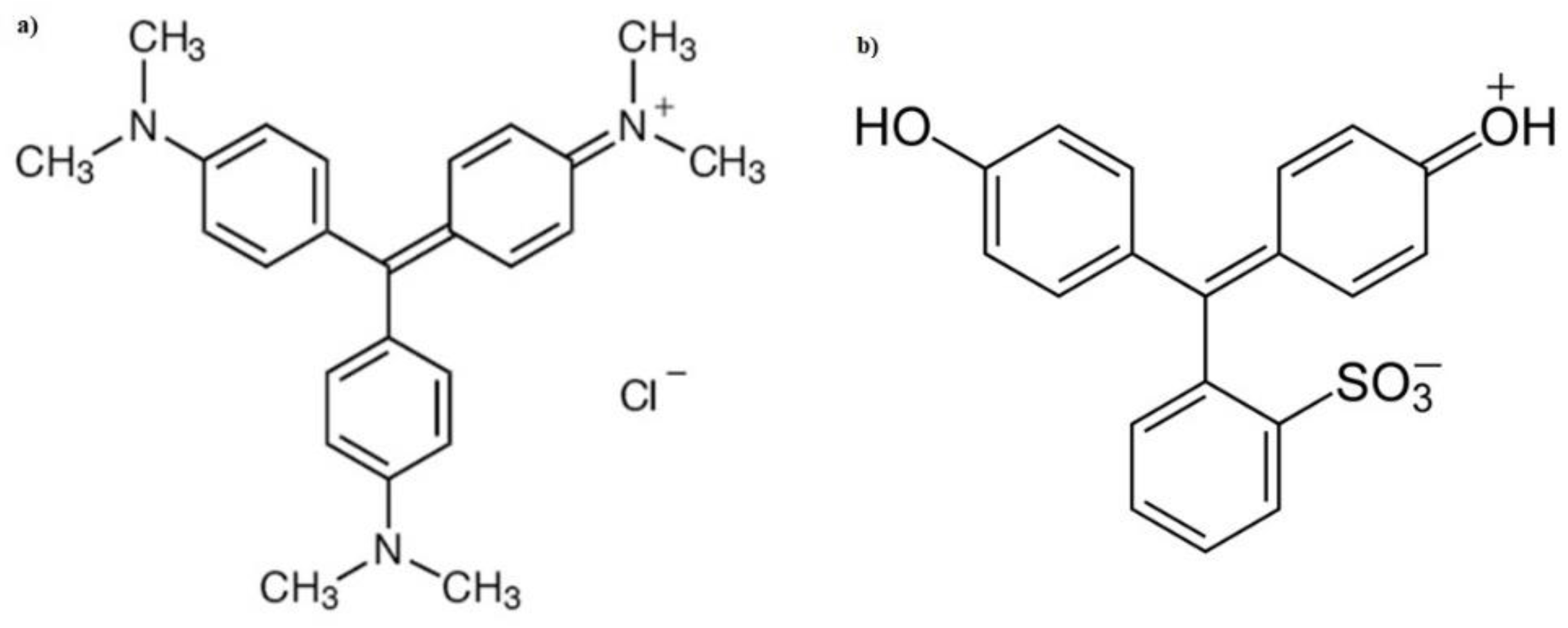
Removal of Anionic and Cationic Dyes Present in Solution Using Biomass of Eichhornia crassipes as Bioadsorbent
Abstract
:1. Introduction
2. Results and Discussion
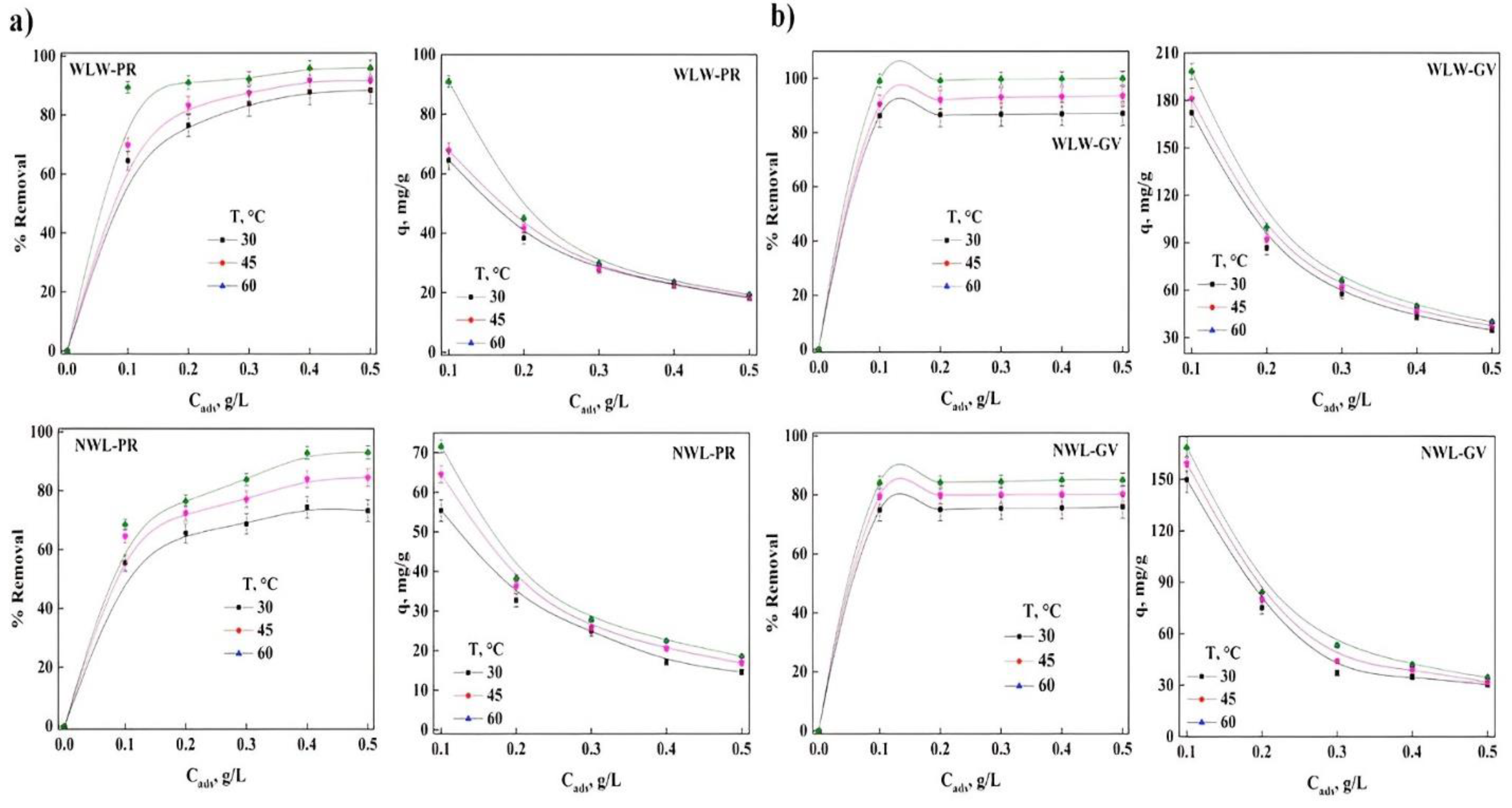
2.1. Effect of the Initial Concentration of Dyes on the Percentage of Removal
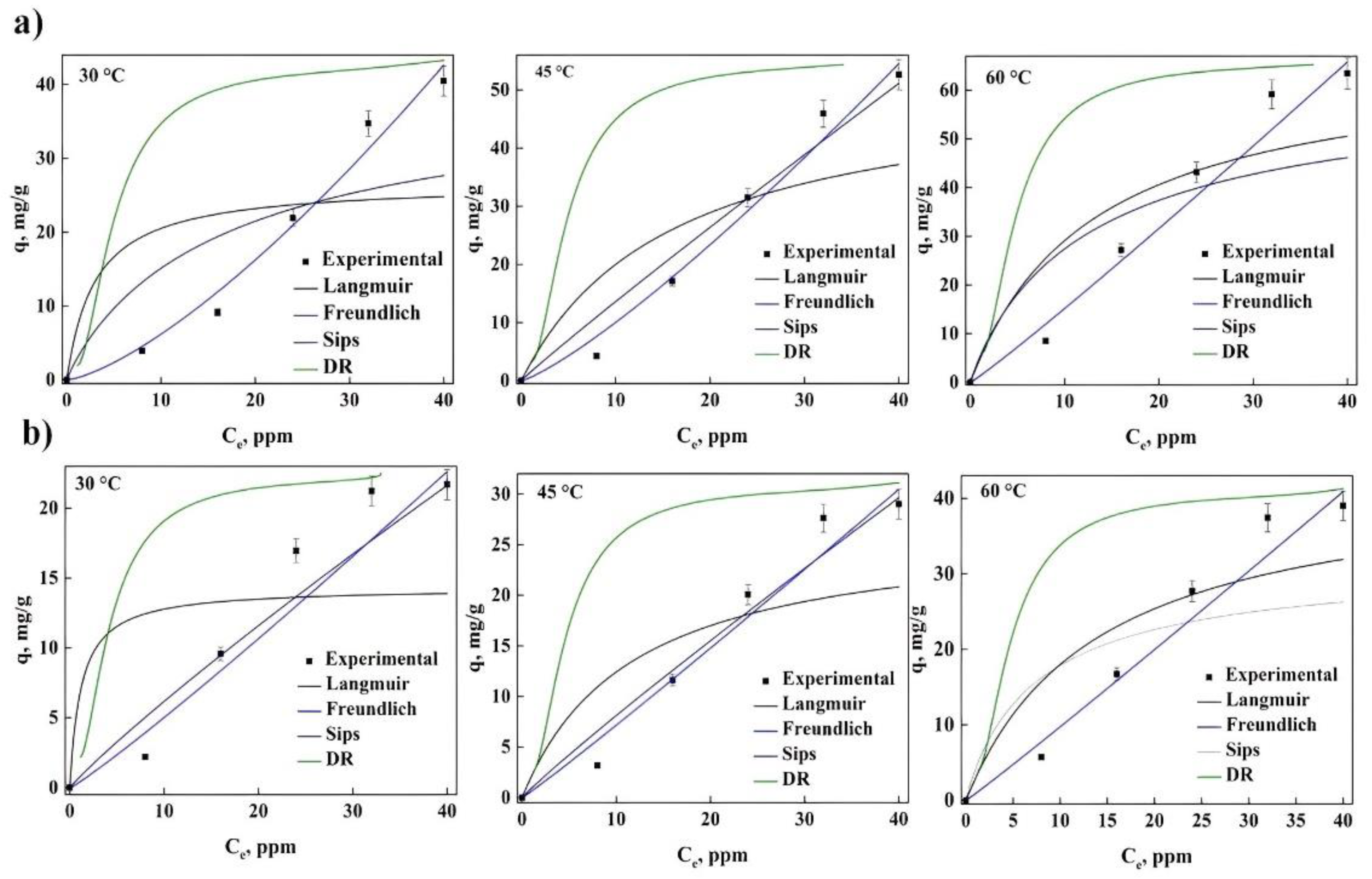
2.2. Equilibrium Dye Adsorption
2.3. Thermodynamic Analysis of the Adsorption Process
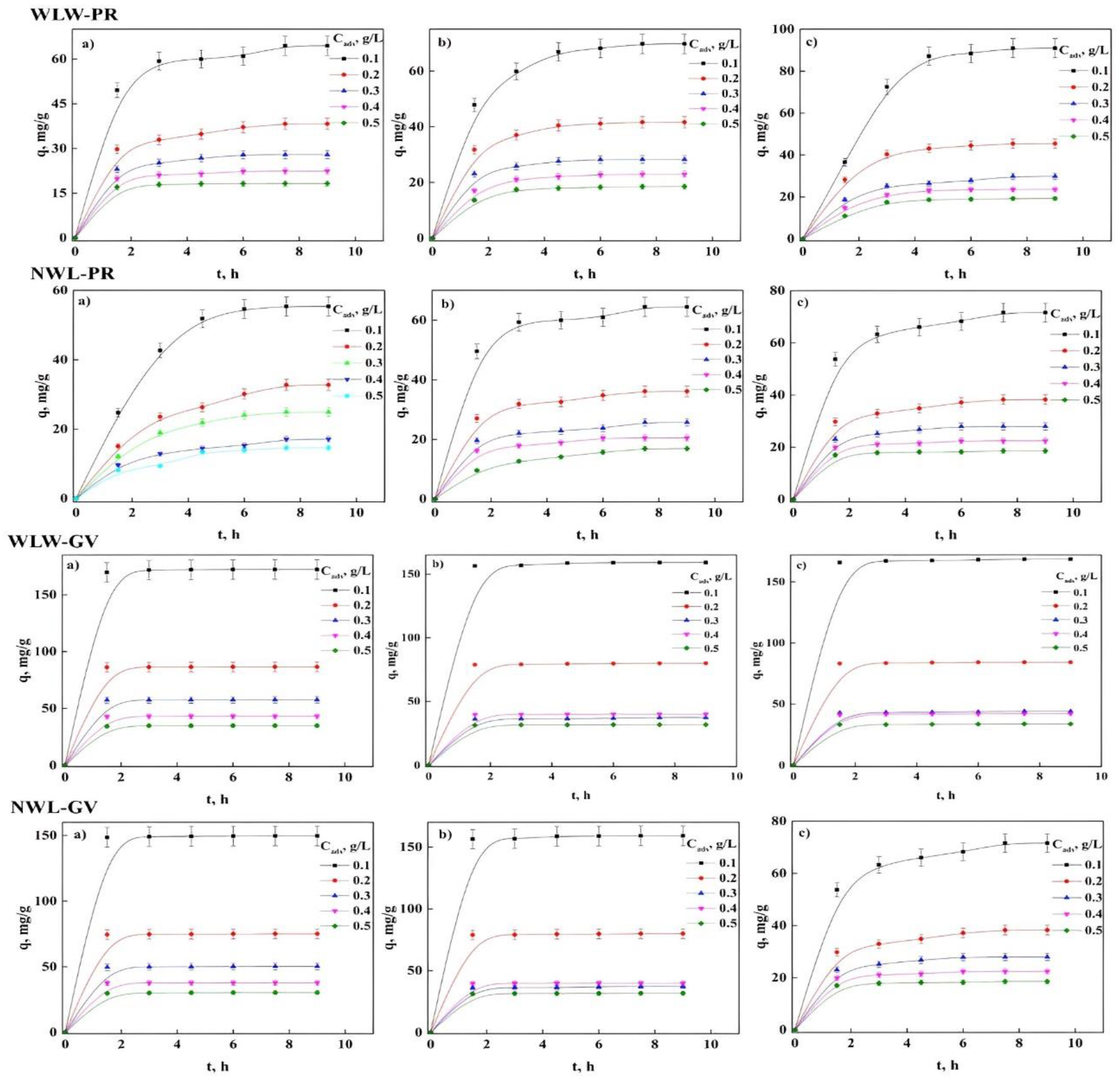
2.4. Contact Time and Concentration of the Bioadsorbent
2.5. Adsorption Kinetics
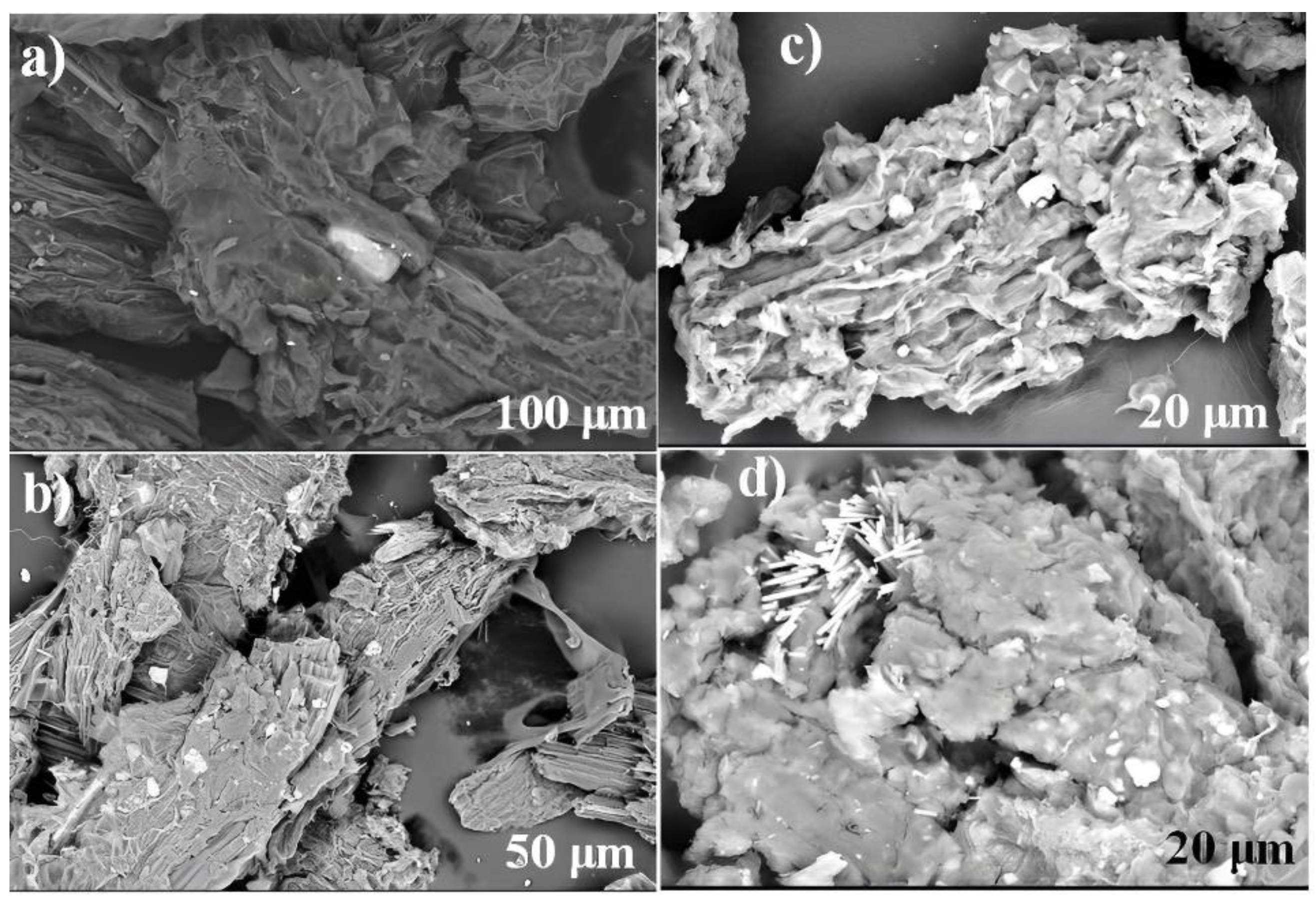
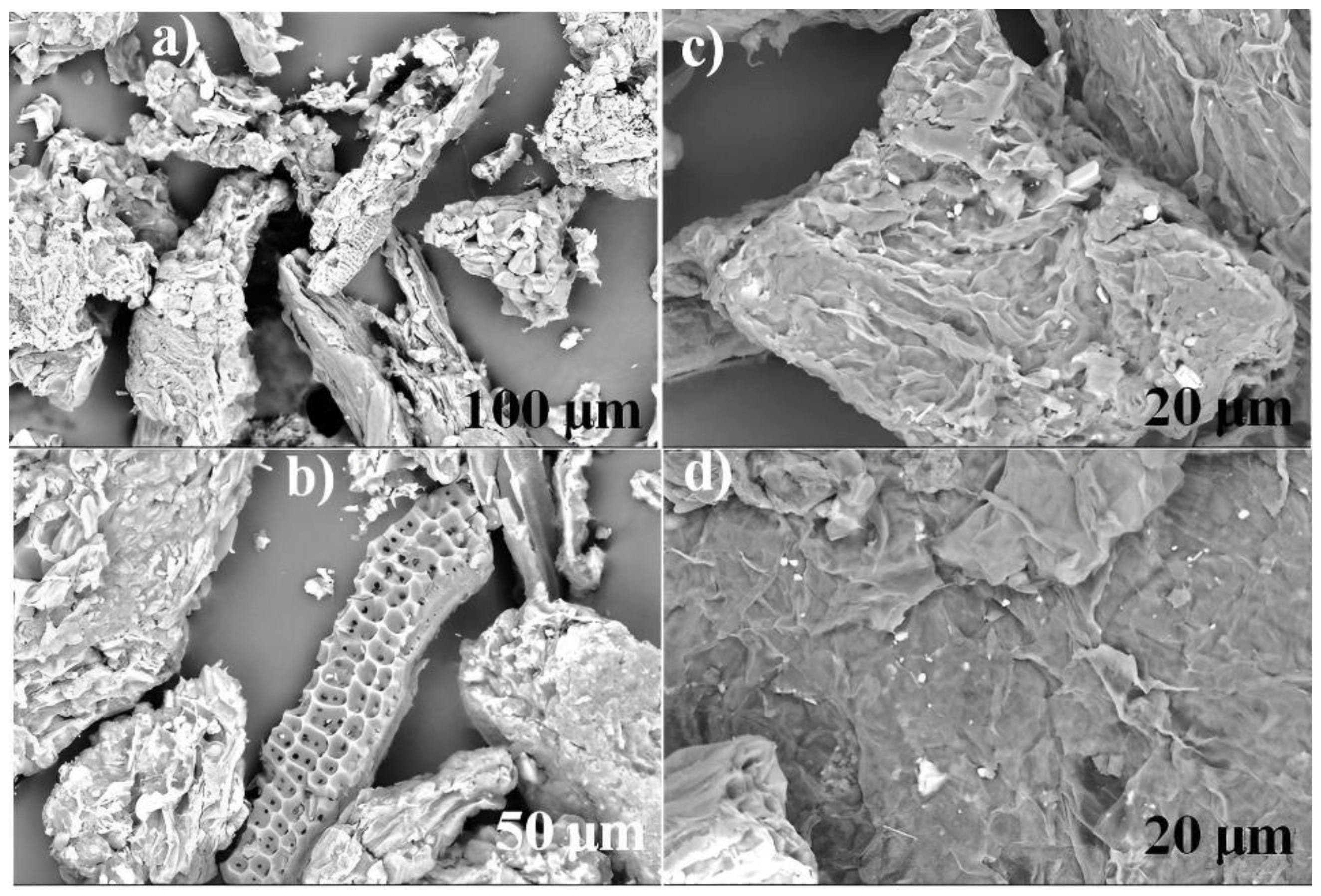
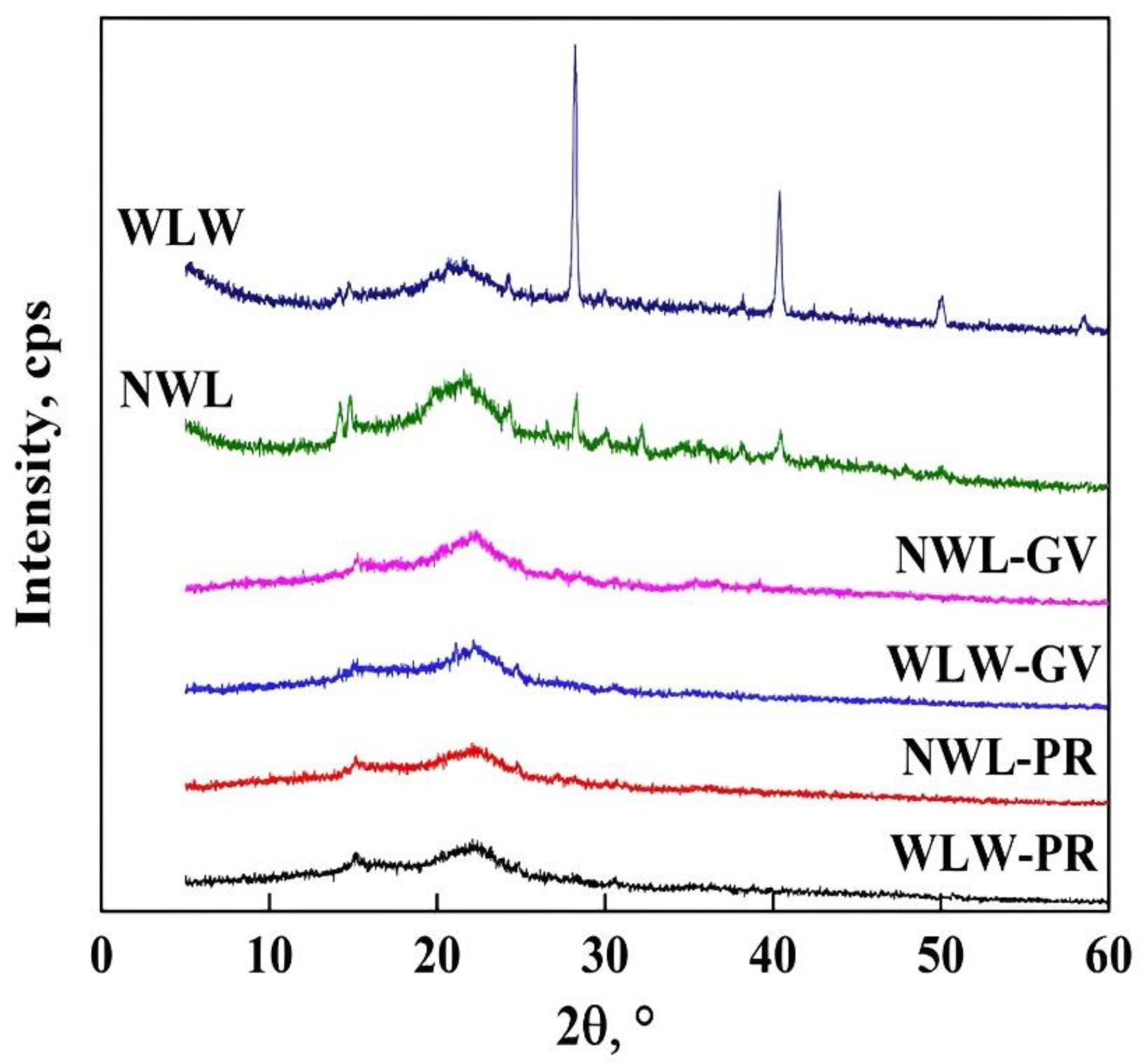
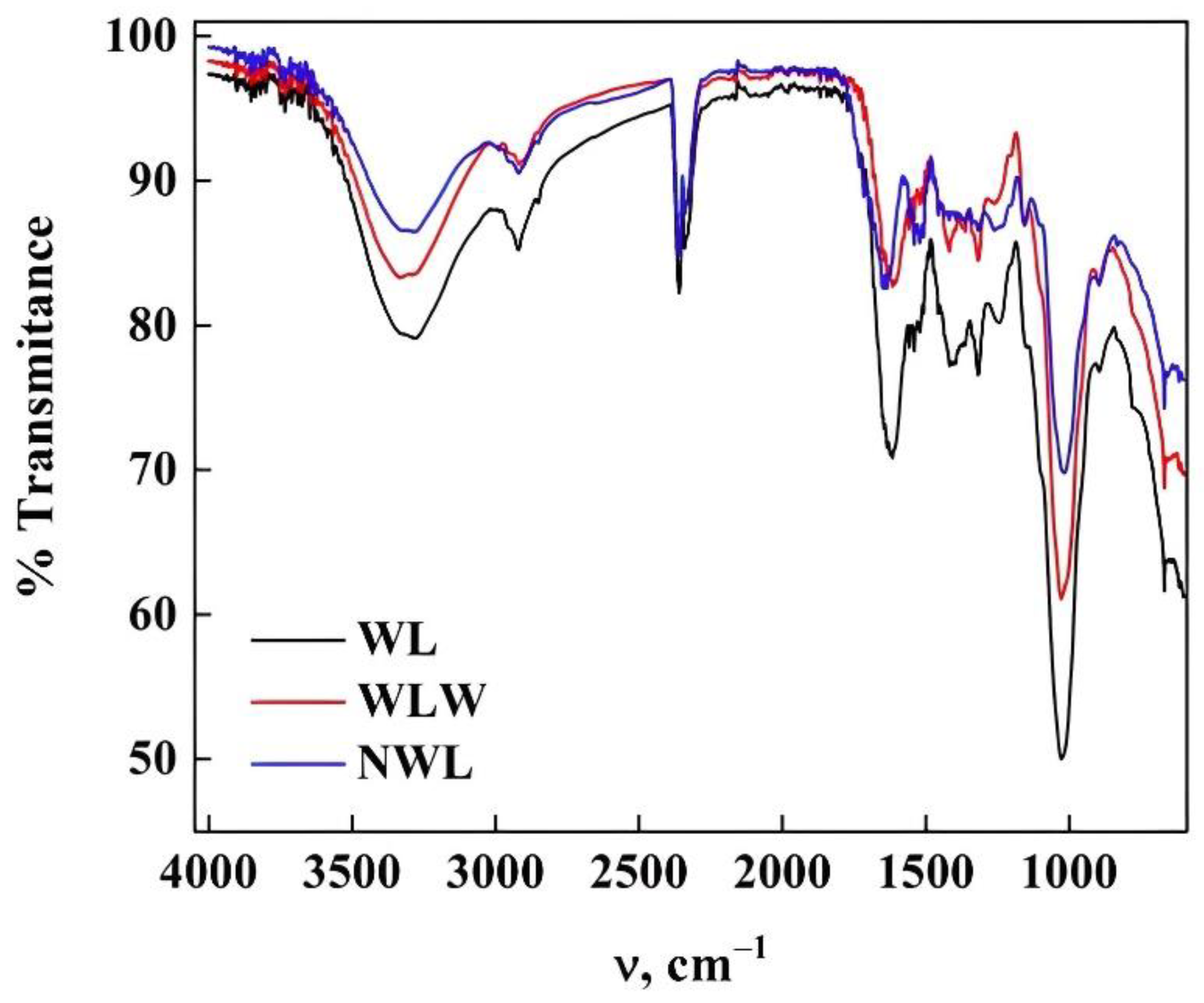
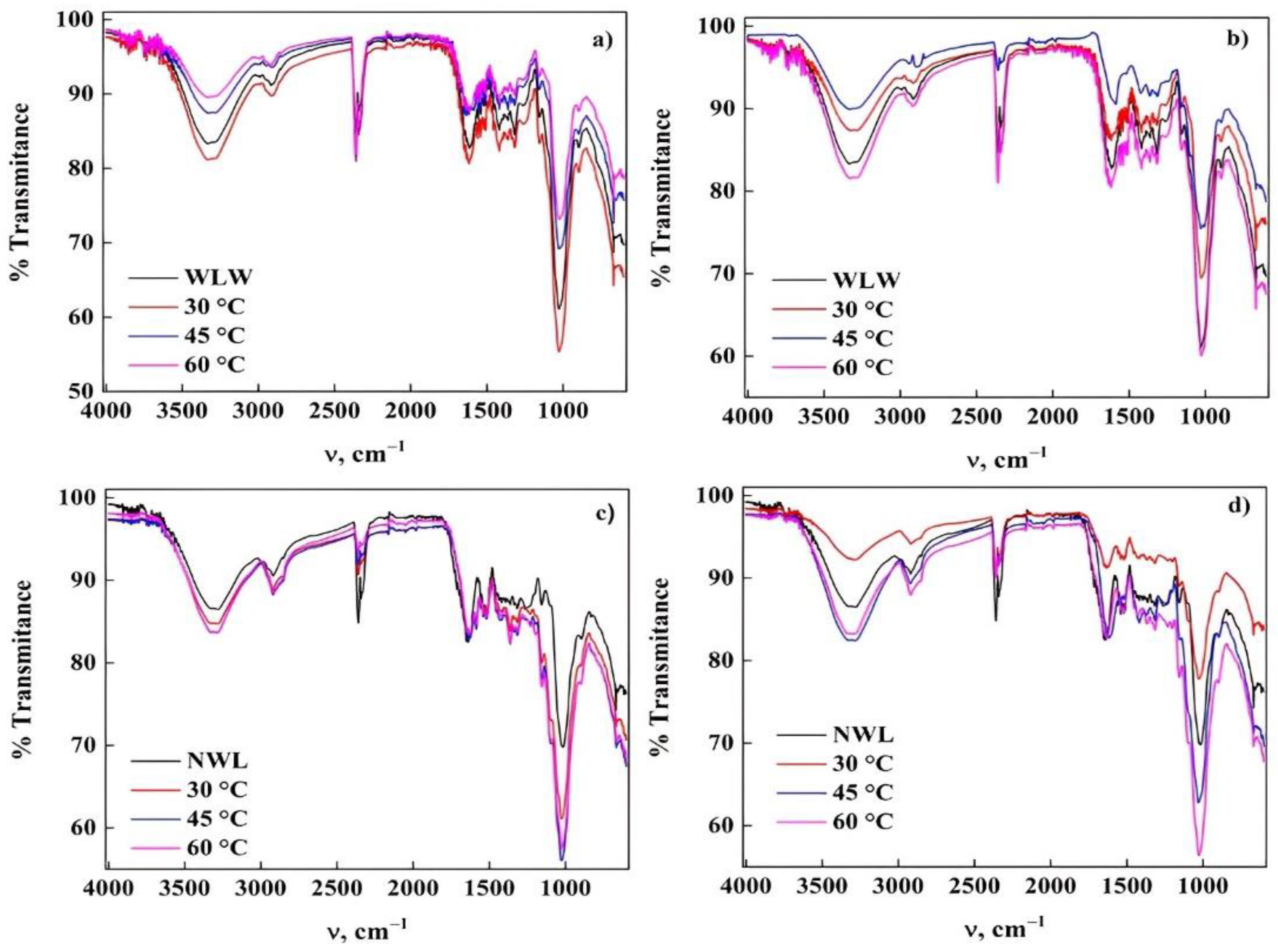
2.6. Characterization of Biomaterials
3. Materials and Methods
3.1. Reagents
3.2. Preparation and Treatment of Water Lily (WL)
3.3. Equilibrium Dye Removal (Adsorption Isotherms)
3.4. Batch Dye Removal (Adsorption Kinetics)
3.5. Characterization of Bioadorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Priya, E.S.; Selvan, P.S. Water hyacinth (Eichhornia Crassipes)—An efficient and economic adsorbent for textile effluent treatment—A review. Arab. J. Chem. 2017, 10, 3548–3558. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Chen, H.; Lu, J.; Yu, G.; Möslang, M.; Zhou, Y. Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy metal ions. J. Hazard. Mater. 2020, 382, 121040. [Google Scholar] [CrossRef] [PubMed]
- Kalam, A.; Rahman, L.; Sarker, A.; Ahmed, N.; Mustofa, M.; Awal, A. Efficient removal of toxic textile dye using petiole part (stem) of Nymphaea alba. Pollution 2021, 7, 643–656. [Google Scholar] [CrossRef]
- Fortas, W.; Djelad, A.; Hasnaoui, M.A.; Sassi, M.; Bengueddach, A. Adsorption of gentian violet dyes in aqueous solution on microporous AlPOs molecular sieves synthesized by ionothermal method. Mater. Res. Express 2018, 5, 025018. [Google Scholar] [CrossRef]
- Abbas, M.; Harrache, Z.; Trari, M. Removal of gentian violet in aqueous solution by activated carbon equilibrium, kinetics, and thermodynamic study. Adsorpt. Sci. Technol. 2019, 37, 566–589. [Google Scholar] [CrossRef]
- Doan, V.D.; Le, V.T.; Ngan-Tran, V.T.; Nguyen, T.K.; Tran, V.A.; Nguyen, T.D. Comparative study on adsorption of cationic and anionic dyes by nanomagnetite supported on biochar derived from Eichhornia crassipes and Phragmites australis stems. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100569. [Google Scholar] [CrossRef]
- Carneiro, M.T.; Barros, A.Z.B.; Morais, A.I.S.; Carvalho Melo, A.L.F.; Bezerra, R.D.S.; Osajima, J.A.; Silva-Filho, E.C. Application of Water Hyacinth Biomass (Eichhornia crassipes) as an Adsorbent for Methylene Blue Dye from Aqueous Medium: Kinetic and Isothermal Study. Polymers 2022, 14, 2732. [Google Scholar] [CrossRef]
- Chong, S.N.; Hadibarata, T. Adsorption of Phenol Red and Remazol Brilliant Blue R by Coconut Shells (Cocos nucifera) and Ambarella Peels (Spondias dulcis). Biointerface Res. Appl. Chem. 2021, 11, 8564–8576. [Google Scholar] [CrossRef]
