Occurrence and Characteristics of Staphylococcus aureus Strains along the Production Chain of Raw Milk Cheeses in Poland
Abstract
:1. Introduction
2. Results
2.1. Occurrence of S. aureus Strains along the Cheese Production Chain
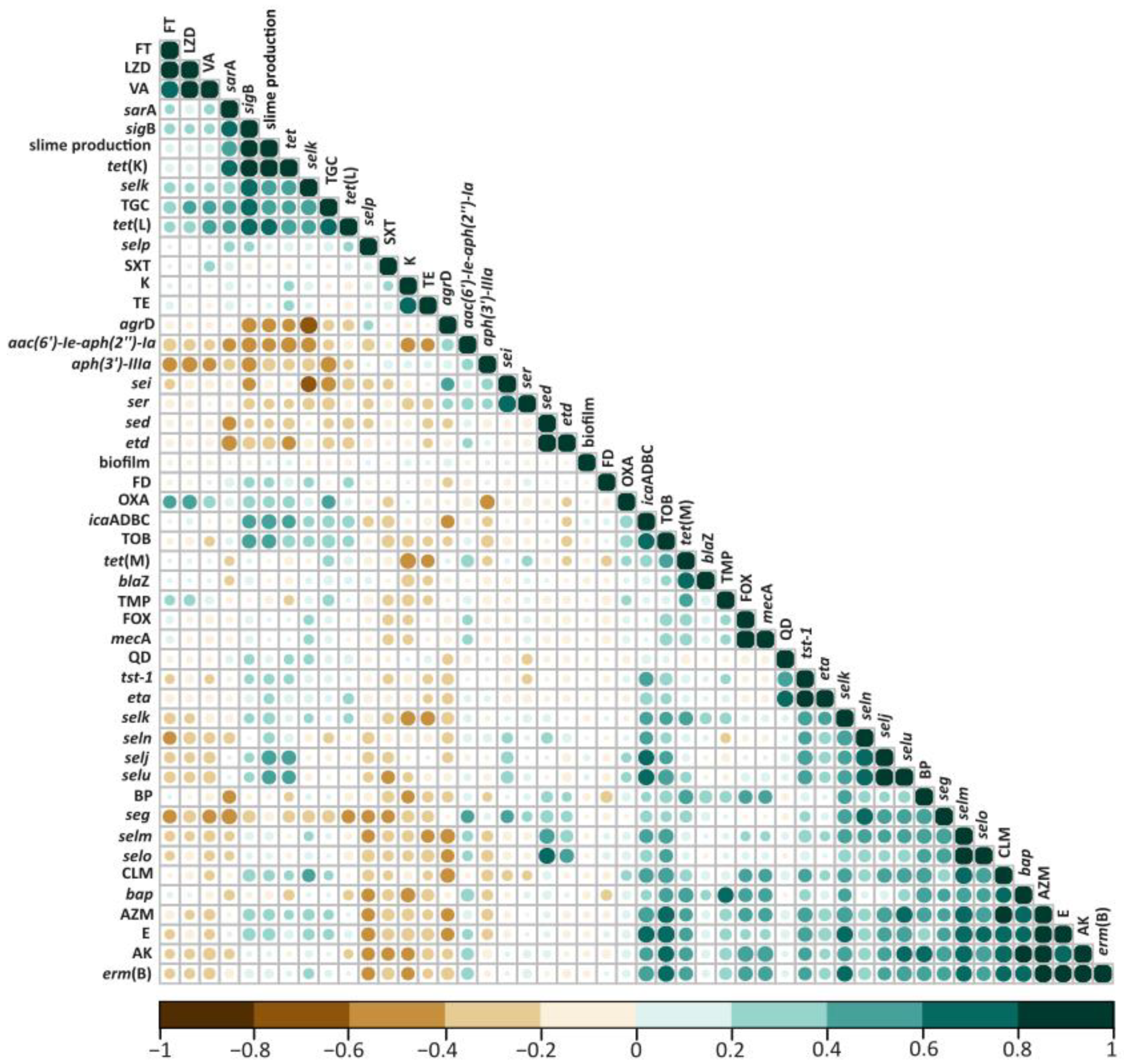
2.2. Antibiotic Resistance Profiles among S. aureus Strains
2.3. Presence of Virulence Factors in S. aureus Isolates
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Isolation and Identification of S. aureus Isolates
4.3. Antimicrobial Susceptibility Testing by Microdilution Broth Assay
4.4. In Vitro Biofilm Production Analysis
4.5. Detection of Antibiotic Resistance, Enterotoxins, and Biofilm-Associated Genes among Isolates
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jianqin, S.; Leiming, X.; Lu, X.; Yelland, G.W.; Ni, J.; Clarke, A.J. Effects of milk containing only A2 β casein versus milk containing both A1 and A2 β casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr. J. 2016, 15, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bórawski, P.; Bórawski, M.B.; Parzonko, A.; Wicki, L.; Rokicki, T.; Perkowska, A.; Dunn, J.W. Development of organic milk production in Poland on the background of the EU. Agriculture 2021, 11, 323. [Google Scholar] [CrossRef]
- Kawęcka, A.; Radkowska, I.; Sikora, J. Concentrations of selected bioactive components in traditional cheeses made from goat’s, cow’s and sheep’s milk. J. Elem. 2020, 25, 431–442. [Google Scholar] [CrossRef]
- Rola, J.G.; Czubkowska, A.; Korpysa-Dzirba, W.; Osek, J. Occurrence of Staphylococcus aureus on farms with small scale production of raw milk cheeses in poland. Toxins 2016, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Haque, Z.F.; Sabuj, A.A.M.; Mahmud, M.M.; Pondit, A.; Islam, M.A.; Saha, S. Characterization of Staphylococcus aureus from Milk and Dairy Products Sold in Some Local Markets of Mymensingh District of Bangladesh. J. Nutr. Food Sci. 2018, 8, 1000743. [Google Scholar] [CrossRef]
- Martínez-Vasallo, A.; Ribot-Enríquez, A.; Riverón-Alemán, Y.; Remón-Díaz, D.; Martínez-García, Y.A.; Jacsens, L.; Uyttendaele, M. Staphylococcus aureus in the production chain of artisan fresh cheese Staphylococcus aureus en la cadena de producción de queso. Rev. Salud Anim. 2019, 41, 1–9. [Google Scholar]
- Stessl, B.; Hein, I.; Wagner, M.E.-S. Staphylococcus aureus in the Dairy Chain. In Rapid Detection, Characterization, and Enumeration of Foodborne Pathogens; Hoorfar, J., Ed.; Wiley: New York, NY, USA, 2011; pp. 291–305. [Google Scholar] [CrossRef]
- Kümmel, J.; Stessl, B.; Gonano, M.; Walcher, G.; Bereuter, O.; Fricker, M.; Grunert, T.; Wagner, M.; Ehling-Schulz, M. Staphylococcus aureus entrance into the Dairy Chain: Tracking S. aureus from dairy cow to cheese. Front. Microbiol. 2016, 7, 1603. [Google Scholar] [CrossRef] [Green Version]
- Şanlıbaba, P. Prevalence, antibiotic resistance, and enterotoxin production of Staphylococcus aureus isolated from retail raw beef, sheep, and lamb meat in Turkey. Int. J. Food Microbiol. 2022, 361, 109461. [Google Scholar] [CrossRef]
- Urmi, M.R.; Ansari, W.K.; Islam, M.S.; Sobur, M.A.; Rahman, M.; Rahman, M.T. Antibiotic Resistance Patterns of Staphylococcus Spp. Isolated from Fast Foods Sold in Different Restaurants of Mymensingh, Bangladesh. J. Adv. Vet. Anim. Res. 2021, 8, 274–281. [Google Scholar] [CrossRef]
- Loomba, P.; Taneja, J.; Mishra, B. Methicillin and vancomycin resistant S. aureus in hospitalized patients. J. Glob. Infect. Dis. 2010, 2, 275. [Google Scholar] [CrossRef]
- Fisher, E.A.; Paterson, G.K. Prevalence and characterisation of methicillin-resistant staphylococci from bovine bulk tank milk in England and Wales. J. Glob. Antimicrob. Resist. 2020, 22, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Founou, L.L.; Founou, R.C.; Essack, S.Y.; Djoko, C.F. Mannitol-fermenting methicillin-resistant staphylococci (MRS) in pig abattoirs in Cameroon and South Africa: A serious food safety threat. Int. J. Food Microbiol. 2018, 285, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Fetsch, A.; Etter, D.; Johler, S. Livestock-Associated Meticillin-Resistant Staphylococcus aureus—Current Situation and Impact From a One Health Perspective. Curr. Clin. Microbiol. Rep. 2021, 8, 103–113. [Google Scholar] [CrossRef]
- Song, J.W.; Yang, S.J.; Shin, S.; Seo, K.S.; Ho Park, Y.; Kun, A.; Park, T. Genotypic and Phenotypic Characterization of Methicillin-Resistant Staphylococcus aureus Isolated from Bovine Mastitic Milk in Korea. J. Food Prot. 2016, 79, 1725–1732. [Google Scholar] [CrossRef]
