Recent Advances and Prospects in Design of Hydrogen Permeation Barrier Materials for Energy Applications—A Review
Abstract
1. Introduction
1.1. Hydrogen Barriers
- ▪
- Reduced hydrogen pick-up to avoid hydrogen transport and formation of hydrides and hydrogen effects
- ▪
- Show mechanical strength
- o
- Coating needs to maintain integrity and to not weaken strength of cladding
- ▪
- Reduced embrittlement due to hydride formation during service
- ▪
- Reduced thermal stress or thermal expansion between cladding and coating
- ▪
- Corrosion resistance (in water and/or oxygen)
- ▪
- Manufacturing feasibility
- ▪
- Reduce hydrogen isotope permeation into the reactor coolant system (RCS)
- ▪
- Reduce hydrogen isotope ingress into nuclear reactor’s structural materials
- ▪
- High-temperature resistance up to loss-of-coolant accident (LOCA) conditions (≥1200 °C)
- ▪
- Neutron transparency
- ▪
- Long term endurance in the radiation environment of a nuclear reactor
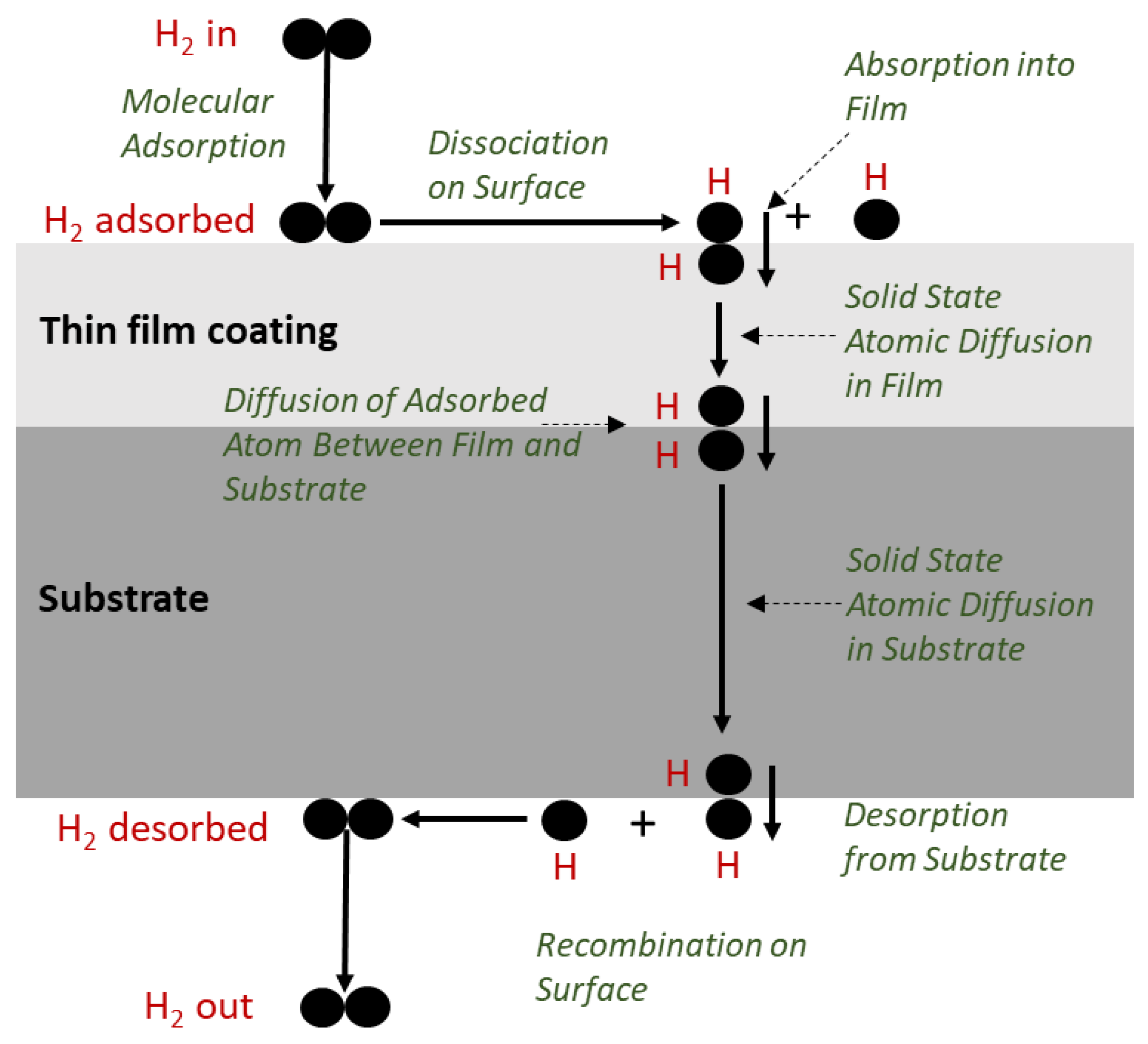
1.2. Mechanism for Hydrogen Permeation
 2H (metal). The pressure dependence follows Sievert’s law for the solubility of diatomic gases (hydrogen isotopes) in metals, where cH is concentration of the atomic hydrogen isotope, S is the Sievert’s solubility constant in mol/m3Pa1/2 and PH is hydrogen pressure (Pa):
