Structural Diversity and Carbon Dioxide Sorption Selectivity of Zinc(II) Metal-Organic Frameworks Based on Bis(1,2,4-triazol-1-yl)methane and Terephthalic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of the MOFs
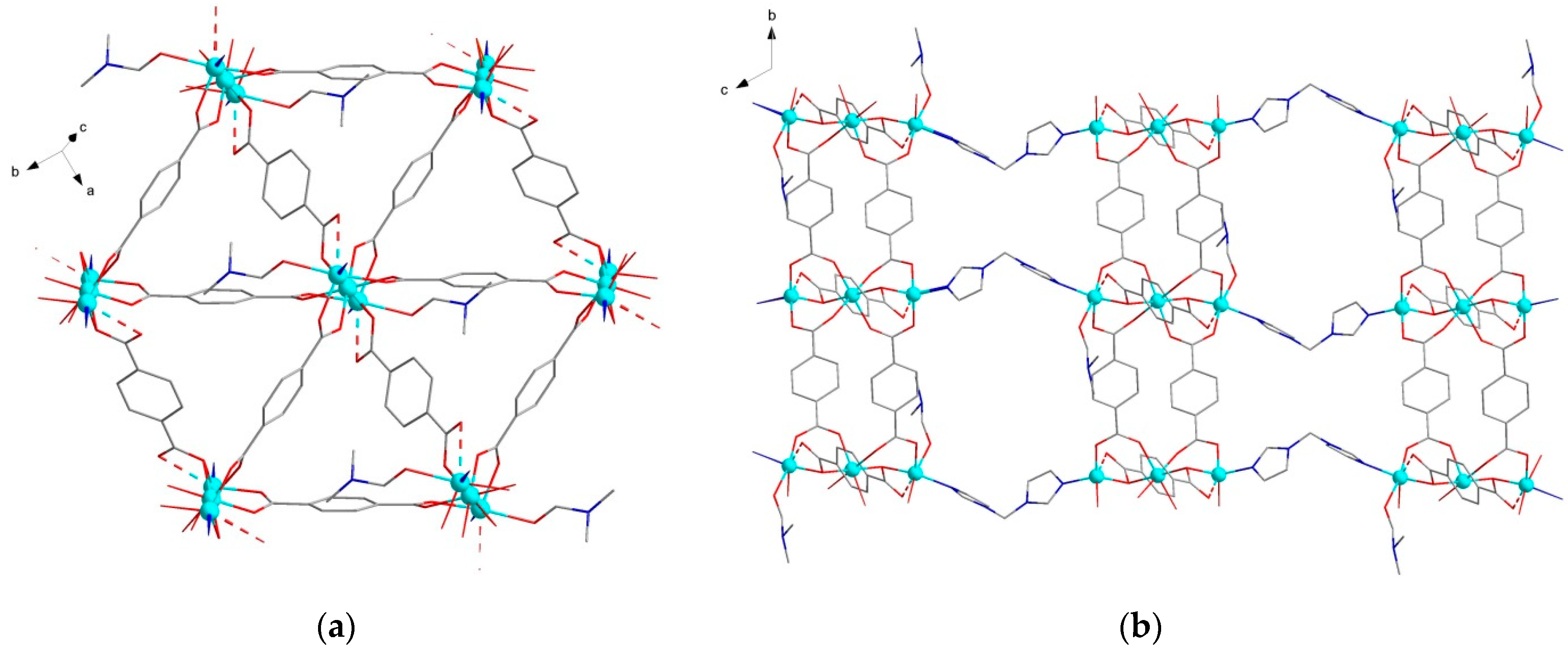
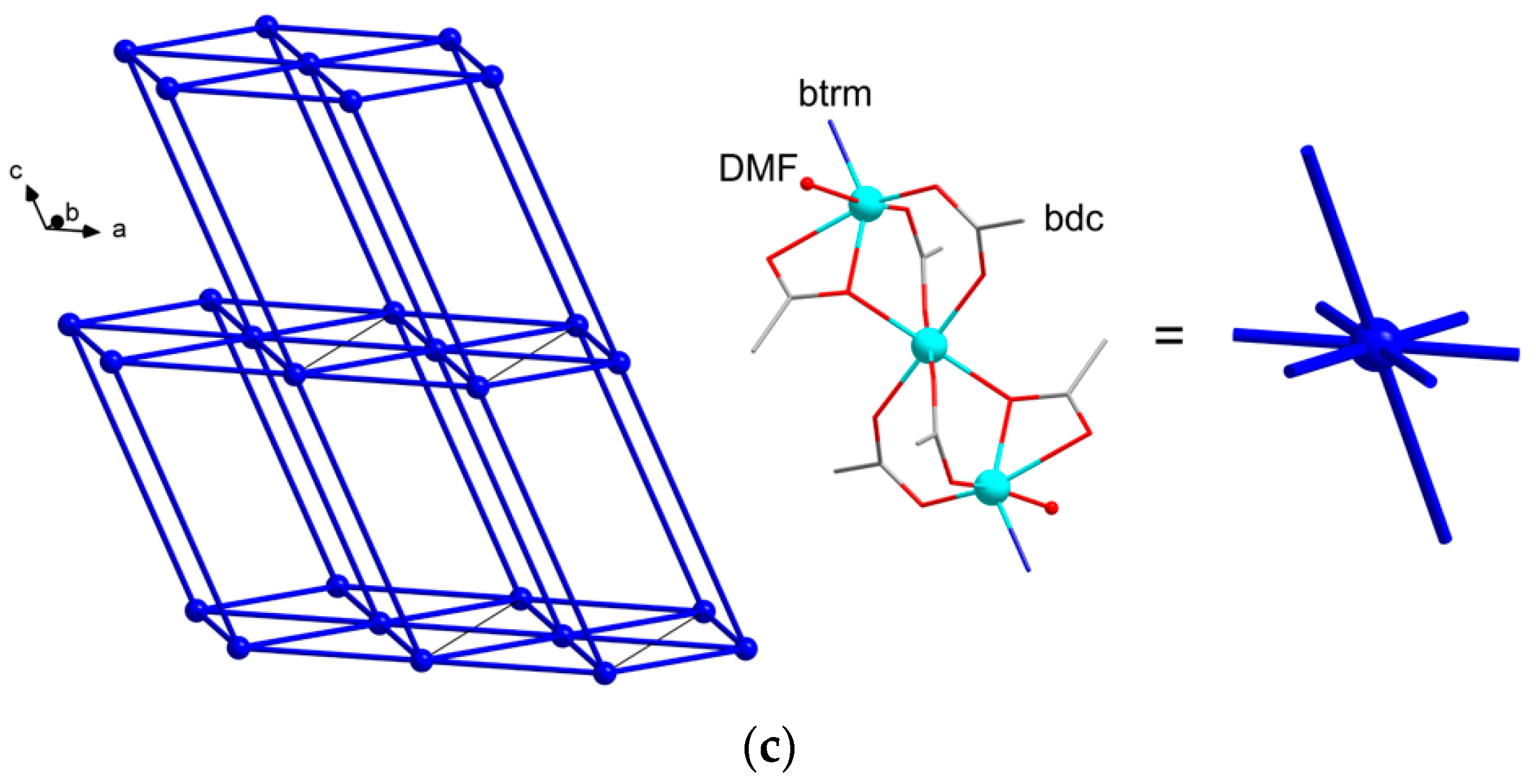
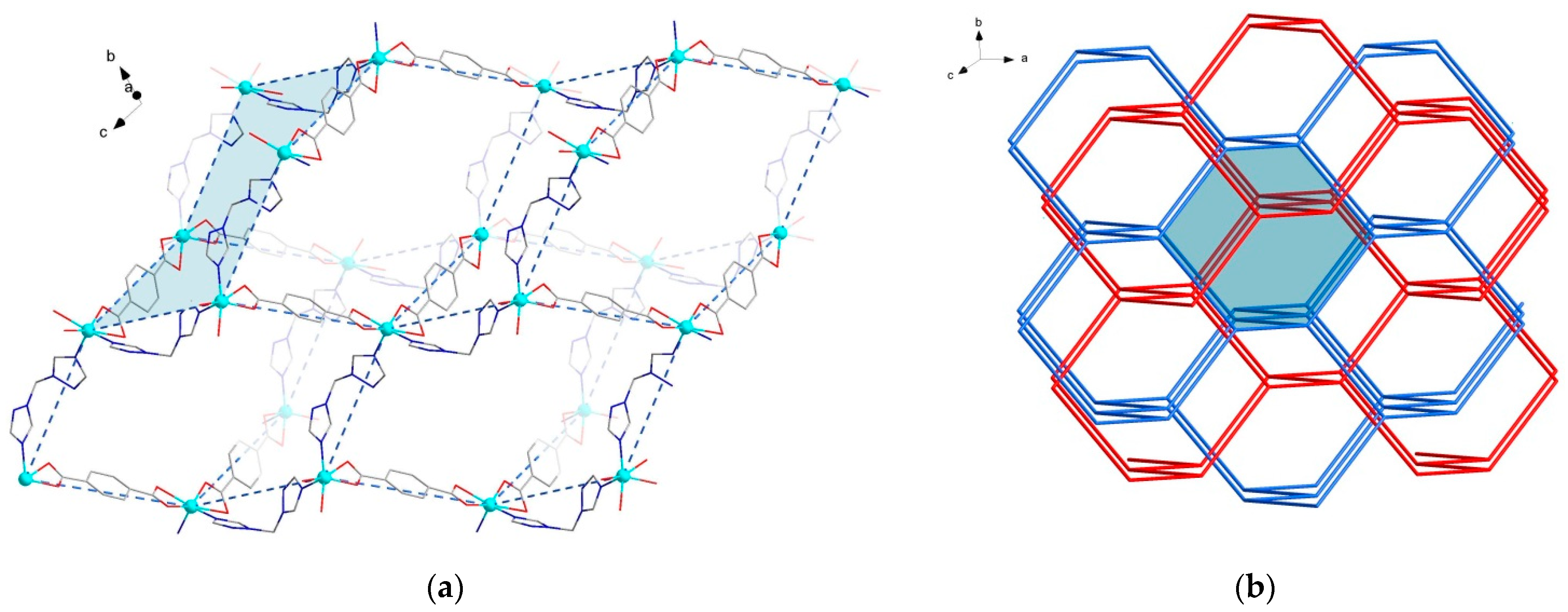
2.2. Crystal Structures of the MOFs
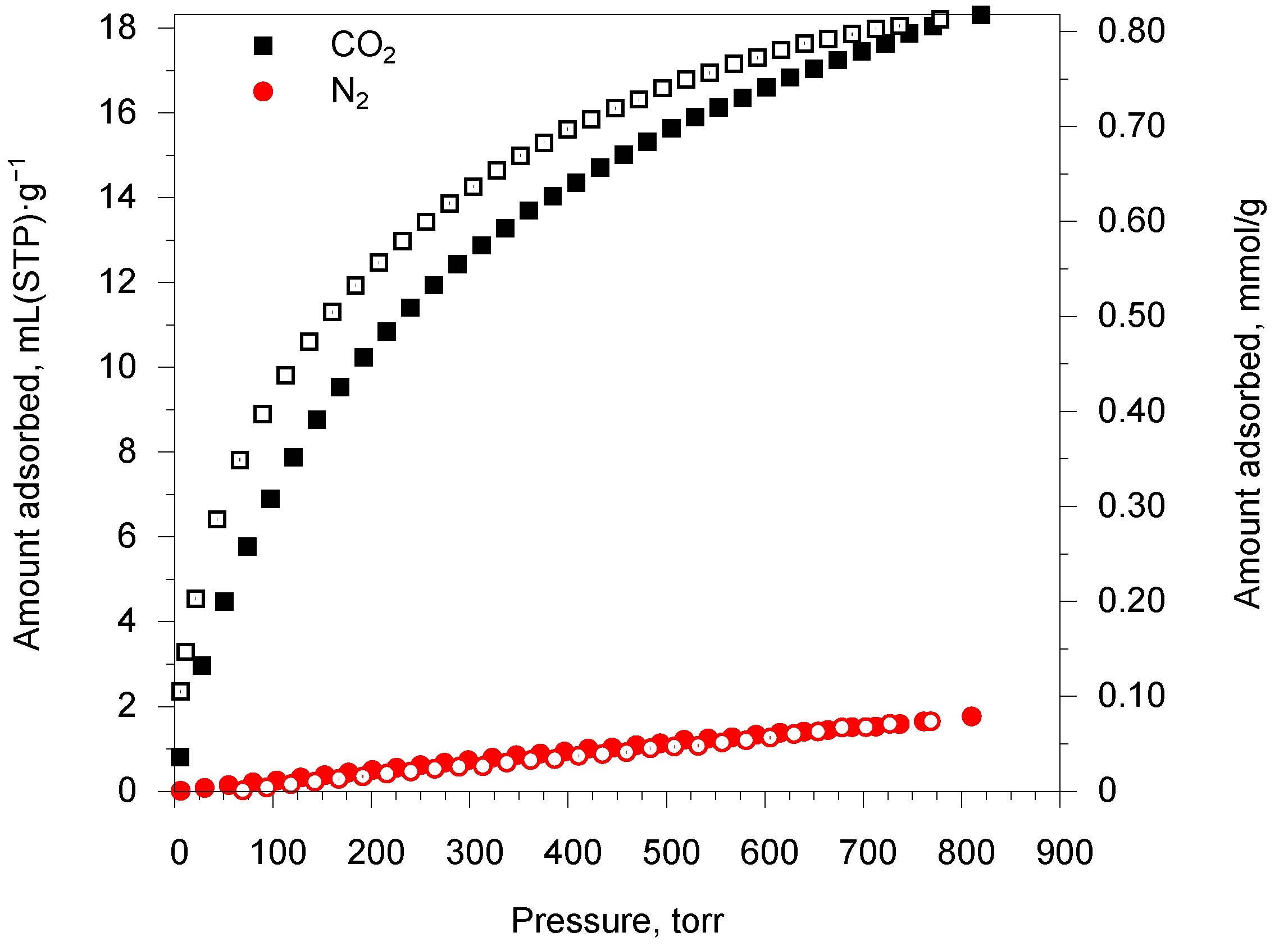
2.3. Sorption Properties of the MOFs
2.4. Luminescent Properties of the MOFs
3. Materials and Methods
3.1. Synthetic Procudures
3.1.1. Synthesis of Compound {[Zn3(btrm)(bdc)3DMF]∙nDMF}∞ (1)
3.1.2. Synthesis of Compound {[Zn3(btrm)(bdc)3]∙nDMF}∞ (2)
3.1.3. Synthesis of Compound {[Zn(btrm)(bdc)]·nDMF}∞ (3)
3.2. Methods of Characterization
3.3. X-ray Structure Determination
3.4. Study of Gas Sorption Properties
3.4.1. Methods for the Experimental Study of Adsorption-Desorption Isotherms
3.4.2. Evaluation of the Adsorption Selectivity
- As the molar ratio of the adsorption quantities at the relevant partial pressures of the gases,
- 2.
- As a ratio of Henry constants which corresponds to the slope of the adsorption isotherm at very low partial pressures,
- 3.
- By an ideal adsorbed solution theory (IAST). The relationship between P, yi and xi (P—the total pressure of the gas phase, yi—mole fraction of the i component in the gas phase, xi—mole fraction of the i component in the absorbed state) is defined according to the IAST theory [56]:
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [Green Version]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Boyd, P.G.; Chidambaram, A.; García-Díez, E.; Ireland, C.P.; Daff, T.D.; Bounds, R.; Gładysiak, A.; Schouwink, P.; Moosavi, S.M.; Maroto-Valer, M.M.; et al. Data-driven design of metal–organic frameworks for wet flue gas CO2 capture. Nature 2019, 576, 253–256. [Google Scholar] [CrossRef] [Green Version]
