Electrochemical Properties of a Rhodium(III) Mono-Terpyridyl Complex and Use as a Catalyst for Light-Driven Hydrogen Evolution in Water
Abstract
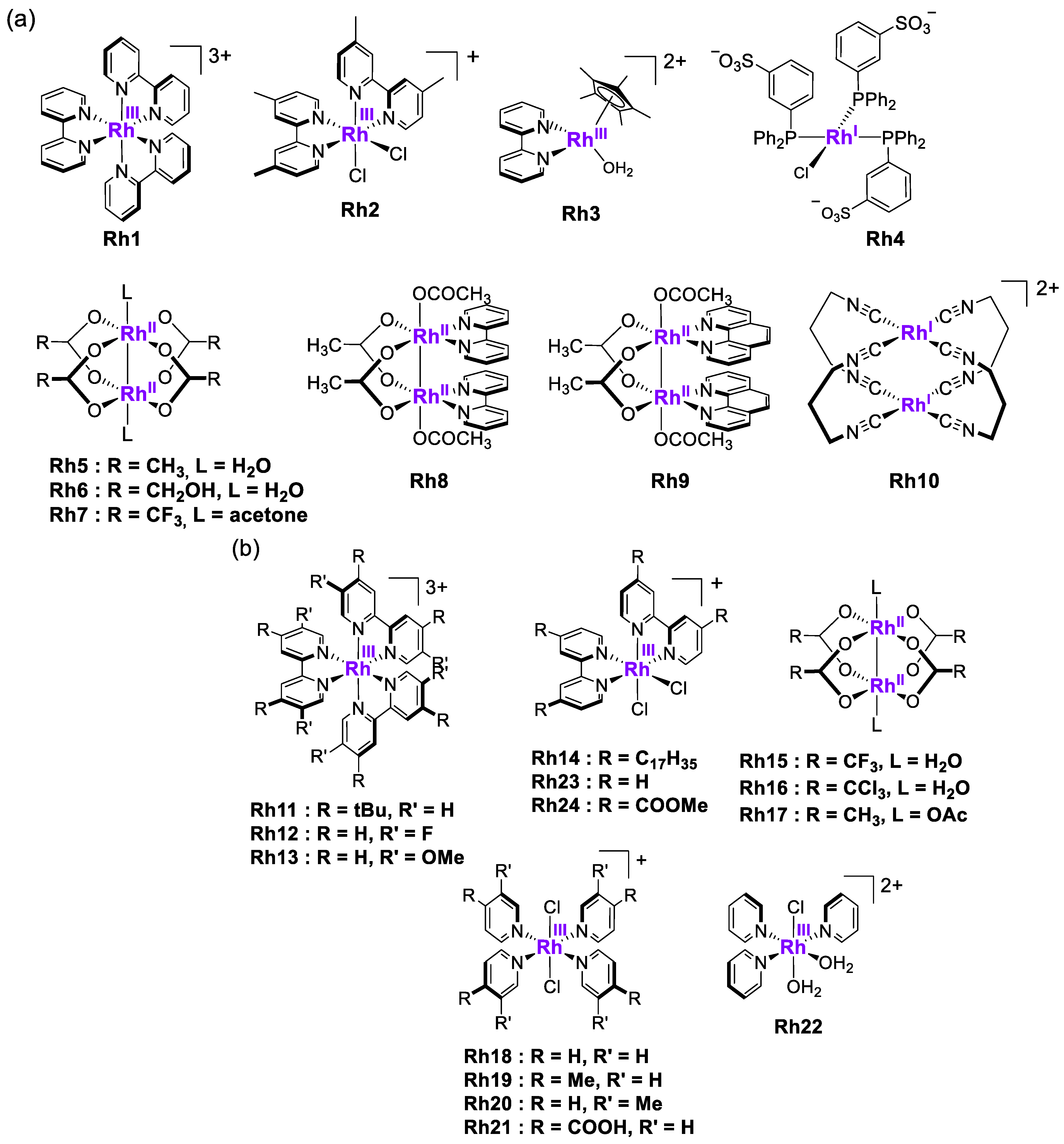
1. Introduction
2. Results and Discussion
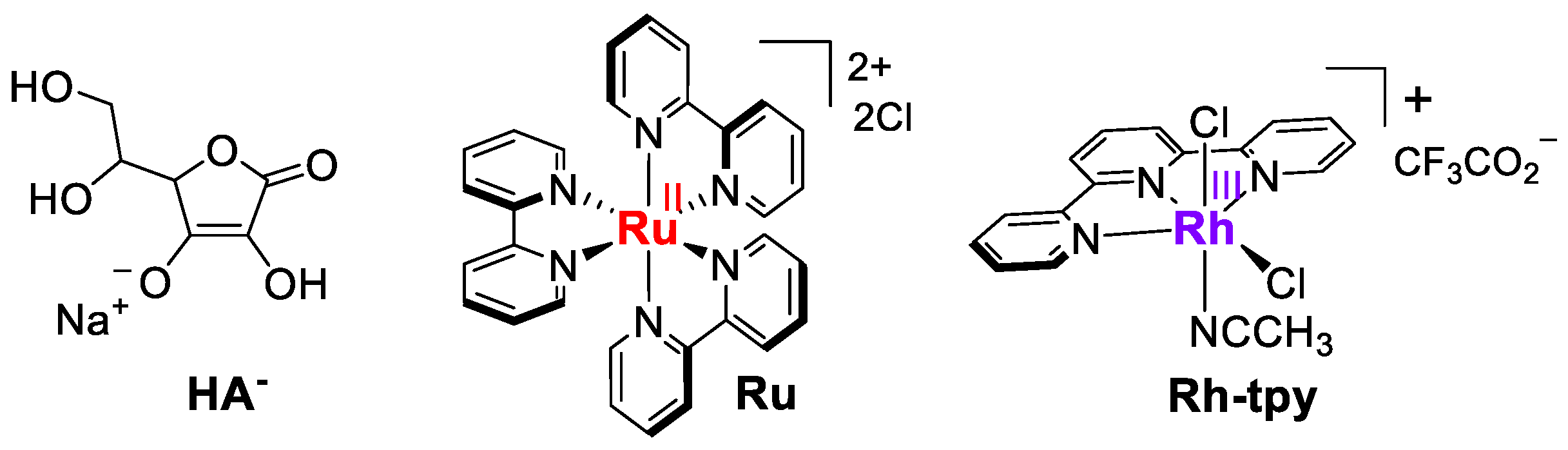
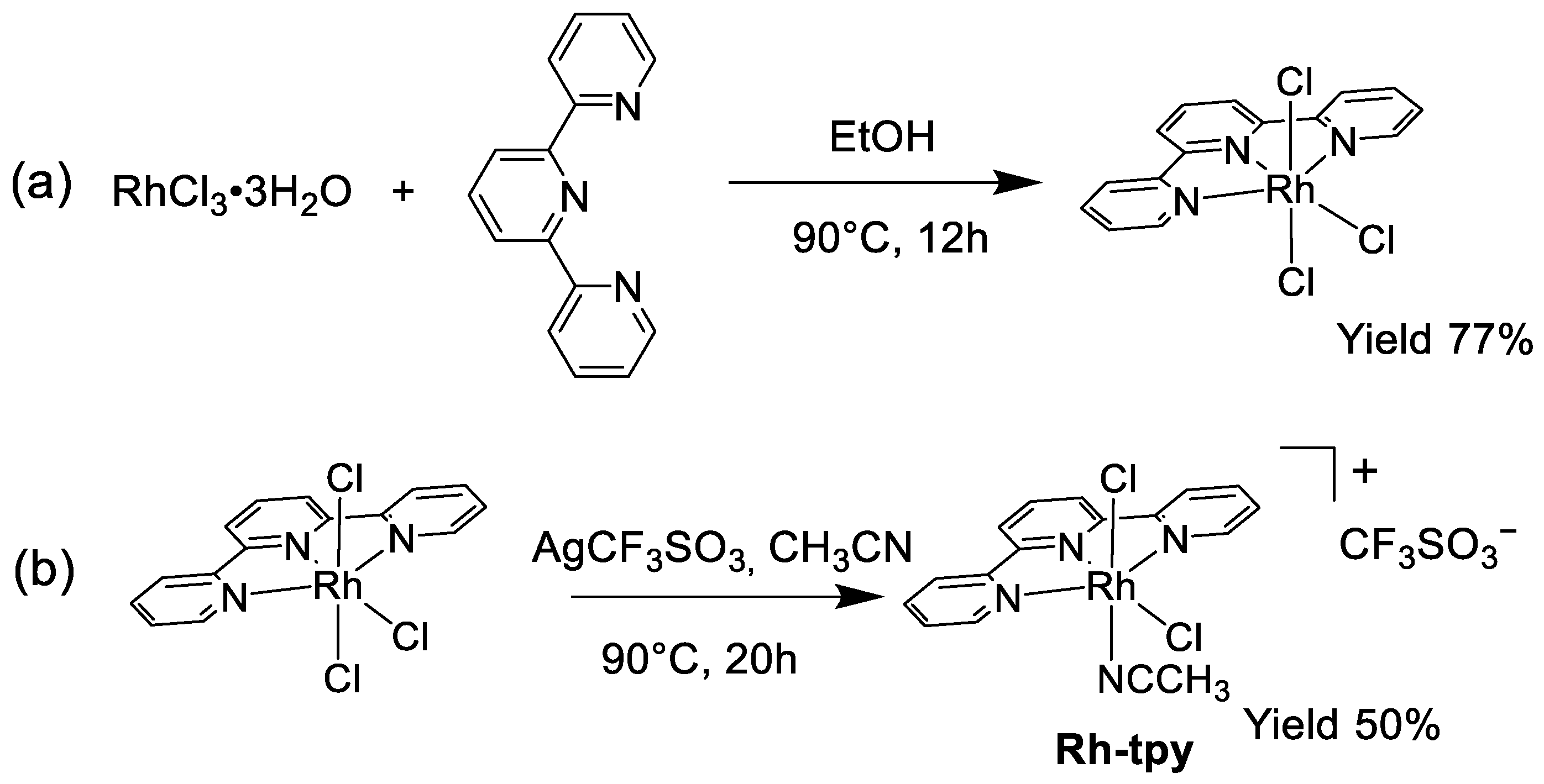
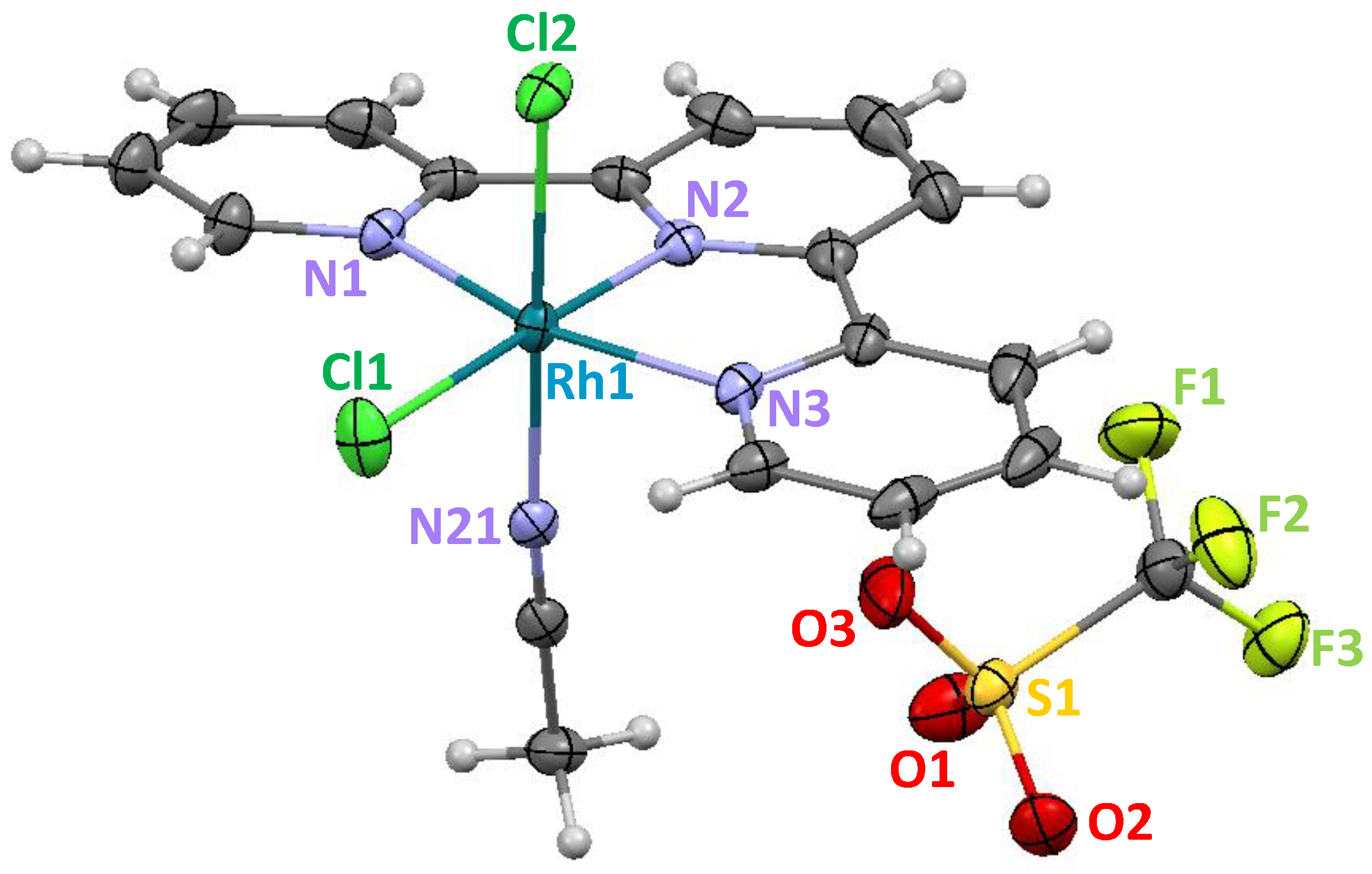
2.1. Synthesis and Crystal Structure of the [RhIII(tpy)(CH3CN)Cl2](CF3SO3) Complex
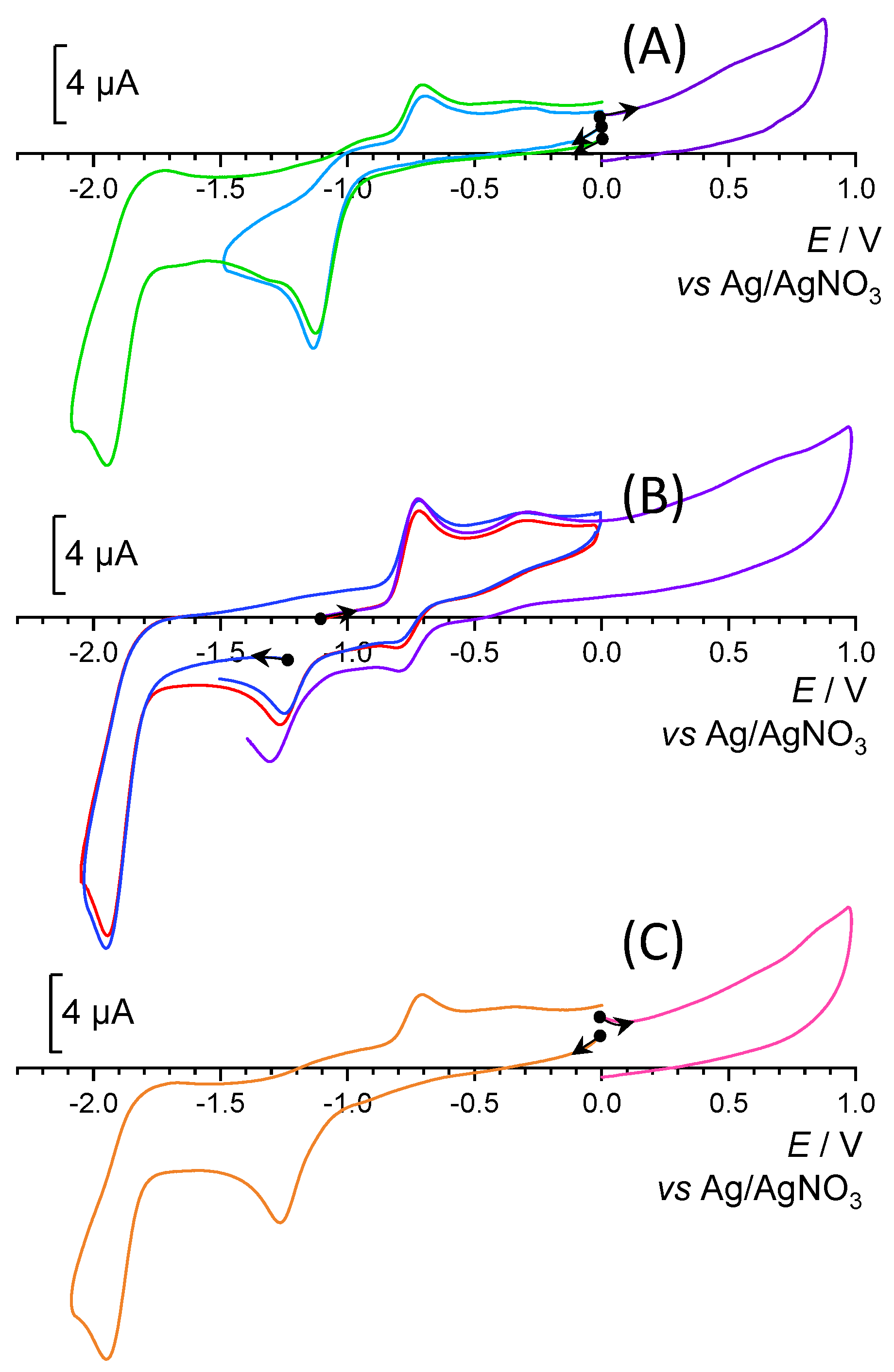
2.2. Spectroelectrochemical Properties of the [RhIII(tpy)(CH3CN)Cl2](CF3SO3) Complex (Rh-tpy) in N,N-Dimethylformamide (DMF)
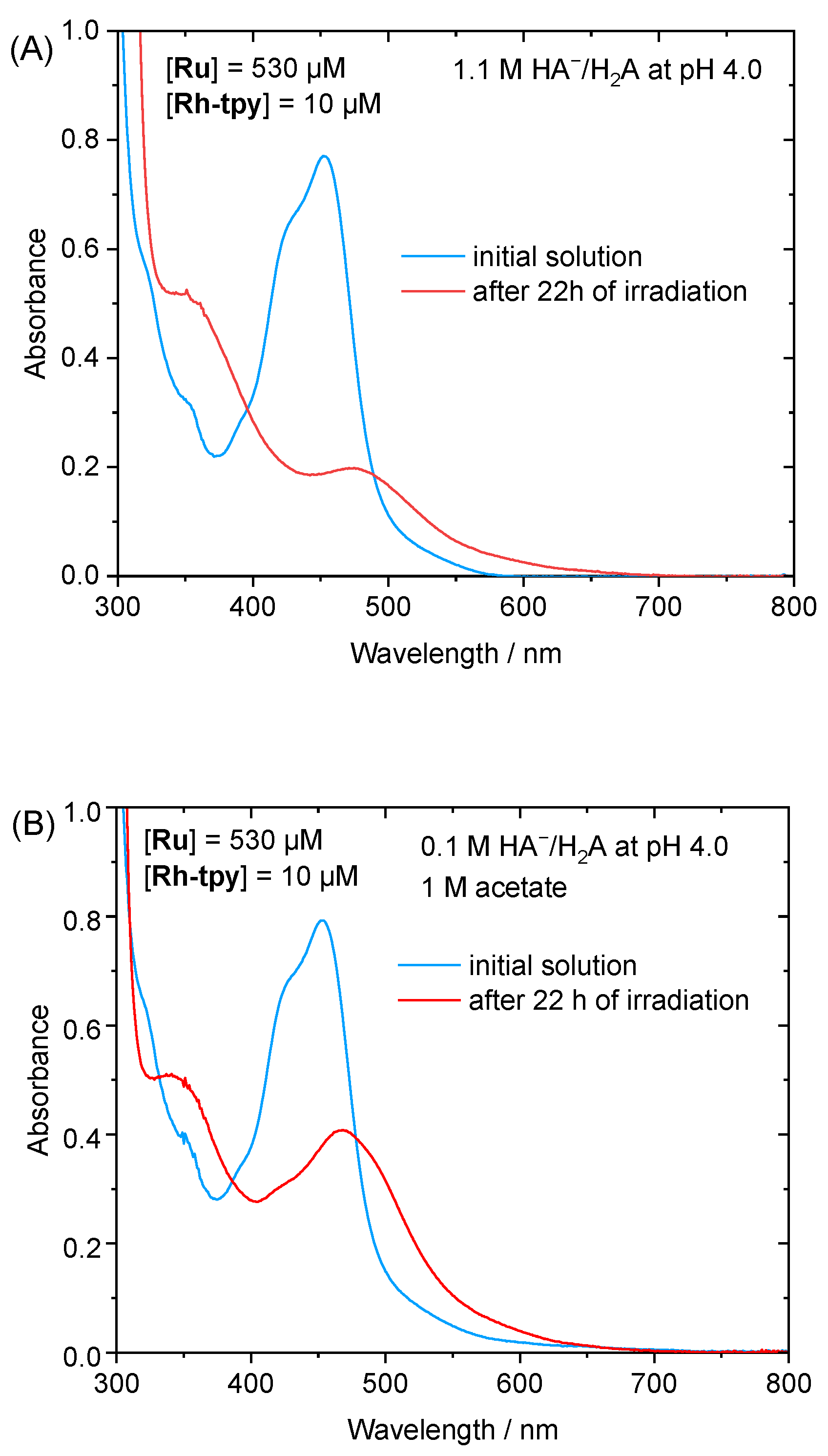
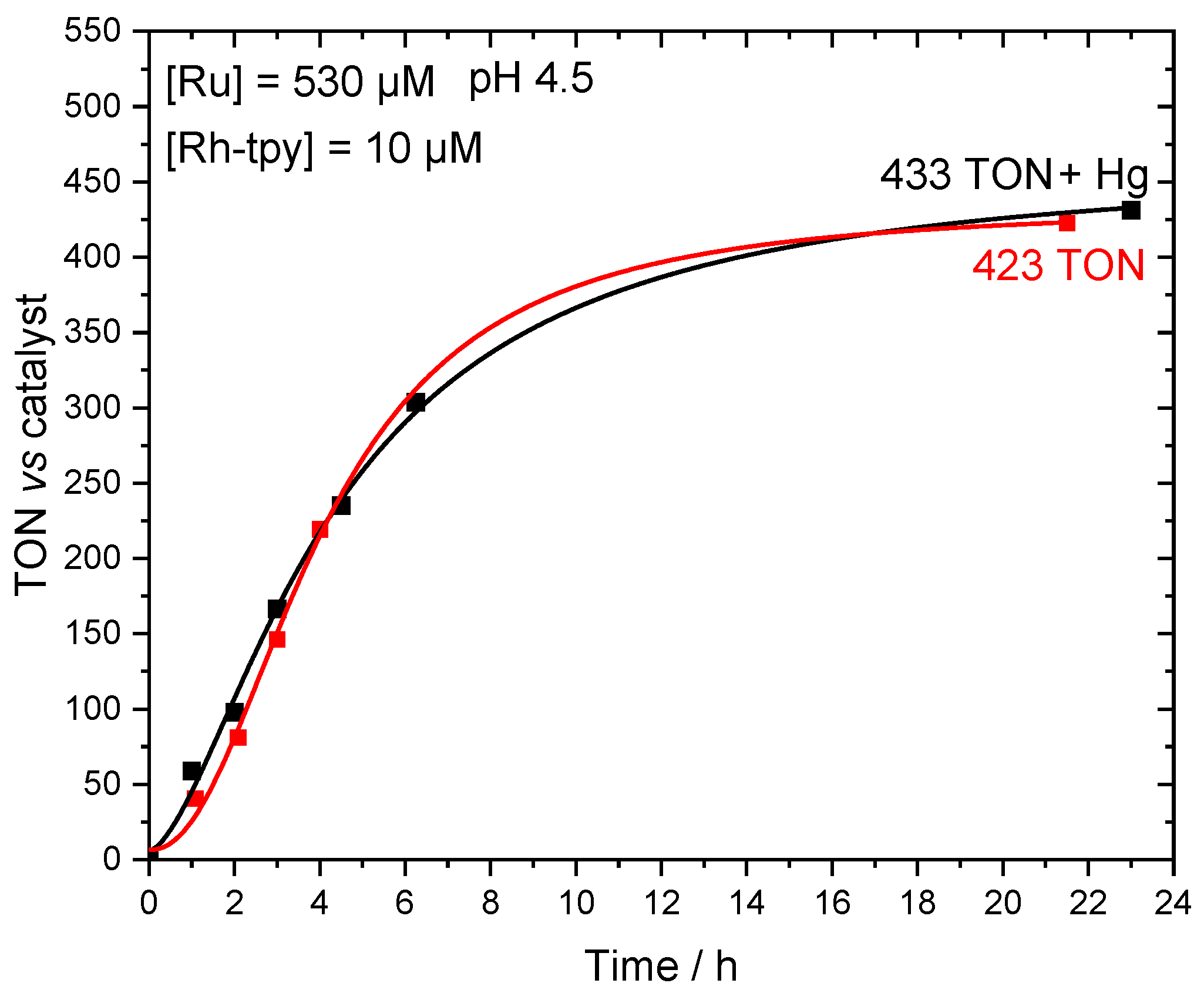
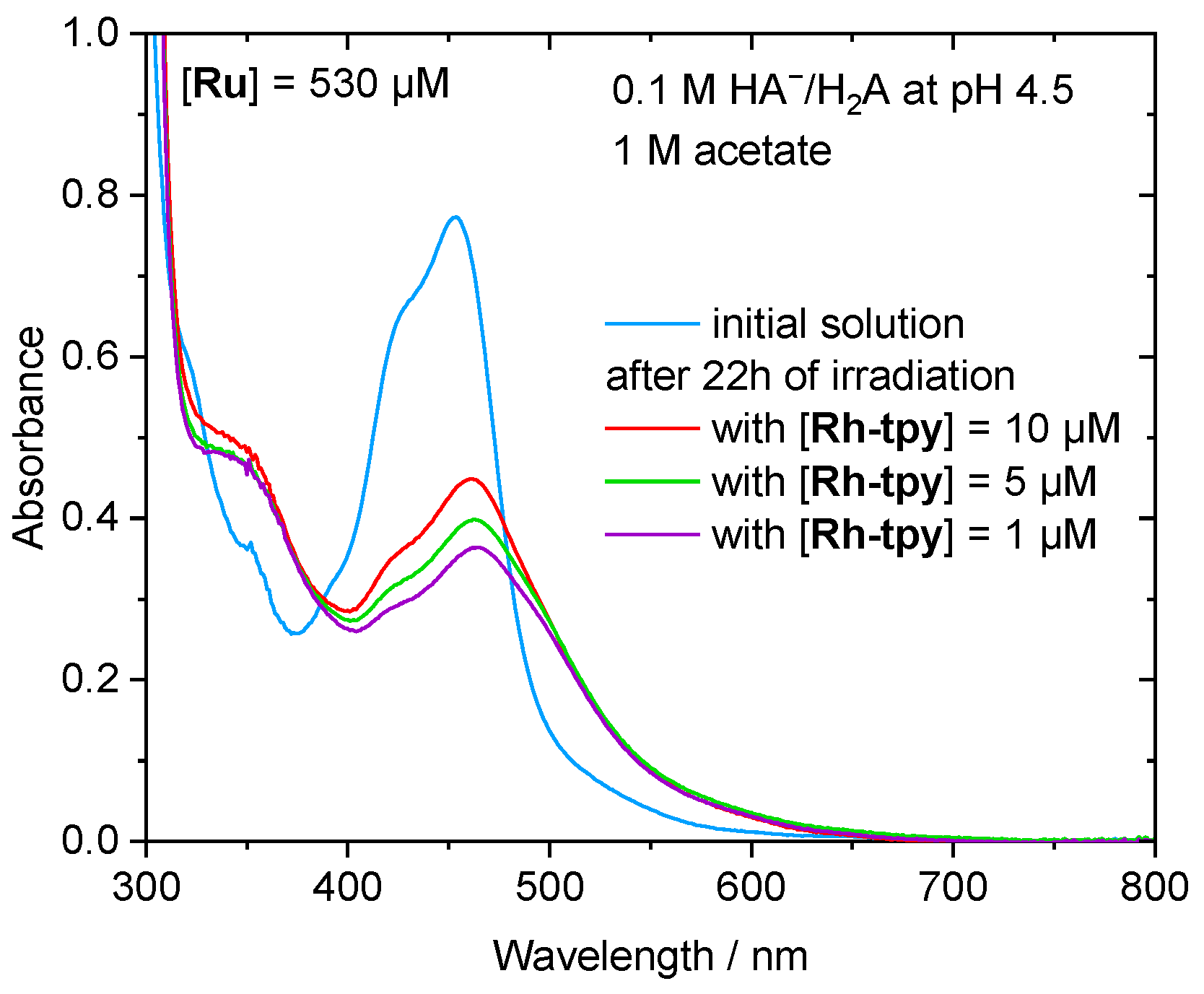
2.3. Photocatalytic Hydrogen Production
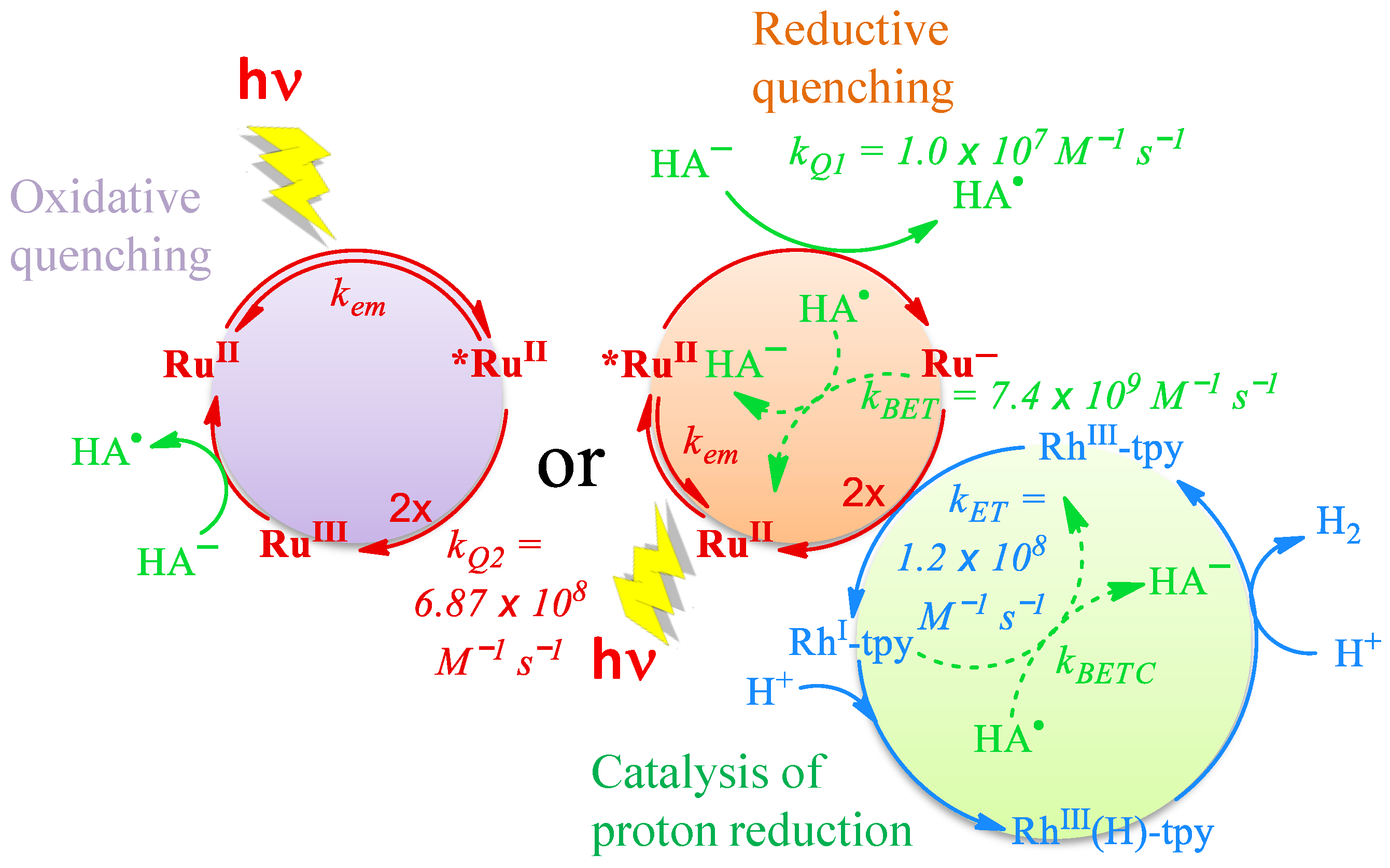
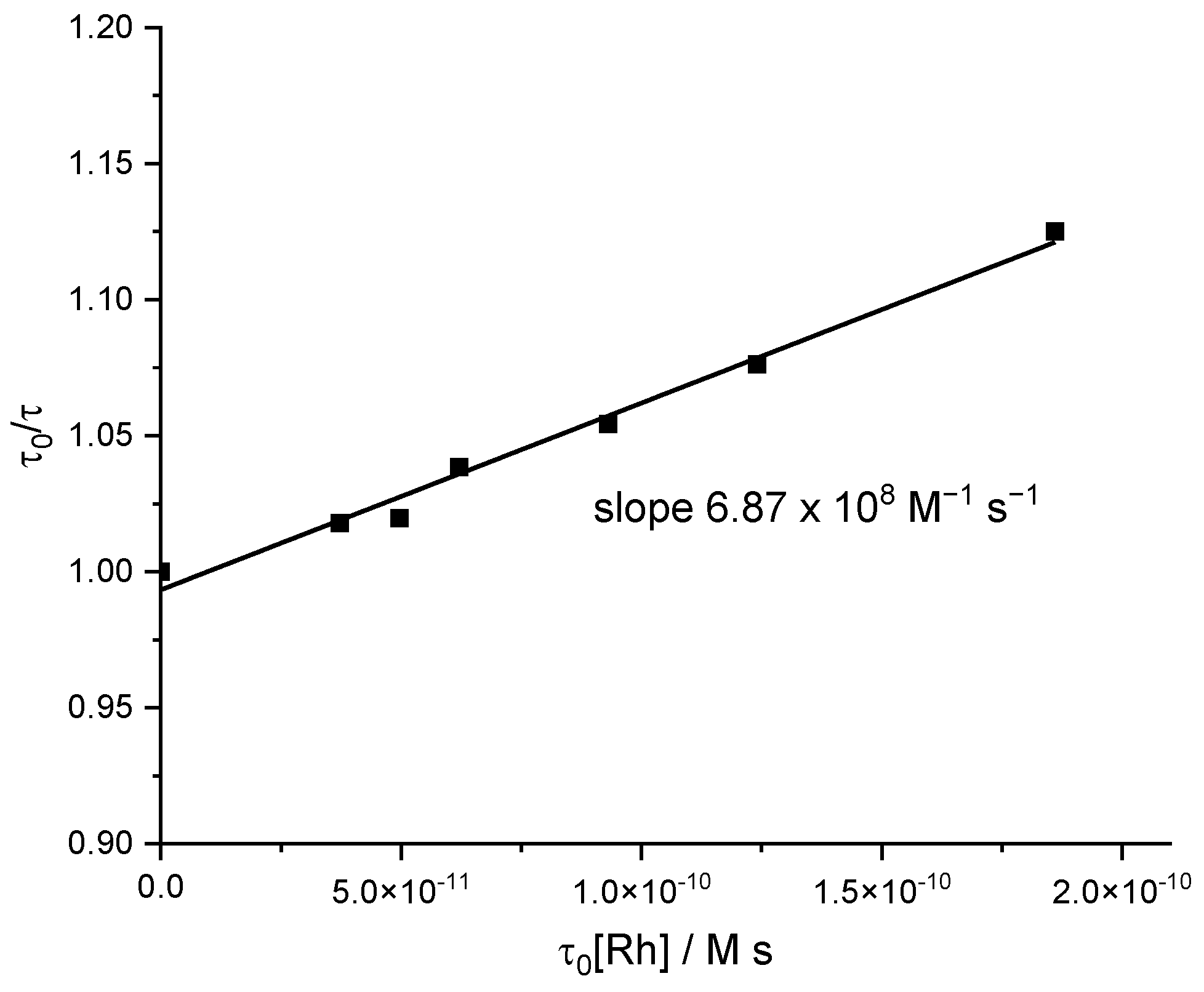
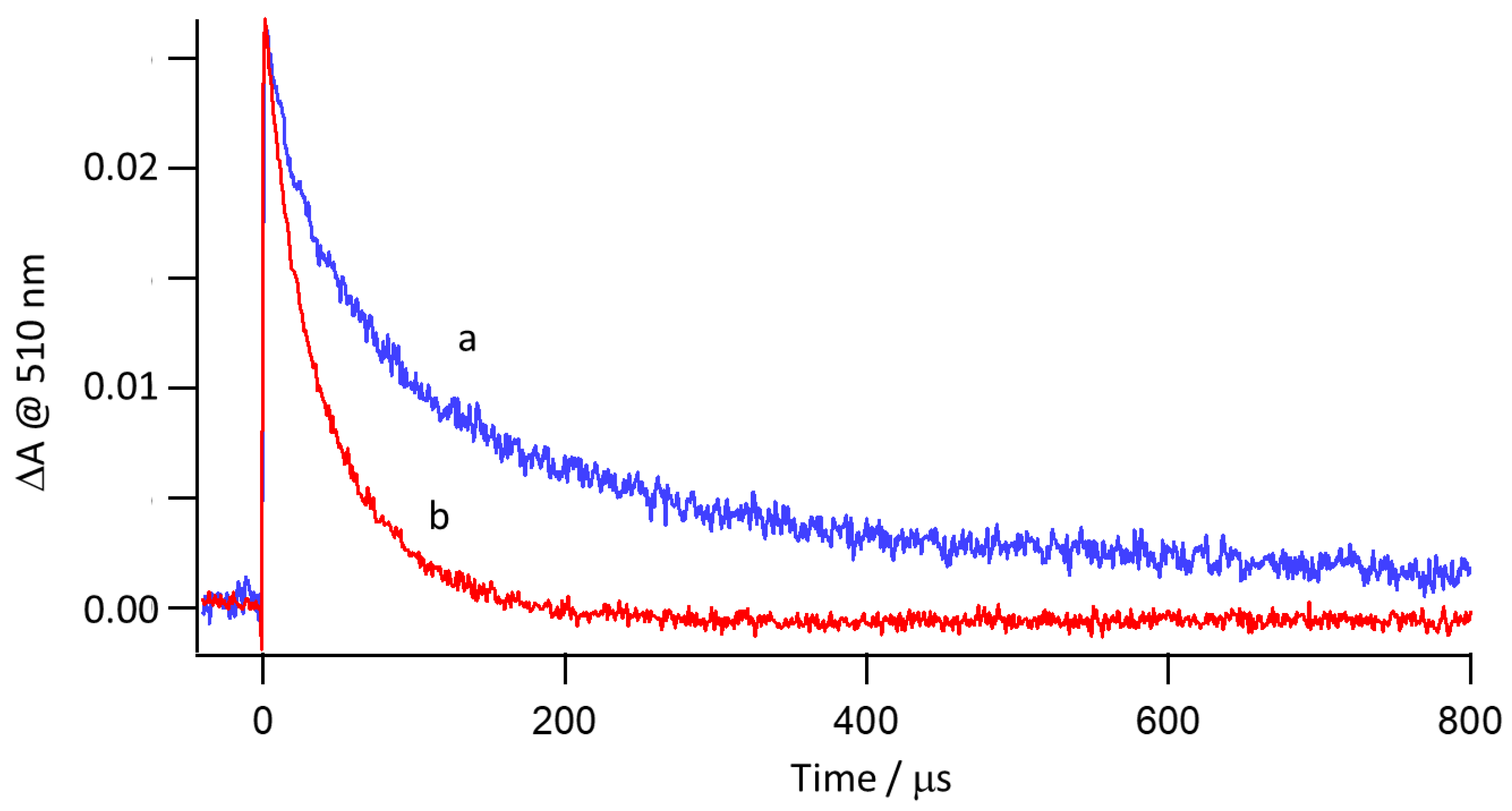
2.4. Mechanistic Insight for the Ru/Rh-tpy/HA−/H2A System from Photophysical Measurements
3. Materials and Methods
3.1. Synthesis of [RhIII(tpy)Cl3]
3.2. Synthesis of [RhIII(tpy)(CH3CN)Cl2](CF3SO3) (Rh-tpy)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Armaroli, N.; Balzani, V. The Hydrogen Issue. ChemSusChem 2011, 4, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.M.; Sauvage, J.P. Chemical Storage of Light energy—Catalytic Generation of Hydrogen by Visible-Light or Sunlight Irradiation of Neutral Aqueous Solution. Nouv. J. Chim. 1977, 1, 449–451. [Google Scholar]
- Kirch, M.; Lehn, J.M.; Sauvage, J.P. Hydrogen Generation by Visible-Light Irradiation of Aqueous-Solutions of Metal-Complexes—Approach to the Photo-Chemical Conversion and Storage of Solar-Energy. Helv. Chim. Acta 1979, 62, 1345–1384. [Google Scholar] [CrossRef]
- Eckenhoff, W.T.; Eisenberg, R. Molecular systems for light driven hydrogen production. Dalton Trans. 2012, 41, 13004–13021. [Google Scholar] [CrossRef]
- Dalle, K.E.; Warnan, J.; Leung, J.J.; Reuillard, B.; Karmel, I.S.; Reisner, E. Electro- and Solar-Driven Fuel Synthesis with First Row Transition Metal Complexes. Chem. Rev. 2019, 119, 2752–2875. [Google Scholar] [CrossRef]
- Oishi, S. A Water-Soluble Wilkinsons Complex as Homogeneous Catalyst for the Photochemical Reduction of Water. J. Mol. Catal. 1987, 39, 225–232. [Google Scholar] [CrossRef]
- Bauer, R.; Werner, H.A.F. Investigations on a Homogeneous Wilkinsons Catalyst for the Water Photolysis. Int. J. Hydrogen Energy 1994, 19, 497–499. [Google Scholar] [CrossRef]