2H (metal). The pressure dependence follows Sievert’s law for the solubility of diatomic gases (hydrogen isotopes) in metals, where cH is concentration of the atomic hydrogen isotope, S is the Sievert’s solubility constant in mol/m3Pa1/2 and PH is hydrogen pressure (Pa):1.3. Permeation Measurements—Gas Transmission Rate
1.4. Permeation Measurements—Electrochemical Technique
1.5. Coating Application Methods
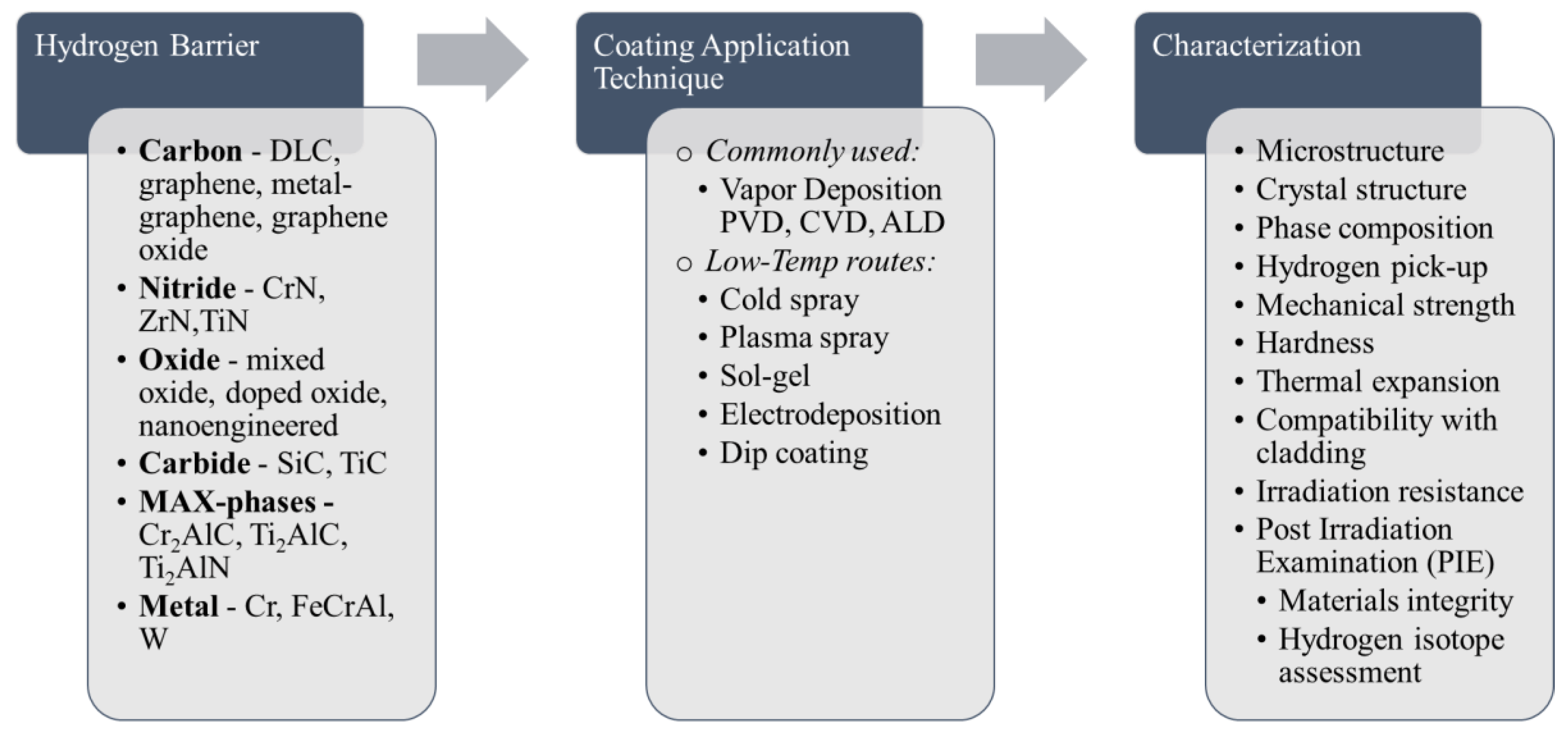
2. Recent Advances in Hydrogen Permeation Barriers
2.1. Oxides
2.1.1. Aluminum Oxide
2.1.2. Zirconium Oxide
2.1.3. Aluminide + Alumina; FeAl-Al2O3
2.1.4. Bipolar Oxide: Chromium Oxide-Aluminum Oxide
2.1.5. Rare-Earth Oxides
2.2. Nitrides
2.2.1. Titanium Nitride
2.2.2. Chromium Nitride
2.2.3. Less Common Nitrides
2.2.4. Irradiation Experiments on Nitrides
2.3. Carbon Materials
2.3.1. Diamond-Like Carbon (DLC)
2.3.2. Graphene and Graphite
2.3.3. Carbides
2.4. MAX-Phases and MXenes
2.5. Metals
2.5.1. Chromium
2.5.2. Tungsten, Gold, Silver, Platinum, etc.
2.6. FeCrAl Alloys
Molybdenum Diffusion Barrier Layer with FeCrAl
3. Summary of Advanced Coatings for Hydrogen Barrier Applications and Future Prospects
- Carbon: diamond like carbon (DLC); graphene, metal-graphene, graphene oxide.
- Carbides: SiC, SiC/SiC, TiC, TiAlC layered phase.
- MAX-phases and MXenes.
- Metals: Cr, FeCrAl alloys, FeCrAl + Mo diffusion barrier.
- Nitrides: CrN, ZrN, TiN.
- Oxides: dopant + ZrO2; bipolar oxide Al2O3/Cr2O3, nano-Al2O3.
Identified Knowledge Gaps—Future Research Prospects
- Perform hydrogen absorption and permeation measurements in environments relevant to the application.
- Correlate permeation rate with hydrogen trapping, solubility, diffusivity, microstructure, phase formation, grain boundaries and fabrication procedure to learn how to tailor materials to reduce hydrogen permeability.
- Utilize nanoscience, structural modifications and recoverable Nano Lattices to reduce hydrogen permeability.
- Advance structurally modified oxide materials; dopants; nano-engineering; nanoceramics.
- Explore combinations of metals and metal alloys with diffusion barrier.
- Exploration of the impact of dopants and alloying elements.
- Explore bipolar oxides.
- Explore diamond like carbon with buffer layer of CrN, etc.
- o
- Micro or nanostructure engineering.
- o
- Key is structural control.
- Study of microstructure related to fabrication method to identify improved performance.
- Exploration of multilayer structures with hybrid architecture for evaluation with respect to mechanical strength and manufacturing feasibility.
- Identification of cost-effective, low-temperature preparation method to avoid HAZ.
- o
- For example; using cold spray, sol-gel routes, chemical spray pyrolysis or nitrates and triblock copolymers for wet chemistry routes.
- Develop new feasible scale-up fabrication techniques of low cost.
- Study of radiation resistance in nuclear reactor applications.
