Electrochemical Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy of Imidazole Ring Functionalized Monolayer on Smooth Gold Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of N-(2-(1H-Imidazol-4-yl)ethyl)-6-mercaptohexanamide (IMHA) and 6-Mercapto-N-methylhexanamide (Fragment Molecule)
2.2. Synthesis of Silicon Dioxide Covered Spherical Silver Nanoparticles (Ag@SiO2)
2.3. Preparation and Characterization of SAM
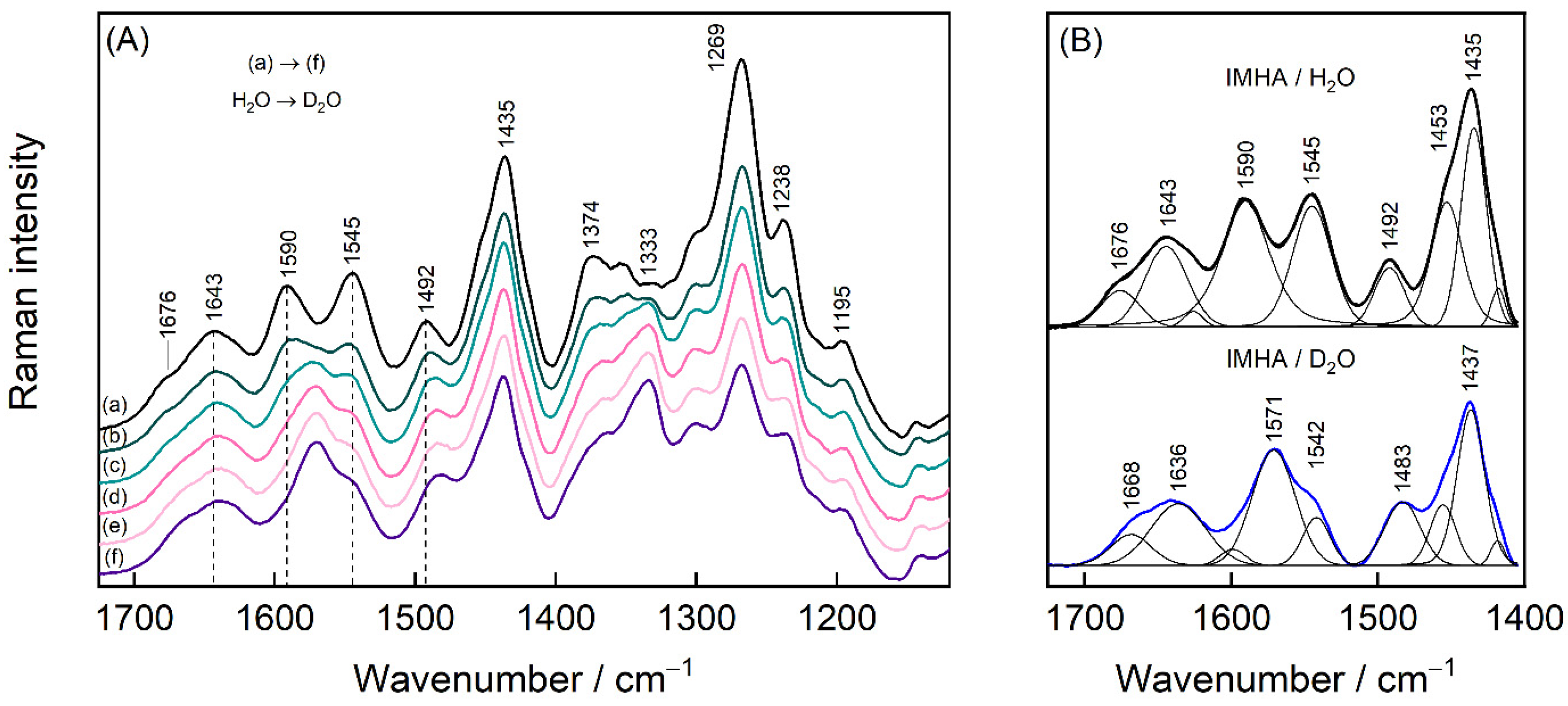
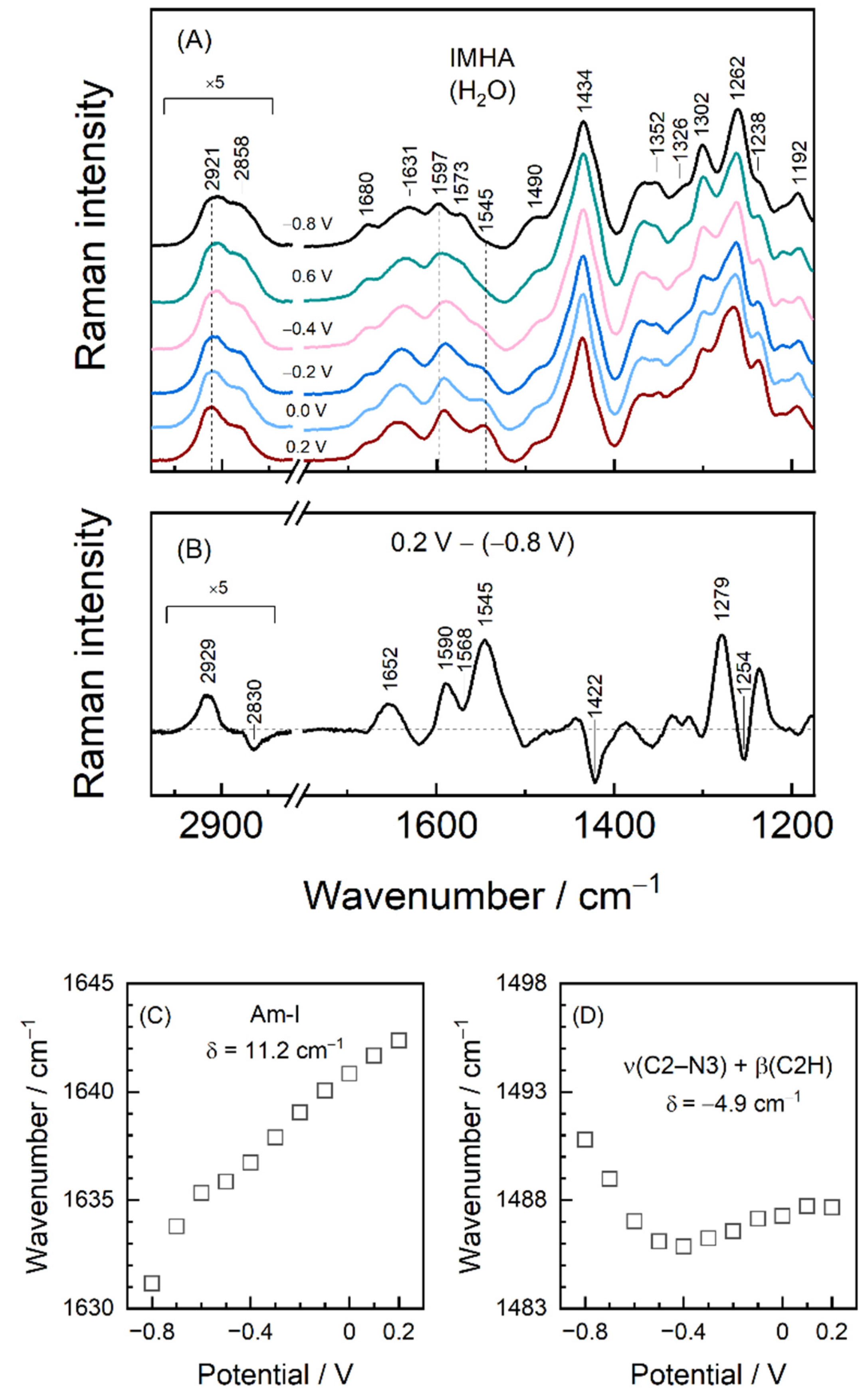
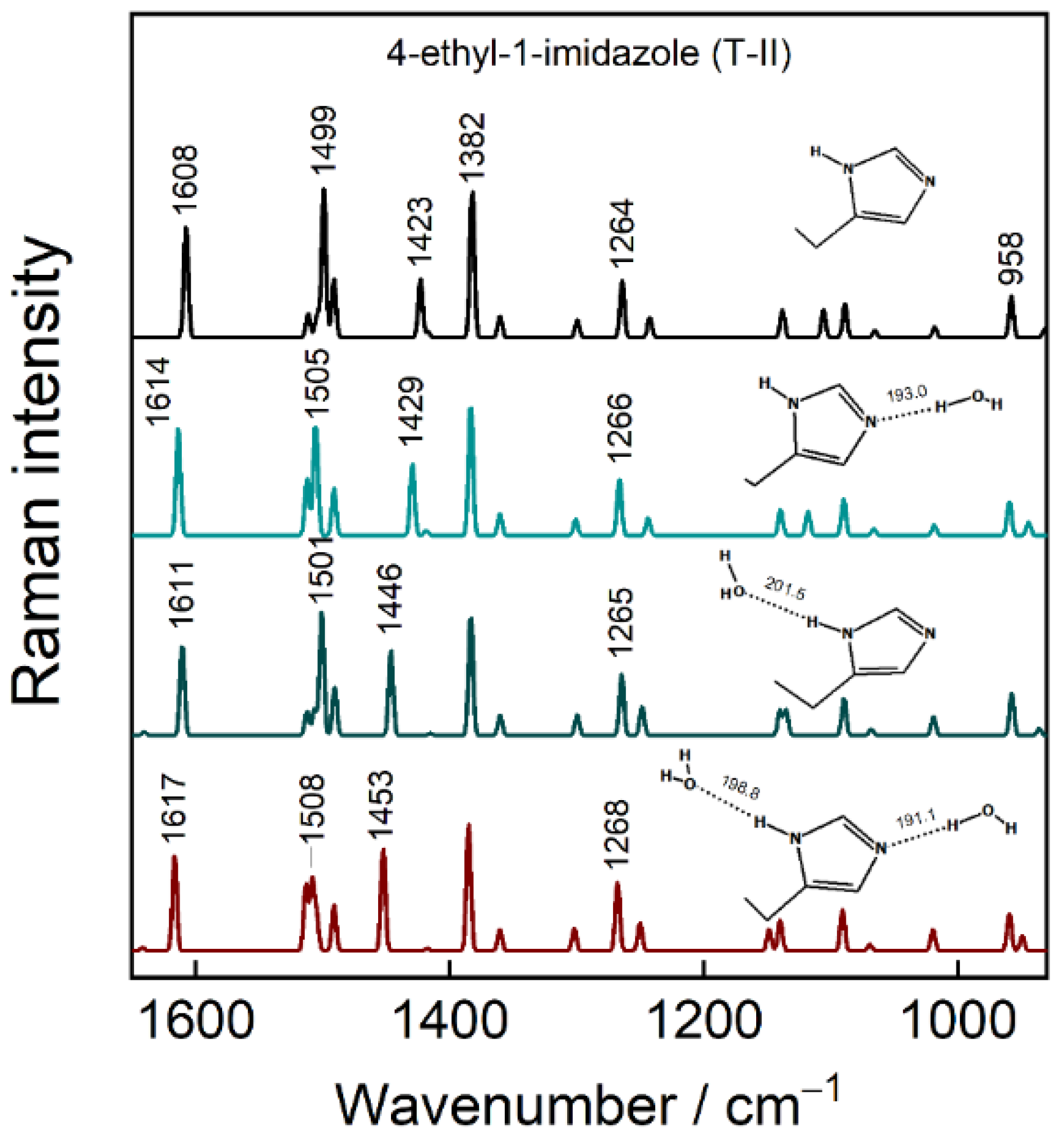
3. Results and Discussion
3.1. Assignments of Raman Bands
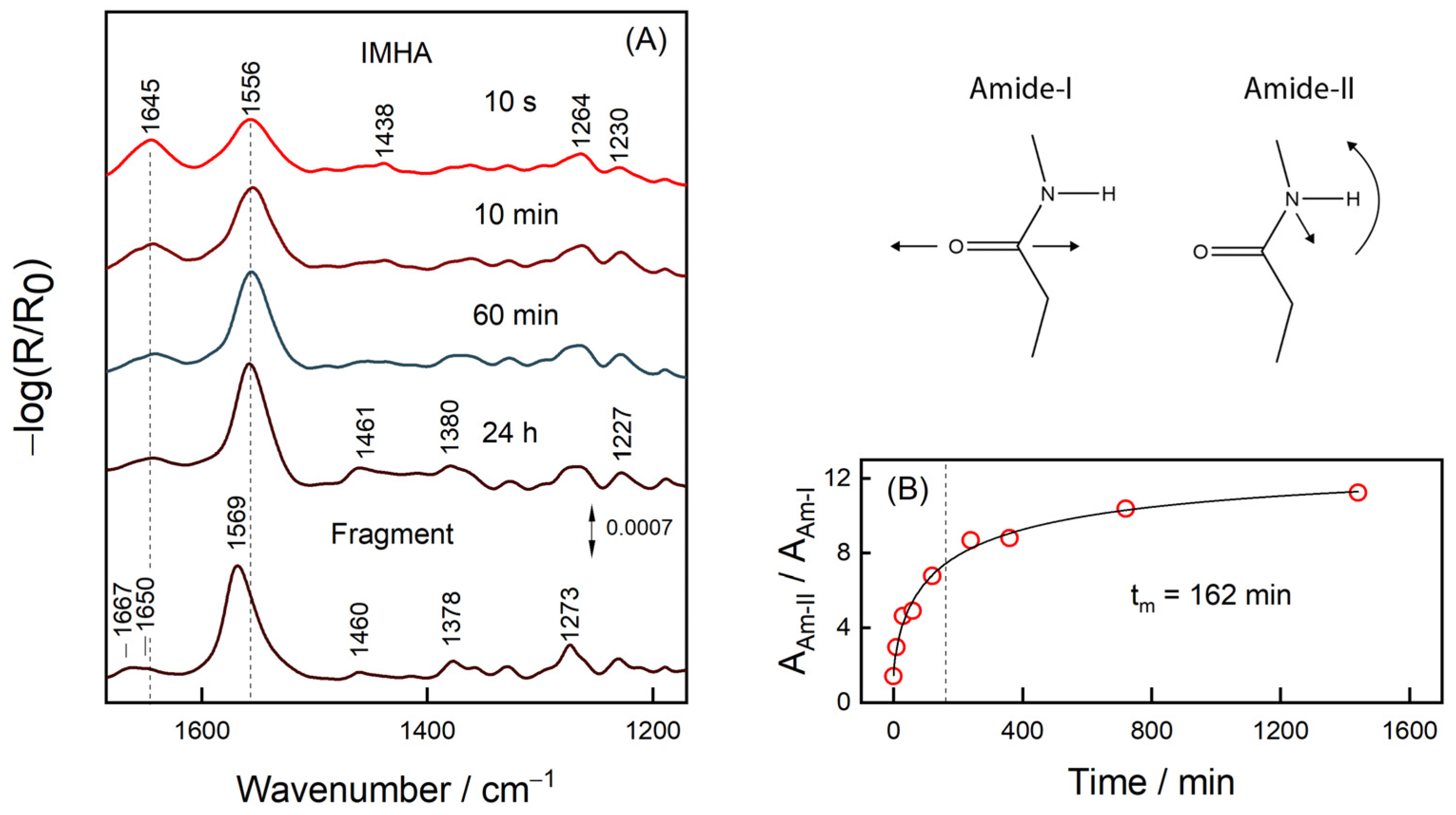
3.2. RAIRS Analysis of the Monolayer Formation
3.3. General Features of IMHA Monolayer SHINERS Spectrum
3.4. Potential-Controlled SHINERS Measurements of IMHA Monolayer
3.5. Raman Markers for H-Bonding Interaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sundberg, R.J.; Martin, R.B. Interactions of histidine and other imidazole derivatives with transition metal ions in chemical and biological systems. Chem. Rev. 1974, 74, 471–517. [Google Scholar] [CrossRef]
- Liao, S.-M.M.; Du, Q.-S.S.; Meng, J.-Z.Z.; Pang, Z.-W.W.; Huang, R.-B.B. The multiple roles of histidine in protein interactions. Chem. Cent. J. 2013, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Zaitouna, A.J.; Lai, R.Y. Design and characterization of a metal ion-imidazole self-assembled monolayer for reversible immobilization of histidine-tagged peptides. Chem. Commun. 2011, 47, 12391–12393. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, B.S.; Chi, Y.S.; Kwak, J.; Choi, I.S.; Lee, S.G. Faradaic impedance titration and control of electron transfer of 1-(12-mercaptododecyl)imidazole monolayer on a gold electrode. Electrochim. Acta 2008, 53, 2630–2636. [Google Scholar] [CrossRef]
- Tappura, K.; Vikholm-Lundin, I.; Albers, W.M. Lipoate-based imprinted self-assembled molecular thin films for biosensor applications. Biosens. Bioelectron. 2007, 22, 912–919. [Google Scholar] [CrossRef]
- Saada, M.C.; Montero, J.L.; Vullo, D.; Scozzafava, A.; Winum, J.Y.; Supuran, C.T. Carbonic anhydrase activators: Gold nanoparticles coated with derivatized histamine, histidine, and carnosine show enhanced activatory effects on several mammalian isoforms. J. Med. Chem. 2011, 54, 1170–1177. [Google Scholar] [CrossRef]
