A Box–Behnken Extraction Design and Hepatoprotective Effect of Isolated Eupalitin-3-O-β-D-Galactopyranoside from Boerhavia diffusa Linn.
Abstract
:1. Introduction
2. Results
2.1. Extract and Percentage Yield of Eupalitin-3-O-β-D-Galactopyranoside
2.2. Development of the Mobile Phase
2.3. Method of Validation
2.4. Box–Behnken Design-Experiment
2.5. Effect of Independent Factors on Eupalitin-3-O-β-D-Galactopyranoside (Y1)
2.6. Identification of Eupalitin-3-O-β-D-Galactopyranoside
2.7. In Vitro Cytotoxicity Study of Eupalitin-3-O-β-D-Galactopyranoside
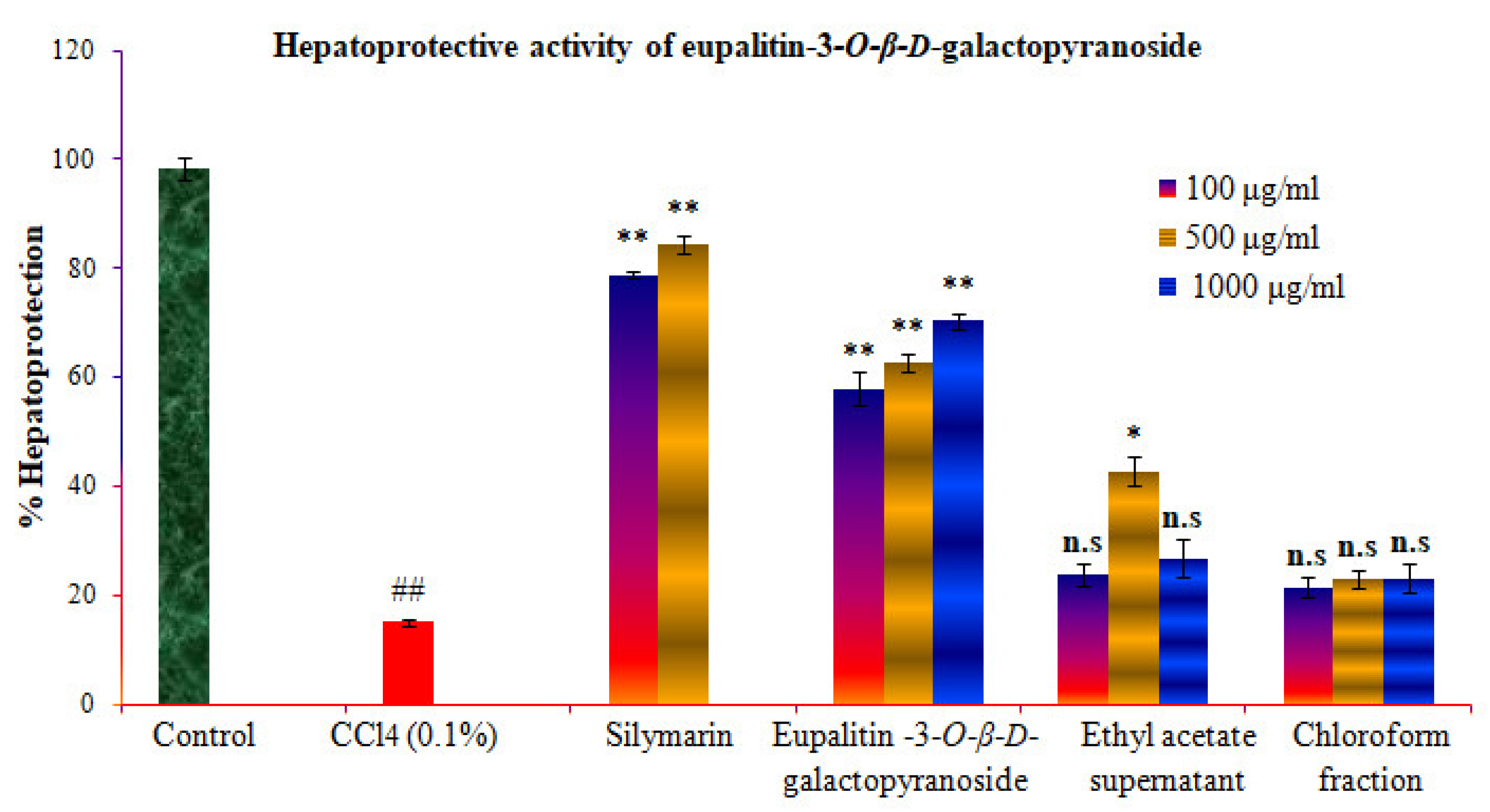
2.8. Hepatoprotective Activity of Eupalitin-3-O-β-D-Galactopyranoside
3. Discussion
4. Materials and Methods
4.1. Chemicals and Equipment
4.2. Collection of Plant Materials
4.3. HPTLC Instrumentation
4.3.1. Extraction of Boerhavia diffusa
4.3.2. Preparation of Standard Solutions
4.3.3. Preparation of the Plate
4.3.4. Chromatography Parameters
4.3.5. Calibration Curve of Eupalitin-3-O-β-D-Galactopyranoside
4.3.6. Analytical Method Evaluation
4.4. Optimization of Eupalitin-3-O-β-D-Galactopyranoside in Hydro-Alcoholic Extracts
4.5. Extraction and Isolation of Eupalitin-3-O-β-D-Galactopyranoside
4.6. Hepatoprotective Activity of Eupalitin-3-O-β-D-Galactopyranoside
4.7. Statistical Analysis of Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Srivastava, R.; Saluja, D.; Dwarakanath, B.S.; Chopra, M. Inhibition of Human Cervical Cancer Cell Growth by Ethanolic Extract of Boerhaviadiffusa Linn. (Punarnava) Root. Evid. Based Complement. Altern. Med. 2011, 2011, 42703. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Baxi, D.; Doshi, A.; Ramachandran, A.V. Antihyperglycaemic and renoprotective effect of Boerhaavia diffusa L. in experimental diabetic rats. J. Complement. Integr. Med. 2011, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Valecha, R. Evidence for involvement of the monoaminergic system in antidepressant-like activity of an ethanol extract of Boerhaavia diffusa and its isolated constituent, punarnavine, in mice. Pharm. Biol. 2014, 52, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Gairola, S.; Gaur, R.D.; Painuli, R.M.; Siddiqi, T.O. Ethnomedicinal plants used for treating epilepsy by indigenous communities of sub-Himalayan region of Uttarakhand, India. J. Ethnopharmacol. 