- o
- Structural modifications to improve radiation resistance in a nuclear reactor.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Locatelli, G.; Mancini, M.; Todeschini, N. Generation IV nuclear reactors: Current status and future prospects. Energy Policy 2013, 61, 1503–1520. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shahd, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463. [Google Scholar] [CrossRef]
- Sapre, S.; Pareek, K.; Vyas, M. Investigation of structural stability of type IV compressed hydrogen storage tank during refueling of fuel cell vehicle. Energy Storage 2020, 2, e150. [Google Scholar] [CrossRef]
- Serp, J.; Allibert, M.; Benes, O.; Delpech, S.; Feynberg, O.; Ghetta, V.; Heuer, D.; Holcomb, D.; Ignatiev, V.; Kloosterman, J.; et al. The molten salt reactor (MSR) in generation IV: Overview and perspectives. Prog. Nucl. Energy 2014, 77, 308–319. [Google Scholar] [CrossRef]
- Wipf, H. Solubility and diffusion of hydrogen in pure metals and alloys. Phys. Scr. 2001, T94, 43. [Google Scholar] [CrossRef]
- Kirchheim, R. Solubility and diffusivity of hydrogen in complex materials. Phys. Scr. 2001, T94, 58–67. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). ISO 15105-1:2007(E); Plastics—Film and Sheeting—Determination of Gas-transmission Rate—Part 1: Differential-Pressure Methods. BSI British Standards: London, UK, 2007.
- ISO 15105-2:2003; Plastics—Film and Sheeting—Determination of Gas-transmission Rate—Part 2: Equal-Pressure Method. International Organization for Standardization (ISO): Geneva, Switzerland, 2003.
- ASTM G148-97; Standard Practice for Evaluation of Hydrogen Uptake, Permeation, and Transport in Metals by an Electrochemical Technique. ASTM International: West Conshohocken, PA, USA, 2018.
- Devanathan, M.A.V.; Stachurski, Z. The adsorption and diffusion of electrolytic hydrogen in palladium. Proc. R. Soc. 1962, A270, 90–102. [Google Scholar]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- VRC Metal Systems, Cold-Spray Technology. Available online: https://vrcmetalsystems.com/ (accessed on 1 September 2022).
- Levin, I.; Brandon, D. Metastable Alumina Polymorphs: Crystal Structures and Transition Sequences. J. Am. Ceram. Soc. 1998, 81, 1995–2012. [Google Scholar] [CrossRef]
- Hollenberg, G.W.; Simonen, E.P.; Kalinin, G. Tritium/hydrogen barrier development. Fusion Eng. Des. 1995, 28, 190–208. [Google Scholar] [CrossRef]
- Iadicicco, D.; Bassini, S.; Vanazzi, M.; Muñoz, P.; Moroño, A.; Hernandez, T.; García-Cortés, I.; Sánchez, F.J.; Utili, M.; Ferré, F.G.; et al. Efficient hydrogen and deuterium permeation reduction in Al2O3 coatings with enhanced radiation tolerance and corrosion resistance. Nucl. Fusion 2018, 58, 126007. [Google Scholar] [CrossRef]
- Youngman, R.A.; Mitchell, T.E.; Clinard, F.W.; Hurley, G.F. High dose neutron irradiation damage in alpha alumina. J. Mater. Res. 1991, 6, 2178–2187. [Google Scholar] [CrossRef]
- Pells, G.P. Radiation Damage Effects in Alumina. J. Am. Ceram. Soc. 1994, 77, 368–377. [Google Scholar] [CrossRef]
- Morono, A.; Hodgson, E.R.; Malo, M. Radiation enhanced deuterium absorption for Al2O3 and macor ceramic. Fusion Eng. Des. 2013, 88, 2488–2491. [Google Scholar] [CrossRef]
