S100 Proteins as Novel Therapeutic Targets in Psoriasis and Other Autoimmune Diseases
Abstract
1. Introduction
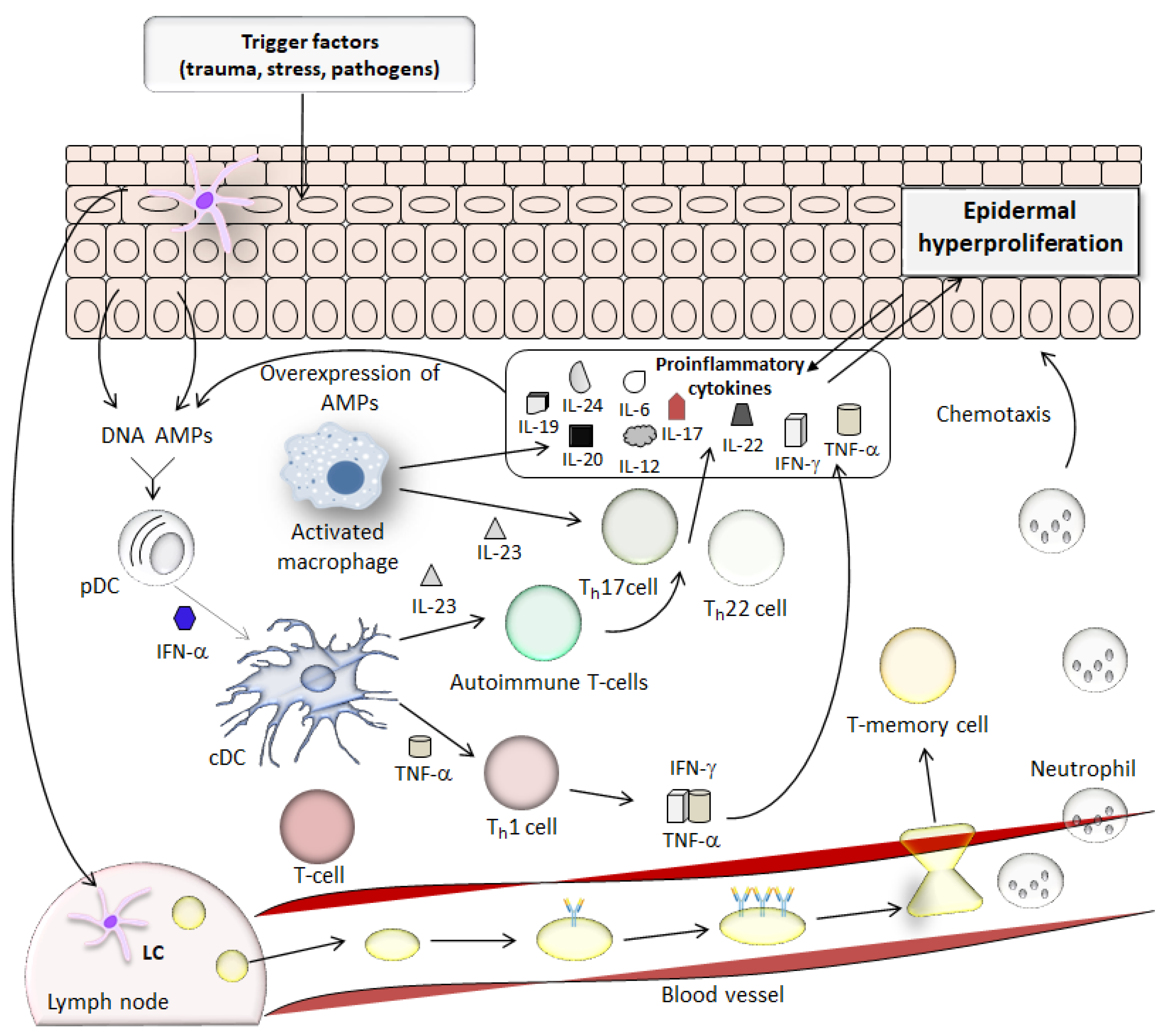
2. Pathogenesis of Psoriasis
3. Antimicrobial Peptides and Proteins
4. Therapeutic Potential of AMPs
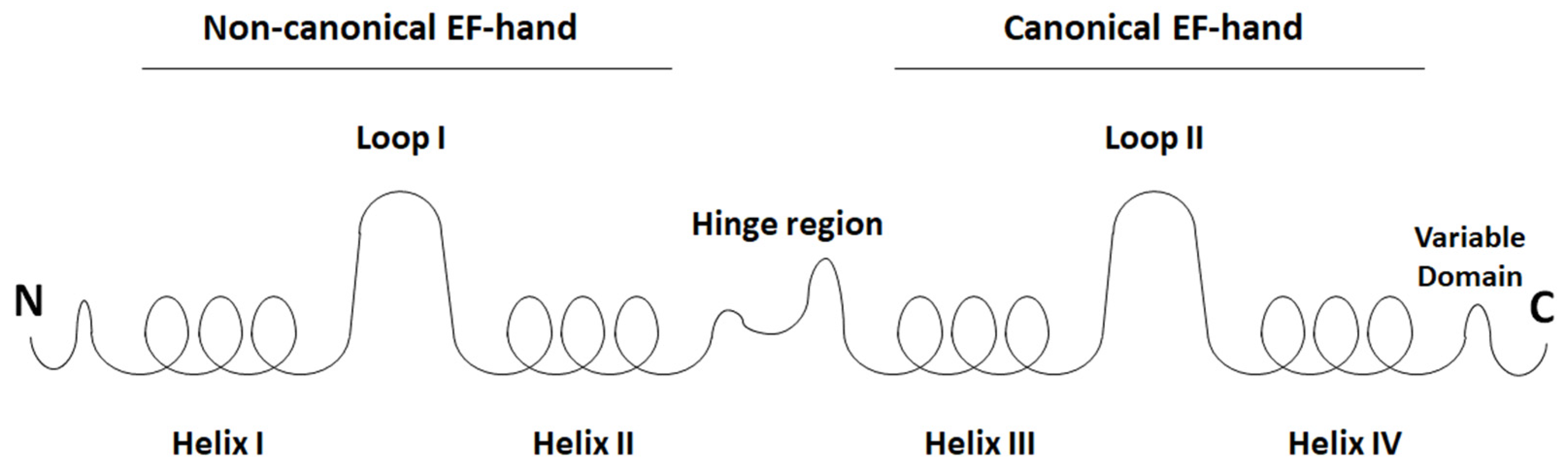
5. S100 Proteins
6. S100 Proteins as Biomarkers and Therapeutic Targets
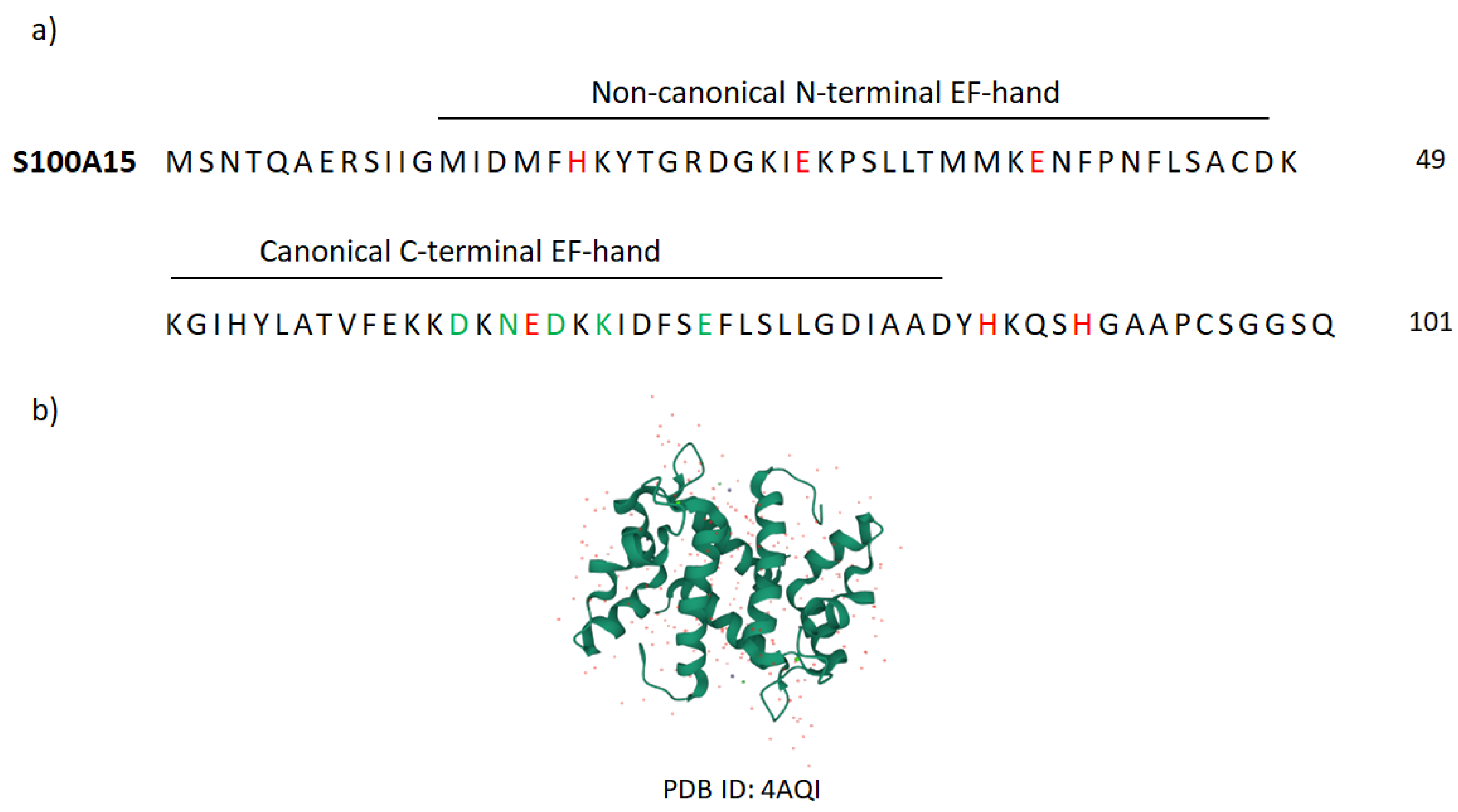
7. Koebnerisin (S100A15)
8. The Function of Koebnerisin in Other Immune-Mediated Inflammatory Diseases
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
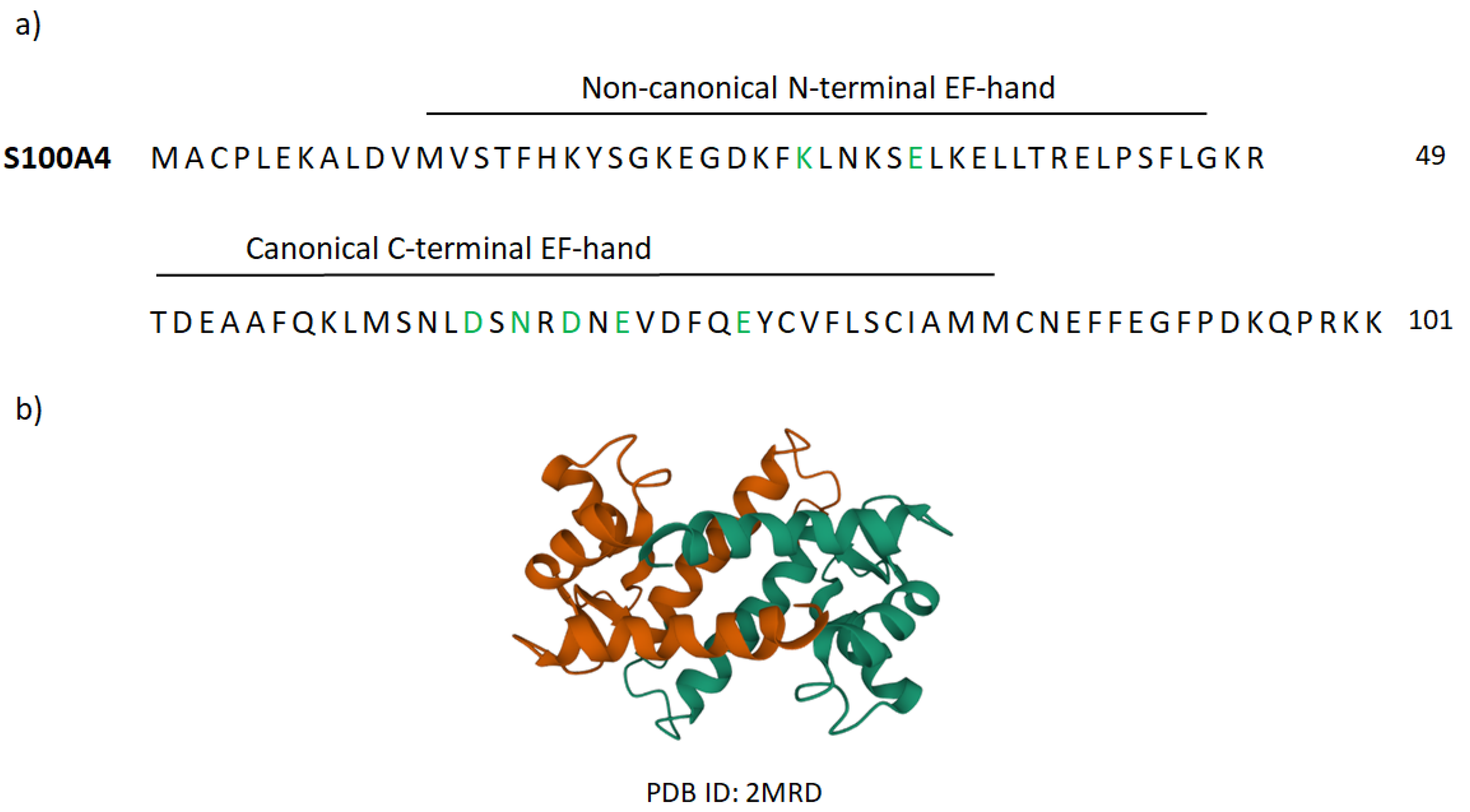
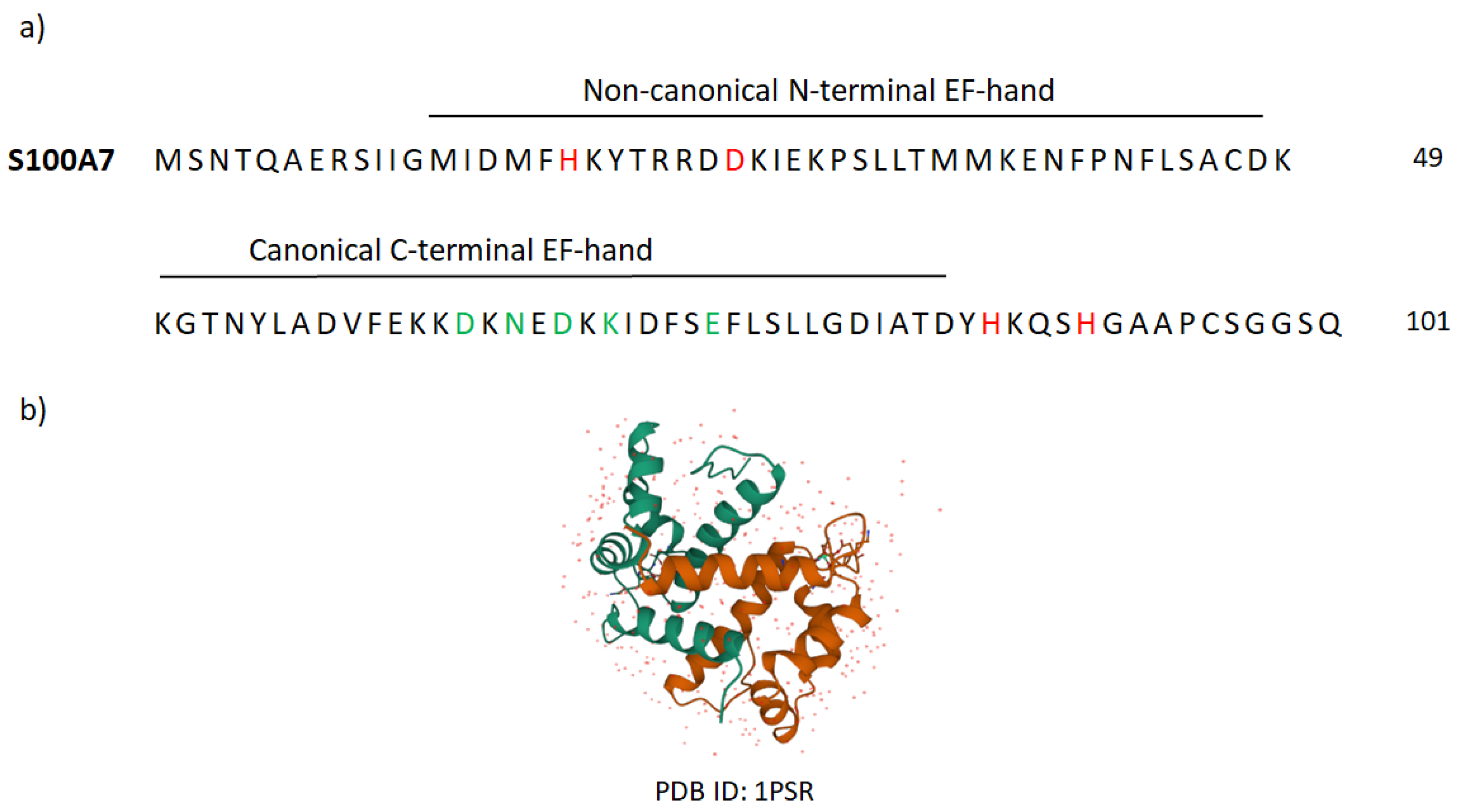
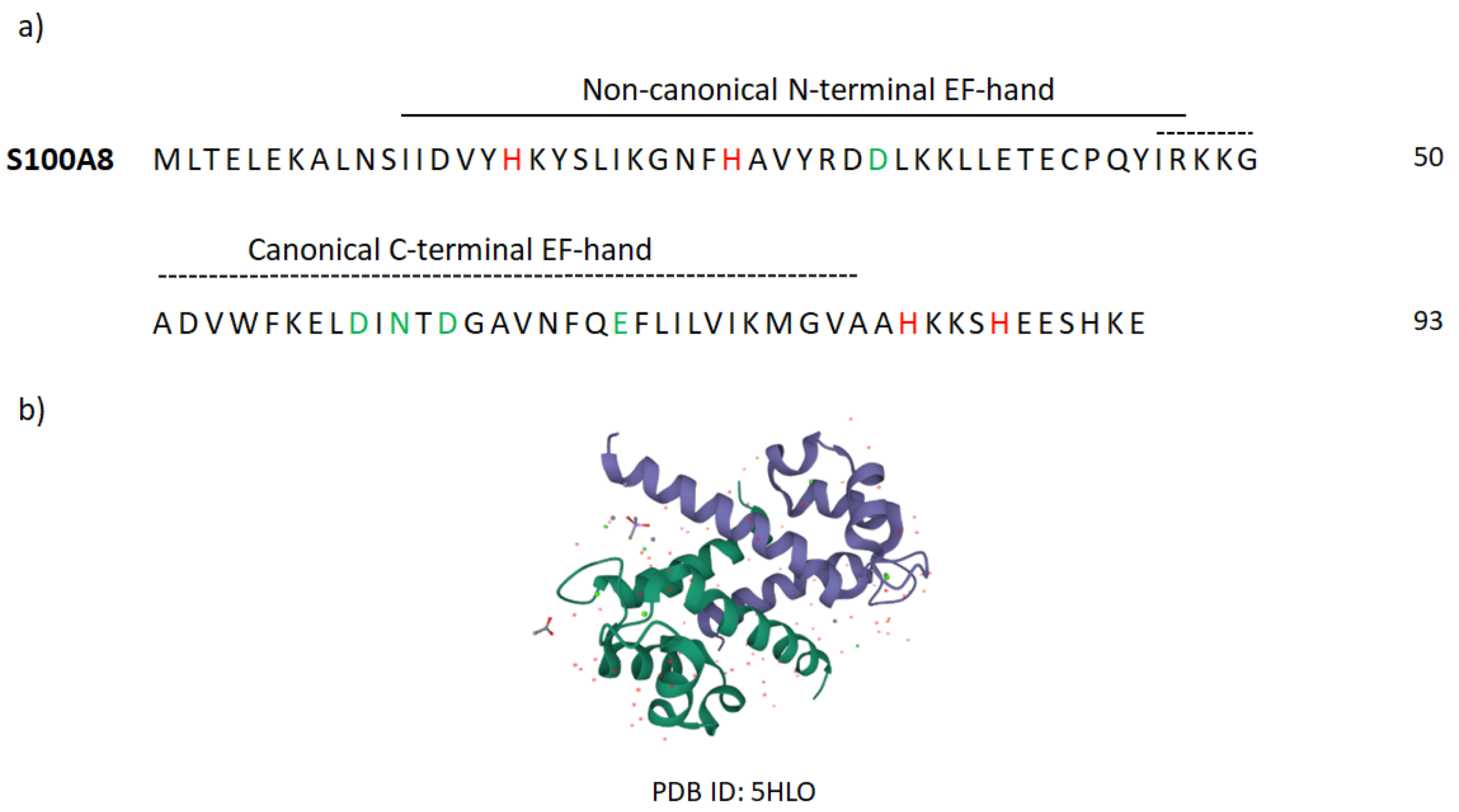
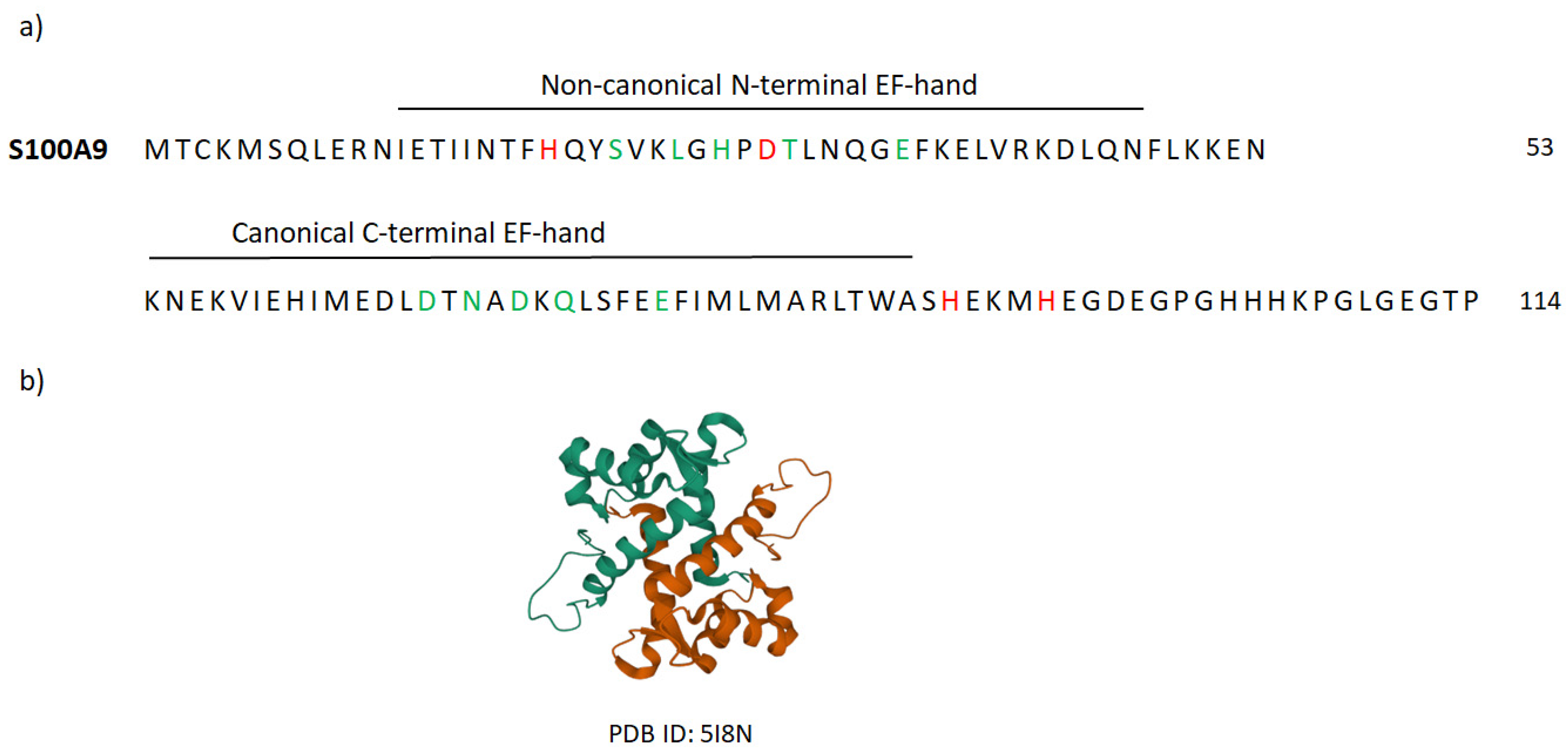
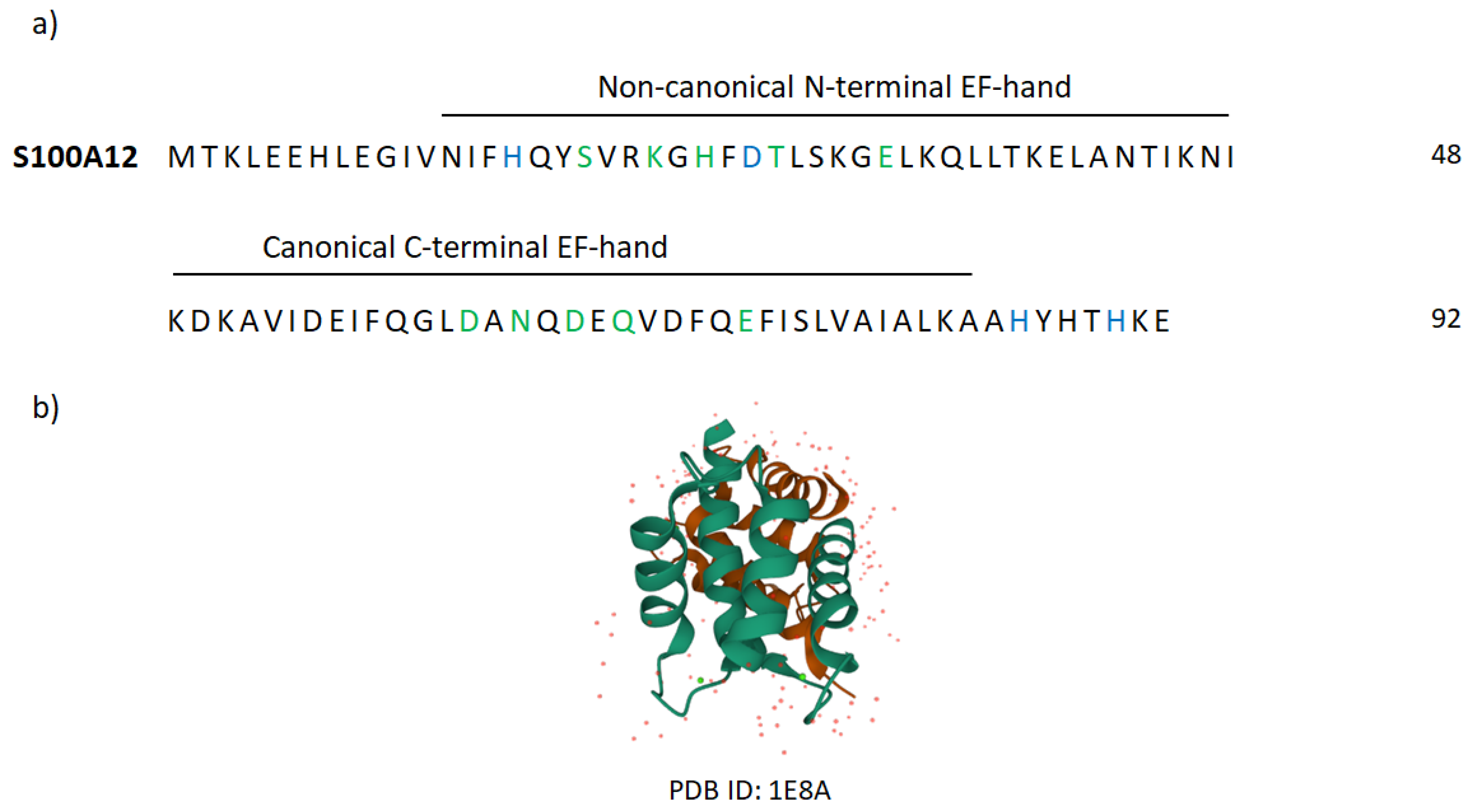

Appendix B
| Protein | Chemical Formula | Molecular Weight [Da] | Net Charge at pH 7.4 | Instability Index | Aliphatic Index | GRAVY | Isoelectric Point |
|---|---|---|---|---|---|---|---|
| S100A4 | C519H813N135O156S9 | 11,728.52 | −1.797 | 37.66 | 62.77 | −0.564 | 5.85 |
| S100A7 | C500H786N136O159S7 | 11,470.98 | −1.681 | 41.30 | 59.01 | −0.750 | 6.27 |
| S100A8 | C492H774N128O141S3 | 10,834.51 | −1.584 | 25.04 | 96.45 | −0.397 | 6.50 |
| S100A9 | C579H909N163O179S7 | 13,241.99 | −6.458 | 38.68 | 68.42 | −0.870 | 5.71 |
| S100A12 | C476H753N127O143S1 | 10,575.04 | −4.513 | 22.45 | 99.67 | −0.400 | 5.81 |
| S100B | C470H723N119O153S7 | 10,713.04 | −14.583 | 36.45 | 74.13 | −0.489 | 4.52 |
References
- Wilsmann-Theis, D.; Wagenpfeil, J.; Holzinger, D.; Roth, J.; Koch, S.; Schnautz, S.; Bieber, T.; Wenzel, J. Among the S100 proteins, S100A12 is the most significant marker for psoriasis disease activity. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Morizane, S.; Gallo, R.L. Antimicrobial peptides in the pathogenesis of psoriasis. J. Dermatol. 2012, 39, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yamasaki, K. Psoriasis and Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 6791. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Mishra, B.; Lau, K.; Lushnikova, T.; Golla, R.; Wang, X. Antimicrobial Peptides in 2014. Pharmaceuticals 2015, 8, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef]
- Costa, F.; Teixeira, C.; Gomes, P.; Martins, M.C.L. Clinical Application of AMPs. Adv. Exp. Med. Biol. 2019, 1117, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Luong, H.X.; Thanh, T.T.; Tran, T.H. Antimicrobial peptides–Advances in development of therapeutic applications. Life Sci. 2020, 260, 118407. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Gläser, R.; Schröder, J.-M. The Role and Potential Therapeutical Applications of Antimicrobial Proteins in Infectious and Inflammatory Diseases. Endocr. Metab. Immune Disord. Targets 2007, 7, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Malik, E.; Dennison, S.R.; Harris, F.; Phoenix, D.A. pH Dependent Antimicrobial Peptides and Proteins, Their Mechanisms of Action and Potential as Therapeutic Agents. Pharmaceuticals 2016, 9, 67. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, H.; Kim, D.; Liu, L.; Ganz, T. A Model for Antimicrobial Gene Therapy: Demonstration of Human b-Defensin 2 Antimicrobial Activities In Vivo. Hum. Gene Ther. 2002, 13, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Heizmann, C.W.; Fritz, G.; Schäfer, B.W. S100 proteins: Structure, functions and pathology. Front. Biosci. 2002, 7, 1356–1368. [Google Scholar] [CrossRef]
- Heizmann, C.W. S100 proteins: Diagnostic and prognostic biomarkers in laboratory medicine. Biochim. Biophys. Acta-Mol. Cell Res. 2019, 1866, 1197–1206. [Google Scholar] [CrossRef]
- Halawi, A.; Abbas, O.; Mahalingam, M. S100 proteins and the skin: A review. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Broome, A.M.; Ryan, D.; Eckert, R.L. S100 protein subcellular localization during epidermal differentiation and psoriasis. J. Histochem. Cytochem. 2003, 51, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Role of S100 proteins in health and disease. Biochim. Biophys. Acta-Mol. Cell Res. 2020, 1867, 118677. [Google Scholar] [CrossRef] [PubMed]
- Zibert, J.R.; Skov, L.; Thyssen, J.P.; Jacobsen, G.K.; Grigorian, M. Significance of the S100A4 protein in psoriasis. J. Investig. Dermatol. 2010, 130, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.; Hassan, E.; Donia, H.; Maamon, Y. Serum calprotectin as a predictive biomarker in the treatment of psoriasis vulgaris with methotrexate. J. Egypt. Women’s Dermatol. Soc. 2019, 16, 112. [Google Scholar] [CrossRef]
