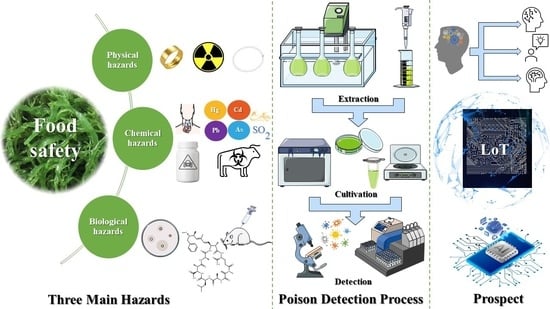
Current Status and Future Trends in Removal, Control, and Mitigation of Algae Food Safety Risks for Human Consumption
Abstract
:1. Introduction
2. Classification of Algal Food and Its Application in Food Industry
2.1. Classification of Algal Food
2.2. Algal Application in Food Industry
3. Physical Factors Affecting Food Safety in Algae
3.1. External Matter in Food Processing
3.2. Radioactive Contamination
4. Chemical Factors Affecting Food Safety in Algae
4.1. Iodine
4.2. Heavy Metals
4.3. Sulfur Dioxide
4.4. Pesticide Residue
4.5. Veterinary Drug Residue
5. Biological Factors Affecting Food Safety in Algae
5.1. Pathogenic Bacteria
5.2. Algal Toxin
5.3. Genetically Modified Seaweeds
6. The Prospect of Food Safety Research on Algae
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahoo, D.; Baweja, P. General Characteristics of Algae; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–29. [Google Scholar]
- Sahoo, D.; Seckbach, J. The Algae World; Springer: Dordrecht, The Netherlands, 2015; Volume 26. [Google Scholar]
- Seckbach, J. Algae and Cyanobacteria in Extreme Environments; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Singh, R.N.; Sharma, S. Development of Suitable Photobioreactor for Algae Production—A Review. Renew. Sustain. Energy Rev. 2012, 16, 2347–2353. [Google Scholar] [CrossRef]
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-C. Solvent Extraction of Fucoxanthin from Phaeodactylum Tricornutum. Sep. Sci. Technol. 2014, 49, 410–415. [Google Scholar] [CrossRef]
- Martín, M.; Grossmann, I.E. Design of an Optimal Process for Enhanced Production of Bioethanol and Biodiesel from Algae Oil via Glycerol Fermentation. Appl. Energy 2014, 135, 108–114. [Google Scholar] [CrossRef]
- Saengsawang, B.; Bhuyar, P.; Manmai, N.; Ponnusamy, V.K.; Ramaraj, R.; Unpaprom, Y. The Optimization of Oil Extraction from Macroalgae, Rhizoclonium Sp. by Chemical Methods for Efficient Conversion into Biodiesel. Fuel 2020, 274, 117841. [Google Scholar] [CrossRef]
- Aravind, S.; Barik, D.; Ragupathi, P.; Vignesh, G. Investigation on Algae Oil Extraction from Algae Spirogyra by Soxhlet Extraction Method. Mater. Today Proc. 2021, 43, 308–313. [Google Scholar] [CrossRef]
- Yadav, G.; Fabiano, L.A.; Soh, L.; Zimmerman, J.; Sen, R.; Seider, W.D. CO2 Process Intensification of Algae Oil Extraction to Biodiesel. AIChE J. 2021, 67, e16992. [Google Scholar] [CrossRef]
- Yu, K.L.; Lau, B.F.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chen, W.-H.; Ng, E.P.; Chang, J.-S. Recent Developments on Algal Biochar Production and Characterization. Bioresour. Technol. 2017, 246, 2–11. [Google Scholar] [CrossRef]
- Chia, S.R.; Nomanbhay, S.B.H.M.; Chew, K.W.; Munawaroh, H.S.H.; Shamsuddin, A.H.; Show, P.L. Algae as Potential Feedstock for Various Bioenergy Production. Chemosphere 2022, 287, 131944. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Algae; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780429095818. [Google Scholar]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2021: Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All; FAO: Rome, Italy, 2021. [Google Scholar]
- Chauton, M.S.; Forbord, S.; Mäkinen, S.; Sarno, A.; Slizyte, R.; Mozuraityte, R.; Standal, I.B.; Skjermo, J. Sustainable Resource Production for Manufacturing Bioactives from Micro- and Macroalgae: Examples from Harvesting and Cultivation in the Nordic Region. Physiol. Plant 2021, 173, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Charoensiddhi, S.; Lorbeer, A.J.; Franco, C.M.M.; Su, P.; Conlon, M.A.; Zhang, W. Process and Economic Feasibility for the Production of Functional Food from the Brown Alga Ecklonia Radiata. Algal Res. 2018, 29, 80–91. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-Value Products from Microalgae-Their Development and Commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Mendes, A.; da Silva, T.L.; Reis, A. DHA Concentration and Purification from the Marine Heterotrophic Microalga Crypthecodinium Cohnii CCMP 316 by Winterization and Urea Complexation. Food Technol. Biotechnol. 2007, 45, 38–44. [Google Scholar]
- Cofrades, S.; López-López, I.; Ruiz-Capillas, C.; Triki, M.; Jiménez-Colmenero, F. Quality Characteristics of Low-Salt Restructured Poultry with Microbial Transglutaminase and Seaweed. Meat Sci. 2011, 87, 373–380. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Recent Developments in the Application of Seaweeds or Seaweed Extracts as a Means for Enhancing the Safety and Quality Attributes of Foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Zailanie, K.; Kartikaningsih, H. Dietary Fiber and Fatty Acids in the Thallus of Brown Alga (Sargassum Duplicatum J.G. Agardh). Int. Food Res. J. 2016, 23, 1584–1589. [Google Scholar]