- Tanaka, S.; Masaoka, S.; Yamauchi, K.; Annaka, M.; Sakai, K. Photochemical and thermal hydrogen production from water catalyzed by carboxylate-bridged dirhodium(II) complexes. Dalton Trans. 2010, 39, 11218–11226. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Kobayashi, T.; Suenobu, T. Photocatalytic Production of Hydrogen by Disproportionation of One-Electron-Reduced Rhodium and Iridium–Ruthenium Complexes in Water. Angew. Chem. Int. Ed. 2011, 50, 728–731. [Google Scholar] [CrossRef]
- Xie, J.; Li, C.; Zhou, Q.; Wang, W.; Hou, Y.; Zhang, B.; Wang, X. Large Improvement in the Catalytic Activity Due to Small Changes in the Diimine Ligands: New Mechanistic Insight into the Dirhodium(II,II) Complex-Based Photocatalytic H2 Production. Inorg. Chem. 2012, 51, 6376–6384. [Google Scholar] [CrossRef]
- Stoll, T.; Gennari, M.; Serrano, I.; Fortage, J.; Chauvin, J.; Odobel, F.; Rebarz, M.; Poizat, O.; Sliwa, M.; Deronzier, A.; et al. [RhIII(dmbpy)2Cl2]+ as a Highly Efficient Catalyst for Visible-Light-Driven Hydrogen Production in Pure Water: Comparison with Other Rhodium Catalysts. Chem.-Eur. J. 2013, 19, 782–792. [Google Scholar] [CrossRef]
- Stoll, T.; Gennari, M.; Fortage, J.; Castillo, C.E.; Rebarz, M.; Sliwa, M.; Poizat, O.; Odobel, F.; Deronzier, A.; Collomb, M.-N. An Efficient RuII–RhIII–RuII Polypyridyl Photocatalyst for Visible-Light-Driven Hydrogen Production in Aqueous Solution. Angew. Chem. Int. Ed. 2014, 53, 1654–1658. [Google Scholar] [CrossRef]
- Kayanuma, M.; Stoll, T.; Daniel, C.; Odobel, F.; Fortage, J.; Deronzier, A.; Collomb, M.-N. A computational mechanistic investigation of hydrogen production in water using the [RhIII(dmbpy)2Cl2]+/[RuII(bpy)3]2+/ascorbic acid photocatalytic system. Phys. Chem. Chem. Phys. 2015, 17, 10497–10509. [Google Scholar] [CrossRef]
- Canterbury, T.R.; Arachchige, S.M.; Brewer, K.J.; Moore, R.B. A new hydrophilic supramolecular photocatalyst for the production of H2 in aerobic aqueous solutions. Chem. Commun. 2016, 52, 8663–8666. [Google Scholar] [CrossRef]
- Cline, E.D.; Adamson, S.E.; Bernhard, S. Homogeneous Catalytic System for Photoinduced Hydrogen Production Utilizing Iridium and Rhodium Complexes. Inorg. Chem. 2008, 47, 10378–10388. [Google Scholar] [CrossRef]
- Miyake, Y.; Nakajima, K.; Sasaki, K.; Saito, R.; Nakanishi, H.; Nishibayashi, Y. Design and Synthesis of Diphosphine Ligands Bearing an Osmium(II) Bis(terpyridyl) Moiety as a Light-Harvesting Unit: Application to Photocatalytic Production of Dihydrogen. Organometallics 2009, 28, 5240–5243. [Google Scholar] [CrossRef]
