pH-Responsive Inorganic/Organic Nanohybrids System for Controlled Nicotinic Acid Drug Release
Abstract
:1. Introduction
2. Results
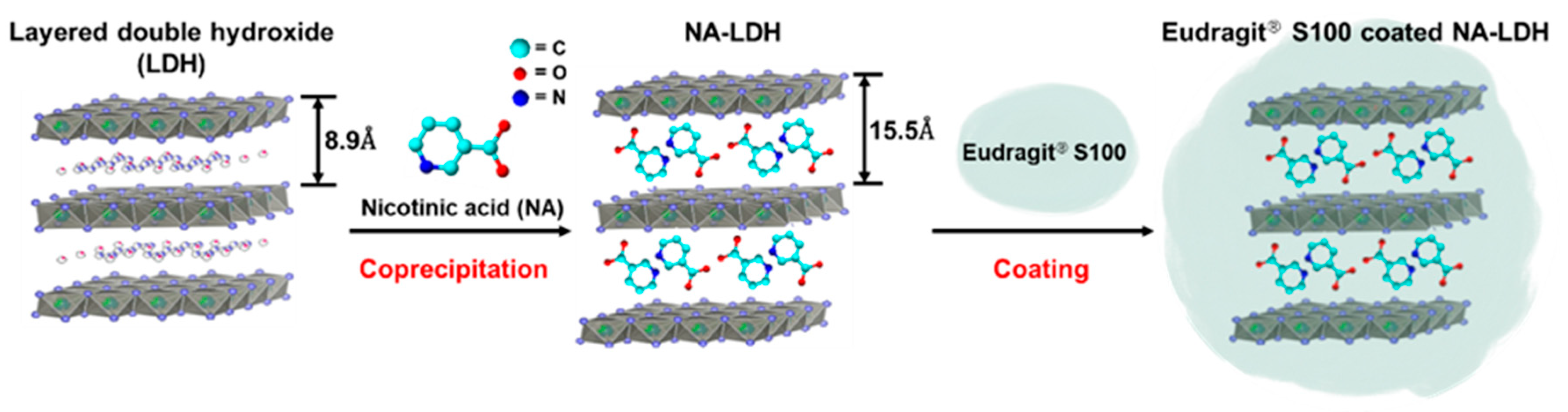
2.1. Powder X-ray Diffraction Analysis
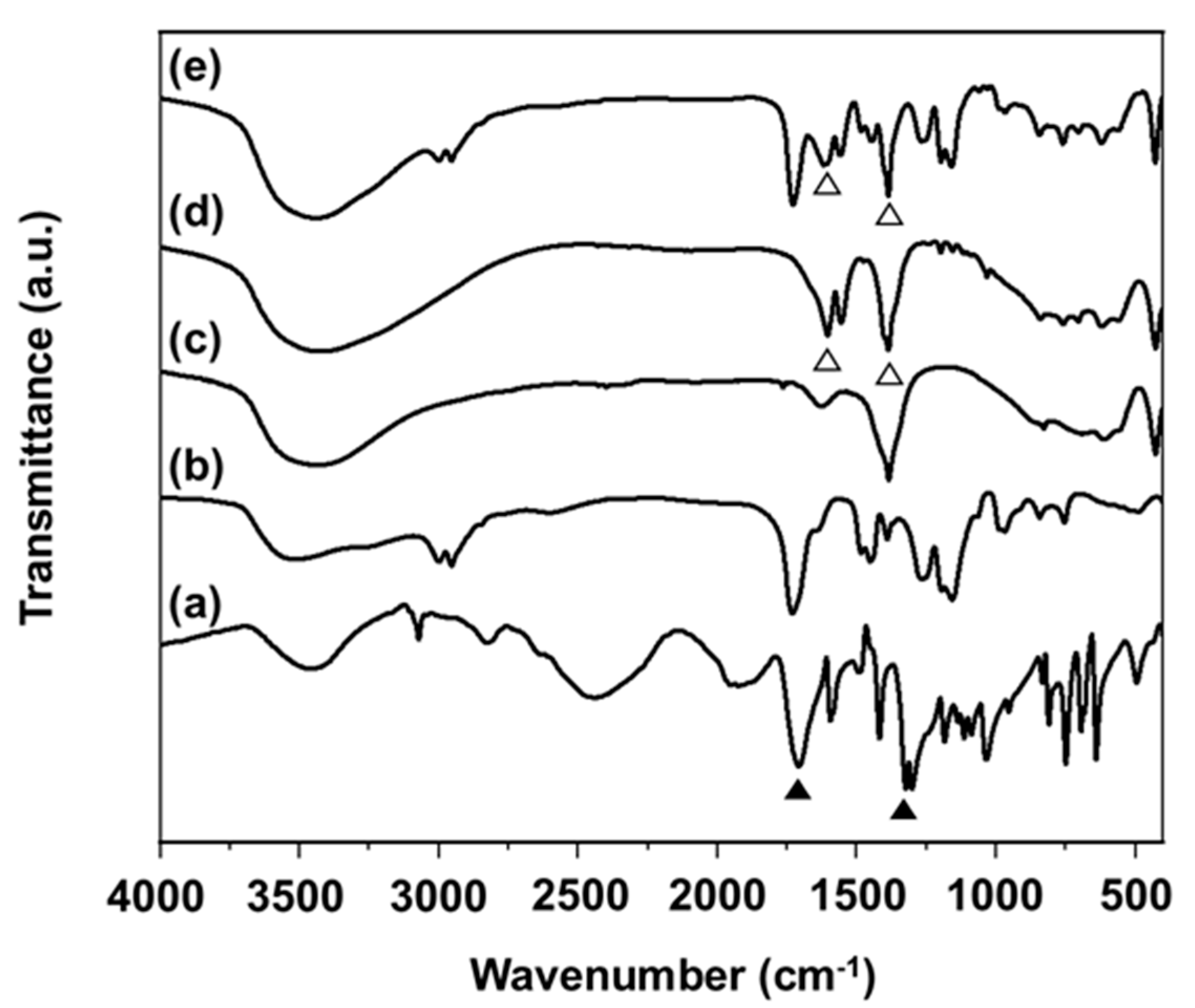
2.2. FT-IR Studies
2.3. FE-SEM and DLS Analysis
2.4. Surface Charge
2.5. Determination of NA Content
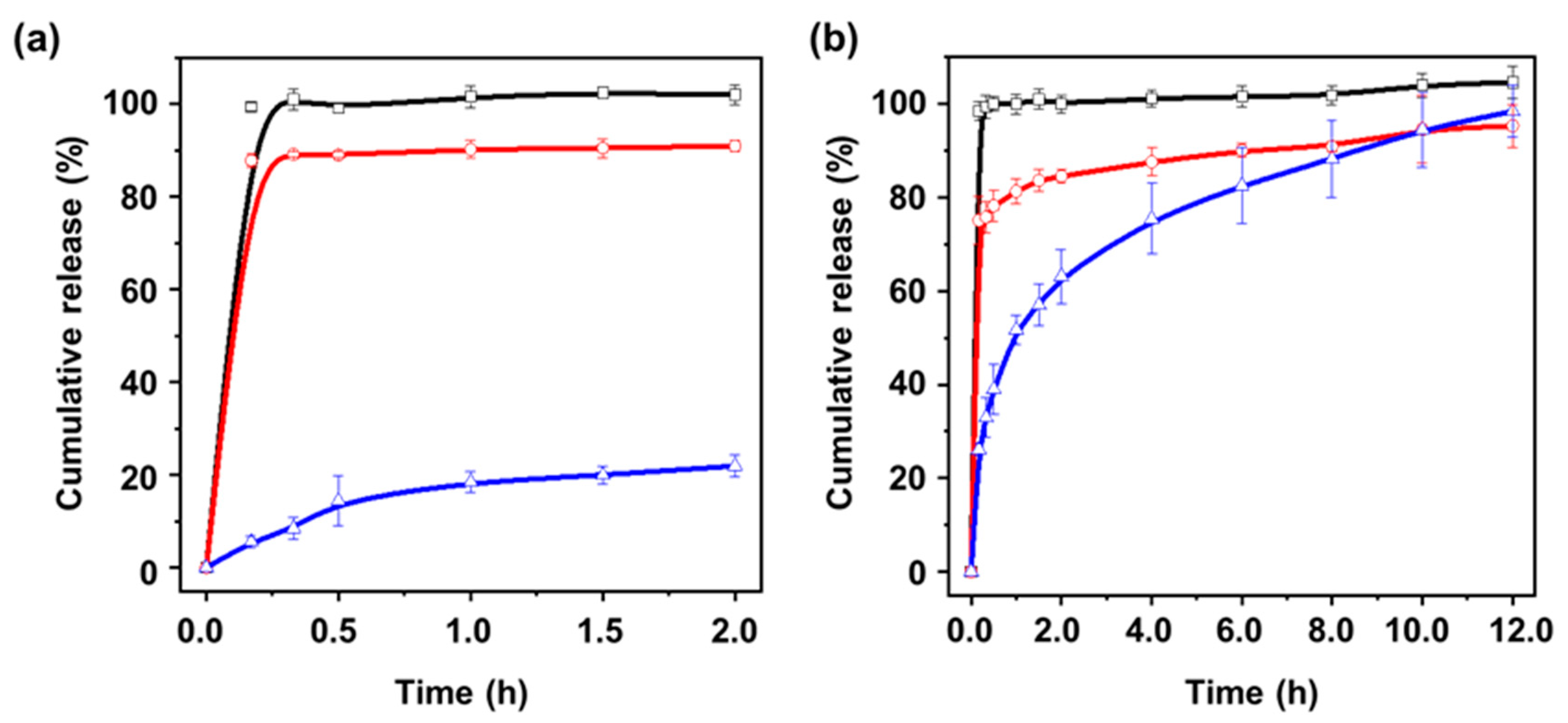
2.6. In Vitro Release Study
2.7. Kinetic Model
2.8. TG Analysis
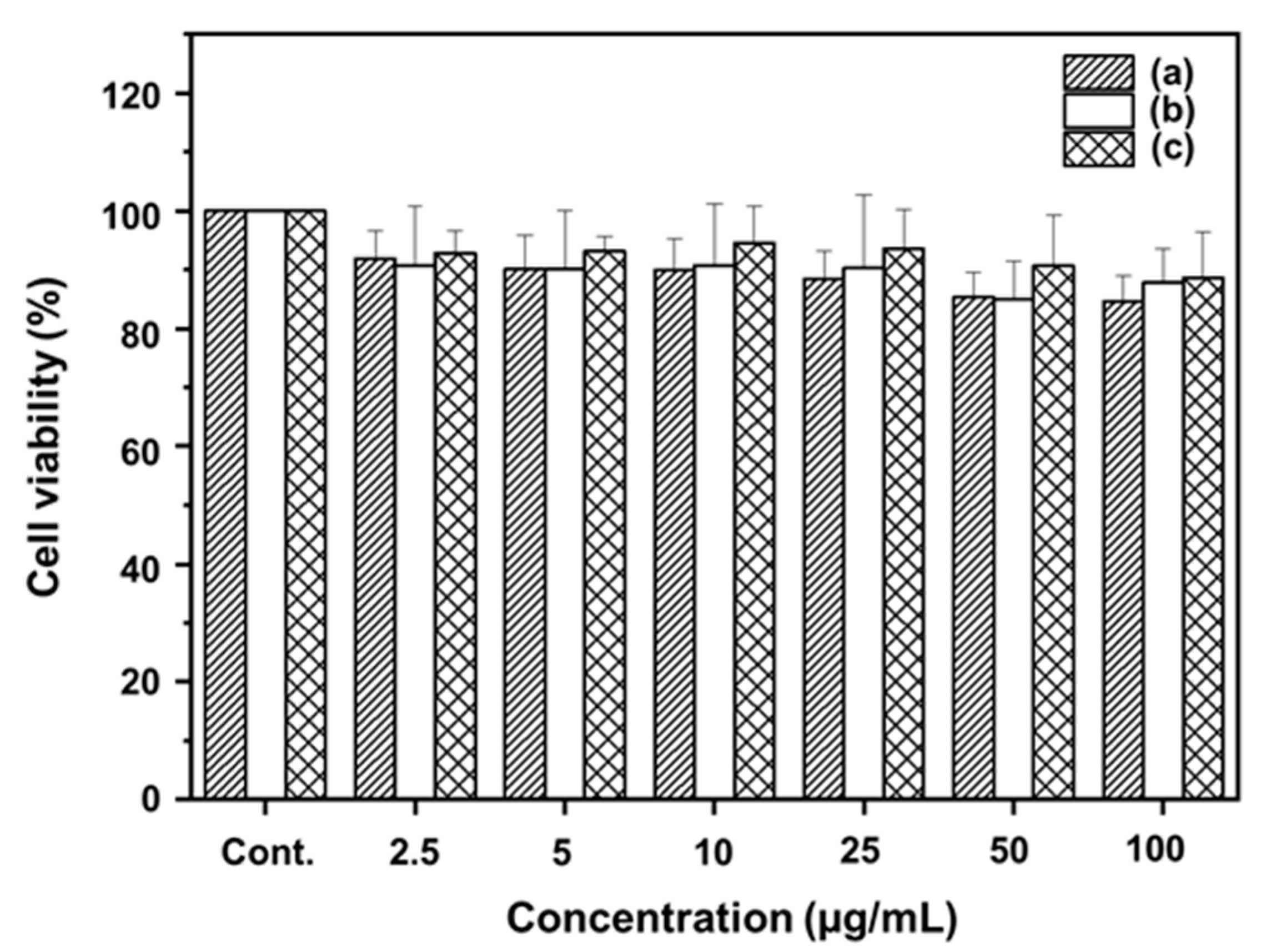
2.9. Cytotoxicity Studies of the LDH Nanohybrids
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of the NA-LDH Nanohybrid
4.2.1. NA-LDH Nanohybrid
4.2.2. Eudragit® S100-coated NA-LDH
4.3. Sample Characterization
4.4. Determination of NA Content
4.5. In Vitro Release Experiment
4.6. MTT Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bodor, E.T.; Offermanns, S. Nicotinic acid: An old drug with a promising future. Br. J. Pharmacol. 2008, 153 (Suppl. S1), S68–S75. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, X.; Kong, L.; Chen, Z. Nicotinic acid regulates glucose and lipid metabolism through lipid independent pathways. Curr. Pharm. Biotechnol. 2015, 16, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Shen, C.; Wang, Z.; Li, S.; Zhang, X.; Song, Z. Protection of nicotinic acid against oxidative stress-induced cell death in hepatocytes contributes to its beneficial effect on alcohol-induced liver injury in mice. J. Nutr. Biochem. 2013, 24, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Kim, H.J.; Rodriguez-Iturbe, B.; Vaziri, N.D. Niacin ameliorates oxidative stress, inflammation, proteinuria, and hypertension in rats with chronic renal failure. Am. J. Physiol. Ren. Physiol. 2009, 297, 106–113. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, L.; Jiang, R.; Liao, C.; Xu, J.; Jiang, S.; Yang, Y.; Lin, L.; Huang, J.; Shen, Y.; et al. Nicotinic Acid against Acetaminophen-Induced Hepatotoxicity via Sirt1/Nrf2 Antioxidative Pathway in Mice. J. Nutr. Sci. Vitam. 2021, 67, 145–152. [Google Scholar] [CrossRef]
- Gasperi, V.; Sibilano, M.; Savini, I.; Catani, M.V. Niacin in the Central Nervous System: An Update of Biological Aspects and Clinical Applications. Int. J. Mol. Sci. 2019, 20, 974. [Google Scholar] [CrossRef]
- Jain, N.; Utreja, D.; Kaur, K.; Jain, P. Novel Derivatives of Nicotinic Acid as Promising Anticancer Agents. Mini Rev. Med. Chem. 2021, 21, 847–882. [Google Scholar] [CrossRef]