- Martins, L.R.; Rodrigues, J.A.V.; Adarme, O.F.H.; Melo, T.M.S.; Gurgel, L.; Gil, L.F. Optimization of cellulose and sugarcane bagasse oxidation: Application for adsorptive removal of crystal violet and auramine-O from aqueous solution. J. Colloid Interface Sci. 2017, 494, 223–241. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, A. Enhanced adsorption of gentian violet dye from water using lignocellulosic agricultural waste modified with di- and tri-carboxylic acids: Artificial intelligence modeling, practical comprehension, mechanistic and regeneration analyses. J. Environ. Chem. Eng. 2021, 9, 105578. [Google Scholar] [CrossRef]
- Lim, L.B.L.; Usman, A.; Hassan, M.H.; Zaida, N.A.H.M. Tropical wild fern (Diplazium esculentum) as a new and effective low-cost adsorbent for removal of toxic crystal violet dye. J. Taibah Univ. Sci. 2020, 14, 621–627. [Google Scholar] [CrossRef]
- Fan, H.; Ma, Y.; Wan, J.; Wang, Y. Removal of gentian violet and rhodamine B using banyan aerial roots after modification and mechanism studies of differential adsorption behaviors. Environ. Sci. Pollut. Res. 2020, 27, 9152–9266. [Google Scholar] [CrossRef] [PubMed]
- Vidya Vijay, E.V.; Jerold, M.; Sankar, R.; Lakshmanan, S.; Sivasubramanian, V. Electrocoagulation using commercial grade aluminium electrode for the removal of crystal violet from aqueous solution. Water Sci. Technol. 2019, 79, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.; Babu, D.; Reiz, B.; Whittal, R.; Suh, L.Y.K.; Siraki, A.G. Caution for the routine use of phenol red—It is more than just a pH indicator. Chem. Biol. Interact. 2019, 310, 108739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, X.; Zhu, J.; Zhao, Q.; Li, Y.; Li, W.; Fan, C.; Huang, Q. Cytotoxicity of Phenol Red in Toxicity Assays for Carbon Nanoparticles. Int. J. Mol. Sci. 2012, 13, 12336–12348. [Google Scholar] [CrossRef]
- My Linh, N.L.; Duong, T.; Van Duc, H.; Anh Thu, N.Y.; Lieu, P.K.; Van Hung, N.; Hoa, L.T.; Khieu, D.Q. Phenol Red Adsorption from Aqueous Solution on the Modified Bentonite. J. Chem. 2020, 2020, 1504805. [Google Scholar] [CrossRef]
- Acosta-Rodríguez, I.; Rodríguez-Pérez, A.; Pacheco-Castillo, N.C.; Enriquez-Domínguez, E.; Cárdenas-González, J.F.; Martínez-Juárez, V.M. Removal of cobalt (II) from waters contaminated by the biomass of Eichhornia Crassipes. Water 2021, 13, 1725. [Google Scholar] [CrossRef]
- Almaraj, R.; Ramsenthil, R.; Durai, G.; Jayakumar, R.; Palaniraj, R. Dyes Removal using novel sorbent—A Review. J. Pharm. Res. Int. 2021, 33, 355–382. [Google Scholar] [CrossRef]
- Saltabaş, Ö.; Teker, M.; Konuk, Z. Biosorption of cationic dyes from aqueous solution by water hyacinth roots. Glob. NEST J. 2012, 14, 24–31. [Google Scholar] [CrossRef]
- Jabar, J.M.; Odusote, Y.A. Utilization of prepared activated biochar from water lily (Nyvmphaea lotus) stem for adsorption of malachite green dye from aqueous solution. Biomass Convers. Biorefin. 2021, 1–12. [Google Scholar] [CrossRef]
- Soltani, A.; Faramarzi, M.; Parsa, S.A.M. A review on adsorbent parameters for removal of dye products from industrial Wastewater. Water Qual. Res. J. 2021, 56, 181–193. [Google Scholar] [CrossRef]
