Chemical Composition, Antioxidant and Antiproliferative Activities of Taraxacum officinale Essential Oil
Abstract
1. Introduction
2. Results
2.1. Chemical Analysis
2.2. Total Phenolic Compounds
2.3. Antioxidant Activity
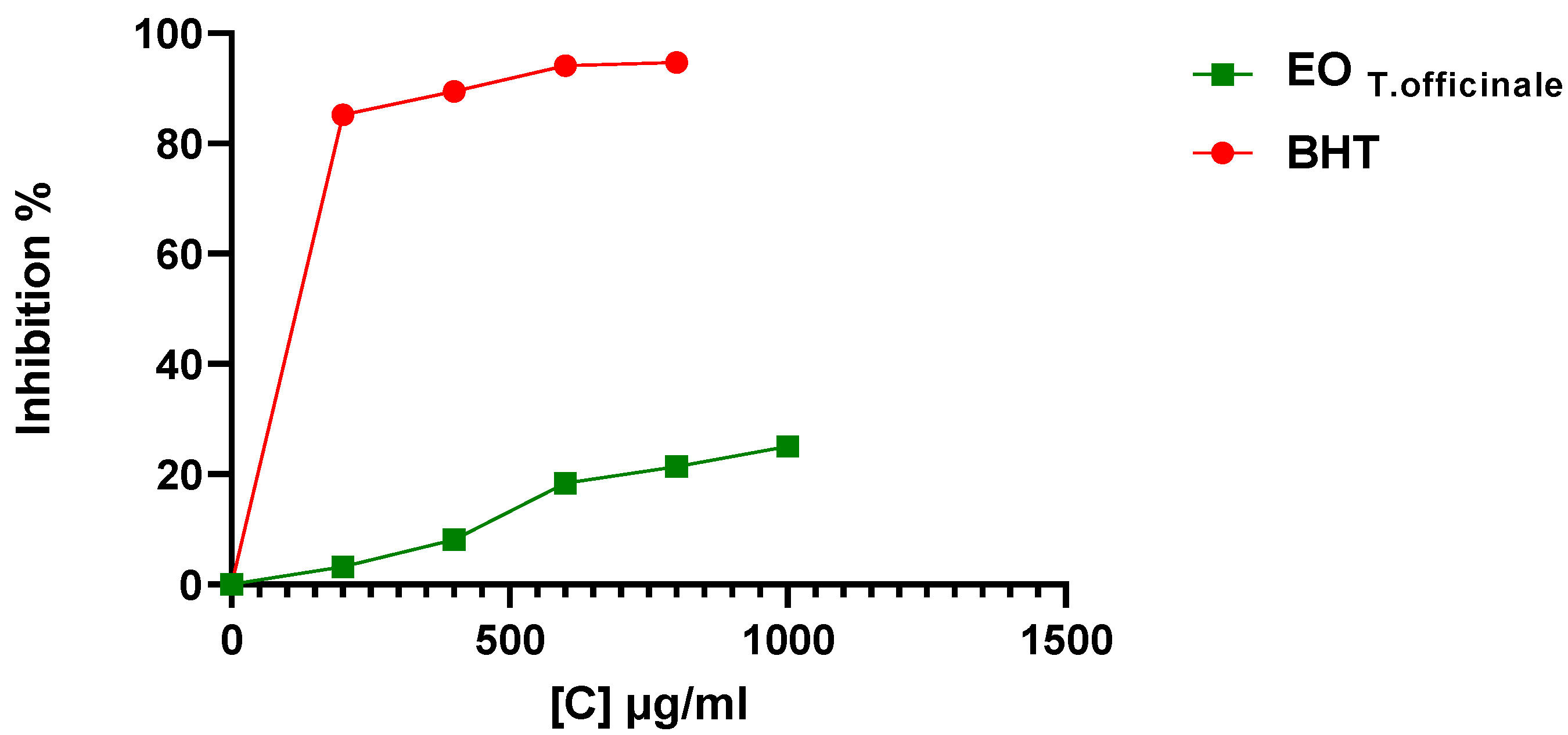
2.3.1. DPPH Assay
2.3.2. Reducing Power Assay
2.4. In Vivo Antioxidant Activity
2.4.1. Effects of Treatments on Body Weight of Treated Mice
2.4.2. Oxidant Stress Parameters Analysis
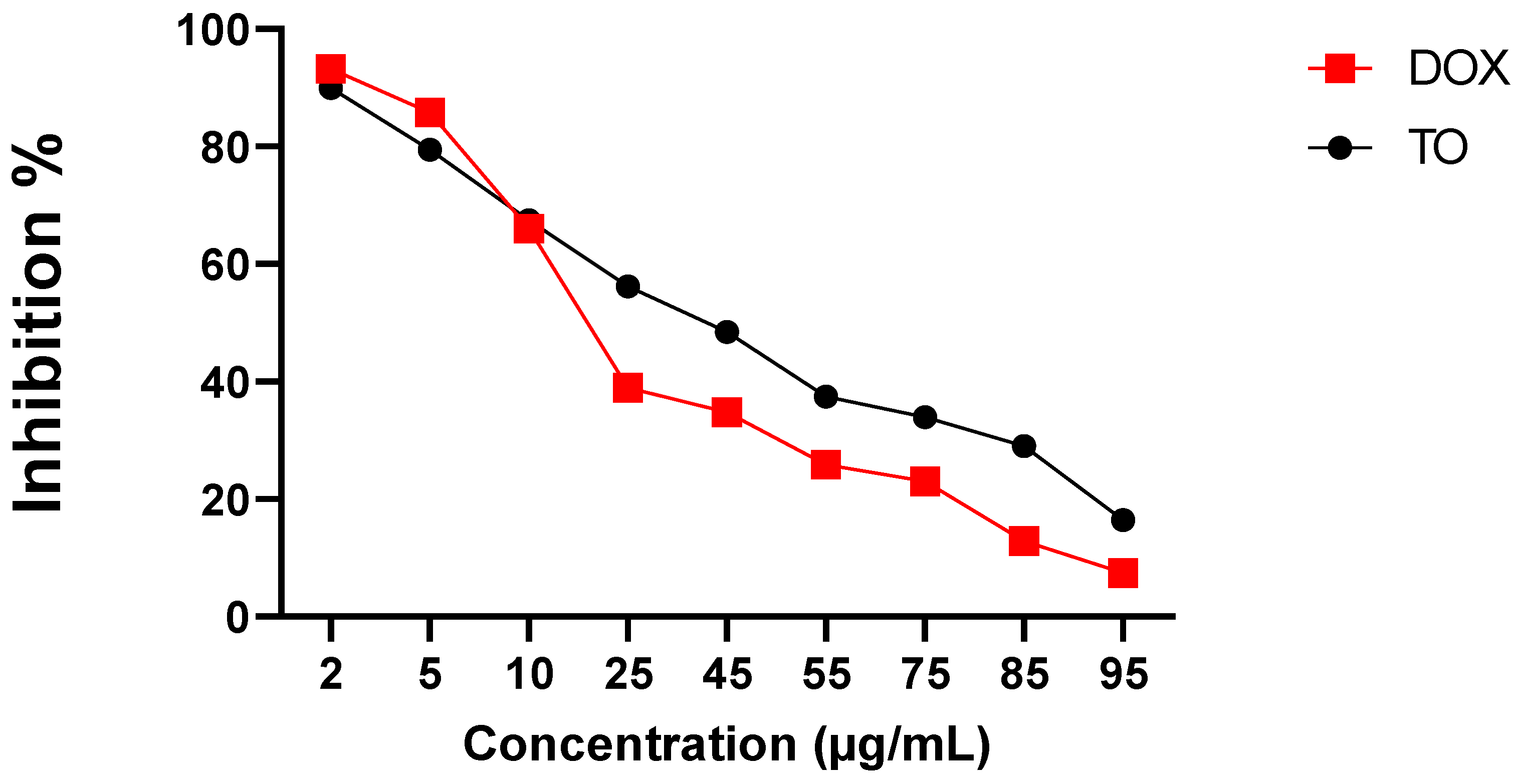
2.5. Cytotoxic Activity
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. Isolation of Essential Oil
4.3. Chemicals and Reagents
4.4. Gas Chromatography–Mass Spectrometry of Essential Oil
4.5. Determination of Total Phenolic Content
4.6. In Vitro Antioxidant Activity
DPPH and Reducing Power (RP) Assays
4.7. In Vivo Antioxidant Activity
4.7.1. Animal Models and Induction of Oxidative Stress
4.7.2. Experimental Design
- Group I was designated as vehicle and was treated with 0.1% CMC;
- Group II (negative control) received no treatment but had free access to water and food;
- Groups III (toxic control), IV, V, and VI received a single intraperitoneal injection of acetaminophen (APAP) (400 mg/kg, ip) before the start of the experiment to induce hepato-renal oxidative injury;
- Group IV served as the standard and received AA, 200 mg/kg body weight;