- Meza, L.R.; Das, S.; Greer, J.R. Strong, lightweight, and recoverable three-dimensional ceramic nanolattices. Science 2014, 345, 1322. [Google Scholar] [CrossRef]
- Ferré, F.G.; Mairov, A.; Ceseracciu, L.; Serruys, Y.; Trocellier, P.; Baumier, C.; Kaïtasov, O.; Brescia, R.; Gastaldi, D.; Vena, P.; et al. Radiation endurance in Al2O3 nanoceramics. Sci. Rep. 2016, 6, 33478. [Google Scholar] [CrossRef]
- Couet, A.; Motta, A.T.; Comstock, R.J. Hydrogen pickup measurements in zirconium alloys: Relation to oxidation kinetics. J. Nucl. Mater. 2014, 451, 1. [Google Scholar] [CrossRef]
- Hashizume, K.; Ogata, K.; Akamaru, S.; Hatano, Y. Solubility of hydrogen isotopes in zirconia ceramics. J. Plasma Fusion Res. Ser. 2013, 10, 33. [Google Scholar]
- Youssef, M.; Yang, M.; Yildiz, B. Doping in the Valley of Hydrogen Solubility: A Route to Designing Hydrogen-Resistant Zirconium Alloys. Phys. Rev. Appl. 2016, 5, 014008. [Google Scholar] [CrossRef]
- Hatano, Y.; Zhang, K.; Hashizume, K. Fabrication of ZrO2 coatings on ferritic steel by wet-chemical methods as a tritium permeation barrier. Phys. Scr. 2011, T145, 1. [Google Scholar] [CrossRef]
- Forsberg, C.W.; Lam, S.; Carpenter, D.M.; Whyte, D.G.; Scarlat, R.; Contescu, C.; Wei, L.; Stempien, J.; Blandford, E. Tritium Control and Capture in Salt-Cooled Fission and Fusion Reactors: Status, Challenges and Path Forward. Nucl. Technol. 2017, 197, 119–139. [Google Scholar] [CrossRef]
- Xiang, X.; Wang, X.; Zhang, G.; Tang, T.; Lai, X. Preparation technique and alloying effect of aluminide coatings as tritium permeation barriers: A review. Int. J. Hydrog. Energy 2015, 40, 3697–3707. [Google Scholar] [CrossRef]
- Yang, F.; Xiang, X.; Lu, G.; Zhang, G.; Tang, T.; Shi, Y.; Wang, X. Tritium permeation characterization of Al2O3/FeAl coatings as tritium permeation barriers on 321 type stainless steel containers. J. Nucl. Mater. 2016, 478, 144–148. [Google Scholar] [CrossRef]
- Xiang, X.; Yang, F.; Zhang, G.; Wang, X. Effect of Steel Substrates on the Formation and Deuterium Permeation Resistance of Aluminide Coatings. Coatings 2019, 9, 95. [Google Scholar] [CrossRef]
- Prakash, U.; Parvathavarthini, N.; Dayal, R.K. Effect of composition on hydrogen permeation in Fe-Al alloys. Intermetallics 2007, 15, 17–1924. [Google Scholar] [CrossRef]
- Xin, Z.; Yin, X.; Ling, Y.; Zhang, Z.; Liu, X.; Liang, H.; Deng, X. Hydrogen permeation behavior and mechanism of Cr2O3/Al2O3 bipolar oxide film under plasma discharging environment. Int. J. Hydrogen Energy 2017, 42, 20869–20878. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Xiang, Q.Y.; Yan, K.; Tang, T.; Rao, Y.; Cao, J.-L. Novel nano-crystalline Er2O3 hydrogen isotopes permeation barriers. J. Eur. Ceram. Soc. 2017, 37, 249–254. [Google Scholar] [CrossRef]
- Mao, W.; Wilde, M.; Chikada, T.; Ogura, S.; Fukutani, K.; Terai, T.; Matsuzaki, H. Fabrication and Hydrogen Permeation Properties of Epitaxial Er2O3 Films Revealed by Nuclear Reaction Analysis. J. Phys. Chem. C 2016, 120, 15147–15152. [Google Scholar] [CrossRef]
- Tamura, M. Hydrogen Permeation Characteristics of TiN-Coated Stainless Steels. J. Mater. Sci. Eng. 2015, A5, 197–201. [Google Scholar]
- Tamura, M.; Eguchi, T. Nanostructured thin films for hydrogen-permeation barrier. J. Vac. Sci. Technol. A Vac. Surf. Films 2015, 33, 041503. [Google Scholar] [CrossRef]