- Remy, M.K.; Vala; Tichagwa, L. Enhancement of the adsorption of phenol red from wastewater onto clinoptilolite by modification with n-terminated siloxanes. Clays Clay Miner. 2013, 61, 532–540. [Google Scholar] [CrossRef]
- Chen, Q.; Dong, N.; Zhang, Q.; Yang, Y.; Wang, Q.; He, Y. Synergetic effect on methylene blue adsorption to biochar with gentian violet in dyeing and printing wastewater under competitive adsorption mechanism. Case Stud. Eng. 2021, 26, 101099. [Google Scholar] [CrossRef]
- Tara, N.; Siddiqui, S.I.; Rathi, G.; Chaudhry, S.A.; Inamuddin; Asiri, A.M. Nano-engineered adsorbent for the removal of dyes from water: A review. Curr. Anal. Chem. 2020, 16, 14–40. [Google Scholar] [CrossRef]
- Alguacil, F.J.; López, F.A. Organic Dyes versus Adsorption Processing. Molecules 2021, 26, 5440. [Google Scholar] [CrossRef]
- El-Zawahry, M.M.; Abdelghaffar, F.; Abdelghaffar, R.A.; Mashaly, H.M. Functionalization of the aquatic weed water hyacinth Eichhornia Crassipes by using zinc oxide nanoparticles for removal of organic dyes effluent. Fibers Polym. 2016, 17, 186–193. [Google Scholar] [CrossRef]
- de Menezes, E.W.; Lima, E.C.; Royer, B.; de Souza, F.E.; dos Santos, B.D.; Gregório, J.R.; Costa, T.M.H.; Gushikem, Y.; Benvenutti, E.V. Ionic silica based hybrid material containing the pyridinium group used as an adsorbent for textile dye. J. Colloid Interface Sci. 2012, 378, 10–20. [Google Scholar] [CrossRef]
- Bellir, K.; Sadok Bouziane, I.; Boutamine, Z.; Bencheikh Lehocine, M.; Meniai, A.-H. Sorption Study of a Basic from Aqueous Solutions Using Activated Bentonite. Energy Procedia 2012, 18, 924–933. [Google Scholar] [CrossRef]
- Enaime, G.; Lübken, M. Agricultural Waste-Based Biochar for Agronomic Applications. Appl. Sci. 2021, 11, 8914. [Google Scholar] [CrossRef]
- Amode, J.O.; Alam, Z.; Mirza, A.H.; Mei, C.C.; Santos, J.H. Synergistic Studies on the Removal of Dyes by Waste (Metroxylon sagu) Based Activated Carbon: An Optimization Study. IOP Conf Ser. Earth Environ. Sci 2018, 159, 012016. [Google Scholar] [CrossRef]
- Nurhadi, M.; Widiyowati, I.I.; Wirhanuddin, W.; Chandren, S. Kinetic of adsorption process of sulfonated carbon derived from Eichhornia Crassipes in the adsorption of methylene blue dye from aqueous solution. Bull. Chem. React. Eng. Catal. 2019, 14, 17–27. [Google Scholar] [CrossRef]
- Mittal, A.; Mittal, J.; Malviya, A.; Kaur, D.; Gupta, V.K. Adsorption of hazardous dye crystal violet from wastewater by waste materials. J. Colloid Interface Sci. 2010, 343, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Tsade Kara, H.; Anshebo, S.T.; Sabir, F.K.; Workineh, G.A. Removal of methylene blue dye from wastewater using periodiated modified nanocellulose. Int. J. Chem. Eng. 2021, 2021, 9965452. [Google Scholar] [CrossRef]
- Sharma, R.; Saini, H.; Paul, D.R.; Chaudhary, S.; Nehra, S.P. Removal of organic dyes from wastewater using Eichhornia Crassipes: A potential phytoremediation option. Environ. Sci. Pollut. Res. 2021, 28, 7116–7122. [Google Scholar] [CrossRef]
- Guimarães Gusmão, K.A.; Sacramento Melo, T.M.; Alves Gurgel, L.V.; Gil, L.F. Application of succinylated sugarcane bagasse as adsorbent to remove methylene blue and gentian violet from aqueous solutions e Kinetic and equilibrium studies. Dye. Pigment. 2012, 92, 967–974. [Google Scholar] [CrossRef]
