Easy and Fast Production of Solketal from Glycerol Acetalization via Heteropolyacids
Abstract
:1. Introduction
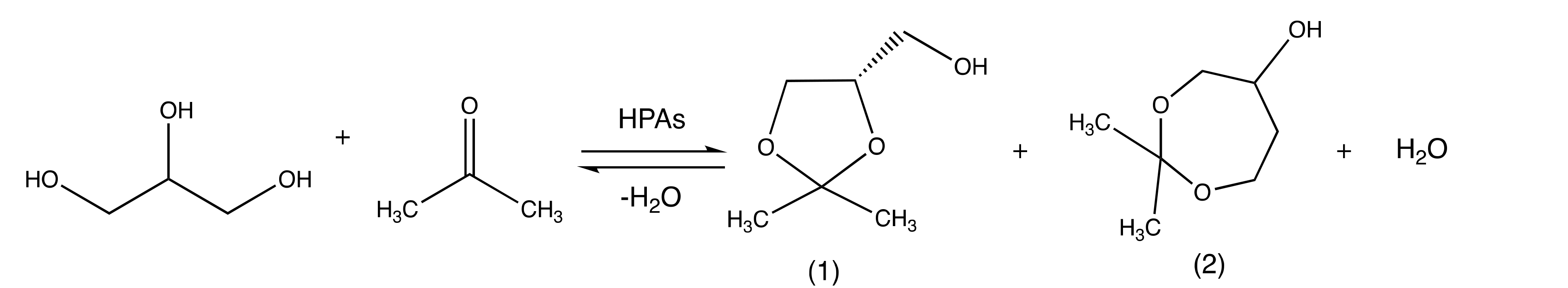
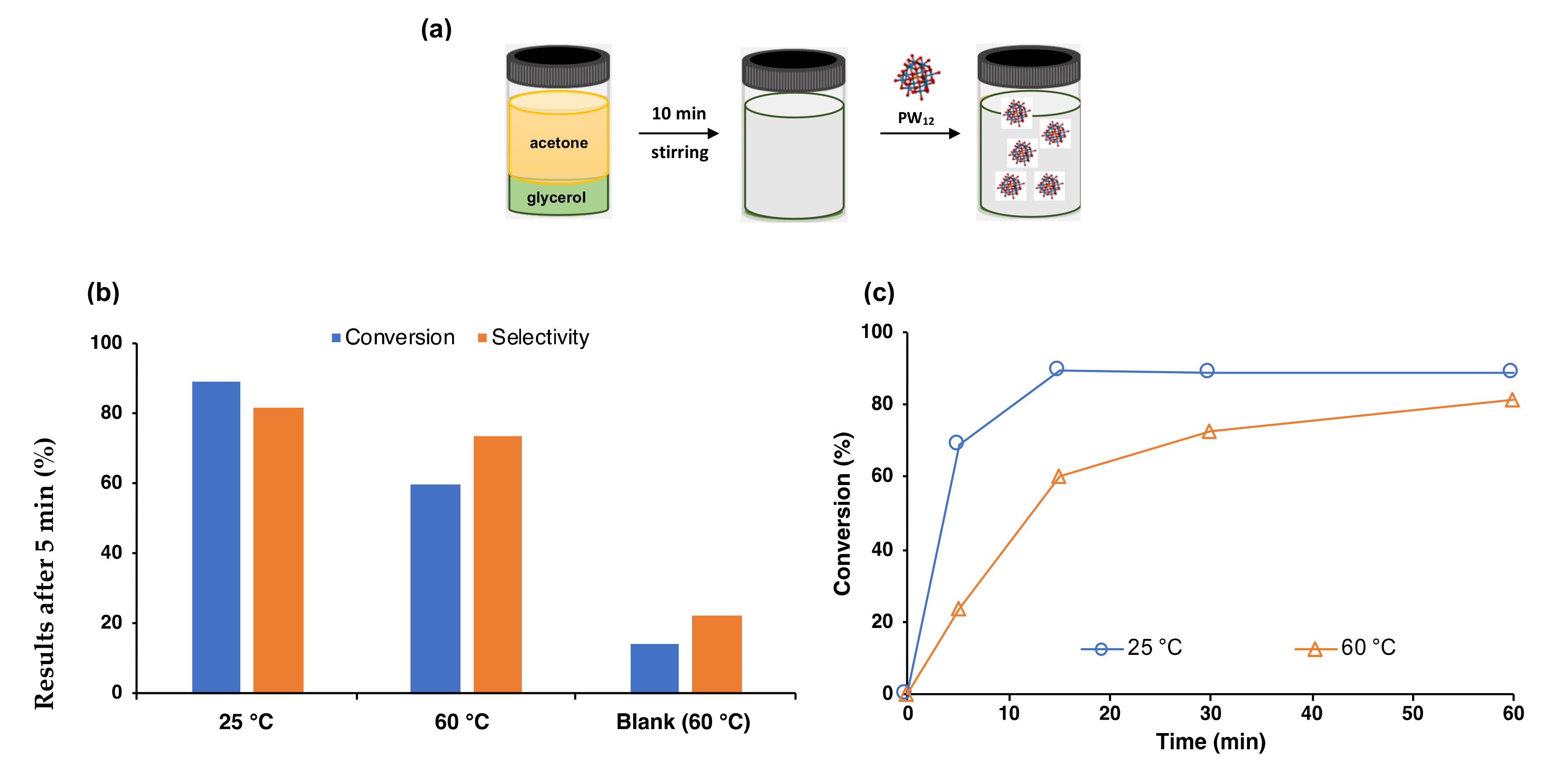
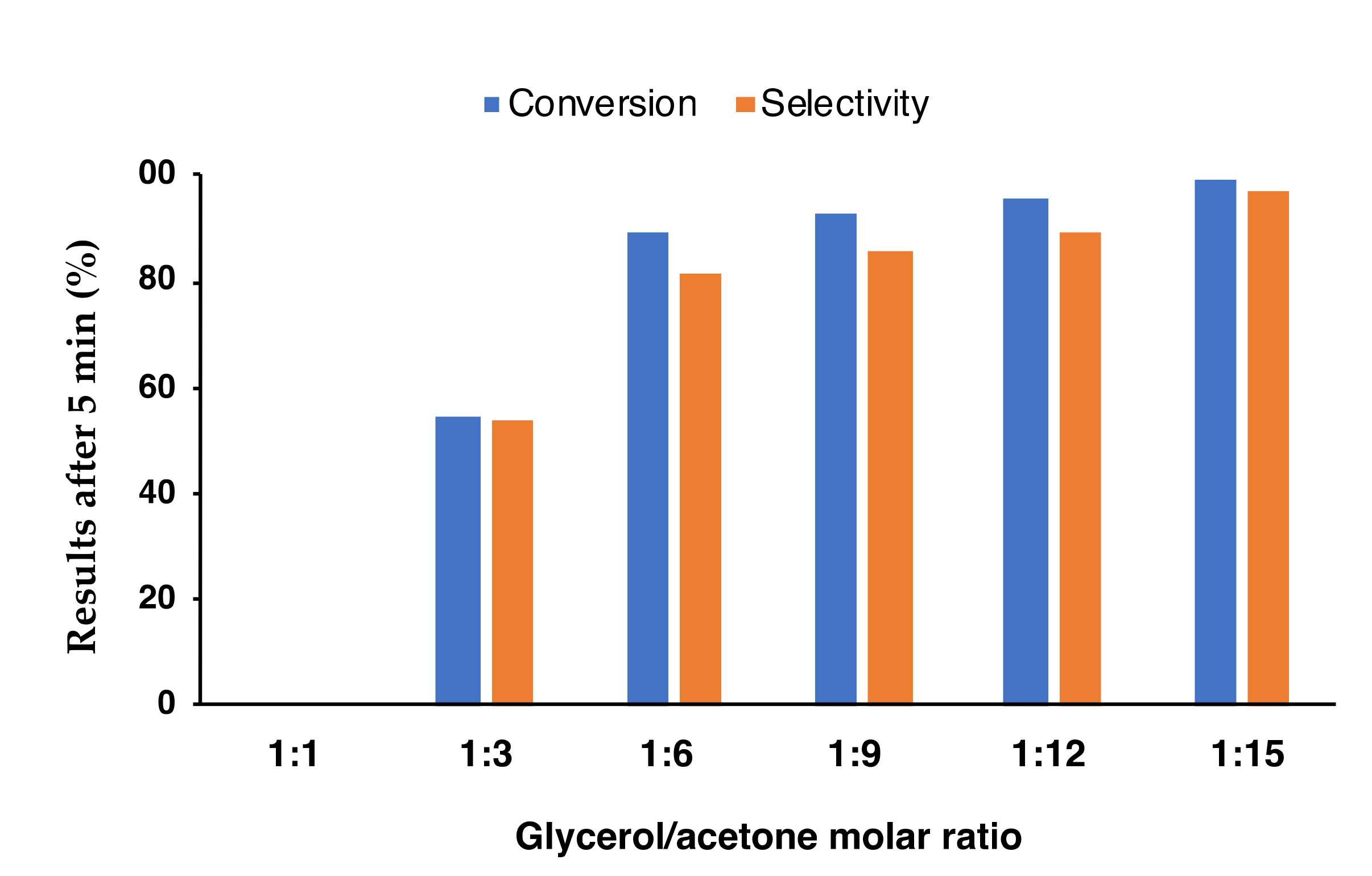
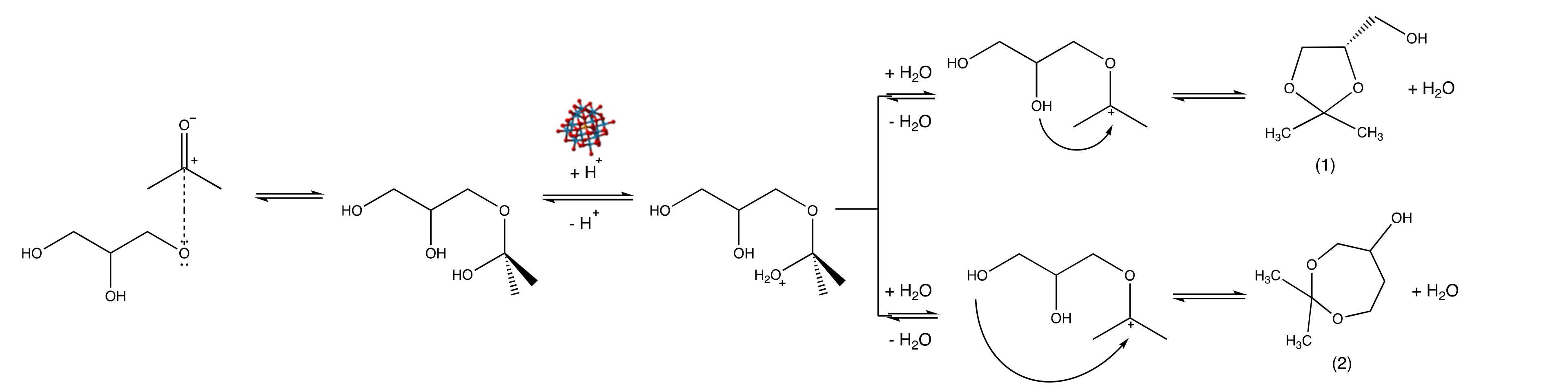
2. Results and Discussion
| Catalyst | Ratio of Glycerol/Acetone | Temp. (°C) | Time (h) | Conversion (%) | Selectivity to Solketal (%) | Ref. |
|---|---|---|---|---|---|---|
| Cs2.5H0.5PW12O40 | 1:6 | RT | 1 | 94 | >90 | [2] |
| Cs2.5H0.5PW12O40@KIT-6 | 1:6 | RT | 0.25 | 95 | >90 | [2] |
| H3PW12O40 | 1:10 | RT | 2 | 58 | 97 | [16] |
| H3PW12O40 | 1:10 | 60 | 1 | 47.8 | 97.5 | [22] |
| H3PW12O40@SiO2 | 1:6 | 70 | 4 | 97 | 97 | [21] |
| ⎨H20⎬-355 | 1:2 | RT | 0.67 | 98 | >98 | [23] |
| H3PW12O40 | 1:15 | RT | 0.08 | 99.2 | 97 | This work |
| H3PMo12O40 | 1:15 | RT | 0.08 | 91.4 | 94 | This work |
| H4SiW12O40 | 1:15 | RT | 0.08 | 90.7 | 85.7 | This work |
3. Materials and Methods
3.1. Materials
3.2. Catalytic Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Grubler, A. Trends in Global Emissions: Carbon, Sulfur, and Nitrogen; IIASA: Laxenburg, Austria, 2002; pp. 35–53. [Google Scholar]
- Chen, L.; Nohair, B.; Zhao, D.; Kaliaguine, S. Highly Efficient Glycerol Acetalization over Supported Heteropoly Acid Catalysts. ChemCatChem 2018, 10, 1918–1925. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Najaafi, N.; Tarighi, S. A Review on the Catalytic Acetalization of Bio-renewable Glycerol to Fuel Additives. Front. Chem. 2018, 6, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Vivek, N.; Sindhu, R.; Madhavan, A.; Anju, A.J.; Castro, E.; Faraco, V.; Pandey, A.; Binod, P. Recent advances in the production of value added chemicals and lipids utilizing biodiesel industry generated crude glycerol as a substrate–Metabolic aspects, challenges and possibilities: An overview. Bioresour. Technol. 2017, 239, 507–517. [Google Scholar] [CrossRef]
