Chinese Cordyceps: Bioactive Components, Antitumor Effects and Underlying Mechanism—A Review
Abstract
:1. Bioactive Components
1.1. Adenosine and Cordycepin
1.2. Polysaccharides
2. Antitumor Activities and Their Participation in Molecular Mechanisms
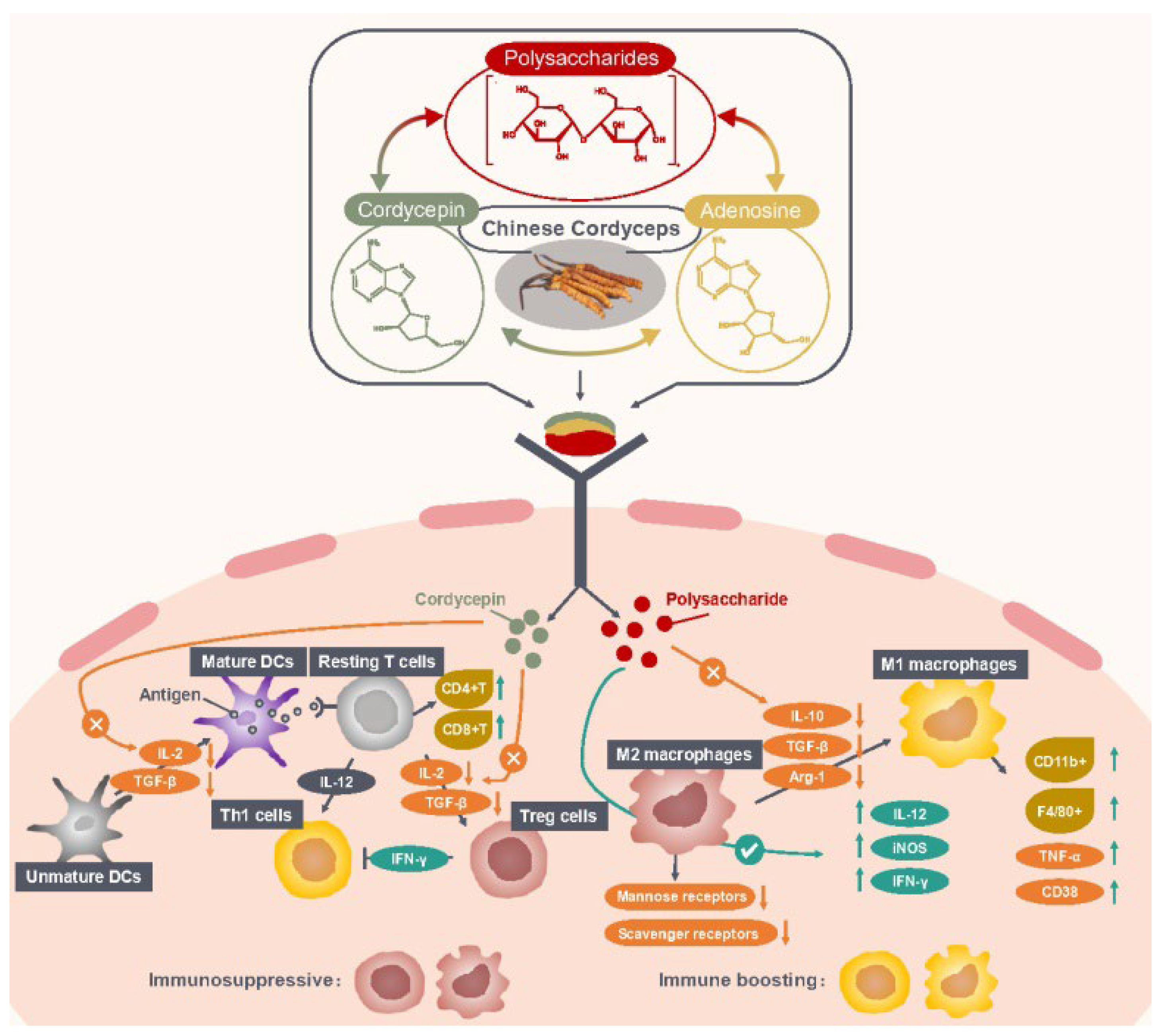
2.1. Enhancing Antitumor Immune Responses
2.2. Direct Antitumor Effects
2.2.1. Inducing Apoptosis and Autophagy
2.2.2. Blocking Cell Cycle
2.2.3. Inhibiting Migration, Invasion and Metastasis
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bhetwal, S.; Chattarjee, S.; Rijal, R.; Rana, M.; Srivastava, S. Cordyceps sinensis: Peculiar caterpillar mushroom, salutary in its medicinal and restorative capabilities. J. Pharm. Innov. 2021, 10, 1045–1054. [Google Scholar]
- Xiao, G.; Miyazato, A.; Abe, Y.; Zhang, T.; Nakamura, K.; Inden, K.; Tanaka, M.; Tanno, D.; Miyasaka, T.; Ishii, K.; et al. Activation of myeloid dendritic cells by deoxynucleic acids from Cordyceps sinensis via a Toll-like receptor 9-dependent pathway. Cell. Immunol. 2010, 263, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.W.; Gong, Z.H.; Su, Y.; Lin, J.; Tang, K.X. Cordyceps fungi: Natural products, pharmacological functions and developmental products. J. Pharm. Pharmacol. 2009, 61, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Ye, M.; Zhou, Z.; Sun, W.; Lin, X. The genus Cordyceps: A chemical and pharmacological review. Pharm. Pharmacol. 2013, 65, 474–493. [Google Scholar] [CrossRef]
- Haskó, G.; Cronstein, B.N. Adenosine: An endogenous regulator of innate immunity. Trends Immunol. 2004, 25, 33–39. [Google Scholar] [CrossRef]
- Ma, Y.F.; Zhang, J.; Zhang, Q.; Chen, P.; Song, J.; Yu, S.; Liu, H.; Liu, F.; Song, C.; Yang, D.; et al. Adenosine induces apoptosis in human liver cancer cells through ROS production and mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 2014, 448, 8–14. [Google Scholar] [CrossRef]
- Yang, D.; Yaguchi, T.; Yamamoto, H.; Nishizaki, T. Intracellularly transported adenosine induces apoptosis in HuH-7 human hepatoma cells by downregulating c-FLIP expression causing caspase-3/-8 activation. Biochem. Pharmacol. 2007, 73, 1665–1675. [Google Scholar] [CrossRef]
- Yang, D.; Yaguchi, T.; Lim, C.R.; Ishizawa, Y.; Nakano, T.; Nishizaki, T. Tuning of apoptosis-mediator gene transcription in HepG2 human hepatoma cells through an adenosine signal. Cancer Lett. 2010, 291, 225–229. [Google Scholar] [CrossRef]
- Kitakaze, M.; Hori, M. Adenosine therapy: A new approach to chronic heart failure. Expert Opin. Investig. Drugs 2000, 9, 2519–2535. [Google Scholar] [CrossRef]
- Cronstein, B.N.; Sitkovsky, M. Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Csoka, B.; Selmeczy, Z.; Koscso, B.; Nemeth, Z.H.; Pacher, P.; Murray, P.J.; Kepka-Lenhart, D.; Morris, S.M., Jr.; Gause, W.C.; Leibovich, J.S.; et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012, 26, 376–386. [Google Scholar] [CrossRef]
- Da Rocha Lapa, F.; da Silva, M.D.; de Almeida Cabrini, D.; Santos, A.R.S. Anti-inflammatory effects of purine nucleosides, adenosine and inosine, in a mouse model of pleurisy: Evidence for the role of adenosine A2 receptors. Purinergic Signal. 2012, 8, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Safarzadeh, E.; Jadidi-Niaragh, F.; Motallebnezhad, M.; Yousefi, M. The role of adenosine and adenosine receptors in the immunopathogenesis of multiple sclerosis. Inflamm. Res. 2016, 65, 511–520. [Google Scholar] [CrossRef]
- Subramanian, M.; Kini, R.; Madasu, M.; Ohta, A.; Nowak, M.; Exley, M.; Sitkovsky, M.; Ohta, A. Extracellular adenosine controls NKT-cell-dependent hepatitis induction. Eur. J. Immunol. 2014, 44, 1119–1129. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Zhao, J.; Zhu, Q.; Li, S.P. Macrophage biospecific extraction and high performance liquid chromatography for hypothesis of immunological active components. J. Pharm. Biomed. 2007, 44, 439–443. [Google Scholar] [CrossRef]
- Welihinda, A.A.; Kaur, M.; Greene, K.; Zhai, Y.; Amento, E.P. The adenosine metabolite inosine is a functional agonist of the adenosine A2A receptor with a unique signaling bias. Cell. Signal. 2016, 28, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Vinadé, E.R.; Schmidt, A.P.; Frizzo, M.E.S.; Izquierdo, I.; Elisabetsky, E.; Souza, D.O. Chronically administered guanosine is anticonvulsant, amnesic and anxiolytic in mice. Brain Res. 2003, 977, 97–102. [Google Scholar] [CrossRef]
- Chen, L.S.; Stellrecht, C.M.; Gandhi, V. RNA-directed agent, cordycepin, induces cell death in multiple myeloma cells. Br. J. Haematol. 2008, 140, 682–687. [Google Scholar] [CrossRef]
- Choi, S.; Lim, M.H.; Kim, K.M.; Jeon, B.H.; Song, W.O.; Kim, T.W. Cordycepin-induced apoptosis and autophagy in breast cancer cells are independent of the estrogen receptor. Toxicol. Appl. Pharmacol. 2011, 257, 165–173. [Google Scholar] [CrossRef]
- Jen, C.Y.; Lin, C.Y.; Huang, B.M.; Leu, S.F. Cordycepin induced MA-10 mouse Leydig tumor cell apoptosis through caspase-9 pathway. Evid. Based Complement. Altern. Med. 2011, 2011, 984537. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.W.; Jin, C.Y.; Park, C.; Han, M.H.; Kim, G.Y.; Moon, S.K.; Kim, C.G.; Jeong, Y.K.; Kim, W.J.; Lee, J.D.; et al. Inhibition of migration and invasion of LNCaP human prostate carcinoma cells by cordycepin through inactivation of Akt. Int. J. Oncol. 2012, 40, 1697–1704. [Google Scholar] [CrossRef]
- Jin, Y.; Meng, X.; Qiu, Z.; Su, Y.; Yu, P.; Qu, P. Antitumor and antimetastatic roles of cordycepin, one bioactive compound of Cordyceps militaris. Saudi J. Biol. Sci. 2018, 25, 991–995. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, W.J.; Moon, S.K. Cordycepin suppresses TNF-alpha-induced invasion, migration and matrix metalloproteinase-9 expression in human bladder cancer cells. Phytother. Res. 2010, 24, 1755–1761. [Google Scholar] [CrossRef]
