Binuclear Triphenylantimony(V) Catecholates through N-Donor Linkers: Structural Features and Redox Properties
Abstract
:1. Introduction
2. Results and Discussion
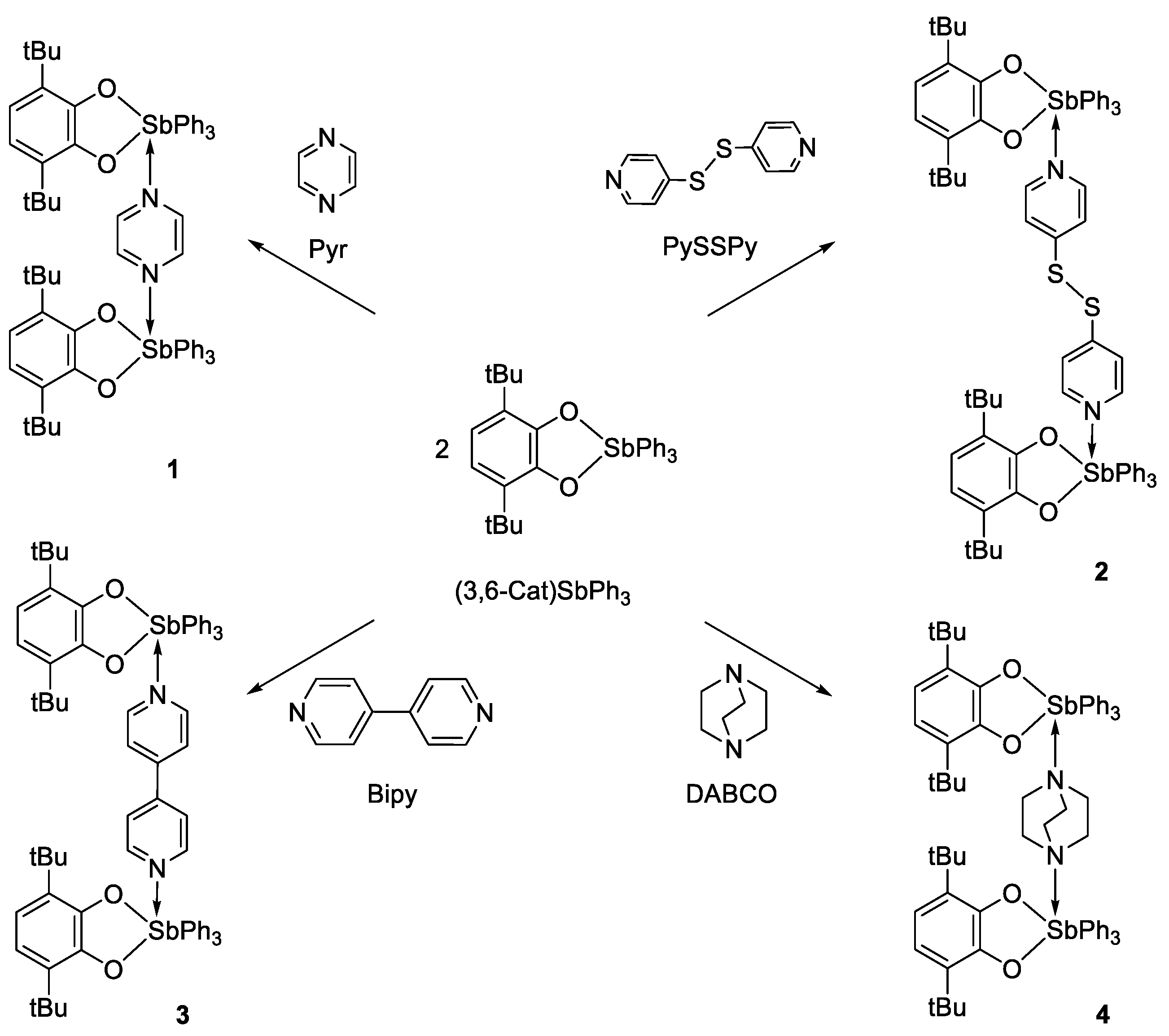
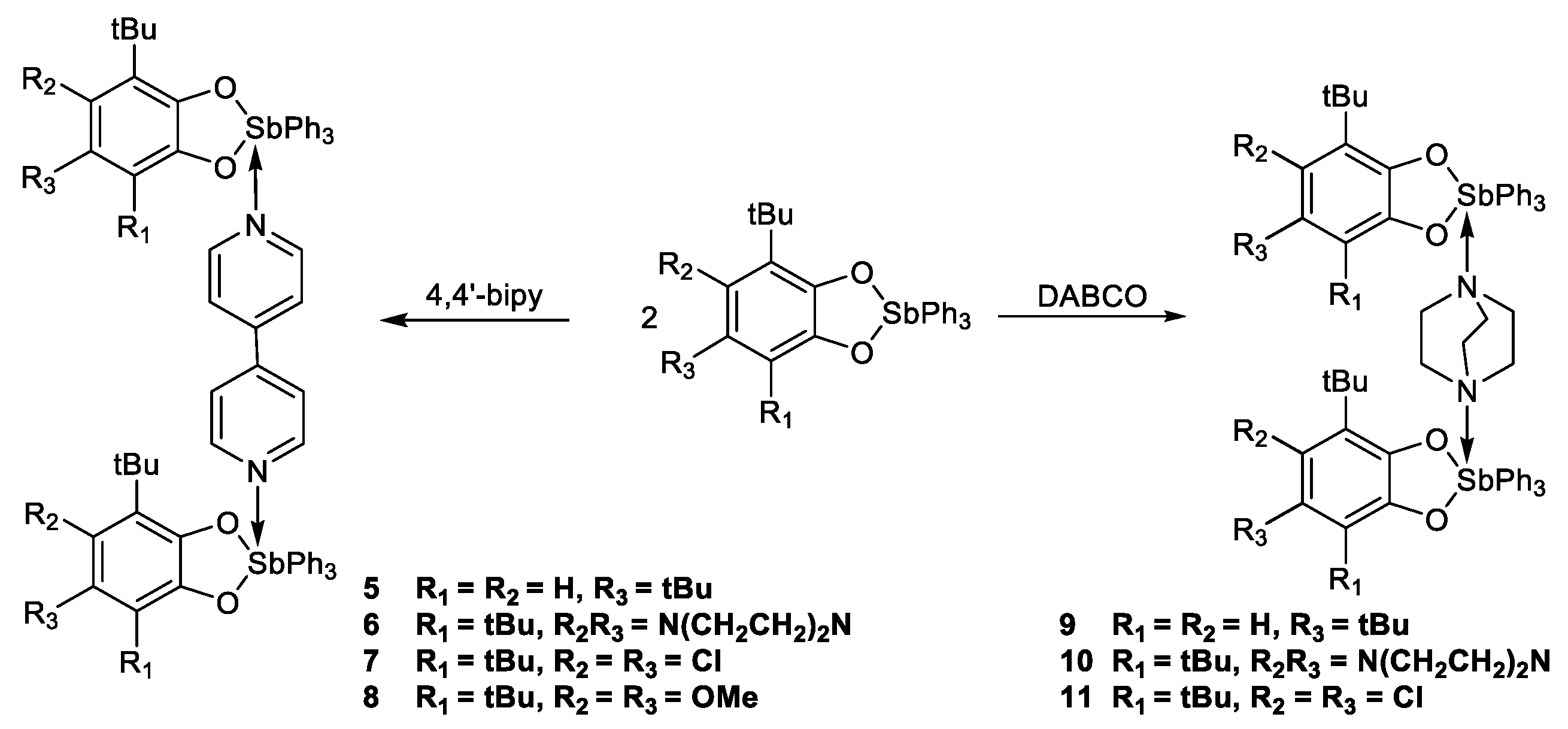
2.1. Synthesis and Characterization
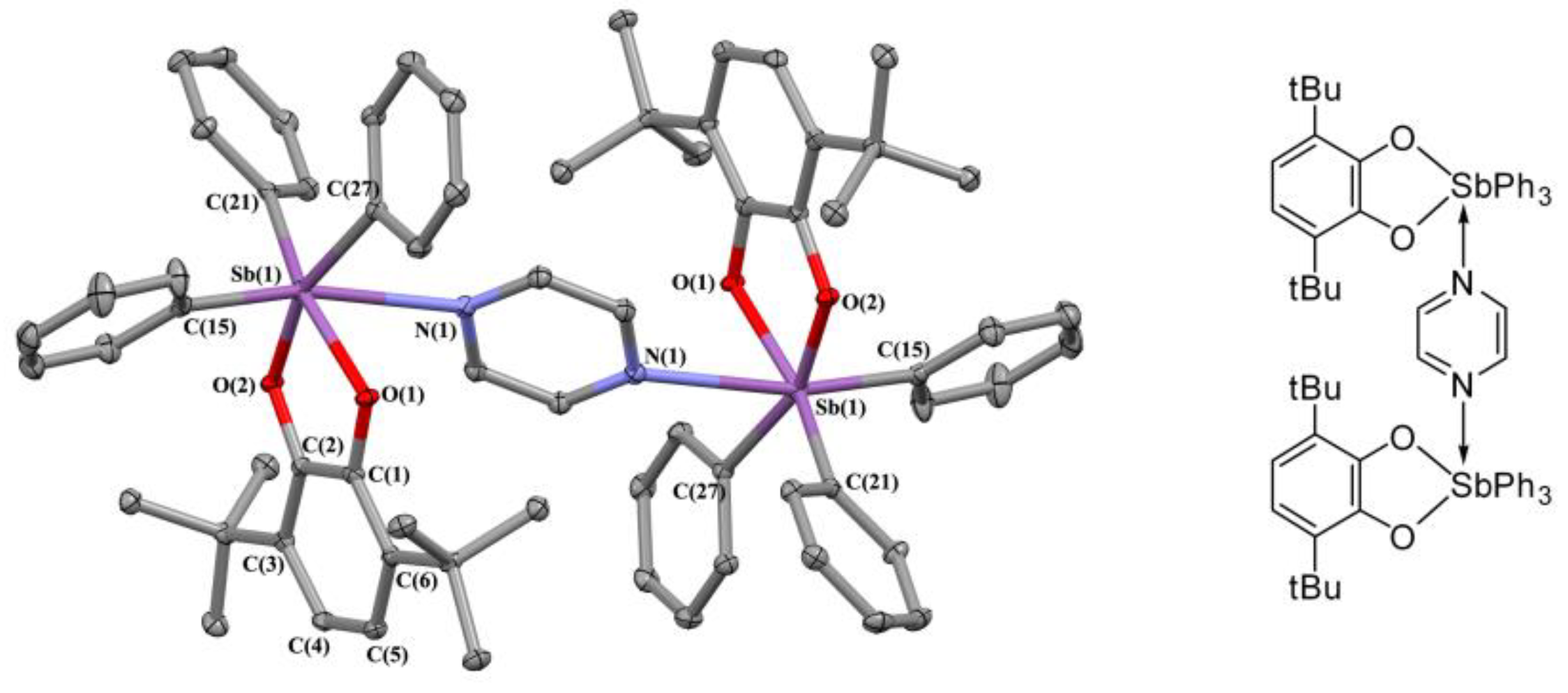
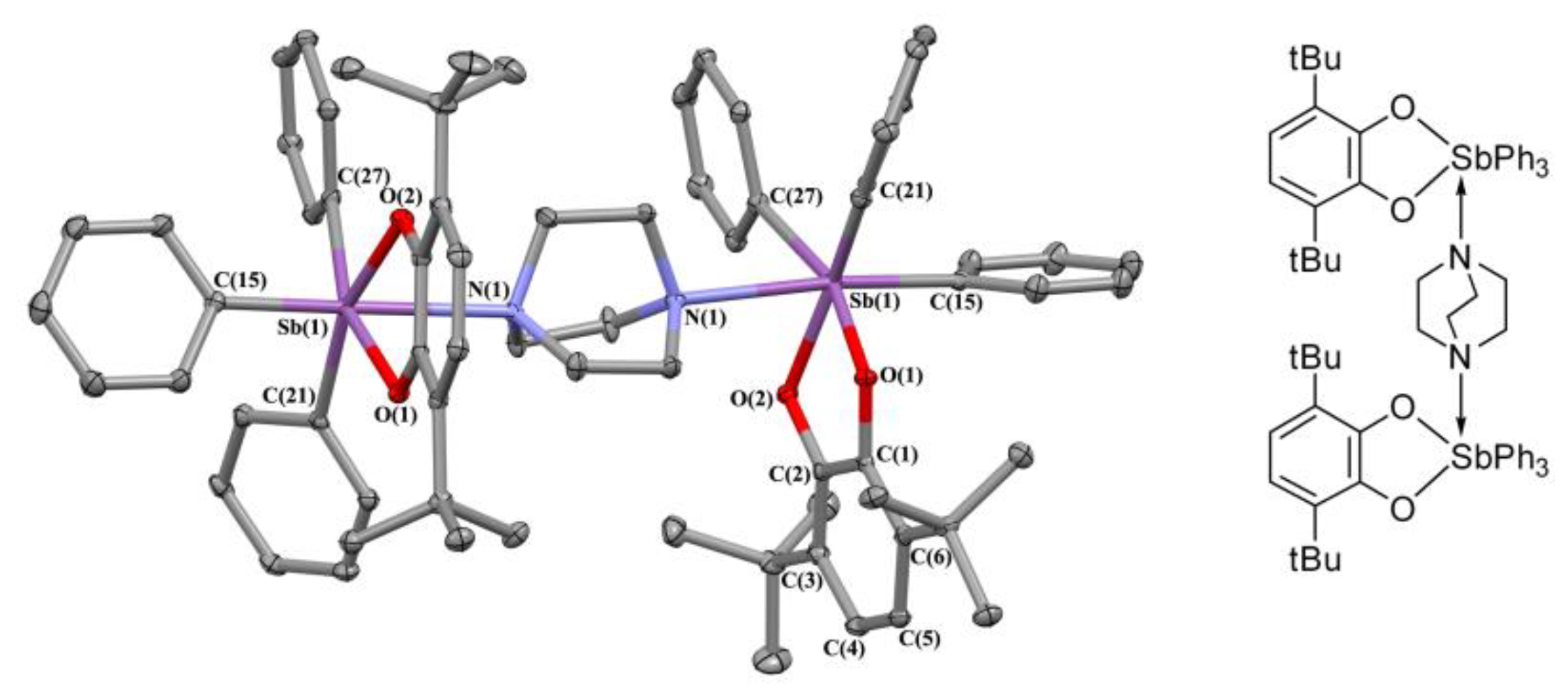
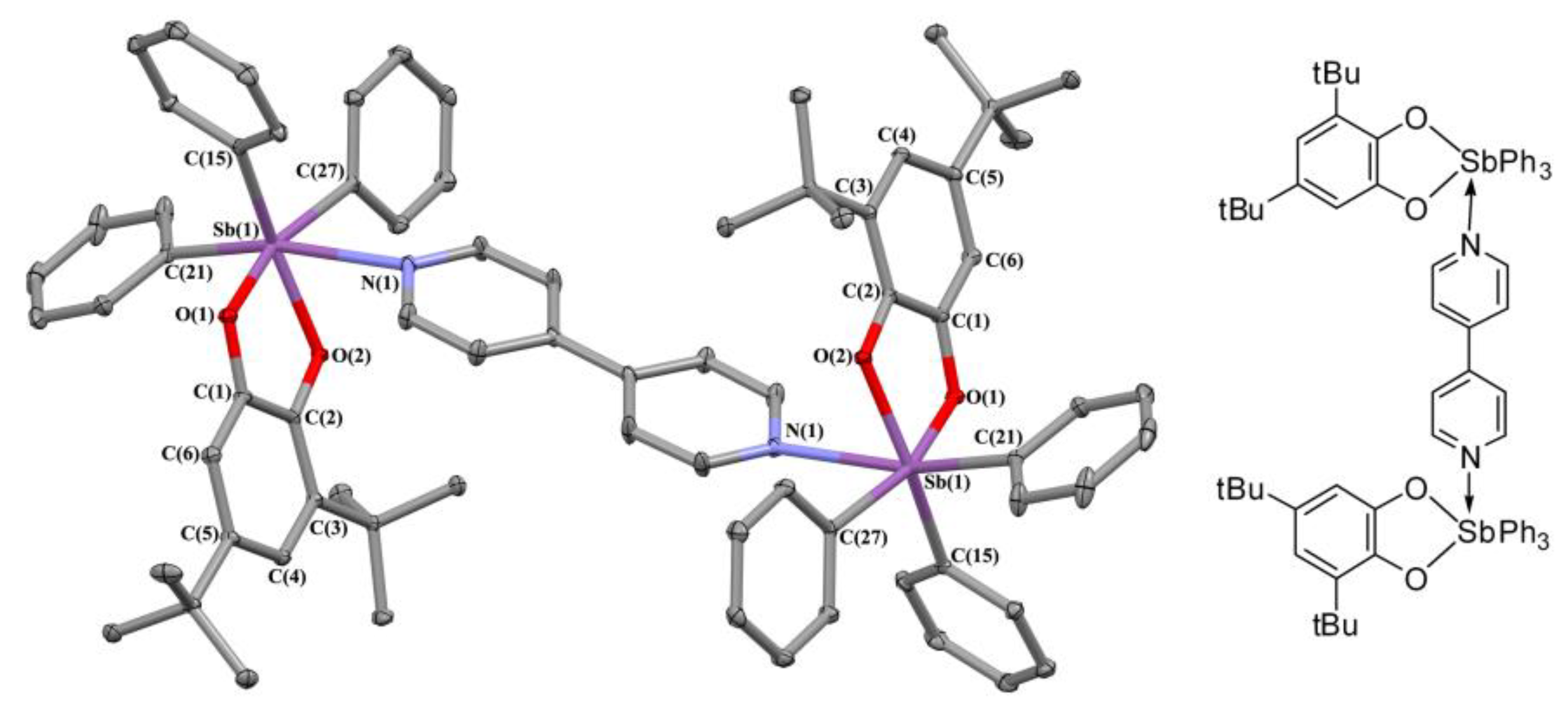
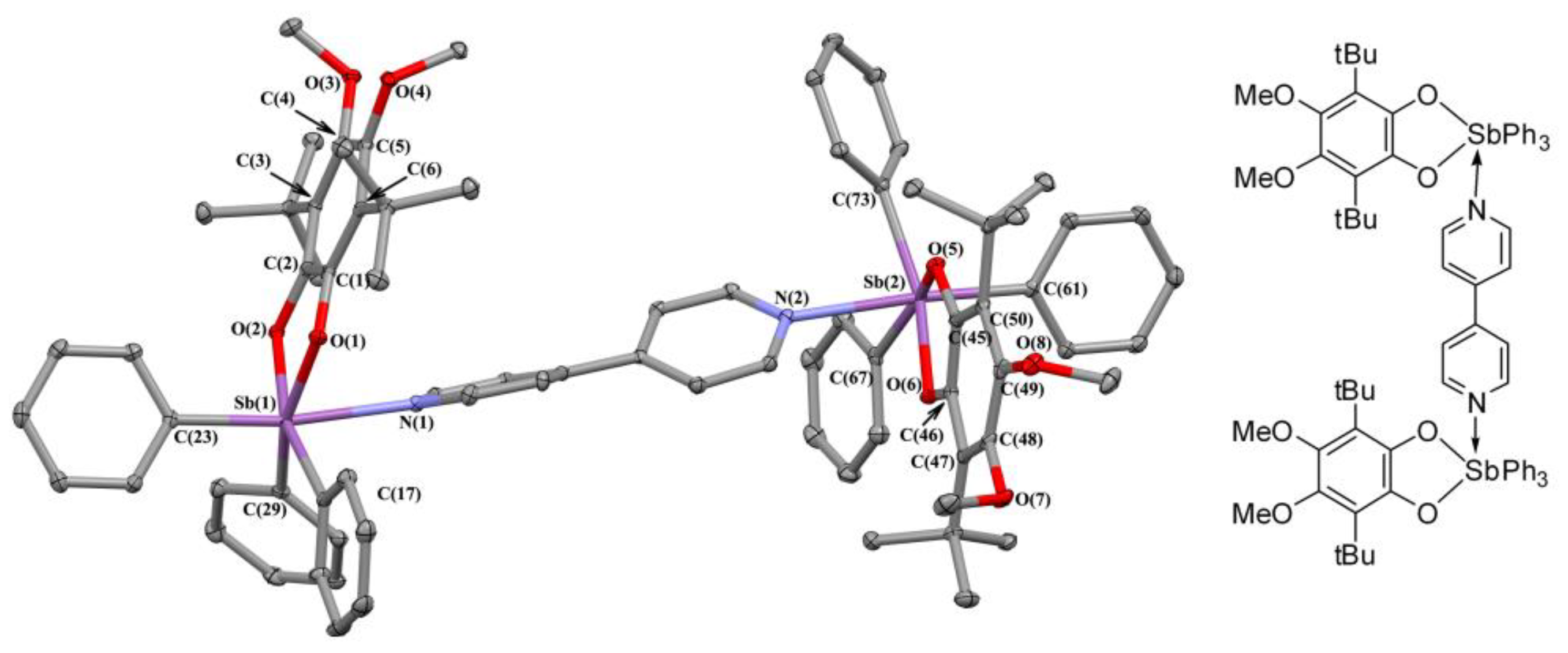
2.2. X-ray Structures
2.3. Charge Density in 5ED
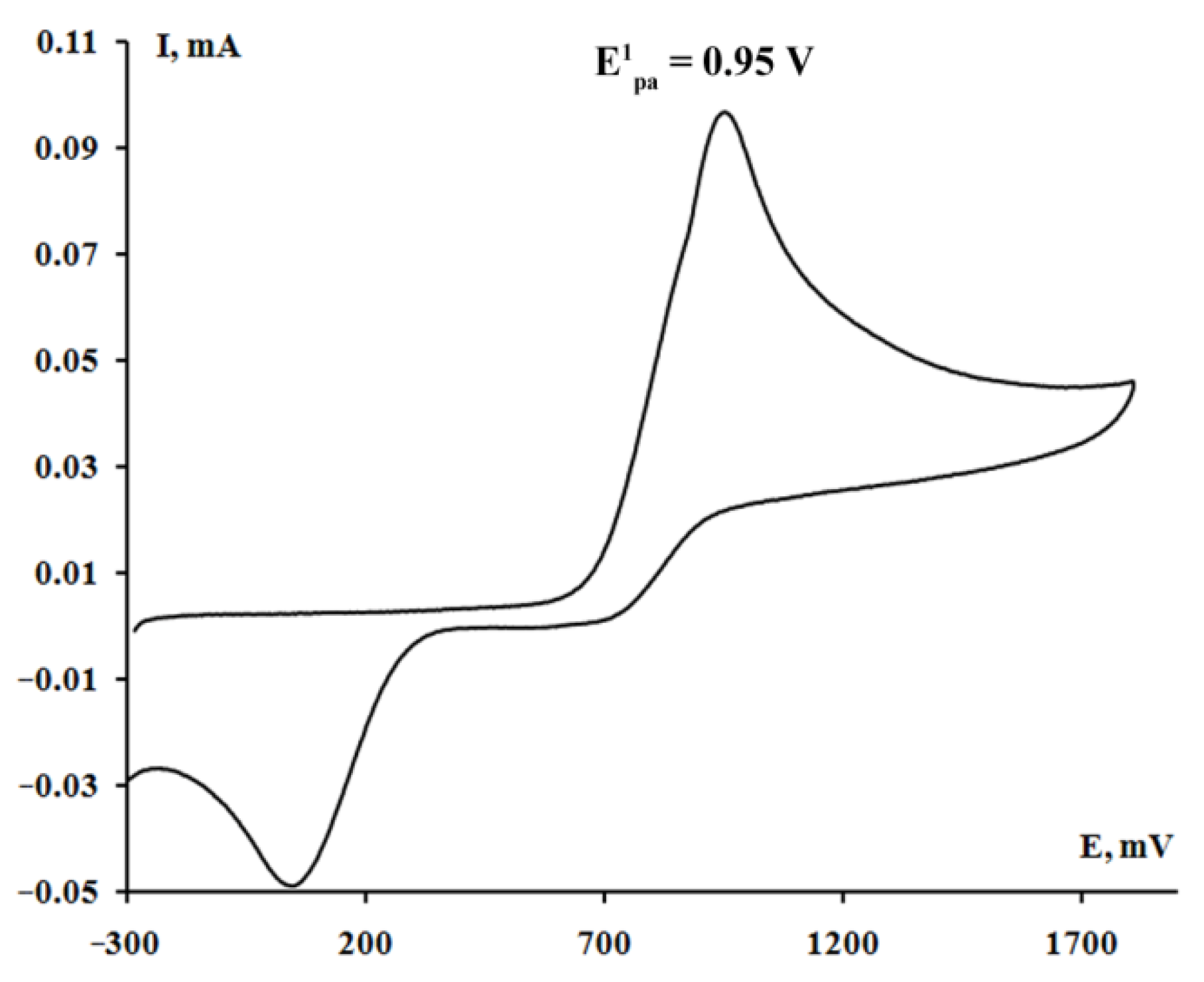
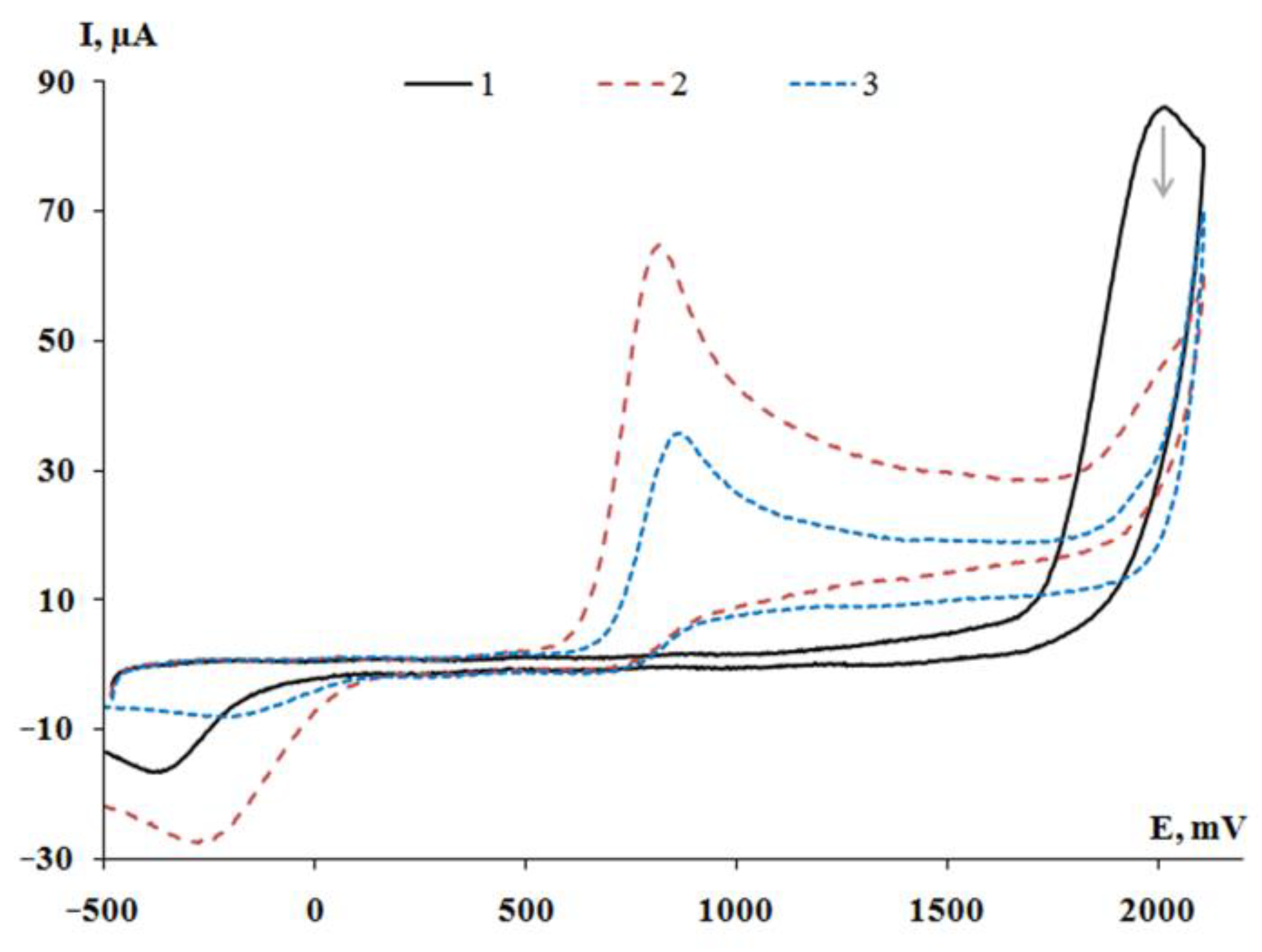
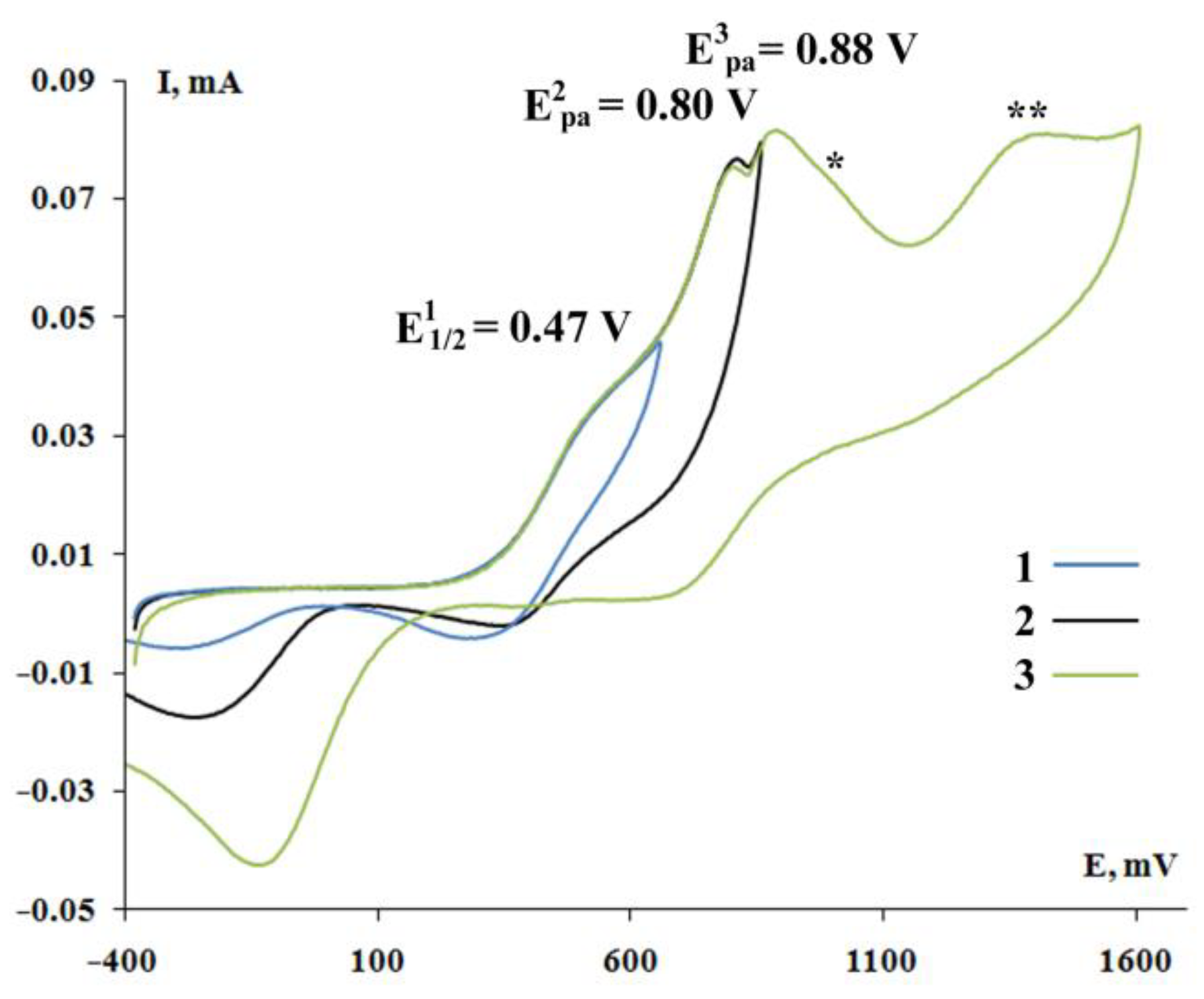
2.4. Cyclic Voltammetry
3. Materials and Methods
3.1. General
3.2. The X-ray Diffraction
3.3. Synthesis and Characterization
3.3.1. The General Synthetic Method of Binuclear Triphenylantimony(V) Complexes with Linker N-Donor Ligands
3.3.2. [(3,6-. DBCat)SbPh3]2(Pyr) (1)
3.3.3. [(3,6-. DBCat)SbPh3]2·(PySSPy) (2)
3.3.4. [(3,6-. DBCat)SbPh3]2·(Bipy) (3)
3.3.5. [(3,6-. DBCat)SbPh3]2·(DABCO) (4)
3.3.6. [(3,5-. DBCat)SbPh3]2·(Bipy) (5)
3.3.7. [(4,5-. pip-3,6-DBCat)SbPh3]2·(Bipy) (6)
3.3.8. [(4,5-. Cl2-3,6-DBCat)SbPh3]2·(Bipy) (7)
3.3.9. [(4,5-(MeO)2-3,6-. DBCat)SbPh3]2·(Bipy) (8)
3.3.10. [(3,5-. DBCat)SbPh3]2·(DABCO) (9)
3.3.11. [(4,5-. pip-3,6-DBCat)SbPh3]2·(DABCO) (10)
3.3.12. [(4,5-. Cl2-3,6-DBCat)SbPh3]2·(DABCO) (11)
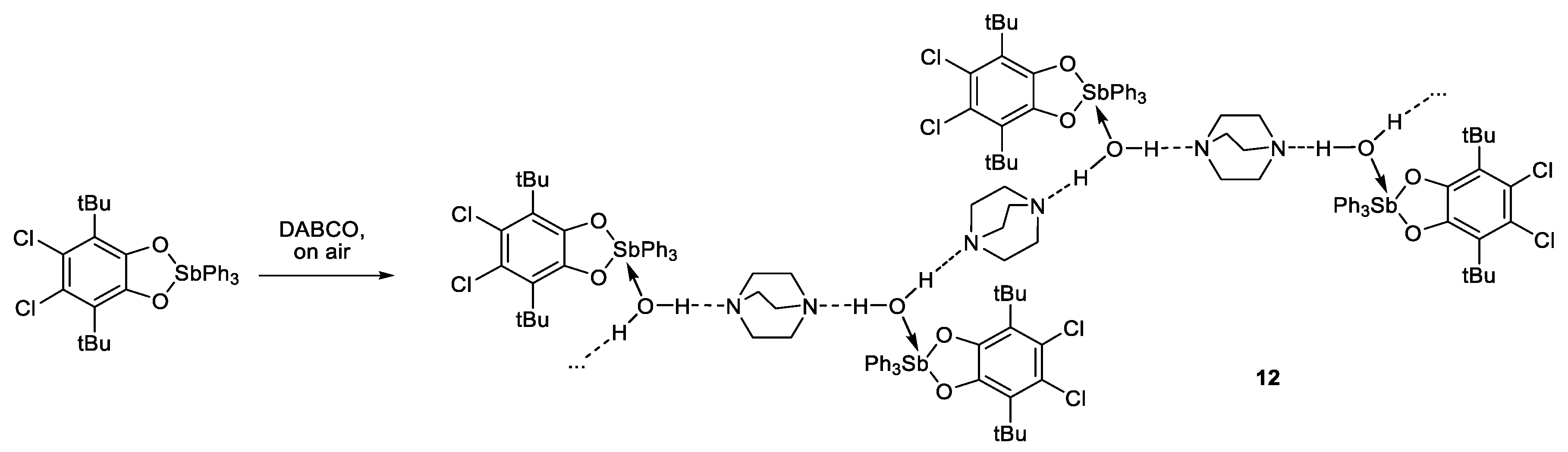
3.3.13. {[(4,5-. Cl2-3,6-DBCat)SbPh3·H2O]·DABCO}n (12)
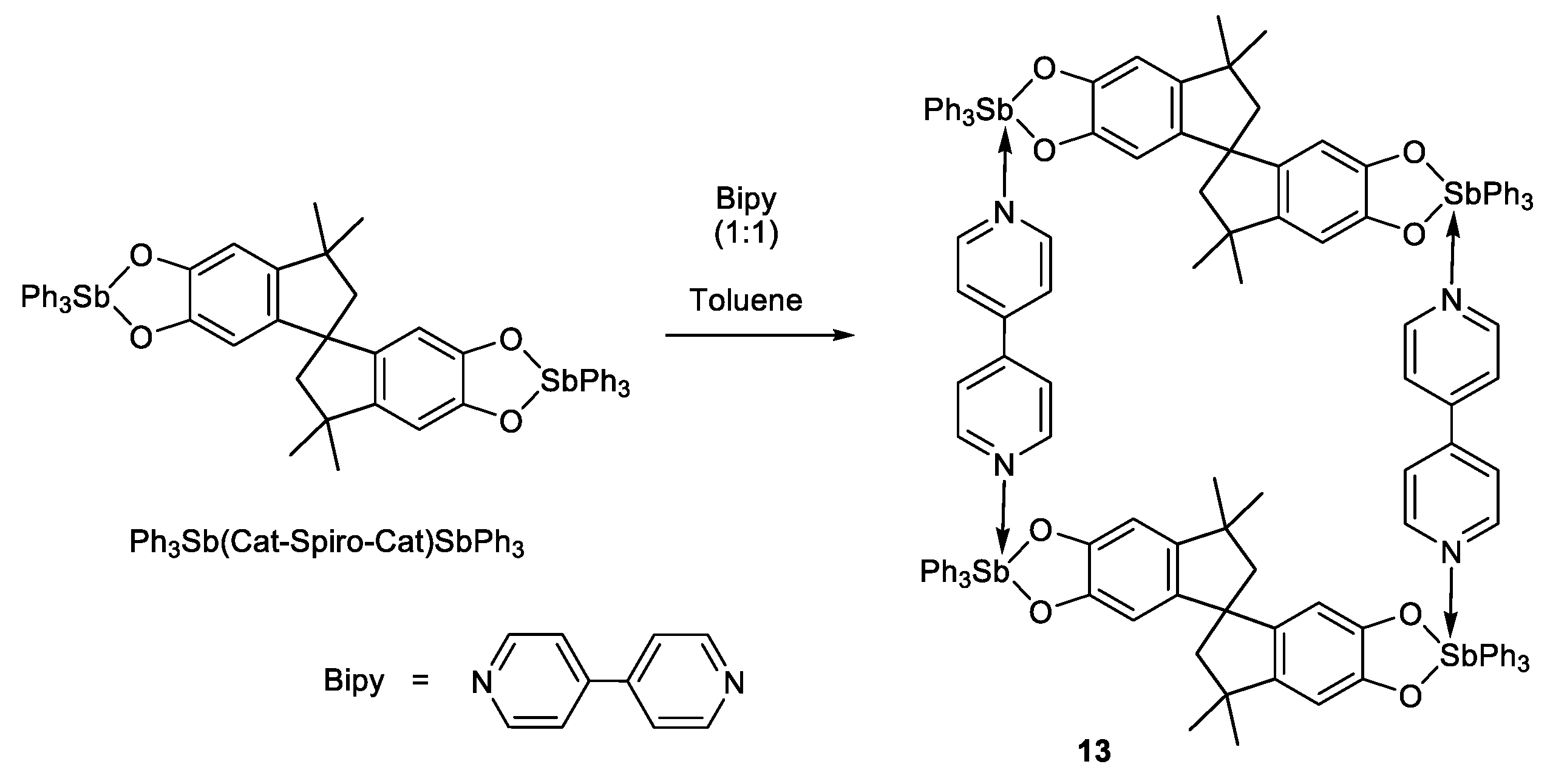
3.3.14. Tetranuclear complex {Ph3Sb(Cat-Spiro-Cat)SbPh3∙(Bipy)}2 (13)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Caracelli, I.; Haiduc, I.; Zukerman-Schpector, J.; Tiekink, E.R.T. Delocalised antimony(lone pair)- and bismuth-(lone pair)…π(arene) interactions: Supramolecular assembly and other considerations. Coord. Chem. Rev. 2013, 257, 2863–2879. [Google Scholar] [CrossRef]
- Ke, I.-S.; Gabbaï, F.P. σ-Donor/Acceptor-Confused Ligands: The Case of a Chlorostibine. Inorg. Chem. 2013, 52, 7145–7151. [Google Scholar] [CrossRef]
- Jones, J.S.; Wade, C.R.; Gabbaï, F.P. Redox and Anion Exchange Chemistry of a Stibine–Nickel Complex: Writing the L, X, Z Ligand Alphabet with a Single Element. Angew. Chem. Int. Ed. 2014, 53, 8876–8879. [Google Scholar] [CrossRef]
- Hirai, M.; Cho, J.; Gabbaï, F.P. Promoting the Hydrosilylation of Benzaldehyde by Using a Dicationic Antimony-Based Lewis Acid: Evidence for the Double Electrophilic Activation of the Carbonyl Substrate. Chem. Eur. J. 2016, 22, 6537–6541. [Google Scholar] [CrossRef]
- Piesch, M.; Gabbaï, F.P.; Scheer, M. Phosphino-Stibine Ligands for the Synthesis of Heterometallic Complexes. Z. Anorg. Allgem. Chem. 2021, 647, 266–278. [Google Scholar] [CrossRef]
- Hering-Junghans, C.; Thomas, M.; Villinger, A.; Schulz, A. Synthesis of Elusive Chloropnictenium Ions. Chem. Eur. J. 2015, 21, 6713–6717. [Google Scholar] [CrossRef]
