Abstract
To address drug resistance to influenza virus neuraminidase inhibitors (NAIs), a series of novel boron-containing N-substituted oseltamivir derivatives were designed and synthesized to target the 150-cavity of neuraminidase (NA). In NA inhibitory assays, it was found that most of the new compounds exhibited moderate inhibitory potency against the wild-type NAs. Among them, compound 2c bearing 4-(3-boronic acid benzyloxy)benzyl group displayed weaker or slightly improved activities against group-1 NAs (H1N1, H5N1, H5N8 and H5N1-H274Y) compared to that of oseltamivir carboxylate (OSC). Encouragingly, 2c showed 4.6 times greater activity than OSC toward H5N1-H274Y NA. Moreover, 2c exerted equivalent or more potent antiviral activities than OSC against H1N1, H5N1 and H5N8. Additionally, 2c demonstrated low cytotoxicity in vitro and no acute toxicity at the dose of 1000 mg/kg in mice. Molecular docking of 2c was employed to provide a possible explanation for the improved anti-H274Y NA activity, which may be due to the formation of key additional hydrogen bonds with surrounding amino acid residues, such as Arg152, Gln136 and Val149. Taken together, 2c appeared to be a promising lead compound for further optimization.
1. Introduction
Influenza (flu) is an acute respiratory infectious disease that continues to baffle humans by its frequently changing nature, seasonal epidemics and occasional pandemics [1]. Influenza viruses belong to the family of Orthomyxoviridae and are categorized as types A, B, C and D, of which only influenza virus A, B and C can infect humans [1,2]. According to the World Health Organization (WHO), influenza affects approximately 1 billion individuals each year resulting in between 290,000 and 650,000 deaths [3,4]. The influenza virus A has caused three major pandemics in the last century, including 1918 Spanish flu (H1N1), 1957 Asian flu (H2N2) and 1968 Hong Kong flu (H3N2) [5]. The 2009 Swine flu (H1N1) caused millions of cases worldwide and had a significant impact on human lives and resources [6,7]. In recent years, highly pathogenic avian influenza viruses of subtype H5N1 and H7N9 exhibited high mortality rates of around 60% and 40%, respectively, resulting in many hospitalizations and deaths [8,9]. Therefore, it is vital to pay more attention to such diseases as a serious and constant threat to global public health [10].
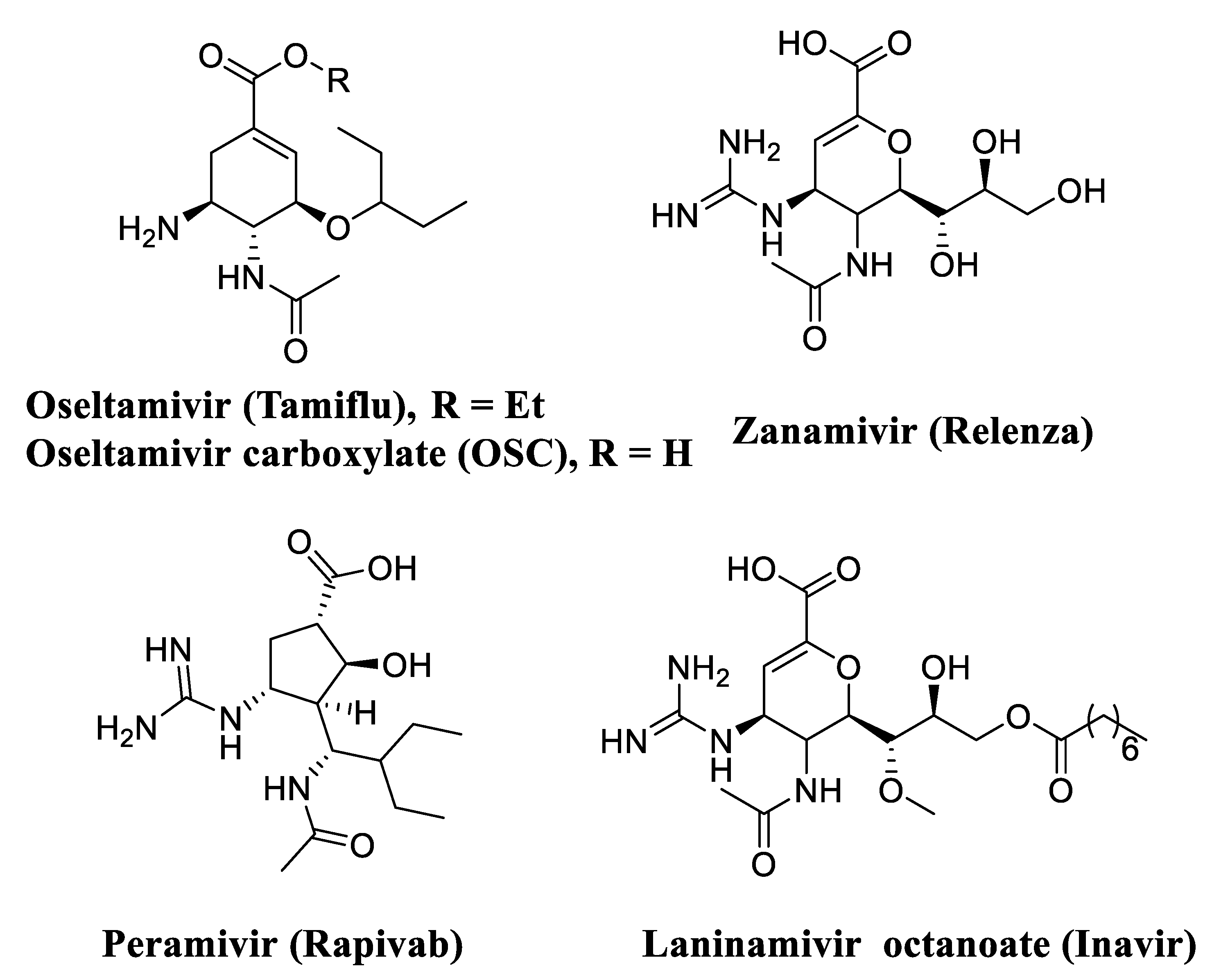
Neuraminidase (NA), a membrane-bound glycoprotein on the viral surface, plays a critical role not only in catalyzing the release of newly formed virions in host cells, but also in facilitating the movement of virions in the respiratory tract [11]. Up to now, four neuraminidase inhibitors (NAIs) were successfully approved to treat influenza virus infections, namely Oseltamivir (Tamiflu) [12], Zanamivir (Relenza) [13], Peramivir (Rapivab) [14] and Laninamivir octanoate (Inavir) (Figure 1) [15]. Among them, oseltamivir is the only agent for oral administration and this NAI has taken over the largest market share. However, with the widespread use of oseltamivir, resistant strains of influenza virus have compromised the therapeutic effects, especially the N1-H274Y mutant [16,17]. Hence, it is urgent to develop a new generation of NA inhibitors via contemporary medicinal chemistry strategies to overcome drug resistance [18,19,20,21].
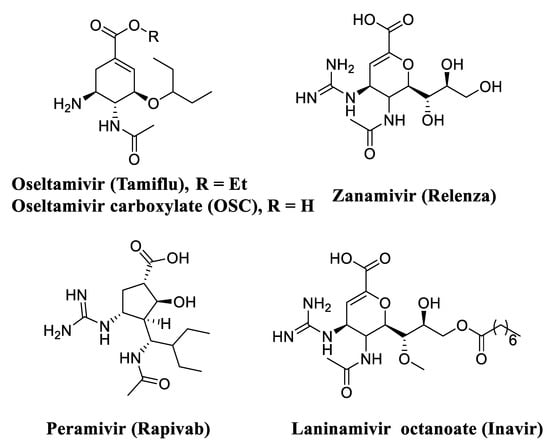
Figure 1.
Structures of approved NA inhibitors used in the treatment of influenza virus infection.
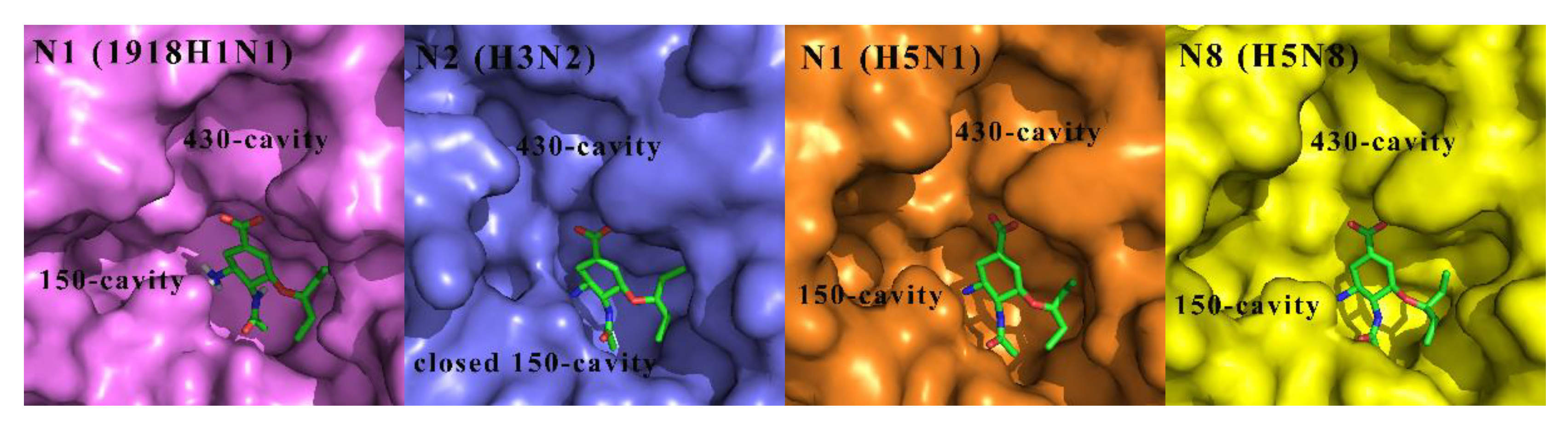
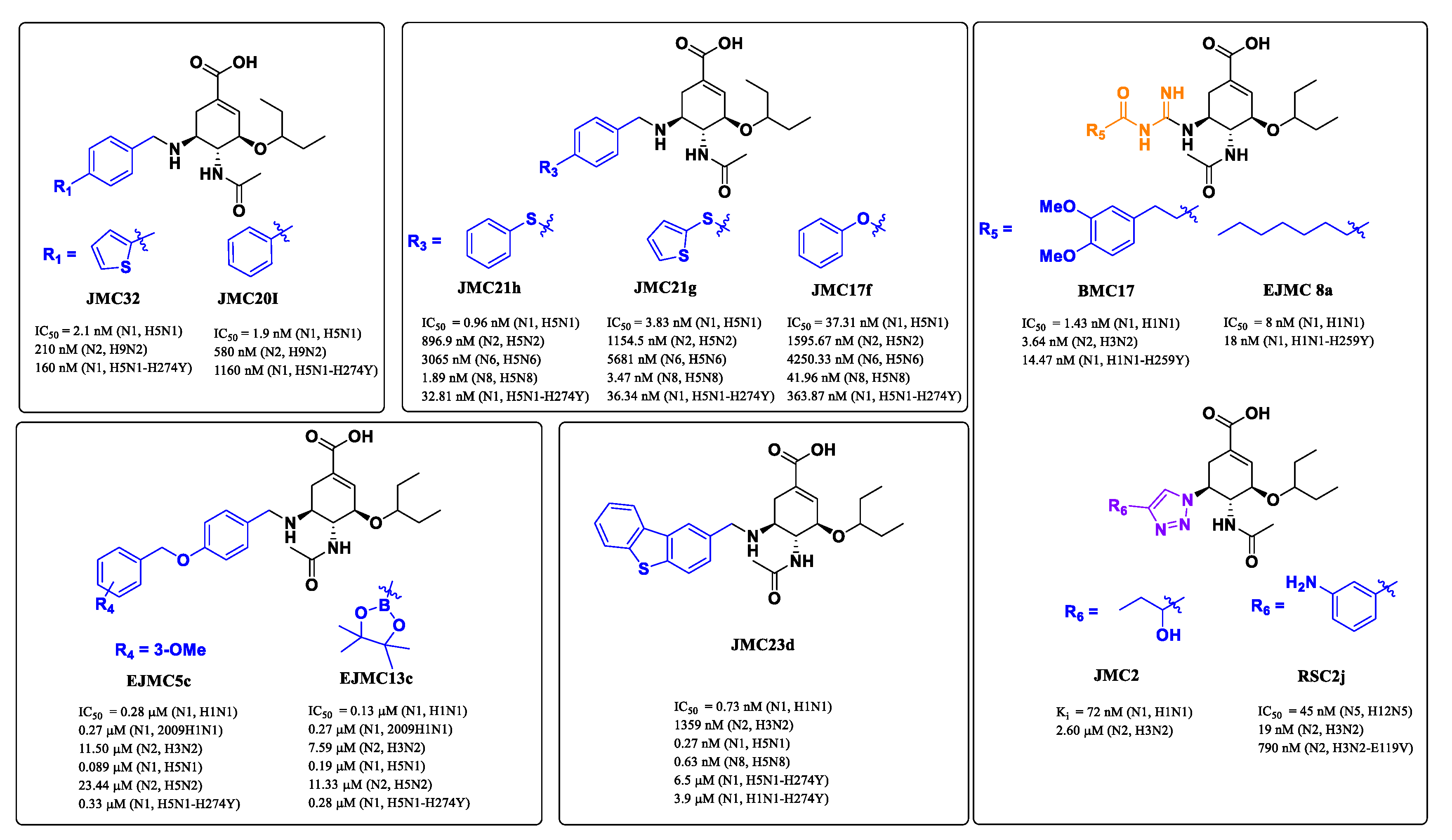
Except for N10 and N11 with no or extremely low sialidase activity, the other nine NAs are categorized into two phylogenetic groups: group 1 NA consists of N1, N4, N5 and N8 subtypes, whereas group 2 NA contains N2, N3, N6, N7 and N9 subtypes [22,23]. In group-1 NAs, with the exception of the 2009 pandemic H1N1 NA [24], a cavity known as the 150-cavity, consisting of residues 147–152, adopts a conformation adjacent to the active site [25]. In contrast, the 150-cavity of group-2 NAs is present in a closed conformation (Figure 2) [26]. The discovery of the 150-cavity in group-1 NAs provides a new strategy for the development of novel NA inhibitors with increased specificity and potency. In recent years, several N-substituted oseltamivir derivatives JMC32, JMC20I [27], JMC21h, JMC21g, JMC17f [28], EJMC5c, EJMC13c [29] and JMC23d [30], BMC17 [31], EJMC8a [32], JMC2 [33] and RSC2j [34] (Figure 3) targeting the 150-cavity have been reported. Most of these inhibitors displayed better or similar inhibitory activities than OSC against both wild-type and mutant NAs. For instance, JMC32, JMC20I, JMC21h, JMC21g and JMC23d showed more potent activity than OSC against H5N1 with IC50 values of 2.1 nM, 1.9 nM, 0.96 nM, 3.83 nM and 0.27 nM, respectively. Moreover, most of the compounds showed in Figure 3 exhibited greater inhibitory activities than OSC against N5N1-H274Y. The discovery of these inhibitors proved that targeting 150-cavity was an effective strategy to enhance the efficacy of OSC derivatives against NAs.
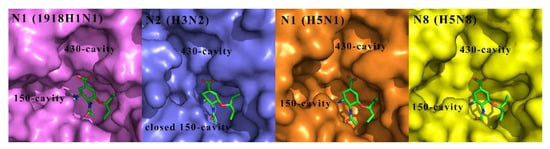
Figure 2.
Comparison of the crystal structures of representative group-1 NAs N1 (1918H1N1, PDB ID: 3BEQ), N1 (H5N1, PDB ID: 2HU0), N8 (H5N8, PDB ID: 2HT7) and group-2 NAs N2 (H3N2, PDB code: 4GZP) used in our inhibition assay, bound to OSC (green).
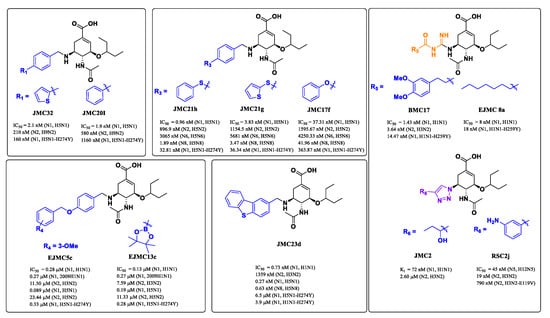
Figure 3.
Structures of previously reported group-1-specific influenza NA inhibitors (JMC32, JMC20I, JMC21h, JMC21g, JMC17f, EJMC5c, EJMC13c and JMC23d) and non-selective NA inhibitors (BMC17, EJMC8a, JMC8a, RSC2j).