- Lee, S.J.; Moon, G.S.; Jung, K.H.; Kim, W.J.; Moon, S.K. c-Jun N-terminal kinase 1 is required for cordycepin-mediated induction of G2/M cell-cycle arrest via p21WAF1 expression in human colon cancer cells. Food Chem. Toxicol. 2010, 48, 277–283. [Google Scholar] [CrossRef]
- Lee, D.; Lee, W.Y.; Jung, K.; Kwon, Y.S.; Kim, D.; Hwang, G.S.; Kim, C.E.; Lee, S.; Kang, K.S. The inhibitory effect of cordycepin on the proliferation of MCF-7 breast cancer cells, and its mechanism/ an investigation using network pharmacology-based analysis. Biomology 2019, 9, 414. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Burger, P.; Vogel, M.; Friese, K.; Bruening, A. The nucleoside antagonist cordycepin causes DNA double strand breaks in breast cancer cells. Investig. New Drugs 2012, 30, 1917–1925. [Google Scholar] [CrossRef]
- Leu, S.F.; Poon, S.L.; Pao, H.Y.; Huang, B.M. The in vivo and in vitro stimulatory effects of cordycepin on mouse Leydig cell steroidogenesis. Biosci. Biotechnol. Biochem. 2011, 75, 723–731. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, N.; Yamada, S.; Takeuchi, C.; Kagota, S.; Shinozuka, K.; Kunitomo, M.; Nakamura, K. Cordycepin (3′-deoxyadenosine) inhibits the growth of B16-BL6 mouse melanoma cells through the stimulation of adenosine A3 receptor followed by glycogen synthase kinase-3β activation and cyclin D1 suppression. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 377, 591–595. [Google Scholar] [CrossRef]
- Zheng, Q.; Sun, J.; Li, W.; Li, S.; Zhang, K. Cordycepin induces apoptosis in human tongue cancer cells in vitro and has anti- tumor effects in vivo. Arch. Oral Biol. 2020, 118, 104846. [Google Scholar] [CrossRef]
- Jiang, Q.; Lou, Z.; Wang, H.; Chen, C. Antimicrobial effect and proposed action mechanism of cordycepin against Escherichia coli and Bacillus subtilis. J. Microbiol. 2019, 57, 288–297. [Google Scholar] [CrossRef]
- Park, E.S.; Kang, D.H.; Yang, M.K.; Kang, J.C.; Jang, Y.C.; Park, J.S.; Shin, H.S. Cordycepin, 3’-deoxyadenosine, prevents rat hearts from ischemia/reperfusion injury via activation of Akt/GSK-3β/p70S6K signaling pathway and HO-1 expression. Cardiovasc. Toxicol. 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, G.Y.; Lee, H.H. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW 264.7 macrophages through Toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-κB signaling pathways. Drug Des. Dev. Ther. 2014, 8, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.W.; Jin, C.Y.; Kim, G.Y.; Lee, J.D.; Park, C.; Kim, G.D.; Kim, W.J.; Jung, W.K.; Seo, S.K.; Choi, I.W.; et al. Anti-inflammatory effects of cordycepin via suppression of inflammatory mediators in BV2 microglial cells. Int. Immunopharmacol. 2010, 10, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Naura, A.S.; Boulares, H. Cordycepin blocks lung injury-associated inflammation and promotes BRCA1-deficient breast cancer cell killing by effectively inhibiting PARP. Mol. Med. 2011, 17, 893–897. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, S.; Xu, L.; Song, B.; Huang, G.; Lu, J.; Guan, S. Suppression of T-cell activation in vitro and in vivo by cordycepin from Cordyceps militaris. J. Surg. Res. 2013, 185, 912–922. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; He, Y.; Li, T.; Wang, W.; Zhang, J.; Wei, J.; Deng, Y.; Lin, R. Cordycepin alleviates airway hyperreactivity in a murine model of asthma by attenuating the inflammatory process. Int. Immunopharm. 2015, 26, 401–408. [Google Scholar] [CrossRef]
- Yang, R.; Wang, X.; Xi, D.; Mo, J.; Wang, K.; Luo, S.; Wei, J.; Ren, Z.; Pang, H.; Luo, Y. Cordycepin attenuates IFN-γ-induced macrophage IP-10 and mig expressions by inhibiting STAT1 activity in CFA-induced inflammation mice model. Inflammtion 2020, 43, 752–764. [Google Scholar] [CrossRef]
- Deng, Q.; Li, X.; Fang, C.; Li, X.; Zhang, J.; Xi, Q.; Li, Y.; Zhang, R. Cordycepin enhances anti-tumor immunity in colon cancer by inhibiting phagocytosis immune checkpoint CD47 expression. Int. Immunopharmacol. 2022, 107, 108695. [Google Scholar] [CrossRef]
- Jeong, M.H.; Lee, C.M.; Lee, S.W.; Seo, S.Y.; Seo, M.J.; Kang, B.W.; Jeong, Y.K.; Choi, Y.J.; Yang, K.M.; Jo, W.S. Cordycepin-enriched Cordyceps militaris induces immunomodulation and tumor growth delay in mouse-derived breast cancer. Oncol. Rep. 2013, 30, 1996–2002. [Google Scholar] [CrossRef] [Green Version]
- Lai, X.; Ning, F.; Yao, Z.; Wang, T.; Zhang, L.; Fang, J.; Ma, J.; Li, G.; Xu, L.; Guo, Y.; et al. Ethylene carbodiimide-fixed donor splenocytes combined with cordycepin induce long-term protection to mice cardiac allografts. Trans. Immunol. 2019, 56, 101196. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Cho, H.D.; Cho, Y.S. Antioxidant and antihyperlipidemic effects of cordycepin-rich Cordyceps militaris in a Sprague-Dawley rat model of alcohol-induced hyperlipidemia and oxidative stress. Bioresour. Bioprocess. 2020, 7, 104846. [Google Scholar] [CrossRef]
- Xiao, L.; Ge, Y.; Sun, L.; Xu, X.; Xie, P.; Zhan, M.; Wang, M.; Dong, Z.; Li, J.; Duan, S.; et al. Cordycepin inhibits albumin-induced epithelial-mesenchymal transition of renal tubular epithelial cells by reducing reactive oxygen species production. Free Radic. Res. 2012, 46, 174–183. [Google Scholar] [CrossRef]
- Guo, P.; Kai, Q.; Gao, J.; Lian, Z.Q.; Wu, C.M.; Wu, C.A.; Zhu, H.B. Cordycepin prevents hyperlipidemia in hamsters fed a high-fat diet via activation of AMP-activated protein kinase. J. Pharmacol. Sci. 2010, 113, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Wong, Y.Y.; Moon, A.; Duffin, R.; Barthet-Barateig, A.; Meijer, H.A.; Clemens, M.J.; de Moor, C.H. Cordycepin inhibits protein synthesis and cell adhesion through effects on signal transduction. J. Biol. Chem. 2010, 285, 2610–2621. [Google Scholar] [CrossRef] [Green Version]
- Nomani, A.Z.; Nabi, Z.; Rashid, H.; Janjua, J.; Nomani, H.; Majeed, A.; Chaudry, S.R.; Mazhar, A.S. Osmotic nephrosis with mannitol: Review article. Ren. Fail. 2014, 36, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