- Duvetorp, A.; Söderman, J.; Assarsson, M.; Skarstedt, M.; Svensson, Å.; Seifert, O. Observational study on swedish plaque psoriasis patients receiving narrowband-UVB treatment show decreased S100A8/A9 protein and gene expression levels in lesional psoriasis skin but no effect on S100A8/A9 protein levels in serum. PLoS ONE 2019, 14, e0213344. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Ruzicka, T.; Yuspa, S.H. Novel S100A7 (psoriasin)/S100A15 (koebnerisin) subfamily: Highly homologous but distinct in regulation and function. Amino Acids 2011, 41, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Mirmohammadsadegh, A.; Walz, M.; Lysa, B.; Tartler, U.; Remus, R.; Hengge, U.; Michel, G.; Ruzicka, T. Molecular cloning and characterization of alternatively spliced mRNA isoforms from psoriatic skin encoding a novel member of the S100 family. FASEB J. 2003, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.Z.; Liu, S.R. Koebner phenomenon leading to the formation of new psoriatic lesions: Evidences and mechanisms. Biosci. Rep. 2019, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Mascia, F.; Dharamsi, A.; Howard, O.M.Z.; Cataisson, C.; Bliskovski, V.; Winston, J.; Feigenbaum, L.; Lichti, U.; Ruzicka, T.; et al. Gene from a psoriasis susceptibility locus primes the skin for inflammation. Sci. Transl. Med. 2010, 2, 61ra90. [Google Scholar] [CrossRef] [PubMed]
- Batycka-Baran, A.; Maj, J.; Wolf, R.; Szepietowski, J.C. The new insight into the role of antimicrobial proteins-alarmins in the immunopathogenesis of psoriasis. J. Immunol. Res. 2014, 2014, 628289. [Google Scholar] [CrossRef]
- Batycka-Baran, A.; Hattinger, E.; Zwicker, S.; Summer, B.; Zack Howard, O.M.; Thomas, P.; Szepietowski, J.C.; Ruzicka, T.; Prinz, J.C.; Wolf, R. Leukocyte-derived koebnerisin (S100A15) and psoriasin (S100A7) are systemic mediators of inflammation in psoriasis. J. Dermatol. Sci. 2015, 79, 214–221. [Google Scholar] [CrossRef]
- Romańska-Gocka, K. Farmakoterapia łuszczycy. Farmakoter. Pol. 2009, 65, 647–654. [Google Scholar]
- Kuchekar, A.B.; Pujari, R.R.; Kuchekar, S.B.; Dhole, S.N.; Mule, P.M.; Vidyapeeth, B.; Wadi, B. Psoriasis: A comprehensive review. Int. J. Pharm. Life Sci. 2011, 2, 857–877. [Google Scholar]
- Unissa, R.; Kumar, P.M.; Pasha, M.; Begum, S.; Maheswari, B. Psoriasis: A Comprehensive Review. Asian J. Res. Pharm. Sci. 2019, 9, 29. [Google Scholar] [CrossRef]
- Yadav, K.; Singh, D.; Singh, M.R. Protein biomarker for psoriasis: A systematic review on their role in the pathomechanism, diagnosis, potential targets and treatment of psoriasis. Int. J. Biol. Macromol. 2018, 118, 1796–1810. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Psoriasis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Kaszuba, A.; Szepietowski, J.; Zygmunt, A. Dermatologia Geriatryczna. T. I, 1st ed.; Wydawnictwo Czelej: Lublin, Poland, 2016; pp. 171–205. [Google Scholar]
- Deng, Y.; Chang, C.; Lu, Q. The Inflammatory Response in Psoriasis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Lanna, C.; Mancini, M.; Gaziano, R.; Cannizzaro, M.V.; Galluzzo, M.; Talamonti, M.; Rovella, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Wang, Y.; et al. Skin immunity and its dysregulation in psoriasis. Cell Cycle 2019, 18, 2581–2589. [Google Scholar] [CrossRef]
- Harden, J.L.; Krueger, J.G.; Bowcock, A.M. The immunogenetics of Psoriasis: A comprehensive review. J. Autoimmun. 2015, 64, 66–73. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Flatz, L.; Conrad, C. Role of T-cell-mediated inflammation in psoriasis: Pathogenesis and targeted therapy. Psoriasis Targets Ther. 2013, 3, 1–10. [Google Scholar] [CrossRef][Green Version]
- Wang, G. Human antimicrobial peptides and proteins. Pharmaceuticals 2014, 7, 545–594. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, S.K.; Ahn, S.K. An update of the defensive barrier function of skin. Yonsei Med. J. 2006, 47, 293–306. [Google Scholar] [CrossRef]
- Clausen, M.L.; Agner, T. Antimicrobial Peptides, Infections and the Skin Barrier. Curr. Probl. Dermatol. 2016, 49, 38–46. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Antimicrobial peptides activity in the skin. Ski. Res. Technol. 2019, 25, 111–117. [Google Scholar] [CrossRef]
- Schauber, J.; Gallo, R.L. Antimicrobial peptides and the skin immune defense system. J. Allergy Clin. Immunol. 2008, 122, 261–266. [Google Scholar] [CrossRef]
- Holland, D.B.; Bojar, R.A.; Farrar, M.D.; Holland, K.T. Differential innate immune responses of a living skin equivalent model colonized by Staphylococcus epidermidis or Staphylococcus aureus. FEMS Microbiol. Lett. 2009, 290, 149–155. [Google Scholar] [CrossRef]
- Divyashree, M.; Mani, M.K.; Reddy, D.; Kumavath, R.; Ghosh, P.; Azevedo, V.; Barh, D. Clinical Applications of Antimicrobial Peptides (AMPs): Where do we Stand Now? Protein Pept. Lett. 2019, 27, 120–134. [Google Scholar] [CrossRef]
- Korting, H.C.; Schöllmann, C.; Stauss-Grabo, M.; Schäfer-Korting, M. Antimicrobial Peptides and Skin: A Paradigm of Translational Medicine. Skin Pharmacol. Physiol. 2012, 25, 323–334. [Google Scholar] [CrossRef]
- Lande, R.; Botti, E.; Jandus, C.; Dojcinovic, D.; Fanelli, G.; Conrad, C.; Chamilos, G.; Feldmeyer, L.; Marinari, B.; Chon, S.; et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat. Commun. 2014, 5, 5621. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, T.; Hirayama, N. Binding Affinity and Interaction of LL-37 with HLA-C*06:02 in Psoriasis. J. Investig. Dermatol. 2016, 136, 1901–1903. [Google Scholar] [CrossRef]
