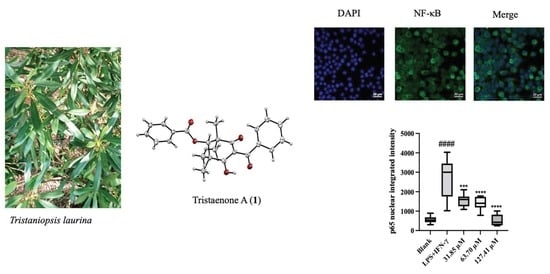
Tristaenone A: A New Anti-Inflammatory Compound Isolated from the Australian Indigenous Plant Tristaniopsis laurina
Abstract
:1. Introduction
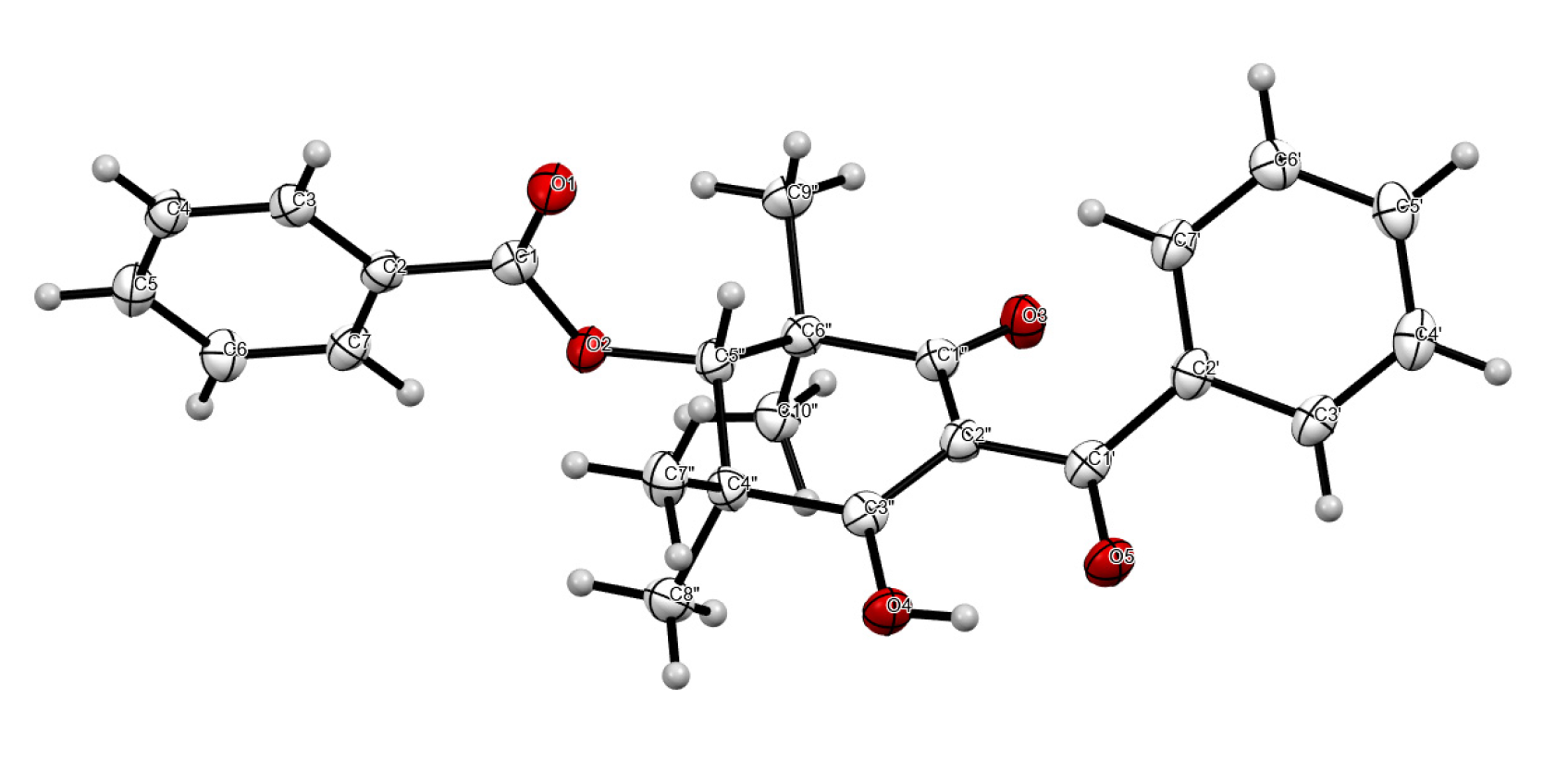
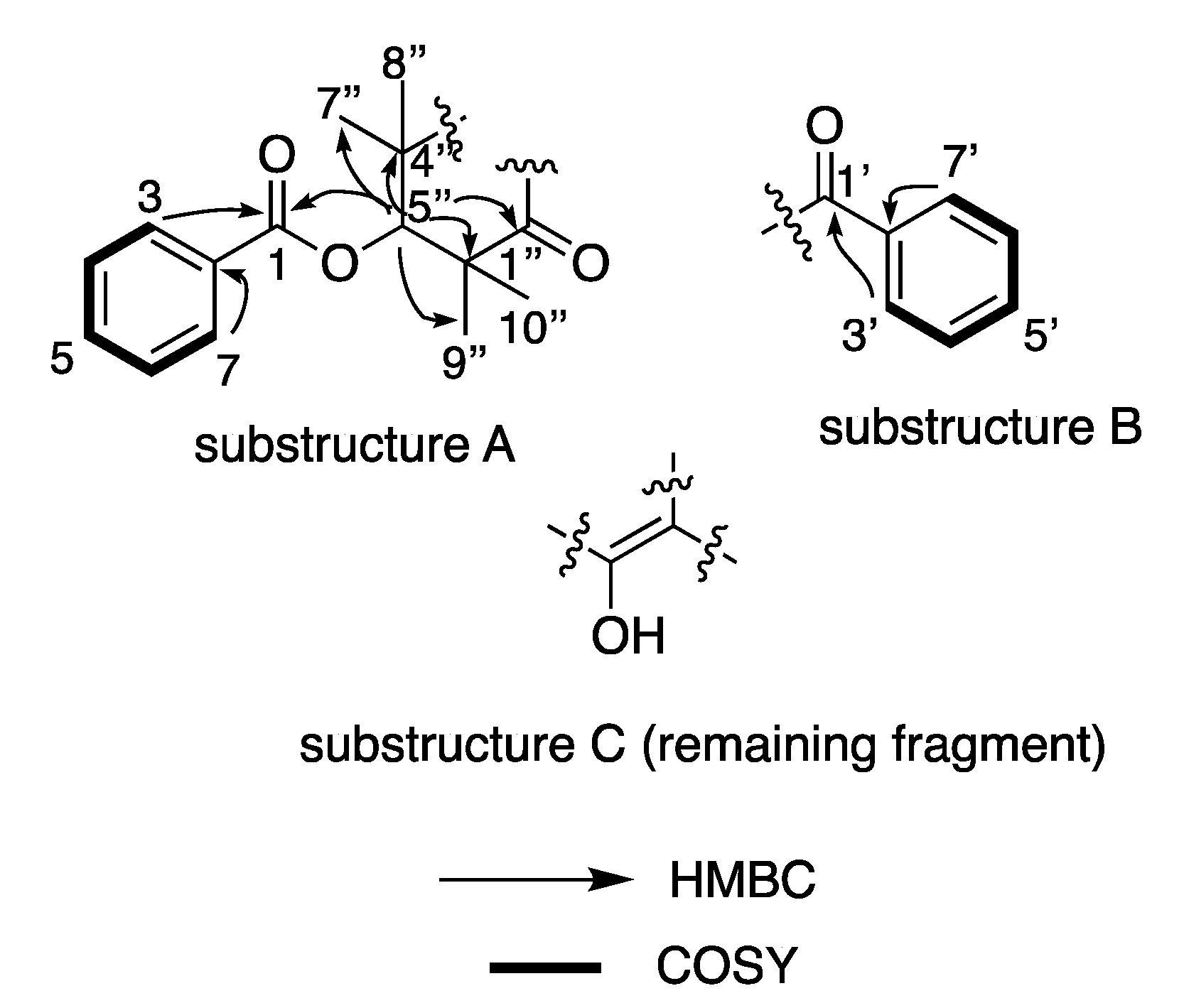
2. Results and Discussion

3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Bioactivity-Guided Isolation of Compounds 1–3
3.4. X-ray Diffraction Analysis
3.5. Maintenance of RAW 264.7 Macrophages
3.6. Pro-Inflammatory Activation of Cells
3.7. Determination of Nitrite by the Griess Assay
3.8. Determination of TNF-α and IL-6 by ELISA
3.9. Determination of NF-kB Translocation
3.10. Determination of Cell Viability by the Alamar Blue Assay
3.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Meek, I.L.; Van de Laar, M.A.; Vonkeman, H.E. Non-Steroidal Anti-Inflammatory Drugs: An Overview of Cardiovascular Risks. Pharmaceuticals 2010, 3, 2146–2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, D.M. Nonsteroidal anti-inflammatory drugs—The clinical dilemmas. Scand. J. Gastroenterol. Suppl. 1992, 192, 9–16. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Sharman, M.J.; Verdile, G.; Kirubakaran, S.; Parenti, C.; Singh, A.; Watt, G.; Karl, T.; Chang, D.; Li, C.G.; Munch, G. Targeting Inflammatory Pathways in Alzheimer’s Disease: A Focus on Natural Products and Phytomedicines. CNS Drugs 2019, 33, 457–480. [Google Scholar] [CrossRef]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef]
- Pennacchio, M.; Kemp, A.S.; Taylor, R.P.; Wickens, K.M.; Kienow, L. Interesting biological activities from plants traditionally used by Native Australians. J. Ethnopharmacol. 2005, 96, 597–601. [Google Scholar] [CrossRef]