- Demakov, P.A.; Volynkin, S.S.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. A selenophene-incorporated metal–organic framework for enhanced CO2 uptake and adsorption selectivity. Molecules 2020, 25, 4396. [Google Scholar] [CrossRef]
- Dubskikh, V.A.; Lysova, A.A.; Samsonenko, D.G.; Lavrov, A.N.; Kovalenko, K.A.; Dybtsev, D.N.; Fedin, V.P. 3D Metal–Organic Frameworks Based on Co(II) and Bithiophendicarboxylate: Synthesis, Crystal Structures, Gas Adsorption, and Magnetic Properties. Molecules 2021, 26, 1269. [Google Scholar] [CrossRef]
- Al-Rowaili, F.N.; Zahid, U.; Onaizi, S.; Khaled, M.; Jamal, A.; AL-Mutairi, E.M. A review for Metal-Organic Frameworks (MOFs) utilization in capture and conversion of carbon dioxide into valuable products. J. CO2 Util. 2021, 53, 101715. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, F.; Kang, D.J.; Pang, H. A Review of Metal-Organic Framework-based Compounds for Environmental Applications. ENERGY Environ. Mater. 2022. [Google Scholar] [CrossRef]
- Jaros, S.W.; Sokolnicki, J.; Wołoszyn, A.; Haukka, M.; Kirillov, A.M.; Smoleński, P. A novel 2D coordination network built from hexacopper(i)-iodide clusters and cagelike aminophosphine blocks for reversible “turn-on” sensing of aniline. J. Mater. Chem. C 2018, 6, 1670–1678. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Matveevskaya, V.; Pavlov, D.; Yakunenkov, A.; Potapov, A. Coordination Polymers Based on Highly Emissive Ligands: Synthesis and Functional Properties. Materials 2020, 13, 2699. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.-Y.; Cheng, C.; Shao, Z.-S.; Wang, H.-S. Strategies to fabricate metal–organic framework (MOF)-based luminescent sensing platforms. J. Mater. Chem. C 2019, 7, 10743–10763. [Google Scholar] [CrossRef]
- Qin, J.-H.; Qin, W.-J.; Xiao, Z.; Yang, J.-K.; Wang, H.-R.; Yang, X.-G.; Li, D.-S.; Ma, L.-F. Efficient Energy-Transfer-Induced High Photoelectric Conversion in a Dye-Encapsulated Ionic Pyrene-Based Metal–Organic Framework. Inorg. Chem. 2021, 60, 18593–18597. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.-H.; Zhang, J.-R.; Xiao, Z.; Wu, Y.-P.; Xu, H.-M.; Yang, X.-G.; Ma, L.-F.; Li, D.-S. Topology- and Guest-Dependent Photoelectric Conversion of 2D Anionic Pyrene-Based Metal–Organic Framework. Cryst. Growth Des. 2022, 22, 4018–4024. [Google Scholar] [CrossRef]