- Wan-Loy, C.; Siew-Moi, P. Marine Algae as a Potential Source for Anti-Obesity Agents. Mar. Drugs 2016, 14, 222. [Google Scholar] [CrossRef] [Green Version]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Yang, T.-H.; Yao, H.-T.; Chiang, M.-T. Red Algae (Gelidium Amansii) Hot-Water Extract Ameliorates Lipid Metabolism in Hamsters Fed a High-Fat Diet. J. Food Drug Anal. 2017, 25, 931–938. [Google Scholar] [CrossRef] [Green Version]
- Banach, J.L.; Hoek-van den Hil, E.F.; van der Fels-Klerx, H.J. Food Safety Hazards in the European Seaweed Chain. Compr. Rev. Food Sci. Food Saf. 2020, 19, 332–364. [Google Scholar] [CrossRef] [PubMed]
- Lozano Muñoz, I.; Díaz, N.F. Minerals in Edible Seaweed: Health Benefits and Food Safety Issues. Crit. Rev. Food Sci. Nutr. 2020, 62, 1592–1607. [Google Scholar] [CrossRef] [PubMed]
- Tanna, B.; Mishra, A. Nutraceutical Potential of Seaweed Polysaccharides: Structure, Bioactivity, Safety, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef]
- Stewart, J.S.; Lignell, Å.; Pettersson, A.; Elfving, E.; Soni, M.G. Safety Assessment of Astaxanthin-Rich Microalgae Biomass: Acute and Subchronic Toxicity Studies in Rats. Food Chem. Toxicol. 2008, 46, 3030–3036. [Google Scholar] [CrossRef] [PubMed]
- van der Spiegel, M.; Noordam, M.Y.; van der Fels-Klerx, H.J. Safety of Novel Protein Sources (Insects, Microalgae, Seaweed, Duckweed, and Rapeseed) and Legislative Aspects for Their Application in Food and Feed Production. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef]
- ElFar, O.A.; Chang, C.-K.; Leong, H.Y.; Peter, A.P.; Chew, K.W.; Show, P.L. Prospects of Industry 5.0 in Algae: Customization of Production and New Advance Technology for Clean Bioenergy Generation. Energy Convers. Manag. X 2021, 10, 100048. [Google Scholar] [CrossRef]
- Baweja, P.; Sahoo, D. Classification of Algae; Springer: Berlin/Heidelberg, Germany, 2015; pp. 31–55. [Google Scholar]
- Cho, T.J.; Rhee, M.S. Health Functionality and Quality Control of Laver (Porphyra, Pyropia): Current Issues and Future Perspectives as an Edible Seaweed. Mar. Drugs 2019, 18, 14. [Google Scholar] [CrossRef] [Green Version]
- Araújo, R.; Peteiro, C. Algae as Food and Food Supplements in Europe. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC125913 (accessed on 4 August 2022).
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary Fiber, Amino Acid, Fatty Acid and Tocopherol Contents of the Edible Seaweeds Ulva Lactuca and Durvillaea Antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Debbarma, J.; Madhusudana Rao, B.; Murthy, L.N.; Mathew, S.; Venkateshwarlu, G.; Ravishankar, C.N. Nutritional Profiling of the Edible Seaweeds Gracilaria Edulis, Ulva Lactuca and Sargassum sp. Indian J. Fish. 2016, 63, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Rasyid, A. Evaluation of Nutritional Composition of The Dried Seaweed Ulva Lactuca from Pameungpeuk Waters, Indonesia. Trop. Life Sci. Res. 2017, 28, 119–125. [Google Scholar] [CrossRef]
- Pangestuti, R.; Haq, M.; Rahmadi, P.; Chun, B.-S. Nutritional Value and Biofunctionalities of Two Edible Green Seaweeds (Ulva Lactuca and Caulerpa Racemosa) from Indonesia by Subcritical Water Hydrolysis. Mar. Drugs 2021, 19, 578. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.A.; Neveux, N.; Magnusson, M.; de Nys, R. Comparative Production and Nutritional Value of “Sea Grapes”—the Tropical Green Seaweeds Caulerpa Lentillifera and C. Racemosa. J. Appl. Phycol. 2014, 26, 1833–1844. [Google Scholar] [CrossRef]
- Delan, G.G.; Legados, J.A.; Pepito, A.R.; Cunado, V.D.; Rica, R.L.; Abdon, H.C.; Ilano, A.S. The Influence of Habitat on the Quality Characteristics of the Green Macro Alga Caulerpa Lentillifera Agardh (Caulerpaceae, Chlorophyta). Trop. Technol. J. 2015, 19, 1–7. [Google Scholar] [CrossRef]
- Tang, G.; Suter, P.M. Vitamin A, Nutrition, and Health Values of Algae: Spirulina, Chlorella, and Dunaliella. J. Pharm. Nutr. Sci. 2011, 1, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Kotrbáček, V.; Doubek, J.; Doucha, J. The Chlorococcalean Alga Chlorella in Animal Nutrition: A Review. J. Appl. Phycol. 2015, 27, 2173–2180. [Google Scholar] [CrossRef]
- Domínguez, H. 1-Algae as a Source of Biologically Active Ingredients for the Formulation of Functional Foods and Nutraceuticals. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Verma, P.; Arun, A.; Sahoo, D. Brown Algae; A.P.H. Publishing Corporation: Delhi, India, 2015; pp. 177–204. [Google Scholar]
- Sappati, P.K.; Nayak, B.; VanWalsum, G.P.; Mulrey, O.T. Combined Effects of Seasonal Variation and Drying Methods on the Physicochemical Properties and Antioxidant Activity of Sugar Kelp (Saccharina latissima). J. Appl. Phycol. 2019, 31, 1311–1332. [Google Scholar] [CrossRef]
