]
- Ma, J.; Xu, R.R.; Lu, Y.; Ren, D.F.; Lu, J. Composition, antimicrobial and antioxidant activity of supercritical fluid extract of Elsholtzia ciliata. J. Essent. Oil-Bear. Plants 2018, 21, 556–562. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, D.M.; Luo, Y.M. Studies on chemical constituents of Mosla Chinensis Maxim.cv. Jiangxiangru. Chin. J. Exp. Tradit. Med. Formulae 2010, 16, 56–59. [Google Scholar]
- Cao, H.; Li, Z.; Chen, X. QSRR study of GC retention indices of volatile compounds emitted from Mosla chinensis Maxim by multiple linear regression. Chin. J. Chem. 2011, 29, 2187–2196. [Google Scholar] [CrossRef]
- Wang, X.; Gong, L.; Jiang, H. Study on the difference between volatile constituents of the different parts from Elsholtzia ciliata by SHS-GC-MS. Am. J. Anal. Chem. 2017, 8, 625–635. [Google Scholar] [CrossRef]
- Dũng, N.X.; Van Hac, L.; Hái, L.H.; Leclercq, P.A. Composition of the essential oils from the aerial parts of Elsholtzia ciliata (Thunb.) Hyland. from vietnam. J. Essent. Oil Res. 1996, 8, 107–109. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Gong, Q.F.; He, Y.; Zhong, L.Y.; Yu, H.; Faan, R.N. Optimization of technology of Moslae herba processed with ginger juice and analysis of its volatile components by HS-GC-MS. Chin. J. Exp. Tradit. Med. Formulae 2019, 25, 162–167. [Google Scholar]
- Liu, X.P.; Jing, X.M. Research of chemical composition and biological activity of the essential oil from Elsholtzia ciliata. J. Heilongjiang Bayi Agric. Univ. 2018, 30, 35–39. [Google Scholar]
- Yu, H.; Hu, J.; Bai, F.P.; Qin, K.M. Analysis of volatiles from bark of Moslae herba by gas chromatography-mass spectroscopy. Guid. J. Tradit. Chin. Med. Pharm. 2017, 23, 82–84. [Google Scholar]
- Liu, H.; Li, G.S.; Luo, Y.M.; Chen, Z.W.; Liu, W.Q. Chemical constituents of petroleum spirit part of Mosla chinensis ‘Jiangxiangru’. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 68–70. [Google Scholar]
- Mao, Y.; Li, Z.G.; Cao, J.L. Analysis of volatile oil composition of Mosla chinensis. J. Zhejiang For. Coll. 2008, 25, 262–265. [Google Scholar]
- Xiang, P. Study and Application of Chemical Constituents and Biological Activity of Medicinal Plant Elsholtzia ciliata from Guizhou. Master’s Thesis, Guizhou University, Guizhou, China, 2018. [Google Scholar]
- Luo, G.M.; Yang, G.Y.; Liu, H.N.; Yang, Y.Q.; Chen, Y.; Li, X.; Zhang, X.Y. Comparative study on chemical constituents of volatile oil of Elsholtzia ciliata. China J. Chin. Mater. Med. 2007, 32, 1483–1485. [Google Scholar]
- Shen, J.J.; Zhang, D.M.; Liu, H.; Luo, Y.M. Studies on polar components of Elsholtzia chinensis Ⅱ. China J. Chin. Mater. Med. 2011, 36, 1779–1781. [Google Scholar]
- Liu, H.; Shen, J.J.; Zhang, D.M.; Luo, Y.M. Studies on polar constituents of Mosla Chinensis ‘Jiangxiangru’. Chin. J. Exp. Tradit. Med. Formulae 2010, 16, 84–86. [Google Scholar]
- Gao, J.P.; Li, D.D.; Qin, L.; Yang, L.; Tian, H.L. Comparative analysis of volatile components in leaves, flowers, and fruits of three Elsholtzia plants. J. Beijing Univ. Agric. 2020, 35, 108–114. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liu, X.; Chen, Y.; An, Y.; Zhao, W.; Wang, L.; Tian, J.; Kong, D.; Xu, Y.; Ba, Y.; et al. Elsholtzia ciliata (Thunb.) Hyland: A Review of Phytochemistry and Pharmacology. Molecules 2022, 27, 6411. https://doi.org/10.3390/molecules27196411
Wang F, Liu X, Chen Y, An Y, Zhao W, Wang L, Tian J, Kong D, Xu Y, Ba Y, et al. Elsholtzia ciliata (Thunb.) Hyland: A Review of Phytochemistry and Pharmacology. Molecules. 2022; 27(19):6411. https://doi.org/10.3390/molecules27196411
Chicago/Turabian StyleWang, Fulin, Xue Liu, Yueru Chen, Ying An, Wei Zhao, Lu Wang, Jinli Tian, Degang Kong, Yang Xu, Yahui Ba, and et al. 2022. "Elsholtzia ciliata (Thunb.) Hyland: A Review of Phytochemistry and Pharmacology" Molecules 27, no. 19: 6411. https://doi.org/10.3390/molecules27196411
APA StyleWang, F., Liu, X., Chen, Y., An, Y., Zhao, W., Wang, L., Tian, J., Kong, D., Xu, Y., Ba, Y., & Zhou, H. (2022). Elsholtzia ciliata (Thunb.) Hyland: A Review of Phytochemistry and Pharmacology. Molecules, 27(19), 6411. https://doi.org/10.3390/molecules27196411





