Chemistry and the Potential Antiviral, Anticancer, and Anti-Inflammatory Activities of Cardiotonic Steroids Derived from Toads †
Abstract
1. Introduction
1.1. Synthesis and Factors Affecting Poison Composition
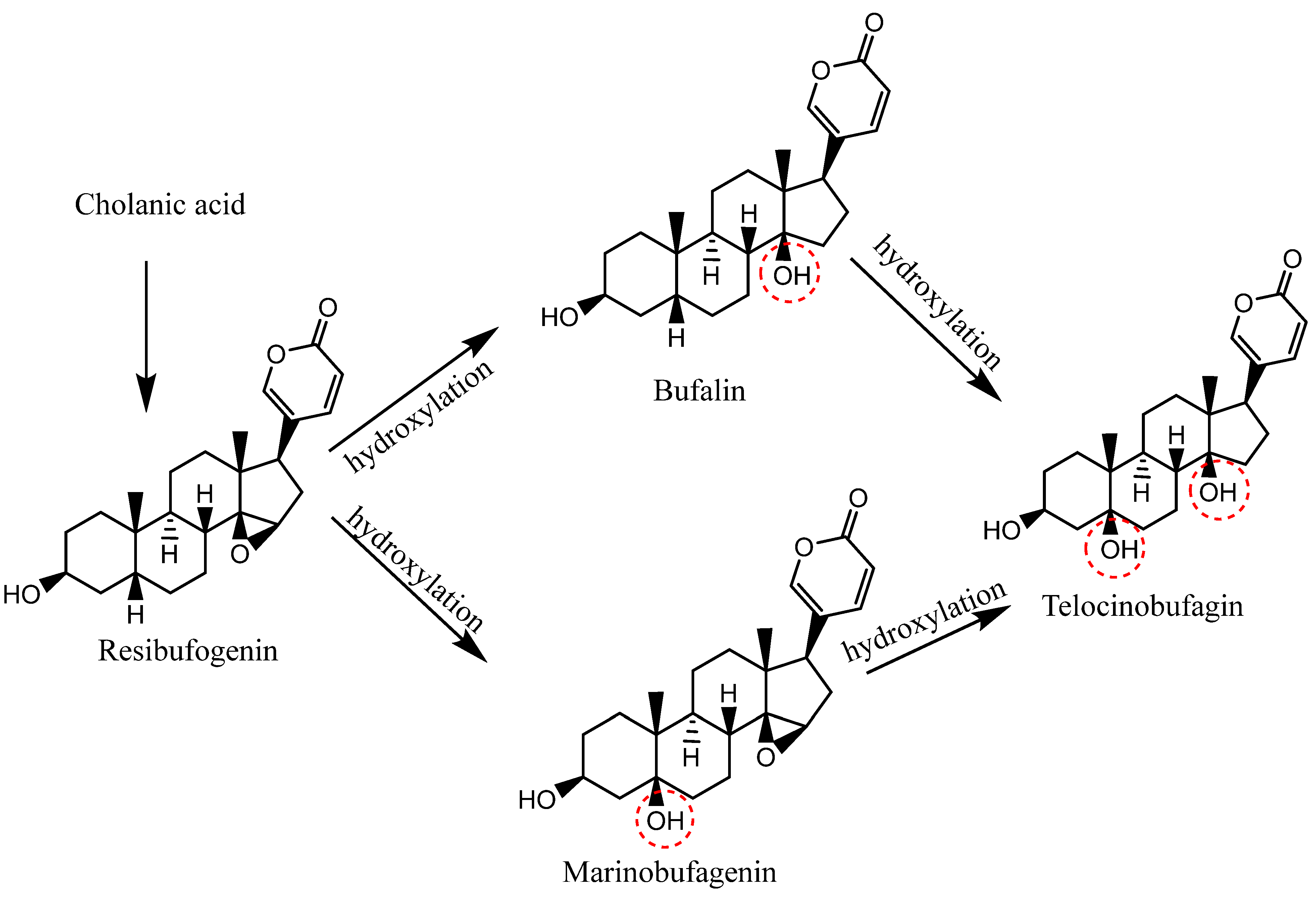
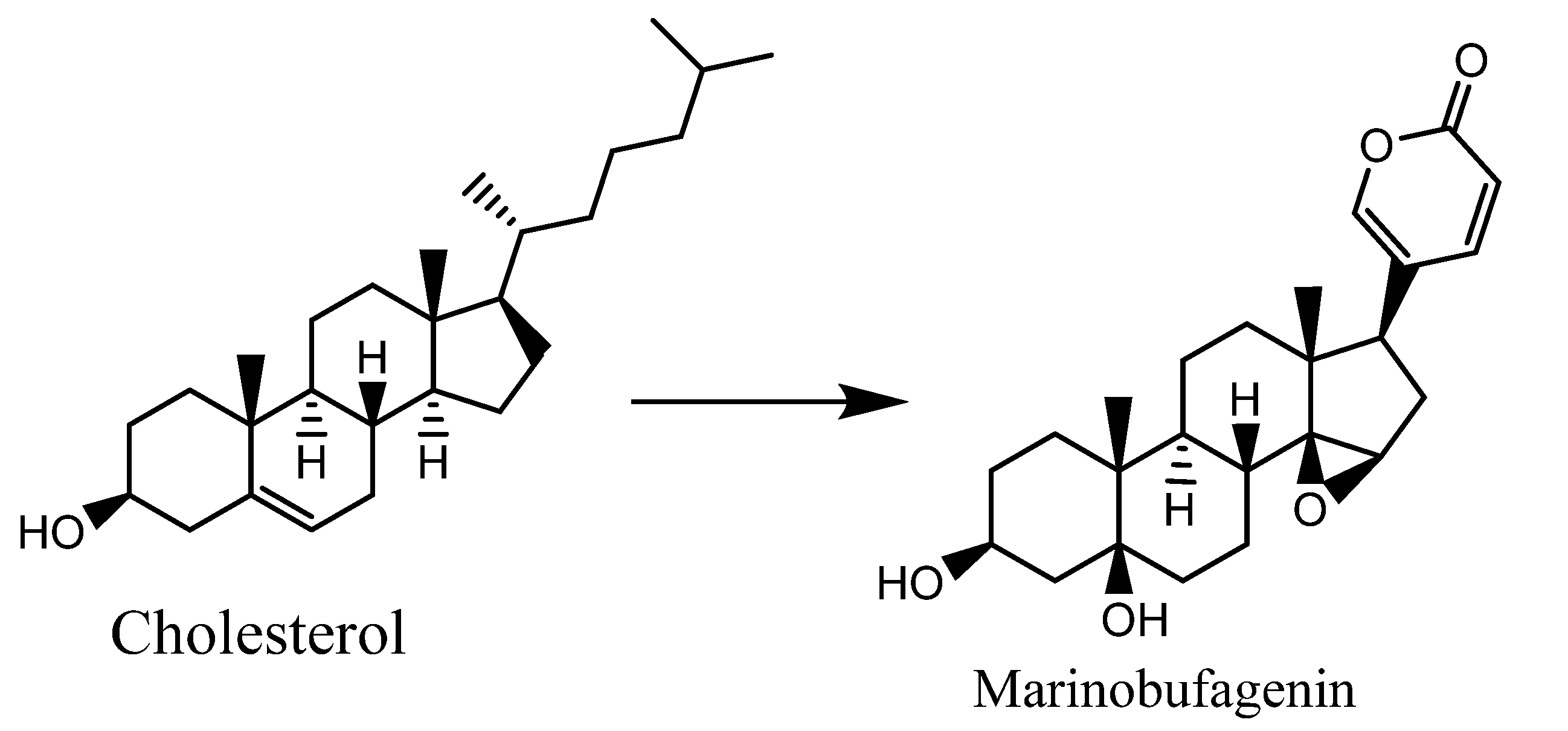
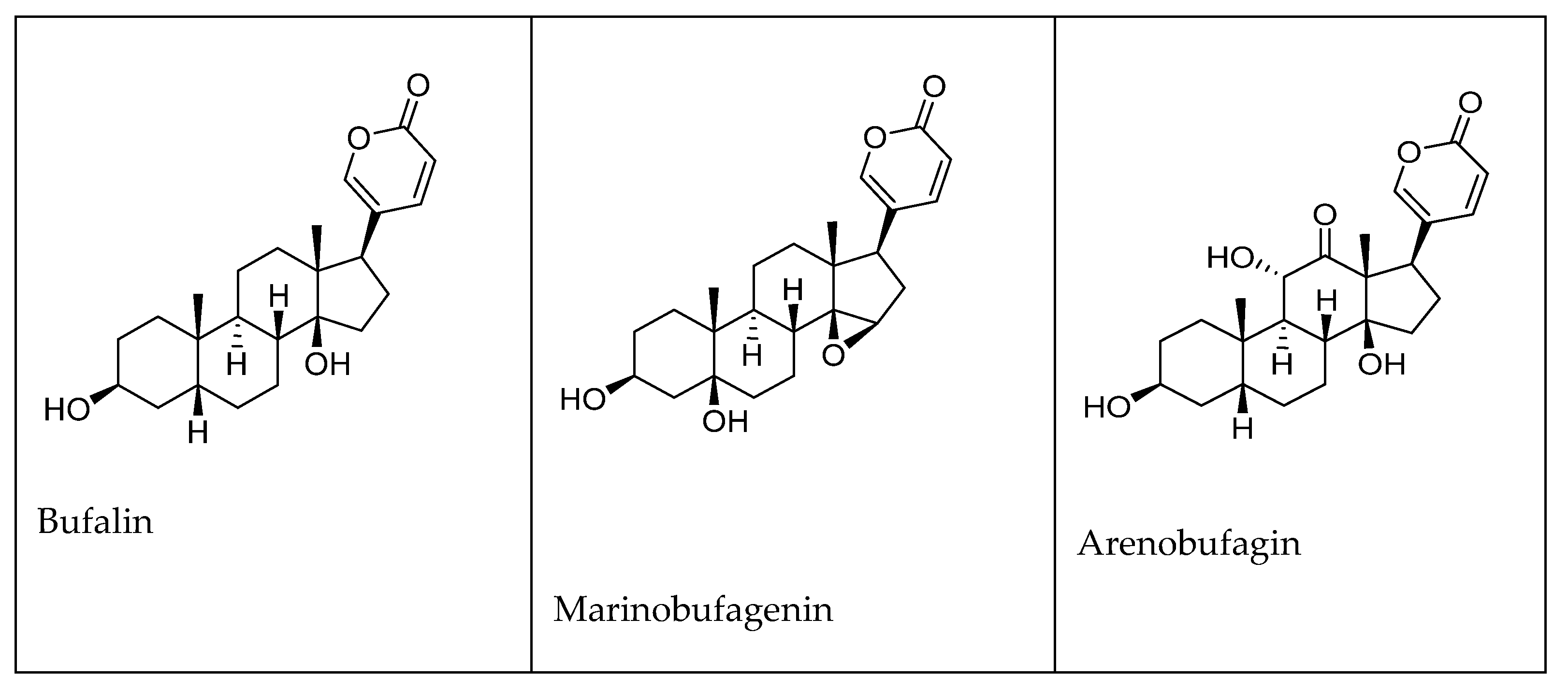
1.2. Biosynthesis of CTS of Toad Origin (Bufadienolides)
1.3. Structure and Morphology of Glands Secreting Venom in Toad
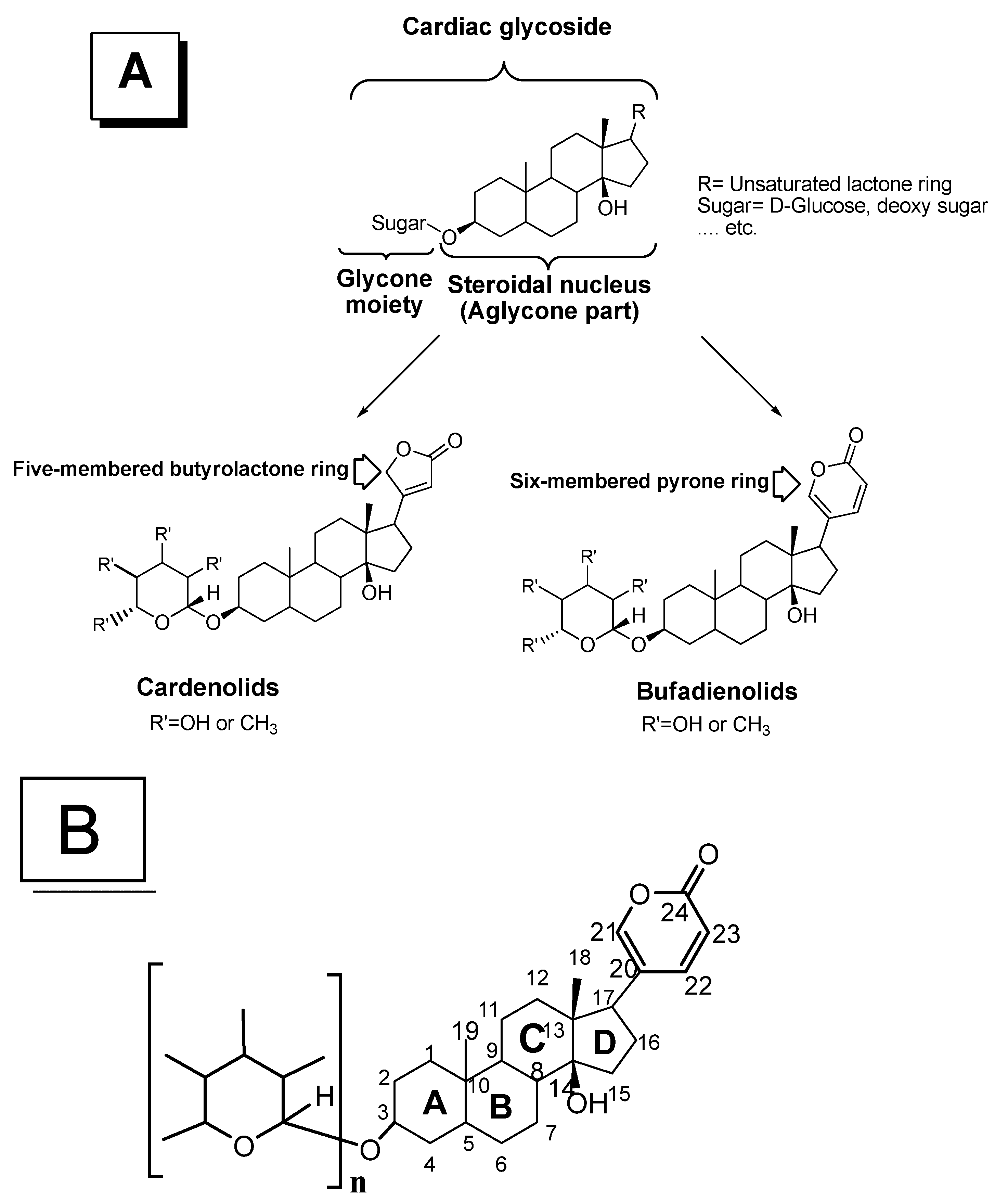
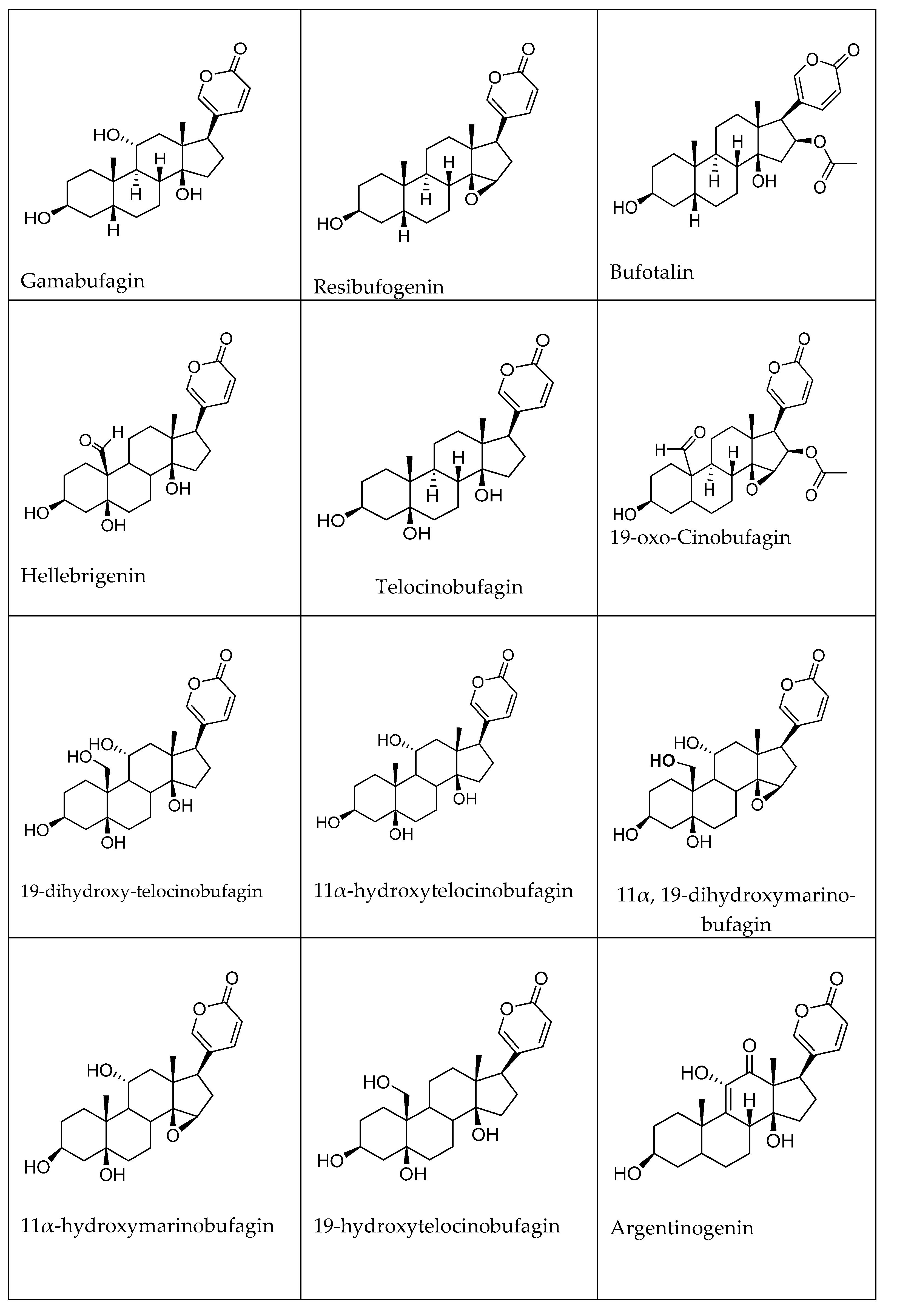
2. Chemistry of Cardiotonic Steroids (CTS)
3. Antiviral Activity
4. Anticancer Activity
5. Anti-Inflammatory Activity
6. CTS with Antiviral, Anticancer, and Anti-Inflammatory Properties
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
References
- El-Mallakh, R.S.; Brar, K.S.; Yeruva, R.R. Cardiac glycosides in human physiology and disease: Update for entomologists. Insects 2019, 10, 102. [Google Scholar] [CrossRef]
- El-Bakry, A.A.; Hammad, I.A.; Galal, T.M.; Ghazi, S.M.; Rafat, F.A. Polymorphism in Calotropis procera: Variation of metabolites in populations from different phytogeographical regions of Egypt. Rend. Lincei 2014, 25, 461–469. [Google Scholar] [CrossRef]
- Mijatovic, T.; Van Quaquebeke, E.; Delest, B.; Debeir, O.; Darro, F.; Kiss, R. Cardiotonic steroids on the road to anti-cancer therapy. Biochim. Biophys. Acta Rev. Cancer 2007, 1776, 32–57. [Google Scholar] [CrossRef]
- Heasley, B. Chemical Synthesis of the Cardiotonic Steroid Glycosides and Related Natural Products. Chem.-A Eur. J. 2012, 18, 3092–3120. [Google Scholar] [CrossRef] [PubMed]
- Bagrov, A.Y.; Shapiro, J.I.; Fedorova, O.V. Endogenous cardiotonic steroids: Physiology, pharmacology, and novel therapeutic targets. Pharmacol. Rev. 2009, 61, 9–38. [Google Scholar] [CrossRef] [PubMed]
- Dvela, M.; Rosen, H.; Feldmann, T.; Nesher, M.; Lichtstein, D. Diverse biological responses to different cardiotonic steroids. Pathophysiology 2007, 14, 159–166. [Google Scholar] [CrossRef]
- Kolmakova, E.V.; Haller, S.T.; Kennedy, D.J.; Isachkina, A.N.; Budny, G.V.; Frolova, E.V.; Piecha, G.; Nikitina, E.R.; Malhotra, D.; Fedorova, O.V.; et al. Endogenous cardiotonic steroids in chronic renal failure. Nephrol. Dial. Transplant. 2011, 26, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, D. The Role of Cardiotonic Steroids in the Pathogenesis of Cardiomyopathy in Chronic Kidney Disease. Nephron Clin. Pract. 2014, 128, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Zhakeer, Z.; Hadeer, M.; Tuerxun, Z.; Tuerxun, K. Bufalin Inhibits the Inflammatory Effects in Asthmatic Mice through the Suppression of Nuclear Factor-Kappa B Activity. Pharmacology 2017, 99, 179–187. [Google Scholar] [CrossRef]
- Abd El-wahed, A.; Yosri, N.; Sakr, H.H.; Du, M.; Algethami, A.F.M.; Zhao, C.; Abdelazeem, A.H.; Tahir, H.E.; Masry, S.H.D.; Abdel-daim, M.M.; et al. A Abd El-Wahed. Toxins 2021, 13, 206. [Google Scholar] [CrossRef]
- Mortari, M.R.; Cunha, A.O.S.; Ferreira, L.B.; dos Santos, W.F. Neurotoxins from invertebrates as anticonvulsants: From basic research to therapeutic application. Pharmacol. Ther. 2007, 114, 171–183. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.; El-Wahed, A.; Yosri, N.; Musharraf, S.G.; Chen, L.; Moustafa, M.; Zou, X.; Al-Mousawi, S.; Guo, Z.; Khatib, A. Antimicrobial properties of Apis mellifera’s bee venom. Toxins 2020, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Toledo, R.C.; Jared, C. Cutaneous granular glands and amphibian venoms. Comp. Biochem. Physiol.-Part A Physiol. 1995, 111, 1–29. [Google Scholar] [CrossRef]
- Van Bocxlaer, I.; Loader, S.P.; Roelants, K.; Biju, S.D.; Menegon, M.; Bossuyt, F. Gradual adaptation toward a range-expansion phenotype initiated the global radiation of toads. Science 2010, 327, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.T. The natural history of amphibian skin secretions, their normal functioning and potential medical applications. Biol. Rev. Camb. Philos. Soc. 1997, 72, 365–379. [Google Scholar] [CrossRef]
- Soumoy, L.; Welles, M.; Najem, A.; Krayem, M.; Ghanem, G.; Hambye, S.; Saussez, S.; Blankert, B.; Journe, F. Toad Venom Antiproliferative Activities on Metastatic Melanoma: Bio-Guided Fractionation and Screening of the Compounds of Two Different Venoms. Biology 2020, 9, 218. [Google Scholar] [CrossRef]
- Tang, H.-J.; Ruan, L.-J.; Tian, H.-Y.; Liang, G.-P.; Ye, W.-C.; Hughes, E.; Esmann, M.; Fedosova, N.U.; Chung, T.-Y.; Tzen, J.T.C. Novel stereoselective bufadienolides reveal new insights into the requirements for Na+, K+-ATPase inhibition by cardiotonic steroids. Sci. Rep. 2016, 6, 29155. [Google Scholar] [CrossRef]
- Baldo, E.C.F.; Anjolette, F.A.P.; Arantes, E.C.; Baldo, M.A. Toad poison and drug discovery. Toxicon 2015, 16, 1–22. [Google Scholar]
- Perry, D. Proteins of parotoid gland secretions from toads of the genus Bufo. Contemp. Herpetol. 2000, 3, 1–7. [Google Scholar] [CrossRef]
- Jared, C.; Antoniazzi, M.M.; Jordão, A.E.C.; Silva, J.R.M.C.; Greven, H.; Rodrigues, M.T. Parotoid macroglands in toad (Rhinella jimi): Their structure and functioning in passive defence. Toxicon 2009, 54, 197–207. [Google Scholar] [CrossRef]
- Gopalakrishnakone, P.; Cruz, L.J.; Luo, S. Toxins and Drug Discovery; Gopalakrishnakone, P., Cruz, L.J., Luo, S., Eds.; Springer: Amsterdam, The Netherlands, 2015. [Google Scholar]
- de Sousa, L.Q.; da Conceição Machado, K.; de Carvalho Oliveira, S.F.; da Silva Araújo, L.; dos Santos Monção-Filho, E.; Amélia, A.; Vieira-Júnior, G.M.; Ferreira, P.M.P. Bufadienolides from amphibians: A promising source of anticancer prototypes for radical innovation, apoptosis triggering and Na+/K+-ATPase inhibition. Toxicon 2017, 127, 63–76. [Google Scholar] [CrossRef]
- Amarelle, L.; Lecuona, E. The Antiviral Effects of Na,K-ATPase Inhibition: A Minireview. Int. J. Mol. Sci. 2018, 19, 2154. [Google Scholar] [CrossRef] [PubMed]