- Markel, A. The resurgence of niacin: From nicotinic acid to niaspan/laropiprant. Isr. Med. Assoc. J. 2011, 13, 368–374. [Google Scholar]
- Minto, C.; Vecchio, M.G.; Lamprecht, M.; Gregori, D. Definition of a tolerable upper intake level of niacin: A systematic review and meta-analysis of the dose-dependent effects of nicotinamide and nicotinic acid supplementation. Nutr. Rev. 2017, 75, 471–490. [Google Scholar] [CrossRef]
- Drexel, H. Nicotinic acid in the treatment of hyperlipidaemia. Fundam. Clin. Pharmacol. 2007, 21 (Suppl. S2), 5–6. [Google Scholar] [CrossRef]
- McKenney, J.M.; Proctor, J.D.; Harris, S.; Chinchili, V.M. A Comparison of the Efficacy and Toxic Effects of Sustained- vs. Immediate-Release Niacin in Hypercholesterolemic Patients. JAMA 1994, 271, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Svedmyr, N.; Harthon, L. Comparison between the absorption of nicotinic acid and pentaerythritol tetranicotinate (Perycit®) from ordinary and enterocoated tablets. Acta Pharmacol. Toxicol. 1970, 28, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Puthoori, H.; Murthy, T.E.G.K.; Kaushik, A.; Karthyek, M. Formulation and evaluation of floating tablets of niacin for sustained release. Asian J. Pharm. 2012, 6, 31. [Google Scholar] [CrossRef]
- Knopp, R.H. Evaluating niacin in its various forms. Am. J. Cardiol. 2000, 86, 51–56. [Google Scholar] [CrossRef]
- Knopp, R.H.; Alagona, P.; Davidson, M.; Goldberg, A.C.; Kafonek, S.D.; Kashyap, M.; Sprecher, D.; Superko, H.R.; Jenkins, S.; Marcovina, S. Equivalent efficacy of a time-release form of niacin (Niaspan) given once-a-night versus plain niacin in the management of hyperlipidemia. Metabolism 1998, 47, 1097–1104. [Google Scholar] [CrossRef]
- Guyton, J.R. Extended-release niacin for modifying the lipoprotein profile. Expert Opin. Pharmacother. 2004, 5, 1385–1398. [Google Scholar] [CrossRef]
- Pieper, J.A. Understanding niacin formulations. Am. J. Manag. Care 2002, 8, S308–S314. [Google Scholar]
- Manne, R.; Devarajan, A. Development of nicotinic acid controlled release tablets with natural phenolic anti-oxidant polymer by encapsulation technique. J. Nat. Remedies 2020, 20, 139–151. [Google Scholar] [CrossRef]
- Carlson, L.A. Niaspan, the prolonged release preparation of nicotinic acid (niacin), the broad-spectrum lipid drug. Int. J. Clin. Pract. 2004, 58, 706–713. [Google Scholar] [CrossRef]
- McKenney, J. New perspectives on the use of niacin in the treatment of lipid disorders. Arch. Intern. Med. 2004, 164, 697–705. [Google Scholar] [CrossRef]