- Pourrostam-Ravadanaq, P.; Safa, K.D.; Abbasi, H. Study of imidazole performance as pseudo-affinity ligand in the purification of IgG from bovine milk. Anal. Biochem. 2020, 597, 113693. [Google Scholar] [CrossRef]
- Durainatarajan, P.; Prabakaran, M.; Ramesh, S.; Periasamy, V. Self-assembly on copper surface by using imidazole derivative for corrosion protection. J. Adhes. Sci. Technol. 2018, 32, 1733–1749. [Google Scholar] [CrossRef]
- Ashikawa, I.; Itoh, K. Raman spectra of polypeptides containing L-histidine residues and tautomerism of imidazole side chain. Biopolymers 1979, 18, 1859–1876. [Google Scholar] [CrossRef]
- Matulaitienė, I.; Kuodis, Z.; Eicher-Lorka, O.; Niaura, G. SERS characterization of imidazole ring terminated self-assembled monolayer formed from lipoic acid histamide on silver electrode. J. Electroanal. Chem. 2013, 700, 77–85. [Google Scholar] [CrossRef]
- Garfinkel, D.; Edsall, J.T. Raman Spectra of Amino Acids and Related Compounds. VIII. Raman and Infrared Spectra of Imidazole, 4-Methylimidazole and Histidine. J. Am. Chem. Soc. 1958, 80, 3807–3812. [Google Scholar] [CrossRef]
- Ashikawa, I.; Itoh, K. Raman Scattering Study on Tautomerism of L-Histidine. Chem. Lett. 1978, 7, 681–684. [Google Scholar] [CrossRef]
- Mesu, J.G.; Visser, T.; Soulimani, F.; Weckhuysen, B.M. Infrared and Raman spectroscopic study of pH-induced structural changes of L-histidine in aqueous environment. Vib. Spectrosc. 2005, 39, 114–125. [Google Scholar] [CrossRef]
- Martusevičius, S.; Niaura, G.; Talaikytė, Z.; Razumas, V. Adsorption of L-histidine on copper surface as evidenced by surface-enhanced Raman scattering spectroscopy. Vib. Spectrosc. 1996, 10, 271–280. [Google Scholar] [CrossRef]
- Carter, D.A.; Pemberton, J.E. Raman spectroscopy and vibrational assignments of 1- and 2-methylimidazole. J. Raman Spectrosc. 1997, 28, 939–946. [Google Scholar] [CrossRef]
- Takeuchi, H. Raman structural markers of tryptophan and histidine side chains in proteins. Biopolymers 2003, 72, 305–317. [Google Scholar] [CrossRef]
- Miura, T.; Satoh, T.; Hori-i, A.; Takeuchi, H. Raman marker bands of metal coordination sites of histidine side chains in peptides and proteins. J. Raman Spectrosc. 1998, 29, 41–47. [Google Scholar] [CrossRef]