- Orellana, A.M.; Kinoshita, P.F.; Leite, J.A.; Kawamoto, E.M.; Scavone, C. Cardiotonic steroids as modulators of neuroinflammation. Front. Endocrinol. 2016, 7, 10. [Google Scholar] [CrossRef]
- Hayes, R.A.; Piggott, A.M.; Dalle, K.; Capon, R.J. Microbial biotransformation as a source of chemical diversity in cane toad steroid toxins. Bioorganic Med. Chem. Lett. 2009, 19, 1790–1792. [Google Scholar] [CrossRef] [PubMed]
- Üveges, B.; Fera, G.; Móricz, Á.M.; Krüzselyi, D.; Bókony, V.; Hettyey, A. Age- and environment-dependent changes in chemical defences of larval and post-metamorphic toads. BMC Evol. Biol. 2017, 17, 137. [Google Scholar] [CrossRef] [PubMed]
- Bókony, V.; Üveges, B.; Móricz, Á.M.; Hettyey, A. Competition induces increased toxin production in toad larvae without allelopathic effects on heterospecific tadpoles. Funct. Ecol. 2018, 32, 667–675. [Google Scholar] [CrossRef]
- Bókony, V.; Móricz, Á.M.; Tóth, Z.; Gál, Z.; Kurali, A.; Mikó, Z.; Pásztor, K.; Szederkényi, M.; Tóth, Z.; Ujszegi, J.; et al. Variation in Chemical Defense Among Natural Populations of Common Toad, Bufo bufo, Tadpoles: The Role of Environmental Factors. J. Chem. Ecol. 2016, 42, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Chiadao, C.; Osuch, M. V Biosynthesis of bufadienolides—3β-hydroxycholanates as precursors in Bufo marinus bufadienolides synthesis. Biochem. Pharmacol. 1969, 18, 1797–1802. [Google Scholar] [CrossRef]
- Siperstein, M.D.; Murray, A.W.; Titus, E. Biosynthesis of cardiotonic sterols from cholesterol in the toad, Bufo marinus. Arch. Biochem. Biophys. 1957, 67, 154–160. [Google Scholar] [CrossRef]
- Porto, A.M.; Baralle, F.E.; Gros, E.G. Biosynthesis of bufadienolides in toads: III—Experiments with [2-14C] mevalonic acid, [20-14C] 3β-hydroxy-5-pregnen-20-one and [20-14C] cholesterol. J. Steroid Biochem. 1972, 3, 11–17. [Google Scholar] [CrossRef]
- Delfino, G.; Nosi, D.; Giachi, F. Secretory granule-cytoplasm relationships in serous glands of anurans: Ultrastructural evidence and possible functional role. Toxicon 2001, 39, 1161–1171. [Google Scholar] [CrossRef]
- Duellman, W.; Trueb, L. Biology of amphibians; Johns Hopkins University Press: Baltimore, MD, USA, 1994; ISBN 9780801847806. [Google Scholar]
- Mailho-Fontana, P.L.; Antoniazzi, M.M.; Toledo, L.F.; Verdade, V.K.; Sciani, J.M.; Barbaro, K.C.; Pimenta, D.C.; Rodrigues, M.T.; Jared, C. Passive and active defense in toads: The parotoid macroglands in Rhinella marina and Rhaebo guttatus. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2014, 321, 65–77. [Google Scholar] [CrossRef]
- Jin, L.; Quan, C.; Hou, X.; Fan, S. Potential pharmacological resources: Natural bioactive compounds from marine-derived fungi. Mar. Drugs 2016, 14, 76. [Google Scholar] [CrossRef]
- Antoniazzi, M.M.; Neves, P.R.; Mailho-Fontana, P.L.; Rodrigues, M.T.; Jared, C. Morphology of the parotoid macroglands in Phyllomedusa leaf frogs. J. Zool. 2013, 291, 42–50. [Google Scholar] [CrossRef]
- Regis-Alves, E.; Jared, S.G.S.; Maurício, B.; Mailho-Fontana, P.L.; Antoniazzi, M.M.; Fleury-Curado, M.C.; Brodie, E.D.; Jared, C. Structural cutaneous adaptations for defense in toad (Rhinella icterica) parotoid macroglands. Toxicon 2017, 137, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Brodie, E.D.; Smatresk, N.J. The antipredator arsenal of fire salamanders: Spraying of secretions from highly pressurized dorsal skin glands. Herpetologica 1990, 46, 1–7. [Google Scholar]
- Mailho-Fontana, P.L.; Antoniazzi, M.M.; Rodrigues, I.; Sciani, J.M.; Pimenta, D.C.; Brodie, E.D.; Rodrigues, M.T.; Jared, C. Parotoid, radial, and tibial macroglands of the frog Odontophrynus cultripes: Differences and similarities with toads. Toxicon 2017, 129, 123–133. [Google Scholar] [CrossRef]
- Tempone, A.G.; Pimenta, D.C.; Lebrun, I.; Sartorelli, P.; Taniwaki, N.N.; de Andrade, H.F.; Antoniazzi, M.M.; Jared, C. Antileishmanial and antitrypanosomal activity of bufadienolides isolated from the toad Rhinella jimi parotoid macrogland secretion. Toxicon 2008, 52, 13–21. [Google Scholar] [CrossRef]
- Gustavo Tempone, A.; de Souza Carvalho Melhem, M.; Oliveira Prado, F.; Motoie, G.; Mitsuyoshi Hiramoto, R.; Maria Antoniazzi, M.; Fernando Baptista Haddad, C.; Jared, C. Amphibian Secretions for Drug Discovery Studies: A Search for New Antiparasitic and Antifungal Compounds. Lett. Drug Des. Discov. 2006, 4, 67–73. [Google Scholar] [CrossRef]
- Dmitrieva, R.I.; Doris, P.A. Cardiotonic steroids: Potential endogenous sodium pump ligands with diverse function. Exp. Biol. Med. 2002, 227, 561–569. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Stonik, V.A. Steroid glycosides from marine organisms. Steroids 2011, 76, 425–454. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Saleem, M.; Yamdagni, R.; Ali, M.A. Steroid and antibacterial steroidal glycosides from marine green alga Codium iyengarii borgesen. Nat. Prod. Lett. 2002, 16, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Saikia, S.; Kolita, B.; Dutta, P.P.; Dutta, D.J.; Neipihoi; Nath, S.; Bordoloi, M.; Quan, P.M.; Thuy, T.T.; Phuong, D.L.; et al. Marine steroids as potential anticancer drug candidates: In silico investigation in search of inhibitors of Bcl-2 and CDK-4/Cyclin D1. Steroids 2015, 102, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Dias, A.C.; Couzinet-Mossion, A.; Ruiz, N.; Lakhdar, F.; Etahiri, S.; Bertrand, S.; Ory, L.; Roussakis, C.; Pouchus, Y.F.; Nazih, E.H.; et al. Steroids from marine-derived fungi: Evaluation of antiproliferative and antimicrobial activities of eburicol. Mar. Drugs 2019, 17, 372. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Khalifa, S.A.M.; Taher, E.A.; Farag, M.A.; Saeed, A.; Gamal, M.; Hegazy, M.E.F.; Youssef, D.; Musharraf, S.G.; Alajlani, M.M.; et al. Cardenolides: Insights from chemical structure and pharmacological utility. Pharmacol. Res. 2019, 141, 123–175. [Google Scholar] [CrossRef]
- Melero, C.P.; Medarde, M.; San Feliciano, A. A short review on cardiotonic steroids and their aminoguanidine analogues. Molecules 2000, 5, 51–81. [Google Scholar] [CrossRef]
