Clay-Catalyzed Ozonation of Hydrotalcite-Extracted Lactic Acid Potential Application for Preventing Milk Fermentation Inhibition
Abstract
:1. Introduction
2. Experimental Section
2.1. Catalyst Preparation and Characterization
2.2. Calibration Curves and Ozonation Experiments
2.3. Reaction Mixture Analysis
2.4. Intermediate Identification by LC-ToF-MS
3. Results and Discussion
3.1. LA UV-Vis Spectrum Changes in Clay Suspension before Ozonation
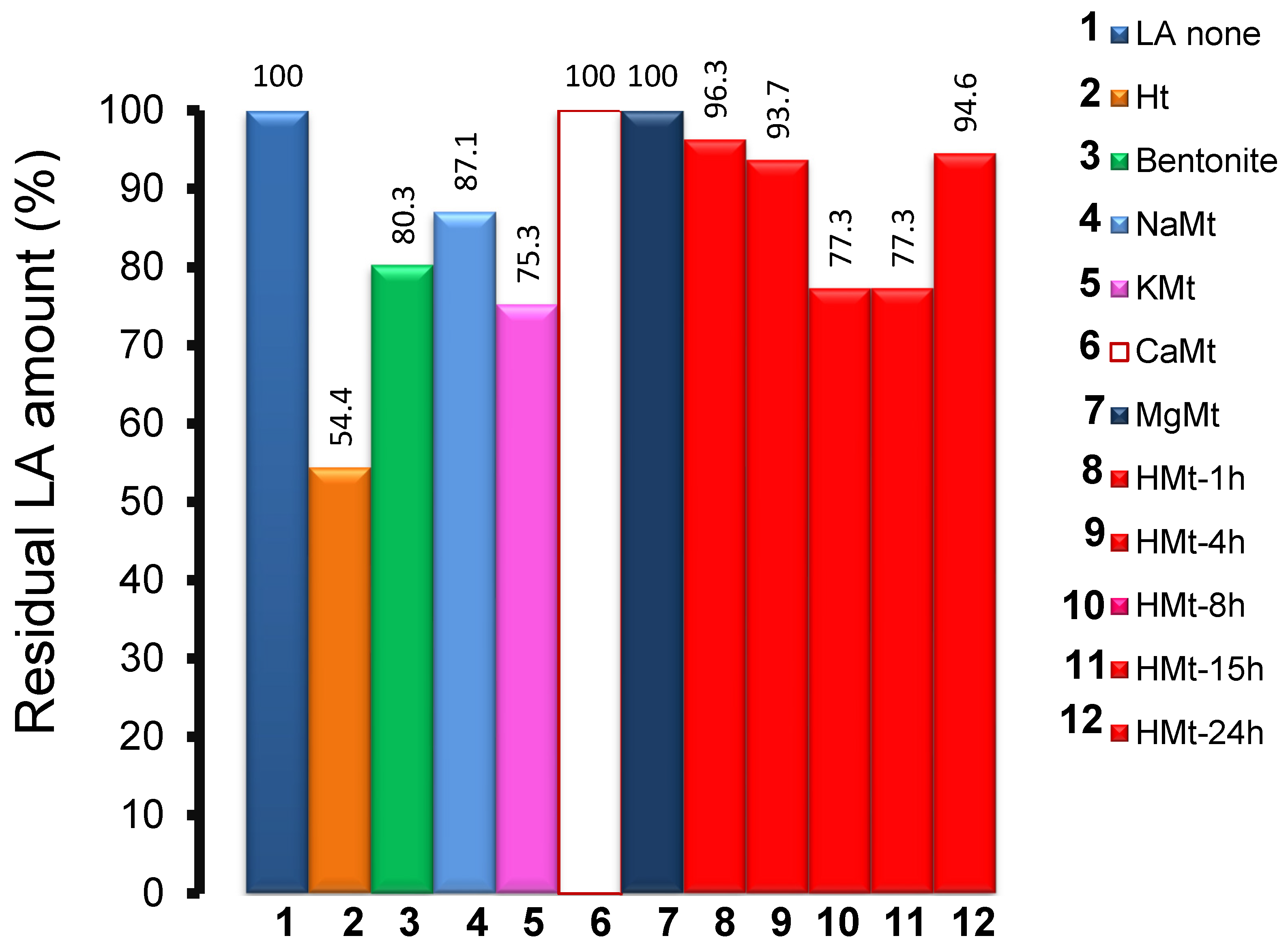
3.2. Effect of Clay-Induced pH on LA Adsorption
3.3. Effect of Catalyst Addition on LA UV-Vis Spectrum
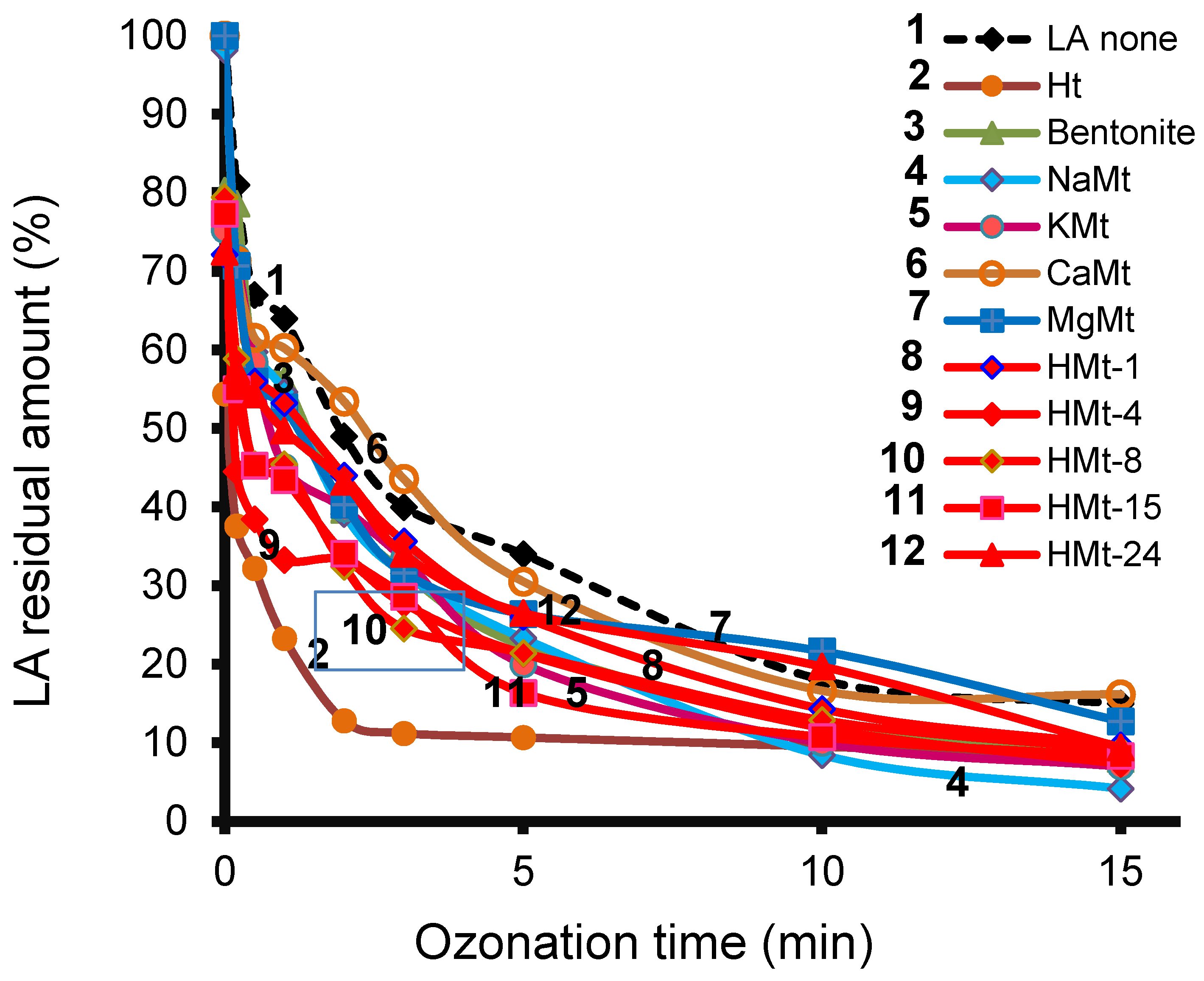
3.4. LA Depletion during Ozonation and Product Identification
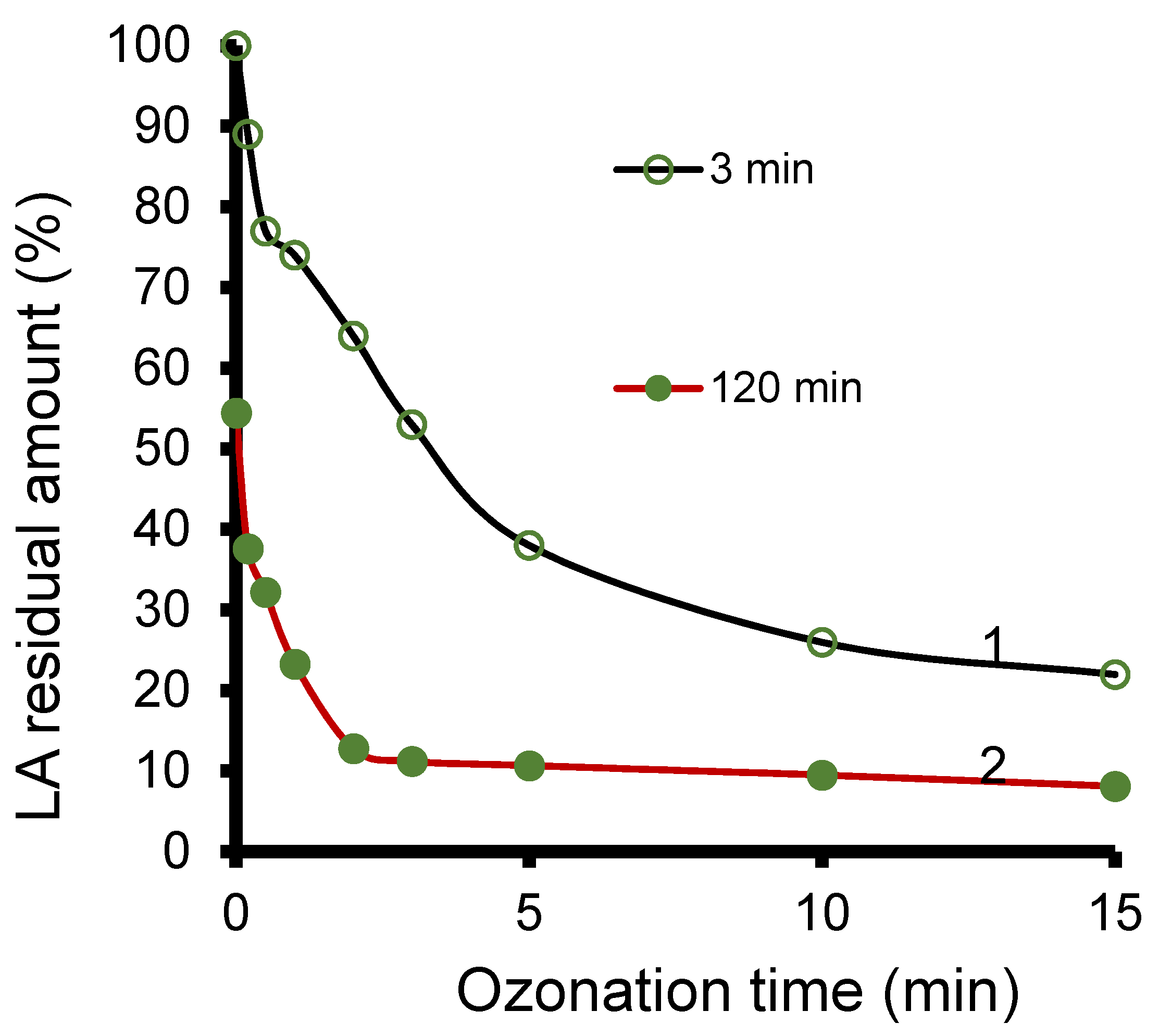
3.5. Ht-Catalyzed Ozonation
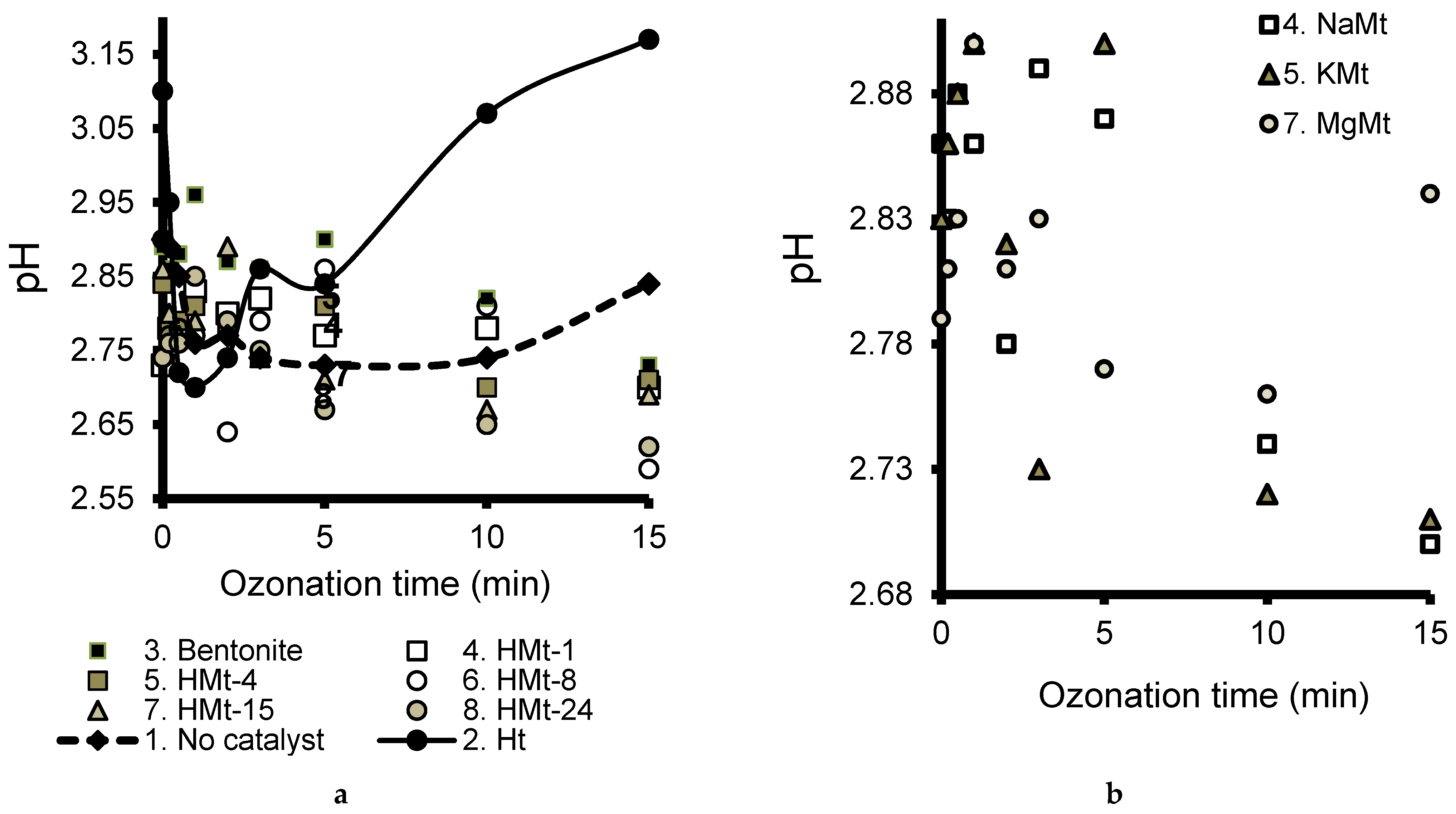
3.6. Effects of pH Evolution
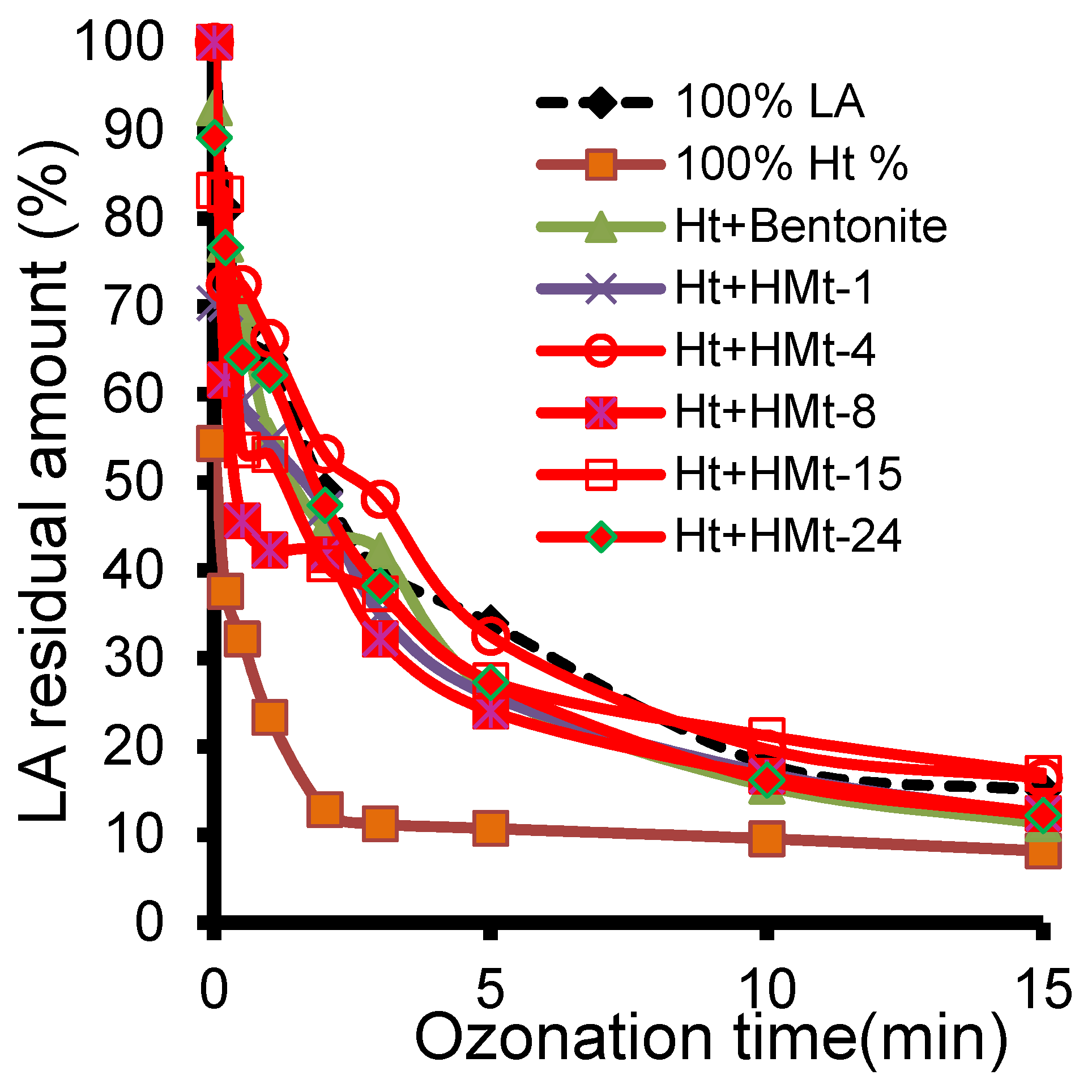
3.7. Ozonation with Ht-cationic Clay Mixtures
3.8. Ozonation Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Datta, R.; Henry, M. Lactic acid: Recent advances in products, processes and technologies—A review. J. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Varadarajan, S.; Miller, D.J. Catalytic Upgrading of Fermentation-Derived Organic Acids. Biotechnol. Prog. 1999, 15, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, T.; Irshad, M.; Anwar, Z.; Aqil, T.; Zulifqar, Z.; Tariq, A.; Kamran, M.; Ehsan, N.; Mehmood, S. Recent trends in lactic acid biotechnology: A brief review on production to purification. J. Radiat. Res. Appl. Sci. 2014, 7, 222–229. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Vidmantiene, D.; Basinskiene, L.; Cernauskas, D.; Bartkiene, E.; Cizeikiene, D. Green metrics for sustainability of biobased lactic acid from starchy biomass vs chemical synthesis. Catal. Today 2015, 239, 11–16. [Google Scholar] [CrossRef]
- Rawlings, A.V.; Davies, A.; Carlomusto, M.; Pillai, S.; Zhang, K.; Kosturko, R.; Verdejo, P.; Feinberg, C.; Nguyen, L.; Chandar, P. Effect of lactic acid isomers on keratinocyte ceramide synthesis, stratum corneum lipid levels and stratum corneum barrier function. Arch. Dermatol. Res. 1996, 288, 383–390. [Google Scholar] [CrossRef]