- Matulaitienė, I.; Pociūtė, E.; Kuodis, Z.; Eicher-Lorka, O.; Niaura, G. Interaction of 4-imidazolemethanol with a copper electrode revealed by isotope-edited SERS and theoretical modeling. Phys. Chem. Chem. Phys. 2015, 17, 16483–16493. [Google Scholar] [CrossRef]
- Toyama, A.; Ono, K.; Hashimoto, S.; Takeuchi, H. Raman spectra and normal coordinate analysis of the N1-H and N3-H tautomers of 4-methylimidazole: Vibrational modes of histidine tautomer markers. J. Phys. Chem. A 2002, 106, 3403–3412. [Google Scholar] [CrossRef]
- Tasumi, M.; Harada, I.; Takamatsu, T.; Takahashi, S. Raman studies of L-histidine and related compounds in aqueous solutions. J. Raman Spectrosc. 1982, 12, 149–151. [Google Scholar] [CrossRef]
- Takeuchi, H.; Kimura, Y.; Koitabashi, I.; Harada, I. Raman bands of N-deuterated histidinium as markers of conformation and hydrogen bonding. J. Raman Spectrosc. 1991, 22, 233–236. [Google Scholar] [CrossRef]
- Wu, Q.; Li, F.; Wang, W.; Hecht, M.H.; Spiro, T.G. UV Raman monitoring of histidine protonation and H-2H exchange in plastocyanin. J. Inorg. Biochem. 2002, 88, 381–387. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, D.; Spiro, T.G. A UV Resonance Raman Monitor of Histidine Protonation in Proteins: Bohr Protons in Hemoglobin. J. Am. Chem. Soc. 1998, 120, 8517–8518. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A. Review of SERS substrates for chemical sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef]
- Langer, J.; De Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Niaura, G.; Gaigalas, A.K.; Vilker, V.L. Surface-enhanced Raman spectroscopy of phosphate anions: Adsorption on silver, gold, and copper electrodes. J. Phys. Chem. B 1997, 101, 9250–9262. [Google Scholar] [CrossRef]
- Silva, E.F.; Wysard, J.S.; Bandeira, M.C.E.; Mattos, O.R. Electrochemical and surface enhanced Raman spectroscopy study of Guanine as corrosion inhibitor for copper. Corr. Sci. 2021, 191, 109714. [Google Scholar] [CrossRef]
- Silva, E.F.; Wysard, J.S.; Bandeira, M.C.E.; Mattos, O.R.; Alves, W.A. On the 4-methylimidazole behavior at a copper electrode: A view from surface-enhnaced Raman scattering. J. Raman Spectrosc. 2019, 50, 1438–1444. [Google Scholar] [CrossRef]
- Gutowski, L.; Liszewska, M.; Bartosewicz, B.; Budner, B.; Weyher, J.L.; Jankiewicz, B.J. Investigation of organic monoradicals reactivity using surface-enhanced Raman spectroscopy. Spectrochim. Acta A 2022, 278, 121312. [Google Scholar] [CrossRef]