- Dombrowski, Y.; Peric, M.; Koglin, S.; Kammerbauer, C.; Göß, C.; Anz, D.; Simanski, M.; Gläser, R.; Harder, J.; Hornung, V.; et al. Cytosolic DNA Triggers Inflammasome Activation in Keratinocytes in Psoriatic Lesions. Sci. Transl. Med. 2011, 3, 82ra38. [Google Scholar] [CrossRef]
- Fitzgerald-Hughes, D.; Devocelle, M.; Humphreys, H. Beyond conventional antibiotics for the future treatment of methicillin-resistant Staphylococcus aureus infections: Two novel alternatives. FEMS Immunol. Med. Microbiol. 2012, 65, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- McInturff, J.E.; Wang, S.J.; Machleidt, T.; Lin, T.R.; Oren, A.; Hertz, C.J.; Krutzik, S.R.; Hart, S.; Zeh, K.; Anderson, D.H.; et al. Granulysin-derived peptides demonstrate antimicrobial and anti-inflammatory effects against Propionibacterium acnes. J. Investig. Dermatol. 2005, 125, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Marta Guarna, M.; Coulson, R.; Rubinchik, E. Anti-inflammatory activity of cationic peptides: Application to the treatment of acne vulgaris. FEMS Microbiol. Lett. 2006, 257, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, M. The Role and Potential Application of Antimicrobial Peptides in Autoimmune Diseases. Front. Immunol. 2020, 11, 859. [Google Scholar] [CrossRef]
- Han, R.; Blencke, H.M.; Cheng, H.; Li, C. The antimicrobial effect of CEN1HC-Br against Propionibacterium acnes and its therapeutic and anti-inflammatory effects on acne vulgaris. Peptides 2018, 99, 36–43. [Google Scholar] [CrossRef]
- Tian, M.; Liu, J.; Chai, J.; Wu, J.; Xu, X. Antimicrobial and Anti-inflammatory Effects of a Novel Peptide From the Skin of Frog Microhyla pulchra. Front. Pharmacol. 2021, 12, 3489. [Google Scholar] [CrossRef] [PubMed]
- van der Does, A.M.; Hiemstra, P.S.; Mookherjee, N. Antimicrobial host defence peptides: Immunomodulatory functions and translational prospects. Adv. Exp. Med. Biol. 2019, 1117, 149–171. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Pinegin, B.; Vorobjeva, N.; Pinegin, V. Neutrophil extracellular traps and their role in the development of chronic inflammation and autoimmunity. Autoimmun. Rev. 2015, 14, 633–640. [Google Scholar] [CrossRef]
- Dwivedi, N.; Radic, M. Burning controversies in NETs and autoimmunity: The mysteries of cell death and autoimmune disease. Autoimmunity 2018, 51, 267–280. [Google Scholar] [CrossRef]
- Hall, J.C.; Rosen, A. Type i interferons: Crucial participants in disease amplification in autoimmunity. Nat. Rev. Rheumatol. 2010, 6, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Németh, T.; Mócsai, A.; Lowell, C.A. Neutrophils in animal models of autoimmune disease. Semin. Immunol. 2016, 28, 174–186. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Kaplan, M.J. Little Peptide, Big Effects: The Role of LL-37 in Inflammation and Autoimmune Disease. J. Immunol. 2013, 191, 4895–4901. [Google Scholar] [CrossRef]
- Lu, X.; Tang, Q.; Lindh, M.; Dastmalchi, M.; Alexanderson, H.; Popovic Silwerfeldt, K.; Agerberth, B.; Lundberg, I.E.; Wick, C. The host defense peptide LL-37 a possible inducer of the type I interferon system in patients with polymyositis and dermatomyositis. J. Autoimmun. 2017, 78, 46–56. [Google Scholar] [CrossRef]
- Dominguez-Villar, M.; Hafler, D.A. Regulatory T cells in autoimmune disease. Nat. Immunol. 2018, 19, 665–673. [Google Scholar] [CrossRef]
- Diana, J.; Simoni, Y.; Furio, L.; Beaudoin, L.; Agerberth, B.; Barrat, F.; Lehuen, A. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat. Med. 2013, 19, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.; Tsomos, E.; Hammerstad, S.S.; Tomer, Y. Interferon alpha: The key trigger of type 1 diabetes. J. Autoimmun. 2018, 94, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Niyonsaba, F.; Ushio, H.; Nagaoka, I.; Ikeda, S.; Okumura, K.; Ogawa, H. Human cathelicidin LL-37 increases vascular permeability in the skin via mast cell activation, and phosphorylates MAP kinases p38 and ERK in mast cells. J. Dermatol. Sci. 2006, 43, 63–66. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Hara, M.; Yokoi, H.; Tominaga, M.; Takamori, K.; Kajiwara, N.; Saito, H.; Nagaoka, I.; Ogawa, H.; et al. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J. Immunol. 2010, 184, 3526–3534. [Google Scholar] [CrossRef]
- Ganguly, D.; Chamilos, G.; Lande, R.; Gregorio, J.; Meller, S.; Facchinetti, V.; Homey, B.; Barrat, F.J.; Zal, T.; Gilliet, M. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009, 206, 1983–1994. [Google Scholar] [CrossRef]
- Morizane, S.; Yamasaki, K.; Mühleisen, B.; Kotol, P.F.; Murakami, M.; Aoyama, Y.; Iwatsuki, K.; Hata, T.; Gallo, R.L. Cathelicidin antimicrobial peptide LL-37 in psoriasis enables keratinocyte reactivity against TLR9 ligands. J. Investig. Dermatol. 2012, 132, 135–143. [Google Scholar] [CrossRef] [PubMed]
- van der Does, A.M.; Beekhuizen, H.; Ravensbergen, B.; Vos, T.; Ottenhoff, T.H.M.; van Dissel, J.T.; Drijfhout, J.W.; Hiemstra, P.S.; Nibbering, P.H. LL-37 Directs Macrophage Differentiation toward Macrophages with a Proinflammatory Signature. J. Immunol. 2010, 185, 1442–1449. [Google Scholar] [CrossRef]