- Wanyonyi, W.C.; Onyari, J.M.; Shiundu, P.M. Adsorption of Methylene Blue Dye from Aqueous Solutions Using Eichhornia crassipes. Bull. Environ. Contam. Toxicol. 2013, 91, 362–366. [Google Scholar] [CrossRef]
- Prasad, R.; Sharma, D.; Yadav, K.D.; Ibrahim, H. Eichhornia Crassipes as biosorbent for industrial wastewater treatment: Equilibrium and kinetic studies. Can. J. Chem. Eng. 2021, 100, 439–450. [Google Scholar] [CrossRef]
- Tamjidi, S.; Ameri, A. A review of the application of sea material shells as low cost and effective bio-adsorbent for removal of heavy metals from wastewater. Environ. Sci. Pollut. Res. 2020, 27, 31105–31119. [Google Scholar] [CrossRef]
- Ranasinghe, S.H.; Navaratne, A.N.; Priyantha, N. Enhancement of adsorption characteristics of Cr(III) and Ni(II) by surface modification of jackfruit peel biosorbent. J. Environ. Chem. Eng. 2018, 6, 5670–5682. [Google Scholar] [CrossRef]
- Mukaratirwa-Muchanyereyi, N.; Kugara, J.; Zaranyika, M.F. Surface composition and surface properties of water hyacinth (Eichhornia crassipes) root biomass: Effect of mineral acid and organic solvent treatment. Afr. J. Biechnol. 2016, 15, 897–909. [Google Scholar] [CrossRef]
- Alebachew, N.; Yadav, O.P.; Lokesh. Removal of Phenol Red Dye from Contaminated Water Using Barley (Hordeum vulgare L.) Husk-Derived Activated Carbon. Sci. Int. 2017, 5, 7–16. [Google Scholar] [CrossRef]
- Tabinda, A.B.; Arif, R.A.; Yasar, A.; Baquir, M.; Rasheed, R.; Ahmood, A.; Iqbal, A. Treatment of textile effluents with Pistia stratiotes, Eichhornia Crassipes and Oedogonium sp. Int. J. Phytorem. 2019, 21, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.S.A.; Naim, M.N.; Che Man, H.; Abu Bakar, N.F.; Mokhtar, M.N. Evaluation of surface water treated with lotus plant; Nelumbo Nucifera. J. Environ. Chem. Eng. 2019, 7, 103048. [Google Scholar] [CrossRef]
- Torab-Mostaedi, M.; Asadollahzadeh, M.; Hemmati, A.; Khosravi, A. Biosorption of lanthanum and cerium from aqueous solutions by grapefruit peel: Equilibrium, kinetic and thermodynamic studies. Res. Chem. Intermed. 2015, 41, 559–573. [Google Scholar] [CrossRef]
- Liu, W.; Jin, L.; Xu, J.; Liu, J.; Li, Y.; Zhou, P.; Wang, C.; Dahlgren, R.A.; Wang, R. Insight into pH Dependent Cr(VI) Removal with Magnetic Fe3S4. Chem. Eng. J. 2018, 359, 564–571. [Google Scholar] [CrossRef]
- Bronzato, G.R.F.; Ziegler, S.M.; Silva, R.C.; Cesarino, I.; Leão, A.L. Characterization of the pre-treated biomass of Eichhornia Crassipes (water hyacinth) for the second-generation ethanol production. Mol. Cryst. Liq. Cryst. 2017, 655, 224–235. [Google Scholar] [CrossRef]
- Márquez, C.O.; García, V.J.; Guaypatin, J.R.; Fernández-Martínez, F.; Ríos, A.C. Cationic and anionic dye adsorption on a natural clayey composite. Appl. Sci. 2021, 11, 5127. [Google Scholar] [CrossRef]
- Hernández-Origel, C.D.; Patiño-Saldivar, L.; Salazar-Hernández, M.; Ardila, A.N.; Talavera-López, A.; Hernández-Soto, R.; Hernández, J.A. Use of Eichhornia Crassipes as a Bioadsorbent for the Removal of Methyl Orange and Methylene Blue Present in Residual Solutions. J. Ecol. Eng. 2022, 23, 193–211. [Google Scholar] [CrossRef]