- Wang, J.; White, T.A.; Arachchige, S.M.; Brewer, K.J. A new structural motif for photoinitiated electron collection: Ru,Rh bimetallics providing insight into H2 production via photocatalysis of water reduction by Ru,Rh,Ru supramolecules. Chem. Commun. 2011, 47, 4451–4453. [Google Scholar] [CrossRef]
- White, T.A.; Higgins, S.L.H.; Arachchige, S.M.; Brewer, K.J. Efficient Photocatalytic Hydrogen Production in a Single-Component System Using Ru,Rh,Ru Supramolecules Containing 4,7-Diphenyl-1,10-Phenanthroline. Angew. Chem. Int. Ed. 2011, 50, 12209–12213. [Google Scholar] [CrossRef]
- White, T.A.; Whitaker, B.N.; Brewer, K.J. Discovering the Balance of Steric and Electronic Factors Needed To Provide a New Structural Motif for Photocatalytic Hydrogen Production from Water. J. Am. Chem. Soc. 2011, 133, 15332–15334. [Google Scholar] [CrossRef]
- Peuntinger, K.; Pilz, T.D.; Staehle, R.; Schaub, M.; Kaufhold, S.; Petermann, L.; Wunderlin, M.; Görls, H.; Heinemann, F.W.; Li, J.; et al. Carbene based photochemical molecular assemblies for solar driven hydrogen generation. Dalton Trans. 2014, 43, 13683–13695. [Google Scholar] [CrossRef]
- Zhou, R.; Manbeck, G.F.; Wimer, D.G.; Brewer, K.J. A new RuIIRhIII bimetallic with a single Rh–Cl bond as a supramolecular photocatalyst for proton reduction. Chem. Commun. 2015, 51, 12966–12969. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.T.; Zhou, R.; Quinn, K.S.; White, T.A.; Wang, J.; Brewer, K.J. Tuning the Photophysical Properties of Ru(II) Monometallic and Ru(II),Rh(III) Bimetallic Supramolecular Complexes by Selective Ligand Deuteration. J. Phys. Chem. A 2015, 119, 6781–6790. [Google Scholar] [CrossRef] [PubMed]
- Mengele, A.K.; Kaufhold, S.; Streb, C.; Rau, S. Generation of a stable supramolecular hydrogen evolving photocatalyst by alteration of the catalytic center. Dalton Trans. 2016, 45, 6612–6618. [Google Scholar] [CrossRef] [PubMed]
- Kagalwala, H.N.; Chirdon, D.N.; Mills, I.N.; Budwal, N.; Bernhard, S. Light-Driven Hydrogen Generation from Microemulsions Using Metallosurfactant Catalysts and Oxalic Acid. Inorg. Chem. 2017, 56, 10162–10171. [Google Scholar] [CrossRef] [PubMed]
- Manbeck, G.F.; Fujita, E.; Brewer, K.J. Tetra- and Heptametallic Ru(II),Rh(III) Supramolecular Hydrogen Production Photocatalysts. J. Am. Chem. Soc. 2017, 139, 7843–7854. [Google Scholar] [CrossRef] [PubMed]