- Chen, J.; Yan, S.; Zhang, X.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Chemical and biological conversion of crude glycerol derived from waste cooking oil to biodiesel. Waste Manag. 2018, 71, 164–175. [Google Scholar] [CrossRef] [Green Version]
- El Roz, A.; Fongarland, P.; Dumeignil, F.; Capron, M. Glycerol to Glyceraldehyde Oxidation Reaction Over Pt-Based Catalysts Under Base-Free Conditions. Front. Chem. 2019, 7, 156. [Google Scholar] [CrossRef] [Green Version]
- Reinoso, D.M.; Boldrini, D.E. Kinetic study of fuel bio-additive synthesis from glycerol esterification with acetic acid over acid polymeric resin as catalyst. Fuel 2020, 264, 116879. [Google Scholar] [CrossRef]
- Ghosh, A.; Singha, A.; Auroux, A.; Das, A.; Sen, D.; Chowdhury, B. A green approach for the preparation of a surfactant embedded sulfonated carbon catalyst towards glycerol acetalization reactions. Catal. Sci. Technol. 2020, 10, 4827–4844. [Google Scholar] [CrossRef]
- Huang, X.; Guo, Y.; Zou, Y.; Jiang, J. Electrochemical oxidation of glycerol to hydroxypyruvic acid on cobalt (oxy)hydroxide by high-valent cobalt redox centers. 2022, 309, 121247. Appl. Catal. B Environ. 2022, 309, 121247. [Google Scholar] [CrossRef]
- Ma, T.; Ding, J.; Shao, R.; Xu, W.; Yun, Z. Dehydration of glycerol to acrolein over Wells–Dawson and Keggin type phosphotungstic acids supported on MCM-41 catalysts. Chem. Eng. J. 2017, 316, 797–806. [Google Scholar] [CrossRef]