- Sahmeddini, M.A.; Zahiri, S.; Khosravi, M.B.; Ghaffaripour, S.; Eghbal, M.H.; Shokrizadeh, S. Effect of mannitol on postreperfusion cardiac output and central venous oxygen saturation during orthotopic liver transplant: A double-blind randomized clinical trial. Prog. Transplant. 2014, 24, 121–125. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wang, W.; Zhang, H.; Zhang, X.; Han, C. The chemical constituents and pharmacological actions of Cordyceps sinensis. Evid. Based Complement. Altern. Med. 2015, 2015, 575063. [Google Scholar] [CrossRef] [Green Version]
- Bi, S.; Huang, W.; Chen, S.; Huang, C.; Li, C.; Guo, Z.; Yang, J.; Zhu, J.; Song, L.; Yu, R. Cordyceps militaris polysaccharide converts immunosuppressive macrophages into M1-like phenotype and activates T lymphocytes by inhibiting the PD-L1/PD-1 axis between TAMs and T lymphocytes. Int. J. Biol. Macromol. 2020, 150, 261–280. [Google Scholar] [CrossRef]
- Jayakumar, T.; Chiu, C.C.; Wang, S.H.; Chou, D.S.; Huang, Y.K.; Sheu, J.R. Anti-cancer effects of CME-1, a novel polysaccharide, purified from the mycelia of Cordyceps sinensis against B16-F10 melanoma cells. J. Cancer Res. Ther. 2014, 10, 43–49. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, J.S.; Yun, J.S.; Pahk, J.W.; Shin, W.C.; Lee, S.Y.; Hong, E.K. Structural characterization of immunostimulating polysaccharide from cultured mycelia of Cordyceps militaris. Carbohydr. Polym. 2010, 80, 1011–1017. [Google Scholar] [CrossRef]
- Qi, W.; Zhou, X.; Wang, J.; Zhang, K.; Zhou, Y.; Chen, S.; Nie, S.; Xie, M. Cordyceps sinensis polysaccharide inhibits colon cancer cells growth by inducing apoptosis and autophagy flux blockage via mTOR signaling. Carbohydr. Polym. 2020, 237, 116113. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Yang, J.Y.; Chen, J.P.; Hou, Y.Y.; Han, X.D. Immunomodulatory and antitumour effects of an exopolysaccharide fraction from cultivated Cordyceps sinensis (Chinese caterpillar fungus) on tumor-bearing mice. Biotechnol. Appl. Biochem. 2005, 42, 9–15. [Google Scholar] [CrossRef]
- Chen, W.X.; Zhang, W.Y.; Shen, W.B.; Wang, K. Effects of the acid polysaccharide fraction isolated from a cultivated C. sinensis on macrophages in vitro. Cell. Immunol. 2010, 262, 69–74. [Google Scholar] [CrossRef]
- He, B.L.; Zheng, Q.W.; Guo, L.Q.; Huang, J.Y.; Yun, F.; Huang, S.S.; Lin, J.F. Structural characterization and immune-enhancing activity of a novel high-molecular-weight polysaccharide from Cordyceps militaris. Int. J. Biol. Macromol. 2020, 145, 11–20. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, D.S.; Lee, K.R.; Park, J.M.; Ha, S.J.; Hong, E.K. Mechanism of macrophage activation induced by polysaccharide from Cordyceps militaris culture broth. Carbohydr. Polym. 2015, 120, 29–37. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.Z.; Li, L.D.J.; Zhou, X.W. Immunostimulatory effects of the intracellular polysaccharides isolated from liquid culture of Ophiocordyceps sinensis (Ascomycetes) on RAW264.7 cells via the MAPK and PI3K/Akt signaling pathways. J. Ethnopharmacol. 2021, 275, 114130. [Google Scholar] [CrossRef]
- Song, D.; Lin, J.Y.; Yuan, F.J.; Zhang, W.Y. Ex vivo stimulation of murine dendritic cells by an exopolysaccharide from one of the anamorph of C. sinensis. Cell Biochem. Funct. 2011, 29, 555–561. [Google Scholar] [CrossRef]
- Sheng, L.; Chen, J.; Li, J.; Zhang, W. An exopolysaccharide from cultivated Cordyceps sinensis and its effects on cytokine expressions of immunocytes. Appl. Microbiol. Biotechnol. 2011, 163, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nie, S.; Cui, S.W.; Wang, Z.; Phillips, A.O.; Phillips, G.O.; Li, Y.; Xie, M. Structural characterization and immunostimulatory activity of a glucan from natural Cordyceps sinensis. Food Hydrocoll. 2017, 67, 139–147. [Google Scholar] [CrossRef]
- Wang, M.; Meng, X.; Yang, R.; Qin, T.; Li, Y.; Zhang, L.; Hu, Y. Cordyceps militaris polysaccharides can improve the immune efficacy of Newcastle disease vaccine in chicken. Int. J. Biol. Macromol. 2013, 59, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, Y.; Zhang, Z.; Ding, Y.; Dai, X.; Li, Y. Effect of polysaccharide from cultured Cordyceps sinensis on immune function and anti-oxidation activity of mice exposed to 60Co. Int. Immunopharmacol. 2011, 11, 2251–2257. [Google Scholar] [CrossRef]
- Li, C.Y.; Chiang, C.S.; Tsai, M.L.; Hseu, R.S.; Shu, W.Y.; Chuang, C.Y.; Sun, Y.C.; Chang, Y.S.; Lin, J.G.; Chen, C.S.; et al. Two-sided effect of Cordyceps sinensis on dendritic cells in different physiological stages. J. Leukocyte Biol. 2009, 85, 987–995. [Google Scholar] [CrossRef]
- Yu, R.; Song, L.; Zhao, Y.; Bin, W.; Wang, L.; Zhang, H.; Wu, Y.; Ye, W.; Yao, X. Isolation and biological properties of polysaccharide CPS-1 from cultured Cordyceps militaris. Fitoterapia 2004, 75, 465–472. [Google Scholar] [CrossRef]
- Lin, R.; Liu, H.; Wu, S.; Pang, L.; Jia, M.; Fan, K.; Jia, L. Production and in vitro antioxidant activity of exopolysaccharide by a mutant, Cordyceps militaris SU5-08. Int. J. Biol. Macromol. 2012, 51, 153–157. [Google Scholar] [CrossRef]
- Wang, J.; Nie, S.; Kan, L.; Chen, H.; Cui, S.W.; Phillips, A.O.; Phillips, G.O.; Xie, M. Comparison of structural features and antioxidant activity of polysaccharides from natural and cultured Cordyceps sinensis. Food Sci. Biotechnol. 2017, 26, 55–62. [Google Scholar] [CrossRef]
- Ohta, Y.; Lee, J.B.; Hayashi, K.; Fujita, A.; Park, D.K.; Hayashi, T. In vivo anti-influenza virus activity of an immunomodulatory acidic polysaccharide isolated from Cordyceps militaris grown on germinated soybeans. J. Agric. Food Chem. 2007, 55, 10194–10199. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Zhao, H.; Jiang, H.; Luo, C.; Wang, M.; Yin, H. Cordyceps sinensis polysaccha-ride CPS-2 protects human mesangial cells from PDGF-BB-induced proliferation through the PDGF/ERK and TGF-β1/Smad pathways. Mol. Cell. Endocrinol. 2014, 382, 979–988. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Ling, Y.; Fan, W.; Wang, Y.; Yin, H. Structural determination and antioxidant activity of a polysaccharide from the fruiting bodies of cultured Cordyceps sinensis. Am. J. Chin. Med. 2009, 37, 977–989. [Google Scholar] [CrossRef]