2013, 150, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Bharali, R.; Azad, M.R.; Tabassum, J. Chemopreventive action of Boerhaavia diffusa on DMBA-induced skin carcinogenesis in mice. Indian J.Physiol.Pharmacol. 2003, 47, 459–464. [Google Scholar]
- Vyas, B.A.; Desai, N.Y.; Patel, P.K.; Joshi, S.V.; Shah, D.R. Effect of Boerhaavia diffusa in experimental prostatic hyperplasia in rats. Indian J. Pharmacol. 2013, 45, 264–269. [Google Scholar] [CrossRef]
- Ahmed-Belkacem, A.; Macalou, S.; Borrelli, F.; Capasso, R.; Fattorusso, E.; Taglialatela-Scafati, O.; Di Pietro, A. Nonprenylated rotenoids, a new class of potent breast cancer resistance protein inhibitors. J. Med. Chem. 2007, 50, 1933–1938. [Google Scholar] [CrossRef]
- Aher, V.; Chattopadhyay, P.; Goyary, D.; Veer, V. Evaluation of the genotoxic and antigenotoxic potential of the alkaloid punarnavine from Boerhaavia diffusa. Planta Med. 2013, 79, 939–945. [Google Scholar] [CrossRef]
- Sreeja, S.; Sreeja, S. An in-vitro study on antiproliferative and antiestrogenic effects of Boerhaavia diffusa L. extracts. J. Ethnopharmacol. 2009, 126, 221–225. [Google Scholar] [CrossRef]
- Apu, A.S.; Liza, M.S.; Jamaluddin, A.T.; Howlader, M.A.; Saha, R.K.; Rizwan, F.; Nasrin, N. Phytochemical screening and in vitro bioactivities of the extracts of aerial part of Boerhavia diffusa Linn. Asian Pac. J.Trop. Biomed. 2012, 2, 673–678. [Google Scholar] [CrossRef]
- Manu, K.A.; Kuttan, G. Boerhaavia diffusa stimulates cell-mediated immune response by upregulating IL-2 and downregulating the pro-inflammatory cytokines and GM-CSF in B16F-10 metastatic melanoma bearing mice. J. Exp. Ther. Oncol. 2008, 7, 17–29. [Google Scholar] [PubMed]