- Packer, J.; Brouwer, N.; Harrington, D.; Gaikwad, J.; Heron, R.; Yaegl Community, E.; Ranganathan, S.; Vemulpad, S.; Jamie, J. An ethnobotanical study of medicinal plants used by the Yaegl Aboriginal community in northern New South Wales, Australia. J. Ethnopharmacol. 2012, 139, 244–255. [Google Scholar] [CrossRef]
- Bodkin, F. Dharawal Pharmacopeia Collection; Western Sydney University: Penrith, Australia, 2021. [Google Scholar]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I. Essential oils of Australian species of the genera Tristaniopsis and Tristania (Myrtaceae). J. Essent. Oil Res. 1999, 11, 661–665. [Google Scholar] [CrossRef]
- Lamberton, J. The occurrence of 5-hydroxy-7, 4′-dimethoxy-6-methylflavone in Eucalyptus waxes. Aust. J. Chem. 1964, 17, 692–696. [Google Scholar] [CrossRef]
- Brezani, V.; Lelakova, V.; Hassan, S.T.S.; Berchova-Bimova, K.; Novy, P.; Kloucek, P.; Marsik, P.; Dall’Acqua, S.; Hosek, J.; Smejkal, K. Anti-Infectivity against Herpes Simplex Virus and Selected Microbes and Anti-Inflammatory Activities of Compounds Isolated from Eucalyptus globulus Labill. Viruses 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowieson, N.P.; Aragao, D.; Clift, M.; Ericsson, D.J.; Gee, C.; Harrop, S.J.; Mudie, N.; Panjikar, S.; Price, J.R.; Riboldi-Tunnicliffe, A.; et al. MX1: A bending-magnet crystallography beamline serving both chemical and macromolecular crystallography communities at the Australian Synchrotron. J. Synchrotron Rad. 2015, 22, 187–190. [Google Scholar] [CrossRef] [Green Version]
- Kabsch, W.X. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Cryst. 1993, 26, 795–800. [Google Scholar] [CrossRef]