- Tenhagen, B.-A.; Alt, K.; Pfefferkorn, B.; Wiehle, L.; Käsbohrer, A.; Fetsch, A. Short communication: Methicillin-resistant Staphylococcus aureus in conventional and organic dairy herds in Germany. J. Dairy Sci. 2018, 101, 3380–3386. [Google Scholar] [CrossRef]
- Ou, Q.; Zhou, J.; Lin, D.; Bai, C.; Zhang, T.; Lin, J.; Zheng, H.; Wang, X.; Ye, J.; Ye, X.; et al. A large meta-analysis of the global prevalence rates of S. aureus and MRSA contamination of milk. Crit. Rev. Food Sci. Nutr. 2018, 58, 2213–2228. [Google Scholar] [CrossRef]
- De Oliveira, A.P.D.; da Costa, M.M.; Nogueira, D.M.; Dias, F.S. Characterisation of Staphylococcus aureus strains from milk and goat cheese and evaluation of their inhibition by gallic acid, nisin and velame of the Brazilian caatinga. Int. J. Dairy Technol. 2020, 73, 345–356. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; Bendary, M.M.; Merwad, A.M.A.; Elsohaby, I.; Mohammad Ghaith, D.; Alshareef, W.A. What is behind phylogenetic analysis of hospital-, community- and livestock-associated methicillin-resistant Staphylococcus aureus? Transbound. Emerg. Dis. 2019, 66, 1506–1517. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; Sewid, A.H.; Samir, M.; H Hegazy, W.A.; Bahnass, M.M.; Mosbah, R.A.; Ghaith, D.M.; Khalifa, E.; Ramadan, H.; Alshareef, W.A.; et al. Clonal Diversity and Epidemiological Characteristics of ST239-MRSA Strains. Front. Cell. Infect. Microbiol. 2022, 12, 241. [Google Scholar] [CrossRef]
- Da Silva Cândido, T.J.; da Silva, A.C.; de Matos, L.G.; da Silva do Nascimento, M.; Camargo, C.H.; Cobo Zanella, R.; Mores Rall, V.L.; Cirone Silva, N.C. Enterotoxigenic potential and molecular typing of Staphylococcus sp. isolated from organic and conventional fresh minas cheese in the state of São Paulo, Brazil. Int. Dairy J. 2020, 102, 104605. [Google Scholar] [CrossRef]
- Avila-Novoa, M.G.; González-Gómez, J.P.; Guerrero-Medina, P.J.; Cardona-López, M.A.; Ibarra-Velazquez, L.M.; Velazquez-Suarez, N.Y.; Morales-del Río, J.A.; Gutiérrez-Lomelí, M. Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) strains isolated from dairy products: Relationship of ica-dependent/independent and components of biofilms produced in vitro. Int. Dairy J. 2021, 119, 105066. [Google Scholar] [CrossRef]
- Gajewska, J.; Chajęcka-Wierzchowska, W. Biofilm formation ability and presence of adhesion genes among coagulase-negative and coagulase-positive staphylococci isolates from raw cow’s milk. Pathogens 2020, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Alghizzi, M.; Shami, A. The prevalence of Staphylococcus aureus and methicillin resistant Staphylococcus aureus in milk and dairy products in Riyadh, Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 7098–7104. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, P.; Papadopoulos, T.; Angelidis, A.S.; Kotzamanidis, C.; Zdragas, A.; Papa, A.; Filioussis, G.; Sergelidis, D. Prevalence, antimicrobial susceptibility and characterization of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus isolated from dairy industries in north-central and north-eastern Greece. Int. J. Food Microbiol. 2019, 291, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, R.A.; Heggebø, R.; Sunde, E.B.; Skjervheim, M. Staphylococcus aureus and Listeria monocytogenes in Norwegian raw milk cheese production. Food Microbiol. 2011, 28, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Sri Prabakusuma, A.; Zhu, J.; Shi, Y.; Ma, Q.; Zhao, Q.; Yang, Z.; Xu, Y.; Huang, A. Prevalence and antimicrobial resistance profiling of Staphylococcus aureus isolated from traditional cheese in Yunnan, China. 3 Biotech 2022, 12, 1. [Google Scholar] [CrossRef]
- Dai, J.; Wu, S.; Huang, J.; Wu, Q.; Zhang, F.; Zhang, J.; Wang, J.; Ding, Y.; Zhang, S.; Yang, X.; et al. Prevalence and Characterization of Staphylococcus aureus Isolated from Pasteurized Milk in China. Front. Microbiol. 2019, 10, 641. [Google Scholar] [CrossRef]
- Cai, H.; Kou, X.; Ji, H.; Wang, X.; Wang, H.; Zhang, Y.; Lu, S.; Li, B.; Dong, J.; Wang, Q.; et al. Prevalence and characteristics of Staphylococcus aureus isolated from Kazak cheese in Xinjiang, China. Food Control 2021, 123, 107759. [Google Scholar] [CrossRef]
- Wang, H.; Shen, J.; Zhu, C.; Ma, K.; Fang, M.; Li, B.; Wang, W.; Xue, T. Antibiotics Resistance and Virulence of Staphylococcus aureus Isolates Isolated from Raw Milk from Handmade Dairy Retail Stores in Hefei City, China. Foods 2022, 11, 2185. [Google Scholar] [CrossRef]