- McHugh, J.; Dumont, S.N.; Paradis, J.; Loan Nguyen, A.; Levesque, S.; Carrier, A. New and Simple HPLC Method for the Determination of Lactic Acid Content in Ciprofloxacin Injection. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1905–1916. [Google Scholar] [CrossRef]
- Halim, M.; Mohd Mustafa, N.A.; Othman, M.; Wasoh, H.; Kapri, M.R.; Ariff, A.B. Effect of encapsulant and cryoprotectant on the viability of probiotic Pediococcus acidilactici ATCC 8042 during freeze-drying and exposure to high acidity, bile salts and heat. LWT Food Sci. Technol. 2017, 81, 210–216. [Google Scholar] [CrossRef]
- Luedeking, R.; Piret, E.L. A kinetic study of the lactic acid fermentation. Batch process at controlled pH. J. Biochem. Microbiol. Technol. Eng. 1959, 1, 393–412. [Google Scholar] [CrossRef]
- Aguirre-Ezkauriatza, E.J.; Aguilar-Yáñez, J.M.; Ramírez-Medrano, A.; Alvarez, M.M. Production of probiotic biomass (Lactobacillus casei) in goat milk whey: Comparison of batch, continuous and fed-batch cultures. Bioresour. Technol. 2010, 101, 2837–2844. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- Broadbent, J.R.; Larsen, R.L.; Deibel, V.; Steele, J.L. Physiological and Transcriptional Response of Lactobacillus casei ATCC 334 to Acid Stress. J. Bacteriol. 2010, 192, 2445. [Google Scholar] [CrossRef] [Green Version]
- Wee, Y.; Kim, J.; Ryu, H. Biotechnological production of lactic acid and its recent applications. Food Technol. Biotechnol. 2006, 44, 163–172. [Google Scholar]
- Boyaval, P.; Corre, C.; Terre, S. Continuous lactic acid fermentation with concentrated product recovery by ultrafiltration and electrodialysis. Biotechnol. Lett. 1987, 9, 207–212. [Google Scholar] [CrossRef]
- Nomura, Y.; Iwahara, M.; Hongo, M. Lactic acid production by electrodialysis fermentation using immobilized growing cells. Biotechnol. Bioeng. 1987, 30, 788–793. [Google Scholar] [CrossRef]
- Yabannavar, V.M.; Wang, D.I.C. Extractive fermentation for lactic acid production. Biotechnol. Bioeng. 1991, 37, 1095–1100. [Google Scholar] [CrossRef]
- Srivastava, A.; Roychoudhury, P.K.; Sahai, V. Extractive lactic acid fermentation using ion-exchange resin. Biotechnol. Bioeng. 1992, 39, 607–613. [Google Scholar] [CrossRef]