- SADABS, version 2014/5; Bruker AXS Inc.: Madison, WI, USA, 2001.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta. Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. Programs for Crystal Structure Analysis; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Raju, R.; Mathew, S.; Reddell, P.; Munch, G. Ternstroenol F: A new pentacyclic triterpenoid saponin isolated from the Australian rainforest plant Ternstroemia cherryi. Nat. Prod. Res. 2022; 1–6, online ahead of print. [Google Scholar] [CrossRef]
- Zhou, X.; Razmovski-Naumovski, V.; Chang, D.; Li, C.; Kam, A.; Low, M.; Bensoussan, A.; Chan, K. Synergistic Effects of Danshen (Salvia Miltiorrhiza Radix et Rhizoma) and Sanqi (Notoginseng Radix et Rhizoma) Combination in Inhibiting Inflammation Mediators in RAW264.7 Cells. BioMed Res. Int. 2016, 2016, 5758195. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Munch, G.; Wohlmuth, H.; Afzal, S.; Kao, M.T.; Al-Khazaleh, A.; Low, M.; Leach, D.; Li, C.G. Synergistic Inhibition of Pro-Inflammatory Pathways by Ginger and Turmeric Extracts in RAW 264.7 Cells. Front. Pharmacol. 2022, 13, 818166. [Google Scholar] [CrossRef]
- Wessel, A.W.; Hanson, E.P. A method for the quantitative analysis of stimulation-induced nuclear translocation of the p65 subunit of NF-kappaB from patient-derived dermal fibroblasts. Methods Mol. Biol. 2015, 1280, 413–426. [Google Scholar] [PubMed]

| Position | δH (J in Hz) | δC a | COSY | HMBC |
|---|---|---|---|---|
| 1 | 166.9 | |||
| 2 | 132.9 | |||
| 3/7 | 8.09, d (7.8) | 130.4 | 4/6 | 1, 2, 3/7 |
| 4/6 | 7.54, dd (7.8, 7.6) | 129.6 | 3/7, 5 | 1, 2, 4/6 |
| 5 | 7.67, dd (7.6, 7.6) | 134.5 | 4/6 | 4/6 |
| 1′ | 199.2 | |||
| 2′ | 139.1 | |||
| 3′/7′ | 7.60, d (7.8) | 129.0 | 4″ | 1′, 2′ |
| 4″ | 7.45, dd (7.8, 7.4) | 128.8 | 3′/7′, 5′ | 2′, 4″ |
| 5′ | 7.54, dd (7.4, 7.4) | 132.9 | 4″ | |
| 1″ | 197.2 | |||