- Manu, K.A.; Kuttan, G. Anti-metastatic potential of Punarnavine, an alkaloid from Boerhaavia diffusa Linn. Immunobiology 2009, 214, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Satheesh, M.A.; Pari, L. Antioxidant effect of Boerhavia diffusa L. in tissues of alloxan induced diabetic rats. Indian J. Exp. Biol. 2004, 42, 989–992. [Google Scholar] [PubMed]
- Yasir, F.; Waqar, M.A. Effect of indigenous plant extracts on calcium oxalate crystallization having a role in urolithiasis. Urol. Res. 2011, 39, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Lami, N.; Kadota, S.; Kikuchi, T.; Momose, Y. Constituents of the roots of Boerhaavia diffusa L. III. Identification of Ca2+ channel antagonistic compound from the methanol extract. Chem. Pharma. Bull. 1991, 39, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Vineetha, V.P.; Prathapan, A.; Soumya, R.S.; Raghu, K.G. Arsenic trioxide toxicity in H9c2 myoblasts damage to cell organelles and possible amelioration with Boerhavia diffusa. Cardiovasc. Toxicol. 2013, 13, 123–137. [Google Scholar] [CrossRef]
- Bairwa, K.; Singh, I.N.; Roy, S.K.; Grover, J.; Srivastava, A.; Jachak, S.M. Rotenoids from Boerhaavia diffusa as potential anti-inflammatory agents. J. Nat. Prod. 2013, 76, 1393–1398. [Google Scholar] [CrossRef]
- Dey, D.; Chaskar, S.; Bhatt, N.; Chitre, D. Hepatoprotective Activity of BV-7310, a Proprietary Herbal Formulation of Phyllanthus niruri, Tephrosia purpurea, Boerhaviadiffusa and Andrographis paniculata, in Alcohol-Induced HepG2 Cells and Alcohol plus a Haloalkane, CCl4, Induced Liver Damage in Rats. Evid. Based Complement. Altern. Med. 2020, 9, 6428906. [Google Scholar]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Pandey, R.; Maurya, R.; Singh, G.; Sathiamoorthy, B.; Naik, S. Immunosuppressive properties of flavonoids isolated from Boerhaviadiffusa Linn. Int. Immunopharmacol. 2005, 3, 541–553. [Google Scholar] [CrossRef]