| Model | Equation | |
|---|---|---|
| Langmuir | qe (mg/g), equilibrium adsorption capacity. Ce (mg/L) is the equilibrium concentration of the dye in the liquid, V (L) is the volume of the dye solution, and m (g), is the mass of the adsorbent. qm, is the maximum adsorbed capacity (mg/g). KL (L/mg), Langmuir equilibrium constant. KF ((mg/g)(L/mg)1/n, Freundlich constant indicating the adsorption capacity of the adsorbent, n (dimensionless), is the exponent of the Freundlich model indicates the intensity of adsorption. kDR (mol/J)2 constant related to the average energy of adsorption, ε (J/mol), Poliani potential. Ks (L/mg) Sips equilibrium constant, β (dimensionless) is the exponent of the Sips model related to the heterogeneity of the system | |
| Freundlich | ||
| Dubinin-Radushkevich (DR) | ||
| Sips |
| Model | WLW | NWL | ||||
|---|---|---|---|---|---|---|
| 30 °C | 45 °C | 60 °C | 30 °C | 45 °C | 60 °C | |
| Langmuir KL (L/mg) qm (mg/g) RL R2 Δq, % | 0.761 14.22 0.55–0.78 0.591 31.681 | 0.196 24.95 0.25–0.51 0.772 3.951 | 0.054 34.57 0.08–0.1 0.852 15.676 | 0.621 21.63 0.43–0.69 0.717 6.235 | 0.218 21.24 0.23–0.47 0.753 5.412 | 0.089 24.11 0.09–0.14 0.812 6.578 |
| Freudlich KF (mg/g) (L/mg)1/n n R2 Δq, % | 1.423 1.052 0.922 1.389 | 1.893 1.088 0.958 0.415 | 1.928 1.026 0.982 2.425 | 1.099 1.048 0.943 3.043 | 1.434 1.052 0.962 4.936 | 2.238 1.194 0.975 3.073 |
| Sips KS (L/mg) qm (mg/g) ns R2 Δq, % | 0.098 22.051 2.823 0.978 6.505 | 0.089 26.415 2.111 0.983 7.509 | 0.103 37.377 1.973 0.991 21.085 | 0.128 15.462 3.676 0.998 5.091 | 0.121 26.764 2.867 0.993 22.023 | 0.142 22.861 2.792 0.999 5.291 |
| DR qm (mg/g) kDR Ea (kJ/mol) R2 Δq, % | 1.787 0.051 3.892 0.936 40.567 | 2.716 0.046 3.202 0.943 39.351 | 3.888 0.033 3.105 0.932 37.933 | 1.801 0.044 3.373 0.908 38.691 | 2.461 0.039 3.269 0.9222 38.585 | 3.753 0.031 3.151 0.899 36.425 |
| Models | WLW | NWL | ||||
|---|---|---|---|---|---|---|
| 30 °C | 45 °C | 60 °C | 30 °C | 45 °C | 60 °C | |
| Langmuir KL (L/mg) qm (mg/g) RL R2 Δq, % | 0.531 26.672 0.24–0.62 0.726 15.433 | 0.199 40.362 0.12–0.40 0.747 10.411 | 0.077 69.991 0.050.20 0.788 4.648 | 0.840 15.554 0.24–0.62 0.761 12.674 | 0.246 28.253 0.09–037 0.739 1.156 | 0.072 45.011 0.03–0.13 0.795 6.652 |
| Freudlich KF (mg/g) (L/mg)1/n n R2 Δq, % | 1.253 0.719 0.982 20.563 | 0.613 0.819 0.984 2.387 | 1.392 0.946 0.977 3.759 | 0.677 0.924 0.949 9.573 | 0.454 0.958 0.9445 11.805 | 0.954 0.967 0.977 4.869 |
| Sips KS (L/mg) qm (mg/g) ns R2 Δq, % | 0.968 40.94 0.916 0.688 0.264 | 0.065 52.09 0.955 0.727 0.434 | 0.077 65.10 0.932 0.774 1.199 | 0.088 21.981 0.914 0.796 0.619 | 0.087 28.985 0.934 0.726 0.031 | 0.136 38.254 0.942 0.685 0.894 |
| DR qm (mg/g) kDR Ea (kJ/mol) R2 Δq, % | 1.956 0.024 4.612 0.969 42.572 | 3.459 0.029 4.158 0.964 41.784 | 6.382 0.035 3.591 0.948 16.178 | 2.409 0.022 4.752 0.967 43.447 | 2.736 0.025 4.435 0.939 40.508 | 4.174 0.026 4.363 0.949 35.933 |
| T, °C | −ΔG, kJ/mol | ΔH, kJ/mol | −ΔS, kJ/mol K | −ΔG, kJ/mol | ΔH, kJ/mol | ΔS, kJ/mol K |
|---|---|---|---|---|---|---|
| WLW-PR | NWL-PR | |||||
| 30 | 41.64 | 83.53 | 0.051 | 41.13 | 98.62 | 0.646 |
| 45 | 40.14 | 40.39 | ||||
| 60 | 38.44 | 39.82 | ||||
