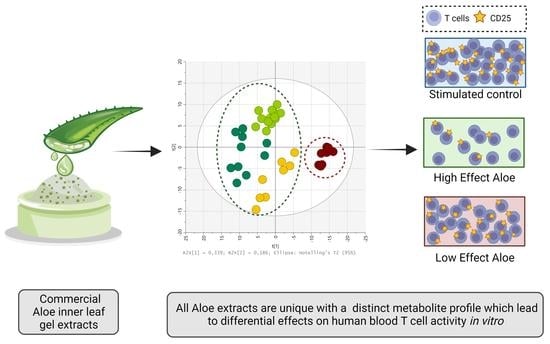
Differences in Metabolite Composition of Aloe barbadensis Mill. Extracts Lead to Differential Effects on Human Blood T Cell Activity In Vitro
Abstract
:1. Introduction
2. Results
2.1. Standard Phytochemical Quality Characteristics of Aloe Extracts
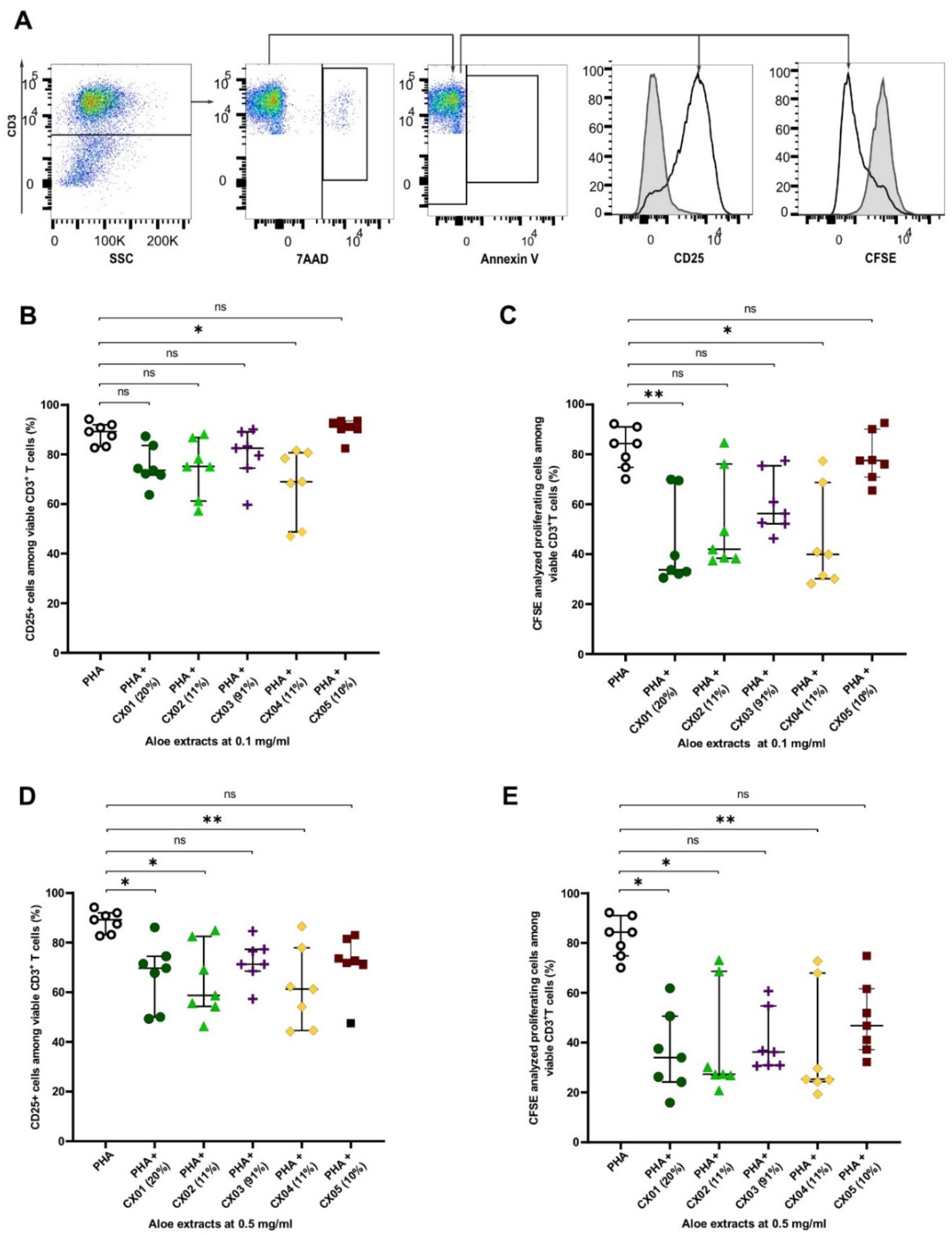
2.2. Aloe Extracts Vary in Their Effect on T Cell Activation and Proliferation, Which Is not Correlated to Their Acemannan Content
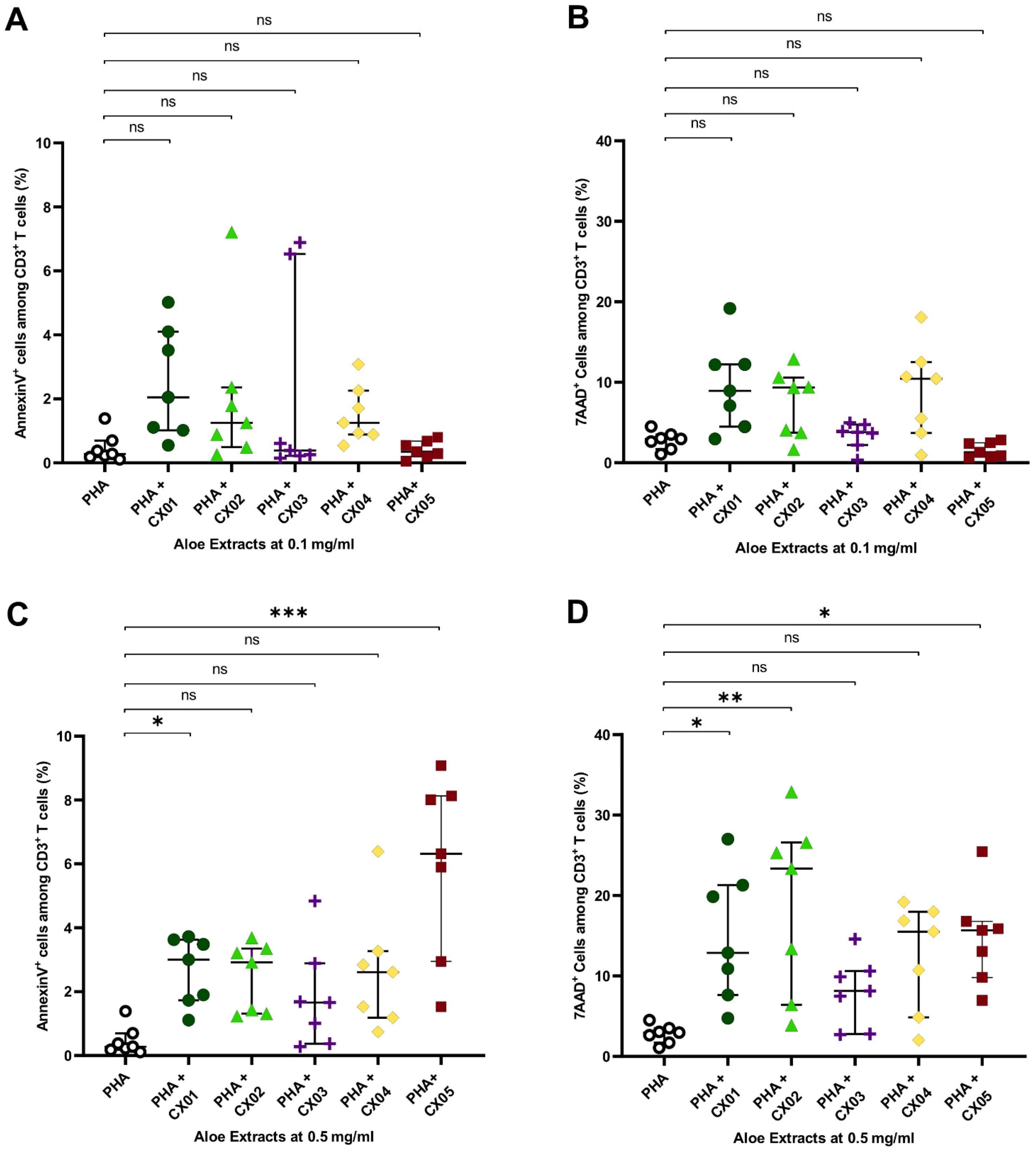
2.3. Aloe Extracts Induce a Concentration Dependent Effect on Apoptosis and Cell Death in Polyclonally Stimulated T Cells
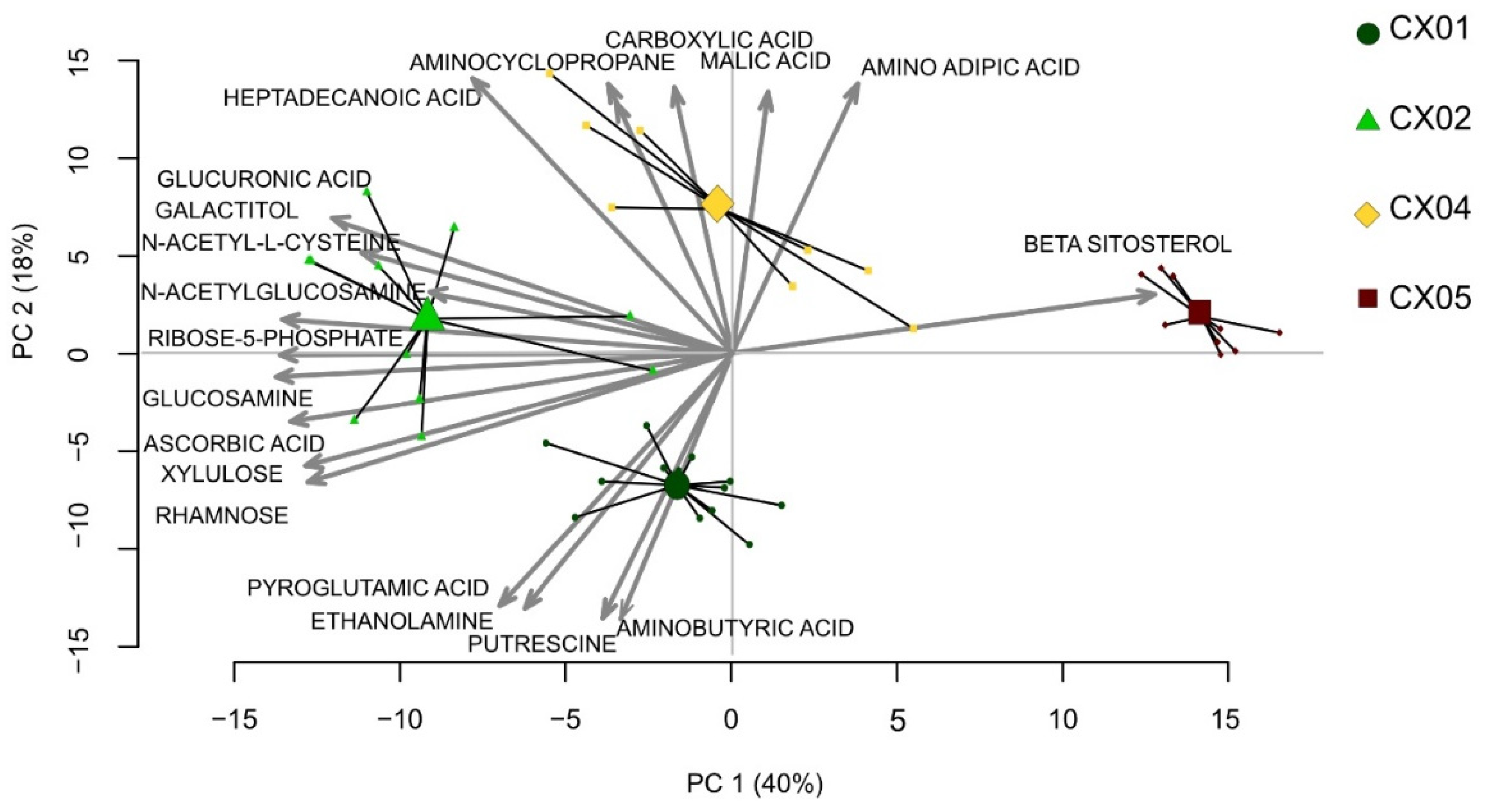
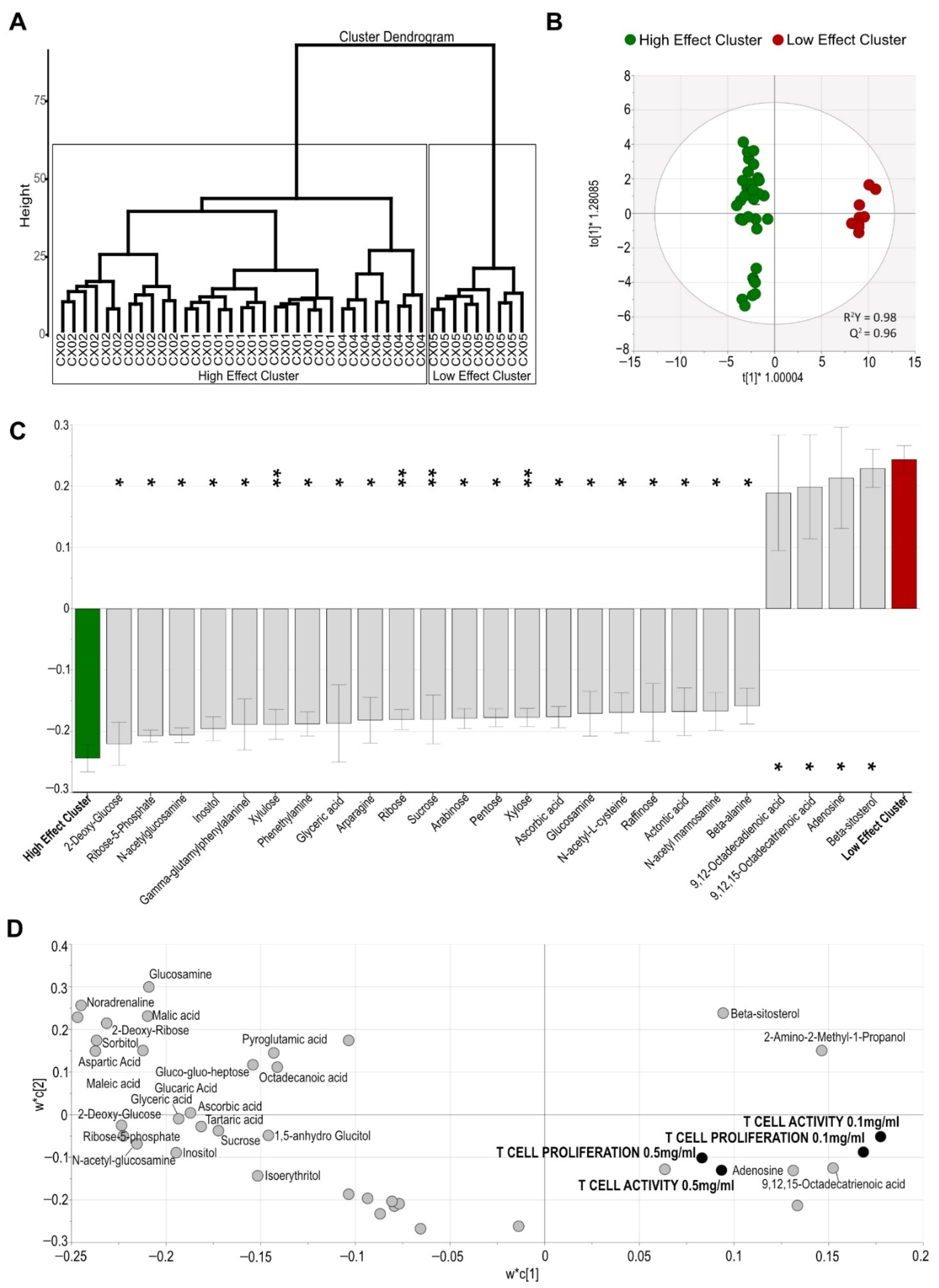
2.4. Aloe Extracts Differ in Their Metabolite Composition
2.5. The Effect of Aloe Extracts on T Cell Activity and Proliferation Is Correlated to Their Distinct Metabolite Composition
3. Discussion
4. Materials and Methods
4.1. Plant Material—Aloe Extracts
4.2. Quality Assessment of Aloe Extracts and Quantification of Standard Aloe Marker Components
4.3. Metabolite Analysis of Aloe Extracts
4.4. Study Subjects and Sample Collection
4.5. Analysis of the Effect of Aloe Extracts on Human Blood T Cell Activity In Vitro