- Kim, Y.H.; Moon, S.-H. Lactic acid recovery from fermentation broth using one-stage electrodialysis. J. Chem. Technol. Biotechnol. 2001, 76, 169–178. [Google Scholar] [CrossRef]
- Hábová, V.; Melzoch, K.; Rychtera, M.; Sekavová, B. Electrodialysis as a useful technique for lactic acid separation from a model solution and a fermentation broth. Desalination 2004, 162, 361–372. [Google Scholar] [CrossRef]
- Boonmee, M.; Cotano, O.; Amnuaypanich, S.; Grisadanurak, N. Improved Lactic Acid Production by In Situ Removal of Lactic Acid During Fermentation and a Proposed Scheme for Its Recovery. Arab. J. Sci. Eng. 2016, 41, 2067–2075. [Google Scholar] [CrossRef]
- Krzyżaniak, A.; Leeman, M.; Vossebeld, F.; Visser, T.J.; Schuur, B.; de Haan, A.B. Novel extractants for the recovery of fermentation derived lactic acid. Sep. Purif. Technol. 2013, 111, 82–89. [Google Scholar] [CrossRef]
- Boontawan, P.; Kanchanathawee, S.; Boontawan, A. Extractive fermentation of l-(+)-lactic acid by Pediococcus pentosaceus using electrodeionization (EDI) technique. Biochem. Eng. J. 2011, 54, 192–199. [Google Scholar] [CrossRef]
- Gao, M.-T.; Shimamura, T.; Ishida, N.; Takahashi, H. pH-Uncontrolled lactic acid fermentation with activated carbon as an adsorbent. Enzym. Microb. Technol. 2011, 48, 526–530. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, A.; Dong, H.; Li, Q.; Niu, C. A novel process for recovery and refining of l-lactic acid from fermentation broth. Bioresour. Technol. 2012, 112, 280–284. [Google Scholar] [CrossRef]
- Gao, M.-T.; Shimamura, T.; Ishida, N.; Nagamori, E.; Takahashi, H.; Umemoto, S.; Omasa, T.; Ohtake, H. Extractive lactic acid fermentation with tri-n-decylamine as the extractant. Enzym. Microb. Technol. 2009, 44, 350–354. [Google Scholar] [CrossRef]
- Othman, M.; Ariff, A.B.; Rios-Solis, L.; Halim, M. Extractive Fermentation of Lactic Acid in Lactic Acid Bacteria Cultivation: A Review. Front. Microbiol. 2017, 8, 2285. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef]