- Li, S.P.; Zhang, G.H.; Zeng, Q.; Huang, Z.G.; Wang, Y.T.; Dong, T.T.; Tsim, K.W. Hypoglycemic activity of polysaccharide, with antioxidation, isolated from cultured Cordyceps mycelia. Phytomedicine 2006, 13, 428–433. [Google Scholar] [CrossRef]
- Qian, G.M.; Pan, G.F.; Guo, J.Y. Anti-inflammatory and antinociceptive effects of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis. Nat. Prod. Res. 2012, 26, 2358–2362. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.M.; Cao, W.; Yao, K.W.; Liu, Z.Q.; Guo, J.Y. Anti-inflammation and antioxidant effect of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Metab. Brain Dis. 2012, 27, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhang, Y.; Yan, Y.B.; Lei, W.; Wu, Z.X.; Liu, N.; Liu, S.; Shi, L.; Fan, Y. The protective effect of cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, on diabetic osteopenia in alloxan-induced diabetic rats. Evid. Based Complement. Altern. Med. 2013, 2013, 985636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, J.M.; Ma, X.C.; Wu, C.F.; Wu, L.J.; Hu, G.S. Cordycedipeptide A, a new cyclodipeptide from the culture liquid of Cordyceps sinensis (BERK.) SACC. Chem. Pharmaceut. Bull. 2005, 53, 582–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.S.; Zhang, D.S.; Zhu, T.J.; Chen, X.Y. A pharmacological analysis of the amino acid components of Cordyceps sinensis Sacc. Yao Xue Xue Bao 1991, 26, 326–330. (In Chinese) [Google Scholar]
- Choi, D.; Cha, W.S.; Park, N.; Kim, H.W.; Lee, J.H.; Park, J.S.; Park, S.S. Purification and characterization of a novel fibrinolytic enzyme from fruiting bodies of Korean Cordyceps militaris. Bioresour. Technol. 2011, 102, 3279–3285. [Google Scholar] [CrossRef]
- Cui, L.; Dong, M.S.; Chen, X.H.; Jiang, M.; Lv, X.; Yan, G. A novel fibrinolytic enzyme from Cordyceps militaris, a Chinese traditional medicinal mushroom. World J. Microbiol. Biotechnol. 2008, 24, 483–489. [Google Scholar] [CrossRef]
- Matsuda, H.; Akaki, J.; Nakamura, S.; Okazaki, Y.; Kojima, H.; Tamesada, M.; Yoshikawa, M. Apoptosis-inducing effects of sterols from the dried powder of cultured mycelium of Cordyceps sinensis. Chem. Pharm. Bull. 2009, 57, 411–414. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Feng, X.; Zheng, D.; Li, A.; Li, C.; Li, S.; Zhao, Z. Ergosterol attenuates cigarette smoke extract-induced COPD by modulating inflammation, oxidative stress and apoptosis In Vitro and In Vivo. Clin. Sci. 2019, 133, 1523–1536. [Google Scholar] [CrossRef]
- Peng, Y.; Tao, Y.; Wang, Q.; Shen, L.; Yang, T.; Liu, Z.; Liu, C. Ergosterol is the active compound of cultured mycelium Cordyceps sinensis on antiliver fibrosis. Evid. Based Complement. Altern. Med. 2014, 2014, 537234. [Google Scholar] [CrossRef] [Green Version]
- Li, S.P.; Yang, F.Q.; Tsim, K.W.K. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J. Pharm. Biomed. Anal. 2006, 41, 1571–1584. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Q.; Leung, P. Inhibitory effects of ethyl acetate extract of Cordyceps sinensis mycelium on various cancer cells in culture and B16 melanoma in C57BL/6 mice. Phytomedicines 2007, 14, 43–49. [Google Scholar] [CrossRef]
- Yang, L.Y.; Huang, W.J.; Hsieh, H.G.; Lin, C.Y. H1-A extracted from Cordyceps sinensis, suppresses the proliferation of human mesangial cells and promotes apoptosis, probably by inhibiting the tyrosine phosphorylation of Bcl-2 and Bcl-XL. J. Lab. Clin. Med. 2003, 141, 74–83. [Google Scholar] [CrossRef]
- Yang, M.L.; Kuo, P.C.; Hwang, T.L.; Wu, T.S. Anti-inflammatory principles from Cordyceps sinensis. J. Nat. Prod. 2011, 74, 1996–2000. [Google Scholar] [CrossRef]
- Shashidhar, M.G.; Giridhar, P.; Sankar, K.U.; Manohar, B. Bioactive principles from Cordyceps sinensis: A potent food supplement—A review. J. Funct. Foods 2013, 5, 1013–1030. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Wang, M.; Zhang, H.; Huang, Z.; Ma, J. Comparative study of the composition of cultivated, naturally grown Cordyceps sinensis, and stiff worms across different sampling years. PLoS ONE 2019, 14, e0225750. [Google Scholar] [CrossRef]
- Li, J.; Feng, C.Q.; Ni, X.M.; Zhang, W.S. Determination of nucleosides of natural Cordyceps sinensis in Qinghai province by capillary electrophoresis. Chin. Pharm. J. 2008, 43, 1105–1107. (In Chinese) [Google Scholar] [CrossRef]
- Ikeda, R.; Nishimura, M.; Sun, Y.; Wada, M.; Nakashima, K. Simple HPLC-UV determination of nucleosides and its application to the authentication of Cordyceps and its allies. Biomed. Chromatogr. 2008, 22, 630–636. [Google Scholar] [CrossRef]
- Yang, F.Q.; Ge, L.; Yong, J.W.; Tan, S.N.; Li, S.P. Determination of nucleosides and nucleobases in different species of Cordyceps by capillary electrophoresis-mass spectrometry. J. Pharm. Biomed. 2009, 50, 307–314. [Google Scholar] [CrossRef]
- Yuan, Y.S.; Zhang, L.; Xu, X.F.; Zhou, Y.X.; Wei, L.X. Determination of nucleosides in Cordyceps by RP-HPLC. Chin. Pharm. J. 2002, 37, 776–778. [Google Scholar] [CrossRef]
- Vannucci, L.; Krizan, J.; Sima, P.; Stakheev, D.; Caja, F.; Rajsiglova, L.; Horak, V.; Saieh, M. Immunostimulatory properties and antitumor activities of glucans (review). Int. J. Oncol. 2013, 43, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.K.; Wang, W.Q.; Li, L.; Wu, J. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J. Funct. Foods 2014, 6, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, Y.; Chen, X.X.; Zheng, S.C.; Chen, P.; Mo, M.H. The mechanisms of pharmacological activities of Ophiocordyceps sinensis fungi. Phytother. Res. 2016, 30, 1572–1583. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Peng, X.; Lee, K.L.; Tang, J.C.; Cheung, P.C.; Wu, J.Y. Structural characterization and immunomodulatory property of an acidic polysaccharide from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 2011, 125, 637–643. [Google Scholar] [CrossRef]