4.6. Statistical and Data Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cock, I.E. The Genus Aloe: Phytochemistry and Therapeutic Uses Including Treatments for Gastrointestinal Conditions and Chronic Inflammation. In Novel Natural Products: Therapeutic Effects in Pain, Arthritis and Gastro-Intestinal Diseases; Rainsford, K., Powanda, M., Whitehouse, M., Eds.; Springer: Basel, Switzerland, 2015; Volume 70, pp. 179–235. [Google Scholar] [CrossRef]
- Eshun, K.; He, Q. Aloe Vera: A Valuable Ingredient for the Food, Pharmaceutical and Cosmetic Industries—A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Hamman, J.H. Composition and Applications of Aloe vera Leaf Gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jettanacheawchankit, S.; Sasithanasate, S.; Sangvanich, P.; Banlunara, W.; Thunyakitpisal, P. Acemannan Stimulates Gingival Fibroblast Proliferation; Expressions of Keratinocyte Growth Factor-1, Vascular Endothelial Growth Factor, and Type I Collagen; and Wound Healing. J. Pharmacol. Sci. 2009, 109, 525–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langmead, L.; Makins, R.; Rampton, D.S. Anti-inflammatory effects of aloe vera gel in human colorectal mucosa in vitro. Aliment. Pharmacol. Ther. 2004, 19, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Modak, D.; Chattaraj, S.; Nandi, D.; Sarkar, A.; Roy, J.; Chaudhuri, T.K.; Bhattacharjee, S. Aloe