- McCullough, B.J.; Neyhouse, B.J.; Schrage, B.R.; Reed, D.T.; Osinski, A.J.; Ziegler, C.J.; White, T.A. Visible-Light-Driven Photosystems Using Heteroleptic Cu(I) Photosensitizers and Rh(III) Catalysts To Produce H2. Inorg. Chem. 2018, 57, 2865–2875. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, S.; Xue, C.; McCullough, B.J.; Roe, S.E.; Neyhouse, B.J.; White, T.A. Probing the Diphosphine Ligand’s Impact within Heteroleptic, Visible-Light-Absorbing Cu(I) Photosensitizers for Solar Fuels Production. ACS Appl. Energy Mater. 2019, 2, 131–143. [Google Scholar] [CrossRef]
- Kataoka, Y.; Yano, N.; Handa, M.; Kawamoto, T. Intrinsic hydrogen evolution capability and a theoretically supported reaction mechanism of a paddlewheel-type dirhodium complex. Dalton Trans. 2019, 48, 7302–7312. [Google Scholar] [CrossRef]
- Kataoka, Y.; Yano, N.; Kohara, Y.; Tsuji, T.; Inoue, S.; Kawamoto, T. Experimental and Theoretical Study of Photochemical Hydrogen Evolution Catalyzed by Paddlewheel-Type Dirhodium Complexes with Electron Withdrawing Carboxylate Ligands. ChemCatChem 2019, 11, 6218–6226. [Google Scholar] [CrossRef]
- Saeedi, S.; White, T.A. Insight into the Reductive Quenching of a Heteroleptic Cu(I) Photosensitizer for Photocatalytic H2 Production. ACS Appl. Energy Mater. 2020, 3, 56–65. [Google Scholar] [CrossRef]
- Whittemore, T.J.; Xue, C.; Huang, J.; Gallucci, J.C.; Turro, C. Single-chromophore single-molecule photocatalyst for the production of dihydrogen using low-energy light. Nat. Chem. 2020, 12, 180–185. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Tkachev, S.; Baidina, I.; Korolkov, I.; Berdyugin, S.; Kozlova, E.; Kozlov, D. Preparation of a rhodium(iii) cis-diaquacomplex by protic acid induced oxalate-release from mer-[Rh(C2O4)Cl(py)3]. New J. Chem. 2018, 42, 19637–19643. [Google Scholar] [CrossRef]
- Vasilchenko, D.B.; Tkachev, S.V.; Kurenkova, A.Y.; Kozlova, E.A.; Kozlov, D.V. Photocatalytic hydrogen evolution by iridium(III)–rhodium(III) system: Effect of the free ligand addition. Int. J. Hydrogen Energy 2016, 41, 2592–2597. [Google Scholar] [CrossRef]
- Stoll, T.; Castillo, C.E.; Kayanuma, M.; Sandroni, M.; Daniel, C.; Odobel, F.; Fortage, J.; Collomb, M.-N. Photo-induced redox catalysis for proton reduction to hydrogen with homogeneous molecular systems using rhodium-based catalysts. Coord. Chem. Rev. 2015, 304–305, 20–37. [Google Scholar] [CrossRef]
- Bakac, A. Aqueous rhodium(III) hydrides and mononuclear rhodium(II) complexes. Dalton Trans. 2006, 13, 1589–1596. [Google Scholar] [CrossRef]
- Castillo, C.E.; Stoll, T.; Sandroni, M.; Gueret, R.; Fortage, J.; Kayanuma, M.; Daniel, C.; Odobel, F.; Deronzier, A.; Collomb, M.-N. Electrochemical Generation and Spectroscopic Characterization of the Key Rhodium(III) Hydride Intermediates of Rhodium Poly(bipyridyl) H2-Evolving Catalysts. Inorg. Chem. 2018, 57, 11225–11239. [Google Scholar] [CrossRef]
