An Eco-Friendly Quaternary Ammonium Salt as a Corrosion Inhibitor for Carbon Steel in 5 M HCl Solution: Theoretical and Experimental Investigation
Abstract
:1. Introduction
2. Materials and Methods
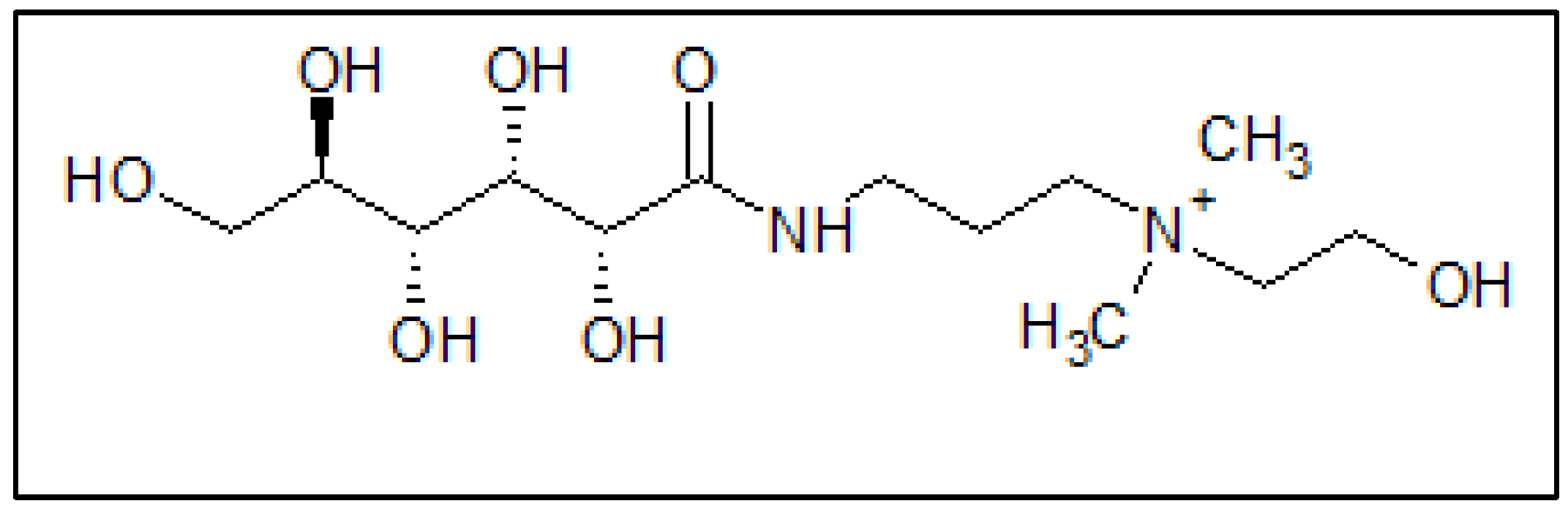
2.1. Materials
2.2. Weight Loss Measurements
2.3. Electrochemical Measurements
2.4. Surface Characterization
2.5. Eco-Toxicity Assessment
2.6. Quantum Chemical Studies
3. Results and Discussion
3.1. Weight Loss Measurements
3.2. Electrochemical Measurements
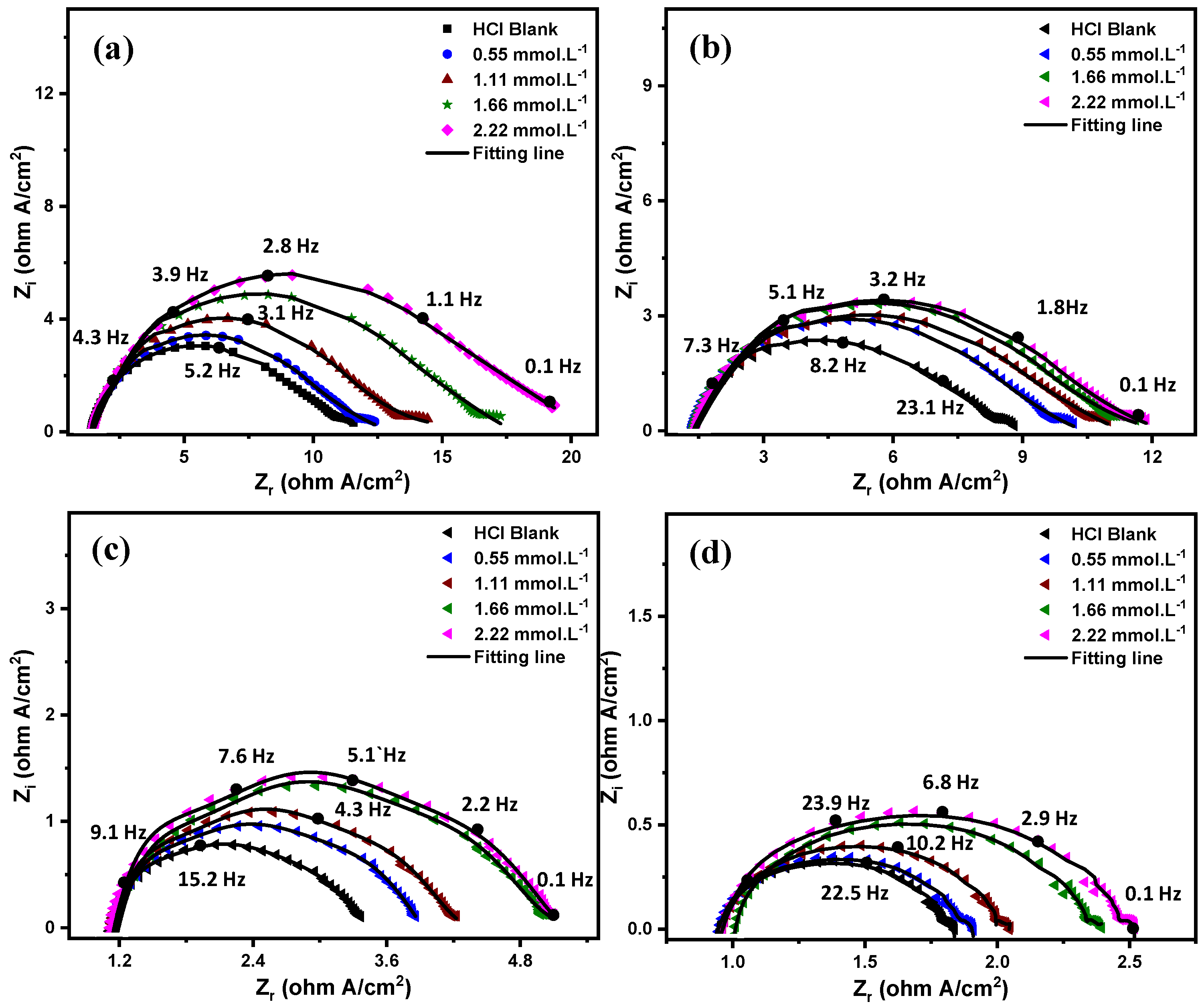
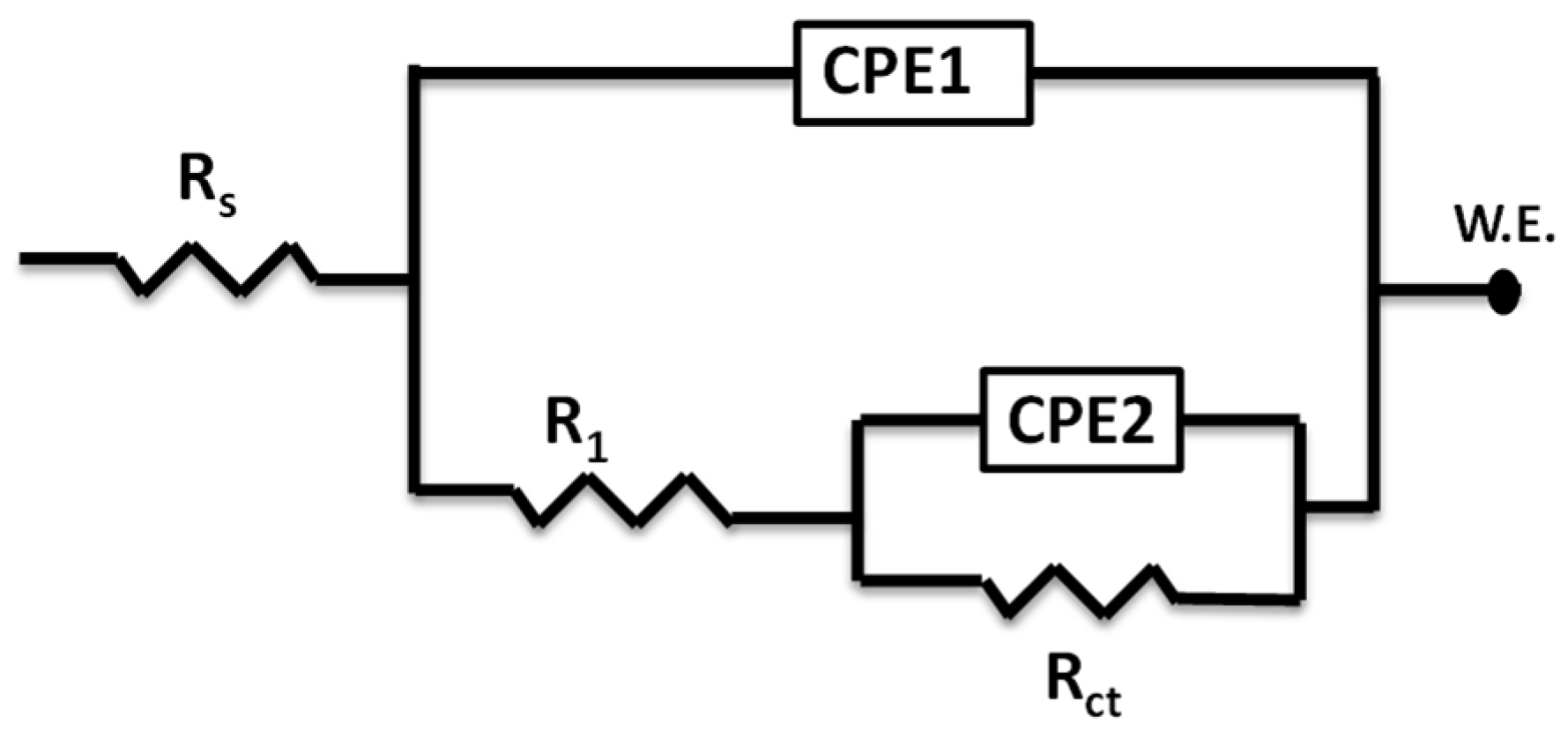
3.2.1. Electrochemical Impedance Spectroscopy (EIS)
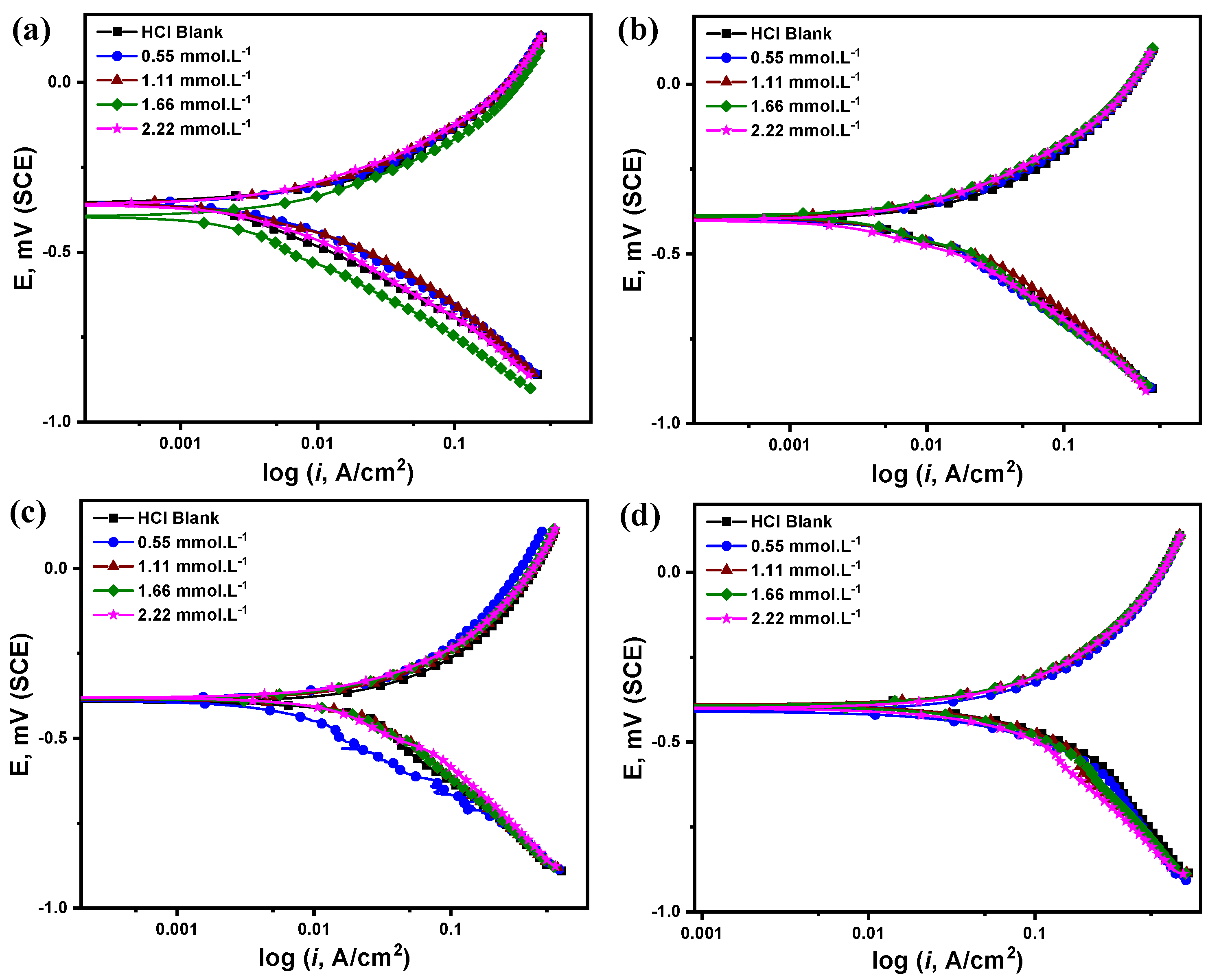
3.2.2. Potentiodynamic Polarization Measurements (PDP)
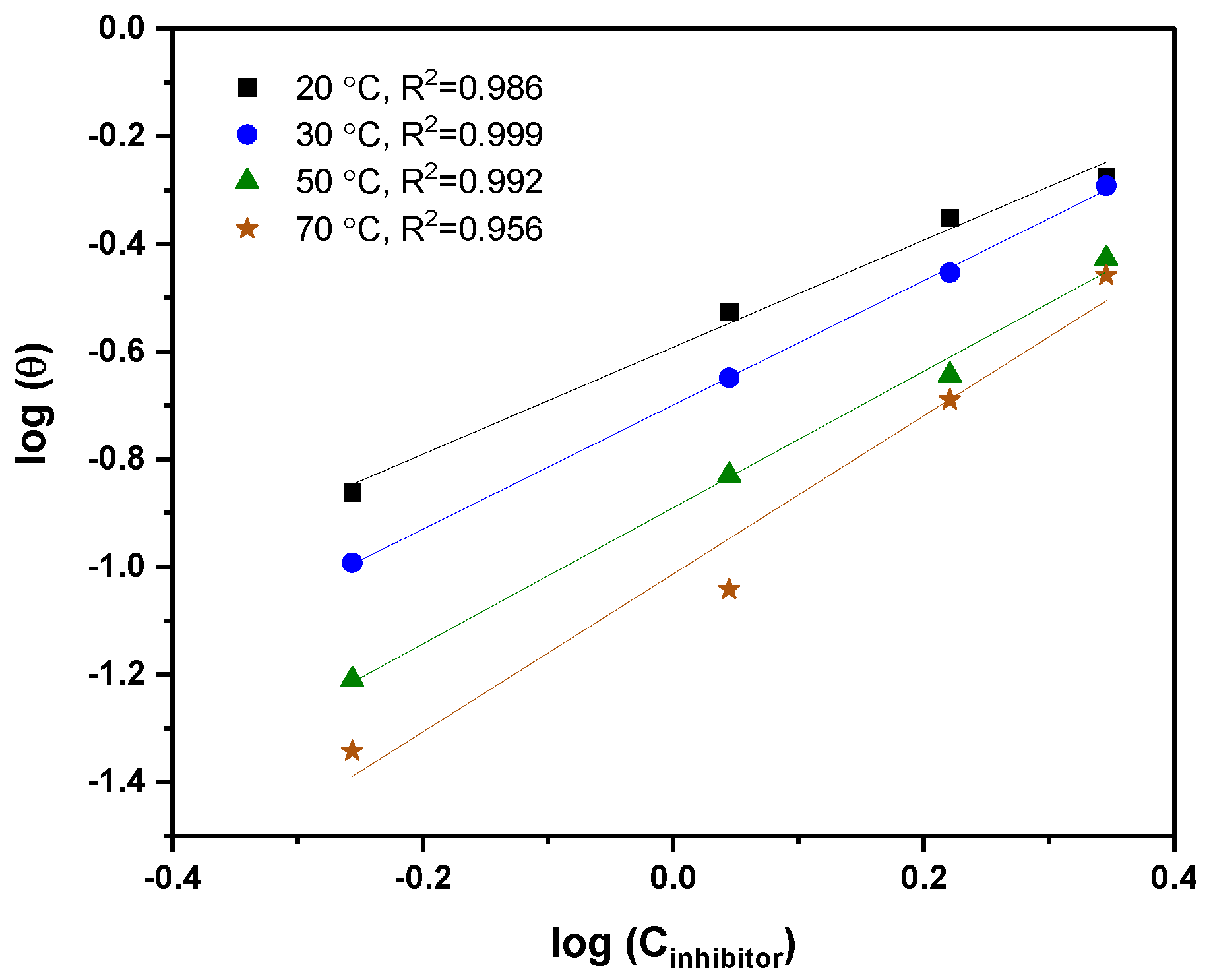
3.3. Adsorption Studies and Thermodynamic Isotherms
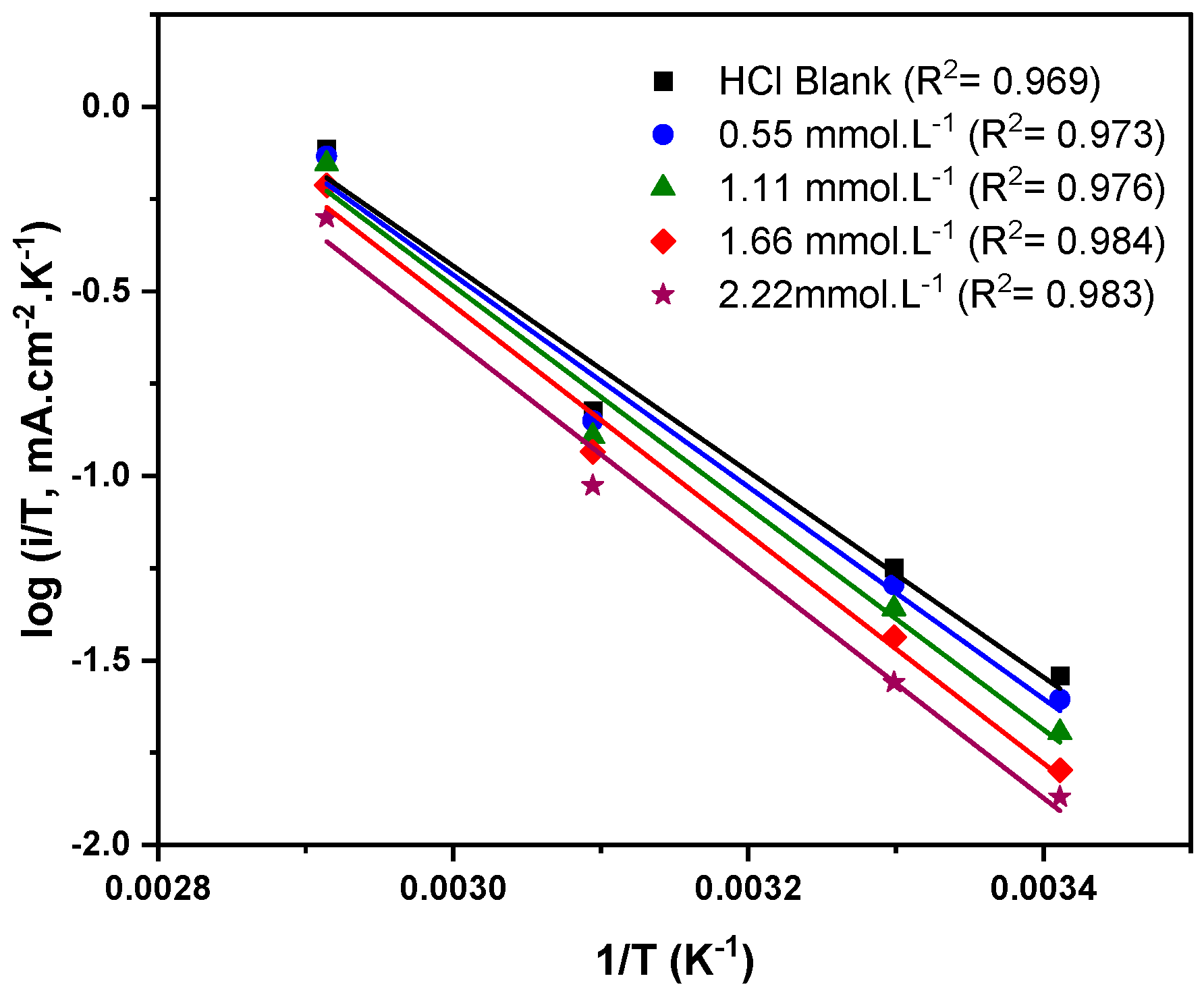
3.4. Corrosion Kinetics Studies
3.5. Surface Characterization
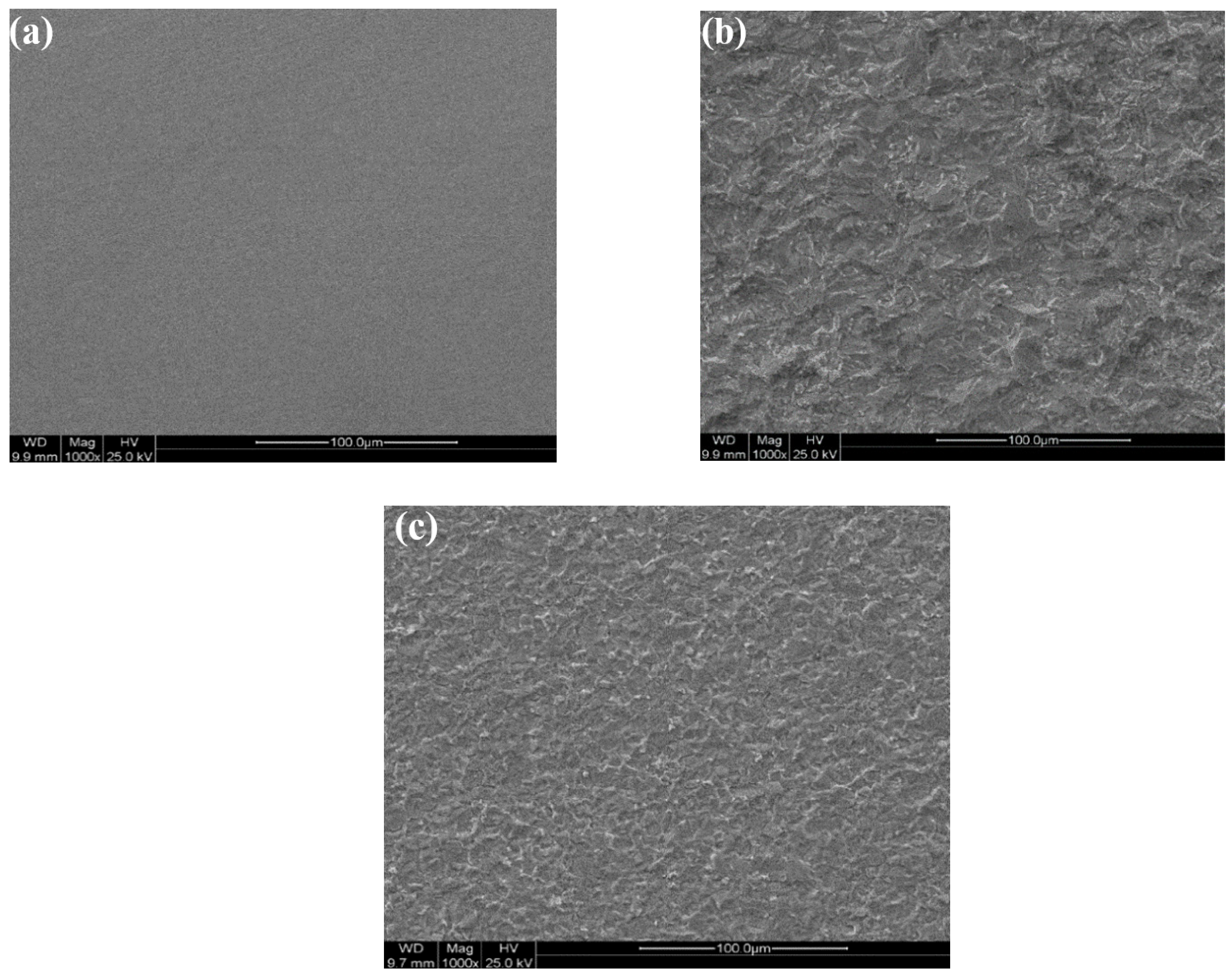
3.5.1. Microscope Analysis
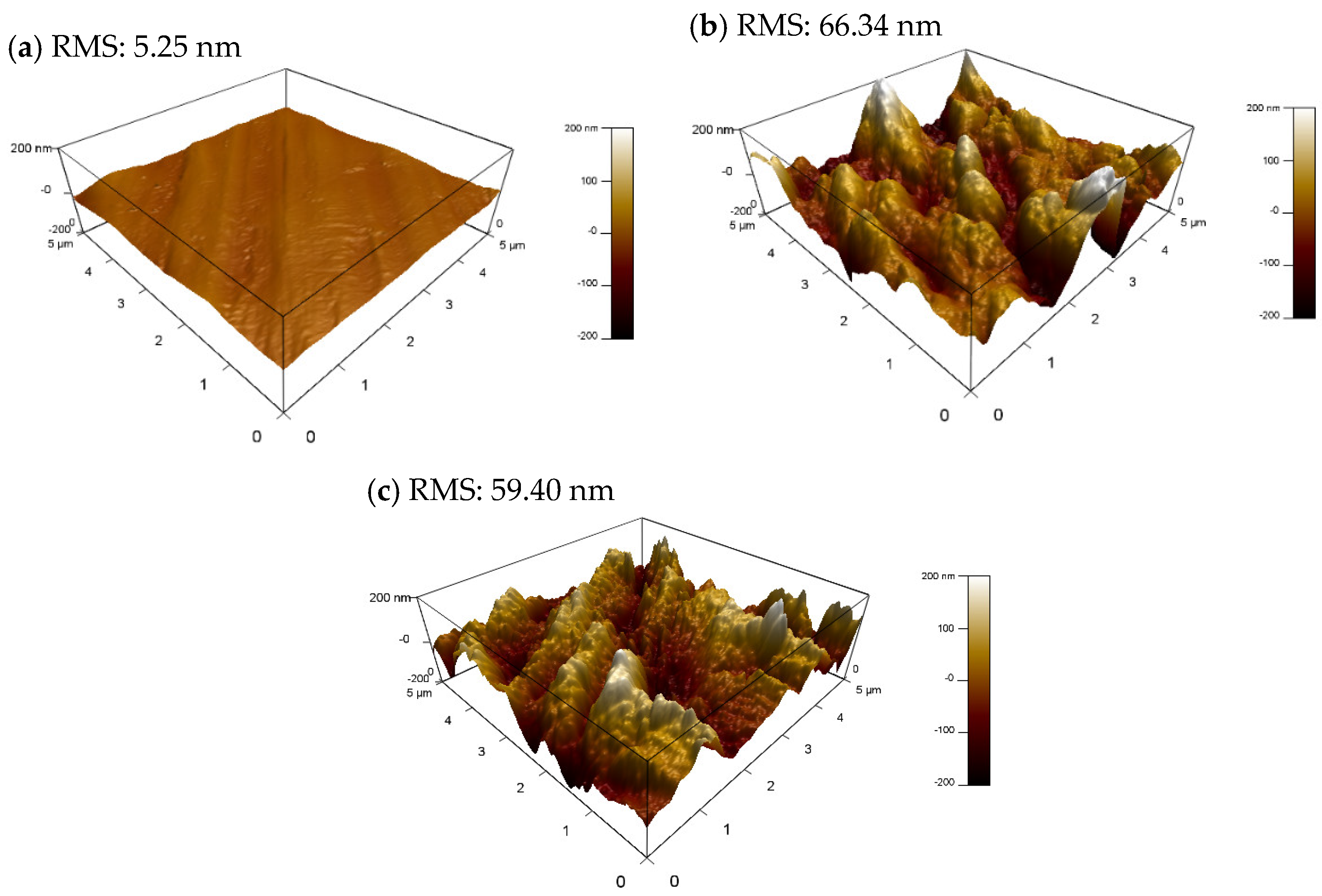
3.5.2. AFM Analysis
3.6. Eco-Toxicity Assessment
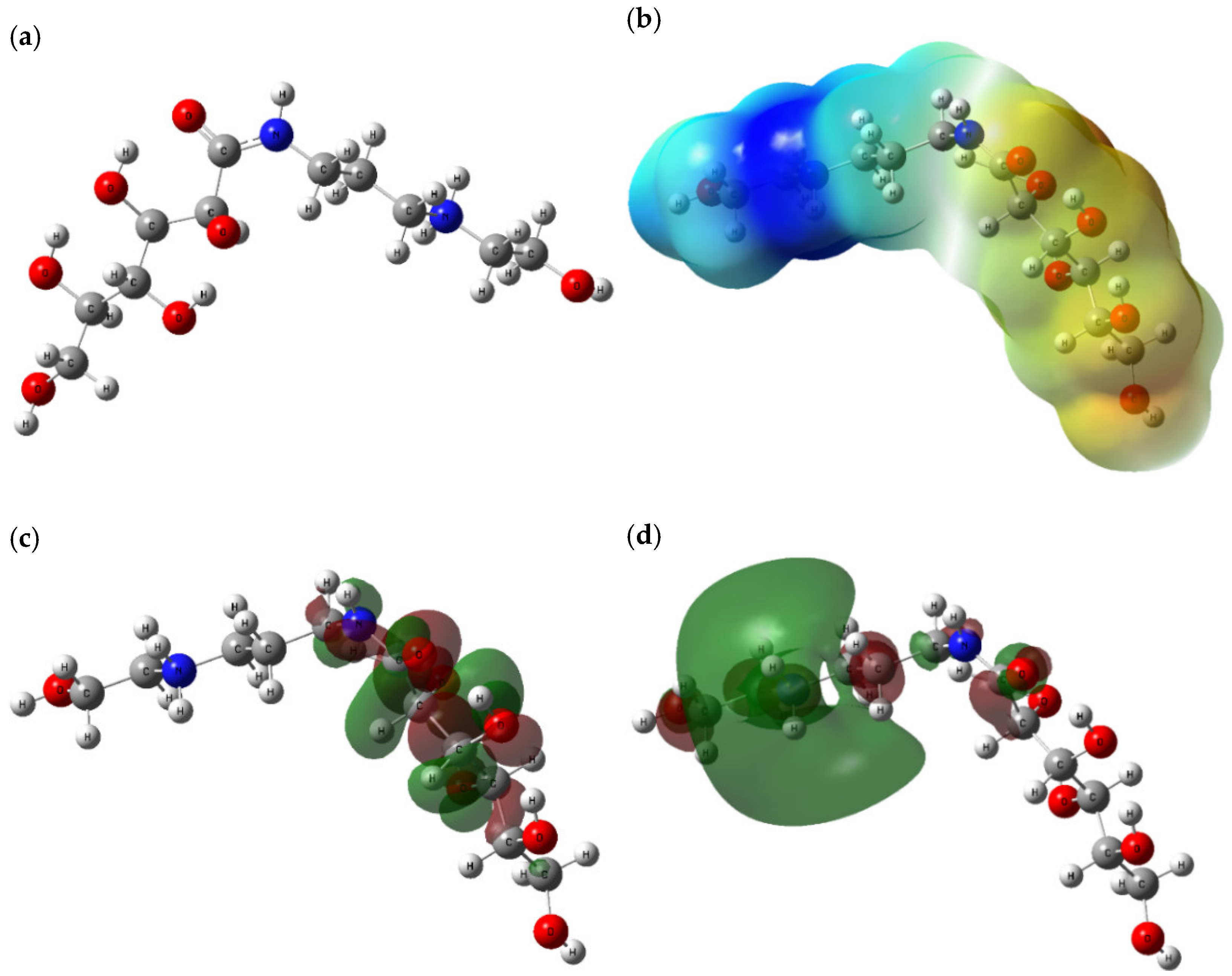
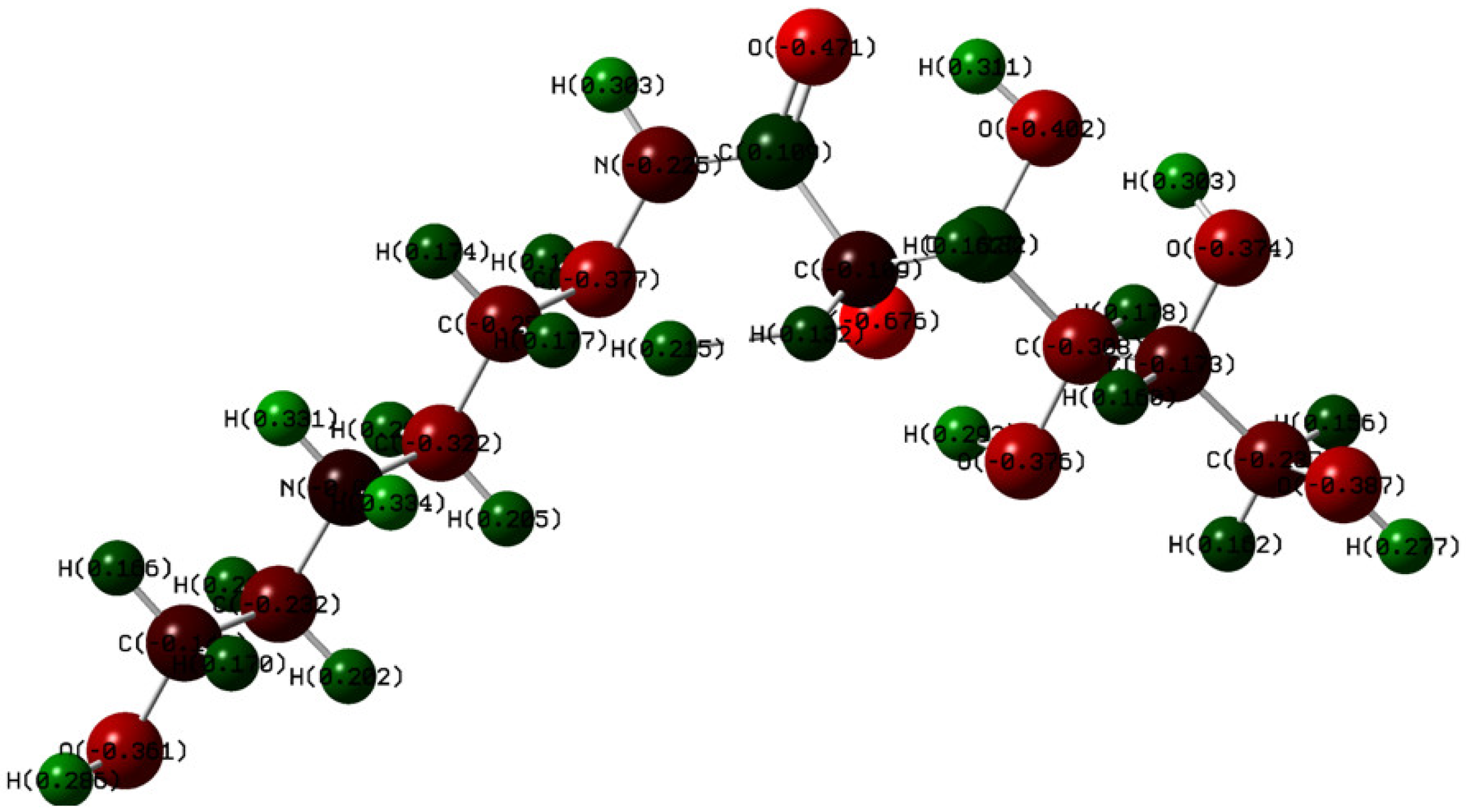
3.7. Quantum Chemical Calculations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saji, V.S.; Umoren, S.A. Corrosion Inhibitors in the Oil and Gas Industry; Saji, V.S., Umoren, S.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Kermani, M.B.; Morshed, A. Carbon Dioxide Corrosion in Oil and Gas Production—A Compendium. Corrosion 2003, 59, 659–683. [Google Scholar] [CrossRef]
- I Fayomi, O.S.; Akande, I.G.; Odigie, S. Economic Impact of Corrosion in Oil Sectors and Prevention: An Overview. J. Phys. Conf. Ser. 2019, 1378, 022037. [Google Scholar] [CrossRef]
- Tiu, B.D.; Advincula, R.C. Polymeric corrosion inhibitors for the oil and gas industry: Design principles and mechanism. React. Funct. Polym. 2015, 95, 25–45. [Google Scholar] [CrossRef]
- Tamalmani, K.; Husin, H. Review on Corrosion Inhibitors for Oil and Gas Corrosion Issues. Appl. Sci. 2020, 10, 3389. [Google Scholar] [CrossRef]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. NPJ Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Harsimran, S.; Santosh, K.; Rakesh, K. Overview of corrosion and its control: A critical review. Proc. Eng. Sci. 2021, 3, 13–24. [Google Scholar] [CrossRef]
- Ríos-Mercado, R.Z.; Borraz-Sánchez, C. Optimization problems in natural gas transportation systems: A state-of-the-art review. Appl. Energy 2015, 147, 536–555. [Google Scholar] [CrossRef]
- Gupta, N.K.; Verma, C.; Quraishi, M.A.; Mukherjee, A.K. Schiff’s bases derived from l-lysine and aromatic aldehydes as green corrosion inhibitors for mild steel: Experimental and theoretical studies. J. Mol. Liq. 2016, 215, 47–57. [Google Scholar] [CrossRef]
- Beidokhti, B.; Dolati, A.; Koukabi, A.H. Effects of alloying elements and microstructure on the susceptibility of the welded HSLA steel to hydrogen-induced cracking and sulfide stress cracking. Mater. Sci. Eng. A 2009, 507, 167–173. [Google Scholar] [CrossRef]