- Anema, J.R.; Li, J.-F.F.; Yang, Z.-L.L.; Ren, B.; Tian, Z.-Q.Q. Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy: Expanding the Versatility of Surface-Enhanced Raman Scattering. Annu. Rev. Anal. Chem. 2011, 4, 129–150. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef]
- Wen, B.Y.; Jin, X.; Li, Y.; Wang, Y.H.; Li, C.Y.; Liang, M.M.; Panneerselvam, R.; Xu, Q.C.; Wu, D.Y.; Yang, Z.L.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy study of the adsorption behaviour of DNA bases on Au(111) electrode surfaces. Analyst 2016, 141, 3731–3736. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Chen, S.Y.; Zheng, Y.L.; Chen, S.P.; Panneerselvam, R.; Chen, S.; Xu, Q.C.; Chen, Y.X.; Yang, Z.L.; Wu, D.Y.; et al. In-situ electrochemical shell-isolated Ag nanoparticles-enhanced Raman spectroscopy study of adenine adsorption on smooth Ag electrodes. Electrochim. Acta 2016, 199, 388–393. [Google Scholar] [CrossRef]
- Barbillon, G. Applications of Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Photonics 2021, 8, 46. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, Y.J.; Ding, S.Y.; Panneerselvam, R.; Tian, Z.Q. Core-shell nanoparticle-enhanced Raman spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, Y.X.; Su, J.Q.; Radjenovic, P.M.; Wang, Y.H.; Zheng, J.F.; Teng, B.; Shao, Y.; Zhou, X.S.; Li, J.F. Probing Interfacial Electronic Effects on Single-Molecule Adsorption Geometry and Electron Transport at Atomically Flat Surfaces. Angew. Chem. Int. Ed. Engl. 2021, 60, 15452–15458. [Google Scholar] [CrossRef]
- Zdaniauskiene, A.; Charkova, T.; Matulaitiene, I.; Eicher-Lorka, O.; Matijoška, A.; Skapas, M.; Selskis, A.; Niaura, G. Electrochemical Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy: Bonding, Structure, and Ion-Pairing of the Positive Charge Bearing Pyridinium Ring Terminated Monolayer at Smooth Gold Electrode. J. Phys. Chem. C 2018, 122, 1234–1242. [Google Scholar] [CrossRef]
- Zdaniauskienė, A.; Ignatjev, I.; Charkova, T.; Talaikis, M.; Lukša, A.; Šetkus, A.; Niaura, G. Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy for Probing Riboflavin on Graphene. Materials 2022, 15, 1636. [Google Scholar] [CrossRef]