- Lee, S.J.; Cho, J.Y.; Hong, E.K. Study on macrophage activation and structural characteristics of purified polysaccharide from the liquid culture broth of Cordyceps militaris. Carbohydr. Polym. 2010, 82, 982–988. [Google Scholar] [CrossRef]
- Bi, S.; Jing, Y.; Zhou, Q.; Hu, X.; Zhu, J.; Guo, Z.; Song, L.; Yu, R. Structural elucidation and immunostimulatory activity of a new polysaccharide from Cordyceps militaris. Food Funct. 2018, 9, 279–293. [Google Scholar] [CrossRef]
- He, L.; Ji, P.; Cheng, J.; Wang, Y.; Qian, H.; Li, W.; Gong, X.; Wang, Z. Structural characterization and immunostimulatory activity of a novel protein-bound polysaccharide produced by Hirsutella sinensis Liu, Guo, Yu & Zeng. Food Chem. 2013, 141, 946–953. [Google Scholar] [CrossRef]
- Yu, R.M.; Yang, W.; Song, L.; Yan, C.; Zhang, Z.; Zhao, Y. Structural characterization and antioxidant activity of a polysaccharide from the fruiting bodies of cultured Cordyceps militaris. Carbohydr. Polym. 2007, 70, 430–436. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, W.Q.; Li, L.; Wu, J.Y. Physiochemical properties and antitumor activities of two α-glucans isolated from hot water and alkaline extracts of Cordyceps (Cs-HK1) fungal mycelia. Carbohydr. Polym. 2011, 85, 753–758. [Google Scholar] [CrossRef]
- Zigler, M.; Shir, A.; Levitzki, A. Targeted cancer immunotherapy. Curr. Opin. Pharmacol. 2013, 13, 504–510. [Google Scholar] [CrossRef]
- Xu, L.; Feng, J.M.; Li, J.X.; Zhu, J.M.; Song, S.S.; Tong, L.J.; Chen, Y.; Yang, X.Y.; Shen, Y.Y.; Lian, F.L.; et al. Tanshinone-1 induces tumor cell killing, enhanced by inhibition of secondary activation of signaling networks. Cell Death Dis. 2013, 4, e905. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Guan, Q.; Guo, Y.; Du, C. Reduction of inflammatory hyperplasia in the intestine in colon cancer-prone mice by water-extract of Cistanche deserticola. Phytother. Res. 2012, 26, 812–819. [Google Scholar] [CrossRef]
- Li, J.; Cai, H.; Sun, H.; Qu, J.; Zhao, B.; Hu, X.; Li, W.; Qian, Z.; Yu, X.; Kang, F.; et al. Extracts of Cordyceps sinensis inhibit breast cancer growth through promoting M1 macrophage polarization via NF-κB pathway activation. J. Ethnopharmacol. 2020, 260, 112969. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, F.; Wang, K.; Song, D.; Zhang, W. Modulatory effects of the acid polysaccharide fraction from one of anamorph of Cordyceps sinensis on Ana-1 cells. J. Ethnopharmacol. 2012, 142, 739–745. [Google Scholar] [CrossRef]
- Guo, Q.; Li, J.; Lin, H. Effect and molecular mechanisms of traditional Chinese medicine on regulating tumor immunosuppressive microenvironment. Biomed. Res. Int. 2015, 2015, 261620. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Luo, F.; Ding, C.; Albeituni, S.; Hu, X.; Ma, Y.; Cai, Y.; Mcnally, L.; Sanders, M.A.; Jain, D.; et al. Dectin-1 activation by a natural product β-glucan converts immunosuppressive macrophages into an M1-like phenotype. J. Immunol. 2015, 195, 5055–5065. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Sun, S.; Xu, A.; Bhargava, P.; Yang, L.; Lam, K.S.L.; Gao, B.; Lee, C.H.; Kersten, S.; Qi, L. Activation of natural killer T cells promotes M2 macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J. Biol. Chem. 2012, 287, 13561–13571. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Fu, X.; Shi, H.; Chen, G.; Dong, P.; Zhang, W. Induction of murine macrophage M2 polarization by cigarette smoke extract via the JAK2/STAT3 pathway. PLoS ONE 2014, 9, e107063. [Google Scholar] [CrossRef]
- Isidro, R.A.; Appleyard, C.B. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G59–G73. [Google Scholar] [CrossRef] [Green Version]
- Tariq, M.; Zhang, J.; Liang, G.; Ding, L.; He, Q.; Yang, B. Macrophage polarization: Anti-cancer strategies to target tumor-associated macrophage in breast cancer. J. Cell. Biochem. 2017, 118, 2484–2501. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, S.H.; Hueng, D.Y.; Syu, J.P.; Liao, C.C.; Wu, Y.C. Cordycepin induces apoptosis of C6 glioma cells through the adenosine 2A receptor-p53-caspase-7-PARP pathway. Chem. Biol. Interact. 2014, 216, 17–25. [Google Scholar] [CrossRef]
- Tania, M.; Shawon, J.; Saif, K.; Kiefer, R.; Khorram, M.S.; Halim, M.A.; Khan, M.A. Cordycepin Downregulates Cdk-2 to Interfere with Cell Cycle and Increases Apoptosis by Generating ROS in Cervical Cancer Cells: In vitro and in silico Study. Curr. Cancer Drug Targets 2019, 19, 152–159. [Google Scholar] [CrossRef]
- Xu, J.; Tan, Z.C.; Shen, Z.Y.; Shen, X.J.; Tang, S.M. Cordyceps cicadae polysaccharides inhibit human cervical cancer hela cells proliferation via apoptosis and cell cycle arrest. Food Chem. Toxicol. 2021, 148, 111971. [Google Scholar] [CrossRef]
- Lee, W.H.; Lee, S.; Lee, K.; Shin, Y.S.; Kang, H.; Cho, H. Anti-cancer effect of Cordyceps militaris in human colorectal carcinoma RKO cells via cell cycle arrest and mitochondrial apoptosis. DARU J. Pharm. Sci. 2015, 23, 35. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.H.; Jeong, J.W.; Choi, Y.H. Inhibition of PI3K/AKT signaling pathway enhances cordycepin-induced apoptosis in human gastric cancer cells. J. Korean Soc. Food Sci. Nutr. 2016, 45, 835–842. [Google Scholar] [CrossRef]
- Xiao, J.H.; Chen, D.X.; Fang, N.; Liu, Z.L.; Zhang, T. Growth arrest of human gastric adenocarcinoma cells by bioactive compounds of Cordyceps jiangxiensis (CaoMuWang) through induction of apoptosis. J. Food Agric. Environ. 2006, 4, 66–73. [Google Scholar]
- Li, Z.; Guo, Z.; Zhu, J.; Bi, S.; Luo, Y.; Yu, R.; Huang, W.; Song, L. Cordyceps militaris fraction inhibits angiogenesis of hepatocellular carcinoma in vitro and in vivo. Pharmacogn. Mag. 2020, 16, 169–176. [Google Scholar] [CrossRef]