- ISO 13680:2010; Petroleum and Natural Gas Industries—Corrosion-Resistant alloy Seamless Tubes for Use as Casing, Tubing and Coupling Stock—Technical Delivery Conditions. BSI: London, UK, 2002.
- Hamza, A.; Hussein, I.A.; Jalab, R.; Saad, M.; Mahmoud, M. Review of iron sulfide scale removal and inhibition in oil and gas wells: Current status and perspectives. Energy Fuels 2021, 35, 14401–14421. [Google Scholar] [CrossRef]
- Sliem, M.H.; Radwan, A.B.; Mohamed, F.S.; Alnuaimi, N.A.; Abdullah, A.M. An efficient green ionic liquid for the corrosion inhibition of reinforcement steel in neutral and alkaline highly saline simulated concrete pore solutions. Sci. Rep. 2020, 10, 14565. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E.E. Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 565–573. [Google Scholar] [CrossRef]
- Raghavendra, N.; Chitnis, S.R.; Sheelimath, D.S. Anti-corrosion investigation of polylysine (amino acid polymer) as efficacious corrosion inhibitor for Al in industrial acidic pickling environment. J. Bio-Tribo-Corrosion 2021, 7, 29. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Quadri, T.W.; Sherif, E.S.M.; Ebenso, E.E. Gravimetric, electrochemical, surface morphology, DFT, and Monte Carlo simulation studies on three N-substituted 2-aminopyridine derivatives as corrosion inhibitors of mild steel in acidic medium. J. Phys. Chem. C 2018, 122, 11870–11882. [Google Scholar] [CrossRef]
- Aly, K.I.; Younis, O.; Mahross, M.H.; Tsutsumi, O.; Mohamed, M.G.; Sayed, M.M. Novel conducting polymeric nanocomposites embedded with nanoclay: Synthesis, photoluminescence, and corrosion protection performance. Polym. J. 2019, 51, 77–90. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y. A Review on Conducting Polymers and Nanopolymer Composite Coatings for Steel Corrosion Protection. Coatings 2019, 9, 807. [Google Scholar] [CrossRef]
- Kumar, R.; Yadav, O.S.; Singh, G. Electrochemical and surface characterization of a new eco-friendly corrosion inhibitor for mild steel in acidic media: A cumulative study. J. Mol. Liq. 2017, 237, 413–427. [Google Scholar] [CrossRef]
- Zhu, Y.; Free, M.L.; Woollam, R.; Durnie, W. A review of surfactants as corrosion inhibitors and associated modeling. Prog. Mater. Sci. 2017, 90, 159–223. [Google Scholar] [CrossRef]
- Tawfik, S.M.; Abd-Elaal, A.A.; Aiad, I. Three gemini cationic surfactants as biodegradable corrosion inhibitors for carbon steel in HCl solution. Res. Chem. Intermed. 2016, 42, 1101–1123. [Google Scholar] [CrossRef]
- Bianchetti, G.O.; Devlin, C.L.; Seddon, K.R. Bleaching systems in domestic laundry detergents: A review. RSC Adv. 2015, 5, 65365–65384. [Google Scholar] [CrossRef]
- Arthur, T.; Harjani, J.R.; Phan, L.; Jessop, P.G.; Hodson, P.V. Effects-driven chemical design: The acute toxicity of CO 2-triggered switchable surfactants to rainbow trout can be predicted from octanol-water partition coefficients. Green Chem. 2012, 14, 357–362. [Google Scholar] [CrossRef]