- Furan, S.; Hupf, E.; Boidol, J.; Brünig, J.; Lork, E.; Mebs, S.; Beckmann, J. Transition metal complexes of antimony centered ligands based upon acenaphthyl scaffolds. Coordination non-innocent or not? Dalton Trans. 2019, 48, 4504–4513. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Poddel’sky, A.I.; Sharutina, O.K. Aryl Compounds of Pentavalent Antimony: Syntheses, Reactions, and Structures. Russ. J. Coord. Chem. 2020, 46, 663–728. [Google Scholar] [CrossRef]
- Christianson, A.M.; Gabbaï, F.P. Antimony-and bismuth-based materials and applications. In Main Group Strategies towards Functional Hybrid Materials; Baumgartner, T., Jaekle, F., Eds.; John Wiley & Sons: Weinheim, Germany, 2018; pp. 405–432. ISBN 9781119235972. [Google Scholar]
- Qiu, J.; Unruh, D.K.; Cozzolino, A.F. Design, Synthesis, and Structural Characterization of a Bisantimony(III) Compound for Anion Binding and the Density Functional Theory Evaluation of Halide Binding through Antimony Secondary Bonding Interactions. J. Phys. Chem. A 2016, 120, 9257–9269. [Google Scholar] [CrossRef]
- Johnson, J.A.; Venugopal, A. Probing the Lewis acidity of heavier pnictogen trichlorides. J. Chem. Sci. 2019, 131, 114. [Google Scholar] [CrossRef] [Green Version]
- Nag, E.; Kulkarni, A.; Gorantla, S.M.N.V.T.; Graw, N.; Francis, M.; Herbst-Irmer, R.; Stalke, D.; Roesky, H.W.; Mondal, K.C.; Roy, S. Fluorescent organo-antimony compounds as precursors for syntheses of redox-active trimeric and dimeric alkali metal antimonides: An insight into electron transfer reduction processes. Dalton Trans. 2022, 51, 1791–1805. [Google Scholar] [CrossRef]
- Jones, J.S.; Gabbaï, F.P. Coordination- and Redox-Noninnocent Behavior of Ambiphilic Ligands Containing Antimony. Acc. Chem. Res. 2016, 49, 857–867. [Google Scholar] [CrossRef]
- You, D.; Zhou, B.; Hirai, M.; Gabbaï, F.P. Distiboranes based on ortho-phenylene backbones as bidentate Lewis acids for fluoride anion chelation. Org. Biomol. Chem. 2021, 19, 4949–4957. [Google Scholar] [CrossRef]
- Kořenková, M.; Hejda, M.; Erben, M.; Jirásko, R.; Jambor, R.; Růžička, A.; Rychagova, E.; Ketkov, S.; Dostál, L. Reversible C=C Bond Activation by an Intramolecularly Coordinated Antimony(I) Compound. Chem. Eur. J. 2019, 25, 12884–12888. [Google Scholar] [CrossRef]
- Astaf’eva, T.V.; Rumyantcev, R.V.; Arsenyev, M.V.; Fukin, G.K.; Cherkasov, V.K.; Poddel’sky, A.I. 1D Coordination polymers based on triphenylantimony(V) 3-formyl-substituted catecholates. J. Organomet. Chem. 2022, 958, 122190. [Google Scholar] [CrossRef]
- Pugh, T.; Chilton, N.F.; Layfield, R.A. Antimony-ligated dysprosium single-molecule magnets as catalysts for stibine dehydrocoupling. Chem. Sci. 2017, 8, 2073–2080. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Peng, L.; Qiu, R.; Liu, Y.; Chen, Y.; Auc, C.-T.; Yin, S.-F. Establishing the correlation between catalytic performance and N→Sb donor–acceptor interaction: Systematic assessment of azastibocine halide derivatives as water tolerant Lewis acids. Dalton Trans. 2019, 48, 8478–8487. [Google Scholar] [CrossRef]