- McMillan, K.; Moore, S.C.; McAuley, C.M.; Fegan, N.; Fox, E.M. Characterization of Staphylococcus aureus isolates from raw milk sources in Victoria, Australia. BMC Microbiol. 2016, 16, 169. [Google Scholar] [CrossRef]
- Nhatsave, N.; Garrine, M.; Messa, A.; Massinga, A.J.; Cossa, A.; Vaz, R.; Ombi, A.; Zimba, T.F.; Alfredo, H.; Mandomando, I.; et al. Molecular characterization of staphylococcus aureus isolated from raw milk samples of dairy cows in manhiça district, southern mozambique. Microorganisms 2021, 9, 1684. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Cai, H.; Huang, S.; Ni, Y.; Luo, B.; Qian, H.; Ji, H.; Wang, X. Prevalence and Characteristics of Staphylococcus aureus Isolated From Retail Raw Milk in Northern Xinjiang, China. Front. Microbiol. 2021, 12, 705947. [Google Scholar] [CrossRef]
- Hassani, S.; Moosavy, M.H.; Gharajalar, S.N.; Khatibi, S.A.; Hajibemani, A.; Barabadi, Z. High prevalence of antibiotic resistance in pathogenic foodborne bacteria isolated from bovine milk. Sci. Rep. 2022, 12, 3878. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lazaro, D.; Lee, L.-H.; Stessl, B.; Ma, A.; Xu, J.; Wang, W.; Baloch, Z.; Jiang, T.; Zhang, C.; Peng, Z.; et al. Enterotoxigenicity and Antimicrobial Resistance of Staphylococcus aureus Isolated from Retail Food in China. Front. Microbiol. 2017, 8, 2256. [Google Scholar] [CrossRef] [Green Version]
- Mroczkowska, A.; Żmudzki, J.; Marszałek, N.; Orczykowska-Kotyna, M.; Komorowska, I.; Nowak, A.; Grzesiak, A.; Czyżewska-Dors, E.; Dors, A.; Pejsak, Z.; et al. Livestock-associated Staphylococcus aureus on Polish pig farms. PLoS ONE 2017, 12, e0170745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johler, S.; Macori, G.; Bellio, A.; Acutis, P.L.; Gallina, S.; Decastelli, L. Short communication: Characterization of Staphylococcus aureus isolated along the raw milk cheese production process in artisan dairies in Italy. J. Dairy Sci. 2018, 101, 2915–2920. [Google Scholar] [CrossRef]
- Papadopoulos, P.; Papadopoulos, T.; Angelidis, A.S.; Boukouvala, E.; Zdragas, A.; Papa, A.; Hadjichristodoulou, C.; Sergelidis, D. Prevalence of Staphylococcus aureus and of methicillin-resistant S. aureus (MRSA) along the production chain of dairy products in north-western Greece. Food Microbiol. 2018, 69, 43–50. [Google Scholar] [CrossRef]
- Zaatout, N.; Ayachi, A.; Kecha, M.; Kadlec, K. Identification of staphylococci causing mastitis in dairy cattle from Algeria and characterization of Staphylococcus aureus. J. Appl. Microbiol. 2019, 127, 1305–1314. [Google Scholar] [CrossRef]
- Brahma, U.; Suresh, A.; Murthy, S.; Bhandari, V.; Sharma, P. Antibiotic Resistance and Molecular Profiling of the Clinical Isolates of Staphylococcus aureus Causing Bovine Mastitis from India. Microorganisms 2022, 10, 833. [Google Scholar] [CrossRef]
- Kayili, E.; Sanlibaba, P. Prevalence, characterization and antibiotic resistance of Staphylococcus aureus isolated from traditional cheeses in Turkey. Int. J. Food Prop. 2020, 23, 1441–1451. [Google Scholar] [CrossRef]
- Wang, W.; Lin, X.; Jiang, T.; Peng, Z.; Xu, J.; Yi, L.; Li, F.; Fanning, S.; Baloch, Z. Prevalence and characterization of Staphylococcus aureus cultured from raw milk taken from dairy cows with mastitis in Beijing, China. Front. Microbiol. 2018, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.; Pinho, E.; Almeida, G.; Azevedo, N.F.; Almeida, C. Prevalence and Diversity of Staphylococcus aureus and Staphylococcal Enterotoxins in Raw Milk From Northern Portugal. Front. Microbiol. 2022, 13, 703. [Google Scholar] [CrossRef]
- Adame-g, R.; Toribio-jimenez, J.; Vences-velazquez, A.; Rodr, E.; Cristina, M.; Dionisio, S.; Ramirez-peralta, A. Methicillin-Resistant Staphylococcus aureus (MRSA) in Artisanal Cheeses in México. Int. J. Microbiol. 2018, 2018, 8760357. [Google Scholar]
- Bulajic, S.; Colovic, S.; Misic, D.; Djordjevic, J.; Savic-Radovanovic, R.; Asanin, J.; Ledina, T. Enterotoxin production and antimicrobial susceptibility in Staphylococci isolated from traditional raw milk cheeses in Serbia. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2017, 52, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Mehli, L.; Hoel, S.; Thomassen, G.M.B.; Jakobsen, A.N.; Karlsen, H. The prevalence, genetic diversity and antibiotic resistance of Staphylococcus aureus in milk, whey, and cheese from artisan farm dairies. Int. Dairy J. 2017, 65, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Panchal, V.V.; Griffiths, C.; Mosaei, H.; Bilyk, B.; Sutton, J.A.F.; Carnell, O.T.; Hornby, D.P.; Green, J.; Hobbs, J.K.; Kelley, W.L.; et al. Evolving MRSA: High-level β-lactam resistance in Staphylococcus aureus is associated with RNA polymerase alterations and fine tuning of gene expression. PLoS Pathog. 2020, 16, e1008672. [Google Scholar] [CrossRef]