- Obot, I.B.; Solomon, M.M.; Umoren, S.A.; Suleiman, R.; Elanany, M.; Alanazi, N.M.; Sorour, A.A. Progress in the development of sour corrosion inhibitors: Past, present, and future perspectives. J. Ind. Eng. Chem. 2019, 79, 1–18. [Google Scholar] [CrossRef]
- Hamed, I.; Osman, M.M.; Abdelraheem, O.H.; Nessim, M.I.; El Mahgary, M.G. Inhibition of API 5L X52 Pipeline Steel Corrosion in Acidic Medium by Gemini Surfactants: Electrochemical Evaluation and Computational Study. Int. J. Corros. 2019, 2019, 4857181. [Google Scholar] [CrossRef]
- Brycki, B.; Szulc, A. Gemini surfactants as corrosion inhibitors. A review. J. Mol. Liq. 2021, 344, 117686. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abdallah, M.; Ahmed, H. Novel cationic gemini surfactants as corrosion inhibitors for carbon steel pipelines. Corros. Sci. 2010, 52, 2897–2904. [Google Scholar] [CrossRef]
- Migahed, M.A.; Shaban, M.M.; Fadda, A.A.; Ali, T.A.; Negm, N.A. Synthesis of some quaternary ammonium gemini surfactants and evaluation of their performance as corrosion inhibitors for carbon steel in oil well formation water containing sulfide ions. RSC Adv. 2015, 5, 104480–104492. [Google Scholar] [CrossRef]
- Allah, M.D.; Abdelhamed, S.; Soliman, K.A.; Mona, A. The performance of three novel Gemini surfactants as inhibitors for acid steel corrosion: Experimental and theoretical studies. RSC Adv. 2021, 11, 37482–37497. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Chen, B.; Zhang, L. Novel surfactants as green corrosion inhibitors for mild steel in 15% HCl: Experimental and theoretical studies. Chem. Eng. J. 2020, 402, 126219. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Tantawy, A.H. Synthesis and evaluation of novel series of Schiff base cationic surfactants as corrosion inhibitors for carbon steel in acidic/chloride media: Experimental and theoretical investigations. RSC Adv. 2016, 6, 8681–8700. [Google Scholar] [CrossRef]
- Assem, R.; Fouda, A.S.; Ibrahim, A.A.; Saadawy, M. Some anionic surfactants as corrosion inhibitors for carbon steel in hydrochloric acid solution. In Key Engineering Materials 2018; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2018; Volume 786, pp. 134–148. [Google Scholar]
- Nallakukkala, S.; Sivabalan, V.; Lal, B.; Mokhtar Che Ismail, N.D. Nonionic surfactants as corrosion inhibitors for carbon steel in hydrochloric acid medium. Test Eng. Manag. 2019, 81, 5830–5835. [Google Scholar]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. AdmetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sun, L.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. ADMETopt: A web server for ADMET optimization in drug design via scaffold hopping. J. Chem. Inf. Model. 2018, 58, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the colle- salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1998, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Tsuneda, T.; Song, J.-W.; Suzuki, S.; Hirao, K. On Koopmans’ theorem in density functional theory. J. Chem. Phys. 2010, 133, 174101. [Google Scholar] [CrossRef]