| 2″ | 111.6 | |||
| 3″ | 197.2 | |||
| 4″/6″ | 46.2 | |||
| 5″ | 5.51, s | 80.2 | 1, 3″, 4″/6″, 7″/9″, 8″/10″ | |
| 7″/9″ | 1.40, s | 22.7 | 1, 3″, 4″/6″, 7″/9″, 8″/10″, 5″ | |
| 8″/10″ | 1.40, s | 25.8 | 1, 3″, 4″/6″, 7″/9″, 8″/10″, 5″ |
| Compounds | Inhibition of Nitric Oxide Production (µM) | Inhibition of TNF-α Production (µM) | Cell Viability (µM) |
|---|---|---|---|
| Tristaenone A (1) | 37.58 ± 2.45 | 80.61 | >250 |
| 8-desmethyleucalyptin (2) | 16.21 ± 1.53 | 46.58 | >250 |
| Eucalyptin (3) | 138.47 ± 3.42 | >250 | >250 |
| Curcumin | 12.6 ± 1.5 | 11.4 ± 1.3 | 29.5 ± 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathew, S.; Zhou, X.; Münch, G.; Bodkin, F.; Wallis, M.; Li, F.; Raju, R. Tristaenone A: A New Anti-Inflammatory Compound Isolated from the Australian Indigenous Plant Tristaniopsis laurina. Molecules 2022, 27, 6592. https://doi.org/10.3390/molecules27196592
Mathew S, Zhou X, Münch G, Bodkin F, Wallis M, Li F, Raju R. Tristaenone A: A New Anti-Inflammatory Compound Isolated from the Australian Indigenous Plant Tristaniopsis laurina. Molecules. 2022; 27(19):6592. https://doi.org/10.3390/molecules27196592
Chicago/Turabian StyleMathew, Shintu, Xian Zhou, Gerald Münch, Francis Bodkin, Matthew Wallis, Feng Li, and Ritesh Raju. 2022. "Tristaenone A: A New Anti-Inflammatory Compound Isolated from the Australian Indigenous Plant Tristaniopsis laurina" Molecules 27, no. 19: 6592. https://doi.org/10.3390/molecules27196592
APA StyleMathew, S., Zhou, X., Münch, G., Bodkin, F., Wallis, M., Li, F., & Raju, R. (2022). Tristaenone A: A New Anti-Inflammatory Compound Isolated from the Australian Indigenous Plant Tristaniopsis laurina. Molecules, 27(19), 6592. https://doi.org/10.3390/molecules27196592