- Aragão, B.B.; Trajano, S.C.; Silva, J.G.; Silva, B.P.; Oliveira, R.P.; Junior, J.W.P.; Peixoto, R.M.; Mota, R.A. Short communication: High frequency of β-lactam-resistant Staphylococcus aureus in artisanal coalho cheese made from goat milk produced in northeastern Brazil. J. Dairy Sci. 2019, 102, 6923–6927. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Meng, L.; Dong, L.; Zhao, S.; Lan, X.; Wang, J.; Zheng, N. Prevalence, antimicrobial susceptibility, and molecular characterization of Staphylococcus aureus isolated from dairy herds in northern China. J. Dairy Sci. 2017, 100, 8796–8803. [Google Scholar] [CrossRef] [Green Version]
- Alves, V.F.; Niño-Arias, F.C.; Pitondo-Silva, A.; de Araújo Frazilio, D.; de Oliveira Gonçalves, L.; Chaul Toubas, L.; Sapateiro Torres, I.M.; Oxaran, V.; Dittmann, K.K.; De Martinis, E.C.P. Molecular characterisation of Staphylococcus aureus from some artisanal Brazilian dairies. Int. Dairy J. 2018, 85, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Massawe, H.F.; Mdegela, R.H.; Kurwijila, L.R. Antibiotic resistance of Staphylococcus aureus isolates from milk produced by smallholder dairy farmers in Mbeya Region, Tanzania. Int. J. One Health 2019, 5, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Arefi, F.; Mohsenzadeh, M.; Razmyar, J. Isolation, antimicrobial susceptibility and mecA gene analysis of methicillin-resistant Staphylococcus aureus in Iranian white cheeses. Iran. J. Vet. Res. 2014, 15, 127–131. [Google Scholar] [CrossRef]
- Silva, V.; Alfarela, C.; Caniça, M.; Manageiro, V.; Nóvoa, M.; Leiva, B.; Kress, M.; Capelo, J.L.; Poeta, P.; Igrejas, G. A One Health Approach Molecular Analysis of Staphylococcus aureus Reveals Distinct Lineages in Isolates from Miranda Donkeys (Equus asinus) and Their Handlers. Antibiotics 2022, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2017 (EMA/294674/2019); European Medicines Agency: Amsterdam, The Netherlands, 2019; ISBN 9789291550685.
- Aliyu, Y.; Reuben, R.C.; Abdullahi, I.O.; Olayinka, B.O.; Abdullahi, M.S. A systematic review on the prevalence of multidrug-resistant Staphylococcus aureus from milk and milk products in Nigeria. PAMJ One Health 2022, 7, 15. [Google Scholar] [CrossRef]
- Ammar, A.M.; Attia, A.M.; Abd El-Hamid, M.I.; El-Shorbagy, I.M.; Abd El-Kader, S.A. Genetic basis of resistance waves among methicillin resistant Staphylococcus aureus isolates recovered from milk and meat products in Egypt. Cell. Mol. Biol. 2016, 62, 7–15. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Castro, R.D.; Pedroso, S.H.S.P.; Sandes, S.H.C.; Silva, G.O.; Luiz, K.C.M.; Dias, R.S.; Filho, R.A.T.; Figueiredo, H.C.P.; Santos, S.G.; Nunes, A.C.; et al. Virulence factors and antimicrobial resistance of Staphylococcus aureus isolated from the production process of Minas artisanal cheese from the region of Campo das Vertentes, Brazil. J. Dairy Sci. 2020, 103, 2098–2110. [Google Scholar] [CrossRef]
- Sugimoto, S.; Sato, F.; Miyakawa, R.; Chiba, A.; Onodera, S.; Hori, S.; Mizunoe, Y. Broad impact of extracellular DNA on biofilm formation by clinically isolated Methicillin-resistant and -sensitive strains of Staphylococcus aureus. Sci. Rep. 2018, 8, 2254. [Google Scholar] [CrossRef] [Green Version]
- Kirmusaoglu, S. Staphylococcal Biofilms: Pathogenicity, Mechanism and Regulation of Biofilm Formation by Quorum-Sensing System and Antibiotic Resistance Mechanisms of Biofilm-Embedded Microorganisms. In Microbial Biofilms—Importance and Applications; InTechOpen: London, UK, 2016; pp. 189–209. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Iandolo, J.J.; Stewart, G.C. The enterotoxin D plasmid of Staphylococcus aureus encodes a second enterotoxin determinant (sej). FEMS Microbiol. Lett. 1998, 168, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, H.J.; Mørk, T.; Rørvik, L.M. The occurrence of Staphylococcus aureus on a farm with small-scale production of raw milk cheese. J. Dairy Sci. 2005, 88, 3810–3817. [Google Scholar] [CrossRef] [Green Version]
- Zakrzewski, A.J.; Zarzecka, U.; Chajęcka-Wierzchowska, W.; Zadernowska, A. A Comparison of Methods for Identifying Enterobacterales Isolates from Fish and Prawns. Pathogens 2022, 11, 410. [Google Scholar] [CrossRef]
- ISO 20776-2:2007; Part 2: Evaluation of Performance of Antimicrobial Susceptibility Test Devices. ISO: Geneva, Switzerland, 2007.