- Koopmans, T. Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
- El Adnani, Z.; Mcharfi, M.; Sfaira, M.; Benzakour, M.; Benjelloun, A.T.; Touhami, M.E. DFT theoretical study of 7-R-3methylquinoxalin-2 (1H)-thiones (RH; CH3; Cl) as corrosion inhibitors in hydrochloric acid. Corros. Sci. 2013, 68, 223–230. [Google Scholar] [CrossRef]
- Udhayakala, P.; Rajendiran, T.V.; Gunasekaran, S. Quantum chemical investigations on some quinoxaline derivatives as effective corrosion inhibitors for mild steel. Der Pharm. Lett. 2012, 4, 1285–1298. [Google Scholar]
- Mulliken, R.S. Electronic population analysis on LCAO–MO molecular wave functions. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef]
- Masroor, S.; Mobin, M. Non-Ionic Surfactant as Corrosion Inhibitor for Aluminium in 1 M HCl and Synergistic Influence of Gemini Surfactant. Chem. Sci. Rev. Lett. 2014, 3, 33–48. [Google Scholar]
- Aslam, R.; Mobin, M.; Aslam, J.; Lgaz, H. Sugar based N, N′-didodecyl-N, N′ digluconamideethylenediamine gemini surfactant as corrosion inhibitor for mild steel in 3.5% NaCl solution-effect of synergistic KI additive. Sci. Rep. 2018, 8, 3690. [Google Scholar] [CrossRef]
- Sliem, M.H.; Afifi, M.; Bahgat Radwan, A.; Fayyad, E.M.; Shibl, M.F.; Heakal, F.E.; Abdullah, A.M. AEO7 surfactant as an eco-friendly corrosion inhibitor for carbon steel in HCl solution. Sci. Rep. 2019, 9, 2319. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.S.; Al Kiey, S.A. Environmentally benign corrosion inhibitors based on cellulose niacin nano-composite for corrosion of copper in sodium chloride solutions. Int. J. Biol. Macromol. 2020, 161, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Sliem, M.H.; Abdullah, A.M.; Youssef, K.M.; Vezin, H.; Al-Qahtani, N.H. Impact of prolonged exposure to sour service on the mechanical properties and corrosion mechanism of NACE carbon steel material used in wet sour gas multiphase pipeline. Sustainability 2022, 14, 8015. [Google Scholar] [CrossRef]
- Radwan, A.B.; Sliem, M.H.; Okonkwo, P.C.; Shibl, M.F.; Abdullah, A.M. Corrosion inhibition of API X120 steel in a highly aggressive medium using stearamidopropyl dimethylamine. J. Mol. Liq. 2017, 236, 220–231. [Google Scholar] [CrossRef]
- Sliem, M.H.; Shahzad, K.; Sivaprasad, V.N.; Shakoor, R.A.; Abdullah, A.M.H. Enhanced mechanical and corrosion protection properties of pulse electrodeposited NiP-ZrO2 nanocomposite coatings. Surf. Coat. Technol. 2020, 403, 126340. [Google Scholar] [CrossRef]
- Olasunkanmi, L.O.; Sebona, M.F.; Ebenso, E.E. Influence of 6-phenyl-3 (2H)-pyridazinone and 3-chloro-6-phenylpyrazine on mild steel corrosion in 0.5 M HCl medium: Experimental and theoretical studies. J. Mol. Struct. 2017, 1149, 549–559. [Google Scholar] [CrossRef]