vera gel homogenate shows anti-inflammatory activity through lysosomal membrane stabilization and downregulation of TNF-α and Cox-2 gene expressions in inflammatory arthritic animals. Futur. J. Pharm. Sci. 2021, 7, 12. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological Update Properties of Aloe Vera and its Major Active Constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef] [Green Version]
- Sierra-Garcia, G.D.; Castro-Rios, R.; Gonzalez-Horta, A.; Lara-Arias, J.; Chavez-Montes, A. Acemannan, an Extracted Polysaccharide from Aloe vera: A Literature Review. Nat. Prod. Commun. 2014, 9, 1217–1221. [Google Scholar] [CrossRef] [Green Version]
- Xing, W.; Guo, W.; Zou, C.-H.; Fu, T.-T.; Li, X.-Y.; Zhu, M.; Qi, J.-H.; Song, J.; Dong, C.-H.; Li, Z.; et al. Acemannan accelerates cell proliferation and skin wound healing through AKT/mTOR signaling pathway. J. Dermatol. Sci. 2015, 79, 101–109. [Google Scholar] [CrossRef]
- Zhang, L.; Tizard, I.R. Activation of a mouse macrophage cell line by acemannan: The major carbohydrate fraction from Aloe vera gel. Immunopharmacology 1996, 35, 119–128. [Google Scholar] [CrossRef]