- Liu, X.; Xi, X.; Jia, A.; Zhang, M.; Cui, T.; Bai, X.; Shi, Y.; Liu, C. A Fucoidan from Sargassum Fusiforme with Novel Structure and Its Regulatory Effects on Intestinal Microbiota in High-Fat Diet-Fed Mice. Food Chem. 2021, 358, 129908. [Google Scholar] [CrossRef]
- Wu, S.; Zuo, J.; Cheng, Y.; Zhang, Y.; Zhang, Z.; Wu, M.; Yang, Y.; Tong, H. Ethanol Extract of Sargarsum Fusiforme Alleviates HFD/STZ-Induced Hyperglycemia in Association with Modulation of Gut Microbiota and Intestinal Metabolites in Type 2 Diabetic Mice. Food Res. Int. 2021, 147, 110550. [Google Scholar] [CrossRef]
- Goswami, G.; Bang, V.; Agarwal, S. Diverse Applications of Algae. Int. J. Adv. Res. Sci. Eng. 2015, 4, 1102–1109. [Google Scholar]
- Weinrich, R.; Elshiewy, O. Preference and Willingness to Pay for Meat Substitutes Based on Micro-Algae. Appetite 2019, 142, 104353. [Google Scholar] [CrossRef]
- Michel, F.; Knaapila, A.; Hartmann, C.; Siegrist, M. A Multi-National Comparison of Meat Eaters’ Attitudes and Expectations for Burgers Containing Beef, Pea or Algae Protein. Food Qual Prefer. 2021, 91, 104195. [Google Scholar] [CrossRef]
- Sadeghi, S.; Jalili, H.; Ranaei Siadat, S.O.; Sedighi, M. Anticancer and Antibacterial Properties in Peptide Fractions from Hydrolyzed Spirulina Protein. J. Agric. Sci. Technol. 2018, 20, 673–683. [Google Scholar]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and Technological Advancements in the Possible Food Applications of Spirulina and Their Health Benefits: A Review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Yukimoto, T. Preparation and Functional Properties of Beverages Made from Sea Algae. Food Chem. 2003, 81, 327–332. [Google Scholar] [CrossRef]
- Samani, S.A.; Jafari, M.; Sahafi, S.M.; Roohinejad, S. Applications of Algae and Algae Extracts in Human Food and Feed. In Recent Advances in Micro and Macroalgal Processing; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Cofrades, S.; Serdaroǧlu, M.; Jiménez-Colmenero, F. Design of Healthier Foods and Beverages Containing Whole Algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, J.; Zhou, D.; Su, L. Research Progress on Applications of Calcium Derived from Marine Organisms. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Panebianco, F.; Giusti, A.; Giarratana, F.; Armani, A. Ethnic Seafood Products Sold on the Italian Market: Labelling Assessment and Biological, Chemical and Physical Risk Characterization. Food Control. 2019, 105, 198–208. [Google Scholar] [CrossRef]
- Kusaba, Y.; Miwa, T.; Ise, M.; Minoda, R. Pharyngocutaneous Fistula Caused by Dried “Kombu” (Edible Seaweed) after Total Laryngectomy. BMJ Case Rep. 2019, 12, e228091. [Google Scholar] [CrossRef]
- Matos, Â.P. The Impact of Microalgae in Food Science and Technology. J. Am. Oil Chem. Soc. 2017, 94, 1333–1350. [Google Scholar] [CrossRef]
- Enzing, C.; Ploeg, M.; Barbosa, M.; Sijtsma, L. Microalgae-Based Products for the Food and Feed Sector: An Outlook for Europe; JRC Scientific and Policy Reports; Joint Research Centre: Luxembourg, 2014. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef] [Green Version]
- Gutow, L.; Eckerlebe, A.; Giménez, L.; Saborowski, R. Experimental Evaluation of Seaweeds as a Vector for Microplastics into Marine Food Webs. Environ. Sci. Technol. 2016, 50, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Akama, A. Radioactive Contamination of Aquatic Insects in a Stream Impacted by the Fukushima Nuclear Power Plant Accident. Hydrobiologia 2014, 722, 19–30. [Google Scholar] [CrossRef]
- Kawai, H.; Kitamura, A.; Mimura, M.; Mimura, T.; Tahara, T.; Aida, D.; Sato, K.; Sasaki, H. Radioactive Cesium Accumulation in Seaweeds by the Fukushima 1 Nuclear Power Plant Accident-Two Years’ Monitoring at Iwaki and Its Vicinity. J. Plant Res. 2014, 127, 23–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M.; Ohte, N.; Suzuki, T.; Ishii, N.; Igarashi, Y.; Tanoi, K. Biological Proliferation of Cesium-137 through the Detrital Food Chain in a Forest Ecosystem in Japan. Sci. Rep. 2014, 4, 3599. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Funaki, H.; Iri, S.; Dohi, T.; Hagiwara, H. Fate of Radiocesium in Freshwater Aquatic Plants and Algae in the Vicinity of the Fukushima Daiichi Nuclear Power Plant. Limnology 2016, 17, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Shigeoka, Y.; Myose, H.; Akiyama, S.; Matsumoto, A.; Hirakawa, N.; Ohashi, H.; Higuchi, K.; Arakawa, H. Temporal Variation of Radionuclide Contamination of Marine Plants on the Fukushima Coast after the East Japan Nuclear Disaster. Environ. Sci. Technol. 2019, 53, 9370–9377. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Li, J.; Zhou, D.; Guo, R.; Ji, R.; Chen, J. The Bioaccumulation, Elimination, and Trophic Transfer of BDE-47 in the Aquatic Food Chain of Chlorella Pyrenoidosa-Daphnia Magna. Environ. Pollut. 2020, 258, 113720. [Google Scholar] [CrossRef]