- Corrêa, I.; Faria, R.P.V.; Rodrigues, A.E. Continuous Valorization of Glycerol into Solketal: Recent Advances on Catalysts, Processes, and Industrial Perspectives. Sustainable Chemistry 2021, 2, 286–324. [Google Scholar] [CrossRef]
- da Silva, M.J.; Teixeira, M.G.; Chaves, D.M.; Siqueira, L. An efficient process to synthesize solketal from glycerol over tin (II) silicotungstate catalyst. Fuel 2020, 281, 118724. [Google Scholar] [CrossRef]
- Domínguez-Barroso, V.; Herrera, C.; Larrubia, M.Á.; González-Gil, R.; Cortés-Reyes, M.; Alemany, L.J. Continuous-Flow Process for Glycerol Conversion to Solketal Using a Brönsted Acid Functionalized Carbon-Based Catalyst. Catalysts 2019, 9, 609. [Google Scholar] [CrossRef] [Green Version]
- Miao, Z.; Li, Z.; Liang, M.; Meng, J.; Zhao, Y.; Xu, L.; Mu, J.; Zhou, J.; Zhuo, S.; Si, W. Ordered mesoporous titanium phosphate material: A highly efficient, robust and reusable solid acid catalyst for acetalization of glycerol. Chem. Eng. J. 2020, 381, 122594. [Google Scholar] [CrossRef]
- da Silva, M.J.; Julio, A.A.; Dorigetto, F.C.S. Solvent-free heteropolyacid-catalyzed glycerol ketalization at room temperature. RSC Adv. 2015, 5, 44499–44506. [Google Scholar] [CrossRef]
- Chandrasekhar, S. Product stability in kinetically-controlled organic reactions. Chem. Soc. Rev. 1987, 16, 313–338. [Google Scholar] [CrossRef] [Green Version]
- Talebian-Kiakalaieh, A.; Tarighi, S. Hierarchical faujasite zeolite-supported heteropoly acid catalyst for acetalization of crude-glycerol to fuel additives. J. Ind. Eng. Chem. 2019, 79, 452–464. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Selishcheva, S.A.; Yakovlev, V.A. Acetalization Catalysts for Synthesis of Valuable Oxygenated Fuel Additives from Glycerol. Catalysts 2018, 8, 595. [Google Scholar] [CrossRef] [Green Version]
