Structural Requirement of hA5G18 Peptide (DDFVFYVGGYPS) from Laminin α5 Chain for Amyloid-like Fibril Formation and Cell Adhesion
Abstract
1. Introduction
2. Result
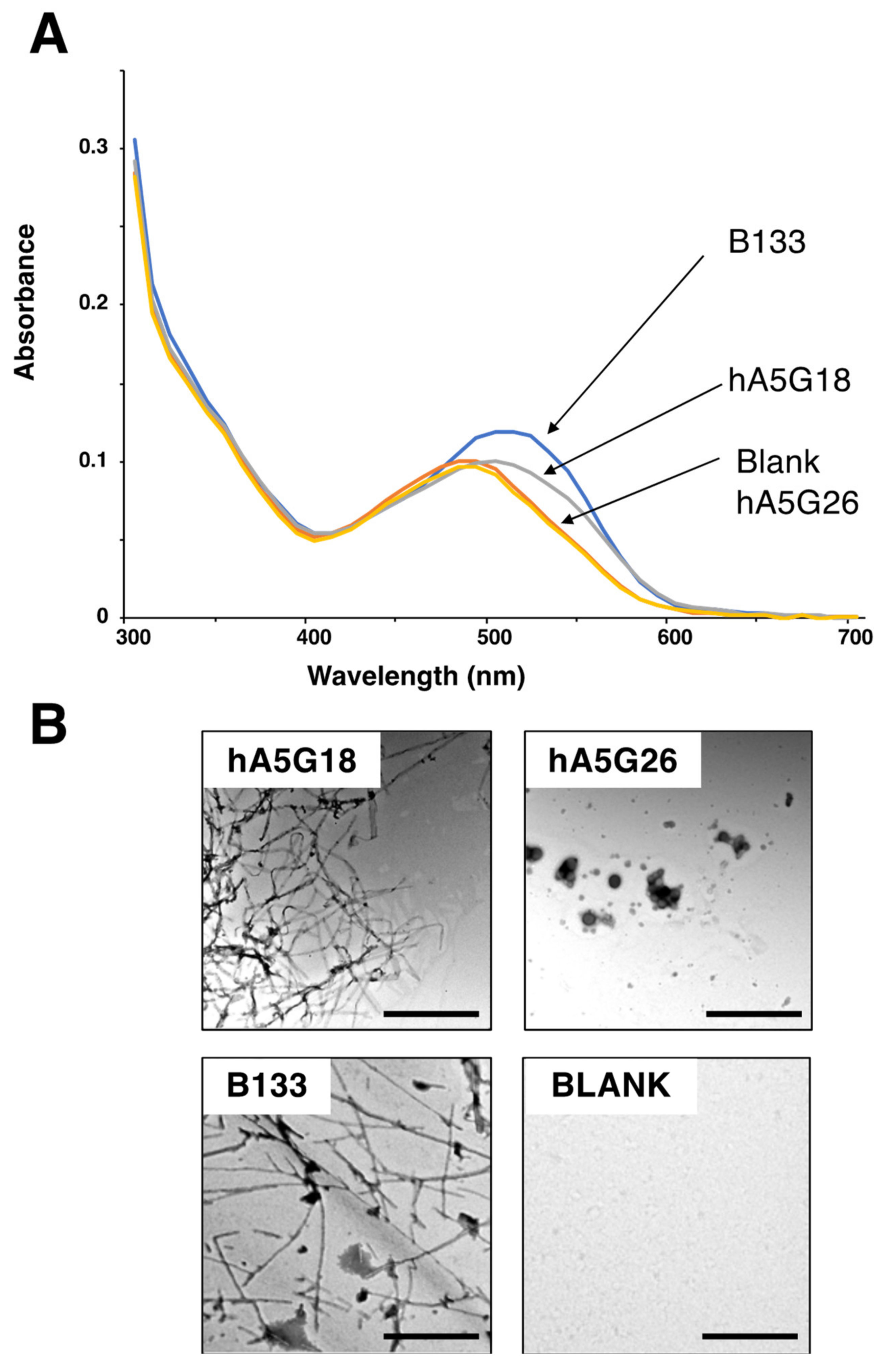
2.1. Amyloidogenic Peptides from the Human Laminin α5 Chain G Domain
2.2. Amyloid-like Fibril Formation and Cell Attachment Activity of Truncated hA5G18 Peptides
2.3. Cell Attachment Activity of hA5G18B Conjugated Sepharose Bead
2.4. Congo Red Analysis and Cell Attachment Activity of Alanine-Substituted hA5G18B Peptides
2.5. Conjugation of an Arg-Gly-Asp (RGD) Sequence and Basic Amino Acids to FVFYV
2.6. Effect of EDTA and Heparin on Cell Attachment to Peptide-Coated Plates
2.7. Effect of Anti-Integrin Antibodies on Cell Attachment and Spreading to Peptide-Coated Plates
3. Discussion
4. Materials and Methods
4.1. Synthetic Peptides
4.2. Antibodies
4.3. Congo Red Binding Analysis
4.4. Transmission Electron Microscopy (TEM)
4.5. Cells and Culture
4.6. Cell Attachment Assay Using Peptide-Coated Plates
4.7. Inhibition Assay
4.8. Cell Attachment Assay Using Peptide-Conjugated Sepharose Beads
4.9. Statistics Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Miner, J.H.; Yurchenco, P.D. Laminin functions in tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 2004, 20, 255–284. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yokoyama, F.; Nomizu, M. Functional sites in the laminin alpha chains. Connect. Tissue Res. 2005, 46, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, K.; Ishikawa, M.; Hayashi, T.; Yamada, Y.; Katagiri, F.; Kikkawa, Y.; Nomizu, M. Identification of cell adhesive sequences in the N-terminal region of the laminin α2 chain. J. Biol. Chem. 2012, 287, 25111–25122. [Google Scholar] [CrossRef] [PubMed]
- Negishi, Y.; Nomizu, M. Laminin-derived peptides: Applications in drug delivery systems for targeting. Pharmacol. Ther. 2019, 202, 91–97. [Google Scholar] [CrossRef]
- Kumai, J.; Yamada, Y.; Hamada, K.; Katagiri, F.; Hozumi, K.; Kikkawa, Y.; Nomizu, M. Identification of active sequences in human laminin α5 G domain. J. Pept. Sci. 2019, 25, e3218. [Google Scholar] [CrossRef]
- Guijarro, J.I.; Sunde, M.; Jones, J.A.; Campbell, I.D.; Dobson, C.M. Amyloid fibril formation by an SH3 domain. Proc. Natl. Acad. Sci. USA 1998, 95, 4224–4228. [Google Scholar] [CrossRef]