- Inoki, D.; Matsumoto, T.; Nakai, H.; Ogo, S. Experimental Study of Reductive Elimination of H2 from Rhodium Hydride Species. Organometallics 2012, 31, 2996–3001. [Google Scholar] [CrossRef]
- Inoki, D.; Matsumoto, T.; Hayashi, H.; Takashita, K.; Nakai, H.; Ogo, S. Establishing the mechanism of Rh-catalysed activation of O2 by H2. Dalton Trans. 2012, 41, 4328–4334. [Google Scholar] [CrossRef]
- Paul, P.; Tyagi, B.; Bilakhiya, A.K.; Bhadbhade, M.M.; Suresh, E.; Ramachandraiah, G. Synthesis and Characterization of Rhodium Complexes Containing 2,4,6-Tris(2-pyridyl)-1,3,5-triazine and Its Metal-Promoted Hydrolytic Products: Potential Uses of the New Complexes in Electrocatalytic Reduction of Carbon Dioxide. Inorg. Chem. 1998, 37, 5733–5742. [Google Scholar] [CrossRef]
- Castillo, C.E.; Gennari, M.; Stoll, T.; Fortage, J.; Deronzier, A.; Collomb, M.N.; Sandroni, M.; Légalité, F.; Blart, E.; Pellegrin, Y.; et al. Visible Light-Driven Electron Transfer from a Dye-Sensitized p-Type NiO Photocathode to a Molecular Catalyst in Solution: Toward NiO-Based Photoelectrochemical Devices for Solar Hydrogen Production. J. Phys. Chem. C 2015, 119, 5806–5818. [Google Scholar] [CrossRef]
- Paul, J.; Spey, S.; Adams, H.; Thomas, J.A. Synthesis and structure of rhodium complexes containing extended terpyridyl ligands. Inorg. Chim. Acta 2004, 357, 2827–2832. [Google Scholar] [CrossRef]
- de Pater, B.C.; Frühauf, H.-W.; Vrieze, K.; de Gelder, R.; Evert, J.B.; McCormack, D.; Lutz, M.; Anthony, L.S.; Hartl, F. Strongly Nucleophilic RhI Centre in Square-Planar Complexes with Terdentate (κ3) 2,2′:6′,2′′-Terpyridine Ligands: Crystallographic, Electrochemical and Density Functional Theoretical Studies. Eur. J. Inorg. Chem. 2004, 2004, 1675–1686. [Google Scholar] [CrossRef]
- Varma, S.; Castillo, C.E.; Stoll, T.; Fortage, J.; Blackman, A.G.; Molton, F.; Deronzier, A.; Collomb, M.-N. Efficient photocatalytic hydrogen production in water using a cobalt(III) tetraaza-macrocyclic catalyst: Electrochemical generation of the low-valent Co(I) species and its reactivity toward proton reduction. Phys. Chem. Chem. Phys. 2013, 15, 17544–17552. [Google Scholar] [CrossRef]
- Gimbert-Surinach, C.; Albero, J.; Stoll, T.; Fortage, J.; Collomb, M.-N.; Deronzier, A.; Palomares, E.; Llobet, A. Efficient and Limiting Reactions in Aqueous Light-Induced Hydrogen Evolution Systems using Molecular Catalysts and Quantum Dots. J. Am. Chem. Soc. 2014, 136, 7655–7661. [Google Scholar] [CrossRef] [PubMed]