- Dang, L.-L.; Li, T.-T.; Zhang, T.-T.; Zhao, Y.; Chen, T.; Gao, X.; Ma, L.-F.; Jin, G.-X. Highly selective synthesis and near-infrared photothermal conversion of metalla-Borromean ring and [2]catenane assemblies. Chem. Sci. 2022, 13, 5130–5140. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Yoo, D.K.; Ahmed, I.; Lee, H.J.; Jhung, S.H. Metal-organic frameworks composed of nitro groups: Preparation and applications in adsorption and catalysis. Chem. Eng. J. 2022, 451, 138538. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Krivosudský, L. Metalloporphyrin Metal-Organic Frameworks: Eminent Synthetic Strategies and Recent Practical Exploitations. Molecules 2022, 27, 4917. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Bryliakov, K.P. Asymmetric catalysis using metal-organic frameworks. Coord. Chem. Rev. 2021, 437, 213845. [Google Scholar] [CrossRef]
- Qin, J.-H.; Xiao, Z.; Xu, P.; Li, Z.-H.; Lu, X.; Yang, X.-G.; Lu, W.; Ma, L.-F.; Li, D.-S. Anionic Porous Zn-Metalated Porphyrin Metal–Organic Framework with PtS Topology for Gas-Phase Photocatalytic CO2 Reduction. Inorg. Chem. 2022, 61, 13234–13238. [Google Scholar] [CrossRef]
- Gorbunova, Y.G.; Enakieva, Y.Y.; Volostnykh, M.V.; Sinelshchikova, A.A.; Abdulaeva, I.A.; Birin, K.P.; Tsivadze, A.Y. Porous porphyrin-based metal-organic frameworks: Synthesis, structure, sorption properties and application prospects. Russ. Chem. Rev. 2022, 91, RCR5038. [Google Scholar] [CrossRef]
- Kovalenko, K.A.; Potapov, A.S.; Fedin, V.P. Micro- and mesoporous metal-organic coordination polymers for separation of hydrocarbons. Russ. Chem. Rev. 2022, 91, RCR5026. [Google Scholar] [CrossRef]