- Groups V and VI received TO EO at doses of 600 and 1200 mg/kg body weight.
4.7.3. Preparation of Tissue Homogenates
4.7.4. Body and Organ Weights
4.7.5. Quantification of Oxidative Stress Biomarkers in Tissue Homogenates
4.8. Cytotoxic Assay
4.8.1. Sample Preparation
4.8.2. Cellular Assays
Cell Lines
4.8.3. Cytotoxic Assay
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juliani, H.R.; Koroch, A.R.; Simon, J.E. Chemical diversity of essential oils of Ocimum species and their associated antioxidant and antimicrobial activity. In Essential Oils and Aromas: Green Extractions and Applications; Har Krishan Bhalla & Sons: Dehradun, India, 2009. [Google Scholar]
- Gautam, N.; Mantha, A.K.; Mittal, S. Essential oils and their constituents as anticancer agents: A mechanistic view. BioMed Res. Int. 2014, 2014, 154106. [Google Scholar] [CrossRef]
- Tayarani-Najaran, Z.; Talasaz-Firoozi, E.; Nasiri, R.; Jalali, N.; Hassanzadeh, M.K. Antiemetic activity of volatile oil from Mentha spicata and Mentha× piperita in chemotherapy-induced nausea and vomiting. Ecancermedicalscience 2013, 7, 290. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- TNAU Agritech. Extraction Methods of Natural Essential Oils. Available online: https://agritech.tnau.ac.in/horticulture/extraction_methods_natural_essential_oil.pdf (accessed on 7 September 2022).