- Gueret, R.; Castillo, C.E.; Rebarz, M.; Thomas, F.; Hargrove, A.-A.; Pécaut, J.; Sliwa, M.; Fortage, J.; Collomb, M.-N. Cobalt(III) tetraaza-macrocyclic complexes as efficient catalyst for photoinduced hydrogen production in water: Theoretical investigation of the electronic structure of the reduced species and mechanistic insight. J. Photochem. Photobiol. B Biol. 2015, 152, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.K.C.; Castillo, C.E.; Gueret, R.; Fortage, J.; Rebarz, M.; Sliwa, M.; Thomas, F.; McAdam, C.J.; Jameson, G.B.; McMorran, D.A.; et al. Synthesis, Characterization, and Photocatalytic H2-Evolving Activity of a Family of [Co(N4Py)(X)]n+ Complexes in Aqueous Solution. Inorg. Chem. 2016, 55, 4564–4581. [Google Scholar] [CrossRef] [PubMed]
- Gueret, R.; Castillo, C.E.; Rebarz, M.; Thomas, F.; Sliwa, M.; Chauvin, J.; Dautreppe, B.; Pécaut, J.; Fortage, J.; Collomb, M.-N. Cobalt(II) Pentaaza-Macrocyclic Schiff Base Complex as Catalyst for Light-Driven Hydrogen Evolution in Water: Electrochemical Generation and Theoretical Investigation of the One-Electron Reduced Species. Inorg. Chem. 2019, 58, 9043–9056. [Google Scholar] [CrossRef] [PubMed]
- Singh, W.M.; Baine, T.; Kudo, S.; Tian, S.L.; Ma, X.A.N.; Zhou, H.Y.; DeYonker, N.J.; Pham, T.C.; Bollinger, J.C.; Baker, D.L.; et al. Electrocatalytic and Photocatalytic Hydrogen Production in Aqueous Solution by a Molecular Cobalt Complex. Angew. Chem. Int. Ed. 2012, 51, 5941–5944. [Google Scholar] [CrossRef]
- Khnayzer, R.S.; Thoi, V.S.; Nippe, M.; King, A.E.; Jurss, J.W.; El Roz, K.A.; Long, J.R.; Chang, C.J.; Castellano, F.N. Towards a comprehensive understanding of visible-light photogeneration of hydrogen from water using cobalt(II) polypyridyl catalysts. Energy Environ. Sci. 2014, 7, 1477–1488. [Google Scholar] [CrossRef]
- Singh, W.M.; Mirmohades, M.; Jane, R.T.; White, T.A.; Hammarstrom, L.; Thapper, A.; Lomoth, R.; Ott, S. Voltammetric and Spectroscopic Characterization of Early Intermediates in the Co(II)-Polypyridyl-Catalyzed Reduction of Water. Chem. Commun. 2013, 49, 8638–8640. [Google Scholar] [CrossRef]
- Natali, M.; Badetti, E.; Deponti, E.; Gamberoni, M.; Scaramuzzo, F.A.; Sartorel, A.; Zonta, C. Photoinduced hydrogen evolution with new tetradentate cobalt(ii) complexes based on the TPMA ligand. Dalton Trans. 2016, 45, 14764–14773. [Google Scholar] [CrossRef]