- Boff, L.; Schreiber, A.; da Rocha Matos, A.; Del Sarto, J.; Brunotte, L.; Munkert, J.; Ottoni, F.M.; Ramos, G.S.; Kreis, W.; Braga, F.C.; et al. Semisynthetic cardenolides acting as antiviral inhibitors of influenza A virus replication by preventing polymerase complex formation. Molecules 2020, 25, 4853. [Google Scholar] [CrossRef]
- Offermanns, S.; Rosenthal, W. Encyclopedia of Molecular Pharmacology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-38916-3. [Google Scholar]
- Michalak, M.; Michalak, K.; Wicha, J. The synthesis of cardenolide and bufadienolide aglycones, and related steroids bearing a heterocyclic subunit. Nat. Prod. Rep. 2017, 34, 361–410. [Google Scholar] [CrossRef]
- Cornelius, F.; Kanai, R.; Toyoshima, C. A structural view on the functional importance of the sugar moiety and steroid hydroxyls of cardiotonic steroids in binding to Na,K-ATPase. J. Biol. Chem. 2013, 288, 6602–6616. [Google Scholar] [CrossRef]
- Morsy, N. Cardiac glycosides in medicinal plants, Aromatic and Medicinal Plants—Back to nature.; El-Shemy, H., Ed.; BoD—Books on Demand: London, UK, 2017; ISBN 9535129775/9789535129776. [Google Scholar]
- Dondoni, A.; Marra, A. Methods for Anomeric Carbon-Linked and Fused Sugar Amino Acid Synthesis: The Gateway to Artificial Glycopeptides. Chem. Rev. 2000, 100, 4395–4422. [Google Scholar] [CrossRef]
- Křen, V. Glycoside vs. Aglycon: The Role of Glycosidic Residue in Biological Activity. In Glycoscience; Springer: Berlin, Heidelberg, 2008; pp. 2589–2644. [Google Scholar]
- Gupta, S.P. Quantitative structure-activity relationships of cardiotonic agents. Prog. Drug Res. 2000, 55, 235–282. [Google Scholar] [PubMed]
- Yang, Q.; Zhou, X.; Zhang, M.; Bi, L.; Miao, S.; Cao, W.; Xie, Y.; Sun, J.; Tang, H.; Li, Y.; et al. Angel of human health: Current research updates in toad medicine. Am. J. Transl. Res. 2015, 7, 1–14. [Google Scholar] [PubMed]
- Klupczynska, A.; Pawlak, M.; Kokot, Z.J.; Matysiak, J. Application of metabolomic tools for studying low molecular-weight fraction of animal venoms and poisons. Toxins 2018, 10, 306. [Google Scholar] [CrossRef]
- Kamano, Y.; Kotake, A.; Hashima, H.; Inoue, M.; Morita, H.; Takeya, K.; Itokawa, H.; Nandachi, N.; Segawa, T.; Yukita, A.; et al. Structure–cytotoxic activity relationship for the toad poison bufadienolides. Bioorg. Med. Chem. 1998, 6, 1103–1115. [Google Scholar] [CrossRef]
- Ye, M.; Qu, G.; Guo, H.; Guo, D. Specific 12β-hydroxylation of cinobufagin by filamentous fungi. Appl. Environ. Microbiol. 2004, 70, 3521–3527. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, X.; Guo, H.; Sun, L.; Wang, A.; Liu, Q.; Wang, X.; Li, J. Bufalin exerts antitumor effects by inducing cell cycle arrest and triggering apoptosis in pancreatic cancer cells. Tumor Biol. 2014, 35, 2461–2471. [Google Scholar] [CrossRef]
- Qian, L.; Su, H.; Wang, G.; Li, B.; Shen, G.; Gao, Q. Anti-tumor activity of bufalin by inhibiting c-MET mediated MEK/ERK and PI3K/AKT signaling pathways in gallbladder cancer. J. Cancer 2020, 11, 3114–3123. [Google Scholar] [CrossRef]
- Su, S.; Dou, H.; Wang, Z.; Zhang, Q. Bufalin inhibits ovarian carcinoma via targeting mTOR/HIF-α pathway. Basic Clin. Pharmacol. Toxicol. 2021, 128, 224–233. [Google Scholar] [CrossRef]
- Qi, J.; Zulfiker, A.H.M.; Li, C.; Good, D.; Wei, M.Q. The development of toad toxins as potential therapeutic agents. Toxins 2018, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.; Williams, J.D.; Opperman, T.J.; Sanchez, R.; Lurain, N.S.; Tortorella, D. Convallatoxin-Induced Reduction of Methionine Import Effectively Inhibits Human Cytomegalovirus Infection and Replication. J. Virol. 2016, 90, 10715–10727. [Google Scholar] [CrossRef]
- Burkard, C.; Verheije, M.H.; Haagmans, B.L.; van Kuppeveld, F.J.; Rottier, P.J.M.; Bosch, B.-J.; de Haan, C.A.M. ATP1A1-Mediated Src Signaling Inhibits Coronavirus Entry into Host Cells. J. Virol. 2015, 89, 4434–4448. [Google Scholar] [CrossRef] [PubMed]
- Laird, G.M.; Eisele, E.E.; Rabi, S.A.; Nikolaeva, D.; Siliciano, R.F. A novel cell-based high-throughput screen for inhibitors of HIV-1 gene expression and budding identifies the cardiac glycosides. J. Antimicrob. Chemother. 2014, 69, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Dodson, A.W.; Taylor, T.J.; Knipe, D.M.; Coen, D.M. Inhibitors of the sodium potassium ATPase that impair herpes simplex virus replication identified via a chemical screening approach. Virology 2007, 366, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.R.; Zhang, Y.N.; Li, X.D.; Zhang, H.Q.; Xiao, S.Q.; Deng, F.; Yuan, Z.M.; Ye, H.Q.; Zhang, B. A cell-based large-scale screening of natural compounds for inhibitors of SARS-CoV-2. Signal Transduct. Target. Ther. 2020, 5, 218. [Google Scholar] [CrossRef]
- Jin, Y.-H.; Jeon, S.; Lee, J.; Kim, S.; Jang, M.S.; Park, C.M.; Song, J.H.; Kim, H.R.; Kwon, S. Broad Spectrum Antiviral Properties of Cardiotonic Steroids Used as Potential Therapeutics for Emerging Coronavirus Infections. Pharmaceutics 2021, 13, 1839. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Inagaki, Y.; Xu, H.; Wang, D.; Qi, F.; Kokudo, N.; Fang, D.; Tang, W. Anti-hepatitis B virus activities of cinobufacini and its active components bufalin and cinobufagin in HepG2.2.15 Cells. Biol. Pharm. Bull. 2010, 33, 1728–1732. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Sun, S.; Zhang, H.; Ma, T.; Su, W.; Jiang, C. Identification of cinobufagin and resibufogenin as inhibitors of enterovirus 71 infection. Chem. Res. Chinese Univ. 2014, 30, 953–958. [Google Scholar] [CrossRef]
- Wong, R.W.; Lingwood, C.A.; Ostrowski, M.A.; Cabral, T.; Cochrane, A. Cardiac glycoside/aglycones inhibit HIV-1 gene expression by a mechanism requiring MEK1/2-ERK1/2 signaling. Sci. Rep. 2018, 8, 850. [Google Scholar] [CrossRef]
- Horisberger, J.D. Recent insights into the structure and mechanism of the sodium pump. Physiology 2004, 19, 377–387. [Google Scholar] [CrossRef]