- Li, J.-F.F.; Ding, S.-Y.Y.; Yang, Z.-L.L.; Bai, M.-L.L.; Anema, J.R.; Wang, X.; Wang, A.; Wu, D.-Y.Y.; Ren, B.; Hou, S.-M.M.; et al. Extraordinary Enhancement of Raman Scattering from Pyridine on Single Crystal Au and Pt Electrodes by Shell-Isolated Au Nanoparticles. J. Am. Chem. Soc. 2011, 133, 15922–15925. [Google Scholar] [CrossRef]
- Krajczewski, J.; Kudelski, A. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Front. Chem. 2019, 7, 410. [Google Scholar] [CrossRef]
- Niciński, K.; Krajczewski, J.; Kudelski, A.; Witkowska, E.; Trzcińska-Danielewicz, J.; Girstun, A.; Kamińska, A. Detection of circulating tumor cells in blood by shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS) in microfluidic device. Sci. Rep. 2019, 9, 9267. [Google Scholar] [CrossRef] [PubMed]
- Krajczewski, J.; Michałowska, A.; Kudelski, A. Star-shaped plasmonic nanostructures: New, simply synthesized materials for Raman analysis of surfaces. Spectrochim. Acta A 2020, 225, 117469. [Google Scholar] [CrossRef] [PubMed]
- Haryanto, A.; Lee, C.W. Shell isolated nanoparticle enhanced Raman spectroscopy for mechanistic investigation of electrochemical reactions. Nano Convergence 2022, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Daublytė, E.; Zdaniauskienė, A.; Talaikis, M.; Drabavičius, A.; Charkova, T. A facile microwave-assisted synthesis of Ag@SiO2 nanoparticles for Raman spectroscopy. New J. Chem. 2021, 45, 10952–10958. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Talaikis, M.; Eicher-Lorka, O.; Valinčius, G.; Niaura, G. Water-induced structural changes in the membrane-anchoring monolayers revealed by isotope-edited SERS. J. Phys. Chem. C 2016, 120, 22489–22499. [Google Scholar] [CrossRef]
- Niaura, G. Raman Spectroscopy in Analysis of Biomolecules. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–34. [Google Scholar] [CrossRef]
- Nelson, P.N. Chain Length and Thermal Sensitivity of the Infrared Spectra of a Homologous Series of Anhydrous Silver(I) n –Alkanoates. Int. J. Spectrosc. 2016, 2016, 3068430. [Google Scholar] [CrossRef]
- Riauba, L.; Niaura, G.; Eicher-Lorka, O.; Butkus, E. A study of cysteamine ionization in solution by Raman spectroscopy and theoretical modeling. J. Phys. Chem. A 2006, 110, 13394–13404. [Google Scholar] [CrossRef]
- Kim, M.; Hohman, J.N.; Serino, A.C.; Weiss, P.S. Structural manipulation of hydrogen-bonding networks in amide-containing alkanethiolate monolayers via electrochemical processing. J. Phys. Chem. C 2010, 114, 19744–19751. [Google Scholar] [CrossRef]