- Lucarini, F.; Pastore, M.; Vasylevskyi, S.; Varisco, M.; Solari, E.; Crochet, A.; Fromm, K.M.; Zobi, F.; Ruggi, A. Heptacoordinate CoII Complex as a New Architecture for Photochemical Hydrogen Production. Chem. Eur. J. 2017, 23, 6768–6771. [Google Scholar] [CrossRef]
- Gueret, R.; Poulard, L.; Oshinowo, M.; Chauvin, J.; Dahmane, M.; Dupeyre, G.; Lainé, P.P.; Fortage, J.; Collomb, M.-N. Challenging the [Ru(bpy)3]2+ Photosensitizer with a Triazatriangulenium Robust Organic Dye for Visible-Light-Driven Hydrogen Production in Water. ACS Catal. 2018, 8, 3792–3802. [Google Scholar] [CrossRef]
- Costentin, C.; Camara, F.; Fortage, J.; Collomb, M.-N. Photoinduced Catalysis of Redox Reactions. Turnover Numbers, Turnover Frequency, and Limiting Processes: Kinetic Analysis and Application to Light-Driven Hydrogen Production. ACS Catal. 2022, 12, 6246–6254. [Google Scholar] [CrossRef]
- Amouyal, E.; Koffi, P. Photochemical Production of Hydrogen from Water. J. Photochem. 1985, 29, 227–242. [Google Scholar] [CrossRef]
- Weddle, K.S.; Aiken, J.D.; Finke, R.G. Rh(0) nanoclusters in benzene hydrogenation catalysis: Kinetic and mechanistic evidence that a putative [(C8H17)3NCH3]+ [RhCl4]− ion-Pair catalyst is actually a distribution of Cl− and [(C8H17)3NCH3]+ stabilized Rh(0) nanoclusters. J. Am. Chem. Soc. 1998, 120, 5653–5666. [Google Scholar] [CrossRef]
- Sandroni, M.; Gueret, R.; Wegner, K.D.; Reiss, P.; Fortage, J.; Aldakov, D.; Collomb, M.-N. Cadmium-Free CuInS2/ZnS Quantum Dots as Efficient and Robust Photosensitizers in combination with a Molecular Catalyst for Visible Light-Driven H2 Production in Water. Energy Environ. Sci. 2018, 11, 1752–1761. [Google Scholar] [CrossRef]
- Bachmann, C.; Guttentag, M.; Spingler, B.; Alberto, R. 3d Element Complexes of Pentadentate Bipyridine-Pyridine-Based Ligand Scaffolds: Structures and Photocatalytic Activities. Inorg. Chem. 2013, 52, 6055–6061. [Google Scholar] [CrossRef]
- Guttentag, M.; Rodenberg, A.; Bachmann, C.; Senn, A.; Hamm, P.; Alberto, R. A highly stable polypyridyl-based cobalt catalyst for homo- and heterogeneous photocatalytic water reduction. Dalton Trans. 2013, 42, 334–337. [Google Scholar] [CrossRef]
- Deponti, E.; Luisa, A.; Natali, M.; Iengo, E.; Scandola, F. Photoinduced hydrogen evolution by a pentapyridine cobalt complex: Elucidating some mechanistic aspects. Dalton Trans. 2014, 43, 16345–16353. [Google Scholar] [CrossRef]
- Natali, M.; Luisa, A.; Lengo, E.; Scandola, F. Efficient photocatalytic hydrogen generation from water by a cationic cobalt(II) porphyrin. Chem. Commun. 2014, 50, 1842–1844. [Google Scholar] [CrossRef]