- Rahimtoola, S.H.; Tak, T. The use of digitalis in heart failure. Curr. Probl. Cardiol. 1996, 21, 781–853. [Google Scholar] [CrossRef]
- Weidemann, H. NA/K-ATPase, endogenous digitalis-like compounds and cancer development—A hypothesis. Front. Biosci. 2005, 10, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Gonta-Grabiec, K.; Rossowski, W.; Szumiel, I. Properties of Na+/K+ ATPase and alkaline phosphatase alter during spontaneous and radiation-induced leukemogenesis in mice. Neoplasma 1986, 33, 141–155. [Google Scholar] [PubMed]
- Kaplan, J.G. Membrane cation transport and the control of proliferation of mammalian cells. Annu. Rev. Physiol. 1978, 40, 19–41. [Google Scholar] [CrossRef]
- Liu, S.H.; Lin, C.H.; Hung, S.K.; Chou, J.H.; Chi, C.W.; Fu, S.L. Fisetin inhibits lipopolysaccharide-induced macrophage activation and dendritic cell maturation. J. Agric. Food Chem. 2010, 58, 10831–10839. [Google Scholar] [CrossRef]
- Shen, S.S.; Hamamoto, S.T.; Bern, H.A.; Steinhardt, R.A. Alteration of Sodium Transport in Mouse Mammary Epithelium Associated with Neoplastic Transformation. Cancer Res. 1978, 38, 1356–1361. [Google Scholar] [PubMed]
- Huang, L.; Li, H.; Xie, Z. Ouabain-induced hypertrophy in cultured cardiac myocytes is accompanied by changes in expression of several late response genes. J. Mol. Cell. Cardiol. 1997, 29, 429–437. [Google Scholar] [CrossRef]
- Davies, R.J.; Sandle, G.I.; Thompson, S.M. Inhibition of the Na+,K(+)-ATPase pump during induction of experimental colon cancer. Cancer Biochem. Biophys. 1991, 12, 81–94. [Google Scholar]
- Kometiani, P.; Li, J.; Gnudi, L.; Kahn, B.B.; Askari, A.; Xie, Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth- related genes in cardiac myocytes: The roles of Ras and mitogen-activated protein kinases. J. Biol. Chem. 1998, 273, 15249–15256. [Google Scholar] [CrossRef]
- Xie, Z.; Kometiani, P.; Liu, J.; Li, J.; Shapiro, J.I.; Askari, A. Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J. Biol. Chem. 1999, 274, 19323–19328. [Google Scholar] [CrossRef]
- Haas, M.; Wang, H.; Tian, J.; Xie, Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J. Biol. Chem. 2002, 277, 18694–18702. [Google Scholar] [CrossRef]
- Cerella, C.; Dicato, M.; Diederich, M. Assembling the puzzle of anti-cancer mechanisms triggered by cardiac glycosides. Mitochondrion 2013, 13, 225–234. [Google Scholar] [CrossRef]
- Menger, L.; Vacchelli, E.; Kepp, O.; Eggermont, A.; Tartour, E.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch: Cardiac glycosides and cancer therapy. Oncoimmunology 2013, 2, e23082. [Google Scholar] [CrossRef]
- Haas, M.; Askari, A.; Xie, Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J. Biol. Chem. 2000, 275, 27832–27837. [Google Scholar] [CrossRef] [PubMed]
- Mekhail, T.; Kaur, H.; Ganapathi, R.; Budd, G.T.; Elson, P.; Bukowski, R.M. Phase 1 trial of AnvirzelTM in patients with refractory solid tumors. Invest. New Drugs 2006, 24, 423–427. [Google Scholar] [CrossRef]
- Feng, B.; Guo, Y.W.; Huang, C.G.; Li, L.; Chen, R.H.; Jiao, B.H. 2′-epi-2′-O-Acetylthevetin B extracted from seeds of Cerbera manghas L. induces cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Chem. Biol. Interact. 2010, 183, 142–153. [Google Scholar] [CrossRef]
- Varbanov, H.P.; Kuttler, F.; Banfi, D.; Turcatti, G.; Dyson, P.J. Repositioning approved drugs for the treatment of problematic cancers using a screening approach. PLoS ONE 2017, 12, e0171052. [Google Scholar] [CrossRef]
- Liang, M.; Tian, J.; Liu, L.; Pierre, S.; Liu, J.; Shapiro, J.; Xie, Z.J. Identification of a pool of non-pumping Na/K-ATPase. J. Biol. Chem. 2007, 282, 10585–10593. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, T.; Roland, I.; Van Quaquebeke, E.; Nilsson, B.; Mathieu, A.; Van Vynckt, F.; Darro, F.; Blanco, G.; Facchini, V.; Kiss, R. The α1 subunit of the sodium pump could represent a novel target to combat non-small cell lung cancers. J. Pathol. 2007, 212, 170–179. [Google Scholar] [CrossRef]
- Mathieu, V.; Pirker, C.; Martin de Lassalle, E.; Vernier, M.; Mijatovic, T.; DeNeve, N.; Gaussin, J.F.; Dehoux, M.; Lefranc, F.; Berger, W.; et al. The sodium pump α1 sub-unit: A disease progression-related target for metastatic melanoma treatment. J. Cell. Mol. Med. 2009, 13, 3960–3972. [Google Scholar] [CrossRef]
- Lefranc, F.; Kiss, R. The sodium pump α1 subunit as a potential target to combat apoptosis-resistant glioblastomas. Neoplasia 2008, 10, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Yim, H.Y.; He, N.; Lee, C.J.; Kim, J.H.; Choi, J.S.; Lee, H.S.; Kim, S.; Jeong, E.; Song, M.; et al. Cardiac glycosides display selective efficacy for STK11 mutant lung cancer. Sci. Rep. 2016, 6, 29721. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, X.; Yang, J.; Sun, X.; Hu, C.; Yan, Z.; Xu, X.; Lu, W.; Wang, X.; Cao, P. Chemoprevention of dietary digitoflavone on colitis-associated colon tumorigenesis through inducing Nrf2 signaling pathway and inhibition of inflammation. Mol. Cancer 2014, 13, 48. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, Q.; Shen, J.J.; Li, D.D.; Jiang, X.J.; Si, S.Y.; Shao, R.G.; Wang, Z. Cardiac glycosides induce autophagy in human non-small cell lung cancer cells through regulation of dual signaling pathways. Int. J. Biochem. Cell Biol. 2012, 44, 1813–1824. [Google Scholar] [CrossRef]
- Juncker, T.; Schumacher, M.; Dicato, M.; Diederich, M. UNBS1450 from Calotropis procera as a regulator of signaling pathways involved in proliferation and cell death. Biochem. Pharmacol. 2009, 78, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pongrakhananon, V.; Chunhacha, P.; Chanvorachote, P. Ouabain Suppresses the Migratory Behavior of Lung Cancer Cells. PLoS ONE 2013, 8, e68623. [Google Scholar] [CrossRef] [PubMed]
- Schneider, N.F.Z.; Geller, F.C.; Persich, L.; Marostica, L.L.; Pádua, R.M.; Kreis, W.; Braga, F.C.; Simões, C.M.O. Inhibition of cell proliferation, invasion and migration by the cardenolides digitoxigenin monodigitoxoside and convallatoxin in human lung cancer cell line. Nat. Prod. Res. 2016, 30, 1327–1331. [Google Scholar] [CrossRef]