- Vardon, P.; Sassi, A.; Zheng, Y.; Birur, D. Fukushima: U.S. Response and the Short-Term Impact on U.S.-Japan Trade in Fish and Seafood. J. Benefit-Cost Anal. 2019, 10, 351–378. [Google Scholar] [CrossRef]
- Pearce, E.N.; Andersson, M.; Zimmermann, M.B. Global Iodine Nutrition: Where Do We Stand in 2013? Thyroid 2013, 23, 523–528. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Andersson, M. Assessment of Iodine Nutrition in Populations: Past, Present, and Future. Nutr. Rev. 2012, 70, 553–570. [Google Scholar] [CrossRef]
- González, A.; Paz, S.; Rubio, C.; Gutiérrez, Á.J.; Hardisson, A. Human Exposure to Iodine from the Consumption of Edible Seaweeds. Biol. Trace Elem. Res. 2020, 197, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Camargo, R.; Knobel, M.; Medeiros-Neto, G. Iodine Nutrition: More Is Better? Arq. Bras. Endocrinol. Metabol. 2007, 51, 2819–2821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Eastman, C.J. The Changing Epidemiology of Iodine Deficiency. Nat. Rev. Endocrinol. 2012, 8, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Gao, Y.; Meng, F.; Liu, S.; Fan, Z.; Wu, J.; Sun, D. Iodine Deficiency and Excess Coexist in China and Induce Thyroid Dysfunction and Disease: A Cross-Sectional Study. PLoS ONE 2014, 9, e111937. [Google Scholar] [CrossRef]
- Watutantrige Fernando, S.; Cavedon, E.; Nacamulli, D.; Pozza, D.; Ermolao, A.; Zaccaria, M.; Girelli, M.E.; Bertazza, L.; Barollo, S.; Mian, C. Iodine Status from Childhood to Adulthood in Females Living in North-East Italy: Iodine Deficiency Is Still an Issue. Eur. J. Nutr. 2016, 55, 335–340. [Google Scholar] [CrossRef]
- Constant, E.L.; de Volder, A.G.; Ivanoiu, A.; Bol, A.; Labar, D.; Seghers, A.; Cosnard, G.; Melin, J.; Daumerie, C. Cerebral Blood Flow and Glucose Metabolism in Hypothyroidism: A Positron Emission Tomography Study. J. Clin. Endocrinol. Metab. 2001, 86, 3864–3870. [Google Scholar] [CrossRef]
- Jeon, M.J.; Kim, W.G.; Kwon, H.; Kim, M.; Park, S.; Oh, H.S.; Han, M.; Kim, T.Y.; Shong, Y.K.; Kim, W.B. Excessive Iodine Intake and Thyrotropin Reference Interval: Data from the Korean National Health and Nutrition Examination Survey. Thyroid 2017, 27, 967–972. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Boelaert, K. Iodine Deficiency and Thyroid Disorders. Lancet Diabetes Endocrinol 2015, 3, 286–295. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, H.K.; Byun, D.W.; Suh, K.; Yoo, M.H.; Min, Y.-K.; Kim, S.W.; Chung, J.H. Iodine Intake as a Risk Factor for BRAF Mutations in Papillary Thyroid Cancer Patients from an Iodine-Replete Area. Eur. J. Nutr. 2018, 57, 809–815. [Google Scholar] [CrossRef]
- Zimmermann, M.B. Iodine: It’s Important in Patients That Require Parenteral Nutrition. Gastroenterology 2009, 137, S36–S46. [Google Scholar] [CrossRef] [Green Version]
- Bouga, M.; Combet, E. Emergence of Seaweed and Seaweed-Containing Foods in the Uk: Focus on Labeling, Iodine Content, Toxicity and Nutrition. Foods 2015, 4, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Nunes, N.; Valente, S.; Ferraz, S.; Barreto, M.C.; de Carvalho, M.A.A.P. Validation of a Spectrophotometric Methodology for a Rapid Iodine Analysis in Algae and Seaweed Casts. Algal Res. 2019, 42, 101613. [Google Scholar] [CrossRef]
- Milinovic, J.; Rodrigues, C.; Diniz, M.; Noronha, J.P. Determination of Total Iodine Content in Edible Seaweeds: Application of Inductively Coupled Plasma-Atomic Emission Spectroscopy. Algal Res. 2021, 53, 102149. [Google Scholar] [CrossRef]
- Badocco, D.; di Marco, V.; Piovan, A.; Caniato, R.; Pastore, P. A Procedure for the Quantification of Total Iodine by Inductively Coupled Plasma Mass Spectrometry, and Its Application to the Determination of Iodine in Algae Sampled in the Lagoon of Venice. Anal. Methods 2016, 8, 7545–7551. [Google Scholar] [CrossRef]
- Yeh, T.S.; Hung, N.H.; Lin, T.C. Analysis of Iodine Content in Seaweed by GC-ECD and Estimation of Iodine Intake. J. Food Drug Anal. 2014, 22, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Murata, K.; Sakamoto, M. Minamata Disease. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Hwang, Y.O.; Park, S.G.; Park, G.Y.; Choi, S.M.; Kim, M.Y. Total Arsenic, Mercury, Lead, and Cadmium Contents in Edible Dried Seaweed in Korea. Food Addit. Contam. Part B Surveill. 2010, 3, 7–13. [Google Scholar] [CrossRef]