- Ilmén, M.; Koivuranta, K.; Ruohonen, L.; Suominen, P.; Penttilä, M. Efficient production of L-lactic acid from xylose by Pichia stipitis. Appl. Environ. Microbiol. 2007, 73, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Schiraldi, C.; Adduci, V.; Valli, V.; Maresca, C.; Giuliano, M.; Lamberti, M.; Cartenì, M.; De Rosa, M. High cell density cultivation of probiotics and lactic acid production. Biotechnol. Bioeng. 2003, 82, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Arus, V.A.; Platon, N.; Nistor, I.D. Lactic Acid Retention by CO3-Mg-Al Layered Double Hydroxides for Improved Milk Clotting An approach through acidity measurements. Rev. Chim. 2016, 67, 1348–1355. [Google Scholar]
- Azzouz, A.; Tudor, S.; Ileana-Denisa, N.; Alexandru, C.; Gheorghe, D. Methods for Cell Growth and Productivity Improvement of Lactic Seeds (Various Proposal). MD 2969 G2. 28 February 2006. [Google Scholar]
- Bergaya, F.; Lagaly, G. Chapter 1 General Introduction: Clays, Clay Minerals, and Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 1–18. [Google Scholar]
- Bejoy, N. Hydrotalcite. Resonance 2001, 6, 57–61. [Google Scholar] [CrossRef]
- Rackley, S.A. 7—Adsorption capture systems. In Carbon Capture and Storage, 2nd ed.; Rackley, S.A., Ed.; Butterworth-Heinemann: Boston, MA, USA, 2017; pp. 151–185. [Google Scholar] [CrossRef]
- Walter, R.H.; Sherman, R.M. Ozonation of Lactic Acid Fermentation Effluent. J. Water Pollut. Control Fed. 1974, 46, 1800–1803. [Google Scholar]
- Walter, R.H.; Sherman, R.M. Kinetics of the lactate-ozone reaction in an open-loop system. J. Water Pollut. Control Fed. 1976, 48, 748–752. [Google Scholar]
- Azzouz, A.; Kotbi, A.; Niquette, P.; Sajin, T.; Ursu, A.; Rami, A.; Monette, F.; Hausler, R. Ozonation of oxalic acid catalyzed by ion-exchanged montmorillonite in moderately acidic media. React. Kinet. Mech. Catal. 2010, 99, 289–302. [Google Scholar] [CrossRef]
- Boudissa, F.; Mirilà, D.; Arus, V.-A.; Terkmani, T.; Semaan, S.; Proulx, M.; Nistor, I.-D.; Roy, R.; Azzouz, A. Acid-treated clay catalysts for organic dye ozonation–Thorough mineralization through optimum catalyst basicity and hydrophilic character. J. Hazard. Mater. 2019, 364, 356–366. [Google Scholar] [CrossRef]
- Shahidi, D.; Moheb, A.; Abbas, R.; Larouk, S.; Roy, R.; Azzouz, A. Total mineralization of sulfamethoxazole and aromatic pollutants through Fe2+-montmorillonite catalyzed ozonation. J. Hazard. Mater. 2015, 298, 338–350. [Google Scholar] [CrossRef]