- Davidson, D.J.; Currie, A.J.; Reid, G.S.D.; Bowdish, D.M.E.; MacDonald, K.L.; Ma, R.C.; Hancock, R.E.W.; Speert, D.P. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J. Immunol. 2004, 172, 1146–1156. [Google Scholar] [CrossRef]
- Scott, M.G.; Vreugdenhil, A.C.E.; Buurman, W.A.; Hancock, R.E.W.; Gold, M.R. Cutting edge: Cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J. Immunol. 2000, 164, 549–553. [Google Scholar] [CrossRef]
- Mookherjee, N.; Hamill, P.; Gardy, J.; Blimkie, D.; Falsafi, R.; Chikatamarla, A.; Arenillas, D.J.; Doria, S.; Kollmann, T.R.; Hancock, R.E.W. Systems biology evaluation of immune responses induced by human host defence peptide LL-37 in mononuclear cells. Mol. Biosyst. 2009, 5, 483–496. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Nagaoka, I.; Ogawa, H.; Okumura, K. Multifunctional antimicrobial proteins and peptides: Natural activators of immune systems. Curr. Pharm. Des. 2009, 15, 2393–2413. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Nagaoka, I.; Ogawa, H. Human defensins and cathelicidins in the skin: Beyond direct antimicrobial properties. Crit. Rev. Immunol. 2006, 26, 545–576. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Tsuruta, D.; Murakami, M.; Kurokawa, I. What is the role of antimicrobial peptides (AMP) in acne vulgaris? Exp. Dermatol. 2013, 22, 386–391. [Google Scholar] [CrossRef]
- Peschel, A.; Jack, R.W.; Otto, M.; Collins, L.V.; Staubitz, P.; Nicholson, G.; Kalbacher, H.; Nieuwenhuizen, W.F.; Jung, G.; Tarkowski, A.; et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 2001, 193, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Spohn, R.; Daruka, L.; Lázár, V.; Martins, A.; Vidovics, F.; Grézal, G.; Méhi, O.; Kintses, B.; Számel, M.; Jangir, P.K.; et al. Integrated evolutionary analysis reveals antimicrobial peptides with limited resistance. Nat. Commun. 2019, 10, 4538. [Google Scholar] [CrossRef]
- Di Somma, A.; Moretta, A.; Canè, C.; Cirillo, A.; Duilio, A. Antimicrobial and Antibiofilm Peptides. Biomolecules 2020, 10, 652. [Google Scholar] [CrossRef]
- Reffuveille, F.; De La Fuente-Núñez, C.; Mansour, S.; Hancock, R.E.W. A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob. Agents Chemother. 2014, 58, 5363–5371. [Google Scholar] [CrossRef]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.W.; Rehm, B.H.A.; Hancock, R.E.W. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef]
- Volejníková, A.; Melicherčík, P.; Nešuta, O.; Vaňková, E.; Bednárová, L.; Rybáček, J.; Čeřovský, V. Antimicrobial peptides prevent bacterial biofilm formation on the surface of polymethylmethacrylate bone cement. J. Med. Microbiol. 2019, 68, 961–972. [Google Scholar] [CrossRef]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef]
- Brandenburg, K.; Schürholz, T. Lack of new antiinfective agents: Passing into the pre-antibiotic age? World J. Biol. Chem. 2015, 6, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Heinbockel, L.; Correa, W.; Lohner, K. Peptides with dual mode of action: Killing bacteria and preventing endotoxin-induced sepsis. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 971–979. [Google Scholar] [CrossRef] [PubMed]
- FDA Wound Healing Clinical Focus Group Guidance for Industry Chronic Cutaneous Ulcer and Burn Wounds-Developing Products for Treatment. Wound Repair Regen. 2001, 9, 258–268. [CrossRef] [PubMed]
- McGregor, D.P. Discovering and improving novel peptide therapeutics. Curr. Opin. Pharmacol. 2008, 8, 616–619. [Google Scholar] [CrossRef]
- Bulger, E.M.; May, A.K.; Robinson, B.R.H.; Evans, D.C.; Henry, S.; Green, J.M.; Toschlog, E.; Sperry, J.L.; Fagenholz, P.; Martin, N.D.; et al. A Novel Immune Modulator for Patients With Necrotizing Soft Tissue Infections (NSTI): Results of a Multicenter, Phase 3 Randomized Controlled Trial of Reltecimod (AB 103). Ann. Surg. 2020, 272, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Scannon, P.J. Recombinant 21 kDa N-terminal fragment of human bactericidal/permeability-increasing protein (rBPI21): Progress in the clinic. J. Endotoxin. Res. 1999, 5, 209–212. [Google Scholar] [CrossRef]
- Neuprex XOMA Corp-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/18465627/ (accessed on 5 September 2022).
- Farokhnia, M.; Grodin, E.N.; Lee, M.R.; Oot, E.N.; Blackburn, A.N.; Stangl, B.L.; Schwandt, M.L.; Farinelli, L.A.; Momenan, R.; Ramchandani, V.A.; et al. Exogenous ghrelin administration increases alcohol self-administration and modulates brain functional activity in heavy-drinking alcohol-dependent individuals. Mol. Psychiatry 2018, 23, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Effects of Ghrelin Administration on Dopamine and Effort-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05318924 (accessed on 5 September 2022).
- Ghrelin Decreases Insulin Sensitivity-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00512525 (accessed on 5 September 2022).