- Kelly, J.W. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr. Opin. Struct. Biol. 1998, 8, 101–106. [Google Scholar] [CrossRef]
- Rochet, J.C.; Lansbury, P.T., Jr. Amyloid fibrillogenesis: Themes and variations. Curr. Opin. Struct. Biol. 2000, 10, 60–68. [Google Scholar] [CrossRef]
- Katagiri, F.; Ueo, D.; Okubo-Gunge, Y.; Usui, A.; Kuwatsuka, S.; Mine, Y.; Hamada, K.; Fujiwara, S.; Sasaki, T.; Nomizu, M.; et al. Fibulin-4 Accelerates Amyloid Formation by Binding with a Keratin 5 Peptide Fragment. JID Innov. 2022, 2, 100114. [Google Scholar] [CrossRef]
- Berhanu, W.M.; Hansmann, U.H. Structure and dynamics of amyloid-β segmental polymorphisms. PLoS ONE 2012, 7, e41479. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Dobson, C.M. The Amyloid Phenomenon and Its Links with Human Disease. Cold Spring Harb. Perspect. Biol. 2017, 9, a023648. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Urushibata, S.; Hozumi, K.; Yokoyama, F.; Ichikawa, N.; Kadoya, Y.; Nishi, N.; Watanabe, N.; Yamada, Y.; Nomizu, M. Identification of multiple amyloidogenic sequences in laminin-1. Biochemistry 2007, 46, 3966–3974. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, F.; Takeyama, K.; Ohga, Y.; Hozumi, K.; Kikkawa, Y.; Kadoya, Y.; Nomizu, M. Amino acid sequence requirements of laminin beta1 chain peptide B133 (DISTKYFQMSLE) for amyloid-like fibril formation, syndecan binding, and neurite outgrowth promotion. Biochemistry 2010, 49, 5909–5918. [Google Scholar] [CrossRef] [PubMed]
- Ohga, Y.; Katagiri, F.; Takeyama, K.; Hozumi, K.; Kikkawa, Y.; Nishi, N.; Nomizu, M. Design and activity of multifunctional fibrils using receptor-specific small peptides. Biomaterials 2009, 30, 6731–6738. [Google Scholar] [CrossRef] [PubMed]
- Klunk, W.E.; Jacob, R.F.; Mason, R.P. Quantifying amyloid beta-peptide (Abeta) aggregation using the Congo red-Abeta (CR-abeta) spectrophotometric assay. Anal. Biochem. 1999, 266, 66–76. [Google Scholar] [CrossRef]
- Nomizu, M.; Kim, W.H.; Yamamura, K.; Utani, A.; Song, S.Y.; Otaka, A.; Roller, P.P.; Kleinman, H.K.; Yamada, Y. Identification of cell binding sites in the laminin alpha 1 chain carboxyl-terminal globular domain by systematic screening of synthetic peptides. J. Biol. Chem. 1995, 270, 20583–20590. [Google Scholar] [CrossRef]