- Sliem, M.H.; Kannan, K.; Maurya, M.R.; Jlassi, K.; Sadasivuni, K.K.; Kumar, B.; Abdullah, A.M. Rational Synthesis of Mixed Metal Oxide Clusters Supported on a Partially Etched MAX Phase for Efficient Electrocatalytic CO2 Conversion. Top. Catal. 2022. [Google Scholar] [CrossRef]
- Ech-Chihbi, E.; Nahlé, A.; Salim, R.; Benhiba, F.; Moussaif, A.; El-Hajjaji, F.; Oudda, H.; Guenbour, A.; Taleb, M.; Warad, I.; et al. Computational, MD simulation, SEM/EDX and experimental studies for understanding adsorption of benzimidazole derivatives as corrosion inhibitors in 1.0 M HCl solution. J. Alloys Compd. 2020, 844, 155842. [Google Scholar] [CrossRef]
- Sliem, M.H.; El Basiony, N.M.; Zaki, E.G.; Sharaf, M.A.; Abdullah, A.M. Corrosion inhibition of mild steel in sulfuric acid by a newly synthesized Schiff base: An electrochemical, DFT and Monte Carlo simulation study. Electroanalysis 2020, 32, 3145–3158. [Google Scholar] [CrossRef]
- Bashir, S.; Lgaz, H.; Chung, I.M.; Kumar, A. Potential of Venlafaxine in the inhibition of mild steel corrosion in HCl: Insights from experimental and computational studies. Chem. Pap. 2019, 73, 2255–2264. [Google Scholar] [CrossRef]
- Parveen, M.; Mobin, M.; Zehra, S.; Aslam, R. L-proline mixed with sodium benzoate as sustainable inhibitor for mild steel corrosion in 1M HCl: An experimental and theoretical approach. Sci. Rep. 2018, 8, 7489. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.; Khaled, K.F.; Fadl-Allah, S.A. Testing validity of the Tafel extrapolation method for monitoring corrosion of cold rolled steel in HCl solutions–experimental and theoretical studies. Corros. Sci. 2010, 52, 140–151. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O. Fluconazole as an inhibitor for aluminium corrosion in 0.1 M HCl. Colloids and surfaces a: Physicochemical and engineering aspects. Colloids Surf. A Physicochem. Eng. Asp. 2008, 330, 207–212. [Google Scholar] [CrossRef]
- Bashir, S.; Thakur, A.; Lgaz, H.; Chung, I.M.; Kumar, A. Corrosion inhibition efficiency of bronopol on aluminium in 0.5 M HCl solution: Insights from experimental and quantum chemical studies. Surf. Interfaces 2020, 20, 100542. [Google Scholar] [CrossRef]
- Abd-Elaal, A.A.; Elbasiony, N.M.; Shaban, S.M.; Zaki, E.G. Studying the corrosion inhibition of some prepared nonionic surfactants based on 3-(4-hydroxyphenyl) propanoic acid and estimating the influence of silver nanoparticles on the surface parameters. J. Mol. Liq. 2018, 249, 304–317. [Google Scholar] [CrossRef]
- Bouklah, M.; Hammouti, B.; Lagrenee, M.; Bentiss, F. Thermodynamic properties of 2, 5-bis (4-methoxyphenyl)-1, 3, 4-oxadiazole as a corrosion inhibitor for mild steel in normal sulfuric acid medium. Corros. Sci. 2006, 48, 2831–2842. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Leiva-Garcia, R.; Akid, R.; Brycki, B.; Kowalczyk, I.; Pospieszny, T. Effectiveness of O-bridged cationic gemini surfactants as corrosion inhibitors for stainless steel in 3 M HCl: Experimental and theoretical studies. J. Mol. Liq. 2018, 249, 1113–1124. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Alvarez, L.X.; dos Santos, N.E.; Barrios, A.C.; Ponzio, E.A. Green synthesis of 1-benzyl-4-phenyl-1H-1, 2, 3-triazole, its application as corrosion inhibitor for mild steel in acidic medium and new approach of classical electrochemical analyses. Corros. Sci. 2019, 149, 185–194. [Google Scholar] [CrossRef]