- Rodríguez, E.R.; Martín, J.D.; Romero, C.D. Aloe vera as a Functional Ingredient in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 305–326. [Google Scholar] [CrossRef]
- Pugh, N.; Ross, S.A.; ElSohly, M.A.; Pasco, D.S. Characterization of Aloeride, a New High-Molecular-Weight Polysaccharide from Aloe vera with Potent Immunostimulatory Activity. J. Agric. Food Chem. 2000, 49, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, B.; Magnusson, M.K.; Isaksson, S.; Larsson, F.; Öhman, L. Effects of Aloe barbadensis Mill. extract (AVH200®) on human blood T cell activity in vitro. J. Ethnopharmacol. 2016, 179, 301–309. [Google Scholar] [CrossRef]
- Budai, M.M.; Varga, A.; Milesz, S.; Tőzsér, J.; Benkő, S. Aloe vera downregulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages. Mol. Immunol. 2013, 56, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habeeb, F.; Stables, G.; Bradbury, F.; Nong, S.; Cameron, P.; Plevin, R.; Ferro, V.A. The inner gel component of Aloe vera suppresses bacterial-induced pro-inflammatory cytokines from human immune cells. Methods 2007, 42, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-Y.; Kwon, H.-J.; Sung, M.-K. Dietary aloin, aloesin, or aloe-gel exerts anti-inflammatory activity in a rat colitis model. Life Sci. 2011, 88, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, D.; Dhandapani, R.; Jayaveni, S.; Jithendra, P.S.; Rose, C.; Mandal, A.B. In vitro anti inflammatory activity of Aloe vera by down regulation of MMP-9 in peripheral blood mononuclear cells. J. Ethnopharmacol. 2012, 141, 542–546. [Google Scholar] [CrossRef]
- Ni, Y.; Turner, D.; Yates, K.M.; Tizard, I. Isolation and characterization of structural components of Aloe vera L. leaf pulp. Int. Immunopharmacol. 2004, 4, 1745–1755. [Google Scholar] [CrossRef]
- IASC. IASC Labeling Guidance. Available online: https://www.iasc.org/files/Publications/22_0506_IASC%20Labeling%20Guidance.pdf (accessed on 29 June 2022).
- IASC. Glossary of Terms Commonly Used in the Aloe Industry. Available online: https://www.iasc.org/files/Publications/22_0506_IASC%20Glossary%20of%20terms.pdf (accessed on 29 June 2022).
- Femenia, A.; Sánchez, E.S.; Simal, S.; Rosselló, C. Compositional features of polysaccharides from Aloe vera (Aloe barbadensis Miller) plant tissues. Carbohydr. Polym. 1999, 39, 109–117. [Google Scholar] [CrossRef]
- Lee, S.; Do, S.-G.; Kim, S.Y.; Kim, J.; Jin, Y.; Lee, C.H. Mass Spectrometry-Based Metabolite Profiling and Antioxidant Activity of Aloe vera (Aloe barbadensis Miller) in Different Growth Stages. J. Agric. Food Chem. 2012, 60, 11222–11228. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Rudaz, S.; Hae Choi, Y.; Kyong Kim, H. Plant Metabolomics: From Holistic Data to Relevant Biomarkers. Curr. Med. Chem. 2013, 20, 1056–1090. [Google Scholar] [CrossRef]
- Aldayel, T.S.; Grace, M.H.; Lila, M.A.; Yahya, M.A.; Omar, U.M.; Alshammary, G. LC-MS characterization of bioactive metabolites from two Yemeni Aloe spp. with antioxidant and antidiabetic properties. Arab. J. Chem. 2020, 13, 5040–5049. [Google Scholar] [CrossRef]
- Cardarelli, M.; Rouphael, Y.; Pellizzoni, M.; Colla, G.; Lucini, L. Profile of bioactive secondary metabolites and antioxidant capacity of leaf exudates from eighteen Aloe species. Ind. Crops Prod. 2017, 108, 44–51. [Google Scholar] [CrossRef]
- Kim, D.M.; Jung, J.Y.; Lee, H.K.; Kwon, Y.S. Determination and Profiling Of Secondary Metabolites in Aloe Vera, Aloe Arborescens and Aloe Saponaria. Biomed. J. Sci. Tech. Res. 2021, 40, 32555–32563. [Google Scholar] [CrossRef]
- Bozzi, A.; Perrin, C.; Austin, S.; Vera, F. Quality and authenticity of commercial Aloe vera gel powders. Food Chem. 2007, 103, 22–30. [Google Scholar] [CrossRef]
- IASC. Aloe Vera Quality Standard. Available online: https://www.iasc.org/files/Publications/22_0506_IASC%20Aloe%20Vera%20Quality%20Standard.pdf (accessed on 29 June 2022).