- Kashkarov, E.; Afornu, B.; Sidelev, D.; Krinitcyn, M.; Gouws, V.; Lider, A. Recent Advances in Protective Coatings for Accident Tolerant Zr-Based Fuel Claddings. Coatings 2021, 11, 557. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Nikitenkov, N.N.; Sutygina, A.N.; Bezmaternykh, A.O.; Kudiiarov, V.N.; Syrtanov, M.S.; Pryamushko, T.S. Hydrogenation behavior of Ti-implanted Zr-1Nb alloy with TiN films deposited using filtered vacuum arc and magnetron sputtering. Appl. Surf. Sci. 2018, 432, 207–213. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Multilayer (TiN, TiAlN) ceramic coatings for nuclear fuel cladding. J. Nucl. Mater. 2016, 478, 236–244. [Google Scholar] [CrossRef]
- Daub, K.; van Nieuwenhove, R.; Nordin, H. Investigation of the impact of coatings on corrosion and hydrogen uptake of Zircaloy-4. J. Nucl. Mater. 2015, 467, 260–270. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Obrosov, A.; Sutygina, A.N.; Uludintceva, E.; Mitrofanov, A.; Weiß, S. Hydrogen Permeation, and Mechanical and Tribological Behavior, of CrNx Coatings Deposited at Various Bias Voltages on IN718 by Direct Current Reactive Sputtering. Coatings 2018, 8, 66. [Google Scholar] [CrossRef]
- Matějíčeka, J.; Veverkaa, J.; Nemanič, V.; Cvrček, L.; Lukáč, F.; Havránek, V.; Illková, K. Characterization of less common nitrides as potential permeation barriers. Fusion Eng. Des. 2019, 139, 74–80. [Google Scholar] [CrossRef]
- Tamura, M.; Takizawa, H. TiAlN/TiMoN Coatings as Hydrogen Barriers. J. Mater. Sci. Eng. 2019, A9, 1–7. [Google Scholar]
- Houben, A.; Rasiński, M.; Gao, L.; Linsmeier, C. Tungsten nitride as tritium permeation barrier. Nucl. Mater. Energy 2020, 24, 100752. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, P.; Panizo-Laiz, M.; González, C.; Iglesias, R.; Martín-Bragado, I.; González-Arrabal, R.; Perlado, J.M.; Peña-Rodríguez, O.; Rivera, A. Direct observation of hydrogen permeation through grain boundaries in tungsten. Emerg. Mater. 2022, 5, 1075–1087. [Google Scholar] [CrossRef]
- van Nieuwenhove, R.; Andersson, V.; Balak, J.; Oberlander, B. In-Pile Testing of CrN, TiAlN and AlCrN Coatings on Zircaloy Cladding in the Halden Reactor, Zirconium in the Nuclear Industry: 18th International Symposium, STP 1597; ASTM International: West Conshohocken, PA, USA, 2016; pp. 965–982. [Google Scholar]
- Tamura, M.; Kumagai, T. Hydrogen permeability of diamondlike amorphous carbons. J. Vac. Sci. Technol. 2017, 35, 04D101. [Google Scholar] [CrossRef]
- Nam, T.-H.; Lee, J.-H.; Choi, S.-R.; Yoo, J.-B.; Kim, J.-G. Graphene coating as a protective barrier against hydrogen embrittlement. Int. J. Hydrog. Energy 2014, 39, 1181–1817. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, Y.; Cui, B.; Zhou, Q. Graphene coating on nickel as effective barriers against hydrogen embrittlement. Surf. Coat. Technol. 2019, 374, 610–616. [Google Scholar] [CrossRef]
- Sagade, A.A.; Aria, A.I.; Edge, S.; Melgari, P.; Gieseking, B.; Bayer, B.C.; Meyer, J.C.; Bird, D.; Brewer, P.; Hofmann, S. Graphene-based nanolaminates as ultra-high permeation barriers. Npj 2D Mater. Appl. 2017, 1, 35. [Google Scholar] [CrossRef]
- Tozzini, V.; Pellegrini, V. Prospects for hydrogen storage in graphene. Phys. Chem. Chem. Phys. 2013, 15, 80. [Google Scholar] [CrossRef]