- Yang, S.Y.; Kim, N.H.; Cho, Y.S.; Lee, H.; Kwon, H.J. Convallatoxin, a dual inducer of autophagy and apoptosis, inhibits angiogenesis in vitro and in vivo. PLoS ONE 2014, 9, e91094. [Google Scholar] [CrossRef]
- Watabe, M.; Masuda, Y.; Nakajo, S.; Yoshida, T.; Kuroiwa, Y.; Nakaya, K. The cooperative interaction of two different signaling pathways in response to bufalin induces apoptosis in human leukemia U937 cells. J. Biol. Chem. 1996, 271, 14067–14073. [Google Scholar] [CrossRef]
- Kurosawa, M.; Numazawa, S.; Tani, Y.; Yoshida, T. ERK signaling mediates the induction of inflammatory cytokines by bufalin in human monocytic cells. Am. J. Physiol.-Cell Physiol. 2000, 278, C500–C508. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Luan, J.; Duan, H.; Zhang, F.; Yagasaki, K.; Zhang, G. Effects of bufalin on the proliferation of human lung cancer cells and its molecular mechanisms of action. Cytotechnology 2010, 62, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Watabe, M.; Ito, K.; Masuda, Y.; Nakajo, S.; Nakaya, K. Activation of AP-1 is required for bufalin-induced apoptosis in human leukemia U937 cells. Oncogene 1998, 16, 779–787. [Google Scholar] [CrossRef]
- Zhang, J.; Sha, J.; Zhou, Y.; Han, K.; Wang, Y.; Su, Y.; Yin, X.; Hu, H.; Yao, Y. Bufalin Inhibits Proliferation and Induces Apoptosis in Osteosarcoma Cells by Downregulating MicroRNA-221. Evid.-Based Complement. Altern. Med. 2016, 2016, 7319464. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Kan, S.F.; Pu, H.F.; Jea Chien, E.; Wang, P.S. Apoptotic signaling in bufalin- and cinobufagin-treated androgen-dependent and -independent human prostate cancer cells. Cancer Sci. 2008, 99, 2467–2476. [Google Scholar] [CrossRef]
- Yamada, K.; Hino, K.I.; Tomoyasu, S.; Honma, Y.; Tsuruoka, N. Enhancement by bufalin of retinoic acid-induced differentiation of acute promyelocytic leukemia cells in primary culture. Leuk. Res. 1998, 22, 589–595. [Google Scholar] [CrossRef]
- Qi, F.; Inagaki, Y.; Gao, B.; Cui, X.; Xu, H.; Kokudo, N.; Li, A.; Tang, W. Bufalin and cinobufagin induce apoptosis of human hepatocellular carcinoma cells via Fas- and mitochondria-mediated pathways. Cancer Sci. 2011, 102, 951–958. [Google Scholar] [CrossRef]
- Chen, X.Y.; Xu, R.C.; Chen, L. Apoptosis of gastric cancer cells induced by bufalin. Basic Med. 2000, 5, 50–54. [Google Scholar]
- Masuda, Y.; Kawazoe, N.; Nakajo, S.; Yoshida, T.; Kuroiwa, Y.; Nakaya, K. Bufalin induces apoptosis and influences the expression of apoptosis-related genes in human leukemia cells. Leuk. Res. 1995, 19, 549–556. [Google Scholar] [CrossRef]
- Watabe, M.; Kawazoe, N.; Masuda, Y.; Nakajo, S.; Nakaya, K. Bcl-2 protein inhibits bufalin-induced apoptosis through inhibition of mitogenactivated protein kinase activation in human leukemia U937 cells. Cancer Res. 1997, 57, 3097–3100. [Google Scholar]
- Han, K.Q.; Huang, G.; Gu, W.; Su, Y.H.; Huang, X.Q.; Ling, C.Q. Anti-tumor activities and apoptosis-regulated mechanisms of bufalin on the orthotopic transplantation tumor model of human hepatocellular carcinoma in nude mice. World J. Gastroenterol. 2007, 13, 3374–3379. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, X.; Zhou, Y.; Wang, Y.; Qian, B.; He, A.; Shen, Z.; Hu, H.; Yao, Y. Bufalin suppresses the migration and invasion of prostate cancer cells through HOTAIR, the sponge of miR-520b. Acta Pharmacol. Sin. 2019, 40, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, X.; Liu, X.; Gong, P. Bufalin inhibits human breast cancer tumorigenesis by inducing cell death through the ROS-mediated RIP1/RIP3/PARP-1 pathways. Carcinogenesis 2018, 39, 700–707. [Google Scholar] [CrossRef]
- Zhen, S.; Hua, L.; Liu, Y.-H.; Wan, D.-Y.; Luo, W.-J. Bufalin attenuates the proliferation of breast cancer MCF-7 cells in vitro and in vivo by inhibiting the PI3K/Akt pathway. Int. J. Clin. Exp. Med. I 2016, 3, 10297–10303. [Google Scholar]
- Nakata, M.; Mori, S.; Kamoshida, Y.; Kawaguchi, S.; Fujita-Yamaguchi, Y.; Gao, B.; Tang, W. Toad skin extract cinobufatini inhibits migration of human breast carcinoma MDA-MB-231 cells into a model stromal tissue. Biosci. Trends 2015, 9, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yin, S.; Li, J.; Jiang, C.; Ye, M.; Hu, H. Bufadienolide compounds sensitize human breast cancer cells to TRAIL-induced apoptosis via inhibition of STAT3/Mcl-1 pathway. Apoptosis 2011, 16, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Feng, L.X.; Sun, P.; Liu, W.; Wu, W.Y.; Jiang, B.H.; Yang, M.; Hu, L.H.; Guo, D.A.; Liu, X. A novel bufalin derivative exhibited stronger apoptosis-inducing effect than bufalin in A549 lung cancer cells and lower acute toxicity in mice. PLoS ONE 2016, 11, e0159789. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, H.; Ma, G.; Wang, Z.; Li, E.; Liu, Y.; Liu, Y. Bufalin induces lung cancer cell apoptosis via the inhibition of PI3K/Akt pathway. Int. J. Mol. Sci. 2012, 13, 2025–2035. [Google Scholar] [CrossRef]
- Pan, Z.; Xie, Y.; Bai, J.; Lin, Q.; Cui, X.; Zhang, N. Bufalin suppresses colorectal cancer cell growth through promoting autophagy: In vivo and in vitro. RSC Adv. 2018, 8, 38910–38918. [Google Scholar] [CrossRef]
- Zhang, N.; Xie, Y.; Tai, Y.; Gao, Y.; Guo, W.; Yu, W.; Li, J.; Feng, X.; Hao, J.; Gao, Y.; et al. Bufalin Inhibits hTERT Expression and Colorectal Cancer Cell Growth by Targeting CPSF4. Cell. Physiol. Biochem. 2016, 40, 1559–1569. [Google Scholar] [CrossRef]
- Yuan, Z.T.; Shi, X.J.; Yuan, Y.X.; Qiu, Y.Y.; Zou, Y.; Liu, C.; Yu, H.; He, X.; Xu, K.; Yin, P.H. Bufalin reverses ABCB1-mediated drug resistance in colorectal cancer. Oncotarget 2017, 8, 48012–48026. [Google Scholar] [CrossRef]
- Qi, H.Y.; Qu, X.J.; Liu, J.; Hou, K.Z.; Fan, Y.B.; Che, X.F.; Liu, Y.P. Bufalin induces protective autophagy by Cbl-b regulating mTOR and ERK signaling pathways in gastric cancer cells. Cell Biol. Int. 2019, 43, 33–43. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, D.; Tan, G.; Liu, Y.; Zhuang, L.; Liu, T. Bufalin promotes apoptosis of gastric cancer by down-regulation of miR-298 targeting bax. Int. J. Clin. Exp. Med. 2015, 8, 3420. [Google Scholar] [PubMed]