- Fu, M.; Wang, Y.; Wang, X.; Sun, D. Metal-Organic Framework Materials for Light Hydrocarbon Separation. Chempluschem 2021, 86, 387–395. [Google Scholar] [CrossRef]
- Mukherjee, S.; Desai, A.V.; Ghosh, S.K. Potential of metal–organic frameworks for adsorptive separation of industrially and environmentally relevant liquid mixtures. Coord. Chem. Rev. 2018, 367, 82–126. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sensharma, D.; Qazvini, O.T.; Dutta, S.; Macreadie, L.K.; Ghosh, S.K.; Babarao, R. Advances in adsorptive separation of benzene and cyclohexane by metal-organic framework adsorbents. Coord. Chem. Rev. 2021, 437, 213852. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Q.-W.; Zhuang, C.; Tang, P.-P.; Lin, N.; Wei, L.-Q. Controlled release of drug molecules in metal–organic framework material HKUST-1. Inorg. Chem. Commun. 2017, 79, 78–81. [Google Scholar] [CrossRef]
- Hamideh, R.A.; Akbari, B.; Fathi, P.; Misra, S.K.; Sutrisno, A.; Lam, F.; Pan, D. Biodegradable MRI Visible Drug Eluting Stent Reinforced by Metal Organic Frameworks. Adv. Healthc. Mater. 2020, 9, 2000136. [Google Scholar] [CrossRef]
- Li, P.; Chen, Q.; Wang, T.C.; Vermeulen, N.A.; Mehdi, B.L.; Dohnalkova, A.; Browning, N.D.; Shen, D.; Anderson, R.; Gómez-Gualdrón, D.A.; et al. Hierarchically Engineered Mesoporous Metal-Organic Frameworks toward Cell-free Immobilized Enzyme Systems. Chem 2018, 4, 1022–1034. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.R.M.; Alexandre, J.Y.N.H.; Souza, J.E.S.; Neto, J.G.L.; de Sousa Júnior, P.G.; Rocha, M.V.P.; dos Santos, J.C.S. The Chemistry and Applications of Metal-Organic Frameworks (MOFs) as Industrial Enzyme Immobilization Systems. Molecules 2022, 27, 4529. [Google Scholar] [CrossRef]
- Chowdhury, M.A. Metal-organic-frameworks for biomedical applications in drug delivery, and as MRI contrast agents. J. Biomed. Mater. Res. Part. A 2017, 105, 1184–1194. [Google Scholar] [CrossRef]
- Osterrieth, J.W.M.; Fairen-Jimenez, D. Metal–Organic Framework Composites for Theragnostics and Drug Delivery Applications. Biotechnol. J. 2021, 16, 2000005. [Google Scholar] [CrossRef]