- Pieper, J.A. Overview of niacin formulations: Differences in pharmacokinetics, efficacy, and safety. Am. J. Health Syst. Pharm. 2003, 60, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Knopp, R.H.; Ginsberg, J.; Albers, J.J.; Hoff, C.; Ogilvie, J.T.; Warnick, G.R.; Burrows, E.; Retzlaff, B.; Poole, M. Contrasting effects of unmodified and time-release forms of niacin on lipoproteins in hyperlipidemic subjects: Clues to mechanism of action of niacin. Metabolism 1985, 34, 642–650. [Google Scholar] [CrossRef]
- Ma, L.; Lee, B.H.; Mao, R.; Cai, A.; Jia, Y.; Clifton, H.; Schaefer, S.; Xu, L.; Zheng, J. Nicotinic acid activates the capsaicin receptor TRPV1: Potential mechanism for cutaneous flushing. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Gille, A.; Bodor, E.T.; Ahmed, K.; Offermanns, S. Nicotinic acid: Pharmacological effects and mechanisms of action. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 79–106. [Google Scholar] [CrossRef] [PubMed]
- Piepho, R.W. The pharmacokinetics and pharmacodynamics of agents proven to raise high-density lipoprotein cholesterol. Am. J. Cardiol. 2000, 86, 351–401. [Google Scholar] [CrossRef]
- Guo, X.; Ren, T.; Ji, J.; Yang, Y.; Di, X. An alternative analytical strategy based on QuEChERS and dissolvable layered double hydroxide dispersive micro-solid phase extraction for trace determination of sulfonylurea herbicides in wolfberry by LC-MS/MS. Food Chem. 2022, 396, 133652. [Google Scholar] [CrossRef]
- Ozturk, E.A.; Ege, Z.R.; Murat, S.; Erdemir, G.; Kuruca, S.; Erkmen, Z.E.; Duygulu, O.; Gunduz, O.; Caykara, T.; Eroglu, M.S. Poly(L-lactic acid)/poly(ethylene oxide) based composite electrospun fibers loaded with magnesium-aluminum layered double hydroxide nanoparticles. Int. J. Biol. Macromol. 2022, 217, 562–571. [Google Scholar] [CrossRef]
- Awassa, J.; Soule, S.; Cornu, D.; Ruby, C.; El-Kirat-Chatel, S. Understanding the role of surface interactions in the anti-bacterial activity of layered double hydroxide nanoparticles by atomic force microscopy. Nanoscale 2022, 14, 10335–10348. [Google Scholar] [CrossRef]
- Ponce, M.D.V.; Cina, M.; Lopez, C.; Cerutti, S. Synthesis and evaluation of a Zn-Al layered double hydroxide for the removal of ochratoxin A. Greenness assessment. Anal. Methods 2022, 14, 2841–2848. [Google Scholar] [CrossRef]