- Bylka, W.; Matlawska, I.; Frański, R. Essential oil composition of Taraxacum officinale. Acta Physiol. Plant. 2010, 32, 231–234. [Google Scholar] [CrossRef]
- Mingarro, D.M.; Plaza, A.; Galán, A.; Vicente, J.A.; Martínez, M.P.; Acero, N. The effect of five Taraxacum species on in vitro and in vivo antioxidant and antiproliferative activity. Food Funct. 2015, 6, 2787–2793. [Google Scholar] [CrossRef] [PubMed]
- Réthy, B.; Csupor-Löffler, B.; Zupkó, I.; Hajdú, Z.; Máthé, I.; Hohmann, J.; Rédei, T.; Falkay, G. Antiproliferative activity of Hungarian Asteraceae species against human cancer cell lines. Part I. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Mondino, A.; Yaneselli, K.; Ingold, A.; Echeverry, C.; Raffaelli, S.; Vázquez, Á.; García y Santos, C. Cytotoxic effect of Senecio madagascariensis (Asteraceae) extracts on cancer derived cell lines. Agrociencia Urug. 2022, 26, e425. [Google Scholar] [CrossRef]
- Aghraz, A.; Gonçalves, S.; Rodríguez-Solana, R.; Dra, L.A.; Di Stefano, V.; Dugo, G.; Cicero, N.; Larhsini, M.; Markouk, M.; Romano, A. Antioxidant activity and enzymes inhibitory properties of several extracts from two Moroccan Asteraceae species. S. Afr. J. Bot. 2018, 118, 58–64. [Google Scholar] [CrossRef]
- Vidic, D.; Ćavar Zeljković, S.; Dizdar, M.; Maksimović, M. Essential oil composition and antioxidant activity of four Asteraceae species from Bosnia. J. Essent. Oil Res. 2016, 28, 445–457. [Google Scholar] [CrossRef]
- Abdolmaleki, Z.; Arab, H.A.; Amanpour, S.; Muhammadnejad, S. Anti-angiogenic effects of ethanolic extract of Artemisia sieberi compared to its active substance, artemisinin. Rev. Bras. Farmacogn. 2016, 26, 326–333. [Google Scholar] [CrossRef][Green Version]
- Asif, M.; Saadullah, M.; Yaseen, H.S.; Saleem, M.; Yousaf, H.M.; Khan, I.U.; Yaseen, M.; Shams, M.U. Evaluation of in vivo anti-inflammatory and anti-angiogenic attributes of methanolic extract of Launaea spinosa. Inflammopharmacology 2020, 28, 993–1008. [Google Scholar] [CrossRef]
- Shakeri, A.; Amini, E.; Asili, J.; Masullo, M.; Piacente, S.; Iranshahi, M. Screening of several biological activities induced by different sesquiterpene lactones isolated from Centaurea behen L. and Rhaponticum repens (L.) Hidalgo. Nat. Prod. Res. 2018, 32, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Wirngo, F.E.; Lambert, M.N.; Jeppesen, P.B. The physiological effects of Dandelion (Taraxacum officinale) in type 2 Diabetes. Rev. Diabet. Stud. 2016, 13, 113–131. [Google Scholar] [CrossRef]
- Brock, M.T. The potential for genetic assimilation of a native dandelion species, Taraxacum ceratophorum (Asteraceae), by the exotic congener T. officinale. Am. J. Bot. 2004, 91, 656–663. [Google Scholar] [CrossRef]
- Escudero, N.L.; de Arellano, M.L.; Fernandez, S.; Albarracin, G.; Mucciarelli, S. Taraxacum officinale as a food source. Plant Foods Hum. Nutr. 2003, 58, 1–10. [Google Scholar] [CrossRef]
- Choi, U.K.; Lee, O.H.; Yim, J.H.; Cho, C.W.; Rhee, Y.K.; Lim, S.I.; Kim, Y.C. Hypolipidemic and antioxidant effects of dandelion (Taraxacum officinale) root and leaf on cholesterol-fed rabbits. Int. J. Mol. Sci. 2010, 11, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Zafar, R. Simultaneous estimation of taraxerol and taraxasterol in root callus cultures of Taraxacum officinale Weber. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 540–546. [Google Scholar]
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse biological activities of dandelion. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Won, Y.K.; Ong, C.N.; Shen, H.M. Anti-cancer potential of sesquiterpene lactones: Bioactivity and molecular mechanisms. Curr. Med. Chem. Anti-Cancer Agents 2005, 5, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, W.; Zhang, M.; Chen, C. Taraxasterol suppresses inflammation in IL-1β-induced rheumatoid arthritis fibroblast-like synoviocytes and rheumatoid arthritis progression in mice. Int. Immunopharmacol. 2019, 70, 274–283. [Google Scholar] [CrossRef]