- Grijalva-Guiza, R.E.; Jimenez-Garduno, A.M.; Hernandez, L.R. Potential Benefits of Flavonoids on the Progression of Atherosclerosis by Their Effect on Vascular Smooth Muscle Excitability. Molecules 2021, 26, 3557. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE 2012, 7, 49493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maaliki, D.; Shaito, A.A.; Pintus, G.; El-Yazbi, A.; Eid, A.H. Flavonoids in hypertension: A brief review of the underlying mechanisms. Curr. Opin. Pharmacol. 2019, 45, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Ascione, V.; Capasso, R.; Izzo, A.A.; Fattorusso, E.; Taglialatela-Scafati, O. Spasmolytic effects of nonprenylated rotenoid constituents of Boerhaviadiffusa roots. J. Nat. Prod. 2006, 69, 903–906. [Google Scholar] [CrossRef]
- Vairetti, M.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Ferrigno, A.; Berardo, C. Changes in Glutathione Content in Liver Diseases: An Update. Antioxidants 2021, 10, 364. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Kadota, S.; Namba, T.; Miyahara, T.; Khan, U.G. Effects on cultured neonatal mouse calvaria of the flavonoids isolated from BoerhaviaRepens. J. Nat. Prod. 1996, 59, 1015–1018. [Google Scholar] [CrossRef]
- Ferreres, F.; Sousa, C.; Justin, M.; Valentão, P.; Andrade, P.B.; Llorach, R.; Rodrigues, A.; Seabra, R.M.; Leitao, A. Characterisation of the phenolic profile of Boerhaviadiffusa L. by HPLC-PAD-MS/MS as a tool for quality control. Phytochem. Anal. 2005, 16, 451–458. [Google Scholar] [CrossRef]
- Conover, C.A.; Lee, P.D. Insulin regulation of insulin-like growth factor-binding protein production in cultured HepG2 cells. J. Clin. Endocrinol. Metab. 1990, 70, 1062–1067. [Google Scholar] [CrossRef]
- Sassa, S.; Sugita, O.; Galbraith, R.A.; Kappas, A. Drug metabolism by the human hepatoma cell, HepG2. Biochem. Biophys. Res. Commun. 1987, 143, 52–57. [Google Scholar] [CrossRef]
- Bouma, M.E.; Rogier, E.; Verthier, N.; Labarre, C.; Feldmann, G. Further cellular investigation of the human hepatoblastoma-derived cell line HepG2: Morphology and immunocytochemical studies of hepatic-secreted proteins. In Vitro Cell. Dev. Biol. 1989, 25, 267–275. [Google Scholar] [CrossRef]