- Sharma, D.; Balasubramaniam, S.; Kumar, S.; Jemmis, E.D.; Venugopal, A. Reversing Lewis acidity from bismuth to antimony. Chem. Commun. 2021, 57, 8889–8892. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, J.; Ding, W.-Y.; Li, S.; Xiang, S.-H.; Tan, B. Asymmetric Pnictogen-Bonding Catalysis: Transfer Hydrogenation by a Chiral Antimony(V) Cation/Anion Pair. J. Am. Chem. Soc. 2021, 143, 6382–6387. [Google Scholar] [CrossRef]
- You, D.; Gabbaï, F.P. Tunable σ-Accepting, Z-Type Ligands for Organometallic Catalysis. Trends Chem. 2019, 1, 485–496. [Google Scholar] [CrossRef]
- Ge, R.; Sun, H. Bioinorganic Chemistry of Bismuth and Antimony: Target Sites of Metallodrugs. Acc. Chem. Res. 2007, 40, 267–274. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C. Chemistry and Some Biological Potential of Bismuth and Antimony Dithiocarbamate Complexes. Molecules 2020, 25, 305. [Google Scholar] [CrossRef] [Green Version]
- Saleem, L.; Altaf, A.A.; Badshah, A.; Rauf, M.K.; Waseem, A.; Danish, M.; Azam, S.S.; Arshad, M.N.; Asiri, A.M.; Ahmad, S.; et al. Structural investigations, anti-leishmanial, antibacterial and docking studies of new pentavalent antimony carboxylates. Inorg. Chim. Acta 2018, 474, 148–155. [Google Scholar] [CrossRef]
- Kapetana, M.; Banti, C.N.; Papachristodoulou, C.; Psycharis, V.; Raptopoulou, C.P.; Hadjikakou, S.K. Conjugation of triphenylantimony(V) with carvacrol against human breast cancer cells. J. Biol. Inorg. Chem. 2022, 27, 373–389. [Google Scholar] [CrossRef]
- Sharma, P.; Perez, D.; Cabrera, A.; Rosas, N.; Arias, J.L. Perspectives of antimony compounds in oncology. Acta Pharmacol. Sin. 2008, 29, 881–890. [Google Scholar] [CrossRef]
- Duffin, R.N.; Blair, V.L.; Kedzierski, L.; Andrews, P.C. Development of new combination anti-leishmanial complexes: Triphenyl Sb(V) mono-hydroxy mono-quinolinolates. J. Inorg. Biochem. 2021, 219, 111385. [Google Scholar] [CrossRef]
- Duffin, R.N.; Blair, V.L.; Kedzierski, L.; Andrews, P.C. Anti-leishmanial activity and cytotoxicity of a series of tris-aryl Sb(V) mandelate cyclometallate complexes. J. Inorg. Biochem. 2020, 203, 110932. [Google Scholar] [CrossRef]
- Duffin, R.N.; Werrett, M.V.; Andrews, P.C. Chapter Seven—Antimony and bismuth as antimicrobial agents. In Advances in Inorganic Chemistry; Sadler, P.J., van Eldik, R., Eds.; Academic Press: Oxford, UK, 2020; Volume 75, pp. 207–255. ISBN 9780128191965. [Google Scholar] [CrossRef]
- Hadjikakou, S.K.; Ozturk, I.I.; Banti, C.N.; Kourkoumelis, N.; Hadjiliadis, N. Recent advances on antimony(III/V) compounds with potential activity against tumor cells. J. Inorg. Biochem. 2015, 153, 293–305. [Google Scholar] [CrossRef]
- Ke, I.-S.; Myahkostupov, M.; Castellano, F.N.; Gabbaï, F.P. Stibonium Ions for the Fluorescence Turn-On Sensing of F– in Drinking Water at Parts per Million Concentrations. J. Am. Chem. Soc. 2012, 134, 15309–15311. [Google Scholar] [CrossRef]
- Wade, C.R.; Ke, I.-S.; Gabbaï, F.P. Sensing of Aqueous Fluoride Anions by Cationic Stibine–Palladium Complexes. Angew. Chem. Int. Ed. 2012, 51, 478–481. [Google Scholar] [CrossRef]
- Hirai, M.; Gabbaï, F.P. Squeezing Fluoride out of Water with a Neutral Bidentate Antimony(V) Lewis Acid. Angew. Chem. Int. Ed. 2015, 54, 1205–1209. [Google Scholar] [CrossRef]