- Tong, L.; Zong, R.; Thummel, R.P. Visible Light-Driven Hydrogen Evolution from Water Catalyzed by A Molecular Cobalt Complex. J. Am. Chem. Soc. 2014, 136, 4881–4884. [Google Scholar] [CrossRef]
- Vennampalli, M.; Liang, G.; Katta, L.; Webster, C.E.; Zhao, X. Electronic Effects on a Mononuclear Co Complex with a Pentadentate Ligand for Catalytic H2 Evolution. Inorg. Chem. 2014, 53, 10094–10100. [Google Scholar] [CrossRef]
- Bachmann, C.; Probst, B.; Guttentag, M.; Alberto, R. Ascorbate as an electron relay between an irreversible electron donor and Ru(II) or Re(I) photosensitizers. Chem. Commun. 2014, 50, 6737–6739. [Google Scholar] [CrossRef] [PubMed]
- Guttentag, M.; Rodenberg, A.; Kopelent, R.; Probst, B.; Buchwalder, C.; Brandstätter, M.; Hamm, P.; Alberto, R. Photocatalytic H2 Production with a Rhenium/Cobalt System in Water under Acidic Conditions. Eur. J. Inorg. Chem. 2012, 2012, 59–64. [Google Scholar] [CrossRef]
- Mulazzani, Q.G.; Emmi, S.; Fuochi, P.G.; Hoffman, M.Z.; Venturi, M. On the nature of tris(2,2′-bipyridine)ruthenium(1+) ion in aqueous solution. J. Am. Chem. Soc. 1978, 100, 981–983. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camara, F.; Gavaggio, T.; Dautreppe, B.; Chauvin, J.; Pécaut, J.; Aldakov, D.; Collomb, M.-N.; Fortage, J. Electrochemical Properties of a Rhodium(III) Mono-Terpyridyl Complex and Use as a Catalyst for Light-Driven Hydrogen Evolution in Water. Molecules 2022, 27, 6614. https://doi.org/10.3390/molecules27196614
Camara F, Gavaggio T, Dautreppe B, Chauvin J, Pécaut J, Aldakov D, Collomb M-N, Fortage J. Electrochemical Properties of a Rhodium(III) Mono-Terpyridyl Complex and Use as a Catalyst for Light-Driven Hydrogen Evolution in Water. Molecules. 2022; 27(19):6614. https://doi.org/10.3390/molecules27196614
Chicago/Turabian StyleCamara, Fakourou, Thomas Gavaggio, Baptiste Dautreppe, Jérôme Chauvin, Jacques Pécaut, Dmitry Aldakov, Marie-Noëlle Collomb, and Jérôme Fortage. 2022. "Electrochemical Properties of a Rhodium(III) Mono-Terpyridyl Complex and Use as a Catalyst for Light-Driven Hydrogen Evolution in Water" Molecules 27, no. 19: 6614. https://doi.org/10.3390/molecules27196614
APA StyleCamara, F., Gavaggio, T., Dautreppe, B., Chauvin, J., Pécaut, J., Aldakov, D., Collomb, M.-N., & Fortage, J. (2022). Electrochemical Properties of a Rhodium(III) Mono-Terpyridyl Complex and Use as a Catalyst for Light-Driven Hydrogen Evolution in Water. Molecules, 27(19), 6614. https://doi.org/10.3390/molecules27196614