- van der Velden, W.J.F.M.; van Iersel, T.M.P.; Blijlevens, N.M.A.; Donnelly, J.P. Safety and tolerability of the antimicrobial peptide human lactoferrin 1-11 (hLF1-11). BMC Med. 2009, 7, 44. [Google Scholar] [CrossRef]
- Reddy, K.V.R.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Steinstraesser, L.; Burghard, O.; Nemzek, J.; Fan, M.-H.; Merry, A.; Remick, D.I.; Su, G.L.; Steinau, H.-U.; Wang, S.C. Protegrin-1 increases bacterial clearance in sepsis but decreases survival. Crit. Care Med. 2003, 31, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Maturana, P.; Martinez, M.; Noguera, M.E.; Santos, N.C.; Disalvo, E.A.; Semorile, L.; Maffia, P.C.; Hollmann, A. Lipid selectivity in novel antimicrobial peptides: Implication on antimicrobial and hemolytic activity. Colloids Surf. B. Biointerfaces 2017, 153, 152–159. [Google Scholar] [CrossRef]
- Di, L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, M.; Lai, R.; Zhang, Z. Chemical modifications to increase the therapeutic potential of antimicrobial peptides. Peptides 2021, 146, 170666. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- White, C.J.; Yudin, A.K. Contemporary strategies for peptide macrocyclization. Nat. Chem. 2011, 3, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Pelay-Gimeno, M.; Tulla-Puche, J.; Albericio, F. “Head-to-side-chain” cyclodepsipeptides of marine origin. Mar. Drugs 2013, 11, 1693–1717. [Google Scholar] [CrossRef]
- Moura, C.B.F.; Brand, G.D.; Fernandes, P.C.; Prates, M.V.; Bloch, C.; Barbosa, A.R.G.; Freitas, N.M.; Restano-Cassulini, R.; Possani, L.D.; Schwartz, E.F. Head-to-tail cyclization after interaction with trypsin: A scorpion venom peptide that resembles plant cyclotides. ACS Publ. 2020, 63, 9511. [Google Scholar] [CrossRef]
- Yang, P.Y.; Zou, H.; Lee, C.; Muppidi, A.; Chao, E.; Fu, Q.; Luo, X.; Wang, D.; Schultz, P.G.; Shen, W. Stapled, Long-Acting Glucagon-like Peptide 2 Analog with Efficacy in Dextran Sodium Sulfate Induced Mouse Colitis Models. J. Med. Chem. 2018, 61, 3218–3223. [Google Scholar] [CrossRef]
- Dougherty, P.G.; Wen, J.; Pan, X.; Koley, A.; Ren, J.-G.; Sahni, A.; Basu, R.; Salim, H.; Kubi, G.A.; Qian, Z.; et al. Enhancing the cell permeability of stapled peptides with a cyclic cell-penetrating peptide. ACS Publ. 2019, 62, 10098–10107. [Google Scholar] [CrossRef]
- Li, C.Y.; Yap, K.; Swedberg, J.E.; Craik, D.J.; de Veer, S.J. Binding loop substitutions in the cyclic peptide SFTI-1 generate potent and selective chymase inhibitors. ACS Publ. 2019, 63, 816–826. [Google Scholar] [CrossRef]
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021, 50, 7820–7880. [Google Scholar] [CrossRef]
- Wang, G. Post-Translational Modifications of Natural Antimicrobial Peptides and Strategies for Peptide Engineering. Curr. Biotechnol. 2012, 1, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Beuerman, R.W.; Dua, H.S.; Lakshminarayanan, R.; Mohammed, I. Strategies in Translating the Therapeutic Potentials of Host Defense Peptides. Front. Immunol. 2020, 11, 983. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.N.; Mitchell, J.P.; Roberts, K.D. The synthesis of cyclic peptides. J. Chem. Soc. Perkin Trans. 1 2001, 5, 471–484. [Google Scholar] [CrossRef]
- Davies, J.S. The cyclization of peptides and depsipeptides. J. Pept. Sci. 2003, 9, 471–501. [Google Scholar] [CrossRef]
- Chu, Q.; Moellering, R.E.; Hilinski, G.J.; Kim, Y.W.; Grossmann, T.N.; Yeh, J.T.H.; Verdine, G.L. Towards understanding cell penetration by stapled peptides. Medchemcomm 2015, 6, 111–119. [Google Scholar] [CrossRef]
- Martí-Centelles, V.; Pandey, M.D.; Burguete, M.I.; Luis, S.V. Macrocyclization reactions: The importance of conformational, configurational, and template-induced preorganization. Chem. Rev. 2015, 115, 8736–8834. [Google Scholar] [CrossRef]
- Cheneval, O.; Schroeder, C.I.; Durek, T.; Walsh, P.; Huang, Y.H.; Liras, S.; Price, D.A.; Craik, D.J. Fmoc-based synthesis of disulfide-rich cyclic peptides. J. Org. Chem. 2014, 79, 5538–5544. [Google Scholar] [CrossRef]
- Falanga, A.; Nigro, E.; Gabriella De Biasi, M.; Daniele, A.; Morelli, G.; Galdiero, S.; Scudiero, O. Cyclic peptides as novel therapeutic microbicides: Engineering of human defensin mimetics. Molecules 2017, 22, 1217. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Joo, S.H. Cyclic Peptides as Therapeutic Agents and Biochemical Tools. Biomol. Ther. (Seoul) 2012, 20, 19. [Google Scholar] [CrossRef]
- Tapeinou, A.; Matsoukas, M.T.; Simal, C.; Tselios, T. Review cyclic peptides on a merry-go-round; towards drug design. Biopolymers 2015, 104, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, J.; Yin, Y.; Wang, G.; Yang, M.; Li, Y.; Zhang, Z.; Lai, R. The antimicrobial peptide ZY4 combats multidrugresistant Pseudomonas aeruginosa and Acinetobacter baumannii infection. Proc. Natl. Acad. Sci. USA 2019, 116, 26516–26522. [Google Scholar] [CrossRef]
- Monaim, S.A.H.A.; Jad, Y.E.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Teixobactin as a scaffold for unlimited new antimicrobial peptides: SAR study. Bioorg. Med. Chem. 2018, 26, 2788–2796. [Google Scholar] [CrossRef]
- Peschel, A.; Sahl, H.G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 2006, 4, 529–536. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Migoń, D.; Neubauer, D.; Kamysz, W. Hydrocarbon Stapled Antimicrobial Peptides. Protein J. 2018, 37, 2–12. [Google Scholar] [CrossRef]
- Walensky, L.D.; Bird, G.H. Hydrocarbon-stapled peptides: Principles, practice, and progress. J. Med. Chem. 2014, 57, 6275–6288. [Google Scholar] [CrossRef]
- Wu, Y.D.; Gellman, S. Peptidomimetics. Acc. Chem. Res. 2008, 41, 1231–1232. [Google Scholar] [CrossRef][Green Version]
- Grishin, D.V.; Zhdanov, D.D.; Pokrovskaya, M.V.; Sokolov, N.N. D-amino acids in nature, agriculture and biomedicine. All Life 2020, 13, 11–22. [Google Scholar] [CrossRef]
- Papo, N.; Oren, Z.; Pag, U.; Sahl, H.G.; Shai, Y. The Consequence of Sequence Alteration of an Amphipathic α-Helical Antimicrobial Peptide and Its Diastereomers. J. Biol. Chem. 2002, 277, 33913–33921. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, S.A.; Shin, S.; Lee, J.Y.; Jeong, K.W.; Nan, Y.H.; Park, Y.S.; Shin, S.Y.; Kim, Y. Structural flexibility and the positive charges are the key factors in bacterial cell selectivity and membrane penetration of peptoid-substituted analog of Piscidin 1. Biochim. Biophys. Acta-Biomembr. 2010, 1798, 1913–1925. [Google Scholar] [CrossRef]
- Jung, H.J.; Park, Y.; Sung, W.S.; Suh, B.K.; Lee, J.; Hahm, K.S.; Lee, D.G. Fungicidal effect of pleurocidin by membrane-active mechanism and design of enantiomeric analogue for proteolytic resistance. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 1400–1405. [Google Scholar] [CrossRef]
- Merrifield, R.B.; Juvvadi, P.; Andreu, D.; Ubach, J.; Boman, A.; Boman, H.G. Retro and retroenantio analogs of cecropin-melittin hybrids. Proc. Natl. Acad. Sci. USA 1995, 92, 3449–3453. [Google Scholar] [CrossRef]
- Cardoso, M.H.; Cândido, E.S.; Oshiro, K.G.N.; Rezende, S.B.; Franco, O.L. Peptides containing d-amino acids and retro-inverso peptides: General applications and special focus on antimicrobial peptides. Pept. Appl. Biomed. Biotechnol. Bioeng. 2018, 131–155. [Google Scholar] [CrossRef]
- Silva, T.; Magalhães, B.; Maia, S.; Gomes, P.; Nazmi, K.; Bolscher, J.G.M.; Rodrigues, P.N.; Bastos, M.; Gomes, M.S. Killing of Mycobacterium avium by lactoferricin peptides: Improved activity of arginine- and D-amino-acid-containing molecules. Antimicrob. Agents Chemother. 2014, 58, 3461–3467. [Google Scholar] [CrossRef]
- Hong, S.Y.; Oh, J.E.; Lee, K.H. Effect of d-amino acid substitution on the stability, the secondary structure, and the activity of membrane-active peptide. Biochem. Pharmacol. 1999, 58, 1775–1780. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Han, H.; Miller, D.W.; Wang, G. Solution structures of human ll-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J. Am. Chem. Soc. 2006, 128, 5776–5785. [Google Scholar] [CrossRef]
- Wiśniewski, K.; Galyean, R.; Tariga, H.; Alagarsamy, S.; Croston, G.; Heitzmann, J.; Kohan, A.; Wiśniewska, H.; Laporte, R.; Rivière, P.J.M.; et al. New, potent, selective, and short-acting peptidic V1a receptor agonists. J. Med. Chem. 2011, 54, 4388–4398. [Google Scholar] [CrossRef]
- Maybauer, M.O.; Maybauer, D.M.; Enkhbaatar, P.; Laporte, R.; Winiewska, H.; Traber, L.D.; Lin, C.; Fan, J.; Hawkins, H.K.; Cox, R.A.; et al. The selective vasopressin type 1a receptor agonist selepressin (FE 202158) blocks vascular leak in ovine severe sepsis. Crit. Care Med. 2014, 42, e525–e533. [Google Scholar] [CrossRef]
- Arias, M.; Piga, K.B.; Hyndman, M.E.; Vogel, H.J. Improving the Activity of Trp-Rich Antimicrobial Peptides by Arg/Lys Substitutions and Changing the Length of Cationic Residues. Biomolecules 2018, 8, 19. [Google Scholar] [CrossRef]
- Oliva, R.; Chino, M.; Pane, K.; Pistorio, V.; De Santis, A.; Pizzo, E.; D’Errico, G.; Pavone, V.; Lombardi, A.; Del Vecchio, P.; et al. Exploring the role of unnatural amino acids in antimicrobial peptides. Sci. Rep. 2018, 8, 8888. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Pawlak, J.; Johnson, L.; Bonilla, H.; Saravolatz, L.D.; Fakih, M.G.; Fugelli, A.; Olsen, W.M. In Vitro Activities of LTX-109, a Synthetic Antimicrobial Peptide, against Methicillin-Resistant, Vancomycin-Intermediate, Vancomycin-Resistant, Daptomycin-Nonsusceptible, and Linezolid-Nonsusceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 2012, 56, 4478–4482. [Google Scholar] [CrossRef]
- Haug, B.E.; Stensen, W.; Kalaaji, M.; Rekdal, Ø.; Svendsen, J.S. Synthetic antimicrobial peptidomimetics with therapeutic potential. J. Med. Chem. 2008, 51, 4306–4314. [Google Scholar] [CrossRef]
- Kuzmin, D.V.; Emelianova, A.A.; Kalashnikova, M.B.; Panteleev, P.V.; Ovchinnikova, T.V. Effect of N- and C-Terminal Modifications on Cytotoxic Properties of Antimicrobial Peptide Tachyplesin I. Bull. Exp. Biol. Med. 2017, 162, 754–757. [Google Scholar] [CrossRef]
- Cabrera, M.P.D.S.; Arcisio-Miranda, M.; Costa, S.T.B.; Konno, K.; Ruggiero, J.R.; Procopio, J.; Neto, J.R. Study of the mechanism of action of anoplin, a helical antimicrobial decapeptide with ion channel-like activity, and the role of the amidated C-terminus. J. Pept. Sci. 2008, 14, 661–669. [Google Scholar] [CrossRef]
- Dennison, S.R.; Phoenix, D.A. Influence of C-terminal amidation on the efficacy of modelin-5. Biochemistry 2011, 50, 1514–1523. [Google Scholar] [CrossRef]
- Mura, M.; Wang, J.; Zhou, Y.; Pinna, M.; Zvelindovsky, A.V.; Dennison, S.R.; Phoenix, D.A. The effect of amidation on the behaviour of antimicrobial peptides. Eur. Biophys. J. 2016, 45, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Irudayam, S.J.; Berkowitz, M.L. Binding and reorientation of melittin in a POPC bilayer: Computer simulations. Biochim. Biophys. Acta-Biomembr. 2012, 1818, 2975–2981. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mangoni, M.L. Temporins, anti-infective peptides with expanding properties. Cell. Mol. Life Sci. C 2006, 63, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, R. The chemistry and biological activities of peptides from amphibian skin secretions. Chem. Rev. 2015, 115, 1760–1846. [Google Scholar] [CrossRef]
- Dennison, S.R.; Mura, M.; Harris, F.; Morton, L.H.G.; Zvelindovsky, A.; Phoenix, D.A. The role of C-terminal amidation in the membrane interactions of the anionic antimicrobial peptide, maximin H5. Biochim. Biophys. Acta 2015, 1848, 1111–1118. [Google Scholar] [CrossRef]
- Datta, A.; Kundu, P.; Bhunia, A. Designing potent antimicrobial peptides by disulphide linked dimerization and N-terminal lipidation to increase antimicrobial activity and membrane perturbation: Structural insights into lipopolysaccharide binding. J. Colloid Interface Sci. 2016, 461, 335–345. [Google Scholar] [CrossRef]
- Teixeira, V.; Feio, M.J.; Rivas, L.; De La Torre, B.G.; Andreu, D.; Coutinho, A.; Bastos, M. Influence of lysine Nε-trimethylation and lipid composition on the membrane activity of the cecropin A-melittin hybrid peptide CA(1-7)M(2-9). J. Phys. Chem. B 2010, 114, 16198–16208. [Google Scholar] [CrossRef]
- Jia, F.; Zhang, Y.; Wang, J.; Peng, J.; Zhao, P.; Zhang, L.; Yao, H.; Ni, J.; Wang, K. The effect of halogenation on the antimicrobial activity, antibiofilm activity, cytotoxicity and proteolytic stability of the antimicrobial peptide Jelleine-I. Peptides 2019, 112, 56–66. [Google Scholar] [CrossRef]