- Miao, Q.; Bi, L.L.; Li, X.; Miao, S.; Zhang, J.; Zhang, S.; Yang, Q.; Xie, Y.H.; Zhang, J.; Wang, S.W. Anticancer effects of bufalin on human hepatocellular carcinoma HepG2 Cells: Roles of apoptosis and autophagy. Int. J. Mol. Sci. 2013, 14, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Schoner, W. Endogenous cardiac glycosides, a new class of steroid hormones. Eur. J. Biochem. 2002, 269, 2440–2448. [Google Scholar] [CrossRef]
- Schoner, W.; Scheiner-Bobis, G. Endogenous cardiac glycosides: Hormones using the sodium pump as signal transducer. Semin. Nephrol. 2005, 25, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and exogenous cardiac glycosides: Their roles in hypertension, salt metabolism, and cell growth. Am. J. Physiol.-Cell Physiol. 2007, 293, C509–C536. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.V.; Talan, M.I.; Agalakova, N.I.; Lakatta, E.G.; Bagrov, A.Y. Endogenous ligand of α1 sodium pump, marinobufagenin, is a novel mediator of sodium chloride-dependent hypertension. Circulation 2002, 105, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Allen, S.R.; Jones, R.O.; Zawieja, D.C.; Kuehl, T.J. Pathogenesis of pre-eclampsia: Marinobufagenin and angiogenic imbalance as biomarkers of the syndrome. Transl. Res. 2012, 160, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Puschett, J.B.; Agunanne, E.; Uddin, M.N. Marinobufagenin, resibufogenin and preeclampsia. Biochim. Biophys. Acta-Mol. Basis Dis. 2010, 1802, 1246–1253. [Google Scholar] [CrossRef]
- Bagrov, A.Y.; Fedorova, O.V.; Dmitrieva, R.I.; Howald, W.N.; Hunter, A.P.; Kuznetsova, E.A.; Shpen, V.M. Characterization of a urinary bufodienolide Na+, K+-ATPase inhibitor in patients after acute myocardial infarction. Hypertension 1998, 31, 1097–1103. [Google Scholar] [CrossRef]
- Bagrov, A.Y.; Roukoyatkina, N.I.; Pinaev, A.G.; Dmitrieva, R.I.; Fedorova, O.V. Effects of two endogenous Na+, K+-ATPase inhibitors, marinobufagenin and ouabain, on isolated rat aorta. Eur. J. Pharmacol. 1995, 274, 151–158. [Google Scholar] [CrossRef]
- Fedorova, O.V.; Doris, P.A.; Bagrov, A.Y. Endogenous marinobufagenin-like factor in acute plasma volume expansion. Clin. Exp. Hypertens. 1998, 20, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Bagrov, A.Y.; Fedorova, O.V.; Dmitriev, R.I.; French, A.W.; Anderson, D.E. Plasma marinobufagenin-like and ouabain-like immunoreactivity during saline volume expansion in anesthetized dogs. Cardiovasc. Res. 1996, 31, 296–305. [Google Scholar] [CrossRef]
- Hauck, C.; Frishman, W.H. Systemic hypertension: The roles of salt, vascular Na+/K+ ATPase and the endogenous glycosides, ouabain and marinobufagenin. Cardiol. Rev. 2012, 20, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Gonick, H.C.; Ding, Y.; Vaziri, N.D.; Bagrov, A.Y.; Fedorova, O.V. Simultaneous Measurement of Marinobufagenin, Ouabain, and Hypertension-associated Protein In Various Disease States. Clin. Exp. Hypertens. 1998, 20, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Lopatin, D.A.; Ailamazian, E.K.; Dmitrieva, R.I.; Shpen, V.M.; Fedorova, O.V.; Doris, P.A.; Bagrov, A.Y. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J. Hypertens. 1999, 17, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.L.; Wang, X.; Lou, J.C.; Xing, J.S.; Zou, S.; Yu, Z.L.; Ma, X.C.; Wang, H.; Zhang, B. Marinobufagenin inhibits glioma growth through sodium pump α1 subunit and ERK signaling-mediated mitochondrial apoptotic pathway. Cancer Med. 2018, 7, 2034–2047. [Google Scholar] [CrossRef]
- Chu, Q.; Xu, H.; Gao, M.; Guan, X.; Liu, H.; Deng, S.; Huo, X.; Liu, K.; Tian, Y.; Ma, X. Liver-targeting resibufogenin-loaded poly(Lactic-co-glycolic acid)-D-α-tocopheryl polyethylene glycol 1000 succinate nanoparticles for liver cancer therapy. Int. J. Nanomed. 2016, 11, 449–463. [Google Scholar]
- Lu, Z.; Xu, A.; Yuan, X.; Chen, K.; Wang, L.; Guo, T. Anticancer effect of resibufogenin on gastric carcinoma cells through the phosphoinositide 3-kinase/protein kinase B/glycogen synthase kinase 3β signaling pathway. Oncol. Lett. 2018, 16, 3297–3302. [Google Scholar] [CrossRef]
- Han, Q.; Ma, Y.; Wang, H.; Dai, Y.; Chen, C.; Liu, Y.; Jing, L.; Sun, X. Resibufogenin suppresses colorectal cancer growth and metastasis through RIP3-mediated necroptosis. J. Transl. Med. 2018, 16, 201. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Liu, X.; Zhang, N.; Mao, G.; Zeng, Q.; Yin, M.; Song, D.; Deng, H. Resibufogenin suppresses transforming growth factor-β-activated kinase 1-mediated nuclear factor-κB activity through protein kinase C-dependent inhibition of glycogen synthase kinase 3. Cancer Sci. 2018, 109, 3611–3622. [Google Scholar] [CrossRef]
- Zhang, D.M.; Liu, J.S.; Deng, L.J.; Chen, M.F.; Yiu, A.; Cao, H.H.; Tian, H.Y.; Fung, K.P.; Kurihara, H.; Pan, J.X.; et al. Arenobufagin, a natural bufadienolide from toad venom, induces apoptosis and autophagy in human hepatocellular carcinoma cells through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis 2013, 34, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-H.; Cao, Y.-T.; Pan, H.-Y.; Wang, L.-H. Identification of Antitumor Constituents in Toad Venom by Spectrum-Effect Relationship Analysis and Investigation on Its Pharmacologic Mechanism. Molecules 2020, 25, 4269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Q.; Zou, G.; Gao, G.; Yue, Q. Arenobufagin, isolated from toad venom, inhibited epithelial-to-mesenchymal transition and suppressed migration and invasion of lung cancer cells via targeting IKKβ/NFκB signal cascade. J. Ethnopharmacol. 2020, 250, 112492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Liu, J.S.; Tang, M.K.; Yiu, A.; Cao, H.H.; Jiang, L.; Yuet-Wa Chan, J.; Tian, H.Y.; Fung, K.P.; Ye, W.C. Bufotalin from Venenum Bufonis inhibits growth of multidrug resistant HepG2 cells through G2/M cell cycle arrest and apoptosis. Eur. J. Pharmacol. 2012, 692, 19–28. [Google Scholar] [CrossRef]