- Bakuru, V.R.; Churipard, S.R.; Maradur, S.P.; Kalidindi, S.B. Exploring the Brønsted acidity of UiO-66 (Zr, Ce, Hf) metal–organic frameworks for efficient solketal synthesis from glycerol acetalization. Dalton Trans. 2019, 48, 843–847. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Valorisation of glycerol by condensation with acetone over silica-included heteropolyacids. Appl. Catal. B Environ. 2010, 98, 94–99. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, R.; Ye, B.; Hou, Z. Acetalization of glycerol over sulfated UiO-66 under mild condition. J. Ind. Eng. Chem. 2022, 110, 357–366. [Google Scholar] [CrossRef]
- Peng, Q.; Zhao, X.; Li, D.; Chen, M.; Wei, X.; Fang, J.; Cui, K.; Ma, Y.; Hou, Z. Synthesis of bio-additive fuels from glycerol acetalization over a heterogeneous Ta/W mixed addenda heteropolyacid catalyst. Fuel Process. Technol. 2021, 214, 106705. [Google Scholar] [CrossRef]
- Mallesham, B.; Sudarsanam, P.; Reddy, B.M. Eco-friendly synthesis of bio-additive fuels from renewable glycerol using nanocrystalline SnO2-based solid acids. Catal. Sci. Technol. 2014, 4, 803–813. [Google Scholar] [CrossRef]
- Fan, C.-N.; Xu, C.-H.; Liu, C.-Q.; Huang, Z.-Y.; Liu, J.-Y.; Ye, Z.-X. Catalytic acetalization of biomass glycerol with acetone over TiO2–SiO2 mixed oxides. React. Kinet. Mech. Catal. 2012, 107, 189–202. [Google Scholar] [CrossRef]
- da Silva, C.X.A.; Gonçalves, V.L.C.; Mota, C.J.A. Water-tolerant zeolite catalyst for the acetalisation of glycerol. Green Chem. 2009, 11, 38–41. [Google Scholar] [CrossRef]