- Du, M.; Li, C.-P.; Liu, C.-S.; Fang, S.-M. Design and construction of coordination polymers with mixed-ligand synthetic strategy. Coord. Chem. Rev. 2013, 257, 1282–1305. [Google Scholar] [CrossRef]
- Butova, V.V.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: Structure, properties, synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280–307. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, Y.-P.; Qin, J.-S.; Lu, W.; Zou, L.; Zhang, Q.; Wang, X.; Sun, X.; Zhou, H.-C. Linker Installation: Engineering Pore Environment with Precisely Placed Functionalities in Zirconium MOFs. J. Am. Chem. Soc. 2016, 138, 8912–8919. [Google Scholar] [CrossRef] [PubMed]
- Agafonov, M.A.; Alexandrov, E.V.; Artyukhova, N.A.; Bekmukhamedov, G.E.; Blatov, V.A.; Butova, V.V.; Gayfulin, Y.M.; Garibyan, A.A.; Gafurov, Z.N.; Gorbunova, Y.G.; et al. Metal-organic frameworks in Russia: From the synthesis and structure to functional properties and materials. J. Struct. Chem. 2022, 63, 671–843. [Google Scholar] [CrossRef]
- Pachfule, P.; Panda, T.; Dey, C.; Banerjee, R. Structural diversity in a series of metal-organic frameworks (MOFs) composed of divalent transition metals, 4,4′-bipyridine and a flexible carboxylic acid. CrystEngComm 2010, 12, 2381–2389. [Google Scholar] [CrossRef]
- Cai, S.L.; Wang, T.; Wang, J.; Xie, B.; Wu, Y. Synthesis, luminescent sensing based on a three-fold interpenetrating network with flexible carboxylates. Russ. J. Coord. Chem. 2017, 43, 50–54. [Google Scholar] [CrossRef]
- Sanram, S.; Boonmak, J.; Youngme, S. Structural diversity, single-crystal to single-crystal transformation and photocatalytic properties of Cu(II)-metal-organic frameworks based on 1,4-phenylenedipropionic acid. Inorg. Chim. Acta 2018, 469, 11–19. [Google Scholar] [CrossRef]