- Setty, S.C.; Horam, S.; Pasupuleti, M.; Haq, W. Modulating the Antimicrobial Activity of Temporin L Through Introduction of Fluorinated Phenylalanine. Int. J. Pept. Res. Ther. 2017, 23, 213–225. [Google Scholar] [CrossRef]
- Veronese, F.M. Peptide and protein PEGylation: A review of problems and solutions. Biomaterials 2001, 22, 405–417. [Google Scholar] [CrossRef]
- Henninot, A.; Collins, J.C.; Nuss, J.M. The Current State of Peptide Drug Discovery: Back to the Future? J. Med. Chem. 2018, 61, 1382–1414. [Google Scholar] [CrossRef]
- Bednarska, N.G.; Wren, B.W.; Willcocks, S.J. The importance of the glycosylation of antimicrobial peptides: Natural and synthetic approaches. Drug Discov. Today 2017, 22, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.V.; Hussein, W.M.; Varamini, P.; Simerska, P.; Toth, I. Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem. Sci. 2016, 7, 2492–2500. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Nishida, M.; Ogawa, Y.; Takakura, Y.; Matsuzaki, K. Action mechanism of tachyplesin I and effects of PEGylation. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Nishida, M.; Matsuzaki, K. Action mechanism of PEGylated magainin 2 analogue peptide. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 2578–2585. [Google Scholar] [CrossRef]
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; He, Y.; Shi, K. Cleavable PEGylation: A strategy for overcoming the “PEG dilemma” in efficient drug delivery Cleavable PEGylation: A strategy for overcoming the “PEG dilemma” in efficient drug delivery. Drug Deliv. 2017, 24, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Andina, D.; Nahar, S.; Leroux, J.C.; Gauthier, M.A. Releasable and traceless PEGylation of arginine-rich antimicrobial peptides. Chem. Sci. 2017, 8, 4082–4086. [Google Scholar] [CrossRef]
- Doores, K.J.; Gamblin, D.P.; Davis, B.G. Exploring and Exploiting the Therapeutic Potential of Glycoconjugates. Chem.–A Eur. J. 2006, 12, 656–665. [Google Scholar] [CrossRef]
- Hagedorn, I.; Schmidbauer, S.; Pleines, I.; Kleinschnitz, C.; Kronthaler, U.; Stoll, G.; Dickneite, G.; Nieswandt, B. Factor xiia inhibitor recombinant human albumin infestin-4 abolishes occlusive arterial thrombus formation without affecting bleeding. Circulation 2010, 121, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Zhu, N.; Zhu, Y.; Liu, T.; Gou, S.; Xie, J.; Yao, J.; Ni, J. Antimicrobial peptides conjugated with fatty acids on the side chain of D-amino acid promises antimicrobial potency against multidrug-resistant bacteria. Eur. J. Pharm. Sci. 2020, 141, 105123. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. Development of a novel hybrid antimicrobial peptide for targeted killing of Pseudomonas aeruginosa. Eur. J. Med. Chem. 2020, 185, 111814. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Teng, D.; Mao, R.; Hao, Y.; Yang, N.; Wang, X.; Li, Z.; Wang, X.; Wang, J. Development of chimeric peptides to facilitate the neutralisation of lipopolysaccharides during bactericidal targeting of multidrug-resistant Escherichia coli. Commun. Biol. 2020, 3. [Google Scholar] [CrossRef]
- Lee, H.; Lim, S.I.; Shin, S.H.; Lim, Y.; Koh, J.W.; Yang, S. Conjugation of cell-penetrating peptides to antimicrobial peptides enhances antibacterial activity. ACS Publ. 2019, 4, 15694–15701. [Google Scholar] [CrossRef]
- Michael Henderson, J.; Lee, K.Y.C. Promising antimicrobial agents designed from natural peptide templates. Curr. Opin. Solid State Mater. Sci. 2013, 17, 175–192. [Google Scholar] [CrossRef]
- Rotem, S.; Mor, A. Antimicrobial peptide mimics for improved therapeutic properties. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 1582–1592. [Google Scholar] [CrossRef]
- Giuliani, A.; Rinaldi, A.C. Beyond natural antimicrobial peptides: Multimeric peptides and other peptidomimetic approaches. Springer 2011, 68, 2255–2266. [Google Scholar] [CrossRef]
- Andreev, K.; Martynowycz, M.W.; Huang, M.L.; Kuzmenko, I.; Bu, W.; Kirshenbaum, K.; Gidalevitz, D. Hydrophobic interactions modulate antimicrobial peptoid selectivity towards anionic lipid membranes. Biochim. Biophys. acta. Biomembr. 2018, 1860, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Choudhary, M.; Vashistt, J.; Shrivastava, R.; Bisht, G.S. Cationic antimicrobial peptide and its poly-N-substituted glycine congener: Antibacterial and antibiofilm potential against A. baumannii. Biochem. Biophys. Res. Commun. 2019, 518, 472–478. [Google Scholar] [CrossRef]
- Tew, G.N.; Clements, D.; Tang, H.; Arnt, L.; Scott, R.W. Antimicrobial activity of an abiotic host defense peptide mimic. Biochim. Biophys. Acta 2006, 1758, 1387–1392. [Google Scholar] [CrossRef]
- Chongsiriwatana, N.P.; Patch, J.A.; Czyzewski, A.M.; Dohm, M.T.; Ivankin, A.; Gidalevitz, D.; Zuckermann, R.N.; Barron, A.E. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2008, 105, 2794–2799. [Google Scholar] [CrossRef]
- Andreev, K.; Martynowycz, M.W.; Ivankin, A.; Huang, M.L.; Kuzmenko, I.; Meron, M.; Lin, B.; Kirshenbaum, K.; Gidalevitz, D. Cyclization Improves Membrane Permeation by Antimicrobial Peptoids. Langmuir 2016, 32, 12905–12913. [Google Scholar] [CrossRef]
- Greco, I.; Emborg, A.P.; Jana, B.; Molchanova, N.; Oddo, A.; Damborg, P.; Guardabassi, L.; Hansen, P.R. Characterization, mechanism of action and optimization of activity of a novel peptide-peptoid hybrid against bacterial pathogens involved in canine skin infections. Sci. Rep. 2019, 9, 3679. [Google Scholar] [CrossRef] [PubMed]
- Molchanova, N.; Hansen, P.R.; Damborg, P.; Nielsen, H.M.; Franzyk, H. Lysine-Based α-Peptide/β-Peptoid Peptidomimetics: Influence of Hydrophobicity, Fluorination, and Distribution of Cationic Charge on Antimicrobial Activity and Cytotoxicity. ChemMedChem 2017, 12, 312–318. [Google Scholar] [CrossRef]
- Carratalá, J.V.; Serna, N.; Villaverde, A.; Vázquez, E.; Ferrer-Miralles, N. Nanostructured antimicrobial peptides: The last push towards clinics. Biotechnol. Adv. 2020, 44, 107603. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, C.M. Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science. Angew. Chem.-Int. Ed. 2001, 40, 4128–4158. [Google Scholar] [CrossRef]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef]
- Klubthawee, N.; Bovone, G.; Marco-Dufort, B.; Guzzi, E.A.; Aunpad, R.; Tibbitt, M.W.; Klubthawee, N.; Aunpad, R.; Bovone, G.; Marco-Dufort, B.; et al. Biopolymer Nano-Network for Antimicrobial Peptide Protection and Local Delivery. Adv. Healthc. Mater. 2022, 11, 2101426. [Google Scholar] [CrossRef]
- Xia, C.; Braunstein, Z.; Toomey, A.C.; Zhong, J.; Rao, X. S100 proteins as an important regulator of macrophage inflammation. Front. Immunol. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Bresnick, A.R. S100 proteins as therapeutic targets. Biophys. Rev. 2018, 10, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.L.; Broome, A.; Ruse, M.; Robinson, N.; Ryan, D.; Lee, K. S100 proteins in the epidermis. J. Investig. Dermatol. 2004, 123, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Chessa, C.; Bodet, C.; Jousselin, C.; Wehbe, M.; Lévêque, N.; Garcia, M. Antiviral and Immunomodulatory Properties of Antimicrobial Peptides Produced by Human Keratinocytes. Front. Microbiol. 2020, 11, 1155. [Google Scholar] [CrossRef]
- Murray, J.I.; Tonkin, M.L.; Whiting, A.L.; Peng, F.; Farnell, B.; Cullen, J.T.; Hof, F.; Boulanger, M.J. Structural characterization of S100A15 reveals a novel zinc coordination site among S100 proteins and altered surface chemistry with functional implications for receptor binding. BMC Struct. Biol. 2012, 12, 16. [Google Scholar] [CrossRef]
- Donato, R. Intracellular and extracellular roles of S100 proteins. Microsc. Res. Tech. 2003, 60, 540–551. [Google Scholar] [CrossRef]
- Mustafa, F.E.Z.A.; Abdel-maksoud, F.M.; Hassan, A.H.S.; Mokhtar, D.M. Melatonin induces a stimulatory action on the scrotal skin components of Soay ram in the non-breeding season. Sci. Rep. 2020, 10, 10154. [Google Scholar] [CrossRef]
- Hussein, M.T.; Mokhtar, D.M.; Hassan, A.H.S. Melatonin activates the vascular elements, telocytes, and neuroimmune communication in the adrenal gland of Soay rams during the non-breeding season. Protoplasma 2020, 257, 353–369. [Google Scholar] [CrossRef]
- Fang, J.; Li, Y.H.; Li, X.H.; Chen, W.W.; He, J.; Xue, M.Z. Effects of melatonin on expressions of β-amyloid protein and S100β in rats with senile dementia. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7526–7532. [Google Scholar] [CrossRef]
- Hegyi, Z.; Zwicker, S.; Bureik, D.; Peric, M.; Koglin, S.; Batycka-Baran, A.; Prinz, J.C.; Ruzicka, T.; Schauber, J.; Wolf, R. Vitamin D analog calcipotriol suppresses the Th17 cytokine-induced proinflammatory S100 alarmins psoriasin (S100A7) and koebnerisin (S100A15) in psoriasis. J. Investig. Dermatol. 2012, 132, 1416–1424. [Google Scholar] [CrossRef]
- Cubillos, S.; Norgauer, J. Low vitamin D-modulated calcium-regulating proteins in psoriasis vulgaris plaques: S100A7 overexpression depends on joint involvement. Int. J. Mol. Med. 2016, 38, 1083–1092. [Google Scholar] [CrossRef]
- Wolf, R.; Lewerenz, V.; Büchau, A.S.; Walz, M.; Ruzicka, T. Human S100A15 splice variants are differentially expressed in inflammatory skin diseases and regulated through Th1 cytokines and calcium. Exp. Dermatol. 2007, 16, 685–691. [Google Scholar] [CrossRef]
- Benoit, S.; Toksoy, A.; Ahlmann, M.; Schmidt, M.; Sunderkötter, C.; Foell, D.; Pasparakis, M.; Roth, J.; Goebeler, M. Elevated serum levels of calcium-binding S100 proteins A8 and A9 reflect disease activity and abnormal differentiation of keratinocytes in psoriasis. Br. J. Dermatol. 2006, 155, 62–66. [Google Scholar] [CrossRef]
- Foell, D.; Seeliger, S.; Vogl, T.; Koch, H.G.; Maschek, H.; Harms, E.; Sorg, C.; Roth, J. Expression of S100A12 (EN-RAGE) in cystic fibrosis. Thorax 2003, 58, 613–617. [Google Scholar] [CrossRef]
- Turnier, J.L.; Fall, N.; Thornton, S.; Witte, D.; Bennett, M.R.; Appenzeller, S.; Klein-Gitelman, M.S.; Grom, A.A.; Brunner, H.I. Urine S100 proteins as potential biomarkers of lupus nephritis activity. Arthritis Res. Ther. 2017, 19, 242. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, K.; Jiang, X.; Zhang, J. S100A6 as a Potential Serum Prognostic Biomarker and Therapeutic Target in Gastric Cancer. Dig. Dis. Sci. 2014, 59, 2136–2144. [Google Scholar] [CrossRef]
- Salem, S.A.M.; Harvy, M.; Abdelaal, W.; El-Hagry, O.O.; El-Khateeb, E.A.; Emam, H.M.E.S.; El Nemr, R. Study of serum levels and skin expression of S100B protein inpsoriasis. An. Bras. Dermatol. 2017, 92, 323. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Pal, H.C.; Siddiqui, I.A.; Adhami, V.M.; Ayehunie, S.; Boylan, B.T.; Noubissi, F.K.; Khan, N.; Syed, D.N.; Elmets, C.A.; et al. Prodifferentiation, anti-inflammatory and antiproliferative effects of delphinidin, a dietary anthocyanidin, in a full-thickness three-dimensional reconstituted human skin model of psoriasis. Skin Pharmacol. Physiol. 2015, 28, 177–188. [Google Scholar] [CrossRef]
- Gambichler, T.; Bechara, F.G.; Scola, N.; Rotterdam, S.; Altmeyer, P.; Skrygan, M. Serum levels of antimicrobial peptides and proteins do not correlate with psoriasis severity and are increased after treatment with fumaric acid esters. Arch. Dermatol. Res. 2012, 304, 471–474. [Google Scholar] [CrossRef]
- Anderson, K.S.; Wong, J.; Polyak, K.; Aronzon, D.; Enerbäck, C. Detection of psoriasin/S100A7 in the sera of patients with psoriasis. Br. J. Dermatol. 2009, 160, 325–332. [Google Scholar] [CrossRef]
- Salama, R.H.M.; Al-Shobaili, H.A.; Al Robaee, A.A.; Alzolibani, A.A. Psoriasin: A novel marker linked obesity with psoriasis. Dis. Markers 2013, 34, 33–39. [Google Scholar] [CrossRef]
- Oesterle, A.; Hofmann Bowman, M.A. S100A12 and the S100/Calgranulins: Emerging Biomarkers for Atherosclerosis and Possibly Therapeutic Targets. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2496–2507. [Google Scholar] [CrossRef]
- Shiotsu, Y.; Mori, Y.; Nishimura, M.; Sakoda, C.; Tokoro, T.; Hatta, T.; Maki, N.; Iida, K.; Iwamoto, N.; Ono, T.; et al. Plasma S100A12 Level Is Associated with Cardiovascular Disease in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 718. [Google Scholar] [CrossRef]
- Wittkowski, H.; Frosch, M.; Wulffraat, N.; Goldbach-Mansky, R.; Kallinich, T.; Kuemmerle-Deschner, J.; Frühwald, M.C.; Dassmann, S.; Pham, T.-H.; Roth, J.; et al. S100A12 is a novel molecular marker differentiating systemic-onset juvenile idiopathic arthritis from other causes of fever of unknown origin. Arthritis Rheum. 2008, 58, 3924–3931. [Google Scholar] [CrossRef] [PubMed]