- Moon, D.O.; Lee, K.J.; Choi, Y.H.; Kim, G.Y. β-Sitosterol-induced-apoptosis is mediated by the activation of ERK and the downregulation of Akt in MCA-102 murine fibrosarcoma cells. Int. Immunopharmacol. 2007, 7, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Lee, J.Y.; Kang, S.K.; Kim, J.K.; Park, W.H.; Kim, J.G.; Moon, S.-K.; Kim, C.-H. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: Isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005, 77, 2760–2769. [Google Scholar] [CrossRef]
- Dandriyal, J.; Singla, R.; Kumar, M.; Jaitak, V. Recent developments of C-4 substituted coumarin derivatives as anticancer agents. Eur. J. Med. Chem. 2016, 119, 141–168. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhou, V.; Pan, S.; Liu, Y.; Hornsby, M.; McMullan, D.; Klock, H.E.; Haugen, J.; Lesley, S.A.; Gray, N.; et al. Identification of coumarin derivatives as a novel class of allosteric MEK1 inhibitors. Bioorganic Med. Chem. Lett. 2005, 15, 5467–5473. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. In vivo formed metabolites of polyphenols and their biological efficacy. Food Funct. 2019, 10, 6999–7021. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, M.; Sarac, I. Essential oils from plants. J. Biotechnol. Biomed. Sci. 2018, 1, 35–43. [Google Scholar] [CrossRef]
- Ngan, T.; Muoi, N.; Quan, P.; Cang, M. Huynh CM. Evaluation of physical and chemical properties of Pomelo (Citrus grandis L.) essential oil using steam distillation process. Asian J. Chem. 2020, 32, 1433. [Google Scholar] [CrossRef]
- Fernández-Sestelo, M.; Carrillo, J.M. Environmental effects on yield and composition of essential oil in wild populations of spike lavender (Lavandula latifolia Medik.). Agriculture 2020, 10, 626. [Google Scholar] [CrossRef]
- Bharath, B.; Perinbam, K.; Devanesan, S.; AlSalhi, M.S.; Saravanan, M. Evaluation of the anticancer potential of Hexadecanoic acid from brown algae Turbinaria ornata on HT–29 colon cancer cells. J. Mol. Struct. 2021, 1235, 130229. [Google Scholar] [CrossRef]
- Bradberry, J.C.; Hilleman, D.E. Overview of omega-3 fatty acid therapies. P and T. Peer Rev. J. Formul. Med. 2013, 38, 681–691. [Google Scholar]
- Marathe, S.J.; Hamzi, W.; Bashein, A.M.; Deska, J.; Seppänen-Laakso, T.; Singhal, R.S.; Shamekh, S. Anti-angiogenic effect of Cantharellus cibarius extracts, its correlation with lipoxygenase inhibition, and role of the bioactives therein. Nutr. Cancer 2021, 74, 1–11. [Google Scholar] [CrossRef]
- Loffredo, S.; Borriello, F.; Iannone, R.; Ferrara, A.L.; Galdiero, M.R.; Gigantino, V.; Esposito, P.; Varricchi, G.; Lambeau, G.; Cassatela, M.A.; et al. Group V secreted phospholipase A2 induces the release of proangiogenic and antiangiogenic factors by human neutrophils. Front. Immunol. 2017, 8, 443. [Google Scholar] [CrossRef]
- Wei, L.S.; Wee, W.; Siong, J.Y.F.; Syamsumir, D.F. Characterization of anticancer, antimicrobial, antioxidant properties and chemical compositions of Peperomia pellucida leaf extract. Acta Med. Iran. 2011, 49, 670–674. [Google Scholar]
- Premathilaka, R.; Silva, M. Bioactive compounds and antioxidant activity of Bunchosia armeniaca. World J. Pharm. Pharm. Sci. 2016, 5, 1237–1247. [Google Scholar]
- Cotrim, B.A.; Joglar, J.; Rojas, M.J.L.; Del Olmo, J.M.D.; Macias-González, M.; Cuevas, M.R.; Fito, M.; Munoz-Aguayo, D.; Covas Planells, M.I.; Farre, I.; et al. Unsaturated fatty alcohol derivatives of olive oil phenolic compounds with potential low-density lipoprotein (LDL) anti-oxidant and anti-obesity properties. J. Agric. Food Chem. 2012, 60, 1067–1074. [Google Scholar] [CrossRef]
- Vasas, A.; Hohmann, J. Euphorbia Diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef]
- Frank, M.B.; Yang, Q.; Osban, J.; Azzarello, J.T.; Saban, M.R.; Saban, R.; Ashley, R.A.; Welter, J.C.; Fung, K.-M.; Lin, H.-K. Frankincense oil derived from Boswellia carteri induces tumor cell specific cytotoxicity. BMC Complement. Altern. Med. 2009, 9, 6. [Google Scholar] [CrossRef]
- De Lima, E.J.; Alves, R.G.; Anunciação, T.A.D.; Silva, V.R.; Santos, L.D.S.; Soares, M.B.; Cardozo, N.M.D.; Costa, E.V.; da Silva, F.M.A.; Koolen, H.H.F.; et al. Antitumor effect of the essential oil from the leaves of Croton matourensis Aubl. (Euphorbiaceae). Molecules 2018, 23, 2974. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and anti-oxidant potential of wild vegetables from western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Petkova, N.; Ivanov, I.; Topchieva, S.; Denev, P.; Pavlov, A. Biologically active substances and in vitro antioxidant activity of different extracts from dandelion (Taraxacum officinale) roots. Sci. Bulletin. Ser. F Biotechnic. 2015, 19, 190–197. [Google Scholar]