- Kuodis, Z.; Matulaitienė, I.; Špandyreva, M.; Labanauskas, L.; Stončius, S.; Eicher-Lorka, O.; Sadzevičienė, R.; Niaura, G. Reflection Absorption Infrared Spectroscopy Characterization of SAM Formation from 8-Mercapto-N-(phenethyl)octanamide Thiols with Phe Ring and Amide Groups. Molecules 2020, 25, 5633. [Google Scholar] [CrossRef]
- Jackson, M.; Mantsch, H.H. The Use and Misuse of FTIR Spectroscopy in the Determination of Protein Structure. Crit. Rev. Biochem. Mol. Biol. 1995, 9238, 95–120. [Google Scholar] [CrossRef]
- Clegg, R.S.; Hutchison, J.E. Hydrogen-bonding, self-assembled monolayers: Ordered molecular films for study of through-peptide electron transfer. Langmuir 1996, 12, 5239–5243. [Google Scholar] [CrossRef]
- Kocherbitov, V.; Latynis, J.; Misiūnas, A.; Barauskas, J.; Niaura, G. Hydration of lysozyme studied by Raman spectroscopy. J. Phys. Chem. B 2013, 117, 4981–4992. [Google Scholar] [CrossRef] [PubMed]
- Myshakina, N.S.; Ahmed, Z.; Asher, S.A. Dependence of amide vibrations on hydrogen bonding. J. Phys. Chem. B 2008, 112, 11873–11877. [Google Scholar] [CrossRef]
- Talaikis, M.; Strazdaitė, S.; Žiaunys, M.; Niaura, G. Far-Off Resonance: Multiwavelength Raman Spectroscopy Probing Amide Bands of Amyloid-β-(37–42). Molecules 2020, 25, 3556. [Google Scholar] [CrossRef]
- Matulaitienė, I.; Kuodis, Z.; Matijoška, A.; Eicher-Lorka, O.; Niaura, G. SERS of the Positive Charge Bearing Pyridinium Ring Terminated Self-Assembled Monolayers: Structure and Bonding Spectral Markers. J. Phys. Chem. C 2015, 119, 26481–26492. [Google Scholar] [CrossRef]
- Holze, R. The adsorption of thiophenol on gold—A spectroelectrochemical study. Phys. Chem. Chem. Phys. 2015, 17, 21364–21372. [Google Scholar] [CrossRef] [PubMed]
- Nyamekye, C.K.A.; Weibel, S.C.; Smith, E.A. Directional Raman scattering spectra of metal–sulfur bonds at smooth gold and silver substrates. J. Raman Spectrosc. 2021, 52, 1246–1255. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Corthey, G.; Pensa, E.; Cortés, E.; Fonticelli, M.H.; Ibañez, F.; Benitez, G.E.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiolates on metals: A review article on sulfur-metal chemistry and surface structures. RSC Adv. 2014, 4, 27730–27754. [Google Scholar] [CrossRef]