- Smith, J.; Summers, G.; Wong, R. Nutrient and Heavy Metal Content of Edible Seaweeds in New Zealand. N. Z. J. Crop Hortic. Sci. 2010, 38, 19–28. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Dawczynski, C.; Duelund, L.; Jahreis, G.; Vetter, W.; Schröder, M. On the Human Consumption of the Red Seaweed Dulse (Palmaria Palmata (L.) Weber & Mohr). J. Appl. Phycol. 2013, 25, 1777–1791. [Google Scholar] [CrossRef]
- Sevillano-Morales, J.S.; Cejudo-Gómez, M.; Ramírez-Ojeda, A.M.; Cámara-Martos, F.; Moreno-Rojas, R. Risk Profile of Methylmercury in Seafood. Curr. Opin. Food Sci. 2015, 6, 53–60. [Google Scholar] [CrossRef]
- Filippini, M.; Baldisserotto, A.; Menotta, S.; Fedrizzi, G.; Rubini, S.; Gigliotti, D.; Valpiani, G.; Buzzi, R.; Manfredini, S.; Vertuani, S. Heavy Metals and Potential Risks in Edible Seaweed on the Market in Italy. Chemosphere 2021, 263, 127983. [Google Scholar] [CrossRef]
- Pétursdóttir, Á.H.; Gunnlaugsdóttir, H.; Krupp, E.M.; Feldmann, J. Inorganic Arsenic in Seafood: Does the Extraction Method Matter? Food Chem. 2014, 150, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Lorenc, W.; Kruszka, D.; Kachlicki, P.; Kozłowska, J.; Barałkiewicz, D. Arsenic Species and Their Transformation Pathways in Marine Plants. Usefulness of Advanced Hyphenated Techniques HPLC/ICP-MS and UPLC/ESI-MS/MS in Arsenic Species Analysis. Talanta 2020, 220, 121384. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto-Tanibuchi, E.; Sugimoto, T.; Kawaguchi, T.; Sakakibara, N.; Yamashita, M. Determination of Inorganic Arsenic in Seaweed and Seafood by LC-ICP-MS: Method Validation. J. AOAC Int. 2019, 102, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Eom, H.; Park, M.; Jang, A.; Kim, S.; Oh, S.-E. A Simple and Rapid Algal Assay Kit to Assess Toxicity of Heavy Metal-Contaminated Water. Environ. Pollut. 2021, 269, 116135. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Lin, L.; Wu, M.; Yu, H.; Shang, T.; Zhang, T.; Zhao, M. Total and Inorganic Arsenic Contents in Seaweeds: Absorption, Accumulation, Transformation and Toxicity. Aquaculture 2018, 497, 49–55. [Google Scholar] [CrossRef]
- Lorenc, W.; Hanć, A.; Sajnóg, A.; Barałkiewicz, D. Lc/Icp-Ms And Complementary Techniques In Bespoke And Nontargeted Speciation Analysis Of Elements In Food Samples. Mass Spectrom. Rev. 2022, 41, 32–50. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.; Liu, D.; Zhang, H.; Liu, W.; Cui, G. SO2/NOx emissions and ash formation from algae biomass combustion: Process characteristics and mechanisms. Energy 2016, 113, 821–830. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Cantos-Villar, E. Demonstrating the Efficiency of Sulphur Dioxide Replacements in Wine: A Parameter Review. Trends Food Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
- Qin, G.; Wu, M.; Sang, N. Sulfur Dioxide and Benzo(a)Pyrene Trigger Apoptotic and Anti-Apoptotic Signals at Different Post-Exposure Times in Mouse Liver. Chemosphere 2015, 139, 318–325. [Google Scholar] [CrossRef]
- Qian, B.; Zhao, J.; He, Y.; Peng, L.; Ge, H.; Han, B. Miniaturized Dielectric Barrier Discharge-Molecular Emission Spectrometer for Determination of Total Sulfur Dioxide in Food. Food Chem. 2020, 317, 126437. [Google Scholar] [CrossRef]
- Zhang, J.B.; Zhang, H.; Wang, H.L.; Zhang, J.Y.; Luo, P.J.; Zhu, L.; Wang, Z.T. Risk Analysis of Sulfites Used as Food Additives in China. Biomed. Environ. Sci. 2014, 27, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and Physical Methodologies for the Replacement/Reduction of Sulfur Dioxide Use during Winemaking: Review of Their Potentialities and Limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Chen, W.; Fang, Q.; Yang, D.; Zhang, H.; Song, X.; Foley, J. Selective, Highly Sensitive Fluorescent Probe for the Detection of Sulfur Dioxide Derivatives in Aqueous and Biological Environments. Anal. Chem. 2015, 87, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Ma, W.; Wei, Q.; Zhang, W.; Jiang, F.; Chen, J. Integrated ABR and UASB System for Dairy Wastewater Treatment: Engineering Design and Practice. Sci. Total Environ. 2020, 749, 142267. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Li, G.; Kang, Q.; Xu, Y.; Fu, Y.; Ye, L. Determination of Sulfur Dioxide in Food by Liquid Chromatography with Pre-Column Derivatization. Food Control. 2022, 132, 108500. [Google Scholar] [CrossRef]
- Araya, M.; García, S.; Rengel, J.; Pizarro, S.; Álvarez, G. Determination of Free and Protein Amino Acid Content in Microalgae by HPLC-DAD with Pre-Column Derivatization and Pressure Hydrolysis. Mar. Chem. 2021, 234, 103999. [Google Scholar] [CrossRef]
- Tien, C.-J.; Chen, C.S. Assessing the Toxicity of Organophosphorous Pesticides to Indigenous Algae with Implication for Their Ecotoxicological Impact to Aquatic Ecosystems. J. Environ. Sci. Health Part B 2012, 47, 901–912. [Google Scholar] [CrossRef]