- Pareek, A.; Godavarthi, A.; Issarani, R.; Nagori, B.P. Antioxidant and hepatoprotective activity of Fagonia schweinfurthii (Hadidi) Hadidi extract in carbon tetrachloride induced hepatotoxicity in HepG2 cell line and rats. J. Ethnopharmacol. 2013, 150, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Krithika, R.; Mohankumar, R.; Verma, R.J.; Shrivastav, P.S.; Mohamad, I.L.; Gunasekaran, P.; Narasimhan, S. Isolation, characterization and antioxidative effect of phyllanthin against CCl4-induced toxicity in HepG2 cell line. Chem. Biol. Interact. 2009, 181, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.T.; Minsky, N.W.; Espinosa, L.E.; Aranda, R.S.; Meseguer, J.P.; Perez, P.C. In vitro assessment of hepatoprotective agents against damage induced by acetaminophen and CCl4. Evid. Based Complement Altern. Med. 2017, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Navabha, J.; Verma, D.L. Eupalitin-3-O-α-rhamnosyl (1→2) α-rhamnosyl (1→6)-β-D-galactopyranoside From The Aerial Parts Of Punarnava. IJCA 2013, 5, 267–273. [Google Scholar]
- Maurya, R.; Sathiamoorthy, B.; Deepak, M. Flavonoids and phenol glycosides from BoerhaviaDiffusa. Nat. Prod. Res. 2007, 2, 126–134. [Google Scholar] [CrossRef]
- Gomes, A.D.; Vaidya, V.V.; Bhagat, R.D.; Gadgil, J.N. Separation and quantification of pharmacologically active markers boeravinone B, eupalitin-3-o-β-d galactopyranoside and β-sitosterol from Boeravia diffusa Linn. and from marketed formulation. Int. J. Pharm. Pharm. Sci. 2013, 5, 953–956. [Google Scholar]
- Ilyas, U.; Katare, D.P.; Aeri, V.; Naseef, P.P. A Review on Hepatoprotective and Immunomodulatory Herbal Plants. Pharmacogn. Rev. 2016, 10, 66–70. [Google Scholar]
- Pandey, M.M.; Rastogi, S.; Rawat, A.K. Indian traditional Ayurvedic system of medicine and nutritional supplementation. Evid. Based Complement Altern. Med. 2013, 2013, 376327. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef]
- Chandan, B.K.; Sharma, A.K.; Anand, K.K. Boerhaviadiffusa: A study of its hepatoprotective activity. J. Ethnopharmacol. 1991, 3, 299–307. [Google Scholar] [CrossRef]