- Shahidi, D.; Roy, R.; Azzouz, A. Total removal of oxalic acid via synergistic parameter interaction in montmorillonite catalyzed ozonation. J. Environ. Chem. Eng. 2014, 2, 20–30. [Google Scholar] [CrossRef]
- Shahidi, D.; Roy, R.; Azzouz, A. Advances in catalytic oxidation of organic pollutants—Prospects for thorough mineralization by natural clay catalysts. Appl. Catal. B Environ. 2015, 174–175, 277–292. [Google Scholar] [CrossRef]
- Larouk, S.; Ouargli, R.; Shahidi, D.; Olhund, L.; Shiao, T.C.; Chergui, N.; Sehili, T.; Roy, R.; Azzouz, A. Catalytic ozonation of Orange-G through highly interactive contributions of hematite and SBA-16–To better understand azo-dye oxidation in nature. Chemosphere 2017, 168, 1648–1657. [Google Scholar] [CrossRef]
- Mirilă, D.-C.; Boudissa, F.; Beltrao-Nuñes, A.-P.; Platon, N.; Didi, M.-A.; Nistor, I.-D.; Roy, R.; Azzouz, A. Organic Dye Ozonation Catalyzed by Chemically Modified Montmorillonite K10– Role of Surface Basicity and Hydrophilic Character. Ozone Sci. Eng. 2020, 42, 517–530. [Google Scholar] [CrossRef]
- Benghaffour, A.; Foka-Wembe, E.N.; Dami, M.; Dewez, D.; Azzouz, A. Insight into natural medium remediation through ecotoxicity correlation with clay catalyst selectivity in organic molecule ozonation. Dalton Trans. 2022, 51, 4366–4376. [Google Scholar] [CrossRef]
- Zekkari, M.; Ouargli-Saker, R.; Boudissa, F.; Lachachi, A.K.; El Houda Sekkal, K.N.; Tayeb, R.; Boukoussa, B.; Azzouz, A. Silica-catalyzed ozonation of 17α -ethinyl-estradiol in aqueous media-to better understand the role of silica in soils. Chemosphere 2022, 298, 134312. [Google Scholar] [CrossRef] [PubMed]
- Pusch, R. Bentonite Clay: Environmental Properties and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Bouberka, Z.; Khenifi, A.; Benderdouche, N.; Derriche, Z. Removal of Supranol Yellow 4GL by adsorption onto Cr-intercalated montmorillonite. J. Hazard. Mater. 2006, 133, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.A.P.; Debacher, N.A.; Downs, A.J.; Cottet, L.; Mello, C.A.D. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J. Colloid Interface Sci. 2009, 332, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, S.; Pramada, P. Rice husk ash as an adsorbent for methylene blue—Effect of ashing temperature. Adsorption 2006, 12, 27. [Google Scholar] [CrossRef]