- Bryant, M.A.; Pemberton, J.E. Surface Raman Scattering of Self-Assembled Monolayers Formed from 1-Alkanethiols at Ag. J. Am. Chem. Soc. 1991, 113, 3629–3637. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Jarzecki, A.A.; Wu, Q.; Kozlowski, P.M.; Wang, D.; Spiro, T.G. Mode recognition in UV resonance Raman spectra of imidazole: Histidine monitoring in proteins. J. Phys. Chem. B 2012, 116, 9387–9395. [Google Scholar] [CrossRef][Green Version]
- Xu, M.; Shashilov, V.; Lednev, I.K. Probing the cross-β core structure of amyloid fibrils by hydrogen-deuterium exchange deep ultraviolet resonance Raman spectroscopy. J. Am. Chem. Soc. 2007, 129, 11002–11003. [Google Scholar] [CrossRef] [PubMed]
- Razmute-Razmė, I.; Kuodis, Z.; Eicher-Lorka, O.; Niaura, G. SERS observation of soft C-H vibrational mode of bifunctional alkanethiol molecules adsorbed at Au and Ag electrodes. Phys. Chem. Chem. Phys. 2010, 12, 4564–4568. [Google Scholar] [CrossRef] [PubMed]

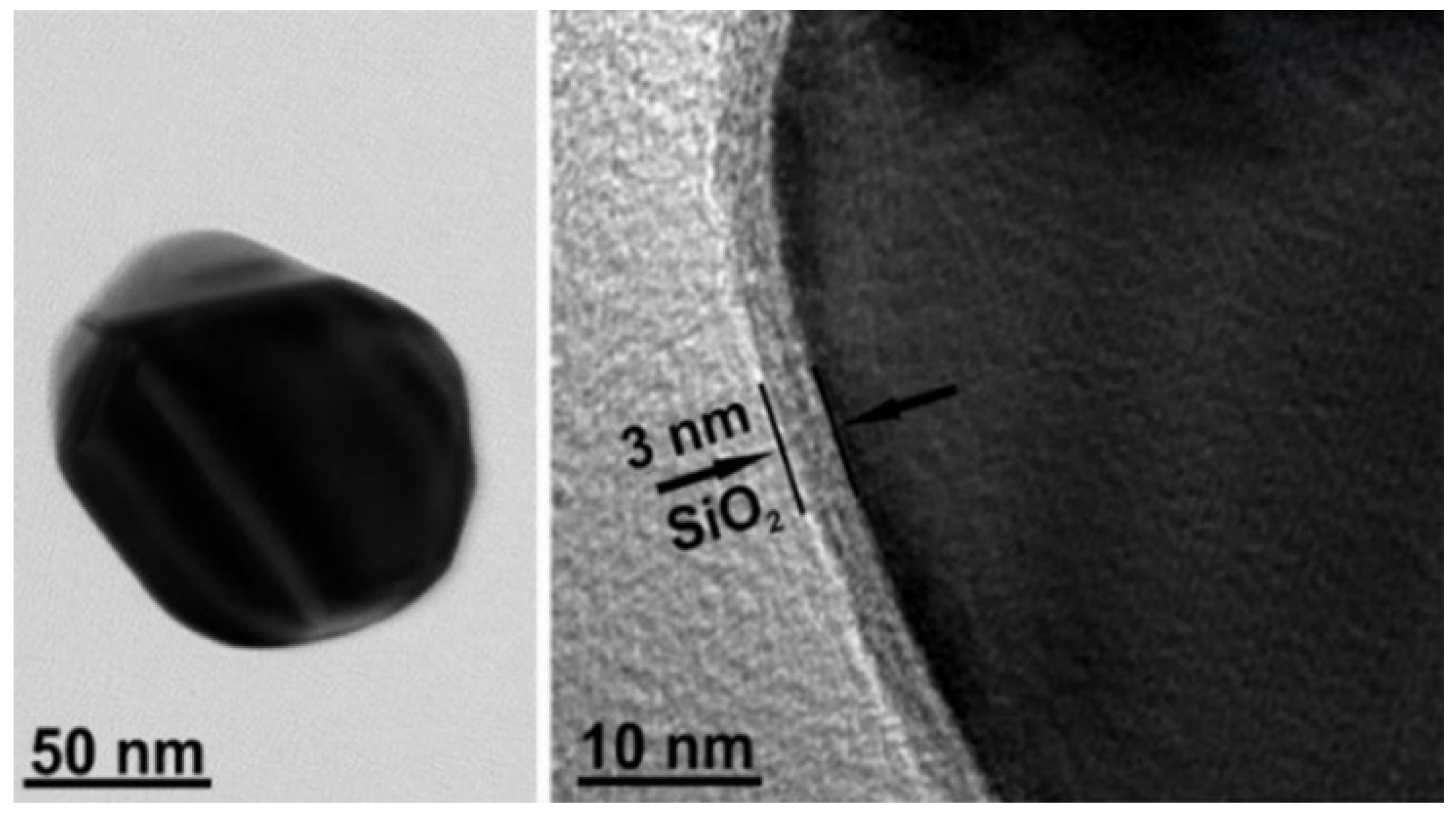
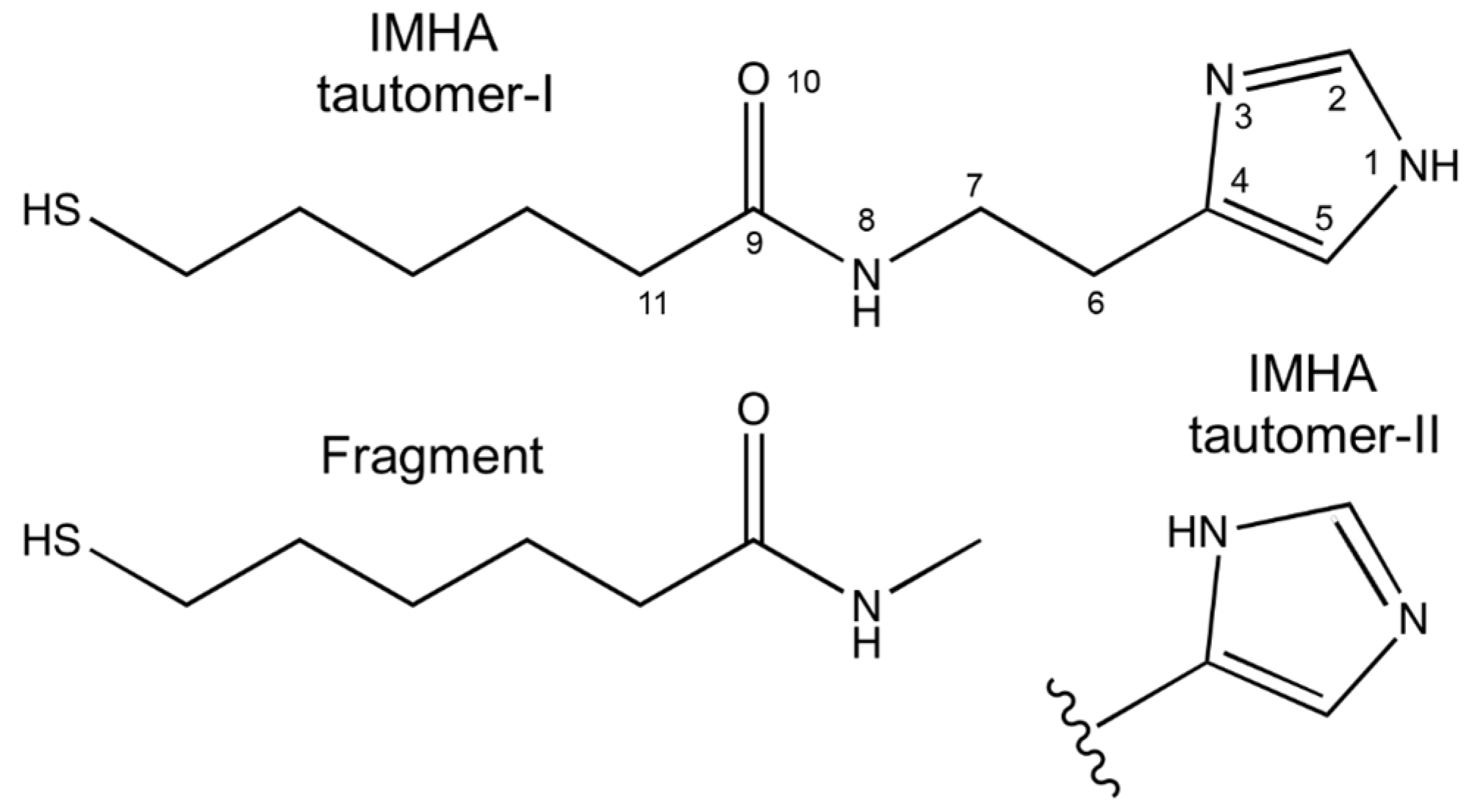
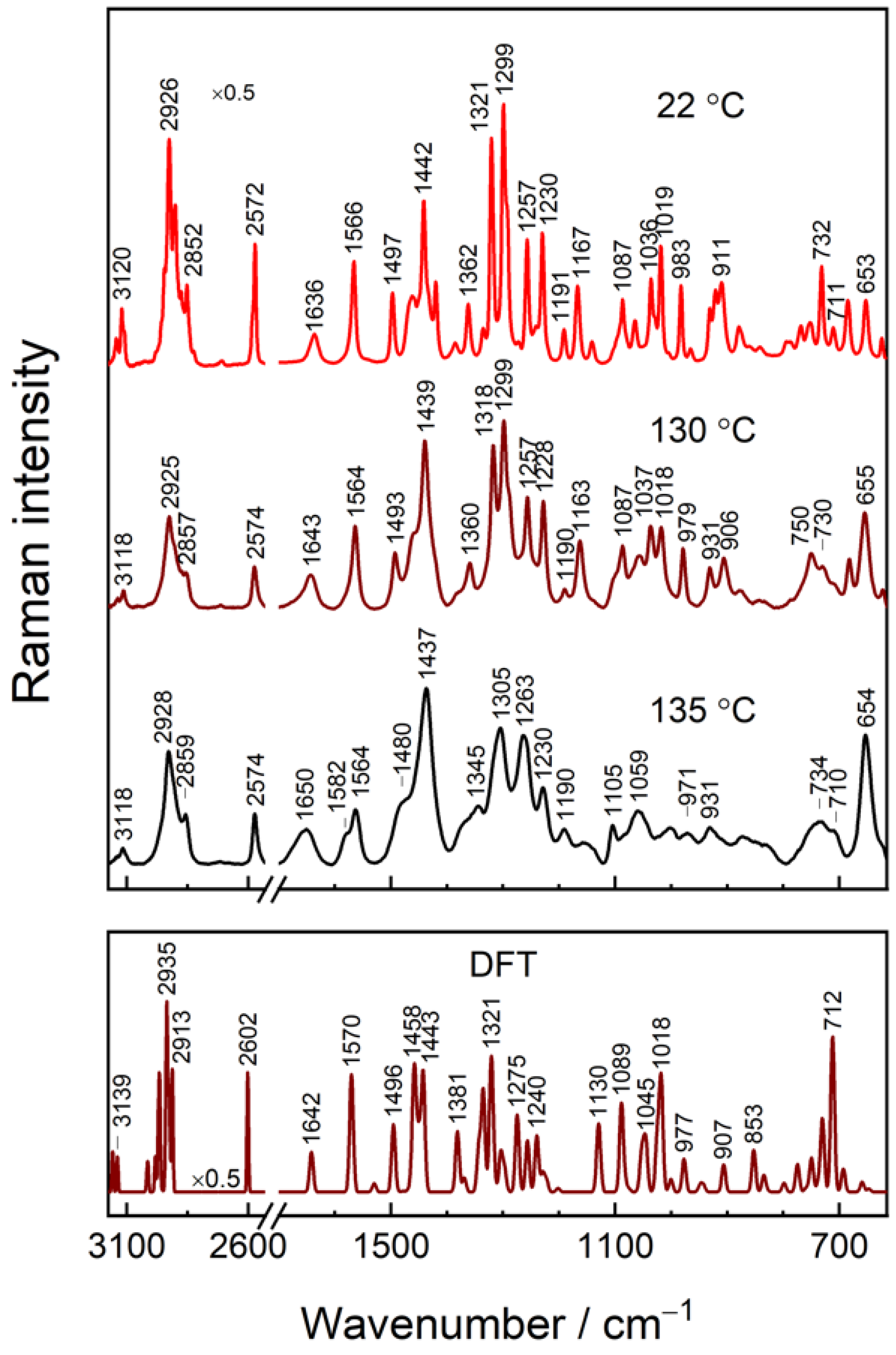
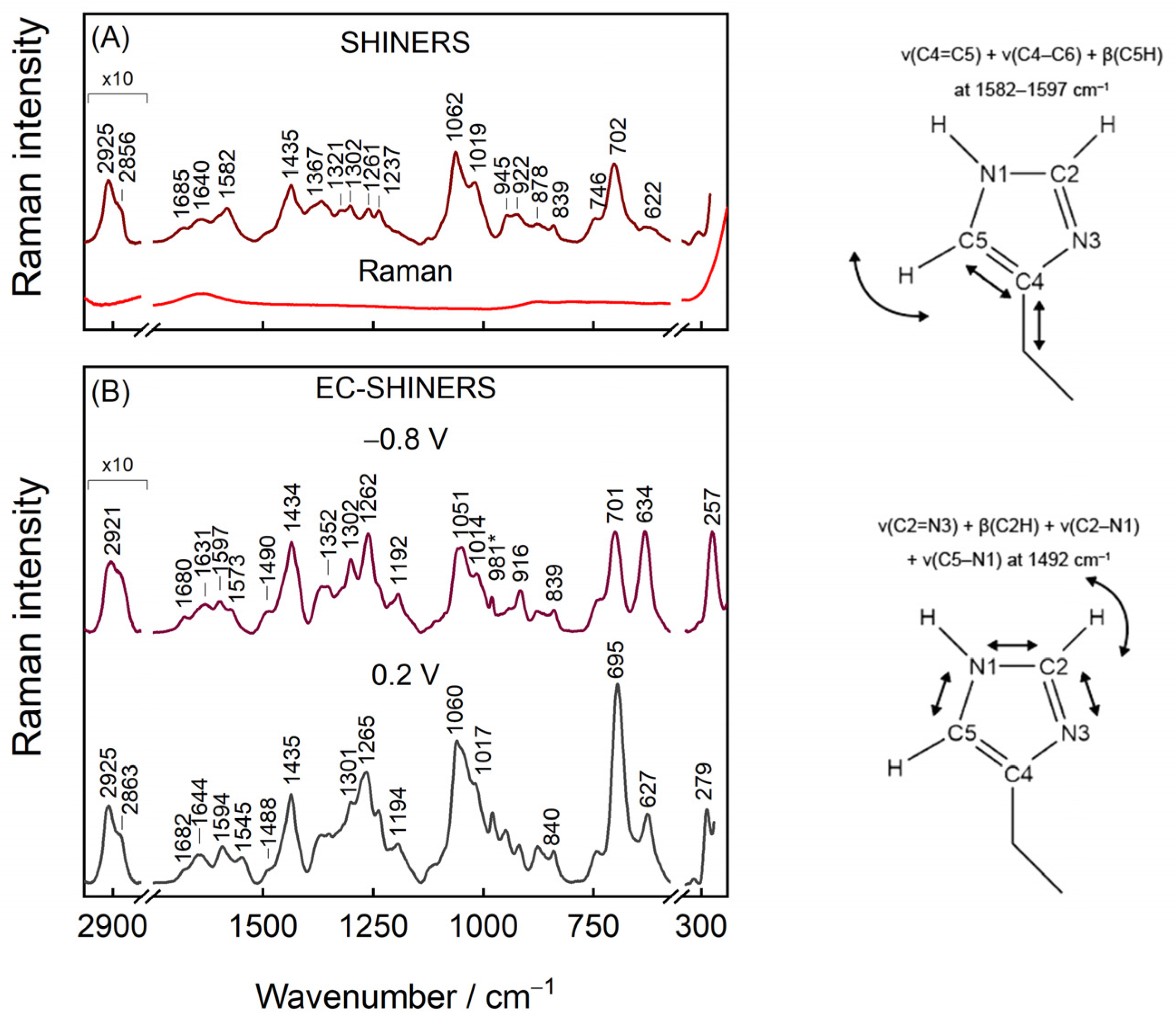
| Raman, cm−1 | SHINERS, cm−1 | DFT | Ref. | Assignment | ||
|---|---|---|---|---|---|---|
| 22 °C | 130 °C | 135 °C | H2O, −0.8 V | |||
| 3142 | 3139 | 3144 sh | 3158 | ν(C5–H) | ||
| 3120 | 3118 | 3118 | 3139 | ν(C2–H) | ||
| 2926 | 2925 | 2928 | 2921 | 2935 | νas(CH2) | |
| 2852 | 2857 | 2859 | 2856 | 2913 | νs(CH2) | |
| 2572 | 2574 | 2574 | n.a. | 2602 | ν(S–H) | |
| 1636 | 1643 | 1650 | 1680 1631 | 1642 | ν(C=O) Amide-I | |
| 1582 T-II | 1597 T-II | 1587 T-II | [9,18,19] | ν(C4=C5) + ν(C4–C6) + β(C5H) | ||
| 1566 T-I | 1564 T-I | 1564 T-I | 1573 T-I | 1570 T-I | ||
| 1530 | ν(C–N) + δ(NH) Amide-II | |||||
| 1497 | 1493 | 1480 | 1490 sh | 1496 | [10,18] | ν(C2–N3) + β(C2H) + ν(C2–N1) + ν(C5–N1) |
| 1464 | 1461 | 1458 | δ(CH2) scissoring | |||
| 1442 | 1439 | 1437 | 1434 | 1443 | δ(CH2) scissoring | |
| 1362 | 1360 | 1372 | 1381 | w(CH2) | ||
| 1345 T-II | 1352 sh T-II | [19] | δ(CH2) + ν(Im) breathing + δ(C5H) | |||
| 1321 T-I | 1318 T-I | 1326 sh T-I | 1335 T-I | |||
| 1299 | 1299 | 1305 | 1302 | 1303 | t(CH2) | |
| 1257 T-II | 1257 T-II | 1263 T-II | 1262 T-II | [9,19,47] | ν(Im) breathing + β(C2H) | |
| 1230 | 1228 | 1230 | 1238 | 1240 | [18] | β(C5H) + β(C2H) + ν(C5–N1) |