- Chakraborty, S.; Marappa, S.; Agarwal, S.; Bagchi, D.; Rao, A.; Vinod, C.P.; Peter, S.C.; Singh, A.; Eswaramoorthy, M. Improvement in Oxygen Evolution Performance of NiFe Layered Double Hydroxide Grown in the Presence of 1T-Rich MoS2. ACS Appl. Mater. Interfaces 2022, 14, 31951–31961. [Google Scholar] [CrossRef]
- Sadeghi Rad, T.; Yazici, E.S.; Khataee, A.; Gengec, E.; Kobya, M. Nanoarchitecture of graphene nanosheets decorated with NiCr layered double hydroxide for sonophotocatalytic degradation of refractory antibiotics. Environ. Res. 2022, 214, 113788. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Gu, Z.; Williams, G.R.; Strimaite, M.; Zha, J.; Zhou, Z.; Zhang, X.; Tan, C.; Liang, R. Layered double hydroxide-based nanomaterials for biomedical applications. Chem. Soc. Rev. 2022, 51, 6126–6176. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Q.; Wang, Q.; Hao, J.; Cui, P.; Cao, J.; Wang, Y. Formation of Cr-based layered double hydroxide: Effect of the amendments. Bull. Environ. Contam. Toxicol. 2022, 109, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Negarestani, M.; Farimaniraad, H.; Mollahosseini, A.; Kheradmand, A.; Shayesteh, H. Facile preparation of sisal-Fe/Zn layered double hydroxide bio-nanocomposites for the efficient removal of rifampin from aqueous solution: Kinetic, equilibrium, and thermodynamic studies. Int. J. Phytoremediation 2022, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.F.; Zeng, H.Y.; Huang, Q.J.; Zhang, W.; Du, J.Z.; Duan, H.Z.; Chen, C.R. Facile Preparation of Modifying Layered Double Hydroxide Nanoparticles for Drug Delivery. J. Nanosci. Nanotechnol. 2018, 18, 5256. [Google Scholar] [CrossRef]
- Usman, M.S.; Hussein, M.Z.; Fakurazi, S.; Ahmad Saad, F.F. Gadolinium-based layered double hydroxide and graphene oxide nano-carriers for magnetic resonance imaging and drug delivery. Chem. Cent. J. 2017, 11, 47. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wang, X. Synthesis and In Vitro Characterization of Fe(3+)-Doped Layered Double Hydroxide Na-norings as a Potential Imageable Drug Delivery System. Materials 2017, 10, 1140. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, M.; Zhang, X.; Huang, L.; Chen, W.; Roy, V.A.L.; Zhang, W.; Chen, X. A Novel Type of Aqueous Dispersible Ultrathin-Layered Double Hydroxide Nanosheets for in Vivo Bioimaging and Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 34185–34193. [Google Scholar] [CrossRef]
- Sani Usman, M.; Hussein, M.Z.; Fakurazi, S.; Masarudin, M.J.; Ahmad Saad, F.F. Gadolinium-Doped Gallic Ac-id-Zinc/Aluminium-Layered Double Hydroxide/Gold Theranostic Nanoparticles for a Bimodal Magnetic Resonance Imaging and Drug Delivery System. Nanomaterials 2017, 7, 244. [Google Scholar] [CrossRef]