- Yamada, Y.; Onda, T.; Hamada, K.; Kikkawa, Y.; Nomizu, M. Octa-arginine and Octa-lysine Promote Cell Adhesion through Heparan Sulfate Proteoglycans and Integrins. Biol. Pharm. Bull. 2022, 45, 207–212. [Google Scholar] [CrossRef]
- Suzuki, N.; Nakatsuka, H.; Mochizuki, M.; Nishi, N.; Kadoya, Y.; Utani, A.; Oishi, S.; Fujii, N.; Kleinman, H.K.; Nomizu, M. Biological activities of homologous loop regions in the laminin alpha chain G domains. J. Biol. Chem. 2003, 278, 45697–45705. [Google Scholar] [CrossRef]
- Hoffman, M.P.; Engbring, J.A.; Nielsen, P.K.; Vargas, J.; Steinberg, Z.; Karmand, A.J.; Nomizu, M.; Yamada, Y.; Kleinman, H.K. Cell type-specific differences in glycosaminoglycans modulate the biological activity of a heparin-binding peptide (RKRLQVQLSIRT) from the G domain of the laminin alpha1 chain. J. Biol. Chem. 2001, 276, 22077–22085. [Google Scholar] [CrossRef]
- Miyazaki, T.; Futaki, S.; Suemori, H.; Taniguchi, Y.; Yamada, M.; Kawasaki, M.; Hayashi, M.; Kumagai, H.; Nakatsuji, N.; Sekiguchi, K.; et al. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat. Commun. 2012, 3, 1236. [Google Scholar] [CrossRef] [PubMed]
- Rodin, S.; Domogatskaya, A.; Ström, S.; Hansson, E.M.; Chien, K.R.; Inzunza, J.; Hovatta, O.; Tryggvason, K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 2010, 28, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.Y.; Miki, T.; Mihara, H.; Tsutsumi, H. Biofunctional supramolecular hydrogels fabricated from a short self-assembling peptide modified with bioactive sequences for the 3D culture of breast cancer MCF-7 cells. Bioorg. Med. Chem. 2021, 46, 116345. [Google Scholar] [CrossRef] [PubMed]
- Nomizu, M.; Song, S.Y.; Kuratomi, Y.; Tanaka, M.; Kim, W.H.; Kleinman, H.K.; Yamada, Y. Active peptides from the carboxyl-terminal globular domain of laminin alpha2 and Drosophila alpha chains. FEBS Lett. 1996, 396, 37–42. [Google Scholar] [CrossRef]







| Peptide | Sequence | Congo Red Staining a | Cell Attachment b | Cell Spreading c |
|---|---|---|---|---|
| hA5G18 | DDFVFYVGGYPS | + | + | + |
| hA5G18A | DFVFYVGGYPS | + | + | + |
| hA5G18B | FVFYVGGYPS | + | + | + |
| hA5G18C | VFYVGGYPS | - | - | - |
| hA5G18BTC1 | FVFYVGGYP | + | - | - |