- Huo, X.; Liu, C.; Bai, X.; Li, W.; Li, J.; Hu, X.; Cao, L. Aqueous extract of Cordyceps sinensis potentiates the antitumor effect of DDP and attenuates therapy-associated toxicity in non-small cell lung cancer via IkBa/NFkB and AKT/MMP2/MMP9 pathways. RSC Adv. 2017, 66, 37743–37754. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.S.; Li, Y.; He, W.; Yi, K.S.; Huang, M. Polysaccharide of Cordyceps sinensis Enhances Cisplatin Cytotoxicity in Non–Small Cell Lung Cancer H157 Cell Line. Integr. Cancer Ther. 2011, 10, 359–367. [Google Scholar] [CrossRef]
- Bizarro, A.; Ferreira, I.C.F.R.; Soković, M.; Van, G.; Leo, J.L.D.; Sousa, D.; Vasconcelos, M.H.; Lima, R.T. Cordyceps militaris (L.) Link Fruiting Body Reduces the Growth of a Non-Small Cell Lung Cancer Cell Line by Increasing Cellular Levels of p53 and p21. Molecules 2015, 20, 13927–13940. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, W.; Shi, P.; Chen, J.; Han, X.; Wang, Y. Effects of exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis fungus on c-Myc, c-Fos, and VEGF expression in B16 melanoma-bearing mice. Pathol. Res. Pract. 2005, 201, 745–750. [Google Scholar] [CrossRef]
- Hsu, P.Y.; Lin, Y.H.; Yeh, E.L.; Lo, H.C.; Hsu, T.H.; Su, C.C. Cordycepin and a preparation from Cordyceps militaris inhibit malignant transformation and proliferation by decreasing EGFR and IL-17RA signaling in a murine oral cancer model. Oncotarget 2017, 8, 93712–93728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.T.; Lai, Y.J.; Wu, S.C.; Hsu, W.H.; Tai, C.J. Optimal conditions for cordycepin production in surface liquid-cultured Cordyceps militaris treated with porcine liver extracts for suppression of oral cancer. J. Food Drug Anal. 2018, 26, 135–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, E.; Jang, H.J.; Yang, K.E.; Jang, M.S.; Huh, Y.H.; Yoo, H.S.; Park, J.S.; Jang, I.S.; Park, S.J. Cordyceps militaris induces apoptosis in ovarian cancer cells through TNF-α/TNFR1-mediated inhibition of NF-κB phosphorylation. BMC Complement. Med. Ther. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Lindroth, A.M.; Kwon, S.; Park, S.J.; Par, Y.J. Adenosine derivatives from Cordyceps exert antitumor effects against ovarian cancer cells through ENT1-mediated transport, induction of AMPK signaling, and consequent autophagic cell death. Biomed. Pharmcother. 2022, 153, 113491. [Google Scholar] [CrossRef] [PubMed]
- Algeciras-Schimnich, A.; Shen, L.; Barnhart, B.C. Molecular ordering of the initial signaling events of CD95. Mol. Cell. Biol. 2002, 22, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Villa-Morales, M.; Fernandez-Piqueras, J. Targeting the Fas FasL signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 85–101. [Google Scholar] [CrossRef]
- Lee, S.Y.; Debnath, T.; Kim, S.K.; Lim, B.O. Anti-cancer effect and apoptosis induction of cordycepin through DR3 pathway in the human colonic cancer cell HT-29. Food Chem. Toxicol. 2013, 60, 439–447. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Abu, K.Y. Hijacking of apoptotic pathways by bacterial pathogens. Microbes Infect. 2002, 2, 1705–1719. [Google Scholar] [CrossRef]
- Detjen, K.M.; Farwig, K.; Welzel, M.; Wiedenmann, B.; Rosewicz, S. Interferon gamma inhibits growth of human pancreatic carcinoma cells via caspase-1 dependent induction of apoptosis. Gut 2001, 49, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.H.; Kim, S.O.; Kim, G.Y.; Moon, S.K.; Kim, W.J.; Jeong, Y.K.; Yoo, Y.H.; Choi, Y.H. Involvement of autophagy in cordycepin-induced apoptosis in human prostate carcinoma LNCaP cells. Environ. Toxicol. Pharmacol. 2014, 38, 239–250. [Google Scholar] [CrossRef]
- Balk, J.S.; Mun, S.W.; Kim, K.S.; Park, S.J.; Yoon, H.K.; Kim, D.H.; Park, M.K.; Kim, C.H.; Lee, Y.C. Apoptotic effects of cordycepin through the extrinsic pathway and p38 MAPK activation in human glioblastoma U87MG cells. J. Microbiol. Biotechnol. 2016, 26, 309–314. [Google Scholar] [CrossRef]
- Cao, H.L.; Liu, Z.J.; Chang, Z. Cordycepin induces apoptosis in human bladder cancer cells via activation of A3 adenosine receptors. Tumor Biol. 2017, 39, 7. [Google Scholar] [CrossRef]
- Marino, G.; Niso-Santano, M.; Baehrecke, E.H.; Guido, K. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Kim, S.K.; Choi, W.S.; Kim, W.J.; Moon, S.K. Cordycepin causes p21WAF1-mediated G2/M cell-cycle arrest by regulating c-Jun N-terminal kinase activation in human bladder cancer cells. Arch. Biochem. Biophys. 2009, 490, 103–109. [Google Scholar] [CrossRef]
- Holbein, S.; Wengi, A.; Decourty, L.; Freimoser, F.M.; Jacquier, A.; Dichtl, B. Cordycepin interferes with 3′ end formation in yeast independently of its potential to terminate RNA chain elongation. RNA 2009, 15, 837–849. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Li, X.; Chen, Y.; Lang, M.; Shen, Z.; Shi, L. Ethanolic extract of Cordyceps cicadae exerts antitumor effect on human gastric cancer SGC-7901 cells by inducing apoptosis, cell cycle arrest and endoplasmic reticulum stress. J. Ethnopharmacol. 2019, 231, 230–240. [Google Scholar] [CrossRef]
- Hunter, K.W.; Crawford, N.P.S.; Alsarraj, J. Mechanisms of metastasis. Breast Cancer Res. 2008, 10, S2. [Google Scholar] [CrossRef] [Green Version]
- Tao, X.; Ning, Y.; Zhao, X.; Pan, T. The effects of cordycepin on the cell proliferation, migration and apoptosis in human lung cancer cell lines A549 and NCI-H460. J. Pharm. Pharmacol. 2016, 68, 901–911. [Google Scholar] [CrossRef]
- Nakamura, K.; Shinozuka, K.; Yoshikawa, N. Anticancer and antimetastatic effects of cordycepin, an active component of Cordyceps sinensis. J. Pharm. Sci. 2015, 127, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Phuchareon, J.; Tokuhisa, T. Deregulated c-Fos/AP-1 modulates expression of the cyclin and the cdk gene in splenic B cells stimulated with lipopolysaccharide. Cancer Lett. 1995, 92, 203–208. [Google Scholar] [CrossRef]


| No. | Bioactive Components | Pharmacological Effects | Ref. |
|---|---|---|---|
| 1 | Adenosine | Antitumor activity | [5,6,7,8] |
| Attenuation of chronic heart failure | [9] | ||
| Anti-inflammation | [10,11,12,13,14] | ||