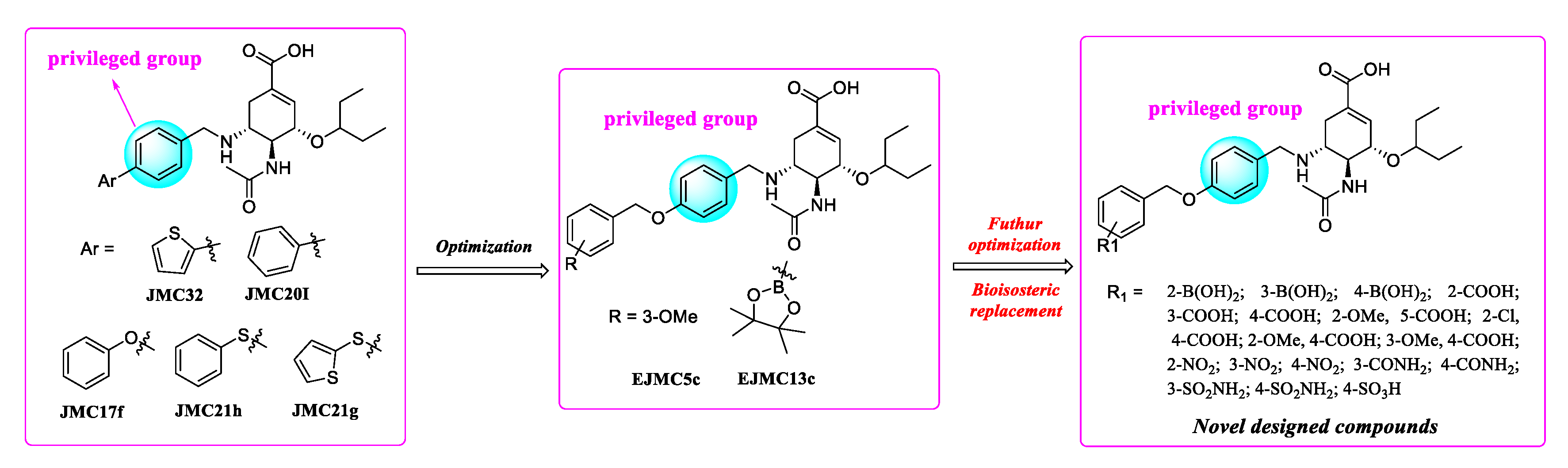
In previous studies, our research efforts explored the substitution on the benzyl group by introducing different hydrophobic groups at the para-position of the benzyl group and led to the discovery of potent inhibitors EJMC5c and EJMC13c. The biological activity of EJMC5c and EJMC13c against H5N1-H274Y NA was 4.85- and 5.71-fold more potent than OSC with IC50 values of 0.33 μM and 0.28 μM, respectively. The present study is an extension of our previous work. Herein, we have kept benzoloxybenzyl unchanged and replaced borate ester with boronic acids, carboxylic acids, nitro groups, amides, sulfonamides and sulfonic acids by utilizing a bioisosteric replacement strategy to obtain novel oseltamivir derivatives, aiming to further explore structure–activity relationships in the 150-cavity (Figure 4). All newly synthesized compounds were evaluated in vitro for their neuraminidase inhibitory activities against H1N1, H3N2, H5N1, H5N8 and H5N1-H274Y. The cell-based antiviral activity and cytotoxicity of selected compounds were also characterized. Furthermore, based on the biological results, we performed molecular docking and predicted the effects of representative compounds on inhibition of CYP enzymes and safety assessment.
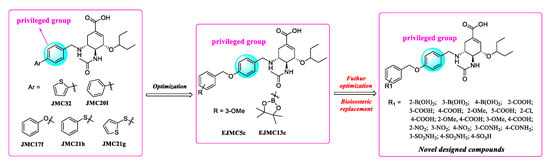
Figure 4.
Further optimization of oseltamivir analogues EJMC5c and EJMC13c targeting 150-cavity via bioisosteric replacement strategy.
2. Results and Discussion
2.1. Chemistry
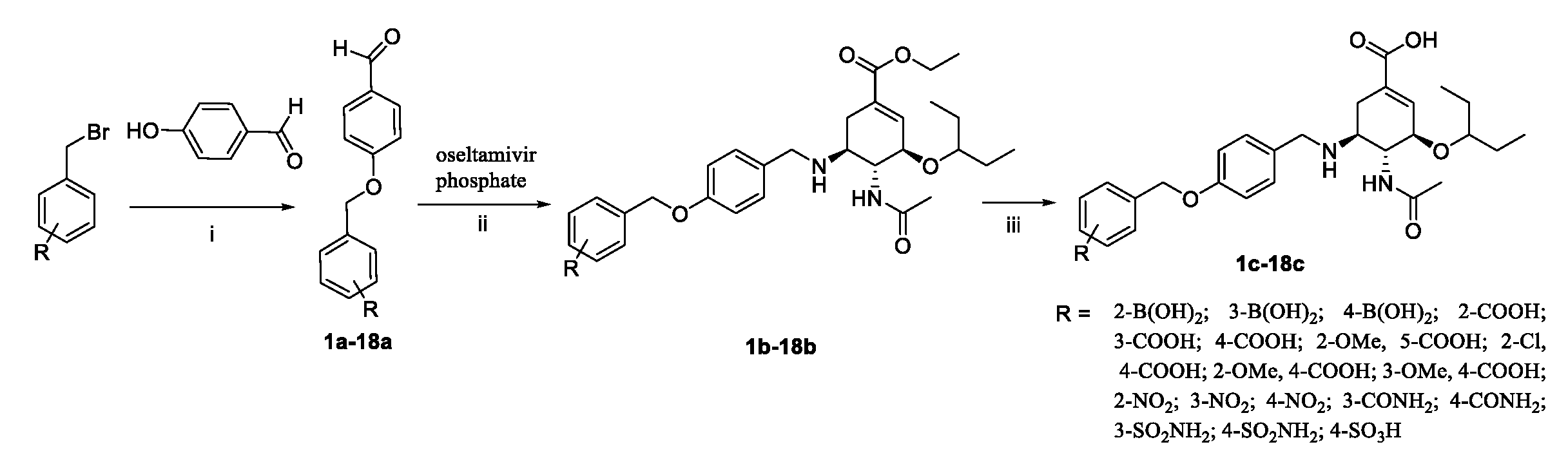
The C-5 N-substituted oseltamivir derivatives (1c–18c) were prepared via a well-established synthetic route as outlined in Scheme 1. The target compounds 1c–18c were synthesized from the commercially available starting material oseltamivir phosphate. Initially, 4-hydroxybenzaldehyde was treated with corresponding benzyl bromide in the presence of potassium carbonate to obtain the intermediates 1a–18a. Then, treatment of oseltamivir phosphate with a series of different aldehydes in the presence of NaBH3CN yielded the intermediates 1b–18b. The intermediates 1b–18b were finally hydrolyzed with NaOH aqueous solution and acidified by HCl aqueous solution to obtain the target compounds 1c–18c. All novel synthesized compounds were fully characterized by electrospray ionization mass spectrometry (ESI-MS), proton nuclear magnetic resonance spectroscopy (1H NMR) (Supplementary Materials), as well as carbon nuclear magnetic resonance spectroscopy (13C NMR).
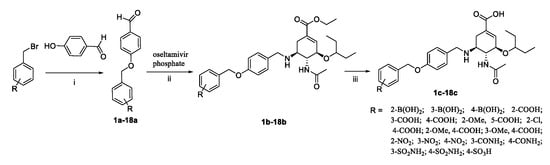
Scheme 1.
Reagents and conditions: (i) K2CO3, DMF, N2, rt., 12 h; (ii) NaBH3CN, CH3OH, rt., 6–7 h; (iii) 1 M NaOH, CH3OH, rt., then 3 M HCl.
2.2. Biological Activity
2.2.1. In Vitro Inhibitory Activities of Influenza Virus Neuraminidase
The synthesized novel oseltamivir derivatives were screened on NAs from H1N1, H3N2, H5N1, H5N8 and H5N1-H274Y according to our previous method [28,30,35]. The inhibition potencies of test compounds and reference compound oseltamivir carboxylate (OSC), are summarized in Table 1. Indeed, OSC showed a greater inhibitory potency toward wild-type NAs with IC50 values of 1.1 × 10−3 μM, 5.2 × 10−3 μM, 0.019 μM and 7.0 × 10−3 μM against H1N1, H3N2, H5N1 and H5N8, respectively. In line with the reported data, OSC exhibited weaker inhibitory activity against mutant H5N1-H274Y (IC50 = 1.25 μM) compared to that against wild-type H5N1 (IC50 = 0.019 μM).

Table 1.
Neuraminidase (NA) inhibition of oseltamivir derivatives in chemiluminescence-based assay.
As reported in Table 1, all the target compounds displayed decreased activities compared to that of OSC towards all wide-type NAs of group-1 (H1N1, H5N1 and H5N8) and group-2 (H3N2). With the exception of compounds 3c, 7c and 14c, the other compounds showed better inhibitory activities against N1 (H1N1, H5N1) and N8 (H5N8) than N2 (H3N2). Intriguingly, compounds with R groups of 4-B(OH)2 (3c), 2-OMe, 5-COOH (7c), 3-CONH2 (14c) demonstrated greater or similar activities against N2 (H3N2, IC50 = 0.058 μM, 8.19 μM and 0.027 μM, respectively) relative to N1 (H1N1, IC50 = 0.045 μM, 14.02 μM and 0.011 μM, respectively), which might be explained by their ability to induce the opening of the 150-loop of group-2 NA.
Regrettably, compounds bearing ortho-boronic acid (1c), carboxylic acid (4c-7c, 9c-10c), nitro (11c, 13c), para-amide (15c), meta-sulfonamide (16c) and para-sulfonic acid (18c) showed sharply decreased potency against NAs compared to that of OSC. Interestingly, 8c featuring 2-Cl, 4-COOH-substituted phenyl group (IC50 = 0.036 μM) displayed relatively similar activity to that of OSC (IC50 = 7.0 × 10−3 μM) against H5N8. Besides, 12c (R = 3-NO2) with IC50 value of 0.023 μM exhibited equivalent inhibitory activity to that of OSC with IC50 value of 0.019 μM against H5N1. Additionally, compound 17c carrying para-sulfonamide displayed comparable activity (IC50 = 0.027 μM, 0.053 μM, respectively) to OSC (IC50 = 1.1 × 10−3 μM, 0.019 μM, respectively) against H1N1 and H5N1. Notably, 3c (R = 4-B(OH)2) and 14c (R = 3-CONH2) exerted slightly weaker or equal inhibitory activities than OSC with IC50 values at the two-digit nanomolar range against both group-1 NAs (H1N1, H5N1 and H5N8) and group-2 NA (H3N2), while 2c (R = 3-B(OH)2) proved to be potent inhibitor of group-1 NAs (H1N1, H5N1 and H5N8).
The inhibitory activity of all compounds against mutant H5N1-H274Y NA were also tested. As shown in Table 1, it was noticed that most of the compounds (except for 2c, 3c, 12c, 14c and 17c) were found to be less active against the mutant H5N1-H274Y NA compared to that of OSC. The inhibitory activities of compounds 12c and 17c (with IC50 values of 0.87 μM and 0.85 μM, respectively) were close to that of OSC (with IC50 value of 1.25 μM). Impressively, 2c, 3c and 14c (IC50 = 0.27 μM, 0.56 μM and 0.57 μM, respectively) were 2.2- to 4.6-fold more potent than OSC. Indeed, these compounds could be used as lead compounds for further optimization.
2.2.2. In Vitro Anti-Influenza Virus Activity
Considering the enzymatic inhibition activity results, the promising compounds 2c, 3c and 14c were further evaluated for antiviral activity and cytotoxicity in Chicken Embryo Fibroblast cells (CEFs) infected with A/Goose/Guangdong/SH7/2013 (H5N1) as well as A/Goose/Jiangsu/1306/2014 (H5N8). Oseltamivir carboxylate (OSC) was used as a reference compound in parallel. The values of EC50 (anti-influenza virus activity) and CC50 (cytotoxicity) of the selected compounds were determined.
As outlined in Table 2, all the tested compounds showed no appreciable cytotoxicity in CEFs. In the case of H5N1 and H5N8 viruses, compounds 3c (EC50 = 3.20 μM and 2.51 μM, respectively) and 14c (EC50 = 1.18 μM and 2.80 μM, respectively) displayed moderate activities compared to that of OSC (EC50 = 0.76 μM and 2.01 μM, respectively). Notably, compound 2c (EC50 = 0.69 μM and 1.57 μM, respectively) exhibited the most potent inhibitory activity, which was better than OSC against H5N1 and H5N8.

Table 2.
Anti-influenza virus activity and cytotoxicity of oseltamivir derivatives in CEF cells.
Then, the representative compounds 2c, 3c and 14c were also tested against A/PR/8/34 (H1N1) and A/Wisconsin/67/05 (H3N2) strains in MDCK cells by means of a plaque reduction assay (PRA). The cytotoxicity was carried out in MDCK cells using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. OSC and zanamivir (ZAN) were included as controls. The EC50 and CC50 values are illustrated in Table 3.

Table 3.
Anti-influenza virus activity and cytotoxicity of oseltamivir derivatives in MDCK cells.
For the test compounds, no cytotoxicity was observed up to the highest tested concentration (250 μM) in MDCK cells. As for the H1N1 strain, compounds 2c and 3c exhibited nearly equivalent potency with EC50 values of 0.03 μM and 0.02 μM, respectively, compared to that of OSC (EC50 = 0.02 μM) and ZAN (EC50 = 9 × 10−3 μM). In contrast, 14c (EC50 = 0.21 μM) exerted decreased activity. Against H3N2 virus, all the tested compounds exhibited weaker potency (EC50 values ranging from 3.08 μM to > 100 μM) compared to that of OSC and ZAN. The antiviral activities of compounds 3c against H3N2 and 14c against H1N1 and H3N2 were inferior to their NA-inhibitory potency, which could be due to the cellular metabolism of the molecules and/or poor membrane permeability.
2.3. Molecular Docking
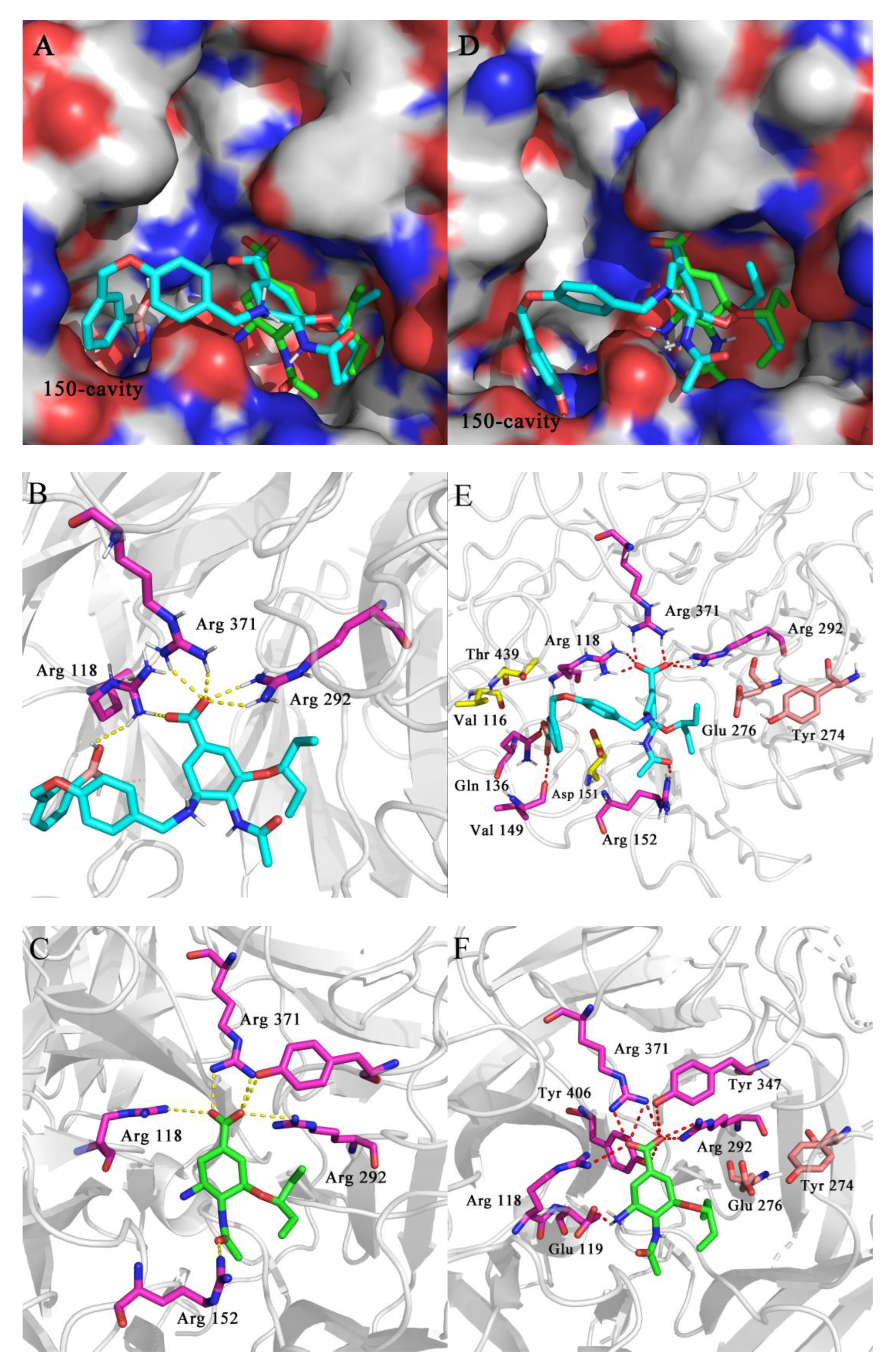
In order to further understand the binding modes of novel oseltamivir derivatives in the 150-cavity of group-1 NAs, molecular docking studies of representative compound 2c to crystal structures of N1 (PDB ID: 2HU0) and N1-H274Y (PDB ID: 3CL0) were performed by using Schrödinger Maestro 12.9 software and the docking results were visualized by PyMOL.