- CLSI M100-ED29: 2021 Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI: Wayne, PA, USA, 2020; Volume 40, ISBN 9781684400324.
- Adzitey, F.; Yussif, S.; Ayamga, R.; Zuberu, S.; Addy, F.; Adu-Bonsu, G.; Huda, N.; Kobun, R. Antimicrobial Susceptibility and Molecular Characterization of Escherichia coli Recovered from Milk and Related Samples. Microorganisms 2022, 10, 1335. [Google Scholar] [CrossRef] [PubMed]
- Kouidhi, B.; Zmantar, T.; Hentati, H.; Bakhrouf, A. Cell surface hydrophobicity, biofilm formation, adhesives properties and molecular detection of adhesins genes in Staphylococcus aureus associated to dental caries. Microb. Pathog. 2010, 49, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Barski, P.; Piechowicz, L.; Galiński, J.; Kur, J. Rapid assay for detection of methicillin-resistant staphylococcus aureus using multiplex PCR. Mol. Cell. Probes 1996, 10, 471–475. [Google Scholar] [CrossRef]
- Małyszko, I.; Schwarz, S.; Hauschild, T. Detection of a new mecC allotype, mecC2, in methicillin-resistant Staphylococcus saprophyticus. J. Antimicrob. Chemother. 2014, 69, 2003–2005. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.C. Epidemiology of tetracycline-resistance determinants. Trends Microbiol. 1994, 2, 353–357. [Google Scholar] [CrossRef]
- Gevers, D.; Danielsen, M.; Huys, G.; Swings, J. Molecular characterization of tet(M) genes in Lactobacillus isolates from different types of fermented dry sausage. Appl. Environ. Microbiol. 2003, 69, 1270–1275. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.; Caniça, M.; Ferreira, E.; Vieira-Pinto, M.; Saraiva, C.; Pereira, J.E.; Capelo, J.L.; Igrejas, G.; Poeta, P. Multidrug-Resistant Methicillin-Resistant Coagulase-Negative Staphylococci in Healthy Poultry Slaughtered for Human Consumption. Antibiotics 2022, 11, 365. [Google Scholar] [CrossRef]
- Kaase, M.; Lenga, S.; Friedrich, S.; Szabados, F.; Sakinc, T.; Kleine, B.; Gatermann, S.G. Comparison of phenotypic methods for penicillinase detection in Staphylococcus aureus. Clin. Microbiol. Infect. 2008, 14, 614–616. [Google Scholar] [CrossRef] [Green Version]
- Padmasini, E.; Padmaraj, R.; Ramesh, S.S. High level aminoglycoside resistance and distribution of aminoglycoside resistant genes among clinical isolates of Enterococcus species in Chennai, India. Sci. World J. 2014, 2014, 329157. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, N.; Mahbub Alam, M.; Nishimoto, Y.; Urasawa, S.; Uehara, N.; Watanabe, N. Distribution of aminoglycoside resistance genes in recent clinical isolates of Enterococcus faecalis, Enterococcus faecium and Enterococcus avium. Epidemiol. Infect. 2001, 126, 197–204. [Google Scholar] [CrossRef]
- Arciola, C.R.; Baldassarri, L.; Montanaro, L. Presence of icaA and icaD genes and slime production in a collection of Staphylococcal strains from catheter-associated infections. J. Clin. Microbiol. 2001, 39, 2151–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heilmann, C.; Thumm, G.; Chhatwal, G.S.; Hartleib, J.; Uekötter, A.; Peters, G. Identification and characterization of a novel autolysin (Aae) with adhesive properties from Staphylococcus epidermidis. Microbiology 2003, 149, 2769–2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tristan, A.; Ying, L.; Bes, M.; Etienne, J.; Vandenesch, F.; Lina, G. Use of multiplex PCR to identify Staphylococcus aureus adhesins involved in human hematogenous infections. J. Clin. Microbiol. 2003, 41, 4465–4467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.R.; Bae, Y.M.; Lee, S.Y. Effect of Environmental Conditions on Biofilm Formation and Related Characteristics of Staphylococcus Aureus. J. Food Saf. 2016, 36, 412–422. [Google Scholar] [CrossRef]