- Wei, X.; He, J.; Gao, B.; Han, L.; Mao, Y.; Zhao, H.; Si, N.; Wang, H.; Yang, J.; Bian, B. Hellebrigenin anti-pancreatic cancer effects based on apoptosis and autophage. PeerJ 2020, 2020, e9011. [Google Scholar] [CrossRef]
- Deng, L.-J.; Hu, L.-P.; Peng, Q.-L.; Yang, X.-L.; Bai, L.-L.; Yiu, A.; Li, Y.; Tian, H.-Y.; Ye, W.-C.; Zhang, D.-M. Hellebrigenin induces cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells through inhibition of Akt. Chem. Biol. Interact. 2014, 219, 184–194. [Google Scholar] [CrossRef]
- Yu, Z.; Guo, W.; Ma, X.; Zhang, B.; Dong, P.; Huang, L.; Wang, X.; Wang, C.; Huo, X.; Yu, W. Gamabufotalin, a bufadienolide compound from toad venom, suppresses COX-2 expression through targeting IKKβ/NF-κB signaling pathway in lung cancer cells. Mol. Cancer 2014, 13, 203. [Google Scholar] [CrossRef]
- Rong, X.; Ni, W.; Liu, Y.; Wen, J.; Qian, C.; Sun, L.; Wang, J. Bufalin, a bioactive component of the Chinese medicine Chansu, inhibits inflammation and invasion of human rheumatoid arthritis fibroblast-like synoviocytes. Inflammation 2014, 37, 1050–1058. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, W.; Jozwik, C.; Lin, Y.; Glasman, M.; Caohuy, H.; Srivastava, M.; Esposito, D.; Gillette, W.; Hartley, J.; et al. Cardiac glycosides inhibit TNF-α/NF-κB signaling by blocking recruitment of TNF receptor-associated death domain to the TNF receptor. Proc. Natl. Acad. Sci. USA. 2005, 102, 9631–9636. [Google Scholar] [CrossRef]
- Carvalho, D.C.M.; Cavalcante-Silva, L.H.A.; De A Lima, É.; Galvão, J.G.F.M.; De A Alves, A.K.; Feijó, P.R.O.; Quintas, L.E.M.; Rodrigues-Mascarenhas, S. Marinobufagenin inhibits neutrophil migration and proinflammatory cytokines. J. Immunol. Res. 2019, 2019, 1094520. [Google Scholar] [CrossRef]
- Qi, F.; Li, A.; Inagaki, Y.; Kokudo, N.; Tamura, S.; Nakata, M.; Tang, W. Antitumor activity of extracts and compounds from the skin of the toad Bufo bufo gargarizans Cantor. Int. Immunopharmacol. 2011, 11, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Huang, Y.; Xie, X.; Huang, W.; Yin, J.; Lin, W.; Jia, Q.; Zeng, W. Anti-inflammatory and antinociceptive activities of bufalin in rodents. Mediators Inflamm. 2014, 2014, 171839. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chen, S.; Maniatis, T. Cardiac glycosides are potent inhibitors of interferon-β gene expression. Nat. Chem. Biol. 2010, 7, 25–33. [Google Scholar] [CrossRef]
- Xie, X.B.; Yin, J.Q.; Wen, L.L.; Gao, Z.H.; Zou, C.Y.; Wang, J.; Huang, G.; Tang, Q.L.; Colombo, C.; He, W.L.; et al. Critical Role of Heat Shock Protein 27 in Bufalin-Induced Apoptosis in Human Osteosarcomas: A Proteomic-Based Research. PLoS ONE 2012, 7, e47375. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Fazal, F. Blocking NF-kB: An inflammatory issue. Proc. Am. Thorac. Soc. 2011, 8, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Ihenetu, K.; Espinosa, R.; De Leon, R.; Planas, G.; Perez-Pinero, A.; Waldbeser, L. Digoxin and digoxin-like immunoreactive factors (DLIF) modulate the release of pro-inflammatory cytokines. Inflamm. Res. 2008, 57, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.L.; Leite, J.A.; Alves, A.K.A.; Rodrigues, Y.K.S.; Amorim, F.M.; Néris, P.L.N.; Oliveira, M.R.; Rodrigues-Mascarenhas, S. Immunomodulatory activity of ouabain in Leishmania leishmania amazonensis-infected Swiss mice. Parasitol. Res. 2013, 112, 1313–1321. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, G.; Fei, J.; Wu, Y.; Yan, J. Bufotalin ameliorates experimental Sjögren’s syndrome development by inhibiting Th17 generation. Naunyn. Schmiedebergs. Arch. Pharmacol. 2020, 393, 1977–1985. [Google Scholar] [CrossRef]
- Zheng, Y.; Deng, L.; Cao, H.; Xu, N.; Zhang, D.; Tian, H.; Li, B.; Lu, Z.; Ye, W.; Yu, L. Screening of bufadienolides from toad venom identifies gammabufotalin as a potential anti-inflammatory agent. Planta Med. 2022, 88, 43–52. [Google Scholar] [CrossRef]
- Takai, N.; Kira, N.; Ishii, T.; Yoshida, T.; Nishida, M.; Nishida, Y.; Nasu, K.; Narahara, H. Bufalin, a traditional oriental medicine, induces apoptosis in human cancer cells. Asian Pacific J. Cancer Prev. 2012, 13, 399–402. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Yang, Y.-K.; Wu, W.-Z. Bufalin attenuates the stage and metastatic potential of hepatocellular carcinoma in nude mice. J. Transl. Med. 2014, 12, 57. [Google Scholar] [CrossRef]
- Wu, S.-H.; Bau, D.-T.; Hsiao, Y.-T.; Lu, K.-W.; Hsia, T.-C.; Lien, J.-C.; Ko, Y.-C.; Hsu, W.-H.; Yang, S.-T.; Huang, Y.-P.; et al. Bufalin induces apoptosis in vitro and has Antitumor activity against human lung cancer xenografts in vivo. Environ. Toxicol. 2017, 32, 1305–1317. [Google Scholar] [CrossRef]
- Yin, P.-H.; Liu, X.; Qiu, Y.-Y.; Cai, J.; Qin, J.; Zhu, H.-R.; Li, Q. Anti-tumor activity and apoptosis-regulation mechanisms of bufalin in various cancers: New hope for cancer patients. Asian Pacific J. cancer Prev. 2012, 13, 5339–5343. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. NF-κB as a Critical Link between Inflammation and Cancer. Cold Spring Harb. Perspect. Biol. 2009, 1, a000141. [Google Scholar] [CrossRef] [PubMed]
| Toad Species | CTS | Target | Ref. |
|---|---|---|---|
| Bufo gargarizans Cantor | Bufalin | Anti-hepatitis B above 10-2 µM Anti-HIV, IC50 = 5 nm Anti-MERS-CoV in vero cells (IC50 = 0.018 µM) after 24 h Anti-MERS-CoV Calu-3 human lung cells (IC50 = 0.544 µM) (in vitro) | [70,71,73] |
| Bufo gargarizans | Cinobufagin | Anti-hepatitis B above 10-1 µM Anti- enterovirus 71 (EV-71) IC50 = 10.94 nmol/L Anti-HIV, IC50 = 24 nm Anti-MERS-CoV in vero cells IC50 = 0.017 µM after 24 h Anti-MERS-CoV Calu-3 human lung cells IC50 = 0.616 µM (in vitro) | [70,71,72,73] |
| Bufo gargarizans | Resibufogenin | Anti-enterovirus 71 (EV71) IC50 = 218 nmol/L Anti-MERS-CoV in vero cells (IC50 = 1.612 µM) after 24 h Anti-MERS-CoV Calu-3 human lung cells IC50 = 15.970 µM (in vitro) | [70,72] |