- Lorenzo, R.A.; Pais, S.; Racamonde, I.; García-Rodríguez, D.; Carro, A.M. Pesticides in Seaweed: Optimization of Pressurized Liquid Extraction and in-Cell Clean-up and Analysis by Liquid Chromatography-Mass Spectrometry. Anal. Bioanal. Chem. 2012, 404, 173–181. [Google Scholar] [CrossRef]
- Li, H.; Watson, J.; Zhang, Y.; Lu, H.; Liu, Z. Environment-Enhancing Process for Algal Wastewater Treatment, Heavy Metal Control and Hydrothermal Biofuel Production: A Critical Review. Bioresour. Technol. 2020, 298, 122421. [Google Scholar] [CrossRef]
- Cai, H.; Liang, J.; Ning, X.-A.; Lai, X.; Li, Y. Algal Toxicity Induced by Effluents from Textile-Dyeing Wastewater Treatment Plants. J. Environ. Sci. 2020, 91, 199–208. [Google Scholar] [CrossRef]
- Baruah, P.; Chaurasia, N. Ecotoxicological Effects of Alpha-Cypermethrin on Freshwater Alga Chlorella Sp.: Growth Inhibition and Oxidative Stress Studies. Environ. Toxicol. Pharmacol. 2020, 76, 103347. [Google Scholar] [CrossRef] [PubMed]
- Narenderan, S.T.; Meyyanathan, S.N.; Babu, B. Review of Pesticide Residue Analysis in Fruits and Vegetables. Pre-Treatment, Extraction and Detection Techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xiao, Y.; Pan, H.; Mei, Y. Toxic Effects of Tetracycline and Its Degradation Products on Freshwater Green Algae. Ecotoxicol. Environ. Saf. 2019, 174, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.; Lemos, M.F.L.; Crespo, D.; Nunes, M.; Freitas, A.; Ramos, F.; Pardal, M.Â.; Leston, S. Integrated Multitrophic Aquaculture Systems—Potential Risks for Food Safety. Trends Food Sci. Technol. 2020, 96, 79–90. [Google Scholar] [CrossRef]
- King, N.J.; Powell, J.; Cressey, P.J.; Rivas, L.; Billington, C.; Soboleva, T. Food Safety during Pregnancy; New Zealand Food Safety (Government Agency), Ministry for Primary Industries: Wellington, New Zealand, 2020; ISBN 9781990043826.
- Hayat Mahmud, Z.; Kassu, A.; Mohammad, A.; Yamato, M.; Bhuiyan, N.A.; Nair, G.B.; Ota, F. Isolation and Molecular Characterization of Toxigenic Vibrio Parahaemolyticus from the Kii Channel, Japan. Microbiol. Res. 2006, 161, 25–37. [Google Scholar] [CrossRef]
- Gasanov, U.; Hughes, D.; Hansbro, P.M. Methods for the Isolation and Identification of Listeria Spp. and Listeria monocytogenes: A Review. FEMS Microbiol. Rev. 2005, 29, 851–875. [Google Scholar] [CrossRef] [Green Version]
- Blikra, M.J.; Løvdal, T.; Vaka, M.R.; Roiha, I.S.; Lunestad, B.T.; Lindseth, C.; Skipnes, D. Assessment of Food Quality and Microbial Safety of Brown Macroalgae (Alaria esculenta and Saccharina latissima). J. Sci. Food Agric. 2019, 99, 1198–1206. [Google Scholar] [CrossRef]
- Quijada, N.M.; Hernández, M.; Rodríguez-Lázaro, D. High-Throughput Sequencing and Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 275–300. [Google Scholar]
- Sekse, C.; Holst-Jensen, A.; Dobrindt, U.; Johannessen, G.S.; Li, W.; Spilsberg, B.; Shi, J. High Throughput Sequencing for Detection of Foodborne Pathogens. Front. Microbiol. 2017, 8, 2029. [Google Scholar] [CrossRef]
- Bergwerff, A.A.; Debast, S.B. Modernization of Control of Pathogenic Micro-Organisms in the Food-Chain Requires a Durable Role for Immunoaffinity-Based Detection Methodology—A Review. Foods 2021, 10, 832. [Google Scholar] [CrossRef]
- Lindon, M.; Heiskary, S. Blue-Green Algal Toxin (Microcystin) Levels in Minnesota Lakes. Lake Reserv. Manag. 2009, 25, 240–252. [Google Scholar] [CrossRef]
- Wang, D.-Z. Neurotoxins from Marine Dinoflagellates: A Brief Review. Mar. Drugs 2008, 6, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Boullot, F.; Fabioux, C.; Hégaret, H.; Boudry, P.; Soudant, P.; Benoit, E. Electrophysiological Evaluation of Pacific Oyster (Crassostrea Gigas) Sensitivity to Saxitoxin and Tetrodotoxin. Mar. Drugs 2021, 19, 380. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Sharma, S.A. Toxicological Effects of Marine Seaweeds: A Cautious Insight for Human Consumption. Crit. Rev. Food Sci. Nutr. 2021, 61, 500–521. [Google Scholar] [CrossRef] [PubMed]
- Aubaeed, M.A.; Abdulkareem, K.F.; Kathim, A.S.; Al-Sultan, Y.A.; AL-Sultan, E. Toxic Effects of Neurotoxins (Anatoxin-a) Purified from Blue-Green Algae Pseudoanbaena Limnetica on Some Organs in Laboratory Mice (Mus Musculus L.). Int. J. Pharm. Res. 2020, 12, 2368–2374. [Google Scholar] [CrossRef]
- Mulvenna, V.; Dale, K.; Priestly, B.; Mueller, U.; Humpage, A.; Shaw, G.; Allinson, G.; Falconer, I. Health Risk Assessment for Cyanobacterial Toxins in Seafood. Int. J. Environ. Res. Public Health 2012, 9, 807–820. [Google Scholar] [CrossRef] [Green Version]
- Vilariño, N.; Louzao, M.C.; Fraga, M.; Rodríguez, L.P.; Botana, L.M. Innovative Detection Methods for Aquatic Algal Toxins and Their Presence in the Food Chain Rapid Detection in Food and Feed. Anal. Bioanal. Chem. 2013, 405, 7719–7732. [Google Scholar] [CrossRef]