- Santos-Vieira, I.C.; Mendes, R.F.; Almeida Paz, F.A.; Rocha, J.; Simões, M.M. Solketal Production via Solvent-Free Acetalization of Glycerol over Triphosphonic-Lanthanide Coordination Polymers. Catalysts 2021, 11, 598. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Phung, T.K.; Hossain, M.A.; Chowdhury, E.; Tulaphol, S.; Lalvani, S.B.; O’Toole, M.; Willing, G.A.; Jasinski, J.B.; Crocker, M.; et al. Hydrophobic functionalization of HY zeolites for efficient conversion of glycerol to solketal. Appl. Catal. A Gen. 2020, 592, 117369. [Google Scholar] [CrossRef]
- Venkatesha, N.J.; Bhat, Y.S.; Jai Prakash, B.S. Dealuminated BEA zeolite for selective synthesis of five-membered cyclic acetal from glycerol under ambient conditions. RSC Adv. 2016, 6, 18824–18833. [Google Scholar] [CrossRef]
- Nair, G.S.; Adrijanto, E.; Alsalme, A.; Kozhevnikov, I.V.; Cooke, D.J.; Brown, D.R.; Shiju, N.R. Glycerol utilization: Solvent-free acetalisation over niobia catalysts. Catal. Sci. Technol. 2012, 2, 1173–1179. [Google Scholar] [CrossRef]
- Gadamsetti, S.; Rajan, N.P.; Rao, G.S.; Chary, K.V. Acetalization of glycerol with acetone to bio fuel additives over supported molybdenum phosphate catalysts. J. Mol. Catal. A Chem. 2015, 410, 49–57. [Google Scholar] [CrossRef]


| Catalyst | Ratio of Glycerol/Acetone | Catalyst/Glycerol (%, w/w) | Temp. (°C) | Time (h) | Conversion (%) | Selectivity to Solketal (%) | Ref. |
|---|---|---|---|---|---|---|---|
| UAV-63 | 1:10 | 5 | 55 | 6 | 84 | 96 | [27] |
| UAV-20 | 1:10 | 5 | 55 | 6 | 56 | 82 | [27] |
| UiO-66(Zr) | 1:10 | wi | 60 | 1 | 0.3 | 60 | [22] |
| UiO-66(Zr) | 1:4 | 10 | Rt | 1 | 2 | 73 | [20] |
| UiO-66(Ce) | 1:4 | 10 | Rt | 1 | 70 | 90 | [20] |
| UiO-66(Hf) | 1:4 | 10 | Rt | 1 | 95 | 97 | [20] |
| UiO-SO3H-0.2 | 1:10 | wi | 60 | 1 | 48,7 | 98.5 | [22] |