- Peng, F.; Wang, D.; Tian, Y.; Cao, H.; Qiao, Y.; Liu, X. Sealing the Pores of PEO Coating with Mg-Al Layered Double Hydroxide: Enhanced Corrosion Resistance, Cytocompatibility and Drug Delivery Ability. Sci. Rep. 2017, 7, 8167. [Google Scholar] [CrossRef]
- Chen, Y.X.; Zhu, R.; Xu, Z.L.; Ke, Q.F.; Zhang, C.Q.; Guo, Y.P. Self-assembly of pifithrin-alpha-loaded layered double hydroxide/chitosan nanohybrid composites as a drug delivery system for bone repair materials. J. Mater. Chem. B 2017, 5, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Gu, Y.; Xu, T.; Cao, F. Multifunctional organic-inorganic hybrid nanoparticles and nanosheets based on chitosan derivative and layered double hydroxide: Cellular uptake mechanism and application for topical ocular drug delivery. Int. J. Nanomed. 2017, 12, 1607–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Z.; Zuo, H.; Wu, A.; Xu, Z.P. Stabilization of layered double hydroxide nanoparticles by bovine serum albumin pre-coating for drug/gene delivery. J. Control. Release 2015, 213, e150–e151. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gu, Z.; Gu, W.; Liu, J.; Xu, Z.P. Efficient drug delivery using SiO2-layered double hydroxide nanocomposites. J. Colloid Interface Sci. 2016, 470, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Zhang, W.; Ryu, H.J.; Choi, G.; Choi, J.Y.; Choy, J.H. Enhanced thermal stability and mechanical property of EVA nanocomposites upon addition of organo-intercalated LDH nanoparticles. Polymer 2019, 177, 274–281. [Google Scholar] [CrossRef]
- Choi, G.; Lee, J.H.; Oh, Y.J.; Choy, Y.B.; Park, M.C.; Chang, H.C.; Choy, J.H. Inorganic-polymer nanohybrid carrier for delivery of a poorly-soluble drug, ursodeoxycholic acid. Int. J. Pharm. 2010, 402, 117–122. [Google Scholar] [CrossRef]
- Choy, J.H.; Kwak, S.Y.; Park, J.S.; Jeong, Y.J.; Portier, J. Intercalative Nanohybrids of Nucleoside Monophosphates and DNA in Layered Metal Hydroxide. J. Am. Chem. Soc. 1999, 121, 1399–1400. [Google Scholar] [CrossRef]
- Choi, G.; Piao, H.; Alothman, Z.A.; Vinu, A.; Yun, C.O.; Choy, J.H. Anionic clay as the drug delivery vehicle: Tumor targeting function of layered double hydroxide-methotrexate nanohybrid in C33A orthotopic cervical cancer model. Int. J. Nanomed. 2016, 11, 337–348. [Google Scholar] [CrossRef]