- Yan, L.; Meng, Q.W.; Kim, I.H. The effects of dietary Houttuynia cordata and Taraxacum officinale extract powder on growth performance, nutrient digestibility, blood characteristics and meat quality in finishing pigs. Livest. Sci. 2011, 141, 188–193. [Google Scholar] [CrossRef]
- Ghaima, K.K.; Hashim, N.M.; Ali, S.A. Antibacterial and antioxidant activities of ethyl acetate extract of nettle (Urtica dioica) and dandelion (Taraxacum officinale). J. Appl. Pharm. Sci. 2013, 3, 96. [Google Scholar]
- Miłek, M.; Marcinčáková, D.; Legáth, J. Polyphenols content, antioxidant activity, and cytotoxicity assessment of Taraxacum officinale extracts prepared through the micelle-mediated extraction method. Molecules 2019, 24, 1025. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Meyers, K.J.; van der Heide, J.; Liu, R.H. Varietal differences in phenolic content and antioxidant and antiproliferative activities of onions. J. Agric. Food Chem. 2004, 52, 6787–6793. [Google Scholar] [CrossRef] [PubMed]
- Tettey, C.O.; Ocloo, A.; Nagajyothi, P.C.; Lee, K.D. Antioxidant Activity of Solvent Fractions of Taraxacum officinale (Dandelion) Leaves. J. Herbs Spices Med. Plants 2014, 20, 329–340. [Google Scholar] [CrossRef]
- Colle, D.; Arantes, L.P.; Gubert, P.; da Luz, S.C.A.; Athayde, M.L.; Teixeira Rocha, J.B.; Soares, F.A.A. Antioxidant Properties of Taraxacum officinale Leaf Extract Are Involved in the Protective Effect Against Hepatoxicity Induced by Acetaminophen in Mice. J. Med. Food 2012, 15, 549–556. [Google Scholar] [CrossRef]
- Ishfaq, S.; Sabir, S.M.; Khurshid, H.; Zaman, T.; Ahmad, Z. Antioxidant activities and inhibitory effect of Taraxacum officinale, Cichorium intybus and Lectuca sativa on prooxidant induced lipid peroxidation in mice liver. Croat. J. Food Sci. Technol. 2018, 10, 16–22. [Google Scholar] [CrossRef]
- Pădureţ, S.E.R.G.I.U.; Amariei, S.; Gutt, G.; Piscuc, B. The evaluation of dandelion (Taraxacum officinale) properties as a valuable food ingredient. Rom. Biotechnol. Lett. 2016, 21, 11569. [Google Scholar]
- Hu, C.; Kitts, D.D. Dandelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro. Phytomedicine 2005, 12, 588–597. [Google Scholar] [CrossRef]
- Thu, K.; Myint, P.P. Pharmacological activities of Cuscuta reflexa (Shwe-nwe) stem and Taraxacum officinale Weber ex FH Wigg. (Dai-Si) leaf extracts. J. Med. Plants 2019, 7, 109–112. [Google Scholar]
- Matthaus, B. Antioxidant activity of extracts obtained from residues of different oilseeds. J. Agric. Food Chem. 2002, 50, 3444–3452. [Google Scholar] [CrossRef]
- Ghorbel Koubaa, F.; Chaâbane, M.; Choura, B.; Turki, M.; Makni-Ayadi, F.; El Feki, A. Hepatoprotective effects of Taraxacum officinale root extract on permethrin-induced liver toxicity in adult mice. Pharm. Biomed. Res. 2020, 6, 223–236. [Google Scholar] [CrossRef]
- Mhamdi, B.; Abbassi, F.; Smaoui, A.; Abdelly, C.; Marzouk, B. Fatty acids, essential oil and phenolics composition of Silybum marianum seeds and their antioxidant activities. Pak. J. Pharm. Sci. 2016, 29, 951–959. [Google Scholar]
- Bourgou, S.; Tammar, S.; Salem, N.; Mkadmini, K.; Msaada, K. Phenolic composition, essential oil, and antioxidant activity in the aerial part of Artemisia herba-alba from several provenances: A comparative study. Int. J. Food Prop. 2016, 19, 549–563. [Google Scholar] [CrossRef]
- Dhouibi, N.; Manuguerra, S.; Arena, R.; Mahdhi, A.; Messina, C.M.; Santulli, A.; Dhaouadi, H. Screening of antioxidant potentials and bioactive properties of the extracts obtained from two Centaurea L. Species (C. kroumirensis Coss. and C. sicula L. subsp sicula). Appl. Sci. 2020, 10, 2267. [Google Scholar] [CrossRef]
- Boukes, G.J.; van de Venter, M. Rooperol as an antioxidant and its role in the innate immune system: An in vitro study. J. Ethnopharmacol. 2012, 144, 692–699. [Google Scholar] [CrossRef]