| WLW-GV | NWL-GV | |||||
| 30 | 41.09 | 35.75 | 0.224 | 42.25 | 67.32 | 0.127 |
| 45 | 40.43 | 41.09 | ||||
| 60 | 39.81 | 39.81 | ||||
| Model | Equation | |
|---|---|---|
| Pseudo first order (PFO) | q (mg/g), adsorption capacity. C0 (mg/L) is the initial concentration of the dye in the liquid, V (L) is the volume of the dye solution and m (g), is the mass of the adsorbent. qmax is the maximum adsorbed capacity (mg/g). k1 (1/h) is the speed constant of the PPO model. k2 (g s/mg) is the speed constant of the PSP model. kext constant kinetic of Avrami model (h−1), nA reflects the changes of the mechanism during the adsorption process. kInt (mg/g h) is the speed constant of the ID model. kExt (1/h) is the speed constant of the model ED. | |
| Pseudosecond order (PSO) | ||
| Avrami | ||
| Intraparticle diffusion (ID) | ||
| External diffusion (ED) |
| Model | 0.1 g/L | 0.3 g/L | 0.5 g/L | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 °C | 45 °C | 60 °C | 30 °C | 45 °C | 60 °C | 30 °C | 45 °C | 60 °C | |
| PFO qmax, mg/g k1, h−1 R2 Δq, % | 45.60 1.017 0.996 1.131 | 62.71 0.742 0.998 0.259 | 97.85 0.332 0.998 2.076 | 27.64 1.139 0.995 0.693 | 27.95 1.118 0.997 0.431 | 29.20 0.656 0.995 0.846 | 18.18 1.861 0.998 0.168 | 18.48 0.904 0.999 0.121 | 19.59 0.612 0.994 0.773 |
| PSO qmax, mg/g k2, h−1 R2 Δq, % | 59.803 8 × 107 0.953 2.963 | 63.72 1 × 108 0.904 3.546 | 73.78 1 × 106 0.675 7.723 | 26.46 3 × 106 0.968 2.160 | 26.88 4 × 106 0.967 1.969 | 26.31 5 × 105 0.874 4.791 | 17.97 6 × 107 0.996 0.633 | 17.41 1 × 107 0.936 2.478 | 17.40 3 × 107 0.831 3.875 |
| Avrami qmax, mg/g kA, h−1 nA R2 Δq, % | 62.69 1.307 0.781 0.996 1.333 | 69.33 0.961 0.771 0.998 0.587 | 97.85 0.571 0.583 0.998 3.654 | 27.46 1.339 0.851 0.995 1.548 | 27.95 1.288 0.868 0.997 0.982 | 29.19 0.839 0.781 0.995 0.999 | 18.18 1.967 0.930 0.999 0.587 | 18.47 0.997 0.906 0.999 1.256 | 19.59 0.756 0.811 0.994 1.587 |
| ID kid, mg/g h 0.5 R2 Δq, % | 25.72 0.776 3.875 | 27.67 0.841 7.738 | 33.21 0.976 3.875 | 11.34 0.729 9.121 | 11.52 0.723 8.896 | 11.49 0.883 0.392 | 7.612 0.591 10.25 | 7.500 0.863 7.686 | 7.616 0.863 9.470 |
| ED kext, h−1 R2 Δq, % | 78.78 0.339 22.45 | 32.38 0.146 17.68 | 1.917 0.000 4.036 | 0.216 0.586 0.999 | 0.226 0.596 1.075 | 0.212 0.801 1.679 | 0.101 0.129 14.27 | 0.098 0.408 14.95 | 0.101 0.613 15.48 |
| Model | 0.1 g/L | 0.3 g/L | 0.5 g/L | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 °C | 45 °C | 60 °C | 30 °C | 45 °C | 60 °C | 30 °C | 45 °C | 60 °C | |
| PFO qmax, mg/g k1, h−1 R2 Δq, % | 28.15 0.450 0.998 1.424 | 39.90 0.469 0.994 0.471 | 51.24 0.847 0.995 0.850 | 9.673 1.081 0.998 0.670 | 18.46 1.122 0.990 1.677 | 23.42 1.117 0.997 1.008 | 5.865 2.015 0.978 2.743 | 12.80 1.222 0.987 1.210 | 16.63 1.273 0.991 1.737 |
| PSO qmax, mg/g k2, h−1 R2 Δq, % | 23.35 3 × 108 0.762 5.780 | 33.55 2 × 107 0.797 6.097 | 47.94 9 × 105 0.929 3.423 | 9.277 1 × 106 0.963 2.313 | 17.77 6 × 106 0.961 3.155 | 22.52 6 × 106 0.966 2.530 | 5.815 1 × 106 0.976 3.067 | 13.71 5 × 106 0.995 1.616 | 16.15 1 × 107 0.964 2.082 |