- Hoseinzadeh, A.R.; Danaee, I.; Maddahy, M.H.; Avei, M.R. Taurine as a green corrosion inhibitor for Aisi 4130 steel alloy in hydrochloric acid solution. Chem. Eng. Commun. 2014, 201, 380–402. [Google Scholar] [CrossRef]
- Noor, E.A.; Al-Moubaraki, A.H. Corrosion behavior of mild steel in hydrochloric acid solutions. Int. J. Electrochem. Sci. 2008, 3, 806–818. [Google Scholar]
- Natarajan, R.; Al Shibli, F. Corrosion inhibition of aluminum under basic conditions using Medicago sativa L. extract—Thermodynamic studies. Korean J. Chem. Eng. 2021, 38, 1903–1912. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abdallah, M.; Alfakeer, M.; Ahmed, H. Corrosion inhibition performance of a novel cationic surfactant for protection of carbon steel pipeline in acidic media. Int. J. Electrochem. Sci. 2018, 13, 6824–6842. [Google Scholar] [CrossRef]
- Shukla, S.K.; Quraishi, M.A.; Prakash, R. A self-doped conducting polymer “polyanthranilic acid”: An efficient corrosion inhibitor for mild steel in acidic solution. Corros. Sci. 2008, 50, 2867–2872. [Google Scholar] [CrossRef]
- Abdel Hameed, R.S.; Al-Bagawi, A.H.; Shehata, H.A.; Shamroukh, A.H.; Abdallah, M. Corrosion inhibition and adsorption properties of some heterocyclic derivatives on C-steel surface in HCl. J. Bio-Tribo-Corros. 2020, 6, 51. [Google Scholar] [CrossRef]
- Hameed, A.; Alfakeer, M.; Abdallah, M. Inhibiting properties of some heterocyclic amide derivatives as potential nontoxic corrosion inhibitors for carbon steel in 1.0 M sulfuric acid. Surf. Eng. Appl. Electrochem. 2018, 54, 599–606. [Google Scholar] [CrossRef]
- Shahzad, K.; Sliem, M.H.; Shakoor, R.A.; Radwan, A.B.; Kahraman, R.; Umer, M.A.; Manzoor, U.; Abdullah, A.M. Electrochemical and thermodynamic study on the corrosion performance of API X120 steel in 3.5% NaCl solution. Sci. Rep. 2020, 10, 4314. [Google Scholar] [CrossRef]
- Sulaiman, K.O.; Onawole, A.T. Quantum chemical evaluation of the corrosion inhibition of novel aromatic hydrazide derivatives on mild steel in hydrochloric acid. Comput. Theor. Chem. 2016, 1093, 73–80. [Google Scholar] [CrossRef]
- Kaya, S.; Tüzün, B.; Kaya, C.; Obot, I.B. Determination of corrosion inhibition effects of amino acids: Quantum chemical and molecular dynamic simulation study. J. Taiwan Inst. Chem. Eng. 2016, 58, 528–535. [Google Scholar] [CrossRef]
- Ansari, K.R.; Sudheer Singh, A.; Quraishi, M.A. Some pyrimidine derivatives as corrosion inhibitor for mild steel in hydrochloric acid. J. Dispers. Sci. Technol. 2015, 36, 908–917. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
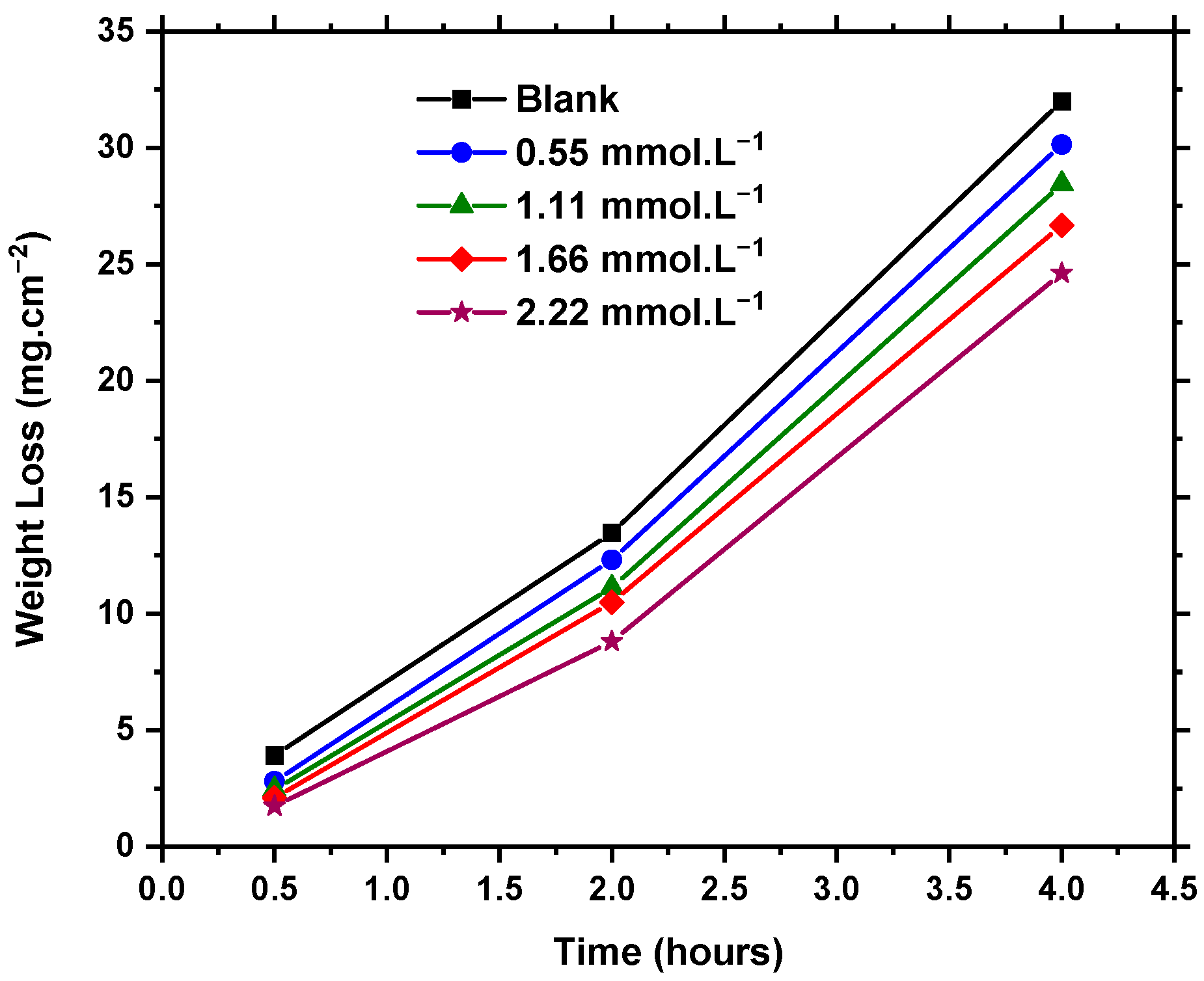


| Surface Coverage 8 | Inhibition Efficiency (IE%) | |||||