- Dentali, S. “Nondecolorized” essential qualifier for NTP aloe vera study material. Toxicol. Sci. 2013, 133, 342. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Aloe vera: A review of toxicity and adverse clinical effects. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 77–96. [Google Scholar] [CrossRef]
- Monakhova, Y.B.; Randel, G.; Diehl, B.W.K. Automated Control of the Organic and Inorganic Composition of Aloe vera Extracts Using H-1 NMR Spectroscopy. J. Aoac Int. 2016, 99, 1213–1218. [Google Scholar] [CrossRef]
- Chien, M.W.; Lin, M.H.; Huang, S.H.; Fu, S.H.; Hsu, C.Y.; Yen, B.L.; Chen, J.T.; Chang, D.M.; Sytwu, H.K. Glucosamine Modulates T Cell Differentiation through Down-regulating N-Linked Glycosylation of CD25. J. Biol. Chem. 2015, 290, 29329–29344. [Google Scholar] [CrossRef] [Green Version]
- de Jesus, T.; Shukla, S.; Ramakrishnan, P. Too sweet to resist: Control of immune cell function by O-GlcNAcylation. Cell. Immunol. 2018, 333, 85–92. [Google Scholar] [CrossRef]
- Zhao, Q.; Chu, Z.; Zhu, L.; Yang, T.; Wang, P.; Liu, F.; Huang, Y.; Zhang, F.; Zhang, X.; Ding, W.; et al. 2-Deoxy-d-Glucose Treatment Decreases Anti-inflammatory M2 Macrophage Polarization in Mice with Tumor and Allergic Airway Inflammation. Front. Immunol. 2017, 8, 637. [Google Scholar] [CrossRef] [Green Version]
- Huston, M.; Smith, T. Plant Succession: Life History and Competition. Am. Nat. 1987, 130, 168–198. [Google Scholar] [CrossRef]
- Kim, J.Y.; Loo, E.P.; Pang, T.Y.; Lercher, M.; Frommer, W.B.; Wudick, M.M. Cellular export of sugars and amino acids: Role in feeding other cells and organisms. Plant Physiol. 2021, 187, 1893–1914. [Google Scholar] [CrossRef] [PubMed]
- Lefsih, K.; Iboukhoulef, L.; Petit, E.; Benouatas, H.; Pierre, G.; Delattre, C. Anti-Inflammatory and Antioxidant Effect of a D-galactose-rich Polysaccharide Extracted From Aloe vera Leaves. Adv. Appl. Chem. Biochem. 2018, 1, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Pimentel-Muiños, F.X.; Muñoz-Fernández, M.A.; Fresno, M. Control of T lymphocyte activation and IL-2 receptor expression by endogenously secreted lymphokines. J. Immunol. 1994, 152, 5714–5722. [Google Scholar]
- Reddy, M.; Eirikis, E.; Davis, C.; Davis, H.M.; Prabhakar, U. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: An in vitro model to monitor cellular immune function. J. Immunol. Methods 2004, 293, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.H.; Donato, J.J.; Hartman, G.M.; Haas, R.C. Anti-inflammatory and wound healing activity of a growth substance in Aloe vera. J. Am. Podiatr. Med. Assoc. 1994, 84, 77–81. [Google Scholar]
- Vázquez, B.; Avila, G.; Segura, D.; Escalante, B. Antiinflammatory activity of extracts from Aloe vera gel. J. Ethnopharmacol. 1996, 55, 69–75. [Google Scholar] [CrossRef]
- Choi, S.H.; Shin, H.S. Anti-inflammatory and Anti-bacterial Effects of Aloe vera MAP against Multidrug-resistant Bacteria. Nat. Prod. Sci. 2017, 23, 286–290. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Cui, Y.; Pi, F.; Cheng, Y.; Guo, Y.; Qian, H. Extraction, Purification, Structural Characteristics, Biological Activities and Pharmacological Applications of Acemannan, a Polysaccharide from Aloe vera: A Review. Molecules 2019, 24, 1554. [Google Scholar] [CrossRef] [Green Version]
- Foster, M.; Hunter, D.; Samman, S. Evaluation of the Nutritional and Metabolic Effects of Aloe vera. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor and Francis Group, LLC.: Boca Raton, FL, USA, 2011. [Google Scholar]
- Babu, S.; Jayaraman, S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef]
- Linnemann, C.; Schildberg, F.A.; Schurich, A.; Diehl, L.; Hegenbarth, S.I.; Endl, E.; Lacher, S.; Müller, C.E.; Frey, J.; Simeoni, L.; et al. Adenosine regulates CD8 T-cell priming by inhibition of membrane-proximal T-cell receptor signalling. Immunology 2009, 128, e728–e737. [Google Scholar] [CrossRef] [PubMed]
- EMA. European Union Herbal Monograph on Aloe Barbadensis Mill, and on Aloe (Various Species, Mainly Aloe Ferox Mill and its Hybrids), Folii Succus Siccatus. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-aloe-barbadensis-mill-aloe-various-species-mainly-aloe-ferox_en.pdf (accessed on 20 June 2022).