From Figure 5, it can be observed that the 4-(3-boronic acid benzyloxy)benzyl group of compound 2c could extend into the 150-cavity of N1 (H5N1, Figure 5A) and N1-H274Y (H5N1-H274Y, Figure 5D), while the oseltamivir carboxylic acid part of this compound was well embedded in the active sites of NAs in line with the binding pattern of OSC. In Figure 5B,C, it could be found that 2c retained key hydrogen bonds between carboxylic acid and Arg292, Arg371 and Arg118, which was consistent with OSC binding. Moreover, an additional H-bond interaction was formed between the terminal boronic acid of 2c and NH2 of Arg118. However, the hydrogen bonds formed by C-1 carboxyl acid and C-4 acetamide of OSC with Tyr347 and Arg152, respectively, disappeared in 2c, which might be the reason why 2c showed 3.9-fold lower potency against H5N1 in the enzymatic assay.
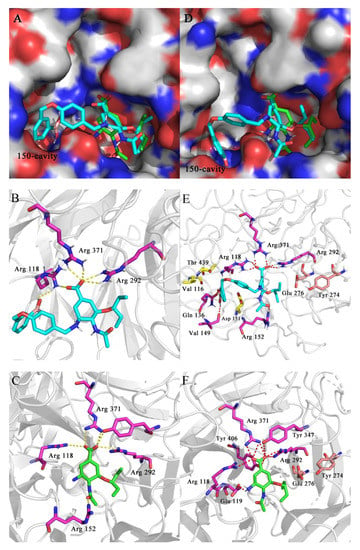
Figure 5.
Putative binding models of 2c (cyan) and OSC (green) with the crystal structures of N1 (H5N1, PDB ID: 2HU0) and N1-H274Y (H5N1-H274Y, PDB ID: 3CL0). (A,D). Superposition of 2c and OSC with H5N1 NA and H5N1-H274Y NA, respectively; (B,E). The key interactions formed between 2c and H5N1 NA and H5N1-H274Y NA, respectively; (C,F). The key interactions formed between OSC and H5N1 NA and H5N1-H274Y NA, respectively. Hydrogen bonds are shown as dashed lines (yellow for H5N1 NA, red for H5N1-H274Y).
According to previous research, the bulker tyrosine residue pushes the carboxyl group of Glu276 close to the binding site due to the mutation of His274Tyr. In this position, the charged carboxyl group destroys the otherwise hydrophobic pocket that normally accommodates the pentyloxy substituent of oseltamivir, thus causing a change in the conformation of OSC, which explained the resistance of the N1-H274Y mutant to oseltamivir. From Figure 5F, it was found that the hydrogen bonds formed by the C-4 amide groups of OSC with Arg152 disappeared, possibly due to the change in the conformation of OSC. However, as shown in Figure 5E, it could be found that 2c retained key hydrogen bonds between amide groups and Arg152, which may be due to the introduction of 4-(3-boronic acid benzyloxy)benzyl group, which extends into the 150-cavity and further distorts the conformation of the parent ring. Although C-1 carboxylic acid and C-5 amino of OSC generated additional hydrogen bonds with Tyr263, Tyr321 and Glu38, respectively, new hydrogen bonds compensated for the lost interactions built by boronic acid of 2c with Gln136 and Val149, and by C-4 acetamide of 2c with Arg152. Meanwhile, benzyl of 2c might produce hydrophobic interactions with Thr439, Val116 and Asp151. This might account for the superior inhibition of 2c against H5N1-H274Y NA compared to that of OSC. Overall, it should be noted that the molecule docking results were consistent with our original design hypothesis that the modification targeting of the 150-cavity of NAs is an effective strategy for overcoming drug resistance profile.
2.4. In Silico Predicted Effects of Representative Compounds on Inhibition of CYP Enzymes
The important metabolic enzyme, cytochrome P450 (CYP), exerts a significant influence in drug metabolism. However, several drugs can also induce or inhibit CYP enzymes, and therefore lead to a decreased therapeutic effect or an adverse reaction profile when co-administrating different drugs, thus causing metabolic-mediated drug–drug interactions (DDI). Thus, we evaluated the inhibitory potential of compounds 2c, 3c, 14c, EJMC5c, EJMC13c and OSC on the main CYP enzymes by utilizing online software (http://admet.scbdd.com/calcpre/index/). As summarized in Table 4, compounds 3c and 14c were not predicted to inhibit CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4. In addition, 2c displayed similar CYP enzymatic inhibition of the five CYP isozymes compared to that of EJMC5c.

Table 4.
Inhibitory ability on CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4 of compounds 2c, 3c, 14c, EJMC5c, EJMC13c and OSC.
2.5. In Silico Prediction of Physicochemical Properties
Additionally, the drug-like properties of representative compounds 2c, 3c, 14c, EJMC5c, EJMC13c and OSC were characterized using a free online software (http://www.molinspiration.com/, accessed on 6 September 2022). As shown in Table 5, the results indicated that various parameters of compounds 2c, 3c and 14c including hydrogen bond acceptors (nON), topological polar surface area (tPSA) and Molinspiration-predicted Log P (miLog P) were all in the acceptable range, except for the slight deviation of hydrogen bond donors (nOHNH) and rotatable bonds (nrotb). Inspiringly, all of the test compounds displayed an acceptable miLog P, while the OSC went beyond the normal criteria. The tPSA values of all compounds were in the range of 101.65 to 139.99 Å2, suggesting their advantage for intestinal absorption (<140 Å2) and inability to pass through the blood–brain barrier (>60 Å2).

Table 5.
Physicochemical properties of some selected compounds and OSC.
2.6. Safety Assessment
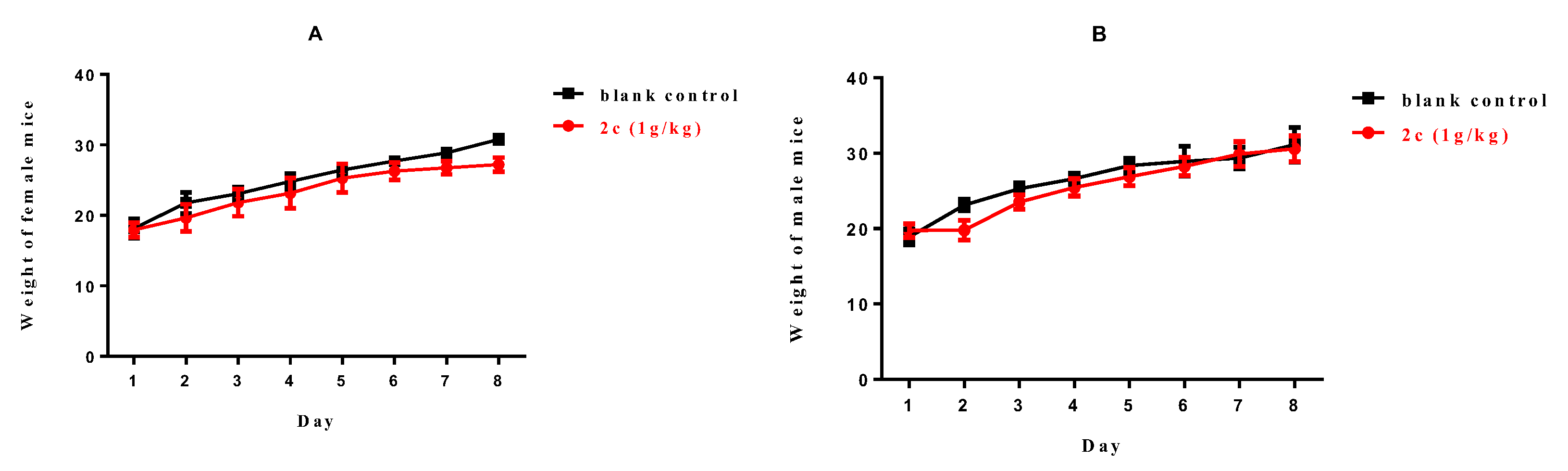
A single-dose toxicity assay of compound 2c was conducted in healthy Kunming mice. After intragastric administration of 2c at a dose of 1000 mg·kg−1, no death occurred, and no significant behavior abnormalities (lethargy, clonic convulsions, anorexia, and ruffled fur) were observed. As illustrated in Figure 6, the body weights of both female and male mice increased gradually within a week. Thus, this would argue that 2c was well-tolerated at a dose of 1000 mg/kg with no acute toxicity.
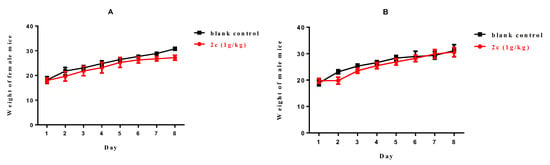
Figure 6.
Body weight of (A) female mice (g) and (B) male mice (g) versus time (day).
3. Conclusions
In summary, we designed and synthesized a novel series of N-substituted oseltamivir derivatives using a bioisosterism strategy for further structural exploration of the 150-cavity. Among the tested series, compounds 2c, 3c and 14c exhibited moderate to remarkable inhibitory potency against N1 (H1N1), N1 (H5N1), N8 (H5N8) and mutant N1 (H5N1-H274Y) compared to that of OSC. In particular, 2c (IC50 = 0.27 μM) showed 4.6-fold more potent activity than OSC (IC50 = 1.25 μM) toward H5N1-H274Y NA. Furthermore, 2c maintained similar activity against H1N1 in MDCK cells, which reached the same level to that of OSC, and also exhibited a slightly improved potency against H5N1 and H5N8 in CEFs over that of OSC. Meanwhile, 2c displayed low cytotoxicity in CEFs and MDCK cells and no acute toxicity in Kunming mice. Molecular docking studies were carried out to rationalize our design hypotheses and offer guidance for further structural optimizations. Collectively, we consider 2c is a valuable lead compound and further modification is in progress.
4. Experimental Section
4.1. Chemistry
The key chemical reactant oseltamivir phosphate and the other chemicals and reagents were purchased from commercial suppliers and used without further purification. Solvents were of reagent grade and were purified and dried with standard methods when necessary. Thin-layer chromatography (TLC) was performed on Silica Gel GF254 for TLC, and spots were visualized by irradiation with UV light (λ = 254 nm and 365 nm). Flash column chromatography was carried out on columns packed with Silica Gel 60 (200–300 mesh), purchased from Qingdao Haiyang Chemical Company. A rotary evaporator was used for the concentration of the reaction solutions under reduced pressure. All melting points (mp) were determined on a micro melting-point apparatus (RY-1G; Tianjin TianGuang Optical Instruments) and were uncorrected. The 1H NMR and 13C NMR spectra were recorded on a Bruker AV-400 spectrometer in the indicated solvent DMSO-d6 and CD3OD with tetramethylsilane (TMS) as the internal standard. Chemical shifts were expressed in δ units (ppm) and J values were presented in hertz (Hz). Mass spectra (MS) were obtained on an API 4000 LC/MS spectrometer (Applied Biosystems, Foster City, CA, USA).
4.1.1. General Procedure for the Preparation of Compounds 1a–18a
A solution of 4-hydroxybenzaldehyde (1 g, 8.2 mmol), substituted benzyl bromide (8.4 mmol, 1.02 eq) and potassium carbonate (1.7 g, 12.3 mmol) in 30 mL of N,N-dimethylformamide (DMF) was stirred overnight under an inert atmosphere at room temperature [29]. Subsequently, 50 mL water was added and the mixture was extracted with ethyl acetate (40 mL × 3). The combined organic phase was washed with a saturated brine (30 mL × 2), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure to give the crude product, which was purified by column chromatography on silica gel (10–50% ethyl acetate in petroleum ether) to obtain the corresponding products, 1a–18a.
(2-((4-Formylphenoxy)methyl)phenyl)boronic acid (1a). White powder, 31%, mp: 185.1–186.7 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.83 (s, J = 27.6 Hz, 1H, CHO), 8.10 (s, 1H, OH), 7.93–7.51 (m, 3H, 3Ph-H), 7.51–6.98 (m, 5H, 5Ph-H), 5.58 (d, J = 15.7 Hz, 1H, CH), 5.36 (s, 1H, CH), 3.42 (s, 1H, OH).
(3-((4-Formylphenoxy)methyl)phenyl)boronic acid (2a). White powder, 24%, mp: 170.1–172.2 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.96–9.76 (m, J = 4.2 Hz, 1H, CHO), 8.09 (s, 1H, OH), 7.98–7.63 (m, 4H, 4Ph-H), 7.50 (d, J = 7.3 Hz, 1H, Ph-H), 7.39 (dt, J = 21.4, 6.6 Hz, 1H, Ph-H), 7.22 (dd, J = 6.6, 1.8 Hz, 2H, 2Ph-H), 5.22 (t, J = 24.0 Hz, 2H, CH2), 3.52 (s, 1H, OH).
(4-((4-Formylphenoxy)methyl)phenyl)boronic acid (3a). White powder, 38%, mp: 129.5–131.7 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.87 (s, 1H, CHO), 7.87 (d, J = 8.1 Hz, 2H, 2Ph-H), 7.81 (d, J = 7.5 Hz, 2H, 2Ph-H), 7.42 (d, J = 7.5 Hz, 2H, 2Ph-H), 7.21 (d, J = 8.2 Hz, 2H, 2Ph-H), 5.24 (s, 2H, CH2), 2.89 (s, 1H, OH), 2.73 (s, 1H, OH).
Methyl 2-((4-formylphenoxy)methyl)benzoate (4a). White powder, 62%, mp: 89.8–91.8 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.88 (s, 1H, CHO), 7.94 (d, J = 7.7 Hz, 1H, Ph-H), 7.89 (d, J = 8.7 Hz, 2H, 2Ph-H), 7.69–7.61 (m, 2H, 2Ph-H), 7.54–7.47 (m, 1H, Ph-H), 7.19 (d, J = 8.7 Hz, 2H, 2Ph-H), 5.54 (s, 2H, CH2), 3.81 (s, 3H, CH3).
Methyl 3-((4-formylphenoxy)methyl)benzoate (5a). White powder, 64%, mp: 79.1–81.2 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.88 (s, 1H, CHO), 8.07 (s, 1H, Ph-H), 7.95 (d, J = 7.8 Hz, 1H, Ph-H), 7.89 (d, J = 8.7 Hz, 2H, 2Ph-H), 7.76 (d, J = 7.7 Hz, 1H, Ph-H), 7.58 (t, J = 7.7 Hz, 1H, Ph-H), 7.23 (d, J = 8.6 Hz, 2H, 2Ph-H), 5.33 (s, 2H, CH2), 3.87 (s, 3H, CH3).
Methyl 4-((4-formylphenoxy)methyl)benzoate (6a). White powder, 61%, mp: 107.1–109.5 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.88 (s, 1H, CHO), 8.00 (d, J = 8.2 Hz, 2H, 2Ph-H), 7.89 (d, J = 8.7 Hz, 2H, 2Ph-H), 7.62 (d, J = 8.1 Hz, 2H, 2Ph-H), 7.23 (d, J = 8.6 Hz, 2H, 2Ph-H), 5.35 (s, 2H, CH2), 3.86 (s, 3H, CH3).
Methyl 3-((4-formylphenoxy)methyl)-4-methoxybenzoate (7a). White powder, 76%, mp: 66.5–68.1 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.89 (s, 1H, CHO), 8.06–7.97 (m, 2H, 2Ph-H), 7.89 (d, J = 8.6 Hz, 2H, 2Ph-H), 7.22 (d, J = 8.5 Hz, 3H, 3Ph-H), 5.23 (s, 2H, CH2), 3.93 (s, 3H, CH3), 3.82 (s, 3H, CH3).
Methyl 3-chloro-4-((4-formylphenoxy)methyl)benzoate (8a). White powder, 73%, mp: 109.8–111.5 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.90 (s, 1H, CHO), 8.01 (d, J = 1.2 Hz, 1H, Ph-H), 7.97 (dd, J = 8.0, 1.3 Hz, 1H, Ph-H), 7.91 (d, J = 8.7 Hz, 2H, 2Ph-H), 7.78 (d, J = 8.0 Hz, 1H, Ph-H), 7.26 (d, J = 8.7 Hz, 2H, 2Ph-H), 5.37 (s, 2H, CH2), 3.89 (s, 3H, CH3).
Methyl 4-((4-formylphenoxy)methyl)-3-methoxybenzoate (9a). White powder, 72%, mp: 92.2–93.8 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.88 (s, 1H, CHO), 7.89 (d, J = 8.7 Hz, 2H, 2Ph-H), 7.64–7.53 (m, J = 19.5, 6.1 Hz, 3H, 3Ph-H), 7.21 (d, J = 8.6 Hz, 2H, 2Ph-H), 5.26 (s, 2H, CH2), 3.92 (s, 3H, CH3), 3.87 (s, 3H, CH3).