- Diotallevi, M.; Checconi, P.; Palamara, A.T.; Celestino, I.; Coppo, L.; Holmgren, A.; Abbas, K.; Peyrot, F.; Mengozzi, M.; Ghezzi, P. Glutathione fine-tunes the innate immune response toward antiviral pathways in a macrophage cell line independently of its antioxidant properties. Front. Immunol. 2017, 8, 1239. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Vodjgani, M.; Salehi, Z.; Izad, M. The Influence of Reactive Oxygen Species in the Immune System and Pathogenesis of Multiple Sclerosis. Autoimmune Dis. 2020, 2020, 5793817. [Google Scholar]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxidative Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutterdige, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Okado-Matsumoto, A.; Fridovich, I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J. Biol. Chem. 2001, 27, 38388–38393. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.W.G.; Elwell, J.H.; Oberley, L.W.; Goeddel, D.V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell 1989, 58, 923–931. [Google Scholar] [CrossRef]
- Weydert, C.; Roling, B.; Liu, J.; Hinkhouse, M.M.; Ritchie, J.M.; Oberley, L.W.; Cullen, J.J. Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase. Mol. Cancer Ther. 2003, 2, 361–369. [Google Scholar] [PubMed]
- Liu, J.; Du, J.; Zhang, Y.; Sun, W.; Smith, B.J.; Oberley, L.W.; Cullen, J.J. Suppression of the malignant phenotype in pancreatic cancer by overexpression of phospholipid hydroperoxide glutathione peroxidase. Hum. Gene Ther. 2006, 17, 105–116. [Google Scholar] [CrossRef]
- Liu, J.; Hinkhouse, M.M.; Sun, W.; Weydert, C.J.; Ritchie, J.M.; Oberley, L.W.; Cullen, J.J. Redox regulation of pancreatic cancer cell growth: Role of glutathione peroxidase in the suppression of the malignant phenotype. Hum. Gene Ther. 2004, 15, 239–250. [Google Scholar] [CrossRef]
- Cullen, J.J.; Mitros, F.A.; Oberley, L.W. Expression of anti-oxidant enzymes in diseases of the human pancreas: Another link between chronic pancreatitis and pancreatic cancer. Pancreas 2003, 26, 23–27. [Google Scholar] [CrossRef]
- Glorieux, C.; Dejeans, N.; Sid, B.; Beck, R.; Calderon, P.B.; Verrax, J. Catalase overexpression in mammary cancer cells leads to a less aggressive phenotype and an altered response to chemotherapy. Biochem. Pharmacol. 2011, 82, 1384–1390. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef]
- Kodydková, J.; Vávrová, L.; Kocík, M.; Zak, A. Human catalase, its polymorphisms, regulation and changes of its activity in different diseases. Folia Biol. 2014, 60, 153. [Google Scholar]
- Nishikawa, M.; Hashida, M.; Takakura, Y. Catalase delivery for inhibiting ROS-mediated tissue injury and tumor metastasis. Adv. Drug Deliv. Rev. 2009, 61, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Chole, R.H.; Patil, R.N.; Basak, A.; Palandurkar, K.; Bhowate, R. Estimation of serum malondialdehyde in oral cancer and precancer and its association with healthy individuals, gender, alcohol, and tobacco abuse. J. Cancer Res. Ther. 2010, 6, 487. [Google Scholar] [CrossRef]
- Gönenç, A.; Özkan, Y.; Torun, M.; Şimşek, B. Plasma malondialdehyde (MDA) levels in breast and lung cancer patients. J. Clin. Pharm. Ther. 2001, 26, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Taysi, S.; Uslu, C.; Akcay, F.; Sutbeyaz, M.Y. Malondialdehyde and nitric oxide levels in the plasma of patients with advanced laryngeal cancer. Surg. Today 2003, 33, 651–654. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Wang, M.; Dhingra, K.; Hittelman, W.N.; Liehr, J.G.; De Andrade, M.; Li, D. Lipid peroxidation-induced putative malondialdehyde-DNA adducts in human breast tissues. Cancer Epidemiol. Prev. Biomark. 1996, 5, 705–710. [Google Scholar]
- Sheweita, S.A.; El-Hosseiny, L.S.; Nashashibi, M.A. Protective effects of essential oils as natural anti-oxidants against hepatotoxicity induced by cyclophosphamide in mice. PLoS ONE 2016, 11, e0165667. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Peng, J.; Wei, H. Oregano essential oil induces SOD1 and GSH expression through Nrf2 activation and alleviates hydrogen peroxide-induced oxidative damage in IPEC-J2 cells. Oxidative Med. Cell. Longev. 2016, 2016, 5987183. [Google Scholar]