|---|---|---|---|---|---|---|
| Concentration | 0.5 h | 2 h | 4 h | 0.5 h | 2 h | 4 h |
| HCl Blank | __ | __ | __ | __ | __ | __ |
| 0.55 mmol·L−1 | 0.28 | 0.09 | 0.06 | 28 | 9 | 6 |
| 1.11 mmol·L−1 | 0.38 | 0.17 | 0.11 | 38 | 17 | 11 |
| 1.66 mmol·L−1 | 0.47 | 0.22 | 0.17 | 47 | 22 | 17 |
| 2.22 mmol·L−1 | 0.56 | 0.35 | 0.23 | 56 | 35 | 23 |
| Temperature (°C) | Conc. (mmol·L−1) | RS (Ω·cm2) | R1 (Ω·cm2) | Y1 (µsn·Ω−1·cm−2) | n1 | Rct (Ω·cm2) | Y2 (µsn·Ω−1·cm−2) | n2 | (µF) | 8 | IE% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | HCl Blank | 0.67 | 1.50 | 2812 | 0.93 | 10.61 | 1102 | 0.83 | 443.0468471 | - | - |
| 0.55 | 0.70 | 1.47 | 2832 | 0.64 | 11.95 | 1090 | 0.82 | 420.34 | 0.112 | 11.21 | |
| 1.11 | 0.70 | 1.41 | 2854 | 0.87 | 14.75 | 928 | 0.83 | 385.33 | 0.280 | 28.06 | |
| 1.66 | 0.71 | 1.40 | 2892 | 0.58 | 17.14 | 963 | 0.82 | 391.17 | 0.380 | 38.09 | |
| 2.22 | 0.70 | 1.37 | 2903 | 0.73 | 19.34 | 847.2 | 0.79 | 284.01 | 0.451 | 45.13 | |
| 30 | HCl Blank | 0.67 | 1.45 | 2826 | 0.67 | 8.51 | 1247 | 0.83 | 491.48 | - | - |
| 0.55 | 0.66 | 1.43 | 2846 | 0.48 | 10.21 | 1091 | 0.84 | 463.22 | 0.166 | 16.65 | |
| 1.11 | 0.69 | 1.37 | 2932 | 0.83 | 10.91 | 1072 | 0.84 | 459.40 | 0.219 | 21.99 | |
| 1.66 | 0.68 | 1.32 | 2975 | 0.46 | 11.32 | 1059 | 0.84 | 455.97 | 0.248 | 24.82 | |
| 2.22 | 0.70 | 1.21 | 3093 | 0.81 | 11.97 | 1002 | 0.84 | 431.47 | 0.289 | 28.90 | |
| 50 | HCl Blank | 0.56 | 1.23 | 2990 | 0.97 | 3.39 | 1942 | 0.83 | 694.10 | - | - |
| 0.55 | 0.57 | 1.17 | 3120 | 0.67 | 4.04 | 1676 | 0.84 | 647.24 | 0.160 | 16.08 | |
| 1.11 | 0.56 | 1.13 | 3170 | 0.96 | 4.23 | 1628 | 0.83 | 587.21 | 0.198 | 19.85 | |
| 1.66 | 0.57 | 1.09 | 3211 | 0.55 | 4.45 | 1586 | 0.83 | 575.00 | 0.238 | 23.82 | |
| 2.22 | 0.56 | 1.06 | 3254 | 0.83 | 4.66 | 1585 | 0.81 | 501.21 | 0.272 | 27.25 | |
| 70 | HCl Blank | 0.48 | 0.97 | 3260 | 0.81 | 1.83 | 2467 | 0.95 | 1856.6 | - | - |
| 0.55 | 0.50 | 0.98 | 3242 | 0.75 | 1.91 | 2385 | 0.94 | 1690.5 | 0.041 | 4.18 | |
| 1.11 | 0.49 | 0.95 | 3580 | 0.98 | 2.04 | 2261 | 0.93 | 1508.2 | 0.102 | 10.29 | |
| 1.66 | 0.49 | 1.01 | 3272 | 0.53 | 2.28 | 2254 | 0.92 | 1425.2 | 0.197 | 19.73 | |
| 2.22 | 0.49 | 0.93 | 3690 | 0.87 | 2.41 | 2201 | 0.91 | 1310.9 | 0.240 | 24.06 |
| Temperature (°C) | Conc. (mmol·L−1) | −Ecorr (mV, SCE) | icorr (mA·cm−2) | (nV/decade) | (nV/decade) | CR (mpy) | 8 | IE% | |
|---|---|---|---|---|---|---|---|---|---|
| 20 | HCl Blank | 354 | 8.44 | 230.50 | 288.40 | 6.59 | 1583 | - | - |
| 0.55 | 357 | 7.28 | 212.50 | 275.90 | 7.16 | 1632 | 0.14 | 14 | |
| 1.11 | 357 | 5.92 | 193.30 | 248.50 | 7.97 | 1163 | 0.30 | 30 | |
| 1.66 | 360 | 4.68 | 185.20 | 254.80 | 9.95 | 1195 | 0.45 | 45 | |
| 2.22 | 360 | 3.96 | 177.20 | 250.30 | 11.38 | 892 | 0.53 | 53 | |
| 30 | HCl Blank | 399 | 17.10 | 235.30 | 321.00 | 3.45 | 3160 | - | - |
| 0.55 | 396 | 15.36 | 235.00 | 328.10 | 3.87 | 3075 | 0.10 | 10 | |
| 1.11 | 392 | 13.26 | 230.70 | 288.10 | 4.20 | 2715 | 0.22 | 22 | |