Methyl 4-((4-formylphenoxy)methyl)-2-methoxybenzoate (10a). White powder, 72%, mp: 79.1–81.5 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.88 (s, 1H, CHO), 7.89 (d, J = 8.7 Hz, 2H, 2Ph-H), 7.68 (d, J = 7.9 Hz, 1H, Ph-H), 7.27 (s, 1H, Ph-H), 7.23 (d, J = 8.6 Hz, 2H, 2Ph-H), 7.11 (d, J = 7.9 Hz, 1H, Ph-H), 5.29 (s, 2H, CH2), 3.84 (s, 3H, CH3), 3.79 (s, 3H, CH3).
4-((2-Nitrobenzyl)oxy)benzaldehyde (11a). White powder, 62%, mp: 85.1–87.2 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.88 (s, 1H, CHO), 8.15 (d, J = 8.1 Hz, 1H, Ph-H), 7.92–7.87 (m, 2H, 2Ph-H), 7.84–7.77 (m, 2H, 2Ph-H), 7.65 (ddd, J = 8.6, 6.8, 4.1 Hz, 1H, Ph-H), 7.22 (d, J = 8.7 Hz, 2H, 2Ph-H), 5.59 (s, 2H, CH2).
4-((3-Nitrobenzyl)oxy)benzaldehyde (12a). White powder, 66%, mp: 79.7–81.5 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.89 (s, 1H, CHO), 8.35 (s, 1H, Ph-H), 8.22 (dd, J = 8.2, 1.4 Hz, 1H, Ph-H), 7.92 (dd, J = 17.7, 8.2 Hz, 3H, 3Ph-H), 7.73 (t, J = 7.9 Hz, 1H, Ph-H), 7.26 (d, J = 8.7 Hz, 2H, 2Ph-H), 5.41 (s, 2H, CH2).
4-((4-Nitrobenzyl)oxy)benzaldehyde (13a). Yellow powder, 65%. 1H NMR (400 MHz, DMSO-d6) δ: 9.89 (s, 1H, CHO), 8.28 (d, J = 8.7 Hz, 2H, 2Ph-H), 7.90 (d, J = 8.7 Hz, 2H, 2Ph-H), 7.75 (d, J = 8.6 Hz, 2H, 2Ph-H), 7.24 (d, J = 8.7 Hz, 2H, 2Ph-H), 5.42 (s, 2H, CH2).
3-((4-Formylphenoxy)methyl)benzamide (14a). Pale yellow oil, 63% yield. 1H NMR (400 MHz, DMSO-d6) δ: 9.87 (s, 1H, CHO), 8.00 (d, J = 12.9 Hz, 2H, 2Ph-H), 7.87 (dd, J = 11.6, 8.6 Hz, 3H, Ph-H, NH2), 7.62 (d, J = 7.6 Hz, 1H, Ph-H), 7.49 (t, J = 7.7 Hz, 1H, Ph-H), 7.41 (s, 1H, Ph-H), 7.23 (d, J = 8.6 Hz, 2H, 2Ph-H), 5.28 (s, 2H, CH2).
4-((4-Formylphenoxy)methyl)benzamide (15a). White powder, 73%, mp: 185.8–188.2 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.88 (s, 1H, CHO), 7.99 (s, 1H, NH), 7.89 (t, J = 7.8 Hz, 4H, 4Ph-H), 7.54 (d, J = 8.1 Hz, 2H, 2Ph-H), 7.39 (s, 1H, NH), 7.22 (d, J = 8.7 Hz, 2H, 2Ph-H), 5.31 (s, 2H, CH2).
3-((4-Formylphenoxy)methyl)benzenesulfonamide (16a). White powder, 67%, mp: 137.2–139.7 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.89 (s, 1H, CHO), 7.95 (s, 1H, Ph-H), 7.90 (d, J = 8.8 Hz, 2H, 2Ph-H), 7.82 (d, J = 7.8 Hz, 1H, Ph-H), 7.70 (d, J = 7.7 Hz, 1H, Ph-H), 7.62 (t, J = 7.7 Hz, 1H, Ph-H), 7.42 (s, 2H, NH2), 7.24 (d, J = 8.7 Hz, 2H, 2Ph-H), 5.34 (s, 2H, CH2).
4-((4-Formylphenoxy)methyl)benzenesulfonamide (17a). White powder, 65%, mp: 124.5–126.5 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.87 (s, 1H, CHO), 7.87 (dd, J = 12.4, 8.5 Hz, 4H, 4Ph-H), 7.65 (d, J = 8.2 Hz, 2H, 2Ph-H), 7.37 (s, 2H, NH2), 7.22 (d, J = 8.6 Hz, 2H, 2Ph-H), 5.34 (s, 2H, CH2).
4-((4-Formylphenoxy)methyl)benzenesulfonyl fluoride (18a). White powder, 72%, mp: 74.8–76.2 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.89 (s, 1H, CHO), 8.19 (d, J = 8.4 Hz, 2H, 2Ph-H), 7.89 (dd, J = 16.0, 8.4 Hz, 4H, 4Ph-H), 7.25 (d, J = 8.6 Hz, 2H, 2Ph-H), 5.47 (s, 2H, CH2).
4.1.2. General Procedure for the Preparation of Compounds 1b–18b
To a solution of oseltamivir phosphate (0.82 g, 2.0 mmol) in 30 mL methanol, different substituted aldehyde (2.0 mmol, 1 eq) was added at room temperature. The reaction mixture was stirred for 0.5 h, and then NaBH3CN (0.31 g, 5.0 mmol, 2.5 eq) was added. The resulting mixture was stirred at room temperature until completion, monitored by TLC. After removal of the excess solvent under reduced pressure, saturated brine (30 mL) and saturated sodium carbonate solution (10 mL) were added. The aqueous phase was extracted with ethyl acetate (3 × 30 mL). Then, the combined organic phase was dried over anhydrous MgSO4, filtered, and purified by flash column chromatography (0–6% methanol in dichloromethane) to provide the corresponding intermediate 1b–18b.
(2-((4-((((1S,5R,6R)-6-acetamido-3-(ethoxycarbonyl)-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)phenyl)boronic acid (1b). Pale yellow powder, 74% yield, mp: 132.5–134.7 °C. 1H NMR (400 MHz, CD3OD) δ: 7.44–7.21 (m, 6H, 6Ph-H), 6.99 (d, J = 7.9 Hz, 2H, 2Ph-H), 6.78 (s, 1H, CH), 5.10 (s, 2H, CH2), 4.21 (q, J = 7.0 Hz, 2H, CH2), 4.07 (d, J = 10.3 Hz, 1H, CH), 3.93 (t, J = 9.4 Hz, 1H, CH), 3.86 (d, J = 12.7 Hz, 1H, CH), 3.70 (d, J = 12.6 Hz, 1H, CH), 3.42–3.36 (m, 1H, CH), 2.95 (td, J = 10.0, 5.4 Hz, 1H, CH), 2.83 (dd, J = 17.7, 5.0 Hz, 1H, CH), 2.34–2.20 (m, 1H, CH), 2.00 (s, 3H, CH3), 1.59–1.42 (m, 4H, 2CH2), 1.29 (t, J = 7.0 Hz, 3H, CH3), 0.90 (q, J = 8.0 Hz, 6H, 2CH3). ESI-MS: m/z 553.3 [M + H]+, C30H41BN2O7 (552.48).
(3-((4-((((1S,5R,6R)-6-acetamido-3-(ethoxycarbonyl)-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)phenyl)boronic acid (2b). White powder, 73% yield, mp: 163.1–165.2 °C. 1H NMR (400 MHz, CD3OD) δ: 7.84–7.70 (m, 1H, Ph-H), 7.70–7.57 (m, 1H, Ph-H), 7.45 (d, J = 7.4 Hz, 1H, Ph-H), 7.33 (t, J = 7.5 Hz, 1H, Ph-H), 7.25 (d, J = 7.8 Hz, 2H, 2Ph-H), 6.97 (d, J = 7.7 Hz, 2H, 2Ph-H), 6.78 (s, 1H, CH), 5.07 (s, 2H, CH2), 4.22 (q, J = 6.9 Hz, 2H, CH2), 4.07 (d, J = 8.5 Hz, 1H, CH), 3.94 (t, J = 9.5 Hz, 1H, CH), 3.88 (d, J = 12.6 Hz, 1H, CH), 3.71 (d, J = 12.7 Hz, 1H, CH), 3.39 (q, J = 5.1 Hz, 1H, CH), 2.99 (dt, J = 15.1, 7.8 Hz, 1H, CH), 2.84 (dd, J = 17.5, 5.0 Hz, 1H, CH), 2.35–2.23 (m, 1H, CH), 1.99 (s, 3H, CH3), 1.57–1.44 (m, 4H, 2CH2), 1.29 (t, J = 6.9 Hz, 3H, CH3), 0.89 (q, J = 8.0 Hz, 6H, 2CH3). ESI-MS: m/z 553.5 [M + H]+, C30H41BN2O7 (552.48).
(4-((4-((((1S,5R,6R)-6-acetamido-3-(ethoxycarbonyl)-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)phenyl)boronic acid (3b). White powder, 69% yield, mp: 189.2–191.1 °C. 1H NMR (400 MHz, CD3OD) δ: 7.70 (s, 2H, 2Ph-H), 7.39 (d, J = 7.5 Hz, 2H, 2Ph-H), 7.25 (d, J = 7.9 Hz, 2H, 2Ph-H), 6.96 (d, J = 7.9 Hz, 2H, 2Ph-H), 6.79 (s, 1H, CH), 5.08 (s, 2H, CH2), 4.22 (q, J = 7.0 Hz, 2H, CH2), 4.08 (d, J = 7.9 Hz, 1H, CH), 4.00–3.85 (m, 2H, 2CH), 3.73 (d, J = 12.7 Hz, 1H, CH), 3.42–3.36 (m, 1H, CH), 3.00 (dt, J = 15.1, 7.7 Hz, 1H, CH), 2.84 (dd, J = 17.4, 5.0 Hz, 1H, CH), 2.30 (dd, J = 17.4, 9.9 Hz, 1H, CH), 2.00 (s, 3H, CH3), 1.59–1.42 (m, 4H, 2CH2), 1.29 (t, J = 7.0 Hz, 3H, CH3), 0.89 (q, J = 8.0 Hz, 6H, 2CH3). ESI-MS: m/z 553.4 [M + H]+, C30H41BN2O7 (552.48).
Methyl 2-((4-((((1S,5R,6R)-6-acetamido-3-(ethoxycarbonyl)-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)benzoate (4b). White powder, 68% yield, mp: 72.7–74.1 °C. 1H NMR (400 MHz, CD3OD) δ: 7.97 (d, J = 7.8 Hz, 1H, Ph-H), 7.69 (d, J = 7.8 Hz, 1H, Ph-H), 7.56 (t, J = 7.6 Hz, 1H, Ph-H), 7.41 (t, J = 7.6 Hz, 1H, Ph-H), 7.24 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.93 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.77 (s, 1H, CH), 5.43 (s, 2H, CH2), 4.21 (q, J = 7.1 Hz, 2H, CH2), 4.05 (d, J = 8.5 Hz, 1H, CH), 3.94–3.85 (m, 4H, CH, CH3), 3.80 (d, J = 12.7 Hz, 1H, CH), 3.64 (d, J = 12.7 Hz, 1H, CH), 3.37 (dt, J = 11.2, 5.6 Hz, 1H, CH), 2.94–2.85 (m, 1H, CH), 2.81 (dd, J = 17.6, 5.0 Hz, 1H, CH), 2.28–2.18 (m, 1H, CH), 1.99 (s, 3H, CH3), 1.56–1.45 (m, 4H, 2CH2), 1.29 (t, J = 7.1 Hz, 3H, CH3), 0.89 (dt, J = 9.9, 7.4 Hz, 6H, 2CH3). ESI-MS: m/z 567.6 [M + H]+, C32H42N2O7 (566.70).
Methyl 3-((4-((((1S,5R,6R)-6-acetamido-3-(ethoxycarbonyl)-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)benzoate (5b). White powder, 72% yield, mp: 75.2–77.1 °C. 1H NMR (400 MHz, CD3OD) δ: 8.09 (s, 1H, Ph-H), 7.96 (d, J = 7.8 Hz, 1H, Ph-H), 7.68 (d, J = 7.6 Hz, 1H, Ph-H), 7.49 (t, J = 7.7 Hz, 1H, Ph-H), 7.25 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.97 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.78 (s, 1H, CH), 5.13 (s, 2H, CH2), 4.21 (q, J = 7.1 Hz, 2H, CH2), 4.06 (d, J = 8.2 Hz, 1H, CH), 3.97–3.87 (m, 4H, CH, CH3), 3.84 (d, J = 12.7 Hz, 1H, CH), 3.67 (d, J = 12.7 Hz, 1H, CH), 3.41–3.34 (m, 1H, CH), 2.93 (d, J = 5.5 Hz, 1H, CH), 2.83 (d, J = 17.6 Hz, 1H, CH), 2.31–2.20 (m, 1H, CH), 1.99 (s, 3H, CH3), 1.57–1.44 (m, 4H, 2CH2), 1.29 (t, J = 7.1 Hz, 3H, CH3), 0.89 (dd, J = 17.0, 7.5 Hz, 6H, 2CH3). ESI-MS: m/z 567.10 [M + H]+, C32H42N2O7 (566.70).
Methyl 4-((4-((((1S,5R,6R)-6-acetamido-3-(ethoxycarbonyl)-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)benzoate (6b). White powder, 67% yield, mp: 141.5–143.5 °C. 1H NMR (400 MHz, CD3OD) δ: 8.01 (d, J = 8.2 Hz, 2H, 2Ph-H), 7.54 (d, J = 8.2 Hz, 2H, 2Ph-H), 7.24 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.96 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.77 (s, 1H, CH), 5.15 (s, 2H, CH2), 4.21 (q, J = 7.1 Hz, 2H, CH2), 4.06 (d, J = 7.9 Hz, 1H, CH), 3.95–3.87 (m, 4H, CH, CH3), 3.81 (d, J = 12.7 Hz, 1H, CH), 3.65 (d, J = 12.7 Hz, 1H, CH), 3.41–3.34 (m, 1H, CH), 2.90 (td, J = 10.0, 5.4 Hz, 1H, CH), 2.81 (dd, J = 17.6, 5.1 Hz, 1H, CH), 2.24 (ddt, J = 15.1, 9.4, 2.6 Hz, 1H, CH), 1.99 (s, 3H, CH3), 1.55–1.45 (m, 4H, 2CH2), 1.29 (t, J = 7.1 Hz, 3H, CH3), 0.89 (dt, J = 9.8, 7.4 Hz, 6H, 2CH3). ESI-MS: m/z 567.5 [M + H]+, C32H42N2O7 (566.70).
Methyl 3-((4-((((1S,5R,6R)-6-acetamido-3-(ethoxycarbonyl)-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)-4-methoxybenzoate (7b). Colorless sticky oil, 70% yield. 1H NMR (400 MHz, CD3OD) δ: 8.06 (d, J = 1.2 Hz, 1H, Ph-H), 8.00 (dd, J = 8.7, 1.8 Hz, 1H, Ph-H), 7.29 (d, J = 8.5 Hz, 2H, 2Ph-H), 7.10 (d, J = 8.7 Hz, 1H, Ph-H), 6.99 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.80 (s, 1H, CH), 5.11 (s, 2H, CH2), 4.22 (q, J = 7.1 Hz, 2H, CH2), 4.10 (d, J = 8.1 Hz, 1H, CH), 4.05–3.93 (m, 5H, CH2, CH3), 3.86 (s, 3H, CH3), 3.82 (d, J = 12.9 Hz, 1H, CH), 3.43–3.36 (m, 1H, CH), 3.09 (td, J = 10.1, 5.6 Hz, 1H, CH), 2.88 (dd, J = 17.0, 4.7 Hz, 1H, CH), 2.42–2.30 (m, 1H, CH), 2.01 (s, 3H, CH3), 1.56–1.45 (m, 4H, 2CH2), 1.30 (t, J = 7.1 Hz, 3H, CH3), 0.89 (dd, J = 16.5, 7.5 Hz, 6H, 2CH3). ESI-MS: m/z 598.05 [M + H]+, C33H44N2O8 (596.72).
Methyl 4-((4-((((1S,5R,6R)-6-acetamido-3-(ethoxycarbonyl)-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)-3-chlorobenzoate (8b). White powder, 73% yield, mp: 159.5–161.5 °C. 1H NMR (400 MHz, CD3OD) δ: 8.03 (d, J = 1.1 Hz, 1H, Ph-H), 7.95 (dd, J = 8.1, 1.1 Hz, 1H, Ph-H), 7.69 (d, J = 8.0 Hz, 1H, Ph-H), 7.26 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.97 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.77 (s, 1H, CH), 5.21 (s, 2H, CH2), 4.21 (q, J = 7.1 Hz, 2H, CH2), 4.05 (d, J = 8.3 Hz, 1H, CH), 3.96–3.86 (m, 4H, CH, CH3), 3.81 (d, J = 12.7 Hz, 1H, CH), 3.64 (d, J = 12.8 Hz, 1H, CH), 3.37 (dt, J = 11.1, 5.5 Hz, 1H, CH), 2.92–2.85 (m, 1H, CH), 2.80 (dd, J = 17.5, 5.2 Hz, 1H, CH), 2.27–2.17 (m, 1H, CH), 1.99 (s, 3H, CH3), 1.57–1.46 (m, 4H, 2CH2), 1.29 (t, J = 7.1 Hz, 3H, CH3), 0.89 (dt, J = 9.7, 7.5 Hz, 6H, 2CH3). ESI-MS: m/z 601.37 [M + H]+, C32H41ClN2O7 (601.14).