- De Sousa, A.C.; Gattass, C.R.; Alviano, D.S.; Alviano, C.S.; Blank, A.F.; Alves, P.B. Melissa officinalis L. essential oil: Anti-tumoral and anti-oxidant activities. J. Agric. Food Chem. 2004, 52, 2485–2489. [Google Scholar]
- Ovadje, P.; Ammar, S.; Guerrero, J.A.; Arnason, J.T.; Pandey, S. Dandelion root extract affects colorectal cancer proliferation and survival through the activation of multiple death signalling pathways. Oncotarget 2016, 7, 73080. [Google Scholar] [CrossRef] [PubMed]
- Sigstedt, S.C.; Hooten, C.J.; Callewaert, M.C.; Jenkins, A.R.; Romero, A.E.; Pullin, M.J.; Kornienko, A.; Lowrey, T.K.; Van Slambrouck, S.; Steelant, W.F. Evaluation of aqueous extracts of Taraxacum officinale on growth and invasion of breast and prostate cancer cells. Int. J. Oncol. 2008, 32, 1085–1090. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Cho, H.S.; Lee, J.J.; Lee, H.J.; Jun, S.Y.; Lee, J.H.; Song, H.-H.; Choi, S.; Saloura, V.; Gil Park, C.; et al. Novel TRAIL sensitizer Taraxacum officinale FH Wigg enhances TRAIL-induced apoptosis in Huh7 cells. Mol. Carcinog. 2016, 55, 387–396. [Google Scholar] [CrossRef]
- Ovadje, P.; Chochkeh, M.; Akbari-Asl, P.; Hamm, C.; Pandey, S. Selective induction of apoptosis and autophagy through treatment with dandelion root extract in human pancreatic cancer cells. Pancreas 2012, 41, 1039–1047. [Google Scholar] [CrossRef]
- Ahn, C.; Lee, J.H.; Park, M.J.; Kim, J.W.; Yang, J.; Yoo, Y.M.; Jeung, E.B. Cytostatic effects of plant essential oils on human skin and lung cells. Exp. Ther. Med. 2020, 19, 2008–2018. [Google Scholar] [CrossRef]
- Russo, R.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Exploitation of cytotoxicity of some essential oils for translation in cancer therapy. Evid.-Based Complement. Altern. Med. 2015, 2015, 397821. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anti-Cancer Res. 2007, 27, 3293–3299. [Google Scholar]
- Sertel, S.; Eichhorn, T.; Plinkert, P.K.; Efferth, T. Cytotoxicity of Thymus vulgaris essential oil towards human oral cavity squamous cell carcinoma. Anti-Cancer Res. 2011, 31, 81–87. [Google Scholar]
- Park, K.R.; Nam, D.; Yun, H.M.; Lee, S.G.; Jang, H.J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Feng-Qing, Y.; Shao-Ping, L.; Jian-Li, G.; Guang, H.; Sin-Cheng, L.; Conceição Leong, E.; Fung, K.-P.; Wang, Y.-T.; Simon, L.M.Y. Furanodiene induces G2/M cell cycle arrest and apoptosis through MAPK signaling and mitochondria-caspase pathway in human hepatocellular carcinoma cells. Cancer Biol. Ther. 2007, 6, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Carnesecchi, S.; Bradaia, A.; Fischer, B.; Coelho, D.; Schöller-Guinard, M.; Gosse, F.; Raul, F. Perturbation by geraniol of cell membrane permeability and signal transduction pathways in human colon cancer cells. J. Pharmacol. Exp. Ther. 2002, 303, 711–715. [Google Scholar] [CrossRef]
- Wang, H.Y.; Cai, B.; Cui, C.B.; Zhang, D.Y.; Yang, B.F. Vitexicarpin, a flavonoid from Vitex trifolia L., induces apoptosis in K562 cells via mitochondria-controlled apoptotic pathway. Yao Xue Xue Bao 2005, 40, 27–31. [Google Scholar]
- Bayala, B.; Bassole, I.H.; Scifo, R.; Gnoula, C.; Morel, L.; Lobaccaro, J.M.A.; Simpore, J. Anti-cancer activity of essential oils and their chemical components—A review. Am. J. Cancer Res. 2014, 4, 591–607. [Google Scholar] [PubMed]
- Salikhov, S.M.; Faizullina, L.K.; Valeev, F.A. Synthesis and cytotoxic activity of isocembrol and its hydroxy derivatives. Russ. Chem. Bull. 2020, 69, 1933–1937. [Google Scholar] [CrossRef]
- Jalili, C.; Taghadosi, M.; Pazhouhi, M.; Bahrehmand, F.; Miraghaee, S.; Pourmand, D.; Rashidi, I. An overview of therapeutic potentials of Taraxacum officinale (Dandelion): A traditionally valuable herb with a reach historical background. World Cancer Res. J. 2020, 7, e1679. [Google Scholar]