| Avrami qmax, mg/g kA, h−1 nA R2 Δq, % | 58.31 0.578 0.731 0.994 2.587 | 62.69 1.063 0.956 0.999 1.587 | 69.53 0.998 0.931 0.995 2.025 | 25.69 0.589 0.740 0.999 1.871 | 24.47 1.007 0.978 0.985 2.987 | 27.46 1.142 0.997 0.999 1.987 | 15.05 0.589 0.728 0.978 3.658 | 16.77 0.640 0.777 0.991 2.587 | 18.35 1.746 1.000 0.999 2.698 |
| ID kid, mg/g h0.5 R2 Δq, % | 10.36 0.942 5.831 | 14.88 0.951 5.390 | 20.74 0.816 7.716 | 3.979 0.734 8.735 | 7.627 0.744 7.692 | 9.660 0.735 8.451 | 2.476 0.647 7.407 | 5.316 0.730 8.529 | 6.916 0.716 7.936 |
| ED kext, h−1 R2 Δq, % | 0.172 0.871 77.10 | 0.413 0.991 60.16 | 1.802 0.822 37.18 | 0.042 0.169 2.806 | 0.101 0.364 1.264 | 0.154 0.471 1.343 | 0.024 0.000 15.37 | 0.060 0.227 17.87 | 0.087 0.281 8.603 |
| Model | 0.1 g/L | 0.3 g/L | 0.5 g/L | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 °C | 45 °C | 60 °C | 30 °C | 45 °C | 60 °C | 30 °C | 45 °C | 60 °C | |
| PFO qmax, mg/g k1, h−1 R2 Δq, % | 172.2 2.835 1.000 0.04 | 180.2 1.906 0.999 0.181 | 196.2 1.742 0.999 0.422 | 57.47 3.885 1.000 0.079 | 61.73 2.488 0.999 0.214 | 65.92 1.996 0.999 0.175 | 34.76 2.861 1.000 0.046 | 37.20 3.464 0.999 0.240 | 39.81 3.095 0.999 0.223 |
| PSO qmax, mg/g k2, h−1 R2 Δq, % | 171.7 3 × 106 0.998 0.140 | 178.4 4 × 106 0.997 0.593 | 193.63 5 × 106 0.994 0.973 | 57.44 0.640 0.999 0.099 | 62.37 0.298 0.998 0.208 | 67.13 0.146 0.998 0.575 | 34.68 6 × 106 0.999 0.139 | 37.16 4 × 106 0.999 0.278 | 39.75 3 × 106 0.999 0.289 |
| Avrami qmax, mg/g kA, h−1 nA R2 Δq, % | 172.2 2.835 1.000 1.000 0.987 | 180.2 2.528 0.754 0.999 2.584 | 196.2 2.165 0.805 0.999 0.898 | 57.47 4.620 0.841 1.000 0.587 | 61.73 3.056 0.814 0.999 0.483 | 65.92 2.230 0.895 0.999 0.258 | 34.76 2.998 0.954 1.000 0.870 | 37.21 3.578 0.968 0.999 0.560 | 39.81 3.174 0.975 0.999 0.287 |
| ID kid, mg/g h0.5 R2 Δq, % | 72.42 0.572 10.64 | 75.53 0.586 10.28 | 82.12 0.607 9.882 | 24.20 0.525 10.67 | 26.96 0.552 10.41 | 27.64 0.575 10.31 | 14.62 0.634 10.64 | 15.67 0.535 10.47 | 16.77 0.538 10.67 |
| ED kext, h−1 R2 Δq, % | 3 × 106 0.000 0.337 | 1 × 106 0.000 4.280 | 2 × 106 0.000 6.555 | 1 × 106 0.472 6.435 | 5 × 108 0.273 3.027 | 2 × 107 0.081 0.282 | 0.843 0.801 6.062 | 1.274 0.921 2.805 | 1.879 0.988 0.044 |
| Model | 0.1 g/L | 0.3 g/L | 0.5 g/L | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 °C | 45 °C | 60 °C | 30 °C | 45 °C | 60 °C | 30 °C | 45 °C | 60 °C | |
| PFO qmax, mg/g k1, h−1 R2 Δq, % | 149.5 3.410 1.000 0.055 | 158.5 2.874 0.999 0.147 | 167.9 2.916 0.999 0.144 | 36.83 2.655 0.999 0.478 | 43.61 2.714 0.999 0.381 | 50.18 3.128 0.999 0.119 | 30.29 2.761 0.999 0.129 | 31.64 2.946 1.000 0.109 | 33.51 3.083 1.000 0.016 |
| PSO qmax, mg/g k2, h−1 R2 Δq, % | 149.3 2 × 106 0.999 0.110 | 158.1 3 × 106 0.999 0.239 | 167.63 3 × 106 0.999 0.231 | 36.71 4 × 106 0.999 0.605 | 43.48 1 × 106 0.999 0.498 | 50.10 1 × 106 0.999 0.498 | 30.21 1 × 107 0.999 0.239 | 31.56 9 × 106 0.999 0.192 | 33.45 7 × 106 0.999 0.039 |
| Avrami qmax, mg/g kA, h−1 nA R2 Δq, % | 149.5 3.801 0.897 1.000 0.874 | 158.5 3.483 0.825 0.999 0.501 | 168.0 3.587 0.813 0.999 1.005 | 74.95 3.514 0.967 1.000 0.981 | 36.83 3.026 0.877 0.999 0.387 | 43.60 3.080 0.881 0.999 0.541 | 30.29 2.790 0.989 0.999 0.365 | 31.64 3.052 0.965 1.000 0.248 | 33.50 3.202 0.963 0.999 0.111 |