| 1191 | 1190 | 1190 | 1192 | 1202 | t(C6H2) + δ(N8H) | |
| 1167 | 1163 | 1130 | [13] | ν(C2–N1) + δ(N1H) | ||
| 1087 | 1087 | 1105 | 1089 | ν(C–C)T + δ(CSH) + δ(CCS) | ||
| 1036 | 1037 | 1059 | 1051 | 1045 | ν(C–C)T | |
| 1019 | 1018 | 1014 | 1018 | ν(C6–C7) | ||
| 983 | 979 | 971 | 977 | [9,19] | β(CH) Im for T-I | |
| 931 | 931 | 931 | 943 | t(CH2) + r(CH2) | ||
| 921 | 916 | 948 | [13] | β(CH) Im | ||
| 911 | 906 | 907 | δ(N8C9C11) | |||
| 841 | 842 | 839 | 835 | γ(C2H) | ||
| 753 | 750 | 750 | [48] | γ(C5H) + r(CH2) | ||
| 732 | 730 | 734 | 730 | [48] | r(CH2) + ν(S–C)T | |
| 711 | 709 | 710 | 701 | 712 | ν(S–C)T | |
| 685 | 682 | 694 | γ(Im) | |||
| 653 | 655 | 654 | 634 | n.a. | [49] | ν(S–C)G + δ(Im) |
| IMHA | Fragment | |||||
|---|---|---|---|---|---|---|
| SAM, 24 h | Powder | δ | SAM, 24 h | Powder | δ | |
| Am-II, cm−1 | 1557, 29 | 1577, 37 | −20 | 1569, 28 | 1556, 52 | 13 |
| Am-I, cm−1 | 1642, 42 | 1638, 27 | 6 | 1650, 28; 1667, 16 | 1647, 41 | 3; 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdaniauskienė, A.; Talaikis, M.; Charkova, T.; Sadzevičienė, R.; Labanauskas, L.; Niaura, G. Electrochemical Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy of Imidazole Ring Functionalized Monolayer on Smooth Gold Electrode. Molecules 2022, 27, 6531. https://doi.org/10.3390/molecules27196531
Zdaniauskienė A, Talaikis M, Charkova T, Sadzevičienė R, Labanauskas L, Niaura G. Electrochemical Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy of Imidazole Ring Functionalized Monolayer on Smooth Gold Electrode. Molecules. 2022; 27(19):6531. https://doi.org/10.3390/molecules27196531
Chicago/Turabian StyleZdaniauskienė, Agnė, Martynas Talaikis, Tatjana Charkova, Rita Sadzevičienė, Linas Labanauskas, and Gediminas Niaura. 2022. "Electrochemical Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy of Imidazole Ring Functionalized Monolayer on Smooth Gold Electrode" Molecules 27, no. 19: 6531. https://doi.org/10.3390/molecules27196531
APA StyleZdaniauskienė, A., Talaikis, M., Charkova, T., Sadzevičienė, R., Labanauskas, L., & Niaura, G. (2022). Electrochemical Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy of Imidazole Ring Functionalized Monolayer on Smooth Gold Electrode. Molecules, 27(19), 6531. https://doi.org/10.3390/molecules27196531