- Karimi, G.; Vahabzadeh, M.; Lari, P.; Rashedinia, M.; Moshiri, M. “Silymarin”, a promising pharmacological agent for treatment of diseases. Iran. J. Basic Med. Sci. 2011, 14, 308–317. [Google Scholar] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 8, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vizcaino, F.; Cesar, G.F. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef] [PubMed]
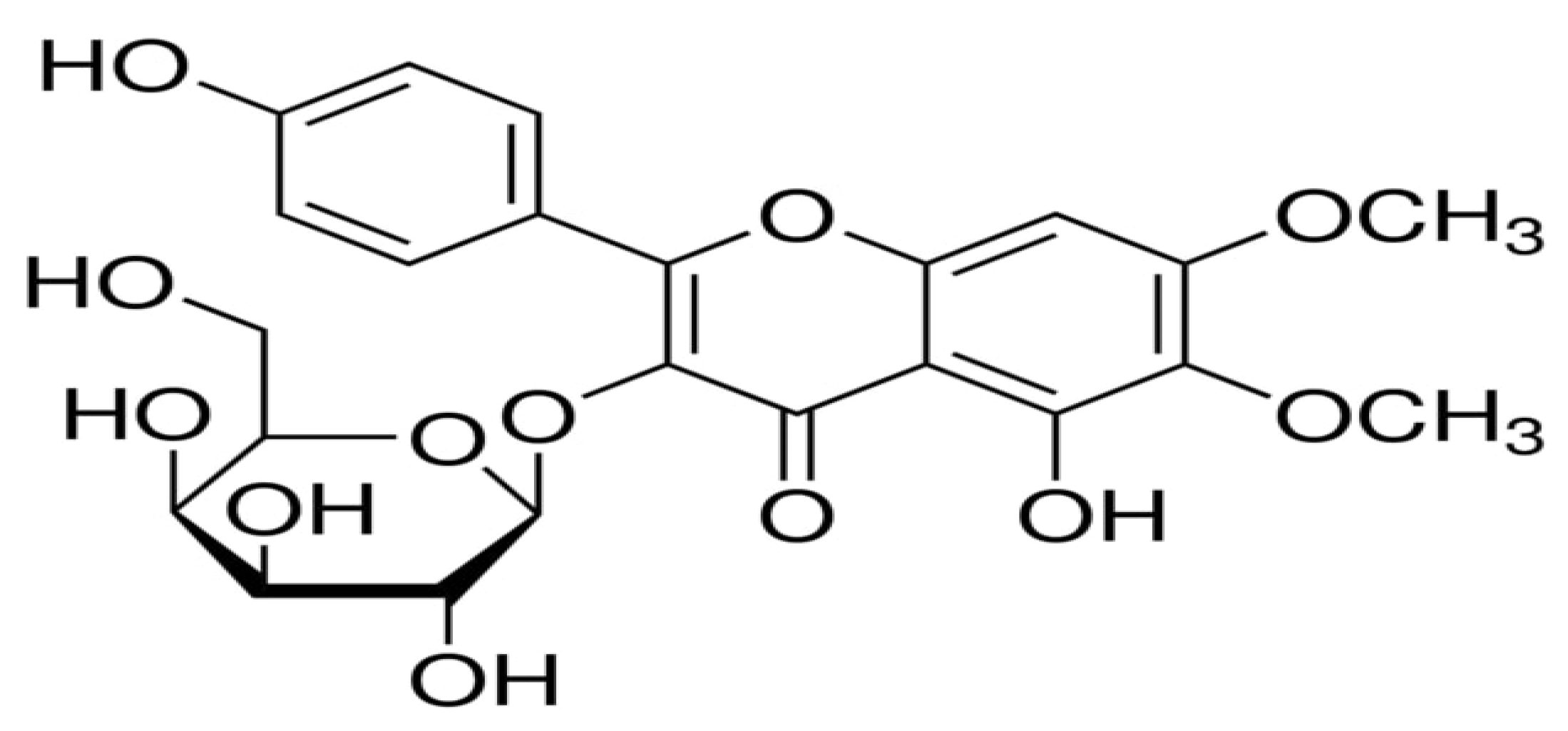
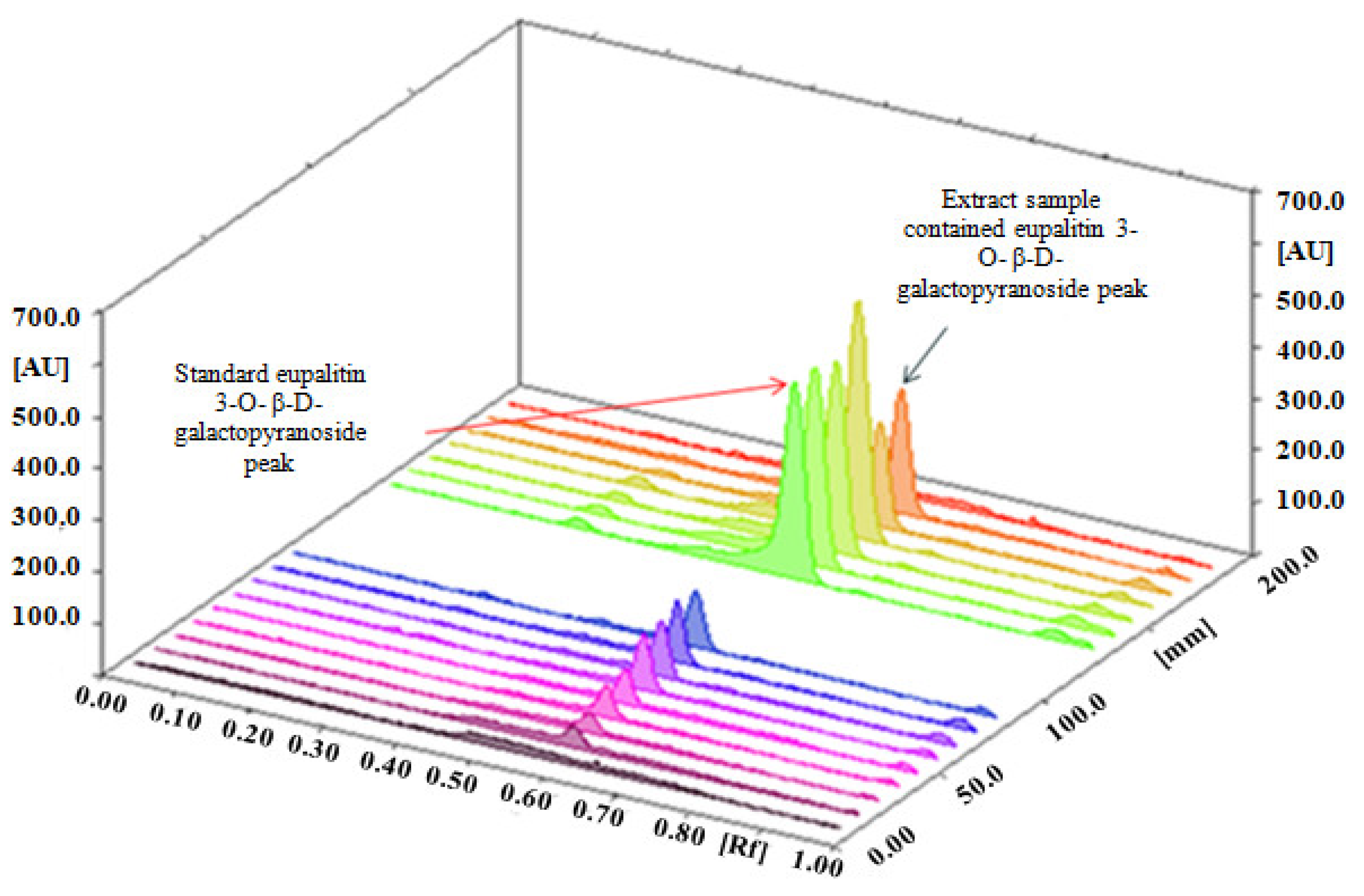


| Parameters | Eupalitin-3-O-β-D-Galactopyranoside |
|---|---|
| LOD (ng) | 30 ng |
| LOQ (ng) | 100 ng |
| Specificity | Specific |
| Inter-day Precision (% RSD) | 0.67 |
| Intra-day precision(% RSD) | 0.991 |
| Regression equation | Y = 942.204 + 2.436 X |
| Linearity Range (Concentration) | 100–5000 ng |
| Average recovery % RSD | 99.78 |
| Rf value | 0.56 |
| Correlation coefficient | 0.9984 |
| Drugs | Models F Value | R2 | Adjusted R2 | Predicted R2 | SD | C.V.% |
|---|---|---|---|---|---|---|
| Eupalitin 3-O-β-D-galactopyranoside (Y1) | Linear | 0.540 | 0.4341 | 0.0799 | 0.00490 | - |
| 2F1 | 0.890 | 0.7761 | 0.0181 | 0.00281 | - | |
| Cubic | 0.960 | 0.8231 | - | 0.00310 | - | |
| Quadratic | 0.8990 | 0.8200 | 0.5941 | 0.00281 | 8.04 |
| Conc. (µg/mL) | Control | CCl4 (0.1%) | Standard Silymarin + CCl4 | Eupalitin-3-O-β-D-Galactopyranoside + CCl4 | Supernatant Ethyl Acetate + CCl4 | Chloroform + CCl4 |
|---|---|---|---|---|---|---|
| 100 | 97.1 ± 2.2 | 15.2 ± 0.6 ## | 80.12 ± 0.7 ** | 57.82 ± 3 ** | 23.84 ± 2.1 ns | 21.32 ± 1.9 ns |
| 500 | - | - | 86.94 ± 0.5 ** | 62.6 ± 1.5 ** | 42.7 ± 2.8 * | 22.99 ± 1.6 ns |
| 1000 | - | - | - | 70.23 ± 1.5 ** | 26.66 ± 3.4 ns | 22.95 ± 2.6 ns |
| Factors Independent Variables | Levels Used | ||
|---|---|---|---|
| Low (−1) | Medium | High (+1) | |
| A1 = Time in min | 30 | 60 | 90 |
| B2 = Temperature (°C) | 30 | 45 | 60 |
| C3 = Solvent ratio (% v/v) | 40 | 60 | 80 |
| Dependent Variables | Goals | ||
| Y1 = Eupalitin-3-O-β-D-galactopyranoside | Maximized | ||