- Ako, A.M.; Hawes, C.S.; Twamley, B.; Schmitt, W. Facile adaptation of 1D Mn(ii) chain motifs to form 3D azo-pyridine-based coordination polymers. CrystEngComm. 2017, 19, 994–1000. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Lin, J.; Wang, L.; Li, C.; Yan, W.; Wu, T. Cuprous Iodide Pseudopolymorphs Based on Imidazole Ligand and Their Luminescence Thermochromism. Cryst. Growth Des. 2016, 16, 2322–2327. [Google Scholar] [CrossRef]
- Barsukova, M.O.; Sapchenko, S.A.; Kovalenko, K.A.; Samsonenko, D.G.; Potapov, A.S.; Dybtsev, D.N.; Fedin, V.P. Exploring the multifunctionality in metal-organic framework materials: How do the stilbenedicarboxylate and imidazolyl ligands tune the characteristics of coordination polymers? New J. Chem. 2018, 42, 6408–6415. [Google Scholar] [CrossRef]
- Senchyk, G.A.; Lysenko, A.B.; Domasevitch, K.V.; Erhart, O.; Henfling, S.; Krautscheid, H.; Rusanov, E.B.; Krämer, K.W.; Decurtins, S.; Liu, S.-X. Exploration of a Variety of Copper Molybdate Coordination Hybrids Based on a Flexible Bis(1,2,4-triazole) Ligand: A Look through the Composition-Space Diagram. Inorg. Chem. 2017, 56, 12952–12966. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Tao, C.-Z.; Zhang, D.-E.; Ma, J.-J.; Liu, L.; Tong, Z.-W.; Yu, X.-Y. Three new three dimensional Zn(II)-benzenetetracarboxylate coordination polymers: Syntheses, crystal structures and luminescent properties. Polyhedron 2017, 123, 69–74. [Google Scholar] [CrossRef]
- Semitut, E.; Sukhikh, T.; Filatov, E.; Ryadun, A.; Potapov, A. Synthesis, Crystal Structure and Luminescent Properties of 2D Zinc Coordination Polymers Based on Bis(1,2,4-triazol-1-yl)methane and 1,3-Bis(1,2,4-triazol-1-yl)propane. Crystals 2017, 7, 354. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Zhu, L.-M.; Qian, L.-L.; Wang, Z.-X.; Li, B.-L.; Li, H.-Y. Syntheses, structural diversity and properties of a series of coordination polymers based on 4-substituted bis(triazole) and multicarboxylate ligands. Polyhedron 2018, 145, 53–62. [Google Scholar] [CrossRef]