- Foell, D.; Kane, D.; Bresnihan, B.; Vogl, T.; Nacken, W.; Sorg, C.; FitzGerald, O.; Roth, J. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology 2003, 42, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.I.; Fernig, D.G.; Rudland, P.S.; Sparks, A.; Wilkinson, M.C.; Barraclough, R. Binding to Intracellular Targets of the Metastasis-Inducing Protein, S100A4 (p9Ka). Biochem. Biophys. Res. Commun. 2001, 286, 1212–1217. [Google Scholar] [CrossRef]
- Chow, K.H.; Park, H.J.; George, J.; Yamamoto, K.; Gallup, A.D.; Graber, J.H.; Chen, Y.; Jiang, W.; Steindler, D.A.; Neilson, E.G.; et al. S100A4 is a biomarker and regulator of glioma stem cells that is critical for mesenchymal transition in glioblastoma. Cancer Res. 2017, 77, 5360–5373. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Bureik, D.; Zwicker, S.; Ruzicka, T.; Wolf, R. The antimicrobial peptides psoriasin (s100a7) and koebnerisin (S100A15) suppress extracellular matrix production and proliferation of human fibroblasts. Skin Pharmacol. Physiol. 2015, 28, 115–123. [Google Scholar] [CrossRef]
- Gläser, R.; Meyer-Hoffert, U.; Harder, J.; Cordes, J.; Wittersheim, M.; Kobliakova, J.; Fölster-Holst, R.; Proksch, E.; Schröder, J.M.; Schwarz, T. The Antimicrobial Protein Psoriasin (S100A7) Is Upregulated in Atopic Dermatitis and after Experimental Skin Barrier Disruption. J. Investig. Dermatol. 2009, 129, 641–649. [Google Scholar] [CrossRef]
- Qin, W.; Ho, L.; Wang, J.; Peskind, E.; Pasinetti, G.M. S100A7, a Novel Alzheimer’s Disease Biomarker with Non-Amyloidogenic α-Secretase Activity Acts via Selective Promotion of ADAM-10. PLoS ONE 2009, 4, e4183. [Google Scholar] [CrossRef]
- LinMei; XiaBairong; QinLing; ChenHong; LouGe S100A7 Regulates Ovarian Cancer Cell Metastasis and Chemoresistance Through MAPK Signaling and Is Targeted by miR-330-5p. DNA Cell Biol. 2018, 37, 491–500. [CrossRef]
- Tian, T.; Li, X.; Hua, Z.; Ma, J.; Wu, X.; Liu, Z.; Chen, H.; Cui, Z.; Tian, T.; Li, X.; et al. S100A7 promotes the migration, invasion and metastasis of human cervical cancer cells through epithelial–mesenchymal transition. Oncotarget 2017, 8, 24964–24977. [Google Scholar] [CrossRef]
- Padilla, L.; Dakhel, S.; Adan, J.; Masa, M.; Martinez, J.M.; Roque, L.; Coll, T.; Hervas, R.; Calvis, C.; Llinas, L.; et al. S100A7: From mechanism to cancer therapy. Oncogene 2017, 36, 6749–6761. [Google Scholar] [CrossRef]
- Cook, M.G.; Massi, D.; Szumera-Ciećkiewicz, A.; Van den Oord, J.; Blokx, W.; van Kempen, L.C.; Balamurugan, T.; Bosisio, F.; Koljenović, S.; Portelli, F.; et al. An updated European Organisation for Research and Treatment of Cancer (EORTC) protocol for pathological evaluation of sentinel lymph nodes for melanoma. Eur. J. Cancer 2019, 114, 1–7. [Google Scholar] [CrossRef]
- Xiong, T.; Pan, F.; Li, D. Expression and clinical significance of S100 family genes in patients with melanoma. Melanoma Res. 2019, 29, 23. [Google Scholar] [CrossRef] [PubMed]
- Jury, C.S.; Mcallister, E.J.; Mackie, R.M. Rising levels of serum S100 protein precede other evidence of disease progression in patients with malignant melanoma. Br. J. Dermatol. 2000, 143, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, F.; Notopoulos, A. S100 protein family and its application in clinical practice. Hippokratia 2008, 12, 198. [Google Scholar]
- Faries, M.B.; Gupta, R.K.; Ye, X.; Lee, C.; Yee, R.; Leopoldo, Z.; Essner, R.; Foshag, L.J.; Elashoff, D.; Morton, D.L. A Comparison of 3 Tumor Markers (MIA, TA90IC, S100B) in Stage III Melanoma Patients. Cancer Investig. 2009, 25, 285–293. [Google Scholar] [CrossRef]
- Strobel, K.; Skalsky, J.; Kalff, V.; Baumann, K.; Seifert, B.; Joller-Jemelka, H.; Dummer, R.; Steinert, H.C. Tumour assessment in advanced melanoma: Value of FDG-PET/CT in patients with elevated serum S-100B. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1366–1375. [Google Scholar] [CrossRef]
- Ionita, M.G.; Vink, A.; Dijke, I.E.; Laman, J.D.; Peeters, W.; Van Der Kraak, P.H.; Moll, F.L.; De Vries, J.P.P.M.; Pasterkamp, G.; De Kleijn, D.P.V. High levels of myeloid-related protein 14 in human atherosclerotic plaques correlate with the characteristics of rupture-prone lesions. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1220–1227. [Google Scholar] [CrossRef]
- Mortensen, O.H.; Nielsen, A.R.; Erikstrup, C.; Plomgaard, P.; Fischer, C.P.; Krogh-Madsen, R.; Lindegaard, B.; Petersen, A.M.; Taudorf, S.; Pedersen, B.K. Calprotectin—A Novel Marker of Obesity. PLoS ONE 2009, 4, e7419. [Google Scholar] [CrossRef]
- De Jong, H.K.; Achouiti, A.; Koh, G.C.K.W.; Parry, C.M.; Baker, S.; Faiz, M.A.; van Dissel, J.T.; Vollaard, A.M.; van Leeuwen, E.M.M.; Roelofs, J.J.T.H.; et al. Expression and Function of S100A8/A9 (Calprotectin) in Human Typhoid Fever and the Murine Salmonella Model. PLoS Negl. Trop. Dis. 2015, 9, e0003663. [Google Scholar] [CrossRef]
- Konikoff, M.R.; Denson, L.A. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 524–534. [Google Scholar] [CrossRef]
- Gebhardt, C.; Németh, J.; Angel, P.; Hess, J. S100A8 and S100A9 in inflammation and cancer. Biochem. Pharmacol. 2006, 72, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Prieto, D.; Sotelo, N.; Seija, N.; Sernbo, S.; Abreu, C.; Durán, R.; Gil, M.; Sicco, E.; Irigoin, V.; Oliver, C.; et al. S100-A9 protein in exosomes from chronic lymphocytic leukemia cells promotes NF-κB activity during disease progression. Blood 2017, 130, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Gunaldi, M.; Okuturlar, Y.; Gedikbasi, A.; Akarsu, C.; Karabulut, M.; Kural, A. Diagnostic importance of S100A9 and S100A12 in breast cancer. Biomed. Pharmacother. 2015, 76, 52–56. [Google Scholar] [CrossRef]
- Topuz, M.F.; Binnetoglu, A.; Yumusakhuylu, A.C.; Sarı, M.; Baglam, T.; Gerin, F. Circulating calprotectin as a biomarker of laryngeal carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 2499–2504. [Google Scholar] [CrossRef]
- Huang, C.H.; Kuo, C.J.; Liang, S.S.; Chi, S.W.; Hsi, E.; Chen, C.C.; Lee, K.T.; Chiou, S.H. Onco-proteogenomics identifies urinary S100A9 and GRN as potential combinatorial biomarkers for early diagnosis of hepatocellular carcinoma. BBA Clin. 2015, 3, 205–213. [Google Scholar] [CrossRef][Green Version]
- Yasar, O.; Akcay, T.; Obek, C.; Turegun, F.A. Significance of S100A8, S100A9 and calprotectin levels in bladder cancer. Scand. J. Clin. Lab. Investig. 2017, 77, 437–441. [Google Scholar] [CrossRef]
- Chromy, B.A.; Gonzales, A.D.; Perkins, J.; Choi, M.W.; Corzett, M.H.; Chang, B.C.; Corzett, C.H.; McCutchen-Maloney, S.L. Proteomic analysis of human serum by two-dimensional differential gel electrophoresis after depletion of high-abundant proteins. J. Proteome Res. 2004, 3, 1120–1127. [Google Scholar] [CrossRef]
- Allgöwer, C.; Kretz, A.L.; von Karstedt, S.; Wittau, M.; Henne-Bruns, D.; Lemke, J. Friend or Foe: S100 Proteins in Cancer. Cancers 2020, 12, 2037. [Google Scholar] [CrossRef]
- MacK, G.S.; Marshall, A. Lost in migration. Nat. Biotechnol. 2010, 28, 214–229. [Google Scholar] [CrossRef]
- Okada, M.; Tokumitsu, H.; Kubota, Y.; Kobayashi, R. Interaction of S100 proteins with the antiallergic drugs, olopatadine, amlexanox, and cromolyn: Identification of putative drug binding sites on S100A1 protein. Biochem. Biophys. Res. Commun. 2002, 292, 1023–1030. [Google Scholar] [CrossRef]
- Shishibori, T.; Oyama, Y.; Matsushita, O.; Yamashita, K.; Furuichi, H.; Okabe, A.; Maeta, H.; Hata, Y.; Kobayashi, R. Three distinct anti-allergic drugs, amlexanox, cromolyn and tranilast, bind to S100A12 and S100A13 of the S100 protein family. Biochem. J. 1999, 338, 583. [Google Scholar] [CrossRef] [PubMed]
- Chiou, J.W.; Fu, B.; Chou, R.H.; Yu, C. Blocking the Interactions between Calcium-Bound S100A12 Protein and the V Domain of RAGE Using Tranilast. PLoS ONE 2016, 11, e0162000. [Google Scholar] [CrossRef]
- Cavalier, M.C.; Pierce, A.D.; Wilder, P.T.; Alasady, M.J.; Hartman, K.G.; Neau, D.B.; Foley, T.L.; Jadhav, A.; Maloney, D.J.; Simeonov, A.; et al. Covalent Small Molecule Inhibitors of Ca2+-Bound S100B. Biochemistry 2014, 53, 6628. [Google Scholar] [CrossRef] [PubMed]
- Dulyaninova, N.G.; Hite, K.M.; Zencheck, W.D.; Scudiero, D.A.; Almo, S.C.; Shoemaker, R.H.; Bresnick, A.R. Cysteine 81 Is Critical for the Interaction of S100A4 and Myosin-IIA. Biochemistry 2011, 50, 7218. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; McDonald, P.R.; Tamewitz, A.; Sikorski, R.P.; Wipf, P.; Skoko, J.J.; Lazo, J.S. A cell-active inhibitor of mitogen-activated protein kinase phosphatases restores paclitaxel-induced apoptosis in dexamethasone-protected cancer cells. Mol. Cancer Ther. 2008, 7, 330–340. [Google Scholar] [CrossRef]
- Brisson, M.; Nguyen, T.; Wipf, P.; Joo, B.; Day, B.W.; Skoko, J.S.; Schreiber, E.M.; Foster, C.; Bansal, P.; Lazo, J.S. Redox regulation of Cdc25B by cell-active quinolinediones. Mol. Pharmacol. 2005, 68, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Sack, U.; Walther, W.; Scudiero, D.; Selby, M.; Aumann, J.; Lemos, C.; Fichtner, I.; Schlag, P.M.; Shoemaker, R.H.; Stein, U. S100A4-induced cell motility and metastasis is restricted by the Wnt/β-catenin pathway inhibitor calcimycin in colon cancer cells. Mol. Biol. Cell 2011, 22, 3344. [Google Scholar] [CrossRef] [PubMed]
- Dahlmann, M.; Kobelt, D.; Walther, W.; Mudduluru, G.; Stein, U. S100A4 in Cancer Metastasis: Wnt Signaling-Driven Interventions for Metastasis Restriction. Cancers 2016, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Stein, U.; Arlt, F.; Smith, J.; Sack, U.; Herrmann, P.; Walther, W.; Lemm, M.; Fichtner, I.; Shoemaker, R.H.; Schlag, P.M. Intervening in β-Catenin Signaling by Sulindac Inhibits S100A4-Dependent Colon Cancer Metastasis. Neoplasia 2011, 13, 131. [Google Scholar] [CrossRef]
- Brufsky, A. Trastuzumab-based therapy for patients with HER2-positive breast cancer: From early scientific development to foundation of care. Am. J. Clin. Oncol. Cancer Clin. Trials 2010, 33, 186–195. [Google Scholar] [CrossRef]
- König, M.; Rharbaoui, F.; Aigner, S.; Dälken, B.; Schüttrumpf, J. Tregalizumab–A Monoclonal Antibody to Target Regulatory T Cells. Front. Immunol. 2016, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W. Anti-VEGF agents in metastatic colorectal cancer (mCRC): Are they all alike? Cancer Manag. Res. 2013, 5, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.; Clauder, A.K.; Manz, R.A. Targeting B cells and plasma cells in autoimmune diseases. Front. Immunol. 2018, 9, 835. [Google Scholar] [CrossRef]
- Björk, P.; Björk, A.; Vogl, T.; Stenström, M.; Liberg, D.; Olsson, A.; Roth, J.; Ivars, F.; Leanderson, T. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009, 7, 0800–0812. [Google Scholar] [CrossRef]
- Austermann, J.; Zenker, S.; Roth, J. S100-alarmins: Potential therapeutic targets for arthritis. Expert Opin. Ther. Targets 2017, 21, 739–751. [Google Scholar] [CrossRef]
- Sekimoto, R.; Fukuda, S.; Maeda, N.; Tsushima, Y.; Matsuda, K.; Mori, T.; Nakatsuji, H.; Nishizawa, H.; Kishida, K.; Kikuta, J.; et al. Visualized macrophage dynamics and significance of S100A8 in obese fat. Proc. Natl. Acad. Sci. USA 2015, 112, E2058–E2066. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Liu, Z.; Xu, M.; Jia, Y.; Wang, Y.; Li, Q.; Yang, W.; Zheng, X.; Tu, K.; Liu, Q. miR-187-3p inhibits the metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting S100A4. Cancer Lett. 2016, 381, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Du, G.; Xu, A.; Xi, X.; Li, D. Expression of miR-149-3p inhibits proliferation, migration, and invasion of bladder cancer by targeting S100A4. Am. J. Cancer Res. 2017, 7, 2209. [Google Scholar]
- Fan, F.; Lu, J.; Yu, W.; Zhang, Y.; Xu, S.; Pang, L.; Zhu, B. MicroRNA-26b-5p regulates cell proliferation, invasion and metastasis in human intrahepatic cholangiocarcinoma by targeting S100A7. Oncol. Lett. 2018, 15, 386. [Google Scholar] [CrossRef]
- Guo, Y.; Fu, W.; Chen, H.; Shang, C.; Zhong, M. miR-24 functions as a tumor suppressor in Hep2 laryngeal carcinoma cells partly through down-regulation of the S100A8 protein. Oncol. Rep. 2012, 27, 1097. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Howard, O.M.Z.; Dong, H.-F.; Voscopoulos, C.; Boeshans, K.; Winston, J.; Divi, R.; Gunsior, M.; Goldsmith, P.; Ahvazi, B.; et al. Chemotactic Activity of S100A7 (Psoriasin) Is Mediated by the Receptor for Advanced Glycation End Products and Potentiates Inflammation with Highly Homologous but Functionally Distinct S100A15. J. Immunol. 2008, 181, 1499–1506. [Google Scholar] [CrossRef]
- S100A7A-Protein S100-A7A-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q86SG5/entry (accessed on 5 September 2022).