- Chajęcka-Wierzchowska, W.; Gajewska, J.; Wiśniewski, P.; Zadernowska, A. Enterotoxigenic potential of coagulase-negative staphylococci from ready-to-eat food. Pathogens 2020, 9, 734. [Google Scholar] [CrossRef]
- Jarraud, S.; Mougel, C.; Thioulouse, J.; Lina, G.; Ne Meugnier, H.; Forey, F.; Nesme, X.; Etienne, J.; Vandenesch, F. Relationships between Staphylococcus aureus Genetic Background, Virulence Factors, agr Groups (Alleles), and Human Disease. Infect. Immun. 2002, 70, 631–641. [Google Scholar] [CrossRef] [Green Version]
- Holtfreter, S.; Grumann, D.; Schmudde, M.; Nguyen, H.T.T.; Eichler, P.; Strommenger, B.; Kopron, K.; Kolata, J.; Giedrys-Kalemba, S.; Steinmetz, I.; et al. Clonal Distribution of Superantigen Genes in Clinical Staphylococcus aureus Isolates. J. Clin. Microbiol. 2007, 45, 2669–2680. [Google Scholar] [CrossRef]
| Source | No. of Samples | No. (%) of S. aureus-Positive Samples |
|---|---|---|
| Sample | ||
| Raw milk | 18 | 10 (55.6%) |
| Heated milk | 18 | 13 (72.2%) |
| Curd | 18 | 15 (83.3%) |
| Whey | 18 | 8 (44.4%) |
| Brine | 18 | 10 (55.6%) |
| Cheese | 18 | 10 (55.6%) |
| Swab | ||
| Tank | 18 | 8 (44.4%) |
| Jar | 18 | 8 (44.4%) |
| Form | 18 | 9 (50.0%) |
| Sink | 18 | 2 (11.1%) |
| Total | 180 | 93 (51.7%) |
| Samples | Swabs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial Class | Antimicrobial Agent | Raw Milk (n = 9) | Heated Milk (n = 14) | Curd (n = 15) | Whey (n = 8) | Brine (n = 10) | Cheese (n = 10) | Tank (n = 8) | Jar (n = 8) | Form (n = 9) | Sink (n = 2) | Total (n = 93) |
| Aminoglycosides | AK | 0 | 4 (28.6%) | 6 (40.0%) | 4 (50.0%) | 0 | 3 (30.0%) | 3 (37.5%) | 1 (12.5%) | 2 (22.2%) | 0 | 23 (24.7%) |
| TOB | 4 (44.4%) | 6 (42.9%) | 5 (33.3%) | 2 (25.0%) | 2 (20.0%) | 4 (40.0%) | 3 (37.5%) | 3 (37.5%) | 2 (22.2%) | 1 (50.0%) | 32 (34.4%) | |
| K | 1 (11.1%) | 0 | 1 (6.7%) | 0 | 1 (10.0%) | 1 (10.0%) | 1 (12.5%) | 0 | 0 | 1 (50.0%) | 6 (6.5%) | |
| Cephalosporins | FOX | 1 (11.1%) | 1 (7.1%) | 3 (20.0%) | 1 (12.5%) | 0 | 2 (20.0%) | 1 (12.5%) | 1 (12.5%) | 2 (22.2%) | 0 | 12 (12.9%) |
| Macrolides, lincosamides and streptogramins | AZM | 0 | 3 (21.4%) | 3 (20.0%) | 2 (25.0%) | 0 | 3 (30.0%) | 1 (12.5%) | 3 (37.5%) | 2 (22.2%) | 0 | 17 (18.3%) |
| CLM | 2 (22.2%) | 3 (21.4%) | 2 (13.3%) | 2 (25.0%) | 0 | 3 (30.0%) | 0 | 0 | 3 (33.3%) | 0 | 15 (16.1%) | |
| E | 2 (22.2%) | 5 (35.7%) | 2 (13.3%) | 2 (25.0%) | 0 | 3 (30.0%) | 2 (25.0%) | 3 (37.5%) | 2 (22.2%) | 0 | 21 (22.6%) | |
| β-lactams | BP | 5 (55.6%) | 8 (57.1%) | 11 (73.3%) | 7 (87.5%) | 5 (50.0%) | 6 (60.0%) | 4 (50.0%) | 4 (50.0%) | 4 (44.4%) | 0 | 54 (58.1%) |
| OXA | 0(0.0%) | 1 (7.1%) | 1 (6.7%) | 1 (10.0%) | 1 (10.0%) | 3 (30.0%) | 1(12.5%) | 0 | 1 (11.1%) | 0 | 9 (9.7%) | |
| Oxazolidinones | LZD | 0 | 0 | 0 | 0 | 0 | 1 (10.0%) | 0 | 0 | 0 | 0 | 1 (1.1%) |
| Nitrofurantoins | FT | 1 (11.1%) | 0 | 0 | 0 | 0 | 1 (10.0%) | 0 | 0 | 0 | 0 | 2 (2.2%) |
| Tetracyclines | TE | 2 (22.2%) | 5 (35.7%) | 2 (13.3%) | 0 | 4 (40.0%) | 2 (20.0%) | 2 (25.0%) | 2(25.0%) | 0 | 0 | 19 (20.4%) |
| TGC | 2 (20.0%) | 3 (21.4%) | 4 (26.7%) | 2 (25.0%) | 1 (10.0%) | 3 (30.0%) | 2 (25.0%) | 3 (37.5%) | 2 (22.2%) | 0 | 22 (23.7%) | |
| Sulfonamides | TMP | 3 (33.3%) | 2 (14.3%) | 1 (6.7%) | 2 (25.0%) | 0 | 2 (20.0%) | 1 (12.5%) | 2 (25.0%) | 2 (22.2%) | 0 | 15 (16.1%) |
| SXT | 1 (11.1%) | 3 (21.4%) | 3 (20.0%) | 0 | 1 (10.0%) | 2 (20.0%) | 1 (12.5%) | 0 | 0 | 0 | 11 (11.8%) | |
| Glycopeptides | VA | 0 | 0 | 1 (6.7%) | 0 | 0 | 1 (10.0%) | 0 | 0 | 0 | 0 | 2 (2.2%) |