| hA5G18BTC2 | FVFYVGGY | + | - | - |
| hA5G18BTC3 | FVFYVGG | + | - | - |
| hA5G18BTC4 | FVFYVG | + | - | - |
| hA5G18BTC5 | FVFYV | + | - | - |
| hA5G18BTC6 | FVFY | - | - | - |
| Peptide | Sequence a | Congo Red Staining b | Amyloid-like Fibril Formation c | Cell Attachment d | Cell Spreading e |
|---|---|---|---|---|---|
| hA5G18B | FVFYVGGYPS | + | + | + | + |
| hA5G18BA1(F) | AVFYVGGYPS | - | - | - | - |
| hA5G18BA2(V) | FAFYVGGYPS | - | - | - | - |
| hA5G18BA3(F) | FVAYVGGYPS | - | - | - | - |
| hA5G18BA4(Y) | FVFAVGGYPS | - | - | - | - |
| hA5G18BA5(V) | FVFYAGGYPS | + | + | + | + |
| hA5G18BA6(G) | FVFYVAGYPS | + | + | + | + |
| hA5G18BA7(G) | FVFYVGAYPS | + | + | + | + |
| hA5G18BA8(Y) | FVFYVGGAPS | + | + | + | + |
| hA5G18BA9(P) | FVFYVGGYAS | + | + | + | - |
| hA5G18BA10(S) | FVFYVGGYPA | + | + | + | + |
| Sequence | Congo Red Staining a | Cell Attachment b | Cell Spreading c |
|---|---|---|---|
| FVFYVGGRGD | + | + | + |
| FVFYVGGRGE | + | + | + |
| FVFYVGGR | + | + | + |
| FVFYVGGK | + | + | + |
| FVFYVGGH | + | - | - |
| RGGFVFYV | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Yamada, Y.; Kumai, J.; Hamada, K.; Kikkawa, Y.; Nomizu, M. Structural Requirement of hA5G18 Peptide (DDFVFYVGGYPS) from Laminin α5 Chain for Amyloid-like Fibril Formation and Cell Adhesion. Molecules 2022, 27, 6610. https://doi.org/10.3390/molecules27196610
Zhang G, Yamada Y, Kumai J, Hamada K, Kikkawa Y, Nomizu M. Structural Requirement of hA5G18 Peptide (DDFVFYVGGYPS) from Laminin α5 Chain for Amyloid-like Fibril Formation and Cell Adhesion. Molecules. 2022; 27(19):6610. https://doi.org/10.3390/molecules27196610
Chicago/Turabian StyleZhang, Guangrui, Yuji Yamada, Jun Kumai, Keisuke Hamada, Yamato Kikkawa, and Motoyoshi Nomizu. 2022. "Structural Requirement of hA5G18 Peptide (DDFVFYVGGYPS) from Laminin α5 Chain for Amyloid-like Fibril Formation and Cell Adhesion" Molecules 27, no. 19: 6610. https://doi.org/10.3390/molecules27196610
APA StyleZhang, G., Yamada, Y., Kumai, J., Hamada, K., Kikkawa, Y., & Nomizu, M. (2022). Structural Requirement of hA5G18 Peptide (DDFVFYVGGYPS) from Laminin α5 Chain for Amyloid-like Fibril Formation and Cell Adhesion. Molecules, 27(19), 6610. https://doi.org/10.3390/molecules27196610