- Christianson, A.M.; Rivard, E.; Gabbaï, F.P. 1λ5-Stibaindoles as Lewis Acidic, π-Conjugated, Fluoride Anion Responsive Platforms. Organometallics 2017, 36, 2670–2676. [Google Scholar] [CrossRef]
- Pan, B.; Gabbaï, F.P. [Sb(C6F5)4][B(C6F5)4]: An Air Stable, Lewis Acidic Stibonium Salt That Activates Strong Element-Fluorine Bonds. J. Am. Chem. Soc. 2014, 136, 9564–9567. [Google Scholar] [CrossRef]
- Jones, J.S.; Gabbai, F.P. Activation of an Au−Cl Bond by a Pendent SbIII Lewis Acid: Impact on Structure and Catalytic Activity. Chem. Eur. J. 2017, 23, 1136–1144. [Google Scholar] [CrossRef]
- Yang, M.; Pati, N.; Bélanger-Chabot, G.; Hirai, M.; Gabbaï, F.P. Influence of the catalyst structure in the cycloaddition of isocyanates to oxiranes promoted by tetraarylstibonium cations. Dalton Trans. 2018, 47, 11843–11850. [Google Scholar] [CrossRef]
- Ugarte, R.A.; Devarajan, D.; Mushinski, R.M.; Hudnall, T.W. Antimony(V) cations for the selective catalytic transformation of aldehydes into symmetric ethers, α,β-unsaturated aldehydes, and 1,3,5-trioxanes. Dalton Trans. 2016, 45, 11150–11161. [Google Scholar] [CrossRef]
- Ugarte, R.A.; Hudnall, T.W. Antimony(V) catalyzed acetalisation of aldehydes: An efficient, solvent-free, and recyclable process. Green Chem. 2017, 19, 1990–1998. [Google Scholar] [CrossRef]
- You, D.; Gabbaï, F.P. Unmasking the Catalytic Activity of a Platinum Complex with a Lewis Acidic, Non-innocent Antimony Ligand. J. Am. Chem. Soc. 2017, 139, 6843–6846. [Google Scholar] [CrossRef]
- Smith, J.E.; Yang, H.; Gabbaï, F.P. An Electrophilic, Intramolecularly Base-Stabilized Platinum–Antimony Complex. Organometallics 2021, 40, 3886–3892. [Google Scholar] [CrossRef]
- You, D.; Smith, J.E.; Sen, S.; Gabbaï, F.P. A Stiboranyl Platinum Triflate Complex as an Electrophilic Catalyst. Organometallics 2020, 39, 4169–4173. [Google Scholar] [CrossRef]
- Tofan, D.; Gabbaï, F.P. Fluorinated antimony(V) derivatives: Strong Lewis acidic properties and application to the complexation of formaldehyde in aqueous solutions. Chem. Sci. 2016, 7, 6768–6778. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-H.; Gabbaï, F.P. Fluoride Anion Complexation by a Triptycene-Based Distiborane: Taking Advantage of a Weak but Observable C−H⋅⋅⋅F Interaction. Angew. Chem. Int. Ed. 2017, 56, 1799–1804. [Google Scholar] [CrossRef]
- Hirai, M.; Gabbaï, F.P. Lewis acidic stiborafluorenes for the fluorescence turn-on sensing of fluoride in drinking water at ppm concentrations. Chem. Sci. 2014, 5, 1886–1893. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, Y.; Li, Y.; Duan, Y.; Qian, Y.; Zhang, P.; Guo, Q.; Ding, J. Polymeric Membrane Fluoride-Selective Electrodes Using Lewis Acidic Organo-Antimony(V) Compounds as Ionophores. ACS Sens. 2020, 5, 3465–3473. [Google Scholar] [CrossRef]
- Abakumov, G.A.; Poddel’sky, A.I.; Grunova, E.V.; Cherkasov, V.K.; Fukin, G.K.; Kurskii, Y.A.; Abakumova, L.G. Reversible Binding of Dioxygen by a Non-transition-Metal Complex. Angew. Chem. Int. Ed. 2005, 44, 2767–2771. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Kurskii, Y.A.; Piskunov, A.V.; Somov, N.V.; Cherkasov, V.K.; Abakumov, G.A. The triphenylantimony(V) o-amidophenolates with unsymmetrical N-aryl group for a reversible dioxygen binding. Appl. Organomet. Chem. 2011, 25, 180–189. [Google Scholar] [CrossRef]