| Streptogramins | QD | 0 | 0 | 0 | 0 | 1 (10.0%) | 0 | 0 | 0 | 1 (11.1%) | 0 | 2 (2.2%) |
| Steroidal | FD | 1 (11.1%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (50.0%) | 2 (2.2%) |
| Samples | Swabs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial Class | Antibiotic Resistance Gene | Raw Milk (n = 9) | Heated Milk (n = 14) | Curd (n = 15) | Whey (n = 8) | Brine (n = 10) | Cheese (n = 10) | tank (n = 8) | Jar (n = 8) | Form (n = 9) | Sink (n = 2) | Total (n = 93) |
| Oxacillin | mecA | 1 (11.1%) | 1 (7.1%) | 3 (20.0%) | 1 (12.5%) | 0 | 2 (20.0%) | 1 (12.5%) | 1 (12.5%) | 2 (22.2%) | 0 | 12 (12.9%) |
| Penicillin | blaZ | 9 (100%) | 13 (92.9%) | 15 (100.0%) | 8 (100.0%) | 8 (80.0%) | 10 (100.0%) | 8 (100.0%) | 8 (100.0%) | 9 (100.0%) | 1 (50.0%) | 89 (95.7%) |
| Tetracyclines | tetK | 5 (55.6%) | 9 (64.3%) | 10 (66.7%) | 4 (50.0%) | 5 (50.0%) | 7 (70.0%) | 5 (62.5%) | 6 (75.0%) | 6 (66.7%) | 2 (100%) | 59 (63.4%) |
| tetM | 6 (66.7%) | 12 (85.7%) | 14 (93.3%) | 6 (75.0%) | 5 (50.0%) | 9 (90.0%) | 7 (87.5%) | 7 (87.5%) | 9 (100.0%) | 1 (50.0%) | 76 (81.7%) | |
| tetL | 1 (11.1%) | 1 (7.1%) | 3 (20.0%) | 2 (25.0%) | 1 (10.0%) | 2 (20.0%) | 1 (12.5%) | 2 (25.0%) | 2 (22.2%) | 1 (50.0%) | 16 (17.2%) | |
| Macrolides, lincosamides, and streptogramins | ermB | 2 (22.2%) | 5 (35.7%) | 5 (33.3%) | 3 (37.5%) | 1 (10.0%) | 2 (20.0%) | 3 (37.5%) | 2 (25.0%) | 3 (33.3%) | 1 (50.0%) | 27 (29.0%) |
| Aminoglycosides | aac(6′)-Ie-aph(2″)-Ia | 3 (33.3%) | 9 (64.3%) | 8 (53.3%) | 3 (37.5%) | 8 (80.0%) | 6 (60.0%) | 8 (100.0%) | 4 (50%) | 7 (77.8%) | 1 (50.0%) | 56 (60.2%) |
| aph(3′)IIIa | 9 (100.0%) | 12 (85.7%) | 12 (80.0%) | 8 (100.0%) | 10 (100.0%) | 9 (90.0%) | 8 (100.0%) | 7 (87.5%) | 9 (100.0%) | 2 (100%) | 86 (92.5%) | |
| Samples | Swabs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw Milk (n = 9) | Heated Milk (n = 14) | Curd (n = 15) | Whey (n = 8) | Brine (n = 10) | Cheese (n = 10) | Tank (n = 8) | Jar (n = 8) | Form (n = 9) | Sink (n = 2) | Total (n = 93) | ||
| Biofilm MTP method | strong | 5 (55.6%) | 9 (64.3%) | 12 (80.0%) | 4 (50.0%) | 7 (70.0%) | 9 (90.0%) | 3 (37.5%) | 3 (37.5%) | 4 (44.4%) | 2 (100%) | 58 (62.4%) |
| intermediate | 1 (11.1%) | 1 (7.1%) | - | - | - | - | - | - | - | - | 2 (2.2%) | |
| weak | 3 (33.3%) | 4 (28.6%) | 3 (20.0%) | 4 (50.0%) | 3 (30.0%) | 1 (10.0%) | 5 (62.5%) | 5 (62.5%) | 5 (55.6%) | 33 (35.4%) | ||
| Slime production | 4 (44.4%) | 7 (50.0%) | 9 (60.0%) | 4 (50.0%) | 4 (40.0%) | 7 (70.0%) | 3 (37.5%) | 5 (62.5%) | 3 (33.3%) | 2 (100%) | 48 (51.6%) | |
| Biofilm-associated genes | icaADBC | 2 (22.2%) | 4 (28.6%) | 4 (26.7%) | 3 (37.5%) | 1 (10.0%) | 4 (40.0%) | 2 (25.0%) | 2 (25.0%) | 2 (22.2%) | 1 (50.0%) | 25 (26.9%) |
| bap | 3 (33.3%) | 7 (50.0%) | 8 (53.3%) | 2 (25.0%) | 1 (10.0%) | 5 (50.0%) | 2 (25.0%) | 1 (12.5%) | 3 (33.3%) | 0 | 32 (34.4%) | |
| eno | 9 (100%) | 14 (100.0%) | 15 (100.0%) | 8 (100.0%) | 10 (100%) | 10 (100.0%) | 8 (100.0%) | 8 (100.0%) | 9 (100.0%) | 2 (100%) | 93 (100.0%) | |
| sigB | 5 (55.6%) | 6 (42.9%) | 8 (53.3%) | 4 (50.0%) | 2 (20.0%) | 6 (60.0%) | 3 (37.5%) | 5 (62.5%) | 7 (77.8%) | 1 (50.0%) | 42 (45.2%) | |
| sarA | 7 (77.8%) | 13 (92.9%) | 12 (80.0%) | 6 (75.0%) | 8 (80.0%) | 8 (80.0%) | 6 (75.0%) | 5 (62.5%) | 7 (77.8%) | 2 (100%) | 74 (79.6%) | |
| agrD | 2 (22.2%) | 6 (42.9%) | 5 (33.3%) | 4 (50.0%) | 5 (50.0%) | 3 (30.0%) | 2 (25.0%) | 3 (37.5%) | 3 (33.3%) | - | 33 (35.5%) | |
| Enterotoxigenic genes | sed | - | 1 (7.1%) | - | - | 1 (10.0%) | 1 (10.0%) | - | - | - | - | 3 (3.2%) |
| seg | 4 (44.4%) | 9 (64.3%) | 8 (53.3%) | 7 (87.5%) | 5 (50.0%) | 6 (60.0%) | 5 (62.5%) | 4 (50.0%) | 5 (55.6%) | 1 (50.0%) | 54 (58.1%) | |