Methyl 4-((4-((((1S,5R,6R)-6-acetamido-3-(ethoxycarbonyl)-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)-3-methoxybenzoate (9b). Colorless sticky oil, 71% yield. 1H NMR (400 MHz, CD3OD) δ: 7.64–7.59 (m, 2H, 2Ph-H), 7.50 (d, J = 7.7 Hz, 1H, Ph-H), 7.34 (d, J = 8.6 Hz, 2H, 2Ph-H), 7.01 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.83 (s, 1H, CH), 5.15 (s, 2H, CH2), 4.23 (q, J = 7.1 Hz, 2H, CH2), 4.12 (dd, J = 20.2, 7.1 Hz, 2H, CH2), 4.08–4.02 (m, 1H, CH), 3.97 (d, J = 13.0 Hz, 1H, CH), 3.93 (s, 3H, CH3), 3.90 (s, 3H, CH3), 3.45–3.38 (m, 1H, CH), 3.30–3.23 (m, 1H, CH), 2.94 (dd, J = 17.5, 5.5 Hz, 1H, CH), 2.53–2.40 (m, 1H, CH), 2.02 (s, 3H, CH3), 1.59–1.46 (m, 4H, 2CH2), 1.30 (t, J = 7.1 Hz, 3H, CH3), 0.90 (q, J = 7.6 Hz, 6H, 2CH3). ESI-MS: m/z 597.16 [M + H]+, C33H44N2O8 (596.72).
Methyl 4-((4-((((1S,5R,6R)-6-acetamido-3-(ethoxycarbonyl)-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)-2-methoxybenzoate (10b). White powder, 67% yield, mp: 133.2–135.3 °C. 1H NMR (400 MHz, CD3OD) δ: 7.75 (d, J = 7.9 Hz, 1H, Ph-H), 7.25 (d, J = 8.6 Hz, 2H, 2Ph-H), 7.18 (s, 1H, Ph-H), 7.06 (d, J = 7.9 Hz, 1H, Ph-H), 6.96 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.77 (s, 1H, CH), 5.13 (s, 2H, CH2), 4.21 (q, J = 7.1 Hz, 2H, CH2), 4.06 (d, J = 8.6 Hz, 1H, CH), 4.01–3.86 (m, 5H, CH2, CH3), 3.86–3.77 (m, 4H, CH, CH3), 3.66 (d, J = 12.7 Hz, 1H, CH), 3.41–3.34 (m, 1H, CH), 2.92 (d, J = 5.5 Hz, 1H, CH), 2.82 (d, J = 17.6 Hz, 1H, CH), 2.31–2.19 (m, 1H, CH), 1.99 (s, 3H, CH3), 1.58–1.45 (m, 4H, 2CH2), 1.29 (t, J = 7.1 Hz, 3H, CH3), 0.89 (dd, J = 17.0, 7.5 Hz, 6H, 2CH3). ESI-MS: m/z 597.83 [M + H]+, C33H44N2O8 (596.72).
Ethyl (3R,4R,5S)-4-acetamido-5-((4-((2-nitrobenzyl)oxy)benzyl)amino)-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate (11b). White powder, 68% yield, mp: 101.8–103.5 °C. 1H NMR (400 MHz, CD3OD) δ: 8.12 (d, J = 8.2 Hz, 1H, Ph-H), 7.83 (d, J = 7.7 Hz, 1H, Ph-H), 7.71 (t, J = 7.6 Hz, 1H, Ph-H), 7.55 (t, J = 7.8 Hz, 1H, Ph-H), 7.25 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.95 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.77 (s, 1H, CH), 5.44 (s, 2H, CH2), 4.21 (q, J = 7.1 Hz, 2H, CH2), 4.05 (d, J = 8.2 Hz, 1H, CH), 3.90 (dd, J = 10.2, 8.7 Hz, 1H, CH), 3.80 (d, J = 12.7 Hz, 1H, CH), 3.64 (d, J = 12.8 Hz, 1H, CH), 3.41–3.33 (m, 1H, CH), 2.93–2.76 (m, 2H, 2CH), 2.28–2.17 (m, 1H, CH), 1.99 (s, 3H, CH3), 1.57–1.43 (m, 4H, 2CH2), 1.29 (t, J = 7.1 Hz, 3H, CH3), 0.89 (dt, J = 9.9, 7.4 Hz, 6H, 2CH3). ESI-MS: m/z 554.5 [M + H]+, C30H39N3O7 (553.66).
Ethyl (3R,4R,5S)-4-acetamido-5-((4-((3-nitrobenzyl)oxy)benzyl)amino)-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate (12b). White powder, 70% yield, mp: 144.2–146.1 °C. 1H NMR (400 MHz, CD3OD) δ: 8.31 (s, 1H, Ph-H), 8.18 (d, J = 8.2 Hz, 1H, Ph-H), 7.84 (d, J = 7.7 Hz, 1H, Ph-H), 7.62 (t, J = 7.9 Hz, 1H, Ph-H), 7.25 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.98 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.77 (s, 1H, CH), 5.21 (s, 2H, CH2), 4.21 (q, J = 7.1 Hz, 2H, CH2), 4.05 (d, J = 8.2 Hz, 1H, CH), 3.95–3.86 (m, 1H, CH), 3.80 (d, J = 12.7 Hz, 1H, CH), 3.64 (d, J = 12.7 Hz, 1H, CH), 3.41–3.33 (m, 1H, CH), 2.88 (td, J = 9.9, 5.4 Hz, 1H, CH), 2.80 (dd, J = 17.5, 5.2 Hz, 1H, CH), 2.22 (ddt, J = 15.0, 9.0, 2.4 Hz, 1H, CH), 1.99 (s, 3H, CH3), 1.56–1.44 (m, 4H, 2CH2), 1.29 (t, J = 7.1 Hz, 3H, CH3), 0.89 (dd, J = 17.2, 7.5 Hz, 6H, 2CH3). ESI-MS: m/z 554.5 [M + H]+, C30H39N3O7 (553.66).
Ethyl (3R,4R,5S)-4-acetamido-5-((4-((4-nitrobenzyl)oxy)benzyl)amino)-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate (13b). White powder, 71% yield, mp: 118.9–120.1 °C. 1H NMR (400 MHz, CD3OD) δ: 8.24 (d, J = 8.7 Hz, 2H, 2Ph-H), 7.68 (d, J = 8.6 Hz, 2H, 2Ph-H), 7.25 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.97 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.77 (s, 1H, CH), 5.22 (s, 2H, CH2), 4.21 (q, J = 7.1 Hz, 2H, CH2), 4.06 (d, J = 8.2 Hz, 1H, CH), 3.90 (dd, J = 10.3, 8.6 Hz, 1H, CH), 3.80 (d, J = 12.7 Hz, 1H, CH), 3.64 (d, J = 12.7 Hz, 1H, CH), 3.41–3.34 (m, 1H, CH), 2.89 (td, J = 9.9, 5.4 Hz, 1H, CH), 2.80 (dd, J = 17.5, 5.2 Hz, 1H, CH), 2.22 (ddt, J = 15.0, 9.4, 2.6 Hz, 1H, CH), 1.99 (s, 3H, CH3), 1.57–1.44 (m, 4H, 2CH2), 1.29 (t, J = 7.1 Hz, 3H, CH3), 0.89 (dt, J = 9.4, 7.5 Hz, 6H, 2CH3). ESI-MS: m/z 554.24 [M + H]+, C30H39N3O7 (553.66).
Ethyl (3R,4R,5S)-4-acetamido-5-((4-((3-carbamoylbenzyl)oxy)benzyl)amino)-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate (14b). Light yellow powder, 62% yield, mp: 119.5–121.3 °C. 1H NMR (400 MHz, CD3OD) δ: 7.89 (s, 1H, Ph-H), 7.75 (d, J = 7.8 Hz, 1H, Ph-H), 7.55 (d, J = 7.7 Hz, 1H, Ph-H), 7.40 (t, J = 7.7 Hz, 1H, Ph-H), 7.23 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.95 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.74 (s, 1H, CH), 5.08 (s, 2H, CH2), 4.16 (q, J = 7.1 Hz, 2H, CH2), 4.04 (t, J = 7.0 Hz, 1H, CH), 4.00–3.88 (m, 2H, 2CH), 3.79 (d, J = 12.9 Hz, 1H, CH), 3.33 (dt, J = 11.3, 5.6 Hz, 1H, CH), 3.08 (td, J = 9.9, 5.4 Hz, 1H, CH), 2.82 (dd, J = 17.6, 5.3 Hz, 1H, CH), 2.39–2.26 (m, 1H, CH), 1.94 (s, 3H, CH3), 1.51–1.38 (m, 4H, 2CH2), 1.23 (t, J = 7.1 Hz, 3H, CH3), 0.88–0.77 (m, 6H, 2CH3). ESI-MS: m/z 552.55 [M + H]+, C31H41N3O6 (551.68).
Ethyl (3R,4R,5S)-4-acetamido-5-((4-((4-carbamoylbenzyl)oxy)benzyl)amino)-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate (15b). White powder, 71% yield, mp: 214.5–216.5 °C. 1H NMR (400 MHz, CD3OD) δ: 7.87 (d, J = 8.2 Hz, 2H, 2Ph-H), 7.53 (d, J = 8.1 Hz, 2H, 2Ph-H), 7.24 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.96 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.77 (s, 1H, CH), 5.14 (s, 2H, CH2), 4.21 (q, J = 7.1 Hz, 2H, CH2), 4.05 (d, J = 8.1 Hz, 1H, CH), 3.90 (dd, J = 10.2, 8.7 Hz, 1H, CH), 3.80 (d, J = 12.7 Hz, 1H, CH), 3.64 (d, J = 12.7 Hz, 1H, CH), 3.37 (dt, J = 11.3, 5.5 Hz, 1H, CH), 2.93–2.85 (m, 1H, CH), 2.81 (dd, J = 17.6, 5.0 Hz, 1H, CH), 2.28–2.18 (m, 1H, CH), 1.99 (s, 3H, CH3), 1.59–1.43 (m, 4H, 2CH2), 1.29 (t, J = 7.1 Hz, 3H, CH3), 0.89 (dt, J = 9.7, 7.4 Hz, 6H, 2CH3). ESI-MS: m/z 552.17 [M + H]+, C31H41N3O6 (551.68).
Ethyl (3R,4R,5S)-4-acetamido-3-(pentan-3-yloxy)-5-((4-((3-sulfamoylbenzyl)oxy) benzyl)amino)cyclohex-1-ene-1-carboxylate (16b). White powder, 64% yield, mp: 127.5–129.7 °C. 1H NMR (400 MHz, CD3OD) δ: 7.93 (s, 1H, Ph-H), 7.79 (d, J = 7.8 Hz, 1H, Ph-H), 7.60 (d, J = 7.7 Hz, 1H, Ph-H), 7.49 (t, J = 7.7 Hz, 1H, Ph-H), 7.22 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.94 (d, J = 8.7 Hz, 2H, 2Ph-H), 6.73 (s, 1H, CH), 5.11 (s, 2H, CH2), 4.16 (q, J = 7.1 Hz, 2H, CH2), 4.02 (d, J = 8.1 Hz, 1H, CH), 3.94–3.83 (m, 2H, 2CH), 3.69 (d, J = 12.8 Hz, 1H, CH), 3.33 (dt, J = 11.3, 5.6 Hz, 1H, CH), 3.00–2.91 (m, 1H, CH), 2.84–2.75 (m, 1H, CH), 2.31–2.20 (m, 1H, CH), 1.94 (s, 3H, CH3), 1.52–1.40 (m, 4H, 2CH2), 1.23 (t, J = 7.1 Hz, 3H, CH3), 0.83 (dt, J = 9.7, 7.4 Hz, 6H, 2CH3). ESI-MS: m/z 588.52 [M + H]+, C30H41N3O7S (587.73).
Ethyl (3R,4R,5S)-4-acetamido-3-(pentan-3-yloxy)-5-((4-((4-sulfamoylbenzyl)oxy) benzyl)amino)cyclohex-1-ene-1-carboxylate (17b). White powder, 69% yield, mp: 76.8–78.9 °C. 1H NMR (400 MHz, CD3OD) δ: 7.83 (d, J = 8.3 Hz, 2H, 2Ph-H), 7.54 (d, J = 8.3 Hz, 2H, 2Ph-H), 7.28 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.97 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.77 (s, 1H, CH), 5.14 (s, 2H, CH2), 4.17 (q, J = 7.1 Hz, 2H, CH2), 4.11–3.95 (m, 3H, 3CH), 3.91 (d, J = 13.0 Hz, 1H, CH), 3.39–3.32 (m, 1H, CH), 3.23–3.16 (m, 1H, CH), 2.87 (dd, J = 17.5, 5.4 Hz, 1H, CH), 2.41 (ddd, J = 15.1, 9.7, 2.3 Hz, 1H, CH), 1.95 (s, 3H, CH3), 1.53–1.38 (m, 4H, 2CH2), 1.24 (t, J = 7.1 Hz, 3H, CH3), 0.83 (q, J = 7.6 Hz, 6H, 2CH3). ESI-MS: m/z 588.49 [M + H]+, C30H41N3O7S (587.73).
Ethyl (3R,4R,5S)-4-acetamido-5-((4-((4-(fluorosulfonyl)benzyl)oxy)benzyl)amino) -3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate (18b). White powder, 72% yield, mp: 109.5–111.3 °C. 1H NMR (400 MHz, CD3OD) δ: 8.07 (d, J = 8.4 Hz, 2H, 2Ph-H), 7.81 (d, J = 8.3 Hz, 2H, 2Ph-H), 7.38 (d, J = 8.6 Hz, 2H, 2Ph-H), 7.08 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.85 (s, 1H, CH), 5.30 (s, 2H, CH2), 4.28–4.15 (m, 4H, CH2, 2CH), 4.13–4.03 (m, 2H, 2CH), 3.46–3.35 (m, 2H, 2CH), 2.96 (dd, J = 17.8, 5.7 Hz, 1H, CH), 2.59–2.48 (m, 1H, CH), 2.02 (s, 3H, CH3), 1.53 (tq, J = 13.5, 6.8 Hz, 4H, 2CH2), 1.30 (t, J = 7.1 Hz, 3H, CH3), 0.90 (q, J = 7.4 Hz, 6H, 2CH3). ESI-MS: m/z 591.51 [M + H]+, C30H39FN2O7S (590.71).
4.1.3. General Procedure for the Preparation of Compounds 1c–18c
The intermediates 1b–18b (0.8 mmol) were dissolved in methanol (30 mL) and 1 mol/L aqueous sodium hydroxide (10 mL) was added. The mixed solution was stirred at room temperature for 3–4 h. After completion of the reaction, the methanol was removed under reduced pressure. The residue was taken up in water (30 mL), and the pH value was adjusted to 2–3 with 3 mol/L HCl aqueous solution while the solid was precipitated from the water solution. After that, the solution was filtered and washed with water and dried under vacuum to afford the target compounds 1c–18c.
(3R,4R,5S)-4-acetamido-5-((4-((2-boronobenzyl)oxy)benzyl)amino)-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylic acid (1c). White powder, 65% yield, mp: 146.3–150.5 °C (along with the decomposition). 1H NMR (400 MHz, CD3OD) δ: 7.51–7.24 (m, 6H, 6Ph-H), 7.14–7.05 (m, J = 9.0 Hz, 2H, 2Ph-H), 6.86 (s, 1H, CH), 5.14 (s, 2H, CH2), 4.34 (d, J = 12.6 Hz, 1H, CH), 4.27–4.14 (m, 3H, 3CH), 3.62–3.52 (m, 1H, CH), 3.49–3.41 (m, 1H, CH), 3.02 (d, J = 16.9 Hz, 1H, CH), 2.70–2.59 (m, 1H, CH), 2.05 (s, 3H, CH3), 1.60–1.46 (m, J = 19.5, 9.7 Hz, 4H, 2CH2), 0.91 (q, J = 7.1 Hz, 6H, 2CH3). 13C NMR (100 MHz, CD3OD) δ: 159.5, 139.8, 131.3, 131.2, 131.2, 131.1, 128.2, 126.9, 126.3, 123.12, 115.1, 82.3, 74.5, 69.9, 54.7, 51.5, 25.7, 25.2, 22.0, 21.7, 8.4, 8.12. ESI-MS: m/z 525.4 [M + H]+, C28H37BN2O7 (524.42).