- Cherkasov, V.K.; Abakumov, G.A.; Grunova, E.V.; Poddel’sky, A.I.; Fukin, G.K.; Baranov, E.V.; Kurskii, Y.A.; Abakumova, L.G. Triphenylantimony(v) Catecholates and o-Amidophenolates: Reversible Binding of Molecular Oxygen. Chem. Eur. J. 2006, 12, 3916–3927. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Kurskii, Y.A.; Fukin, G.K.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. New morpholine- and piperazine-functionalised triphenylantimony(V) catecholates: The spectroscopic and electrochemical studies. J. Organomet. Chem. 2010, 695, 1215–1224. [Google Scholar] [CrossRef]
- Fukin, G.K.; Baranov, E.V.; Poddel’sky, A.I.; Cherkasov, V.K.; Abakumov, G.A. Reversible Binding of Molecular Oxygen to Catecholate and Amidophenolate Complexes of SbV: Electronic and Steric Factors. ChemPhysChem 2012, 13, 3773–3776. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Antonova, N.A.; Poddel’sky, A.I.; Smolyaninova, S.A.; Osipova, V.P.; Berberova, N.T. Radical scavenging activity of sterically hindered catecholate and o-amidophenolate complexes of LSbVPh3 type. J. Organomet. Chem. 2011, 696, 2611–2620. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Poddel’skii, A.I.; Antonova, N.A.; Smolyaninova, S.A.; Berberova, N.T. Antiradical activity of morpholine- and piperazine-functionalized triphenylantimony(V) catecholates. Russ. J. Coord. Chem. 2013, 39, 165–174. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Fukin, G.K.; Berberova, N.T.; Poddel’sky, A.I. Triphenylantimony(V) Catecholates of the type (3-RS-4,6-DBCat)SbPh3—Catechol Thioether Derivatives: Structure, Electrochemical Properties and Antiradical Activity. Molecules 2021, 26, 2171. [Google Scholar] [CrossRef] [PubMed]
- Smolyaninov, I.V.; Antonova, N.A.; Poddel’sky, A.I.; Osipova, V.P.; Berberova, N.T.; Pimenov, Y.T. The influence of some triphenylantimony(V) catecholates and o-amidophenolates on the lipid peroxidation in vitro. Appl. Organomet. Chem. 2012, 26, 277–283. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Antonova, N.A.; Poddel’sky, A.I.; Smolyaninova, S.A.; Osipova, V.P.; Luzhnova, S.A.; Berberova, N.T.; Pimenov, Y.T. The influence of triphenylantimony(V) catecholate and its spiroendoperoxide on lipid peroxidation. Appl. Organomet. Chem. 2014, 28, 274–279. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Kurskii, Y.A.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. New dioxygen-inert triphenylantimony(V) catecholate complexes based on o-quinones with electron-withdrawing groups. Russ. Chem. Bull. Int. Ed. 2009, 58, 532–537. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Vavilina, N.N.; Kurskii, Y.A.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Triaryl- and trialkylantimony(V) Bis(catecholates) based on 1,1′-spirobis[3,3-dimethylindanequinone-5,6]: Spectroscopic and electrochemical studies. Russ. J. Coord. Chem. 2012, 38, 284–294. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Fukin, G.K.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. 3,6-Di-tert-butylcatecholates of trialkyl/triarylantimony(V). J. Organomet. Chem. 2018, 867, 238–245. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Berberova, N.T.; Fukin, G.K.; Cherkasov, V.K.; Abakumov, G.A. Triaryl/trialkylantimony(V) catecholates with electron-acceptor groups. J. Organomet. Chem. 2015, 789–790, 8–13. [Google Scholar] [CrossRef]
- Poddel’skii, A.I.; Ilyakina, E.V.; Smolyaninov, I.V.; Fukin, G.K.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Complexes of triphenylantimony(V) catecholates with ammonium salts. Spectroscopic and electrochemical investigations. Russ. Chem. Bull. Int. Ed. 2014, 63, 923–929. [Google Scholar] [CrossRef]
- Okhlopkova, L.S.; Poddel’sky, A.I.; Smolyaninov, I.V.; Fukin, G.K.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Triphenylantimony(V) Catecholato Complexes with 4-(2,6-Dimethylphenyliminomethyl)pyridine. Structure, Redox Properties: The Influence of Pyridine Ligand. J. Organomet. Chem. 2019, 897, 32–41. [Google Scholar] [CrossRef]
- Okhlopkova, L.S.; Smolyaninov, I.V.; Baranov, E.V.; Poddel’skii, A.I. Mononuclear Antimony(V) Catecholate Complexes with Additional Pyridine Ligands. Rus. J. Coord. Chem. 2020, 46, 466–476. [Google Scholar] [CrossRef]
- Tian, Z.; Tuck, D.G. Oxidation of elemental antimony by substituted ortho-benzoquinones. J. Chem. Soc. Dalton Trans. 1993, 9, 1381–1385. [Google Scholar] [CrossRef]
- Holmes, R.R.; Day, R.O.; Chandrasekhar, V.; Holmes, J.M. Pentacoordinated molecules. 67. Formation and structure of cyclic five-coordinated antimony derivatives. The first square-pyramidal geometry for a bicyclic stiborane. Inorg. Chem. 1987, 26, 157–163. [Google Scholar] [CrossRef]
- Dodonov, V.A.; Fedorov, A.Y.; Fukin, G.K.; Zaburdyaeva, S.N.; Zakharov, L.N.; Ignatenko, A.V. Synthesis and Structural Characterization of Some Complexes of Hexa-coordinated Antimony. Main Group Chem. 1999, 3, 15–22. [Google Scholar] [CrossRef]
- Hall, M.; Sowerby, D.B. Synthesis and crystal structure of bis(triphenylantimony catecholate)hydrate. A new sqare-pyramidal antimony(V) compound. J. Am. Chem. Soc. 1980, 102, 628–632. [Google Scholar] [CrossRef]
- Gibbons, M.N.; Begley, M.J.; Blake, A.J.; Sowerby, D.B. New square-pyramidal organoantimony(V) compounds; crystal structures of (biphenyl-2,2′-diyl)phenylantimony(V) dibromide, dichloride and diisothiocyanate, Sb(2,2′-C12H8)PhX2 (X = Br, Cl or NCS), and of octahedral SbPh(o-O2C6Cl4)Cl2 OEt2. J. Chem. Soc. Dalton Trans. 1997, 14, 2419–2426. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Astaf’eva, T.V.; Smolyaninov, I.V.; Arsenyev, M.V.; Fukin, G.K.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Triphenylantimony(V) 6-alkoxymethyl-3,5-di-tert-butylcatecholates. Structure and redox-properties. J. Organomet. Chem. 2018, 873, 57–65. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Poddel’sky, A.I.; Smolyaninova, S.A.; Arsenyev, M.V.; Fukin, G.K.; Berberova, N.T. Polyfunctional sterically hindered catechols with additional phenolic group and their triphenylantimony(V) catecholates: Synthesis, structure, and redox properties. Molecules 2020, 25, 1770. [Google Scholar] [CrossRef]