| Run | Factor-1 (A1): Time (Min) | Factor-2 (B2): Temperature (°C) | Factor-3 (C3): Solvent Ratio (%) | % Yield Eupalitin-3-O-β-D-Galactopyranoside (Y1) |
|---|---|---|---|---|
| 01 | 30 | 30 | 60 | 0.0331 |
| 02 | 90 | 30 | 60 | 0.0234 |
| 03 | 30 | 60 | 60 | 0.0381 |
| 04 | 90 | 60 | 60 | 0.0429 |
| 05 | 30 | 45 | 40 | 0.0369 |
| 06 | 90 | 45 | 40 | 0.0259 |
| 07 | 30 | 45 | 80 | 0.0339 |
| 08 | 90 | 45 | 80 | 0.042 |
| 09 | 60 | 30 | 40 | 0.022 |
| 10 | 60 | 60 | 40 | 0.041 |
| 11 | 60 | 30 | 60 | 0.0369 |
| 12 | 60 | 60 | 80 | 0.0389 |
| 13 | 60 | 45 | 60 | 0.0351 |
| 14 | 60 | 45 | 60 | 0.0351 |
| 15 | 60 | 45 | 60 | 0.0339 |
| 16 | 60 | 45 | 60 | 0.0341 |
| 17 | 60 | 45 | 60 | 0.0359 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thajudeen, K.Y.; Asiri, Y.I.; Salam, S.; Thorakkattil, S.A.; Rahamathulla, M.; Uoorakkottil, I. A Box–Behnken Extraction Design and Hepatoprotective Effect of Isolated Eupalitin-3-O-β-D-Galactopyranoside from Boerhavia diffusa Linn. Molecules 2022, 27, 6444. https://doi.org/10.3390/molecules27196444
Thajudeen KY, Asiri YI, Salam S, Thorakkattil SA, Rahamathulla M, Uoorakkottil I. A Box–Behnken Extraction Design and Hepatoprotective Effect of Isolated Eupalitin-3-O-β-D-Galactopyranoside from Boerhavia diffusa Linn. Molecules. 2022; 27(19):6444. https://doi.org/10.3390/molecules27196444
Chicago/Turabian StyleThajudeen, Kamal Y., Yahya I. Asiri, Shahana Salam, Shabeer Ali Thorakkattil, Mohamed Rahamathulla, and Ilyas Uoorakkottil. 2022. "A Box–Behnken Extraction Design and Hepatoprotective Effect of Isolated Eupalitin-3-O-β-D-Galactopyranoside from Boerhavia diffusa Linn." Molecules 27, no. 19: 6444. https://doi.org/10.3390/molecules27196444
APA StyleThajudeen, K. Y., Asiri, Y. I., Salam, S., Thorakkattil, S. A., Rahamathulla, M., & Uoorakkottil, I. (2022). A Box–Behnken Extraction Design and Hepatoprotective Effect of Isolated Eupalitin-3-O-β-D-Galactopyranoside from Boerhavia diffusa Linn. Molecules, 27(19), 6444. https://doi.org/10.3390/molecules27196444