- Stewart, I.; McLeod, C. The Laboratory Mouse in Routine Food Safety Testing for Marine Algal Biotoxins and Harmful Algal Bloom Toxin Research: Past, Present and Future. J. AOAC Int. 2014, 97, 356–372. [Google Scholar] [CrossRef]
- Bickman, S.R.; Campbell, K.; Elliott, C.; Murphy, C.; O’Kennedy, R.; Papst, P.; Lochhead, M.J. An Innovative Portable Biosensor System for the Rapid Detection of Freshwater Cyanobacterial Algal Bloom Toxins. Environ. Sci. Technol. 2018, 52, 11691–11698. [Google Scholar] [CrossRef] [Green Version]
- Dridi, F.; Marrakchi, M.; Gargouri, M.; Saulnier, J.; Jaffrezic-Renault, N.; Lagarde, F. Nanomaterial-Based Electrochemical Biosensors for Food Safety and Quality Assessment. In Nanobiosensors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 167–204. [Google Scholar]
- Tran, N.H.; Li, Y.; Reinhard, M.; He, Y.; Gin, K.Y.-H. A Sensitive and Accurate Method for Simultaneous Analysis of Algal Toxins in Freshwater Using UPLC-MS/MS and 15N-Microcystins as Isotopically Labelled Internal Standards. Sci. Total Environ. 2020, 738, 139727. [Google Scholar] [CrossRef]
- Zhang, W.; Dixon, M.B.; Saint, C.; Teng, K.S.; Furumai, H. Electrochemical Biosensing of Algal Toxins in Water: The Current State-of-the-Art. ACS Sens. 2018, 3, 1233–1245. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, B.; Furumai, H. Fabrication of Graphene Film Composite Electrochemical Biosensor as a Pre-Screening Algal Toxin Detection Tool in the Event of Water Contamination. Sci. Rep. 2018, 8, 10686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogiazi, V.; de La Cruz, A.; Mishra, S.; Shanov, V.; Heineman, W.R.; Dionysiou, D.D. A Comprehensive Review: Development of Electrochemical Biosensors for Detection of Cyanotoxins in Freshwater. ACS Sens. 2019, 4, 1151–1173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luo, J.; Beloglazova, N.; Yang, S.; de Saeger, S.; Mari, G.M.; Zhang, S.; Shen, J.; Wang, Z.; Yu, X. Portable Multiplex Immunochromatographic Assay for Quantitation of Two Typical Algae Toxins Based on Dual-Color Fluorescence Microspheres. J. Agric. Food Chem. 2019, 67, 6041–6047. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, J.; He, X.; Hao, S.; Wang, Y.; Zheng, X.; Wang, B. Simple Determination of Six Groups of Lipophilic Marine Algal Toxins in Seawater by Automated On-Line Solid Phase Extraction Coupled to Liquid Chromatography-Tandem Mass Spectrometry. Chemosphere 2021, 262, 128374. [Google Scholar] [CrossRef]
- Panda, D.; Dash, B.P.; Manickam, S.; Boczkaj, G. Recent Advancements in LC-MS Based Analysis of Biotoxins: Present and Future Challenges. Mass Spectrom. Rev. 2021. [Google Scholar] [CrossRef]
- Wu, X.; Hou, L.; Lin, X.; Xie, Z. Application of Novel Nanomaterials for Chemo- and Biosensing of Algal Toxins in Shellfish and Water. In Novel Nanomaterials for Biomedical, Environmental and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Kordasht, H.K.; Hassanpour, S.; Baradaran, B.; Nosrati, R.; Hashemzaei, M.; Mokhtarzadeh, A.; de la Guardia, M. Biosensing of Microcystins in Water Samples; Recent Advances. Biosens. Bioelectron. 2020, 165, 112403. [Google Scholar] [CrossRef]
- Rodríguez, I.; Alfonso, A.; González-Jartín, J.M.; Vieytes, M.R.; Botana, L.M. A Single Run UPLC-MS/MS Method for Detection of All EU-Regulated Marine Toxins. Talanta 2018, 189, 622–628. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S. Algae-Made Nutraceuticals Produced Using Genetic Engineering Approaches. In Algae-Based Biopharmaceuticals; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Nethravathy, M.U.; Mehar, J.G.; Mudliar, S.N.; Shekh, A.Y. Recent Advances in Microalgal Bioactives for Food, Feed, and Healthcare Products: Commercial Potential, Market Space, and Sustainability. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1882–1897. [Google Scholar] [CrossRef] [Green Version]
- Abdelmoteleb, M.; Zhang, C.; Furey, B.; Kozubal, M.; Griffiths, H.; Champeaud, M.; Goodman, R.E. Evaluating potential risks of food allergy of novel food sources based on comparison of proteins predicted from genomes and compared to www.AllergenOnline.org. Food Chem. Toxicol. 2021, 147, 111888. [Google Scholar] [CrossRef]
- Beacham, T.A.; Sweet, J.B.; Allen, M.J. Large Scale Cultivation of Genetically Modified Microalgae: A New Era for Environmental Risk Assessment. Algal Res. 2017, 25, 90–100. [Google Scholar] [CrossRef]
- Ariawan, E.; Stanley Makalew, A. Smart Micro Farm: Sustainable Algae Spirulina Growth Monitoring System. In Proceedings of the 2018 10th International Conference on Information Technology and Electrical Engineering (ICITEE), Bali, Indonesia, 24–26 July 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 587–591. [Google Scholar]