- Hamdaoui, O. Batch study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick. J. Hazard. Mater. 2006, 135, 264–273. [Google Scholar] [CrossRef]
- Boudissa, F.; Zekkari, M.; Arus, V.-A.; Ouargli-Saker, R.; Nabil, B.; Roy, R.; Azzouz, A. Clay-catalyzed ozonation of endocrine-disrupting compounds in solvent-free media–to better understand soil catalytic capacity. Dalton Trans. 2020, 49, 16693–16706. [Google Scholar] [CrossRef]
- Tyagi, B.; Chudasama, C.D.; Jasra, R.V. Determination of structural modification in acid activated montmorillonite clay by FT-IR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 273–278. [Google Scholar] [CrossRef]
- Komadel, P.; Janek, M.; Madejová, J.; Weekes, A.; Breen, C. Acidity and catalytic activity of mildly acid-treated Mg-rich montmorillonite and hectorite. J. Chem. Soc. Faraday Trans. 1997, 93, 4207–4210. [Google Scholar] [CrossRef]
- Arus, V.A.; Nousir, S.; Sennour, R.; Shiao, T.C.; Nistor, I.D.; Roy, R.; Azzouz, A. Intrinsic affinity of acid-activated bentonite towards hydrogen and carbon dioxide. Int. J. Hydrog. Energy 2018, 43, 7964–7972. [Google Scholar] [CrossRef]
- Ouargli, R.; Larouk, S.; Terrab, I.; Hamacha, R.; Benharrats, N.; Bengheddach, A.; Azzouz, A. Intrinsic activity of SBA-like silica in the catalytic ozonation of organic pollutants. Ozone: Sci. Eng. 2016, 38, 48–61. [Google Scholar] [CrossRef]
- Torii, T.; Kanemitsu, K.; Wada, T.; Itoh, S.; Kinugawa, K.; Hagiwara, A. Measurement of short-chain fatty acids in human faeces using high-performance liquid chromatography: Specimen stability. Ann. Clin. Biochem. 2010, 47, 447–452. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J. Lactic acid can be easily and precisely determined by reversed-phase high performance liquid chromatography with pre-column derivatization. J. Anim. Feed Sci. 2008, 17, 268–279. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 54178311, Lactoyllactic Acid. Retrieved 29 November 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lactoyllactic-acid (accessed on 8 September 2022).