- Bukhovets, A.V.; Fotaki, N.; Khutoryanskiy, V.V.; Moustafine, R.I. Interpolymer Complexes of Eudragit® Copolymers as Novel Carriers for Colon-Specific Drug Delivery. Polymers 2020, 12, 1459. [Google Scholar] [CrossRef]
- Luo, F.; Wang, M.; Huang, L.; Wu, Z.; Wang, W.; Zafar, A.; Tian, Y.; Hasan, M.; Shu, X. Synthesis of Zinc Oxide Eudragit FS30D Nanohybrids: Structure, Characterization, and Their Application as an Intestinal Drug Delivery System. ACS Omega 2020, 5, 11799–11808. [Google Scholar] [CrossRef]
- Jain, S.K.; Jain, A.K.; Rajpoot, K. Expedition of Eudragit(R) Polymers in the Development of Novel Drug Delivery Systems. Curr. Drug Deliv. 2020, 17, 448–469. [Google Scholar] [CrossRef] [PubMed]
- Dieng, S.M.; Omran, Z.; Anton, N.; Thioune, O.; Djiboune, A.R.; Sy, P.M.; Messaddeq, N.; Ennahar, S.; Diarra, M.; Vandamme, T. Pickering nano-emulsions stabilized by Eudragit RL100 nanoparticles as oral drug delivery system for poorly soluble drugs. Colloids Surf. B Biointerfaces 2020, 191, 111010. [Google Scholar] [CrossRef] [PubMed]
- Salvi, V.; Pawar, P. Eudragit RL100 Based Moxifloxacin Hydrochloride and Ketorolac Tromethamine Combination Nanoparticulate System for Ocular Drug Delivery. Pharm. Nanotechnol. 2020, 8, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; De Marco, I. Eudragit: A Novel Carrier for Controlled Drug Delivery in Supercritical Antisolvent Coprecipitation. Polymers 2020, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, G.; Mirzaeei, S.; Taghe, S.; Mohammadi, P. Preparation and Evaluation of Eudragit(R) L100 Nanoparticles Loaded Impregnated with KT Tromethamine Loaded PVA -HEC Insertions for Ophthalmic Drug Delivery. Adv. Pharm. Bull. 2019, 9, 593–600. [Google Scholar] [CrossRef]
- Kolev, I.N.; Ivanova Ncapital, A.C.; Marinov, M.K.; Alexieva, G.E.; Strashilov, V.L. A QCM-based assay of drug content in Eudragit RS 100-based delivery systems. Talanta 2019, 202, 531–539. [Google Scholar] [CrossRef]
- Jafri, I.; Shoaib, M.H.; Yousuf, R.I.; Ali, F.R. Effect of permeation enhancers on in vitro release and transdermal delivery of lamotrigine from Eudragit®RS100 polymer matrix-type drug in adhesive patches. Prog. Biomater. 2019, 8, 91–100. [Google Scholar] [CrossRef]
- Porfiryeva, N.N.; Nasibullin, S.F.; Abdullina, S.G.; Tukhbatullina, I.K.; Moustafine, R.I.; Khutoryanskiy, V.V. Acrylated Eudragit(R) E PO as a novel polymeric excipient with enhanced mucoadhesive properties for application in nasal drug delivery. Int. J. Pharm. 2019, 562, 241–248. [Google Scholar] [CrossRef]