(3R,4R,5S)-4-acetamido-5-((4-((3-boronobenzyl)oxy)benzyl)amino)-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylic acid (2c). White powder, 53% yield, mp: 209.8–214.3 °C (along with the decomposition). 1H NMR (400 MHz, CD3OD) δ: 7.73–7.34 (m, 5H, 5Ph-H), 7.08 (t, J = 8.6 Hz, 2H, 2Ph-H), 6.97–6.79 (m, 2H, Ph-H, CH), 5.11 (d, J = 28.6 Hz, 2H, CH2), 4.33 (d, J = 13.0 Hz, 1H, CH), 4.27–4.15 (m, J = 15.2, 4.9 Hz, 3H, 3CH), 3.55 (td, J = 9.6, 5.9 Hz, 1H, CH), 3.46 (dt, J = 11.2, 5.5 Hz, 1H, CH), 3.03 (dd, J = 17.3, 5.5 Hz, 1H, CH), 2.64 (ddd, J = 9.5, 8.5, 4.6 Hz, 1H, CH), 2.06 (s, 3H, CH3), 1.55 (tq, J = 14.0, 6.8 Hz, 4H, 2CH2), 0.92 (q, J = 7.4 Hz, 6H, 2CH3). 13C NMR (100 MHz, CD3OD) δ: 173.5, 167.9, 159.8, 138.5, 136.5, 131.1, 130.6, 129.2, 128.1, 127.4, 122.9, 118.1, 115.4, 114.4, 113.8, 82.3, 74.6, 69.5, 54.6, 51.5, 47.1, 26.1, 25.7, 25.2, 22.0, 8.4, 8.2. ESI-MS: m/z 523.4 [M − H]−, C28H37BN2O7 (524.42).
(3R,4R,5S)-4-acetamido-5-((4-((4-boronobenzyl)oxy)benzyl)amino)-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylic acid (3c). White powder, 63% yield, mp: 168.3–172.1 °C (along with the decomposition). 1H NMR (400 MHz, CD3OD) δ: 7.70–7.55 (m, 1H, Ph-H), 7.41 (dd, J = 8.5, 3.9 Hz, 3H, 3Ph-H), 7.26 (d, J = 8.4 Hz, 1H, Ph-H), 7.08 (t, J = 8.3 Hz, 2H, 2Ph-H), 6.87 (d, J = 8.0 Hz, 1H, CH), 6.79 (d, J = 8.5 Hz, 1H, Ph-H), 5.16 (s, 1H, CH), 5.01 (s, 1H, CH), 4.33 (d, J = 13.0 Hz, 1H, CH), 4.26–4.15 (m, 3H, 3CH), 3.60–3.50 (m, J = 4.2 Hz, 1H, CH), 3.50–3.42 (m, 1H, CH), 3.08–2.97 (m, J = 8.5, 7.0 Hz, 1H, CH), 2.71–2.59 (m, 1H, CH), 2.06 (s, 3H, CH3), 1.55 (dq, J = 14.0, 7.0 Hz, 4H, 2CH2), 0.92 (q, J = 7.4 Hz, 6H, 2CH3). 13C NMR (100 MHz, CD3OD) δ: 159.9, 159.8, 157.1, 137.8, 133.7, 131.1, 131.1, 129.1, 127.5, 126.2, 124.7, 122.8, 122.6, 115.4, 115.3, 114.8, 82.3, 74.6, 69.7, 69.5, 54.6, 51.5, 29.5, 25.7, 25.2, 22.0, 8.4, 8.2. ESI-MS: m/z 523.4 [M − H]−, C28H37BN2O7 (524.42).
2-((4-((((1S,5R,6R)-6-acetamido-3-carboxy-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)benzoic acid (4c). White powder, 63% yield, mp: 177.1–178.9 °C. 1H NMR (400 MHz, CD3OD) δ: 8.06–7.97 (m, 1H, Ph-H), 7.66 (d, J = 7.7 Hz, 1H, Ph-H), 7.57–7.50 (m, 1H, Ph-H), 7.48–7.36 (m, 3H, 3Ph-H), 7.05 (d, J = 8.7 Hz, 2H, 2Ph-H), 6.85 (s, 1H, CH), 5.50 (s, 2H, CH2), 4.31 (d, J = 13.0 Hz, 1H, CH), 4.28–4.13 (m, 3H, 3CH), 3.60 (td, J = 10.3, 5.6 Hz, 1H, CH), 3.49–3.40 (m, 1H, CH), 3.02 (dd, J = 17.3, 5.4 Hz, 1H, CH), 2.71–2.58 (m, 1H, CH), 2.05 (s, 3H, CH3), 1.61–1.45 (m, 4H, 2CH2), 0.97–0.85 (m, 6H, 2CH3). 13C NMR (100 MHz, CD3OD) δ: 173.5, 169.2, 167.5, 159.8, 138.6, 137.0, 131.9, 131.2, 130.6, 129.2, 127.7, 127.2, 127.2, 122.9, 115.2, 82.3, 74.6, 68.0, 54.6, 51.5, 47.0, 26.0, 25.7, 25.2, 22.0, 8.4, 8.2. HRMS calcd. For C29H36N2O7 [M + H]+: 525.2595. Found: m/z 525.2595.
3-((4-((((1S,5R,6R)-6-acetamido-3-carboxy-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)benzoic acid (5c). White powder, 70% yield, mp: 146.2–148.5 °C. 1H NMR (400 MHz, CD3OD) δ: 8.08 (s, 1H, Ph-H), 7.96 (d, J = 7.8 Hz, 1H, Ph-H), 7.66 (d, J = 7.7 Hz, 1H, Ph-H), 7.52–7.38 (m, 3H, 3Ph-H), 7.08 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.84 (s, 1H, CH), 5.19 (s, 2H, CH2), 4.32 (d, J = 13.0 Hz, 1H, CH), 4.28–4.09 (m, 3H, 3CH), 3.58 (td, J = 10.1, 5.6 Hz, 1H, CH), 3.47–3.40 (m, 1H, CH), 3.01 (dd, J = 17.3, 5.4 Hz, 1H, CH), 2.72–2.56 (m, 1H, CH), 2.04 (s, 3H, CH3), 1.62–1.46 (m, 4H, 2CH2), 0.90 (q, J = 7.6 Hz, 6H, 2CH3). 13C NMR (100 MHz, CD3OD) δ: 173.5, 168.3, 167.7, 159.6, 137.5, 136.7, 131.5, 131.3, 128.9, 128.3, 127.9, 123.1, 115.4, 82.3, 74.6, 69.0, 54.6, 51.5, 47.1, 26.0, 25.9, 25.2, 22.0, 8.4, 8.2. HRMS calcd for C29H36N2O7 [M + H]+: 525.2596. Found: m/z 525.2595.
4-((4-((((1S,5R,6R)-6-acetamido-3-carboxy-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)benzoic acid (6c). White powder, 71% yield, mp: 193.3–195.5 °C (along with the decomposition). 1H NMR (400 MHz, CD3OD) δ: 8.01 (d, J = 8.3 Hz, 2H, 2Ph-H), 7.53 (d, J = 8.2 Hz, 2H, 2Ph-H), 7.42 (d, J = 8.6 Hz, 2H, 2Ph-H), 7.07 (d, J = 8.7 Hz, 2H, 2Ph-H), 6.84 (s, 1H, CH), 5.20 (s, 2H, CH2), 4.32 (d, J = 13.0 Hz, 1H, CH), 4.27–4.13 (m, J = 18.8, 8.6 Hz, 3H, 3CH), 3.63–3.54 (m, J = 10.1, 5.6 Hz, 1H, CH), 3.48–3.40 (m, 1H, CH), 3.01 (dd, J = 17.3, 5.4 Hz, 1H, CH), 2.71–2.58 (m, 1H, CH), 2.04 (s, 3H, CH3), 1.60–1.45 (m, 4H, 2CH2), 0.90 (q, J = 7.6 Hz, 6H, 2CH3). 13C NMR (100 MHz, CD3OD) δ; 173.5, 168.3, 167.6, 159.5, 142.2, 136.7, 131.2, 130.3, 129.6, 127.8, 126.8, 123.1, 115.3, 82.3, 74.6, 68.9, 54.6, 51.5, 47.1, 26.0, 25.7, 25.2, 22.0, 8.4, 8.2. HRMS calcd for C29H36N2O7 [M + H]+: 525.2596. Found: m/z 525.2595.
3-((4-((((1S,5R,6R)-6-acetamido-3-carboxy-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)-4-methoxybenzoic acid (7c). White powder, 68% yield, mp: 152.1–154.1 °C. 1H NMR (400 MHz, CD3OD) δ: 8.06–7.95 (m, 2H, 2Ph-H), 7.40 (d, J = 8.6 Hz, 2H, 2Ph-H), 7.14–7.02 (m, 3H, 3Ph-H), 6.81 (s, 1H, CH), 5.14 (s, 2H, CH2), 4.31 (d, J = 13.0 Hz, 1H, CH), 4.24–4.13 (m, J = 10.5, 6.4 Hz, 3H, 3CH), 3.94 (s, 3H, CH3), 3.57–3.48 (m, 1H, CH), 3.47–3.40 (m, 1H, CH), 3.03 (d, J = 5.4 Hz, 1H, CH), 2.71–2.56 (m, J = 17.5, 9.9 Hz, 1H, CH), 2.04 (s, 3H, CH3), 1.60–1.47 (m, 4H, 2CH2), 0.90 (q, J = 7.4 Hz, 6H, 2CH3). 13C NMR (100 MHz, CD3OD) δ: 173.4, 168.4, 168.3, 160.7, 159.7, 135.9, 131.3, 131.2, 129.7, 128.6, 125.0, 123.0, 115.2, 109.8, 82.2, 74.6, 64.4, 55.0, 54.6, 51.56, 26.2, 25.7, 25.2, 22.0, 8.4, 8.2. HRMS calcd for C30H38N2O8 [M + H]+: 555.2703. Found: m/z 555.2701.
4-((4-((((1S,5R,6R)-6-acetamido-3-carboxy-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)-3-chlorobenzoic acid (8c). White powder, 66% yield, mp: 189.3–192.1 °C (along with the decomposition). 1H NMR (400 MHz, CD3OD) δ: 8.01–7.96 (m, J = 3.5 Hz, 1H, Ph-H), 7.89 (dd, J = 8.0, 1.3 Hz, 1H, Ph-H), 7.61 (d, J = 8.0 Hz, 1H, Ph-H), 7.43 (d, J = 8.6 Hz, 2H, 2Ph-H), 7.06 (d, J = 8.7 Hz, 2H, 2Ph-H), 6.80 (s, 1H, CH), 5.22 (s, 2H, CH2), 4.31 (d, J = 13.0 Hz, 1H, CH), 4.24–4.14 (m, 3H, 3CH), 3.56 (td, J = 9.9, 5.6 Hz, 1H, CH), 3.47–3.40 (m, 1H, CH), 3.00 (dd, J = 17.1, 5.7 Hz, 1H, CH), 2.69–2.58 (m, 1H, CH), 2.04 (s, 3H, CH3), 1.59–1.46 (m, 4H, 2CH2), 0.90 (q, J = 7.4 Hz, 6H, 2CH3). 13C NMR (100 MHz, CD3OD) δ: 173.4, 168.6, 168.2, 159.3, 138.3, 135.7, 134.2, 132.4, 131.2, 130.0, 129.0, 128.5, 127.8, 123.9, 115.2, 82.3, 74.8, 66.7, 54.7, 51.7, 47.1, 26.4, 25.8, 25.2, 22.0, 8.4, 8.2. HRMS calcd for C29H35ClN2O7 [M + H]+: 559.2210. Found: m/z 559.2206.
4-((4-((((1S,5R,6R)-6-acetamido-3-carboxy-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)-3-methoxybenzoic acid (9c). White powder, 68% yield, mp: 171.2–175.7 °C (along with the decomposition). 1H NMR (400 MHz, DMSO-d6) δ: 7.98–7.88 (m, 1H, Ph-H), 7.61–7.45 (m, 3H, 2Ph-H, NH), 7.29 (d, J = 8.4 Hz, 2H, 2Ph-H), 6.97 (d, J = 8.4 Hz, 2H, 2Ph-H), 6.61 (s, 1H, CH), 5.10 (s, 2H, CH2), 4.06 (d, J = 7.4 Hz, 1H, CH), 3.93–3.72 (m, 6H, CH3, 3CH), 3.39–3.32 (m, 1H, CH), 2.92 (dd, J = 12.0, 7.0 Hz, 1H, CH), 2.75–2.67 (m, 1H, CH), 2.22 (dd, J = 16.6, 10.2 Hz, 1H, CH), 1.87 (s, 3H, CH3), 1.50–1.32 (m, 4H, 2CH2), 0.87–0.75 (m, 6H, 2CH3). 13C NMR (100 MHz, DMSO-d6) δ: 170.5, 167.9, 167.7, 158.1, 157.0, 137.8, 132.5, 130.4, 130.2, 129.4, 128.8, 122.0, 115.0, 111.5, 81.4, 75.6, 64.8, 56.1, 54.6, 53.6, 48.3, 29.5, 26.1, 25.6, 23.6, 9.9, 9.4. HRMS calcd for C30H38N2O8 [M + H]+: 555.2706. Found: m/z 555.2701.
4-((4-((((1S,5R,6R)-6-acetamido-3-carboxy-5-(pentan-3-yloxy)cyclohex-3-en-1-yl)amino)methyl)phenoxy)methyl)-2-methoxybenzoic acid (10c). White powder, 72% yield, mp: 190.5–195.1 °C (along with the decomposition). 1H NMR (400 MHz, CD3OD) δ: 7.68 (d, J = 7.8 Hz, 1H, Ph-H), 7.39 (d, J = 8.5 Hz, 2H, 2Ph-H), 7.13 (s, 1H, Ph-H), 7.03 (d, J = 8.3 Hz, 3H, 3Ph-H), 6.75 (s, 1H, CH), 5.14 (s, 2H, CH2), 4.26 (d, J = 13.0 Hz, 1H, CH), 4.21–4.07 (m, J = 17.9, 10.8 Hz, 3H, 3CH), 3.85 (s, 3H, CH3), 3.51 (dd, J = 14.5, 9.1 Hz, 1H, CH), 3.46–3.38 (m, 1H, CH), 2.97 (dd, J = 17.3, 5.0 Hz, 1H, CH), 2.61 (dd, J = 17.2, 9.7 Hz, 1H, CH), 2.03 (s, 3H, CH3), 1.61–1.42 (m, 4H, 2CH2), 0.89 (dd, J = 13.5, 7.2 Hz, 6H, 2CH3). 13C NMR (100 MHz, CD3OD) δ: 173.3, 169.8, 169.4, 159.4, 158.5, 142.3, 134.9, 131.0, 130.9, 129.9, 123.8, 122.0, 118.6, 115.3, 110.4, 82.2, 74.9, 69.0, 55.0, 54.6, 51.8, 48.1, 26.7, 25.8, 25.2, 22.0, 8.4, 8.2. HRMS calcd for C30H38N2O8 [M + H]+: 555.2705. Found: m/z 555.2701.
(3R,4R,5S)-4-acetamido-5-((4-((2-nitrobenzyl)oxy)benzyl)amino)-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylic acid (11c). White powder, 68% yield, mp: 150.8–152.7 °C. 1H NMR (400 MHz, CD3OD) δ: 8.00 (d, J = 8.1 Hz, 1H, Ph-H), 7.70 (d, J = 7.7 Hz, 1H, Ph-H), 7.60 (t, J = 7.5 Hz, 1H, Ph-H), 7.45 (t, J = 7.5 Hz, 1H, Ph-H), 7.31 (d, J = 8.4 Hz, 2H, 2Ph-H), 6.95 (d, J = 8.4 Hz, 2H, 2Ph-H), 6.64 (s, 1H, CH), 5.36 (s, 2H, CH2), 4.17 (d, J = 12.9 Hz, 1H, CH), 4.11–3.98 (m, 3H, 3CH), 3.41–3.28 (m, 2H, 2CH), 2.86 (dd, J = 17.1, 4.3 Hz, 1H, CH), 2.49 (dd, J = 17.2, 9.2 Hz, 1H, CH), 1.92 (s, 3H, CH3), 1.48–1.37 (m, 4H, 2CH2), 0.79 (dd, J = 13.0, 7.3 Hz, 6H, 2CH3). 13C NMR (100 MHz, CD3OD) δ: 173.3, 169.5, 159.2, 147.8, 134.6, 133.4, 132.6, 131.1, 130.0, 128.9, 128.7, 124.5, 124.4, 115.2, 82.1, 74.7, 66.8, 54.7, 51.9, 48.1, 26.7, 25.8, 25.2, 21.9, 8.4, 8.2. HRMS calcd for C28H35N3O7 [M + H]+: 526.2551. Found: m/z 526.2548.