| 1.66 | 388 | 11.08 | 228.60 | 316.90 | 5.20 | 2598 | 0.35 | 35 | |
| 2.22 | 401 | 8.36 | 222.10 | 274.60 | 6.38 | 1813 | 0.51 | 51 | |
| 50 | HCl Blank | 393 | 48.60 | 382.70 | 579.50 | 2.06 | 37130 | - | - |
| 0.55 | 388 | 45.60 | 366.30 | 520.70 | 2.05 | 25240 | 0.06 | 6 | |
| 1.11 | 385 | 41.40 | 345.70 | 499.20 | 2.14 | 17760 | 0.15 | 15 | |
| 1.66 | 384 | 37.56 | 323.00 | 441.30 | 2.16 | 13170 | 0.23 | 23 | |
| 2.22 | 380 | 30.42 | 249.40 | 344.80 | 2.07 | 9277 | 0.37 | 37 | |
| 70 | HCl Blank | 390 | 264.00 | 992.00 | 1041.00 | 0.84 | 121300 | - | - |
| 0.55 | 391 | 252.00 | 904.50 | 1038.00 | 0.83 | 93680 | 0.05 | 5 | |
| 1.11 | 390 | 240.00 | 830.40 | 1159.00 | 0.88 | 65410 | 0.09 | 9 | |
| 1.66 | 384 | 210.00 | 763.40 | 913.40 | 0.86 | 62210 | 0.20 | 20 | |
| 2.22 | 380 | 172.00 | 631.80 | 927.20 | 0.95 | 59860 | 0.35 | 35 |
| Concentration (mmol·L−1) | |||
|---|---|---|---|
| HCl Blank | 55.94 | 53.31 | 151.67 |
| 0.55 | 57.62 | 54.98 | 156.22 |
| 1.11 | 60.14 | 57.51 | 163.22 |
| 1.66 | 61.97 | 59.34 | 167.70 |
| 2.22 | 62.05 | 59.41 | 166.15 |
| Carcinogenicity | Eye Irritation | Ames Mutagenesis | Acute Oral Toxicity (Class III) |
|---|---|---|---|
| 0.9 (safe) | 0.95 (safe) | 0.67 (safe) | 0.62 (slightly toxic) |
| Honey bee toxicity | Biodegradability | Fish aquatic toxicity | Water solubility (LogS) |
| 0.72 (safe) | 0.58 (safe) | 0.79 (safe) | −1.86 (soluble) |
| Q-22 (This Study) | QBBD [31] | AEO7 [46] | |
|---|---|---|---|
| HOMO (eV) | −5.57 | −8.17 | - |
| LUMO (eV) | −0.44 | −6.12 | - |
| (eV) | 5.13 | 2.05 | 8.21 |
| I (eV) | 5.57 | - | 6.89 |
| A (eV) | 0.44 | - | −1.31 |
| ɳ(eV) | 2.57 | 2.05 | 4.10 |
| Χ (eV) | 3.01 | 7.12 | 2.79 |
| ω (eV) | 11.60 | - | - |
| TNC (eV) | −5.59 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalab, R.; Saad, M.A.; Sliem, M.H.; Abdullah, A.M.; Hussein, I.A. An Eco-Friendly Quaternary Ammonium Salt as a Corrosion Inhibitor for Carbon Steel in 5 M HCl Solution: Theoretical and Experimental Investigation. Molecules 2022, 27, 6414. https://doi.org/10.3390/molecules27196414
Jalab R, Saad MA, Sliem MH, Abdullah AM, Hussein IA. An Eco-Friendly Quaternary Ammonium Salt as a Corrosion Inhibitor for Carbon Steel in 5 M HCl Solution: Theoretical and Experimental Investigation. Molecules. 2022; 27(19):6414. https://doi.org/10.3390/molecules27196414
Chicago/Turabian StyleJalab, Rem, Mohammed A. Saad, Mostafa H. Sliem, Aboubakr M. Abdullah, and Ibnelwaleed A. Hussein. 2022. "An Eco-Friendly Quaternary Ammonium Salt as a Corrosion Inhibitor for Carbon Steel in 5 M HCl Solution: Theoretical and Experimental Investigation" Molecules 27, no. 19: 6414. https://doi.org/10.3390/molecules27196414
APA StyleJalab, R., Saad, M. A., Sliem, M. H., Abdullah, A. M., & Hussein, I. A. (2022). An Eco-Friendly Quaternary Ammonium Salt as a Corrosion Inhibitor for Carbon Steel in 5 M HCl Solution: Theoretical and Experimental Investigation. Molecules, 27(19), 6414. https://doi.org/10.3390/molecules27196414