- Tian, S.; Li, J.; Tao, Q.; Zhao, Y.; Lv, Z.; Yang, F.; Duan, H.; Chen, Y.; Zhou, Q.; Hou, D. Controlled drug delivery for glaucoma therapy using montmorillonite/Eudragit microspheres as an ion-exchange carrier. Int. J. Nanomed. 2018, 13, 415–428. [Google Scholar] [CrossRef]
- Kumar, V.S.; Rijo, J.; Sabitha, M. Guargum and Eudragit (R) coated curcumin liquid solid tablets for colon specific drug delivery. Int. J. Biol. Macromol. 2018, 110, 318–327. [Google Scholar] [CrossRef]
- Khattab, A.; Shalaby, S. Optimized Ciclopirox-Based Eudragit RLPO Nail Lacquer: Effect of Endopeptidase Enzyme as Permeation Enhancer on Transungual Drug Delivery and Efficiency Against Onychomycosis. AAPS PharmSciTech 2018, 19, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Huanbutta, K.; Nernplod, T.; Akkaramongkolporn, P.; Sriamornsak, P. Design of porous Eudragit® L beads for floating drug delivery by wax removal technique. Asian J. Pharm. Sci. 2017, 12, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Moustafine, R.I.; Sitenkov, A.Y.; Bukhovets, A.V.; Nasibullin, S.F.; Appeltans, B.; Kabanova, T.V.; Khutoryanskiy, V.V.; Van den Mooter, G. Indomethacin-containing interpolyelectrolyte complexes based on Eudragit® E PO/S 100 copolymers as a novel drug delivery system. Int. J. Pharm. 2017, 524, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Zakeri-Milani, P.; Barzegar-Jalali, M.; Jelvehgari, M. Design of eudragit RL nanoparticles by nanoemulsion method as carriers for ophthalmic drug delivery of ketotifen fumarate. Iran. J. Basic Med. Sci. 2016, 19, 550–560. [Google Scholar] [PubMed]
- Zhang, F. Melt-Extruded Eudragit(R) FS-Based Granules for Colonic Drug Delivery. AAPS PharmSciTech 2016, 17, 56–67. [Google Scholar] [CrossRef]
- She, X.; Chen, L.; Velleman, L.; Li, C.; Zhu, H.; He, C.; Wang, T.; Shigdar, S.; Duan, W.; Kong, L. Fabrication of high specificity hollow mesoporous silica nanoparticles assisted by Eudragit for targeted drug delivery. J. Colloid Interface Sci. 2015, 445, 151–160. [Google Scholar] [CrossRef]
- Jeganathan, B.; Prakya, V. Interpolyelectrolyte complexes of Eudragit(R) EPO with hypromellose acetate succinate and Eudragit(R) EPO with hypromellose phthalate as potential carriers for oral controlled drug delivery. AAPS PharmSciTech 2015, 16, 878–888. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Inactive Ingredient Search for Approved Drug Products. 2022. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm?event=BasicSearch.page (accessed on 22 September 2022).