- Menke, K.; Schwermer, M.; Felenda, J.; Beckmann, C.; Stintzing, F.; Schramm, A.; Zuzak, T.J. Taraxacum officinale extract shows antitumor effects on pediatric cancer cells and enhance mistletoe therapy. Complement. Ther. Med. 2018, 40, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Kamal, F.Z.; Stanciu, G.D.; Lefter, R.; Cotea, V.V.; Niculaua, M.; Ababei, D.C.; Ciobica, A.; Ech-Chahad, A. Chemical Composition and Antioxidant Activity of Ammi visnaga L. Essential Oil. Antioxidants 2022, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, B.; He, J.; Han, L.; Zhan, Y.; Wang, Y. In vitro and in vivo antioxidant effects of the ethanolic extract of Swertia chirayita. J. Ethnopharmacol. 2011, 136, 309–315. [Google Scholar]
- Barbarić, M.; Mišković, K.; Bojić, M.; Lončar, M.B.; Smolčić-Bubalo, A.; Debeljak, Ž.; Medić-Šarić, M. Chemical composition of the ethanolic propolis extracts and its effect on HeLa cells. J. Ethnopharmacol. 2011, 135, 772–778. [Google Scholar] [CrossRef]

| Name | a RI | b RI | % Area |
|---|---|---|---|
| Pentadecanoic acid | 1762 | 1777 | 2.28 |
| Tetradecanoic acid | 1774 | 1774 | 0.99 |
| n-Hexadecanoic acid | 1987 | 1980 | 26.11 |
| Thunbergol | 2051 | 2047 | 0.66 |
| Heptadecanoic acid | 2081 | 2080 | 0.81 |
| Heptadecanolide | 2094 | 2051 | 0.95 |
| 9,12-Octadecadienoic acid | 2105 | 2152 | 34.19 |
| n-Nonadecanol-1 | 2157 | 2153 | 1.36 |
| Octadecanoic acid | 2205 | 2165 | 1.11 |
| Linoelaidic acid | 2206 | - | 2.57 |
| Correlation Pearson r | Phenolic Content | DPPH |
|---|---|---|
| Phenolic content | 1 | 0.966 |
| DPPH | 0.966 | 1 |
| Concentration (μg/mL) | Ascorbic Acid | EO |
|---|---|---|
| 1000 | 1.52 ± 0.005 | 0.64 ± 0.003 * |
| 800 | 1.21 ± 0.01 | 0.55 ± 0.04 * |
| 600 | 0.95 ± 0.03 | 0.48 ± 0.03 * |
| 400 | 0.71 ± 0.01 | 0.42 ± 0.05 * |
| 200 | 0.43 ± 0.01 | 0.34 ± 0.04 * |
| 0 | 0 | 0 |
| EC50 (mg/mL) | 0.034 ± 0.28 | 0.963 ± 0.006 |
| Treatments | Mean Body Weight in Grams ± SD | |
|---|---|---|
| Day 0 | Day 14 | |
| C | 29.39 ± 0.29 | 29.58 ± 0.24 |
| CMC | 30.48 ± 0.31 | 30.71 ± 0.30 |
| APAP | 32.54 ± 0.43 | 29.78 ± 0.65 * |
| AA | 27.47 ± 0.28 | 27.92 ± 0.72 |
| TO 1 | 30.24 ± 0.22 | 31.8 ± 0.71 |
| TO 2 | 23.39 ± 0.27 | 23.81 ± 0.25 |
| Groups/Organs | Relative Weight of Liver and Kidney (g/100 g) | |
|---|---|---|
| Liver | Kidneys | |
| C | 5.26 ± 0.26 | 1.37 ± 0.11 |
| CMC | 5.05 ± 0.11 | 1.36 ± 0.17 |
| APAP | 3.88 ± 0.13 *** | 1.04 ± 0.14 ** |
| AA | 4.66 ± 0.16 **; ### | 1.24 ± 0.08 *; # |
| TO 1 | 4.35 ± 0.55 ****, ### | 1.20 ± 0.13 *; # |
| TO 2 | 4.61 ± 0.07 ***; ### | 1.22 ± 0.09 *; # |
| Groups | Examples of Compounds Identified in Our Study | Bioactive Potential | Reference |
|---|---|---|---|
| Fatty acid | n-Hexadecanoic acid; 9,12-Octa-decadienoic acid; Octadecanoic acid; Linoelaidic acid; Tetradecanoic acid; Pentadecanoic acid | Anticancer, antioxidative, immunostimulatory, anti-inflammatory, and anti-obesity | [31,32,33,34,35,36] |
| Fatty alcohol | n-Nonadecanol-1 | Antioxidative and anti-obesity | [37] |
| Diterpene monocyclic alcohol | Thunbergol | Anticancer, antiproliferative, anti-inflammatory, and cardioprotective | [38,39,40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, F.Z.; Lefter, R.; Mihai, C.-T.; Farah, H.; Ciobica, A.; Ali, A.; Radu, I.; Mavroudis, I.; Ech-Chahad, A. Chemical Composition, Antioxidant and Antiproliferative Activities of Taraxacum officinale Essential Oil. Molecules 2022, 27, 6477. https://doi.org/10.3390/molecules27196477
Kamal FZ, Lefter R, Mihai C-T, Farah H, Ciobica A, Ali A, Radu I, Mavroudis I, Ech-Chahad A. Chemical Composition, Antioxidant and Antiproliferative Activities of Taraxacum officinale Essential Oil. Molecules. 2022; 27(19):6477. https://doi.org/10.3390/molecules27196477
Chicago/Turabian StyleKamal, Fatima Zahra, Radu Lefter, Cosmin-Teodor Mihai, Hanane Farah, Alin Ciobica, Ahmad Ali, Iulian Radu, Ioannis Mavroudis, and Abdellah Ech-Chahad. 2022. "Chemical Composition, Antioxidant and Antiproliferative Activities of Taraxacum officinale Essential Oil" Molecules 27, no. 19: 6477. https://doi.org/10.3390/molecules27196477
APA StyleKamal, F. Z., Lefter, R., Mihai, C.-T., Farah, H., Ciobica, A., Ali, A., Radu, I., Mavroudis, I., & Ech-Chahad, A. (2022). Chemical Composition, Antioxidant and Antiproliferative Activities of Taraxacum officinale Essential Oil. Molecules, 27(19), 6477. https://doi.org/10.3390/molecules27196477