| ID kid, mg/g h0.5 R2 Δq, % | 62.93 0.527 10.70 | 66.73 0.541 10.55 | 70.70 0.538 10.54 | 15.51 0.558 10.15 | 18.36 0.553 10.26 | 21.12 0.533 10.58 | 12.75 0.541 10.54 | 13.31 0.536 10.58 | 14.11 0.539 10.49 |
| ED kext, h−1 R2 Δq, % | 2 × 106 0.000 13.73 | 4 × 106 0.000 10.50 | 1 × 106 0.000 7.607 | 285.5 0.987 21.01 | 113.6 0.908 32.20 | 92.11 0.802 13.64 | 0.340 0.518 10.39 | 0.434 0.594 9.608 | 0.653 0.720 7.522 |
| Adsorbent | q, mg/g | Dyes | Reference |
|---|---|---|---|
| Nymphaea alba | 9.66 | Phenol Red | [3] |
| Diplazium esculentum stems | 44.88 | Gentian Violet | [12] |
| Water Lily roots | 42.65 | Methylene Blue | [20] |
| Raw corn stalk biochar | 325.1 387.7 | Methylene Blue Gentian Violet | [24] |
| Eichhornia crassipes/ZnO NP | 40 39.9 | C. I: Blue Red 43 C. I. Basic Blue 3 | [27] |
| Metroxylon sagu | 238.82 | Methylene Blue | [31] |
| Sulfanated carbon from Eichhornia crassipes | 19.5 | Methylene Blue | [32] |
| Eichhornia crassipes/NC | 20.83 | Methylene Blue | [34] |
| Eichhornia crassipes untreatment | 98.8 87.7 44.4 62.8 | Methylene Blue Crystal Violet Phenol Red Methyl Orange | [35] |
| Eichhornia crassipes roots | 9.95 | Methylene Blue | [37] |
| Eichhornia crassipes untreatment | 161.64 | Methylene Blue | [38] |
| Hordeum vulgare L. | 3.50 | Phenol Red | [42] |
| Eichhornia crassipes treated with water | 200.5 193.5 | Methylene Blue Methyl Orange | [49] |
| Eichhornia crassipes treated with NaOH | 226.5 158.5 | Methylene Blue Methyl Orange | [49] |
| Eichhornia crassipes treated with water | 73.78 196.1 | Phenol Red Gentian Violet | This study |
| Eichhornia crassipes treated with NaOH | 47.94 167.9 | Phenol Red Gentian Violet |
| Bioadsorbent | wt.% | ||||
|---|---|---|---|---|---|
| C | O | Al | Si | Ca | |
| WLW | 59.64 | 38.05 | 0.20 | 0.72 | 2.08 |
| NWL | 62.16 | 26.49 | 0.18 | 0.52 | 0.71 |
| WLW-PR | 56.67 | 39.33 | 0.44 | 1.47 | 2.16 |
| NWL-PR | 48.51 | 43.21 | 0.79 | 2.17 | 0.28 |
| WLW-GV | 48.35 | 45.95 | 0.30 | 3.05 | 1.64 |
| NWL-GV | 50.61 | 45.99 | 1.01 | 0.52 | 1.24 |
| Biomaterials | a0, nm |
|---|---|
| WLW | 0.829 |
| NWL | 0.824 |
| WLW-PR | 0.856 |
| NLW-PR | 0.815 |
| WLW-GV | 0.849 |
| NWL-GV | 0.813 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Ahumada, E.; Salazar-Hernández, M.; Talavera-López, A.; Solis-Marcial, O.J.; Hernández-Soto, R.; Ruelas-Leyva, J.P.; Hernández, J.A. Removal of Anionic and Cationic Dyes Present in Solution Using Biomass of Eichhornia crassipes as Bioadsorbent. Molecules 2022, 27, 6442. https://doi.org/10.3390/molecules27196442
López-Ahumada E, Salazar-Hernández M, Talavera-López A, Solis-Marcial OJ, Hernández-Soto R, Ruelas-Leyva JP, Hernández JA. Removal of Anionic and Cationic Dyes Present in Solution Using Biomass of Eichhornia crassipes as Bioadsorbent. Molecules. 2022; 27(19):6442. https://doi.org/10.3390/molecules27196442
Chicago/Turabian StyleLópez-Ahumada, Eunice, Mercedes Salazar-Hernández, Alfonso Talavera-López, O. J. Solis-Marcial, Rosa Hernández-Soto, Jose P. Ruelas-Leyva, and José Alfredo Hernández. 2022. "Removal of Anionic and Cationic Dyes Present in Solution Using Biomass of Eichhornia crassipes as Bioadsorbent" Molecules 27, no. 19: 6442. https://doi.org/10.3390/molecules27196442