(3R,4R,5S)-4-acetamido-5-((4-((3-nitrobenzyl)oxy)benzyl)amino)-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylic acid (12c). White powder, 79% yield, mp: 152.5–155.1 °C. 1H NMR (400 MHz, CD3OD) δ: 8.33 (s, 1H, Ph-H), 8.20 (d, J = 8.2 Hz, 1H, Ph-H), 7.87 (d, J = 7.6 Hz, 1H, Ph-H), 7.65 (t, J = 7.9 Hz, 1H, Ph-H), 7.45 (d, J = 8.5 Hz, 2H, 2Ph-H), 7.12 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.80 (s, 1H, CH), 5.28 (s, 2H, CH2), 4.31 (d, J = 13.0 Hz, 1H, CH), 4.17 (dd, J = 10.4, 7.2 Hz, 3H, 3CH), 3.53 (dd, J = 14.8, 9.4 Hz, 1H, CH), 3.49–3.42 (m, 1H, CH), 3.00 (dd, J = 16.2, 6.1 Hz, 1H, CH), 2.63 (dd, J = 17.4, 9.5 Hz, 1H, CH), 2.06 (s, 3H, CH3), 1.61–1.48 (m, 4H, 2CH2), 0.92 (dd, J = 13.2, 7.3 Hz, 6H, 2CH3). 13C NMR (100 MHz, CD3OD) δ: 173.4, 169.0, 159.2, 148.4, 139.5, 135.1, 133.1, 131.2, 129.5, 123.9, 122.4, 121.6, 115.3, 82.2, 74.7, 68.2, 54.7, 51.7, 47.1, 26.5, 25.7, 25.2, 22.0, 8.4, 8.2. HRMS calcd for C28H35N3O7 [M + H]+: 526.2550. Found: m/z 526.2548.
(3R,4R,5S)-4-acetamido-5-((4-((4-nitrobenzyl)oxy)benzyl)amino)-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylic acid (13c). khaki powder, 68% yield, mp: 184.1–187.5 °C (along with the decomposition). 1H NMR (400 MHz, CD3OD) δ: 8.26 (d, J = 8.7 Hz, 2H, 2Ph-H), 7.71 (d, J = 8.6 Hz, 2H, 2Ph-H), 7.46 (d, J = 8.6 Hz, 2H, 2Ph-H), 7.12 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.87 (s, 1H, CH), 5.29 (s, 2H, CH2), 4.35 (d, J = 13.0 Hz, 1H, CH), 4.29–4.15 (m, 3H, 3CH), 3.62 (td, J = 10.0, 5.6 Hz, 1H, CH), 3.50–3.43 (m, 1H, CH), 3.03 (dd, J = 17.4, 5.3 Hz, 1H, CH), 2.72–2.60 (m, 1H, CH), 2.07 (s, 3H, CH3), 1.62–1.48 (m, 4H, 2CH2), 0.92 (q, J = 7.3 Hz, 6H, 2CH3). 13C NMR (100 MHz, CD3OD) δ: 173.5, 167.6, 159.3, 147.6, 144.6, 136.8, 131.3, 127.8, 127.7, 123.4, 123.3, 115.3, 82.3, 74.6, 68.3, 54.6, 51.5, 26.0, 25.7, 25.2, 22.0, 8.4, 8.2. HRMS calcd for C28H35N3O7 [M + H]+: 526.2549. Found: m/z 526.2548.
(3R,4R,5S)-4-acetamido-5-((4-((3-carbamoylbenzyl)oxy)benzyl)amino)-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylic acid (14c). White powder, 62% yield, mp: 138.2–140.5 °C. 1H NMR (400 MHz, CD3OD) δ: 7.98 (s, 1H, Ph-H), 7.84 (d, J = 7.7 Hz, 1H, Ph-H), 7.64 (d, J = 7.5 Hz, 1H, Ph-H), 7.57–7.38 (m, 3H, 3Ph-H), 7.11 (d, J = 8.5 Hz, 2H, 2Ph-H), 6.88 (s, 1H, CH), 5.20 (s, 2H, CH2), 4.35 (d, J = 13.0 Hz, 1H, CH), 4.22 (dt, J = 23.4, 9.4 Hz, 3H, 3CH), 3.60 (td, J = 10.0, 6.1 Hz, 1H, CH), 3.51–3.43 (m, 1H, CH), 3.04 (dd, J = 17.3, 4.7 Hz, 1H, CH), 2.66 (dd, J = 17.1, 9.6 Hz, 1H, CH), 2.07 (s, 3H, CH3), 1.63–1.47 (m, 4H, 2CH2), 1.00–0.84 (m, 6H, 2CH3). 13C NMR (100 MHz, CD3OD) δ: 173.5, 170.6, 159.6, 137.6, 136.9, 133.9, 131.2, 130.5, 128.4, 126.8, 126.4, 124.7, 123.0, 115.4, 82.3, 74.5, 69.2, 67.5, 54.7, 51.5, 29.5, 25.7, 25.2, 22.0, 8.4, 8.1. HRMS calcd for C29H37N3O6 [M + H]+: 524.2758. Found: m/z 524.2755.
(3R,4R,5S)-4-acetamido-5-((4-((4-carbamoylbenzyl)oxy)benzyl)amino)-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylic acid (15c). White powder, 73% yield, mp: 172.5–174.7 °C. 1H NMR (400 MHz, CD3OD) δ: 7.87 (d, J = 8.2 Hz, 2H, 2Ph-H), 7.52 (d, J = 8.2 Hz, 2H, 2Ph-H), 7.41 (d, J = 8.6 Hz, 2H, 2Ph-H), 7.07 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.82 (s, 1H, CH), 5.19 (s, 2H, CH2), 4.31 (d, J = 13.0 Hz, 1H, CH), 4.26–4.13 (m, 3H, 3CH), 3.57 (td, J = 10.0, 5.7 Hz, 1H, CH), 3.44 (p, J = 5.6 Hz, 1H, CH), 3.00 (dd, J = 17.8, 4.9 Hz, 1H, CH), 2.63 (dd, J = 17.4, 9.8 Hz, 1H, CH), 2.04 (s, 3H, CH3), 1.59–1.46 (m, 4H, 2CH2), 0.90 (q, J = 7.3 Hz, 6H, 2CH3). 13C NMR (100 MHz, CD3OD) δ: 173.4, 170.5, 168.2, 159.5, 141.0, 136.2, 133.1, 131.2, 128.4, 127.5, 126.4, 123.3, 115.3, 82.3, 74.6, 68.9, 54.7, 51.6, 47.9, 26.2, 25.7, 25.2, 22.0, 8.4, 8.2. HRMS calcd for C29H37N3O6 [M + H]+: 524.2758. Found: m/z 524.2755.
(3R,4R,5S)-4-acetamido-3-(pentan-3-yloxy)-5-((4-((3-sulfamoylbenzyl)oxy) benzyl)amino)cyclohex-1-ene-1-carboxylic acid (16c). White powder, 70% yield, mp: 193.2–198.1 °C (along with the decomposition). 1H NMR (400 MHz, DMSO-d6) δ: 7.84 (d, J = 8.2 Hz, 2H, 2Ph-H), 7.61 (d, J = 8.3 Hz, 2H, 2Ph-H), 7.35–7.26 (m, 2H, 2Ph-H), 6.97 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.61 (s, 1H, CH), 5.19 (s, 2H, CH2), 4.07 (d, J = 7.4 Hz, 1H, CH), 3.88–3.66 (m, J = 18.9, 13.8 Hz, 3H, 3CH), 3.40–3.32 (m, 1H, CH), 2.90 (d, J = 7.7 Hz, 1H, CH), 2.75–2.65 (m, J = 15.4, 4.7 Hz, 1H, CH), 2.27–2.12 (m, 1H, CH), 1.86 (s, 3H, CH3), 1.42 (qd, J = 14.0, 7.2 Hz, 4H, 2CH2), 0.87–0.74 (m, 6H, 2CH3). 13C NMR (100 MHz, DMSO-d6) δ: 170.3, 167.8, 157.7, 143.9, 143.8, 141.7, 137.9, 130.2, 129.3, 128.1, 126.3, 115.1, 81.4, 75.6, 68.8, 65.5, 54.5, 53.6, 30.9, 26.0, 25.6, 23.6, 9.9, 9.3. HRMS calcd for C28H37N3O7S [M + H]+: 560.2427. Found: m/z 560.2425.
(3R,4R,5S)-4-acetamido-3-(pentan-3-yloxy)-5-((4-((4-sulfamoylbenzyl)oxy) benzyl)amino)cyclohex-1-ene-1-carboxylic acid (17c). White powder, 69% yield, mp: 158.2–160.5 °C. 1H NMR (400 MHz, CD3OD) δ: 7.99 (s, 1H, Ph-H), 7.85 (d, J = 7.6 Hz, 1H, Ph-H), 7.66 (d, J = 7.5 Hz, 1H, Ph-H), 7.55 (t, J = 7.7 Hz, 1H, Ph-H), 7.42 (d, J = 8.4 Hz, 2H, 2Ph-H), 7.09 (d, J = 8.4 Hz, 2H, 2Ph-H), 6.83 (s, 1H, CH), 5.21 (s, 2H, CH2), 4.31 (d, J = 13.1 Hz, 1H, CH), 4.25–4.12 (m, 3H, 3CH), 3.53 (td, J = 9.7, 6.2 Hz, 1H, CH), 3.48–3.41 (m, 1H, CH), 3.00 (dd, J = 17.4, 5.1 Hz, 1H, CH), 2.62 (dd, J = 17.4, 9.6 Hz, 1H, CH), 2.04 (s, 3H, CH3), 1.60–1.46 (m, 4H, 2CH2), 0.90 (q, J = 7.1 Hz, 6H, 2CH3). 13C NMR (100 MHz, CD3OD) δ: 173.4, 168.0, 159.5, 144.1, 138.4, 136.1, 131.1, 130.6, 128.9, 128.3, 125.2, 124.5, 123.5, 115.4, 82.3, 74.5, 68.8, 54.6, 51.6, 47.9, 26.1, 25.7, 25.2, 21.9, 8.4, 8.1. HRMS calcd for C28H37N3O7S [M + H]+: 560.2428. Found: m/z 560.2425.
(3R,4R,5S)-4-acetamido-3-(pentan-3-yloxy)-5-((4-((4-sulfobenzyl)oxy)benzyl) amino)cyclohex-1-ene-1-carboxylic acid (18c). Off-white powder, 58% yield, mp: 244.5–248.7 °C (along with the decomposition). 1H NMR (400 MHz, DMSO-d6) δ: 12.55 (s, 1H, COOH), 8.77 (d, J = 165.8 Hz, 1H, NH), 8.01 (d, J = 9.1 Hz, 1H, NH), 7.60 (d, J = 8.1 Hz, 2H, 2Ph-H), 7.45–7.30 (m, 4H, 4Ph-H), 7.06 (d, J = 8.6 Hz, 2H, 2Ph-H), 6.64 (s, 1H, CH), 5.18 (s, 2H, CH2), 4.23–4.13 (m, 2H, overlapped, 2CH), 4.08 (d, J = 13.1 Hz, 1H, CH), 3.99 (dd, J = 20.0, 8.9 Hz, 1H, CH), 3.31–3.20 (m, 2H, 2CH), 2.83 (dd, J = 17.0, 4.9 Hz, 1H, CH), 2.58 (dd, J = 16.5, 11.0 Hz, 1H, CH), 1.91 (s, 3H, CH3), 1.51–1.34 (m, 4H, 2CH2), 0.81 (dt, J = 17.9, 7.3 Hz, 6H, 2CH3). 13C NMR (100 MHz, DMSO-d6) δ: 171.3, 167.3, 158.9, 148.2, 137.9, 137.6, 131.9, 127.98, 127.4, 126.1, 115.7, 81.7, 75.0, 69.2, 54.5, 51.2, 46.2, 26.2, 26.0, 25.5, 23.9, 9.8, 9.4. HRMS calcd for C28H36N2O8S [M + H]+: 561.2267. Found: m/z 561.2265
4.2. Neuraminidase Enzyme Inhibitory Assay In Vitro
The influenza neuraminidase activity was measured according to the standard method [27,28,29,35]. The NAs (A/PuertoRico/8/1934 (H1N1), A/Babol/36/2005 (H3N2) and A/Anhui/1/2005 (H5N1-H274Y)) were purchased from Sino Biological Inc., and influenza virus suspensions of A/Goose/Guangdong/SH7/2013 (H5N1) and/Goose/Jiangsu/1306/2014 (H5N8) were harvested from the allantoic fluid of influenza virus-infected chicken embryo layer. The substrate, named 2’-(4-methylumbelliferyl)-α-D-acetylneuraminic acid sodium salt hydrate (4-MUNANA) (Sigma (St. Louis, MO, USA); M8639), was cleaved by NA to yield a quantifiable fluorescent product. The compounds were first dissolved in DMSO, and then diluted to the corresponding concentrations with MES buffer (1.27 g 2-(N-morpholino)-ethanesulfonic acid and 0.09 g CaCl2 in 200 mL Milli-Q water). To a 96-well fluorescent plate, 10 μL of diluted virus supernatant or NA assay diluent, 70 μL of MES buffer and 10 μL of test compounds at different concentrations were added successively. After incubation for 10 min at 37 °C, 10 μL of substrate were added to each well to start the reaction and the plate was further incubated for 40 min. The reaction was stopped by the addition of 150 μL of termination solution (3 g glycine (40 mmol) and 1.6 g NaOH (40 mmol) in 200 mL Milli-Q water). Fluorescence was measured (Ex = 365 nm and Em = 460 nm) with a microplate reader (Molecular Devices; SpectraMax iD5), and substrate blanks were subtracted from the sample readings. The values of IC50 (50%-inhibitory concentration) were determined from the dose–response curves by plotting the percentage inhibition of NA activity versus the concentration of the compounds.
4.3. In Vitro Anti-Influenza Virus Assay and Cytotoxicity Assay in Chicken Embryo Fibroblast (CEF)
The anti-influenza activity (EC50) and cytotoxicity (CC50) of the novel synthesized compounds were evaluated with H5N1 and H5N8 strains in Chicken Embryo Fibroblast cells (CEF) using Cell Counting Kit-8 (CCK-8; Dojindo Laboratories) method as previously described [28,29,35]. The tested compounds and positive control were dissolved in DMSO in advance and diluted in assay media (1% FBS in DMEM). Aliquots of 50 μL of influenza virus suspension (H5N1, H5N8) were mixed with equal volumes of solutions of the tested compounds. The mixtures were added to CEFs in 96-well plates and incubated at 37 °C under 5% CO2 for 48 h. After incubation, 100 μL per well of CCK-8 reagent solution (10 μL of CCK-8 in 90 μL of media) was added according to the manufacturer’s manual. Incubating at 37 °C for 90 min, the absorbance at 450 nm was read on a microplate reader. The EC50 values were calculated by fitting the curve of percent CPE (cytopathic effect) versus inhibitor concentration. The values of CC50 (50% cytotoxic concentration) of the compounds to CEFs were determined in the same manner as EC50 but without virus infection.
4.4. Plaque Reduction Assay (PRA) in MDCK Cells
The antiviral activity of selected compounds against the H1N1 and H3N2 viruses was investigated by PRA, as previously described [36,37,38,39,40,41,42]. Briefly, MDCK cells were seeded at 5 × 105 cells/well into 12-well plates, and then incubated at 37 °C for 24 h. The following day, the culture medium was removed and the monolayers were first washed with serum-free DMEM and then infected with FluA virus (PR8 or WSN strain, 40 PFU/well) in DMEM supplemented with 1 mg/mL of TPCK-treated trypsin (Worthington Biochemical Corporation) and 0.14% BSA in the presence of different concentrations of test compounds for 1 h at 37 °C. After virus adsorption, serum-free medium containing 1 mg/mL of TPCK-treated trypsin, 0.14% BSA, 1.2% Avicel, and test compounds at the same concentrations was then added to cells. Oseltamivir carboxylic acid (OSC) and zanamivir (ZAN) were included in each of the experiments as the reference compounds. At 48 h post-infection, cells’ monolayers were fixed with 4% formaldehyde and stained with 0.1% toluidine blue. The viral plaques were counted, and the mean plaque number in the DMSO-treated control was set at 100%.
4.5. Cytotoxicity Assay in MDCK Cells
Cytotoxicity of representative compounds was assessed in MDCK cells by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method as previously reported [36,37,38,39,40,41,42]. MDCK cells (seeded at density of 2 × 104 cells per well) were cultured in 96-well plates for 24 h and then treated with serial dilutions of test compounds, or the same volume of DMSO as a control, in DMEM supplemented with 10% FBS. After 48 h of incubation at 37 °C, MTT solution (5 mg/mL in PBS) was added to each well and plates were incubated for further 4 h at 37 °C in a CO2 incubator. Successively, a solubilization solution (10% SDS, 0.01 N HCl) was added to lyse cells and incubated for 3 h at 37 °C. Finally, absorbance was read at the wavelength of 620 nm on a microtiter plate reader (Tecan Sunrise). Values obtained from the wells treated with no compound were set as 100% of viable cells.
4.6. Molecular Docking
The docking simulation studies of compound 2c were performed using Schrödinger Maestro 11.8 software. Then, 2c was drawn using ChemDraw professional software, imported to Maestro and then optimized using the LigPrep module. LigPrep performed the 3D structure conversion from 2D with accurate chiralities and an OPLS force field was applied to generate stable conformer with minimum potential energy [43]. The crystal structures of N1 (H5N1, PDB code: 2HU0) and N1-H274Y (H5N1-H274Y, PDB code: 3CL0) were retrieved from molecular dynamics (MD) simulations using Amber14 software as previously described [28]. Before docking, the protein structures were prepared using the “Protein Preparation Wizard” tool of Maestro 11.8 by the addition of hydrogen atoms, and the removal of unwanted water molecules [44]. Furthermore, the grid was generated in the protein using the receptor grid generation tool of the Glide module. Molecular docking of 2c was conducted using standard Glide protocol (Schrödinger Maestro 11.8) [45]. Docking results were analyzed according to Glide score or Glide G-score scoring function and visualized using the software of PyMOL version 1.5.