- Witjaksono, G.; Saeed Rabih, A.A.; Yahya, N.B.; Alva, S. IOT for Agriculture: Food Quality and Safety. IOP Conf. Ser. Mater. Sci. Eng. 2018, 343, 012023. [Google Scholar] [CrossRef]
- Ganjewar, P.D.; Barani, S.; Wagh, S.J.; Sonavane, S.S. Food Monitoring Using Adaptive Naïve Bayes Prediction in IoT. In International Conference on Intelligent Systems Design and Applications; Advances in Intelligent Systems and Computing; Springer: Berlin, Germany, 2020; Volume 940. [Google Scholar]
- Josić, D.; Peršurić, Ž.; Rešetar, D.; Martinović, T.; Saftić, L.; Kraljević Pavelić, S. Use of Foodomics for Control of Food Processing and Assessing of Food Safety. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2017; Volume 81. [Google Scholar]


| Testing Item | Technology of Detecting Seaweeds | Advantages | Limitations | References |
|---|---|---|---|---|
| Iodine | ICP-MS/ICP-AES | High specificity and low detection limit | Pre-treatment is complicated, and the dilution sample easily leads to errors | [84,85] |
| GC-ECD | Low detection limit | Consumable reagents are expensive | [86] |
| Testing Item | Technology of Detecting Seaweeds | Advantages | Limitations | References |
|---|---|---|---|---|
| Arsenic | Electrospray mass spectrometry | Distinguish organic and inorganic arsenic | Intolerant to complex matrix and high salt | [94,97] |
| LC-ICP-MS (Liquid chromatography-inductively coupled plasma mass spectrometry) | High sensitivity, low detection limit, good precision, and wide linear range | Large volume and weight, high price, slow detection speed and high maintenance cost | [95,98] | |
| Mercury | A simple and rapid detection kit | Simple, rapid, low-cost | Only be qualitative, not quantitative | [96] |
| Testing Item | Technology of Detecting Seaweeds | Advantages | Limitations | References |
|---|---|---|---|---|
| Sulfur dioxide | Miniaturized dielectric barrier discharge—molecular emission spectrometry | Good linear relationship, accurate detection results, low cost, compact detection equipment | Detection time is long, instrument is complex and expensive | [102] |
| Liquid chromatography with pre-column derivatization | Short detection time, high sensitivity, and specificity | Consider using HPLC rather than LC | [107,108] | |
| Electrospray mass spectrometry | Distinguish organic and inorganic arsenic | Intolerant to complex matrix and high salt | [94,97] |
| Testing Item | Technology of Detecting Seaweeds | Advantages | Limitations | References |
|---|---|---|---|---|
| Algal toxins | Photochemical and biosensor | Smaller sample numbers and shorter response times | Pre-treatment complex, susceptible to environmental | [130,135,136,137] |
| Fluorescence microsphere-based | Low cost, simple and low interference, and can detect a variety of toxins | Nonspecific fluorescence limits, sensitivity low sensitivity | [130,138,139] | |
| Liquid chromatography-mass spectrometry (LC-MS) | High sensitivity, good detection limit, convenience, and effectiveness | Price Instruments are expensive and costly to maintain | [130,140,141] | |
| Portable biosensor | Portable, rapid, and simple sample preparation | A short service life span | [132,142,143] | |
| UPLC-MS/MS and 15 N isotope labelling | High analysis speed, high specificity, high sensitivity, high accuracy, high stability | Chromatographic column high pressure, easy to block | [134,144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, G.; Zhuang, D.; Chew, K.W.; Ling, T.C.; Khoo, K.S.; Van Quyen, D.; Feng, S.; Show, P.L. Current Status and Future Trends in Removal, Control, and Mitigation of Algae Food Safety Risks for Human Consumption. Molecules 2022, 27, 6633. https://doi.org/10.3390/molecules27196633
Wu G, Zhuang D, Chew KW, Ling TC, Khoo KS, Van Quyen D, Feng S, Show PL. Current Status and Future Trends in Removal, Control, and Mitigation of Algae Food Safety Risks for Human Consumption. Molecules. 2022; 27(19):6633. https://doi.org/10.3390/molecules27196633
Chicago/Turabian StyleWu, Guowei, Dingling Zhuang, Kit Wayne Chew, Tau Chuan Ling, Kuan Shiong Khoo, Dong Van Quyen, Shuying Feng, and Pau Loke Show. 2022. "Current Status and Future Trends in Removal, Control, and Mitigation of Algae Food Safety Risks for Human Consumption" Molecules 27, no. 19: 6633. https://doi.org/10.3390/molecules27196633
APA StyleWu, G., Zhuang, D., Chew, K. W., Ling, T. C., Khoo, K. S., Van Quyen, D., Feng, S., & Show, P. L. (2022). Current Status and Future Trends in Removal, Control, and Mitigation of Algae Food Safety Risks for Human Consumption. Molecules, 27(19), 6633. https://doi.org/10.3390/molecules27196633