- Tian, L.; Zhou, S.Y. Copper(II) coordination polymers constructed from aromatic polycarboxylate and flexible triazole ligands with different spacer lengths: Syntheses, structures, and properties. J. Coord. Chem. 2013, 66, 2863–2874. [Google Scholar] [CrossRef]
- Tian, L.; Yan, L.; Liu, S.-Y. Crystal structures and luminescence of two cadmium(II) polymers constructed from aromatic polycarboxylate and bis(1,2,4-triazol-1-yl)methane ligands. J. Coord. Chem. 2011, 64, 2945–2952. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Tian, L. Poly[hemi(hexaaquazinc) [[µ2-1,3-bis(1,2,4-triazol-1-yl)methane](µ2-5-sulfonatobenzene-1,3-dicarboxylato)zinc] sesquihydrate]. Acta Crystallogr. Sect. E 2011, 67, m950–m951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Semitut, E.Y.; Sukhikh, T.S.; Filatov, E.Y.; Anosova, G.A.; Ryadun, A.A.; Kovalenko, K.A.; Potapov, A.S. Synthesis, Crystal Structure, and Luminescent Properties of Novel Zinc Metal–Organic Frameworks Based on 1,3-Bis(1,2,4-triazol-1-yl)propane. Cryst. Growth Des. 2017, 17, 5559–5567. [Google Scholar] [CrossRef]
- Barthel, S.; Alexandrov, E.V.; Proserpio, D.M.; Smit, B. Distinguishing Metal–Organic Frameworks. Cryst. Growth Des. 2018, 18, 1738–1747. [Google Scholar] [CrossRef] [Green Version]
- Effendy; Marchetti, F.; Pettinari, C.; Pettinari, R.; Skelton, B.W.; White, A.H. Synthesis and Spectroscopic Characterization of Silver(I) Complexes with the Bis(1,2,4-triazol-1-yl)alkane Ligand tz2(CH2). X-ray Structures of Two- and Three-Dimensional Coordination Polymers. Inorg. Chem. 2003, 42, 112–117. [Google Scholar] [CrossRef]
- Bruker AXS, Inc. APEX2 (Version 2.0), SAINT (Version 8.18c), and SADABS (Version 2.11), Bruker Advanced X-ray Solutions; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D. Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevchenko, A.P.; Shabalin, A.A.; Karpukhin, I.Y.; Blatov, V.A. Topological representations of crystal structures: Generation, analysis and implementation in the TopCryst system. Sci. Technol. Adv. Mater. Methods 2022, 2, 250–265. [Google Scholar] [CrossRef]