| sei | 2 (22.2%) | 4 (28.6%) | 1 (6.7%) | 1 (12.5%) | - | - | 2 (25.0%) | 2 (25.0%) | 1 (11.1%) | - | 13 (14.0%) | |
| selj | 1 (11.1%) | 3 (37.5%) | 4 (26.7%) | 3 (37.5%) | 2 (20.0%) | 4 (40.0%) | 2 (25.0%) | 2 (25.0%) | 3 (33.3%) | - | 26 (28.0%) | |
| selk | 7 (77.8%) | 10 (71.4%) | 10 (66.7%) | 4 (50.0%) | 4 (40.0%) | 7 (70.0%) | 3 (37.5%) | 5 (62.5%) | 5 (55.6%) | 2 (100.0%) | 57 (61.3%) | |
| selm | - | 2 (14.3%) | 3 (20.0%) | 2 (25.0%) | 3 (30.0%) | 3 (30.0%) | 3 (37.5%) | - | 1 (11.1%) | 1 (50.0%) | 18 (19.4%) | |
| seln | 2 (22.2%) | 7 (50.0%) | 4 (26.7%) | 6 (75.0%) | 4 (40.0%) | 6 (60.0%) | 4 (40.0%) | 3 (37.5%) | 5 (55.6%) | 1 (50.0%) | 42 (45.2%) | |
| selo | - | 1 (7.1%) | 3 (20.0%) | 1 (12.5%) | 2 (20.0%) | 3 (30.0%) | 3 (37.5%) | - | - | 1 (50.0%) | 14 (15.1%) | |
| selp | 2 (22.2%) | - | 4 (26.7%) | 2 (25.0%) | 3 (30.0%) | 1 (10.0%) | - | 3 (37.5%) | 1 (11.1%) | - | 16 (17.2%) | |
| ser | 1 (11.1%) | 4 (28.6%) | 3 (20.0%) | 1 (12.5%) | - | - | 2 (25.0%) | 1 (12.5%) | 1 (11.1%) | 1 (50.0%) | 14 (15.1%) | |
| selq | 3 (33.3%) | 8 (57.1%) | 8 (53.3%) | 6 (75.0%) | 2 (20.0%) | 6 (60.0%) | 3 (37.5%) | 3 (37.5%) | 3 (33.3%) | 1 (50.0%) | 43 (46.2%) | |
| selu | 5 (35.7%) | 5 (33.3%) | 3 (37.5%) | 2 (20.0%) | 4 (40.0%) | 3 (37.5%) | 2 (25.0%) | 4 (44.4%) | - | 29 (31.2%) | ||
| eta | - | 1 (7.1%) | - | - | - | - | - | 1 (12.5%) | 1 (11.1%) | - | 3 (3.2%) | |
| etd | - | - | - | - | 1 (10.0%) | 1 (10.0%) | - | - | - | - | 2 (2.2%) | |
| tst-1 | - | - | - | 1 (10.0%) | 1 (12.5%) | 2 (22.2%) | - | 4 (4.3%) | ||||
| Antimicrobial Class | Antimicrobials Agent | Dilution Range (mg/L) | Cut-Off Values (mg/L); Resistant > R |
|---|---|---|---|
| Aminoglycosides | Amikacin (AK) | 0.25–128 | 16 |
| Tobramycin (TOB) | 0.063–16 | 2 | |
| Gentamycin (CN) | 1–4 | 2 | |
| Kanamycin (K) | 0.25–128 | 16 | |
| Cephalosporins | Cefoxitin (FOX) | 1–32 | 4 |
| Macrolides | Azithromycin (AZM) | 0.25–16 | 2 |
| Clarithromycin (CLM) | 0.063–16 | 4 | |
| Erythromycin (E) | 0.125–16 | 1 | |
| β-lactams | Benzylpenicillin (BP) | 0.063–8 | 0.125 |
| Oxacillin (OXA) | 0.063–16 | 2 | |
| Oxazolidinones | Linezolid (LZD) | 0.5–16 | 4 |
| Nitrofurantoins | Nitrofurantoin (FT) | 2–128 | 64 |
| Tetracyclines | Tetracycline (TE) | 0.063–32 | 2 |
| Tigecycline (TGC) | 0.016–8 | 0.5 | |
| Sulfonamides | Trimethoprim (TMP) | 0.125–32 | 2 |
| Trimethoprim/sulfamethoxazole (SXT) | 0.125:2.375–32:608 | 2 | |
| Glycopeptides | Vancomycin (VA) | 0.25–64 | 2 |
| Streptogramins | Quinupristin/dalfopristin (QD) | 0.125–8 | 2 |
| Lincosamides | Clindamycin (DA) | 0.031–32 | 2 |
| Rifamycins | Rifampicin (RD) | 0.002–8 | 0.06 |
| Steroidal | Fusidic acid (FD) | 0.5–4 | 1 |
| Fluoroquinolones | Moxifloxacin (MXF) | 0.004–8 | 0.25 |
| Phenicols | Chloramphenicol (C) | 2–32 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajewska, J.; Chajęcka-Wierzchowska, W.; Zadernowska, A. Occurrence and Characteristics of Staphylococcus aureus Strains along the Production Chain of Raw Milk Cheeses in Poland. Molecules 2022, 27, 6569. https://doi.org/10.3390/molecules27196569
Gajewska J, Chajęcka-Wierzchowska W, Zadernowska A. Occurrence and Characteristics of Staphylococcus aureus Strains along the Production Chain of Raw Milk Cheeses in Poland. Molecules. 2022; 27(19):6569. https://doi.org/10.3390/molecules27196569
Chicago/Turabian StyleGajewska, Joanna, Wioleta Chajęcka-Wierzchowska, and Anna Zadernowska. 2022. "Occurrence and Characteristics of Staphylococcus aureus Strains along the Production Chain of Raw Milk Cheeses in Poland" Molecules 27, no. 19: 6569. https://doi.org/10.3390/molecules27196569