| Immunomodulatory activity | [12,15] | ||
| 2 | Inosine | Anti-inflammation | [16] |
| 3 | Guanosine | Seizure prevention | [17] |
| Immunomodulatory activity | [15] | ||
| 4 | Cordycepin | Antitumor activity | [18,19,20,21,22,23,24,25,26,27,28,29] |
| Antibacterial activity | [20] | ||
| Treatment for ischemic/reperfusion (IR) injury | [21] | ||
| Anti-inflammation | [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] | ||
| Immunomodulatory activity | [38,39,40] | ||
| Antioxidant activity | [41,42] | ||
| Cholesterol lowering effect | [43] | ||
| Anti-fibroblast activity | [44] | ||
| 5 | Cordycepic acid | Diuretic effect | [45] |
| Attenuating postreperfusion syndrome | [46] | ||
| Anti-fibrosis and anti-inflammation | [47] | ||
| 6 | Polysaccharides | Antitumor activity | [48,49,50,51,52] |
| Immunomodulatory activity | [48,53,54,55,56,57,58,59,60,61] | ||
| Anti-inflammation | [56,62,63] | ||
| Antioxidant activity | [61,64,65] | ||
| Antiviral activity | [66] | ||
| Protective effects on kidney | [67,68] | ||
| Hypoglycemic effect | [69] | ||
| 7 | Cordymin | Analgesic effect | [70] |
| Anti-inflammation | [71] | ||
| Antioxidant | [71] | ||
| Hypoglycemic effect | [72] | ||
| 8 | Cordycedipeptide A | Antitumor activity | [73] |
| 9 | Tryptophan | Sedative hypnotic effect | [74] |
| 10 | Fibrinolytic enzymes | Treatment for thrombosis | [75,76] |
| 11 | Ergosterol | Cytotoxicity | [77] |
| Anti-inflammation | [78] | ||
| Anti-fibroblast activity | [79] | ||
| Antiviral activity | [80] | ||
| 12 | β-Sitosterol | Cytotoxicity | [77,81] |
| 13 | 5α,8α-epidioxy-22E-ergosta-6,22-dien-3β-ol | Cytotoxicity | [77] |
| 14 | 5α,8α-epidioxy-22E-ergosta-6,9(11),22-trien-3β-ol | Cytotoxicity | [77] |
| 15 | 5α,6α-epoxy-5α-ergosta-7,22-dien-3β-ol | Cytotoxicity | [77] |
| 16 | H1-A | Cytotoxicity | [82] |
| 17 | Cordysinin A | Anti-inflammatory | [83] |
| 18 | Cordysinin B | Anti-inflammatory | [83] |
| 19 | Cordysinin C | Anti-inflammatory | [83] |
| 20 | Cordysinin D | Anti-inflammatory | [83] |
| 21 | Cordysinin E | Anti-inflammatory | [83] |
| No. | Name | MW | Components | Glycosyl Linkage and Branches (Characteristic Signals) | Bioactivities | Source | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | AEPS-1 | 36 kDa | Glcp:GlcUp = 8:1 (M ratio), plus a trace amount of mannose | A main chain of (1→3)-linked α-d-Glcp with α-d-Glcp and α-d-GlcUp branches attached to the main chain by (1→6) glycosidic bonds at every seventh α-d-Glcp unit | Anti-inflammatory; immunomodulatory | Mycelial fermentation of C. sinensis (Cs-HK1) | [93] |
| 2 | EPS | 104 kDa | Man:Glc:Gal = 23:1:2.6 (M ratio) | Immunomodulatory | Mycelial fermentation of C. sinensis (G1) | [58] | |
| 3 | NCSP-50 | 976 kDa | Glucose | A main chain of (1→4)-linked α-d-Glcp with α-d-Glcp branch attached to the C-6 | Immunomodulatory | C. sinensis | [59] |
| 4 | CSP | 28 kDa | Gal:Glc:Man:Ara:GalA = 36.40:28.99:24.81:3.34:7.55 (percentage ratio) | A main chain of (1→4)-linked α-d-Glc and (1→4)-linked α-d-Gal | Antitumor | Cultured mycelia of C. sinensis | [51,65] |
| 5 | CME-1 | 27.6 kDa | Man:Gal:Glc = 39.1:59.2:1.7 (M ratio) | A backbone of (1→4)-linked β-d-Man with Gal branches attached to the O- 6 | Antitumor | Cultured mycelia of C. sinensis | [49] |
| 6 | APSP | Man:Glc:Gal = 3.5:1:1.5 (M ratio) | Immunomodulatory | Mycelia of liquid cultured C. sinensis | [53] | ||
| 7 | PLCM (CPSN Fr II) | 36 kDa | Man:Gal:Glc:Protein:Hexosamine:Uronic acid = 65.12:28.72:6.12:0.20:0.06:0.29 (percentage ratio) | A backbone of (1→2)-, (1→6)-linked β-d-Man with (1→4)-linked β-d-Gal branches attached to the O- 6 | Immunomodulatory | C. militaris liquid culture broth | [55,94] |
| 8 | CMP-III | 4.796 × 104 kDa | Glc:Man:Gal = 8.09:1.00:0.25 (M ratio) | A backbone of (1→4)-linked α-d-Glc with (1→4,6)-linked α-d-Man and (1→2,6)-α-d-Man branches attached to the O- 6 | Immunomodulatory | Cultured fruiting bodies of C. militaris | [54] |
| 9 | CMPB90-1 | 5.8 kD | Gal:Glc:Man = 3.04:1:1.45 (M ratio) | A main chain of (1→6)-linked α-d-Glc and (1→3)- linked α-d-Glc, with branching at O-6, which consists of (1→4)-linked β-d-Man and (1→6)-linked α-d-Glc, respectively, and β-d-Man as the terminal unit | Immunomodulatory | Cultured fruiting bodies of C. militaris | [95] |
| 10 | CPMN Fr III | 210 kDa | Glc:Gal:Man = 9.17:18.61:72.22 (M ratio) | A backbone of (1→6)- linked β-d-Man and (1→6)- linked β-d-Glc with branches of (1→4)- linked β-d-Man terminated with d-Gal and d-Man, respectively | Immunomodulatory | Cultured mycelia of C. militaris | [50] |
| 11 | HS002-II | 44 kDa | D-Man:D-Rib:L-Rha:D-GlcUA:D-GalUA:D-Glc:D-Gal:D-Xyl:L-Ara = 6.47:2.27:1.25:0.69:0.42:65.89:16.17:2.13:4.26 (M ratio) polysaccharide:protein = 57.9:42.1 (percentage ratio) | A long backbone of (1→3)-linked α-d-Ribf, (1→4)-linked α-d-Xylp and approximately 1/31 of (1→4)-linked β-d-Glcp, which was substituted at C-6. The two branches were (1→6)-linked β-d-Manp and (1→6)-linked β-d-Galp terminated with α-L-Arap, respectively | Immunomodulatory | Mycelial fermentation of Hirsutella sinensis Liu, Guo, Yu and Zeng | [96] |
| 12 | P70-1 | 42 k Da | Man:Gal:Glc = 3.12:1.45:1.00 (M ratio) | A backbone of (1→6)-linked α-d-Manp with branching points at O-3, and the branches composed of (1→4)-linked α-d-Glcp and (1→6)-linked β-d-Galp, and terminated with β-d-Galp and α-d-Glcp | Antioxidant | Fruiting bodies of cultured C. militaris | [97] |
| 13 | CPS-1 | 23 kDa | Rha:Xyl:Man:Glc:Gla = 1:6.43:25.6:16.0:13.8 (M ratio) | Composed of (1→2)-linked Man, (1→4)-linked Xyl and (1→2)-linked or (1→3)-linked Rha or Gal | Anti-inflammatory | Cultured C. militaris | [63] |
| 14 | AIPS | 1.15 × 103 kDa | Glucose | α-d-(1→4) glucan | Antitumor | Mycelial fermentation of C. sinensis (Cs-HK1) | [98] |
| Bioactive Component | Pharmacological Effects | Models | Major Mediating Signaling Pathways | Mechanism of Action | Ref. |
|---|---|---|---|---|---|