- Pereira, C.S.; Silva, V.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Matsuura, Y.; Onda, A.; Yanagisawa, K. Selective conversion of lactic acid into acrylic acid over hydroxyapatite catalysts. Catal. Commun. 2014, 48, 5–10. [Google Scholar] [CrossRef]
- Chahal, S.P.; Starr, J.N. Lactic acid. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Splittstoesser, D.; Wilkison, M. Some factors affecting the activity of diethylpyrocarbonate as a sterilant. Appl. Microbiol. 1973, 25, 853–857. [Google Scholar] [CrossRef]
- Ehrenberg, L.; Fedorcsak, I.; Solymosy, F. Diethyl pyrocarbonate in nucleic acid research. In Progress in Nucleic Acid Research and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 1976; Volume 16, pp. 189–262. [Google Scholar]
- Lowenstein, J. Ammonia production in muscle and other tissues: The purine nucleotide cycle. Physiol. Rev. 1972, 52, 382–414. [Google Scholar] [CrossRef]
- Psaltou, S.; Zouboulis, A. Catalytic ozonation and membrane contactors—A review concerning fouling occurrence and pollutant removal. Water 2020, 12, 2964. [Google Scholar] [CrossRef]
- Azzouz, A.; Belkhadem, F.; Benselka, N.; Drici, N.; Hadj-Abdelkader, N.E.H.; Ouargli-Saker, R. Structure-properties correlation with LDH applications. In Applications of Layered Double Hydroxides; Goswamee, R.L., Saikia, P., Eds.; Nova Science Publisher: New York, NY, USA, 2021; pp. 193–244. [Google Scholar] [CrossRef]
- Foster, C.; Shaw, S.; Neill, T.S.; Bryan, N.; Sherriff, N.; Natrajan, L.S.; Wilson, H.; Lopez-Odriozola, L.; Rigby, B.; Haigh, S.J.; et al. Hydrotalcite Colloidal Stability and Interactions with Uranium(VI) at Neutral to Alkaline pH. Langmuir 2022, 38, 2576–2589. [Google Scholar] [CrossRef]
- Sable, S.S.; Medina, F.; Contreras, S. Clofibric acid degradation by catalytic ozonation using hydrotalcite-derived catalysts. Appl. Catal. B Environ. 2014, 150–151, 30–36. [Google Scholar] [CrossRef]
- Fu, X.; Huang, Y.; Wang, Y.; Liang, M.; Yang, Y.; Jin, Z.; Yang, J.; Hu, S.; Li, L. Ozonation Catalyzed by CoxFe1 Layered Double Hydroxide for the Degradation of P-toluenesulfonic Acid. Ozone: Sci. Eng. 2021, 43, 163–172. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, J.; Tang, M.; Wang, D.; Nie, Y.; Yang, L.; Dai, C.; Yang, C.; Lu, L. Surface acidity and basicity of Mg/Al hydrotalcite for 2, 4-dichlorophenoxyacetic acid degradation with ozone: Mineralization, mechanism, and implications to practical water treatment. J. Hazard. Mater. 2021, 402, 123475. [Google Scholar] [CrossRef] [PubMed]
- Sulpizi, M.; Gaigeot, M.-P.; Sprik, M. The Silica–Water Interface: How the Silanols Determine the Surface Acidity and Modulate the Water Properties. J. Chem. Theory Comput. 2012, 8, 1037–1047. [Google Scholar] [CrossRef] [PubMed]



| Sample | Si/Al Mole Ratio | pH a | Average Particle Size (µm) e | Average Zeta Potential (mv) | ||||
|---|---|---|---|---|---|---|---|---|
| Water b | LA 0 min c | LA 1 min d | H2O | LA | H2O | LA | ||
| Bentonite | 2.50 | 6.23 | 2.80 | 2.84 | 1.78 | 1.01 | −30.87 | −25.73 |
| HMt-1 | 2.69 | 5.27 | 2.69 | 2.87 | 2.81 | 1.98 | −18.72 | −24.46 |
| HMt-4 | 3.00 | 4.13 | 2.67 | 2.74 | 2.69 | 0.78 | −24.93 | −25.53 |
| HMt-8 | 3.47 | 4.38 | 2.67 | 2.69 | 2.98 | 1.24 | −27.10 | −22.09 |
| HMt-15 | 4.03 | 3.82 | 2.42 | 2.73 | 3.32 | 2.28 | −35.93 | −31.88 |