- Su, Y.; Kravets, V.G.; Wong, S.L.; Waters, J.; Geim, A.K.; Nair, R.R. Impermeable barrier films and protective coatings based on reduced graphene oxide. Nat. Commun. 2014, 5, 4843. [Google Scholar] [CrossRef]
- Kim, Y.; Baek, J.; Kim, S.; Kim, S.; Ryu, S.; Jeon, S.; Han, S.M. Radiation Resistant Vanadium Graphene Nanolayered Composite. Sci. Rep. 2015, 6, 24785. [Google Scholar] [CrossRef]
- Yang, H.; Shaoa, Z.; Wang, W.; Jia, X.; Li, C. A composite coating of GO-Al2O3 for tritium permeation barrier. Fusion Eng. Des. 2020, 156, 111689. [Google Scholar] [CrossRef]
- Atsumi, H.; Shibata, N.; Tanabe, T.; Shikama, T. Hydrogen absorption into neutron-irradiated graphite and estimation of the trapping effect. Phys. Scr. 2007, T128, 72–75. [Google Scholar] [CrossRef]
- Nemanič, V. Hydrogen permeation barriers: Basic requirements, materials selection, deposition methods, and quality evaluation. Nucl. Mater. Energy 2019, 19, 451–457. [Google Scholar] [CrossRef]
- Barrett, K.; Bragg-Sitton, S.; Galicki, D. Advanced LWR Nuclear Fuel Cladding System Development Trade-off Study; INL/EXT-12-27090; Idaho National Laboratory Light Water Reactor Sustainability Program; Idaho National Laboratory: Idaho Falls, ID, USA, 2012. [Google Scholar]
- Kashkarov, E.B.; Syrtanov, M.S.; Murashkina, T.L.; Kurochkin, A.V.; Shanenkova, Y.; Obrosov, A. Hydrogen Sorption Kinetics of SiC-Coated Zr-1Nb Alloy. Coatings 2019, 9, 31. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Baba, T.; Jiang, H.; Liu, C.; Guan, Y.; Elleuch, O.; Kuech, T.; Morgan, D.; Idrobo, J.-C.; et al. Radiation-induced segregation in a ceramic. Nat. Mater. 2020, 19, 992–998. [Google Scholar] [CrossRef]
- Hu, X.; Koyanagi, T.; Katoh, Y. Report on Hydrogen Isotope Permeability/Hermeticity of SiC-Based Cladding; ORNL/TM-2019/1187; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 2019. [Google Scholar]
- Causey, R.A.; Wampler, W.R.; Retelle, J.R.; Kaae, J.L. Tritium migration in vapor-deposited beta-silicon carbide. J. Nucl. Mater. 1993, 203, 196–205. [Google Scholar] [CrossRef]
- Terrani, K.; Jolly, B.; Trammell, M. 3D printing of high-purity silicon carbide. J. Am. Ceram. Soc. 2020, 103, 1575–1581. [Google Scholar] [CrossRef]
- Sokol, M.; Natu, V.; Kota, S.; Barsoum, M.W. On the Chemical Diversity of the MAX Phases. Trends Chem. 2019, 1, 210–223. [Google Scholar] [CrossRef]
- Ward, J.; Middleburgh, S.; Topping, M.; Garner, A.; Stewart, D.; Barsoum, M.W.; Preuss, M.; Frankel, P. Crystallographic evolution of MAX phases in proton irradiating environments. J. Nucl. Mater. 2018, 502, 220–227. [Google Scholar] [CrossRef]
- Tang, C.; Grosse, M.; Trtik, P.; Steinbrueck, M.; Stueber, M.; Seifert, H.J. H2 Permeation Behaviour of Cr2AlC and Ti2AlC MAX Phase Coated Zircaloy-4 by Neutron Radiography. Acta Polytech. 2018, 58, 69–76. [Google Scholar] [CrossRef]
- Gröner, L.; Mengis, L.; Galetz, M.; Kirste, L.; Daum, P. Investigations of the Deuterium Permeability of As-Deposited and Oxidized Ti2AlN Coatings. Materials 2020, 13, 2085. [Google Scholar] [CrossRef]
- Tunes, M.A.; Imtyazuddin, M.; Kainz, C.; Pogatscher, S.; Vishnyakov, V.M. Deviating from the pure MAX phase concept: Radiation-tolerant nanostructured dual-phase Cr2AlC. Sci. Adv. 2021, 7, eabf6771. [Google Scholar] [CrossRef]