4.7. Acute Toxicity Experiment
Kunming mice (18–22 g and 4–5 weeks old) were purchased from the Animal Experimental Center of Shandong University. The research protocol complied strictly in accordance with the institutional guidelines of Animal Care and Use Committee (AEWC) at Shandong University. Animals were fed at 25 ± 1 °C, and relative humidity was 60 ± 10%, 12 h of light and 12 h of darkness for every day and the mice were given free access to food and water. To evaluate the acute toxicity of 2c in mice, we divided 20 healthy Kunming mice into two groups (5 female mice and 5 male mice per group). Compound 2c was suspended in a mixture of 5% DMSO, 20% PEG-400 and 75% water at a concentrations of 0.1 g/mL, administered intra-gastrically by gavage after the mice had fasted for 12 h. A dose of 1 g/kg of 2c was administered to 10 mice (5 males and 5 females), while the mice in control groups (another 5 male and 5 female mice) were given the same volume of the vehicle solution without 2c. Death, abnormal behaviors and body weight were monitored every day for one week. At the end of the experiment, all animals were sacrificed for subsequent experimental studies [28,29,35].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27196426/s1, The 1H NMR and 13C NMR spectra of compound 1c–18c.
Author Contributions
Conceptualization, R.J. and J.Z. (Jiwei Zhang); methodology, R.J. and J.Z. (Jian Zhang); software, J.Z. (Jiwei Zhang) and Z.G.; validation, R.J., C.B., A.B., L.G. and X.M.; formal analysis, C.L., X.J. and H.J.; investigation, R.J. and X.J.; resources, R.J. and X.L.; data curation, R.J. and Z.L.; writing—original draft preparation, R.J. and A.L.; writing—review and editing, R.J., J.Z. (Jiwei Zhang), P.Z. and A.L.; visualization, R.J.; supervision, X.L. and P.Z.; project administration, X.L. and P.Z.; funding acquisition, X.L., P.Z., B.H. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (NSFC no. 81773574); Shandong Provincial Key research and development project (2019JZZY021011); Science Foundation for Outstanding Young Scholars of Shandong Province (ZR2020JQ31); Foreign cultural and educational experts Project (GXL20200015001); Shandong modern agricultural technology & industry system (SDAIT-21-06); Key Research and Development Program of Shandong Province (2022CXGC010606); Qilu Young Scholars Program of Shandong University and the Taishan Scholar Program at Shandong Province; Associazione Italiana per la Ricerca sul Cancro, AIRC, grants IG 2016—ID. 18855 and IG 2021—ID. 25899 (to A.L.); British Society for Antimicrobial Chemotherapy, UK, BSAC-2018-0064 (to A.L.); Ministero dell’Istruzione, dell’Università e della Ricerca, PRIN 2017-cod. 2017KM79NN (to A.L.); and Fondazione Cassa di Risparmio di Padova e Rovigo-Bando Ricerca COVID-2019 No. 55777 2020.0162-ARREST-COV: AntiviRal PROTAC-Enhanced Small-molecule Therapeutics against COronaViruses (to A.L.).
Institutional Review Board Statement
All animal treatments were performed strictly in accordance with the institutional guidelines of Animal Care and Use Committee at Shandong University, after gaining approval from the Animal Ethical and Welfare Committee (AEWC).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.K. Influenza: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 751–758. [Google Scholar] [PubMed]
- Kim, Y.; Hong, K.; Kim, H.; Nam, J. Influenza vaccines: Past, present, and future. Rev. Med. Virol. 2021, 32, e2243. [Google Scholar] [CrossRef] [PubMed]
- Lampejo, T. Influenza and antiviral resistance: An overview. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Gu, Z.; Mao, L.; Ge, Y.; Hou, D.; Fang, J.; Wei, Z.; Wang, Z. Influenza-existing drugs and treatment prospects. Eur. J. Med. Chem. 2022, 232, 114189. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Kawaoka, Y. Seasonality of influenza and other respiratory viruses. EMBO Mol. Med. 2022, 14, e15352. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.M.; Poon, L.L.M.; Guan, Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J. Clin. Virol. 2009, 45, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Novel Swine-Origin Influenza, A.V.I.T.; Dawood, F.S.; Jain, S.; Finelli, L.; Shaw, M.W.; Lindstrom, S.; Garten, R.J.; Gubareva, L.V.; Xu, X.; Bridges, C.B.; et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 2009, 360, 2605–2615. [Google Scholar]
- Khurana, S.; Sasono, P.; Fox, A.; Nguyen, V.K.; Le, Q.M.; Pham, Q.T.; Nguyen, T.H.; Nguyen, T.L.; Horby, P.; Golding, H. H5N1-SeroDetect EIA and Rapid Test: A Novel Differential Diagnostic Assay for Serodiagnosis of H5N1 Infections and Surveillance. J. Virol. 2011, 85, 12455–12463. [Google Scholar] [CrossRef]
- Li, Y.H.; Hu, C.Y.; Cheng, L.F.; Wu, X.X.; Weng, T.H.; Wu, N.P.; Yao, H.P.; Li, L.J. Highly pathogenic H7N9 avian influenza virus infection associated with up-regulation of PD-1/PD-Ls pathway-related molecules. Int. Immunopharmacol. 2020, 85, 106558. [Google Scholar] [CrossRef] [PubMed]
- Javanian, M.; Barary, M.; Ghebrehewet, S.; Koppolu, V.; Vasigala, V.R.; Ebrahimpour, S. A brief review of influenza virus infection. J. Med. Virol. 2021, 93, 4638–4646. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, S.; Du, L.; Jiang, S. A new role of neuraminidase (NA) in the influenza virus life cycle: Implication for developing NA inhibitors with novel mechanism of action. Rev. Med. Virol. 2016, 26, 242–250. [Google Scholar] [CrossRef] [PubMed]
- McClellan, K.; Perry, C.M. Oseltamivir: A review of its use in influenza. Drugs 2001, 61, 263–283. [Google Scholar] [CrossRef]
- Dunn, C.J.; Goa, K.L. Zanamivir—A review of its use in influenza. Drugs 1999, 58, 761–784. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Peramivir: A Review in Uncomplicated Influenza. Drugs 2018, 78, 1363–1370. [Google Scholar] [CrossRef]
- Kubo, S.; Tomozawa, T.; Kakuta, M.; Tokumitsu, A.; Yamashita, M. Laninamivir Prodrug CS-8958, a Long-Acting Neuraminidase Inhibitor, Shows Superior Anti-Influenza Virus Activity after a Single Administration. Antimicrob. Agents Chemother. 2010, 54, 1256–1264. [Google Scholar] [CrossRef]
- Memoli, M.J.; Hrabal, R.J.; Hassantoufighi, A.; Eichelberger, M.C.; Taubenberger, J.K. Rapid Selection of Oseltamivir- and Peramivir-Resistant Pandemic H1N1 Virus during Therapy in 2 Immunocompromised Hosts. Clin. Infect. Dis. 2010, 50, 1252–1255. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.J.; Haire, L.F.; Lin, Y.P.; Liu, J.; Russell, R.J.M.; Walker, P.A.; Skehel, J.J.; Martin, S.R.; Hay, A.J.; Gamblin, S.J. Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature 2008, 453, 1258–1261. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, Z.; Liu, Z.; Li, X.; Zhang, Y.; Zhang, Z.; Cen, S. A cell-based high-throughput approach to identify inhibitors of influenza A virus. Acta Pharm. Sin. B 2014, 4, 301–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shen, Z.; Lou, K.; Wang, W. New small-molecule drug design strategies for fighting resistant influenza A. Acta Pharm. Sin. B 2015, 5, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Zhang, J.; Ju, H.; Kang, D.; Fang, Z.; Liu, X.; Zhan, P. Discovery of novel anti-influenza agents via contemporary medicinal chemistry strategies (2014–2018 update). Futur. Med. Chem. 2019, 11, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Guo, J.; Kang, D.; Li, Z.; Wang, G.; Wu, J.; Zhang, Z.; Fang, H.; Hou, X.; Huang, Z.; et al. New techniques and strategies in drug discovery. Chin. Chem. Lett. 2020, 31, 1695–1708. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Y.; Tefsen, B.; Shi, Y.; Gao, G.F. Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol. 2014, 22, 183–191. [Google Scholar] [CrossRef]
- Russell, R.J.; Haire, L.F.; Stevens, D.J.; Collins, P.J.; Lin, Y.P.; Blackburn, G.M.; Hay, A.J.; Gamblin, S.; Skehel, J.J. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature 2006, 443, 45–49. [Google Scholar] [CrossRef]
- Li, Q.; Qi, J.; Zhang, W.; Vavricka, C.J.; Shi, Y.; Wei, J.; Feng, E.; Shen, J.; Chen, J.-L.; Liu, D.; et al. The 2009 pandemic H1N1 neuraminidase N1 lacks the 150-cavity in its active site. Nat. Struct. Mol. Biol. 2010, 17, 1266–1268. [Google Scholar] [CrossRef]
- Han, N.; Mu, Y. Plasticity of 150-loop in influenza neuraminidase explored by Hamiltonian replica exchange molecular dynamics simulations. PLoS ONE 2013, 8, e60995. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, G.; Gao, F.; Liu, Y.; Vavricka, C.J.; Qi, J.; Jiang, H.; Yu, K.; Gao, G.F. Induced opening of influenza virus neuraminidase N2 150-loop suggests an important role in inhibitor binding. Sci. Rep. 2013, 3, 1551. [Google Scholar] [CrossRef]
- Xie, Y.C.; Xu, D.; Huang, B.; Ma, X.; Qi, W.; Shi, F.; Liu, X.; Zhang, Y.; Xu, W. Discovery of N-Substituted Oseltamivir Derivatives as Potent and Selective Inhibitors of H5N1 Influenza Neuraminidase. J. Med. Chem. 2014, 57, 8445–8458. [Google Scholar] [CrossRef]
- Zhang, J.; Murugan, N.A.; Tian, Y.; Bertagnin, C.; Fang, Z.; Kang, D.; Kong, X.; Jia, H.; Sun, Z.; Jia, R.; et al. Structure-Based Optimization of N-Substituted Oseltamivir Derivatives as Potent Anti-Influenza A Virus Agents with Significantly Improved Potency against Oseltamivir-Resistant N1-H274Y Variant. J. Med. Chem. 2018, 61, 9976–9999. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Zhang, J.; Bertagnin, C.; Cherukupalli, S.; Ai, W.; Ding, X.; Li, Z.; Zhang, J.; Ju, H.; Ma, X.; et al. Discovery of highly potent and selective influenza virus neuraminidase inhibitors targeting 150-cavity. Eur. J. Med. Chem. 2020, 212, 113097. [Google Scholar] [CrossRef]
- Ju, H.; Murugan, N.A.; Hou, L.; Li, P.; Guizzo, L.; Zhang, Y.; Bertagnin, C.; Kong, X.; Kang, D.; Jia, R.; et al. Identification of C5-NH2 Modified Oseltamivir Derivatives as Novel Influenza Neuraminidase Inhibitors with Highly Improved Antiviral Activities and Favorable Druggability. J. Med. Chem. 2021, 64, 17992–18009. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Meng, Y.; Xu, S.; Shen, W.; Meng, Z.; Wang, Z.; Ding, G.; Huang, W.; Xiao, W.; Xu, J. Discovery of acylguanidine oseltamivir carboxylate derivatives as potent neuraminidase inhibitors. Bioorganic Med. Chem. 2017, 25, 2772–2781. [Google Scholar] [CrossRef]
- Hsu, P.-H.; Chiu, D.-C.; Wu, K.-L.; Lee, P.-S.; Jan, J.-T.; Cheng, Y.-S.E.; Tsai, K.-C.; Cheng, T.-J.; Fang, J.-M. Acylguanidine derivatives of zanamivir and oseltamivir: Potential orally available prodrugs against influenza viruses. Eur. J. Med. Chem. 2018, 154, 314–323. [Google Scholar] [CrossRef]
- Mohan, S.; McAtamney, S.; Haselhorst, T.; von Itzstein, M.; Pinto, B.M. Carbocycles Related to Oseltamivir as Influenza Virus Group-1-Specific Neuraminidase Inhibitors. Binding to N1 Enzymes in the Context of Virus-like Particles. J. Med. Chem. 2010, 53, 7377–7391. [Google Scholar] [CrossRef]
- Wang, P.; Oladejo, B.O.; Li, C.; Fu, L.; Zhang, S.; Qi, J.; Lv, X.; Li, X. Structure-based design of 5′-substituted 1,2,3-triazolylated oseltamivir derivatives as potent influenza neuraminidase inhibitors. RSC Adv. 2021, 11, 9528–9541. [Google Scholar] [CrossRef]
- Zhang, J.; Poongavanam, V.; Kang, D.W.; Bertagnin, C.; Lu, H.M.; Kong, X.J.; Ju, H.; Lu, X.Y.; Gao, P.; Tian, Y.; et al. Optimization of N-Substituted Oseltamivir Derivatives as Potent Inhibitors of Group-1 and-2 Influenza A Neuraminidases, Including a Drug-Resistant Variant. J. Med. Chem. 2018, 61, 6379–6397. [Google Scholar] [CrossRef]
- D’Agostino, I.; Giacchello, I.; Nannetti, G.; Fallacara, A.L.; Deodato, D.; Musumeci, F.; Grossi, G.; Palu, G.; Cau, Y.; Trist, I.M.; et al. Synthesis and biological evaluation of a library of hybrid derivatives as inhibitors of influenza virus PA-PB1 interaction. Eur. J. Med. Chem. 2018, 157, 743–758. [Google Scholar] [CrossRef]
- Desantis, J.; Nannetti, G.; Massari, S.; Barreca, M.L.; Manfroni, G.; Cecchetti, V.; Palù, G.; Goracci, L.; Loregian, A.; Tabarrini, O. Exploring the cycloheptathiophene-3-carboxamide scaffold to disrupt the interactions of the influenza polymerase subunits and obtain potent anti-influenza activity. Eur. J. Med. Chem. 2017, 138, 128–139. [Google Scholar] [CrossRef]
- Massari, S.; Bertagnin, C.; Pismataro, M.C.; Donnadio, A.; Nannetti, G.; Felicetti, T.; di Bona, S.; Nizi, M.G.; Tensi, L.; Manfroni, G.; et al. Synthesis and characterization of 1,2,4-triazolo1,5-a. pyrimidine-2-carboxamide-based compounds targeting the PA-PB1 interface of influenza A virus polymerase. Eur. J. Med. Chem. 2021, 209, 112944. [Google Scholar] [CrossRef]
- Massari, S.; Nannetti, G.; Goracci, L.; Sancineto, L.; Muratore, G.; Sabatini, S.; Manfroni, G.; Mercorelli, B.; Cecchetti, V.; Facchini, M.; et al. Structural Investigation of Cycloheptathiophene-3-carboxamide Derivatives Targeting Influenza Virus Polymerase Assembly. J. Med. Chem. 2013, 56, 10118–10131. [Google Scholar] [CrossRef]
- Muratore, G.; Mercorelli, B.; Goracci, L.; Cruciani, G.; Digard, P.; Palù, G.; Loregian, A. Human Cytomegalovirus Inhibitor AL18 Also Possesses Activity against Influenza A and B Viruses. Antimicrob. Agents Chemother. 2012, 56, 6009–6013. [Google Scholar] [CrossRef]
- Nannetti, G.; Massari, S.; Mercorelli, B.; Bertagnin, C.; Desantis, J.; Palù, G.; Tabarrini, O.; Loregian, A. Potent and broad-spectrum cycloheptathiophene-3-carboxamide compounds that target the PA-PB1 interaction of influenza virus RNA polymerase and possess a high barrier to drug resistance. Antivir. Res. 2019, 165, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Pismataro, M.C.; Felicetti, T.; Bertagnin, C.; Nizi, M.G.; Bonomini, A.; Barreca, M.L.; Cecchetti, V.; Jochmans, D.; de Jonghe, S.; Neyts, J.; et al. 1,2,4-Triazolo1,5-a. pyrimidines: Efficient one-step synthesis and functionalization as influenza polymerase PA-PB1 interaction disruptors. Eur. J. Med. Chem. 2021, 221, 113494. [Google Scholar] [CrossRef] [PubMed]
- Moorkoth, S.; Prathyusha, N.S.; Manandhar, S.; Xue, Y.; Sankhe, R.; Pai, K.S.R.; Kumar, N. Antidepressant-like effect of dehydrozingerone from Zingiber officinale by elevating monoamines in brain: In silico and in vivo studies. Pharmacol. Rep. 2021, 73, 1273–1286. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Mishra, N. In silico validation of coumarin derivatives as potential inhibitors against Main Protease, NSP10/NSP16-Methyltransferase, Phosphatase and Endoribonuclease of SARS CoV-2. J Biomol Struct Dyn. 2021, 39, 7306–7321. [Google Scholar] [CrossRef]
- Sinha, S.K.; Shakya, A.; Prasad, S.K.; Singh, S.; Gurav, N.S.; Prasad, R.S.; Gurav, S.S. An in-silico evaluation of different Saikosaponins for their potency against SARS-CoV-2 using NSP15 and fusion spike glycoprotein as targets. J. Biomol Struct Dyn. 2021, 39, 3244–3255. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).