- Brown, S.N. Metrical Oxidation States of 2-Amidophenoxide and Catecholate Ligands: Structural Signatures of Metal−Ligand π Bonding in Potentially Noninnocent Ligands. Inorg. Chem. 2012, 51, 1251–1260. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Arsenyev, M.V.; Astaf’eva, T.V.; Chesnokov, S.A.; Fukin, G.K.; Abakumov, G.A. New sterically-hindered 6th-substituted 3,5-di-tert-butylcatechols/oquinones with additional functional groups and their triphenylantimony(V) catecholates. J. Organomet. Chem. 2017, 835, 17–24. [Google Scholar] [CrossRef]
- Batsanov, S.S. The atomic radii of the elements. Russ. J. Inorg. Chem. 1991, 36, 1694–1706. [Google Scholar]
- Cordero, B.; Gómez, V.; Platero-Prats, A.E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 21, 2832–2838. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Mantina, M.; Chamberlin, A.C.; Valero, R.; Cramer, C.J.; Truhlar, D.G. Consistent van der Waals Radii for the Whole Main Group. J. Phys. Chem. A 2009, 113, 5806–5812. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, M.; Näther, K.; van Leusen, J.; Kögerler, P.; Zhechkov, N.; Heine, T.; Bensch, W. Covalent Co−O−V and Sb−N Bonds Enable Polyoxovanadate Charge Control. Inorg. Chem. 2017, 56, 7120–7126. [Google Scholar] [CrossRef] [Green Version]
- Fukin, G.K.; Baranov, E.V.; Jelsch, C.; Guillot, B.; Poddelskii, A.I.; Cherkasov, V.K.; Abakumov, G.A. Experimental and Theoretical Investigation of Topological and Energetic Characteristics of Sb Complexes Reversibly Binding Molecular Oxygen. J. Phys. Chem. A 2011, 115, 8271–8281. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in Molecules—A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Poddel’skiy, A.I.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Electrochemical transformations of catecholate and o-amidophenolate complexes with triphenylantimony(V). Russ. J. Coord. Chem. 2010, 36, 644–650. [Google Scholar] [CrossRef]
- Gordon, A.; Ford, R. The Chemist’s Companion; Russian translation; Mir: Moscow, Russia, 1976; pp. 437–444. [Google Scholar]
- Cherkasov, V.K.; Grunova, E.V.; Poddel’sky, A.I.; Fukin, G.K.; Kurskii, Y.A.; Abakumova, L.G.; Abakumov, G.A. Oxidative addition reaction of o-quinones to triphenylantimony. Novel triphenylantimony catecholate complexes. J. Organomet. Chem. 2005, 690, 1273–1281. [Google Scholar] [CrossRef]
- SAINT; Data Reduction and Correction Program; Bruker AXS: Madison, WI, USA, 2014.
- Rigaku Oxford Diffraction. CrysAlis Pro Software System, version 1.171.38.46; Rigaku Corporation: Wroclaw, Poland, 2015. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, N.K.; Coppens, P.H.I.L.I.P. Testing aspherical atom refinements on small-molecule data sets. Acta Crystallogr. Sect. A Found. Adv. 1978, 34, 909–921. [Google Scholar] [CrossRef]