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A technology evaluation. Expert Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef]
- Khan, M.Z.I.; Prebeg, Ž.; Kurjaković, N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers: I. Manipulation of drug release using Eudragit® L100-55 and Eudragit® S100 combinations. J. Control. Release 1999, 58, 215–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, I.; Lu, Y.; Xu, Y.; Yu, D.-G.; Song, W. Intelligent poly (l-histidine)-based nanovehicles for controlled drug delivery. J. Control. Release 2022, 349, 963–982. [Google Scholar] [CrossRef]
- Silva Neto, L.D.; Anchieta, C.G.; Duarte, J.L.S.; Meili, L.; Freire, J.T. Effect of Drying on the Fabrication of MgAl Layered Double Hydroxides. ACS Omega 2021, 6, 21819. [Google Scholar] [CrossRef] [PubMed]
- Koczoń, P.; Dobrowolski, J.C.; Lewandowski, W.; Mazurek, A.P. Experimental and theoretical IR and Raman spectra of picolinic, nicotinic and isonicotinic acids. J. Mol. Struct. 2003, 655, 89–95. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G.; Bairwa, K.; Jana, S. Spectroscopic Characterization of Disulfiram and Nicotinic Acid after Biofield Treatment. J. Anal. Bioanal. Tech. 2015, 6, 1000265. [Google Scholar]
- Hasanin, M.; Labeeb, A.M. Dielectric properties of nicotinic acid/methyl cellulose composite via “green” method for anti-static charge applications. Mater. Sci. Eng. B 2021, 263, 114797. [Google Scholar] [CrossRef]
- Hasanin, M.S. Sustainable hybrid silica extracted from rice husk with polyvinyl alcohol and nicotinic acid as multi adsorbent for textile wastewater treatment. Environ. Sci. Pollut. Res. Int. 2020, 27, 26742–26749. [Google Scholar] [CrossRef]
- Choi, G.; Yang, J.-H.; Park, G.-Y.; Vinu, A.; Elzatahry, A.; Yo, C.H.; Choy, J.-H. Intercalative Ion-Exchange Route to Amino Acid Layered Double Hydroxide Nanohybrids and Their Sorption Properties. Eur. J. Inorg. Chem. 2015, 2015, 925–930. [Google Scholar] [CrossRef]
- Koczoń, P.; Hrynaszkiewicz, T.; Świsłocka, R.; Samsonowicz, M.; Lewandowski, W. Spectroscopic (Raman, FT-IR, and NMR) study of alkaline metal nicotinates and isonicotinates. Vib. Spectrosc. 2003, 33, 215–222. [Google Scholar] [CrossRef]
- Lewandowski, W.; Dasiewicz, B.; Koczoń, P.; Skierski, J.; Dobrosz-Teperek, K.; Świsłocka, R.; Fuks, L.; Priebe, W.; Mazurek, A.P. Vibrational study of alkaline metal nicotinates, benzoates and salicylates. J. Mol. Struct. 2002, 604, 189–193. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yang, J.H.; Lee, J.H.; Choi, G.; Park, D.H.; Jo, M.R.; Choi, S.J.; Choy, J.H. 2D Inorganic-Antimalarial Drug-Polymer Hybrid with pH-Responsive Solubility. Chem. Asian J. 2015, 10, 2264–2271. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, G.J.; Choi, A.J.; Kim, T.H.; Kim, T.I.; Oh, J.M. Layered Double Hydroxide Nanomaterials Encapsulating Angelica gigas Nakai Extract for Potential Anticancer Nanomedicine. Front. Pharmacol. 2018, 9, 723. [Google Scholar] [CrossRef]
- Asfour, M.H.; Mohsen, A.M. Formulation and evaluation of pH-sensitive rutin nanospheres against colon carcinoma using HCT-116 cell line. J. Adv. Res. 2017, 129, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.A.C.; Abdelsadig, M.S.E.; Conway, B.R.; Merchant, H.A. Using zeta potential to study the ionisation behaviour of polymers employed in modified-release dosage forms and estimating their pKa. Int. J. Pharm. X 2019, 1, 100024. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-H.; Han, Y.-S.; Park, M.; Park, T.; Hwang, S.-J.; Choy, J.-H. New Inorganic-Based Drug Delivery System of Indole-3-Acetic Acid-Layered Metal Hydroxide Nanohybrids with Controlled Release Rate. Chem. Mater. 2007, 19, 2679–2685. [Google Scholar] [CrossRef]
- Kleyi, P.E.; Mudaly, P.; Kesavan Pillai, S.; de Beer, M. Zn/Al Layered double hydroxides nanostructure as effective controlled release vehicle of nicotinic acid for topical applications. Appl. Clay Sci. 2021, 215, 106304. [Google Scholar] [CrossRef]
- Gök, A. Enhanced adsorption of nicotinic acid by different types of Mg/Al layered double hydroxides: Synthesis, equilibrium, kinetics, and thermodynamics. J. Dispers. Sci. Technol. 2020, 41, 779–786. [Google Scholar] [CrossRef]
- Choi, S.-J.; Oh, J.-M.; Choy, J.-H. Anticancer drug-layered hydroxide nanohybrids as potent cancer chemotherapy agents. J. Phys. Chem. Solids 2008, 69, 1528–1532. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Piao, H.; Rejinold, N.S.; Lee, H.; Choi, G.; Choy, J.-H. pH-Responsive Inorganic/Organic Nanohybrids System for Controlled Nicotinic Acid Drug Release. Molecules 2022, 27, 6439. https://doi.org/10.3390/molecules27196439
Yu S, Piao H, Rejinold NS, Lee H, Choi G, Choy J-H. pH-Responsive Inorganic/Organic Nanohybrids System for Controlled Nicotinic Acid Drug Release. Molecules. 2022; 27(19):6439. https://doi.org/10.3390/molecules27196439
Chicago/Turabian StyleYu, Seungjin, Huiyan Piao, N. Sanoj Rejinold, Hanna Lee, Goeun Choi, and Jin-Ho Choy. 2022. "pH-Responsive Inorganic/Organic Nanohybrids System for Controlled Nicotinic Acid Drug Release" Molecules 27, no. 19: 6439. https://doi.org/10.3390/molecules27196439