| HMt-24 | 4.36 | 4.48 | 2.60 | 2.67 | 3.71 | 1.16 | −21.48 | −23.75 |
| NaMt | 2.45 | 6.88 | 2.76 | 2.82 | 0.63 | 0.63 | −25.07 | −37.07 |
| MgMt | 2.45 | 5.72 | 2.83 | 2.84 | 1.68 | 4.25 | −15.85 | −12.06 |
| KMt | 2.45 | 6.22 | 2.74 | 2.76 | 0.90 | 0.91 | −18.91 | −30.67 |
| CaMt | 2.45 | 6.07 | 2.74 | 2.74 | 1.27 | 1.12 | −23.14 | −19.62 |
| Ht | Mg/Al=2 | 6.27 | 4.64 | 4.19 | 1.40 | 9.98 | −09.33 | +16.23 |
| Ht Content (%) | LARA (%) after 60 min Ozonation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bent | HMt-1 | HMt-4 | HMt-8 | HMt-15 | HMt-24 | NaMt | KMt | CaMt | MgMt | |
| 100 | 3.31 | 3.31 | 3.31 | 3.31 | 3.31 | 3.31 | 3.31 | 3.31 | 3.31 | 3.31 |
| 80 | 3.93 | 3.40 | 5.30 | 3.20 | 4.06 | 3.19 | 5.65 | - | - | - |
| 60 | 3.85 | 6.35 | 4.25 | 2.93 | 2.67 | 2.66 | 3.92 | - | - | - |
| 40 | 2 | 4.14 | 3.95 | 3.68 | 2.67 | 3.80 | 3.5 | - | - | - |
| 20 | 4.98 | 3.21 | 3.57 | 3.28 | 2.63 | 2.99 | 3.97 | - | - | - |
| 0 | 1.99 | 1.55 | 1.65 | 2.54 | 3.64 | 1.62 | 1.97 | 2.00 | 2.59 | 1.60 |
| Catalyst | Time Range (min) | Reaction Order | R2 | Rate Constant, K * |
|---|---|---|---|---|
| Ht | [0–3] | 2 | 0.9613 | 1.039 |
| [5–15] | 1 | 0.9999 | 0.136 | |
| Bentonite | [0–5] | 2 | 0.9909 | 0.266 |
| [5–15] | 2 | 0.9972 | 0.324 | |
| NaMt | [0–5] | 2 | 0.9889 | 0.251 |
| [5–15] | 2 | 0.9824 | 0.785 | |
| CaMt | [0–5] | 2 | 0.9562 | 0.158 |
| KMt | [0–5] | 2 | 0.9672 | 0.288 |
| [5–15] | 2 | 0.998 | 0.37 | |
| MgMt | [0–3] | 2 | 0.9566 | 0.36 |
| [5–15] | 1 | 0.9346 | 0.162 | |
| HMt-1 | [0–5] | 2 | 0.9448 | 0.195 |
| [5–15] | 2 | 0.9997 | 0.26 | |
| HMt- 4 | [5–15] | 2 | 0.9905 | 0.36 |
| HMt-8 | [0–5] | 2 | 0.9286 | 0.28 |
| [5–15] | 2 | 0.9999 | 0.251 | |
| HMt-15 | [0–5] | 2 | 0.9513 | 0.355 |
| [5–15] | 2 | 0.9971 | 0.234 | |
| HMt-24 | [0–5] | 2 | 0.941 | 0.192 |
| [5–15] | 1 | 0.9428 | 0.274 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Baktaoui, M.; Hadj-Abdelkader, N.E.H.; Benghaffour, A.; Arus, V.-A.; Bennani-Daouadji, N.; Belkhadem, F.; Roy, R.; Azzouz, A. Clay-Catalyzed Ozonation of Hydrotalcite-Extracted Lactic Acid Potential Application for Preventing Milk Fermentation Inhibition. Molecules 2022, 27, 6502. https://doi.org/10.3390/molecules27196502
El Baktaoui M, Hadj-Abdelkader NEH, Benghaffour A, Arus V-A, Bennani-Daouadji N, Belkhadem F, Roy R, Azzouz A. Clay-Catalyzed Ozonation of Hydrotalcite-Extracted Lactic Acid Potential Application for Preventing Milk Fermentation Inhibition. Molecules. 2022; 27(19):6502. https://doi.org/10.3390/molecules27196502
Chicago/Turabian StyleEl Baktaoui, Meriem, Nour El Houda Hadj-Abdelkader, Amina Benghaffour, Vasilica-Alisa Arus, Nadia Bennani-Daouadji, Fatiha Belkhadem, René Roy, and Abdelkrim Azzouz. 2022. "Clay-Catalyzed Ozonation of Hydrotalcite-Extracted Lactic Acid Potential Application for Preventing Milk Fermentation Inhibition" Molecules 27, no. 19: 6502. https://doi.org/10.3390/molecules27196502
APA StyleEl Baktaoui, M., Hadj-Abdelkader, N. E. H., Benghaffour, A., Arus, V.-A., Bennani-Daouadji, N., Belkhadem, F., Roy, R., & Azzouz, A. (2022). Clay-Catalyzed Ozonation of Hydrotalcite-Extracted Lactic Acid Potential Application for Preventing Milk Fermentation Inhibition. Molecules, 27(19), 6502. https://doi.org/10.3390/molecules27196502