| Cordycepin | ↑Antitumor immunity responses ↓CT 26 cell migration ↑CT 26 cell apoptosis | CT 26 cells in mice | ↑CD4+ T, CD8+ T cells ↑NK cells ↑M1 macrophages ↑CD11b+, F4/80+ ↓CD47 | [38] | |
| JLM 0636 (cordycepin-enriched extract of C. militaris) | ↑Th 1 cells ↑Immune responses ↓Treg cells ↓Immunosuppression | FM3A murine breast cancer cells, derived from C3H/He mouse | ↑CD8+ T cells ↑IFN-γ ↓CD4+CD25+ T cells ↓IL-2 ↓TGF-β | [39] | |
| WECS (Nucleoside extract of C. sinensis) | ↓MDA-MB-231 cells ↓4T1 cells ↑M1 macrophages ↑Immune responses | MDA-MB-231, 4T1 breast cancer cells co-cultured with macrophages | NF-κB | ↑CD38 ↑iNOS ↑IL-1β ↑IL-12p70 ↑TNF-α ↑IL-6 ↑IFN-γ ↑NO | [102] |
| EPSP | ↑M1 macrophages ↑Spleen lymphocyte ↑Immune response ↓Tumor migration | B16 melanoma-bearing mice | ↓Bcl-2 | [52] | |
| APSF | ↑M1 macrophages ↑Immune response ↓M2 macrophages ↓Immunosuppression | Ana-1 mouse macrophages co-cultured with H22 cells | NF-κB | ↑TNF-α ↑IL-12 ↑iNOS ↓IL-10 ↓SR ↓MR | [103] |
| CMPB90-1 | ↑M1 macrophages ↑Immune response ↓M2 macrophages ↓Immunosuppression | IL-4, tumor cell supernatant-induced RAW264.7 cells | NF-κB Akt MAPK (p38 and ERK) | ↓IL-10 ↓TGF-β ↓Arg-1 ↑IL-12 ↑iNOS | [48] |
| Cancer | Bioactive Component | Pharmacological Effects | Cell line | Major Mediating Signaling Pathways | Mechanism of Action | Ref. |
|---|---|---|---|---|---|---|
| Bladder cancer | ||||||
| Cordycepin | ↓Migration and invasion | TNF-α-induced 5637 and T-24 cells | NF-κB AP-1 | ↓MMP-9 | [23] | |
| Breast cancer | ||||||
| Cordycepin | ↑Apoptosis | MDA-MB-231 cells | Caspase | ↑Bax (mitochondria) ↑Cytochrome c (cytosol) ↑PARP ↑c-caspases-9, -3 ↑DNA fragmentation | [19] | |
| Cordycepin | ↑Autophagy | MCF-7 cells | Autophagy | ↑LC3-II ↑Autophagosome-like structure | [19] | |
| Cordycepin | ↑Apoptosis | MDA-MB-435 and T47D cells | ↑DNA fragmentation ↑Histone γH2AX ↓RNA synthesis | [26] | ||
| C. militaris extract | ↑Apoptosis | MCF-7 cells | Caspase | ↑Bax/Bcl-2 ↑c-caspase-7, -8 | [25] | |
| Cordycepin | ↑Apoptosis | C6 glioma cells | A2AR Caspase | ↑Caspase-7 ↑p-p53 ↑PARP | [110] | |
| Cervical cancer | ||||||
| Cordycepin | ↑Apoptosis ↓Cell cycle | SiHa cells HeLa cells | ↓CDK-2 ↓Cyclin-E1 ↓Cyclin-A2 ↑ROS | [111] | ||
| CCP (C. cicadae polysaccharides) | ↑Apoptosis ↓Cell cycle | hela cells | Akt | ↑Bak ↑Bax ↑Caspase-3, -7, -9 ↓P21 ↓P27 ↓CDK2 ↓Cyclin E1 ↓Cyclin A2 ↓Bcl-2 ↓Bcl-xl ↓PARP | [112] | |
| Colon cancer | ||||||
| CSP | ↑Autophagy, ↑Apoptosis | HCT116 cells | Autophagy mTOR Caspase | ↑LC3B-II ↑Caspase-8, -3 | [51,65] | |
| Cordycepin | ↓Cell cycle | HCT116 cells | JNK MAPK | ↑p21WAF1 ↓Cyclin B1 ↓Cdc25c ↓Cdc2 | [24] | |
| Colorectal cancer | ||||||
| C. militaris extract | ↓Cell cycle | RKO cells | ↑Bax ↑Bim ↑Bak ↑Bad ↑PARP ↑p-p53 ↑c-caspase -9, -3 | [113] | ||
| Gastric cancer | ||||||
| Cordycepin | ↑Apoptosis | AGS cells | PI3K/Akt | ↑Caspase-9, -3, -7 ↑Bax ↓Bcl-2 | [114] | |
| CECJ (C. jiangxiensis extract) | ↑Apoptosis ↓Cell cycle | SGC-7901 cells | Caspase | ↑Caspase-3 | [115] | |
| Liver cancer | ||||||
| Adenosine | ↑Apoptosis | HepG2 cells | Caspase | ↑TNF ↑TRADD ↑TRAIL-R2 ↑FADD ↑Caspase-9, -8, -3 | [8] | |
| Adenosine | ↑Apoptosis | BEL-7404 cells | Caspase | ↑Caspase-8, -9, -3 ↑c-PARP ↑Bak ↑Mcl1 ↑Bcl-xl | [6] | |
| Adenosine | ↑Apoptosis | HuH-7 Fas-deficient cells | Caspase | ↑AMP ↓Caspase-3, -8 ↓c-FLIP | [7] | |
| CMF (C. militaris extract) | ↓Migration and invasion ↓Tumor growth | SMMC-7721 cells | Akt ERK | ↓p-VEGFR2 ↓p-Akt ↓p-ERK | [116] | |
| Lung cancer | ||||||
| AECS1, AECS2 (C. sinensis nucleosides extract) | ↓Tumor growth | Lewis xenograft mouse | Akt NF-κB | ↓p-Akt ↓MMP2 ↓MMP9 ↓p-IκBα ↓TNF-α ↓COX-2 ↓Bcl2 ↓Bcl-xl ↑Bax | [117] | |
| CS (C. sinensis extract) | ↑Apoptosis | H157 NSCLC cells | ↓VEGF ↓bFGF | [118] | ||
| C. militaris extract | ↑Apoptosis ↓Cell cycle | NCI-H460 cells | ↑P53 ↑P21 ↑53BP1 | [119] | ||
| Mouse melanoma | ||||||
| Cordycepin | ↓Proliferation | B16-BL6 cells | A3R | ↑GSK-3β ↓Cyclin D1 protein | [28] | |
| CME-1 | ↓Tumor migration | B16-F10 cells | NF-κB MAPK (ERK and p38) | ↓MMP-1 | [49] | |
| EPSP | ↓Tumor migration | B16 cells | ↓c-Myc ↓c-Fos ↓VEGF | [120] | ||
| Myeloma cancer | ||||||
| Cordycepin | ↑Apoptosis | MM.1S cells | Caspase | ↑Caspase-9, -3, -8 ↓RNA synthesis | [8] | |
| Oral cancer | ||||||
| CMP (C. militaris polysaccharides) | ↑Apoptosis ↓Cell cycle | 4NAOC-1 cells | STAT3 ERK | ↓ki-67 ↓EGFR ↓IL-17A ↓Cyclin B1 ↓DNA synthesis | [121] | |
| WECM (C. militaris extract) | Apoptosis ↓Cell cycle | SCC-4 cells | ↓PCNA ↓VEGF ↓Caspase-3 ↓c-fos | [122] | ||
| Ovarian cancer | ||||||
| CME (C. militaris extract) | ↑Apoptosis ↓Migration | SKOV-3 cells | NF-κB | ↓TNF-1R ↓Bcl-2 ↑Bcl-xl | [123] | |
| ↑Autophagy ↓Tumor growth | A2780 and OVCAR3 cells | ENT1-AMPK-mTOR | ↑LC3II/LC3I ↑p-AMPK | [124] | ||
| Prostate cancer | ||||||
| Cordycepin | ↓Migration and invasion | LNCaP cells | PI3K/Akt | ↑TIMP-1 ↑TIMP-2 ↓MMP-2 ↓MMP-9 | [21] | |
| Testicular cancer | ||||||
| Cordycepin | ↑Apoptosis ↓Cell cycle | MA-10 cells | Caspase | ↑Caspase-9, -3, -7 ↑DNA fragmentation | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Guo, Z.-J.; Zhou, X.-W. Chinese Cordyceps: Bioactive Components, Antitumor Effects and Underlying Mechanism—A Review. Molecules 2022, 27, 6576. https://doi.org/10.3390/molecules27196576
Liu Y, Guo Z-J, Zhou X-W. Chinese Cordyceps: Bioactive Components, Antitumor Effects and Underlying Mechanism—A Review. Molecules. 2022; 27(19):6576. https://doi.org/10.3390/molecules27196576
Chicago/Turabian StyleLiu, Yan, Zhi-Jian Guo, and Xuan-Wei Zhou. 2022. "Chinese Cordyceps: Bioactive Components, Antitumor Effects and Underlying Mechanism—A Review" Molecules 27, no. 19: 6576. https://doi.org/10.3390/molecules27196576
APA StyleLiu, Y., Guo, Z.-J., & Zhou, X.-W. (2022). Chinese Cordyceps: Bioactive Components, Antitumor Effects and Underlying Mechanism—A Review. Molecules, 27(19), 6576. https://doi.org/10.3390/molecules27196576