- Jelsch, C.; Guillot, B.; Lagoutte, A.; Lecomte, C. Advances in protein and small-molecule charge-density refinement methods using MoPro. J. Appl. Crystallogr. 2005, 38, 38–54. [Google Scholar] [CrossRef] [Green Version]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 1987, 2, S1–S19. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Can X-ray data distinguish bonding effects from vibrational smearing? Acta Crystallogr. Sect. A Found. Adv 1976, 32, 239–244. [Google Scholar] [CrossRef]
- Stash, A.; Tsirelson, V. WinXPRO: A program for calculating crystal and molecular properties using multipole parameters of the electron density. J. Appl. Crystallogr. 2002, 35, 371–373. [Google Scholar] [CrossRef] [Green Version]

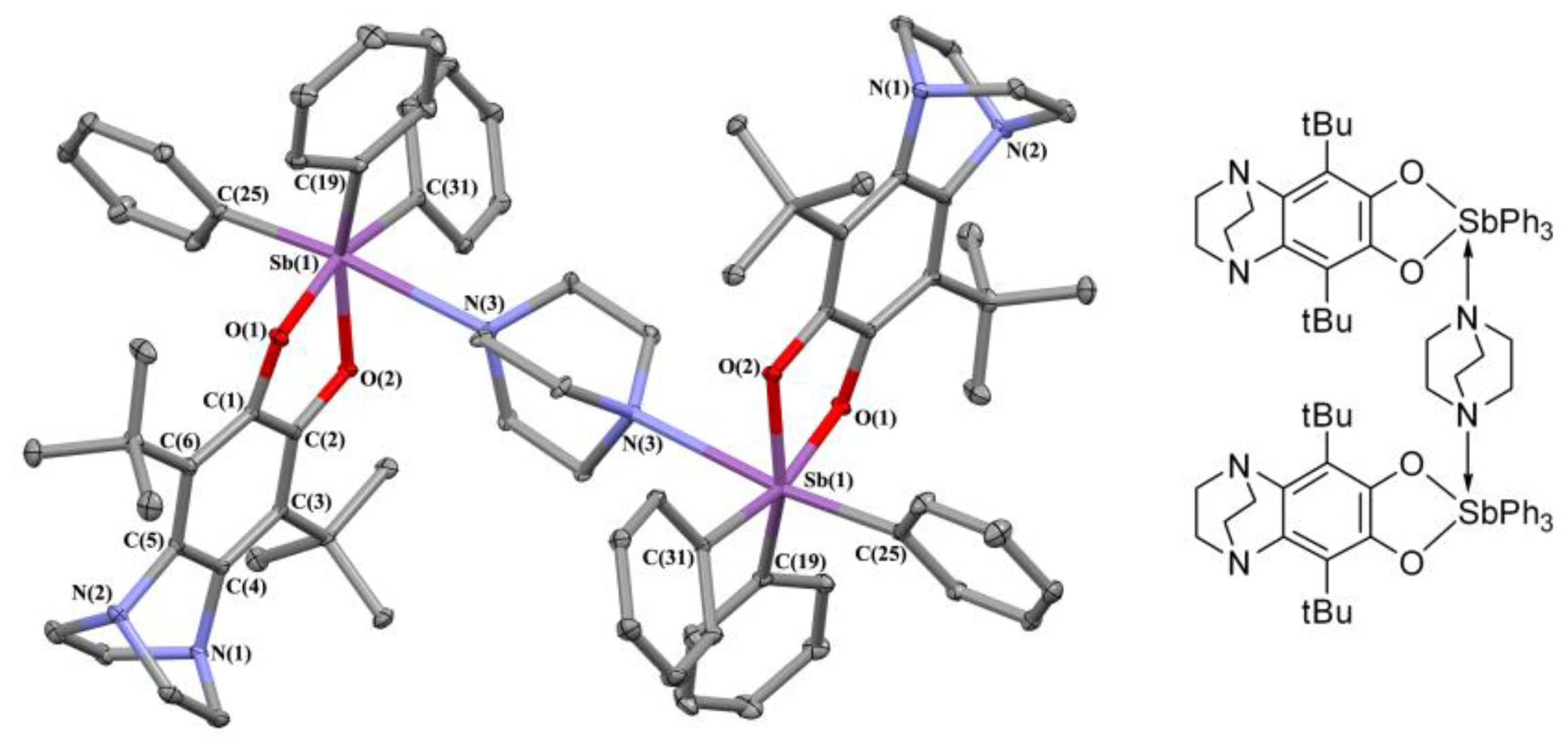



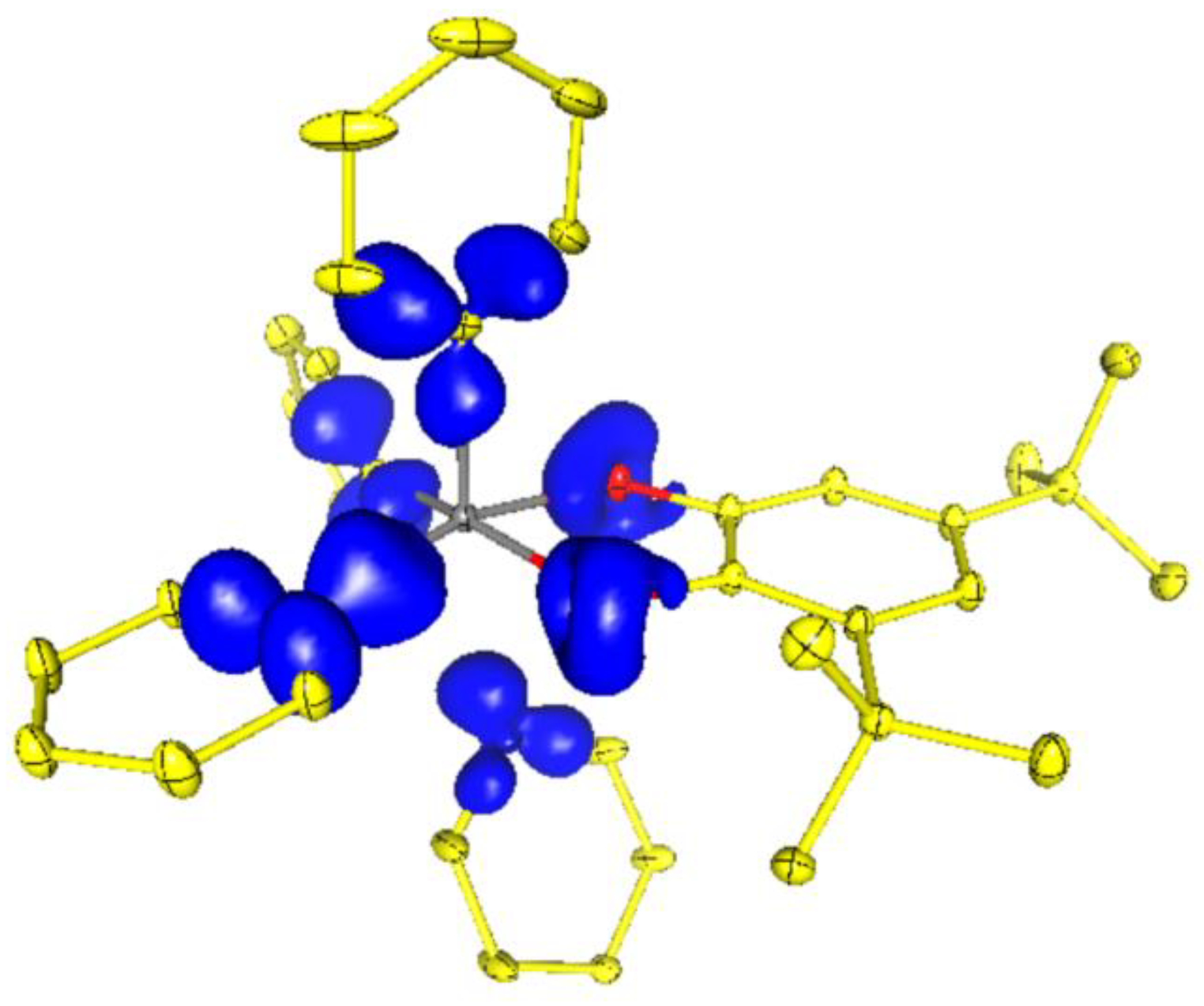
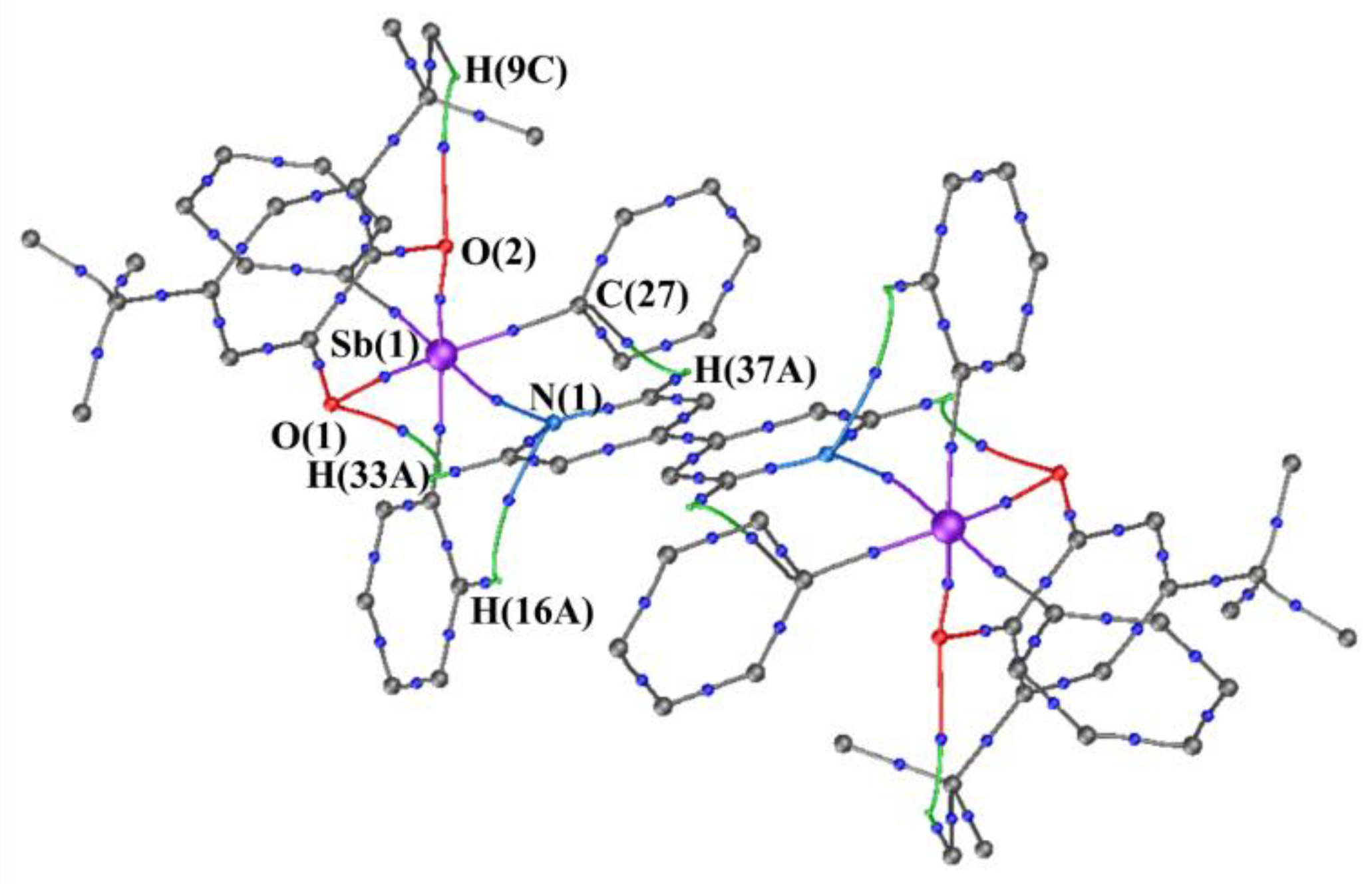
| Bond | ν(r), a.e. | ρ(r), a.e. | ∇2ρ(r), a.e. | he(r), a.e. |
|---|---|---|---|---|
| Sb(1)–O(1) | −0.178 | 0.112 | 0.332 | −0.047 |
| Sb(1)–O(2) | −0.144 | 0.092 | 0.422 | −0.019 |
| Sb(1)–N(1) | −0.024 | 0.030 | 0.083 | −0.002 |
| Sb(1)–C(15) | −0.132 | 0.098 | 0.141 | −0.048 |
| Sb(1)–C(21) | −0.156 | 0.111 | 0.105 | −0.065 |
| Sb(1)–C(27) | −0.188 | 0.126 | 0.064 | −0.086 |
| O(1)–C(1) | −0.711 | 0.300 | −0.741 | −0.448 |
| O(2)–C(2) | −0.726 | 0.304 | −0.796 | −0.462 |
| C(1)–C(2) | −0.772 | 0.315 | −0.781 | −0.484 |
| Complex | E1pa, V | E2pa, V | E3pa, V |
|---|---|---|---|
| 1 [(3,6-DBCat)SbPh3]2·(Pyr) | 0.93 | - | - |
| 2 [(3,6-DBCat)SbPh3]2·(PySSPy) | 0.97 | - | - |
| 3 [(3,6-DBCat)SbPh3]2·(Bipy) | 0.96 | - | - |
| 4 [(3,6-DBCat)SbPh3]2·(DABCO) | 0.45 * | 0.76 | 0.91 |
| 5 [(3,5-DBCat)SbPh3]2·(Bipy) | 0.95 | - | - |
| 6 [(4,5-pip-3,6-DBCat)SbPh3]2·(Bipy) | 0.73 | 1.15 | 1.46 |
| 7 [(4,5-Cl2-3,6-DBCat)SbPh3]2·(Bipy) | 1.05 | - | - |
| 9 [(3,5-DBCat)SbPh3]2·(DABCO) | 0.47 * | 0.80 | 0.88 |
| 13{Ph3Sb(Cat-Spiro-Cat)SbPh3∙(Bipy)}2 | 0.83 | 1.05 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poddel’sky, A.I.; Smolyaninov, I.V.; Shataeva, A.I.; Baranov, E.V.; Fukin, G.K. Binuclear Triphenylantimony(V) Catecholates through N-Donor Linkers: Structural Features and Redox Properties. Molecules 2022, 27, 6484. https://doi.org/10.3390/molecules27196484
Poddel’sky AI, Smolyaninov IV, Shataeva AI, Baranov EV, Fukin GK. Binuclear Triphenylantimony(V) Catecholates through N-Donor Linkers: Structural Features and Redox Properties. Molecules. 2022; 27(19):6484. https://doi.org/10.3390/molecules27196484
Chicago/Turabian StylePoddel’sky, Andrey I., Ivan V. Smolyaninov, Aleksandra I. Shataeva, Evgenii V. Baranov, and Georgy K. Fukin. 2022. "Binuclear Triphenylantimony(V) Catecholates through N-Donor Linkers: Structural Features and Redox Properties" Molecules 27, no. 19: 6484. https://doi.org/10.3390/molecules27196484
APA StylePoddel’sky, A. I., Smolyaninov, I. V., Shataeva, A. I., Baranov, E. V., & Fukin, G. K. (2022). Binuclear Triphenylantimony(V) Catecholates through N-Donor Linkers: Structural Features and Redox Properties. Molecules, 27(19), 6484. https://doi.org/10.3390/molecules27196484