| HY | 1:12 | 5 | 30 | 10 | 89 | 98 | [28] |
| HY | 1:12 | 5 | 50 | 10 | 88 | 98 | [28] |
| OTS-HY | 1:12 | 5 | 30 | 8 | 89 | 98 | [28] |
| HZSM-5 | 1:10 | wi | 60 | 1 | 19.9 | 77.7 | [22] |
| ZrHP | 1:10 | wi | 60 | 1 | 23.9 | 77.3 | [22] |
| Zeolite Beta | 1:1.2 | 19 | 70 | 0.67 | 90 | wi | [26] |
| Zeolite ZSM-5 | 1:1.2 | wi | 70 | 0.6 | 20 | 99 | [26] |
| Dealuminated BEA zeolite | 1:1 | 5 | 30 | 0.5 | 80 | wi | [29] |
| Amberlyst-15 | 1:1.2 | wi | 70 | 0.25 | 95 | 99 | [26] |
| Amberlyst-45 | 1:10 | wi | 60 | 1 | 42.3 | 92.5 | [22] |
| PTSA | 1:1.2 | wi | 70 | 0.83 | 80 | 99 | [26] |
| SO42−/ZrO2 | 1:6 | 5 | RT | 1.5 | 98 | 97 | [24] |
| Nb2O5 | 1:1.5 | 5 | 70 | 6 | 80 | 98 | [30] |
| MoPO@SBA-15 SiO2 | 1:3 | 5 | RT | 2 | 69 | 98 | [31] |
| TiO2@SiO2 | 1:4 | wi | 90 | 3 | >95 | 90 | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julião, D.; Mirante, F.; Balula, S.S. Easy and Fast Production of Solketal from Glycerol Acetalization via Heteropolyacids. Molecules 2022, 27, 6573. https://doi.org/10.3390/molecules27196573
Julião D, Mirante F, Balula SS. Easy and Fast Production of Solketal from Glycerol Acetalization via Heteropolyacids. Molecules. 2022; 27(19):6573. https://doi.org/10.3390/molecules27196573
Chicago/Turabian StyleJulião, Diana, Fatima Mirante, and Salete S. Balula. 2022. "Easy and Fast Production of Solketal from Glycerol Acetalization via Heteropolyacids" Molecules 27, no. 19: 6573. https://doi.org/10.3390/molecules27196573
APA StyleJulião, D., Mirante, F., & Balula, S. S. (2022). Easy and Fast Production of Solketal from Glycerol Acetalization via Heteropolyacids. Molecules, 27(19), 6573. https://doi.org/10.3390/molecules27196573