| Bufo gargarizans | Telocinobufagin | Anti-MERS-CoV in vero cells IC50 = 0.027 µM after 24 h Anti-MERS-CoV Calu-3 human lung cells IC50 = 0.465 µM (in vitro) | [70] |
| Bufo gargarizans Cantor | Bufotalin | Anti-MERS-CoV in vero cells IC50 = 0.063 µM Anti-MERS-CoV Calu-3 human lung cells IC50 = 1.630 µM (in vitro) | [70] |
| Bufo gargarizans Cantor | Cinobufotalin | Anti-MERS-CoV in vero cells IC50 = 0.23 µM after 24 h Anti-MERS-CoV Calu-3 human lung cells IC50 = 3.958 µM (in vitro) | [70] |
| Toad Species | Detected CTS | Target Cell and Mechanism of Action | Ref. |
|---|---|---|---|
| Bufo gargarizans Cantor/ Bufo melanostictus Suhneider (Toad venom) | Arenobufagin | HepG2 cells (IC50 = 20.24 ± 3.84 nM) HepG2/ADM cells (IC50 = 7.46 ± 2.89 nM) HL-60 cells (IC50 = 27.70 ± 8.77 nM) after 72 h Induces apoptosis and autophagy, inhibition of the PI3K/Akt/mTOR pathway (in vitro, in vivo) | [146] |
| Bufo gargarizans | Arenobufagin | Non-small cell lung cancer cell (A549) IC50 = 12.530 ng/mL after 72 h Induces apoptosis in A549 cells with the enhanced expression of cleaved PARP (poly ADP-ribose polymerase (in vitro) | [147] |
| Bufo melanostictus Schneider | Arenobufagin | Lung cancer (A549) At (0.5, 1 and 2 nM) inhibited the mobility of A549 cells (59.9%, 41.1%, and 24.7%, respectively) At (0.5, 1, and 2 nM) inhibited the mobility of H1299 cells (72.3%, 47.4%, and 22.4%, respectively) after 48 h Target IKKβ to inactive NFκB signaling cascade and change protein expression related to EMT (in vitro and in vivo) | [148] |
| Bufo gargarizans Cantor | Bufalin | Non-small cell lung cancer NSCLC A549 cells At 2.5–10 µM, bufalin- induced apoptosis and cell cycle arrest in G1 phase | [105,149] |
| HepG2 cell (IC50 = 0.61 ± 0.06 µM) R-HepG2 cells (IC50 = 0.24 ± 0.02 µM) Induces cell cycle arrest at G2/M phase after 48 h (in vitro) | |||
| Bufo gargarizans Cantor and Bufo melanostictus Schneider | Bufotalin | HepG2 cell (IC50 = 0.43 ± 0.07 µM) R-HepG2 cells(IC50 = 0.13 ± 0.01 µM) Induces cell cycle arrest at G2/M phase after 48 h (in vitro) | [149] |
| Bufo gargarizans Cantor and Bufo melanostictus Schneider | Hellebrigenin | HepG2 cells (IC50 = 0.40 ± 0.05 µmol/L) After 24 h (IC50 = 0.13 ± 0.01µmol/L) After 48 h (IC50 = 0.10 ± 0.01 µmol/L) After 72 h Induces cell cycle arrest at G2/M phase (in vitro) | [150,151] |
| Bufo gargarizans Cantor | Gamabufotalin (CS-6) | NSCLC (IC50 = 50 nM) Inhibit NSCLC cells growth and enhance apoptosis induction (in vitro) | [152] |
| Bufo gargarizans | Cinobufatolin | H157 cancer cells IC50 = 131.12 ng/mL A549 cancer cells IC50 = 23.08 ng/mL after 72 h (in vitro) | [147,149] |
| HepG2 cell (IC50 = 1.58 ± 0.21 µM) R-HepG2 cells(IC50 = 0.74 ± 0.07 µM) Induces cell cycle arrest at G2/M phase after 48 h (in vitro) | |||
| Bufo gargarizans | Telocinobufagin | H157 cancer cells IC50 = 23.60 ng/mL A549 cancer cells IC50 = 27.882 ng/mL after 72 h (in vitro) | [147,149] |
| HepG2 cell (IC50 = 1.28 ± 0.19 µM) R-HepG2 cells(IC50 = 0.49 ± 0.05 µM) after 48 h Induces cell cycle arrest at G2/M phase (in vitro) | |||
| Bufo gargarizans | Resibufogenin | Gastric carcinoma cells (MGC-803) (4 and 8 µM) for 24 h and 48 h Increased Bax/Bcl-2 expression, and suppressed cyclin D1, cyclin E, PI3K, phosphorylated AKT, phosphorylated GSK3β, and β-catenin protein expression in MGC-803 cells. (in vitro) | [143] |
| Toad Species | Detected CTS | Activity | Ref. |
|---|---|---|---|
| Rhinella schneideri | Marinobufagenin | Anti-inflammatory (10, 100, 1000, and 10,000 nM), decreased IL-1β, IL-6, and TNF-α levels (in vitro, in vivo) | [155] |
| Bufo gargarizans Cantor | Bufotalin | Anti-inflammatory against chronic inflammatory autoimmune disease 100 μg/kg in vivo and 200 nM in vitro inhibiting proinflammatory Th17 population and secretion of inflammatory cytokines | [163] |
| Bufo gargarizans Cantor | Bufalin | Anti-inflammatory against carrageenan-induced paw edema model (0.3 and 0.6 mg/kg, i.p.) Downregulation of expression levels of nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), 1β (IL-1β), (IL-6), (TNF-α), and inhibited the activation of NF-κB signaling (in vivo) | [157] |
| Bufo gargarizans | Gammabufotalin | Anti-inflammatory (1, 4, and 12 (50 μM); 2, 13, and 14 (10 μM); 3 and 6 (5 μM); 5 and 8 (1 μM); 7, 9, and 11 (0.5 μM); 10 (4 μM)) Inhibits LPS-induced inflammation by suppressing myeloid differentiation primary response 88/nuclear factor-kappa B and STAT3 signal pathways. (in vivo) | [164] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Seedi, H.R.; Yosri, N.; El-Aarag, B.; Mahmoud, S.H.; Zayed, A.; Du, M.; Saeed, A.; Musharraf, S.G.; El-Garawani, I.M.; Habib, M.R.; et al. Chemistry and the Potential Antiviral, Anticancer, and Anti-Inflammatory Activities of Cardiotonic Steroids Derived from Toads. Molecules 2022, 27, 6586. https://doi.org/10.3390/molecules27196586
El-Seedi HR, Yosri N, El-Aarag B, Mahmoud SH, Zayed A, Du M, Saeed A, Musharraf SG, El-Garawani IM, Habib MR, et al. Chemistry and the Potential Antiviral, Anticancer, and Anti-Inflammatory Activities of Cardiotonic Steroids Derived from Toads. Molecules. 2022; 27(19):6586. https://doi.org/10.3390/molecules27196586
Chicago/Turabian StyleEl-Seedi, Hesham R., Nermeen Yosri, Bishoy El-Aarag, Shaymaa H. Mahmoud, Ahmed Zayed, Ming Du, Aamer Saeed, Syed G. Musharraf, Islam M. El-Garawani, Mohamed R. Habib, and et al. 2022. "Chemistry and the Potential Antiviral, Anticancer, and Anti-Inflammatory Activities of Cardiotonic Steroids Derived from Toads" Molecules 27, no. 19: 6586. https://doi.org/10.3390/molecules27196586
APA StyleEl-Seedi, H. R., Yosri, N., El-Aarag, B., Mahmoud, S. H., Zayed, A., Du, M., Saeed, A., Musharraf, S. G., El-Garawani, I. M., Habib, M. R., Tahir, H. E., Hegab, M. M., Zou, X., Guo, Z., Efferth, T., & Khalifa, S. A. M. (2022). Chemistry and the Potential Antiviral, Anticancer, and Anti-Inflammatory Activities of Cardiotonic Steroids Derived from Toads. Molecules, 27(19), 6586. https://doi.org/10.3390/molecules27196586