- Thermophysical Properties of Fluid Systems. NIST Chemistry Webbook. Available online: http://webbook.nist.gov/chemistry/fluid/ (accessed on 20 September 2022).
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]




| Compound | Gas/Temperature | Specific Surface Area, m2/g | Vpore, cm3/g * | Vads., cm3/g ** | |
|---|---|---|---|---|---|
| Langmuir | BET | ||||
| 1 | N2/77 K | – *** | 20 | 0.0213 | 13.8 |
| CO2/195 K | 109 | 92 | 0.0603 | 38.6 | |
| 3 | CO2/195 K | 139 | 92 | 0.0788 | 37.7 |
| Method | V1/V2 | KH1/KH2 | IAST |
|---|---|---|---|
| Value | 11.2 | 41.8 | 21.1 |
| Compound | λmax, nm | Quantum Yield, % |
|---|---|---|
| {[Zn3(DMF)(btrm)(bdc)3]·nDMF}∞ (1) | 440 | 12 |
| {[Zn3(btrm)(bdc)3]∙DMF}∞ (2) | 435 | 10 |
| {[Zn(bdc)(btrm)]∙2DMF}∞ (3) | 435 | <0.5 |
| btrm | 430 | 5 |
| [Zn(bdc)(btrp)]∙1.5DMF} [47] | 440 | 15 |
| {[Zn3(bdc)3(btrp)]∙nDMF} [47] | 445 | <0.5 |
| btrp [47] | 410 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhikh, T.S.; Filatov, E.Y.; Ryadun, A.A.; Kovalenko, K.A.; Potapov, A.S. Structural Diversity and Carbon Dioxide Sorption Selectivity of Zinc(II) Metal-Organic Frameworks Based on Bis(1,2,4-triazol-1-yl)methane and Terephthalic Acid. Molecules 2022, 27, 6481. https://doi.org/10.3390/molecules27196481
Sukhikh TS, Filatov EY, Ryadun AA, Kovalenko KA, Potapov AS. Structural Diversity and Carbon Dioxide Sorption Selectivity of Zinc(II) Metal-Organic Frameworks Based on Bis(1,2,4-triazol-1-yl)methane and Terephthalic Acid. Molecules. 2022; 27(19):6481. https://doi.org/10.3390/molecules27196481
Chicago/Turabian StyleSukhikh, Taisiya S., Evgeny Yu. Filatov, Alexey A. Ryadun, Konstantin A. Kovalenko, and Andrei S. Potapov. 2022. "Structural Diversity and Carbon Dioxide Sorption Selectivity of Zinc(II) Metal-Organic Frameworks Based on Bis(1,2,4-triazol-1-yl)methane and Terephthalic Acid" Molecules 27, no. 19: 6481. https://doi.org/10.3390/molecules27196481