- Büchau, A.S.; Hassan, M.; Kukova, G.; Lewerenz, V.; Kellermann, S.; Würthner, J.U.; Wolf, R.; Walz, M.; Gallo, R.L.; Ruzicka, T. S100A15, an antimicrobial protein of the skin: Regulation by E. coli through toll-like receptor 4. J. Investig. Dermatol. 2007, 127, 2596–2604. [Google Scholar] [CrossRef]
- ExPASy-ProtParam Tool. Available online: https://web.expasy.org/protparam/ (accessed on 5 September 2022).
- Awad, S.M.; Attallah, D.A.; Salama, R.H.; Mahran, A.M.; Abu El-Hamed, E. Serum levels of psoriasin (S100A7) and koebnerisin (S100A15) as potential markers of atherosclerosis in patients with psoriasis. Clin. Exp. Dermatol. 2018, 43, 262–267. [Google Scholar] [CrossRef]
- Rodríguez-Cerdeira, C.; Cordeiro-Rodríguez, M.; Carnero-Gregorio, M.; López-Barcenas, A.; Martínez-Herrera, E.; Fabbrocini, G.; Sinani, A.; Arenas-Guzmán, R.; González-Cespón, J.L. Biomarkers of Inflammation in Obesity-Psoriatic Patients. Mediat. Inflamm. 2019, 2019, 7353420. [Google Scholar] [CrossRef]
- Batycka-Baran, A.; Hattinger, E.; Marchenkov, A.; Koziol, M.; Bieniek, A.; Szepietowski, J.; Ruzicka, T.; Wolf, R. Koebnerisin (S100A15): A novel player in the pathogenesis of rosacea. J. Am. Acad. Dermatol. 2019, 80, 1753–1755. [Google Scholar] [CrossRef]
- Batycka-Baran, A.; Koziol-Galczynska, M.; Bieniek, A.; Wolf, R.; Łaczmański; Szepietowski, J. C. Expression of koebnerisin (S100A15) and calgranulin A (S100A8) in lesional and perilesional skin in patients suffering from hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e402–e404. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Nogueira da Costa, A.; Makrantonaki, E.; Hou, X.X.; Almansouri, D.; Dudley, J.T.; Edwards, H.; Readhead, B.; Balthasar, O.; Jemec, G.B.E.; et al. Alterations in innate immunity and epithelial cell differentiation are the molecular pillars of hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 846–861. [Google Scholar] [CrossRef] [PubMed]
- Batycka-Baran, A.; Matusiak, Ł.; Nowicka-suszko, D.; Szepietowski, J.C.; Baran, W. Increased serum levels of s100a4 and s100a15 in individuals suffering from hidradenitis suppurativa. J. Clin. Med. 2021, 10, 5320. [Google Scholar] [CrossRef]
- Hattinger, E.; Zwicker, S.; Ruzicka, T.; Yuspa, S.H.; Wolf, R. Opposing functions of Psoriasin (S100A7) and Koebnerisin (S100A15) in epithelial carcinogenesis. Curr. Opin. Pharmacol. 2013, 13, 588. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Lopez-Beltran, A.; Maclennan, G.T.; Montironi, R.; Eble, J.N.; Cheng, L. Expression of S100 Protein Family Members in the Pathogenesis of Bladder Tumors. Anticancer Res. 2007, 27, 3051–3058. [Google Scholar] [PubMed]
- Briso, E.M.; Guinea-Viniegra, J.; Bakiri, L.; Rogon, Z.; Petzelbauer, P.; Eils, R.; Wolf, R.; Rincón, M.; Angel, P.; Wagner, E.F. Inflammation-mediated skin tumorigenesis induced by epidermal c-Fos. Genes Dev. 2013, 27, 1959. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lin, M.C.; Hsiao, C.C.; Zheng, Y.X.; Chen, K.D.; Sung, M.T.; Chen, C.J.; Wang, T.Y.; Lin, Y.Y.; Chang, H.C.; et al. Increased S100A15 expression and decreased DNA methylation of its gene promoter are involved in high metastasis potential and poor outcome of lung adenocarcinoma. Oncotarget 2017, 8, 45710–45724. [Google Scholar] [CrossRef]
- Wolf, R.; Voscopoulos, C.; Winston, J.; Dharamsi, A.; Goldsmith, P.; Gunsior, M.; Vonderhaar, B.K.; Olson, M.; Watson, P.H.; Yuspa, S.H. Highly homologous hS100A15 and hS100A7 proteins are distinctly expressed in normal breast tissue and breast cancer. Cancer Lett. 2009, 277, 101. [Google Scholar] [CrossRef]
- Cho, C.C.; Hung, K.W.; Gorja, D.R.; Yu, C. The solution structure of human calcium-bound S100A4 mutated at four cysteine loci. J. Biomol. NMR 2015, 62, 233–238. [Google Scholar] [CrossRef]
- Brodersen, D.E.; Etzerodt, M.; Madsen, P.; Celis, J.E.; Thøgersen, H.C.; Nyborg, J.; Kjeldgaard, M. EF-hands at atomic resolution: The structure of human psoriasin (S100A7) solved by MAD phasing. Structure 1998, 6, 477–489. [Google Scholar] [CrossRef]
- Lin, H.; Andersen, G.R.; Yatime, L. Crystal structure of human S100A8 in complex with zinc and calcium. BMC Struct. Biol. 2016, 16, 8. [Google Scholar] [CrossRef]
- Chang, C.C.; Khan, I.; Tsai, K.L.; Li, H.; Yang, L.W.; Chou, R.H.; Yu, C. Blocking the interaction between S100A9 and RAGE V domain using CHAPS molecule: A novel route to drug development against cell proliferation. Biochim. Biophys. Acta 2016, 1864, 1558–1569. [Google Scholar] [CrossRef]
- Moroz, O.V.; Antson, A.A.; Murshudov, G.N.; Maitland, N.J.; Dodson, G.G.; Wilson, K.S.; Skibshøj, I.; Lukanidin, E.M.; Bronstein, I.B. The three-dimensional structure of human S100A12. Acta Crystallogr. D Biol. Crystallogr. 2001, 57, 20–29. [Google Scholar] [CrossRef]
- Ostendorp, T.; Leclerc, E.; Galichet, A.; Koch, M.; Demling, N.; Weigle, B.; Heizmann, C.W.; Kroneck, P.M.H.; Fritz, G. Structural and functional insights into RAGE activation by multimeric S100B. EMBO J. 2007, 26, 3868–3878. [Google Scholar] [CrossRef]
- Prot Pi|Protein Tool. Available online: https://www.protpi.ch/Calculator/ProteinTool (accessed on 5 September 2022).

| AMP | Source | Medical Use | Formulation/Route of Administration | FDA Approval/Stage of Development | Company |
|---|---|---|---|---|---|
| Oritavancin | Semisynthetic glycopeptide | Acute bacterial skin and skin structure infections | Intravenous | 2014 | Orbactiv |
| Multiferon | Leukocyte fraction of human blood | Metastatic renal cell carcinoma | Subcutaneous | 2006 | Intron/Roferon-A/Roche |
| Boceprevir | Synthetic peptide | Hepatitis C virus genotype 1 | Oral | 2011 | Victrelis/Merck |
| Daptomycin (lipopeptide) | Streptomyces roseosporus | Complicated skin infections caused by susceptible strains of Gram-positive microorganisms | Intravenous injection | 2003 | Cubist Pharmaceuticals LLC (Merck & Co.) |
| PAC-113 | Human histatin 3 | Oral candidiasis in HIV seropositive patients | Mouth rinse | Phase II | Pacgen Biopharmaceuticals Corporation/Quintiles, Inc. |
| DPK-060 | Human kininogen | Bacterial infections in atopic dermatitis and acute external otitis | Ointment for local application | Phase II | DermaGen AB/Pergamum AB |
| LL-37 | Human (derived from hCAP18) | Hard-to-heal venous leg ulcers | Polyvinyl alcohol-based solution for administration in the wound bed | Phase II | Lipopeptide AB |
| DiaPep277 | Human Heat Shock Protein 60 (Hsp60) | Type 1 Diabetes mellitus | Subcutaneously | Phase III | Andromeda Biotech Ltd. |
| Omiganan | Bovine indolicidin | Catheter infections, atopic dermatitis, genital warts, acne vulgaris, and rosacea | Topical gel | Phase III/Phase II | Cutanea Life Sciences/Mallinacckrodt |
| PM060184 | Lithop locamialithistoides | Cancer | Intravenous | Phase I | PharmaMar (Colmenar Viejo, Madrid, Spain) |
| Pexiganan | Analog of magainin from African clawed frog | Infected diabetic foot ulcers | Topical cream | Phase III | Dipexium Pharmaceuticals (has merged with PLx Pharma) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurpet, K.; Chwatko, G. S100 Proteins as Novel Therapeutic Targets in Psoriasis and Other Autoimmune Diseases. Molecules 2022, 27, 6640. https://doi.org/10.3390/molecules27196640
Kurpet K, Chwatko G. S100 Proteins as Novel Therapeutic Targets in Psoriasis and Other Autoimmune Diseases. Molecules. 2022; 27(19):6640. https://doi.org/10.3390/molecules27196640
Chicago/Turabian StyleKurpet, Katarzyna, and Grażyna Chwatko. 2022. "S100 Proteins as Novel Therapeutic Targets in Psoriasis and Other Autoimmune Diseases" Molecules 27, no. 19: 6640. https://doi.org/10.3390/molecules27196640
APA StyleKurpet, K., & Chwatko, G. (2022). S100 Proteins as Novel Therapeutic Targets in Psoriasis and Other Autoimmune Diseases. Molecules, 27(19), 6640. https://doi.org/10.3390/molecules27196640