- Naguib, M.; Barsoum, M.W.; Gogotsi, Y. Ten Years of Progress in the Synthesis and Development of MXenes. Adv. Mater. 2021, 33, 2103393. [Google Scholar] [CrossRef]
- Shi, K.; Meng, X.; Xiao, S.; Chen, G.; Wu, H.; Zhou, C.; Jiang, S.; Chu, P.K. MXene Coatings: Novel Hydrogen Permeation Barriers for Pipe Steels. Nanomaterials 2021, 11, 2737. [Google Scholar] [CrossRef]
- Bischoff, J.; Delafoy, C.; Vauglin, C.; Barberis, P.; Roubeyrie, C.; Perche, D.; Duthoo, D.; Schuster, F.; Brachet, J.-C.; Schweitzer, E.W.; et al. AREVA NP’s enhanced accident-tolerant fuel developments: Focus on Cr-coated M5 cladding. Nucl. Eng. Technol. 2018, 50, 223–228. [Google Scholar] [CrossRef]
- Sevecek, M.; Gurgen, A.; Seshadri, A.; Che, Y.; Wagih, M.; Phillips, B.; Champagne, V.; Shirvan, K. Development of Cr cold spray-coated fuel cladding with enhanced accident tolerance. Nucl. Eng. Technol. 2018, 50, 229–236. [Google Scholar] [CrossRef]
- Henager, C.H., Jr. Hydrogen Permeation Barrier Coatings. In Materials for the Hydrogen Economy; Jones, R.H., Thomas, G.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; Chapter 8. [Google Scholar]
- REB Research and Consulting. 2011. Available online: https://www.rebresearch.com/H2perm2.htm (accessed on 1 September 2022).
- Field, K.G.; Snead, M.A.; Yamamoto, Y.; Terrani, K.A. Handbook on the Material Properties of FeCrAl Alloys for Nuclear Power Production Applications; ORNL/TM-2018/905; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2018.
- Garud, Y.S.; Hoffman, A.K.; Rebak, R.B. Hydrogen Isotopes Permeation in Clean or Unoxidized FeCrAl Alloys: A Review. Metall. Mater. Trans. A 2022, 53, 773–793. [Google Scholar] [CrossRef]
- Yeom, H.; Maier, B.; Johnson, G.; Dabney, T.; Walters, J.; Sridharan, K. Development of cold spray process for oxidation-resistant FeCrAl and Mo diffusion barrier coatings on optimized ZIRLOTM. J. Nucl. Mater. 2018, 507, 306–315. [Google Scholar] [CrossRef]


| Metal | Permeability mol H2/m/s/Pa0.5 |
|---|---|
| Vanadium | 2.9 × 10−8 |
| Niobium | 7.5 × 10−9 |
| Titanium | 7.5 × 10−9 |
| Nickel | 1.2 × 10−10 |
| Ferritic Steels | 3 × 10−11 |
| Austenitic Steels | 0.7–1.2 × 10−11 |
| Molybdenum | 1.2 × 10−11 |
| Tungsten | 4.3 × 10−15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rönnebro, E.C.E.; Oelrich, R.L.; Gates, R.O. Recent Advances and Prospects in Design of Hydrogen Permeation Barrier Materials for Energy Applications—A Review. Molecules 2022, 27, 6528. https://doi.org/10.3390/molecules27196528
Rönnebro ECE, Oelrich RL, Gates RO. Recent Advances and Prospects in Design of Hydrogen Permeation Barrier Materials for Energy Applications—A Review. Molecules. 2022; 27(19):6528. https://doi.org/10.3390/molecules27196528
Chicago/Turabian StyleRönnebro, Ewa C. E., Robert L. Oelrich, and Robert O. Gates. 2022. "Recent Advances and Prospects in Design of Hydrogen Permeation Barrier Materials for Energy Applications—A Review" Molecules 27, no. 19: 6528. https://doi.org/10.3390/molecules27196528
APA StyleRönnebro, E. C. E., Oelrich, R. L., & Gates, R. O. (2022). Recent Advances and Prospects in Design of Hydrogen Permeation Barrier Materials for Energy Applications—A Review. Molecules, 27(19), 6528. https://doi.org/10.3390/molecules27196528