- IARC. ALOE VERA- IARC Monographs. Int. Agency Res. Cancer 2016, 108, 37–71. [Google Scholar]
- WHO. WHO Monographs on Selected Medicinal Plants. Available online: https://apps.who.int/iris/handle/10665/42052 (accessed on 20 June 2022).
- Boudreau, M.D.; Beland, F.A.; Nichols, J.A.; Pogribna, M. Toxicology and carcinogenesis studies of a nondecolorized [corrected] whole leaf extract of Aloe barbadensis Miller (Aloe vera) in F344/N rats and B6C3F1 mice (drinking water study). Natl. Toxicol. Program Tech. Rep. Ser. 2013, 577, 1–266. [Google Scholar]
- Boudreau, M.D.; Mellick, P.W.; Olson, G.R.; Felton, R.P.; Thorn, B.T.; Beland, F.A. Clear evidence of carcinogenic activity by a whole-leaf extract of Aloe barbadensis miller (aloe vera) in F344/N rats. Toxicol. Sci. 2013, 131, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Jia, Q.; Randel, G.; Diehl, B.; Weaver, S.; Milligan, G. Quantitative 1H-NMR spectrometry method for quality control of Aloe vera products. J. AOAC Int. 2010, 93, 842–848. [Google Scholar]
- Burmistrova, N.A.; Krivets, O.O.; Monakhova, Y.B. UV Spectroscopic Determination of Aloin in Aloe vera (A. vera) Samples Based on Chemometric Data Processing. J. Anal. Chem. 2020, 75, 1137–1142. [Google Scholar] [CrossRef]
- Savolainen, O.I.; Sandberg, A.S.; Ross, A.B. A Simultaneous Metabolic Profiling and Quantitative Multimetabolite Metabolomic Method for Human Plasma Using Gas-Chromatography Tandem Mass Spectrometry. J. Proteome Res. 2016, 15, 259–265. [Google Scholar] [CrossRef]
- Jonsson, P.; Johansson, A.I.; Gullberg, J.; Trygg, J.; A, J.; Grung, B.; Marklund, S.; Sjöström, M.; Antti, H.; Moritz, T. High-Throughput Data Analysis for Detecting and Identifying Differences between Samples in GC/MS-Based Metabolomic Analyses. Anal. Chem. 2005, 77, 5635–5642. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 29 June 2022).
- Shaffer, R.E. Multi- and Megavariate Data Analysis. Principles and Applications, I. Eriksson, E. Johansson, N. Kettaneh-Wold and S. Wold, Umetrics Academy, Umeå, 2001, ISBN 91-973730-1-X, 533pp. J. Chemom. 2002, 16, 261–262. [Google Scholar] [CrossRef]
- Umetrics. SIMCA –OPLS vs PCA: Explaining Differences or Grouping Data. Available online: https://www.sartorius.com/en/knowledge/science-snippets/explaining-differences-or-grouping-data-opls-da-vs-pca-data-analysis-507204 (accessed on 29 July 2022).
- Umetrics. SIMCA® 15 User Guide. PLS-Partial Least Squares Projections to Latent Structures. Available online: https://www.sartorius.com/download/544940/simca-15-user-guide-en-b-00076-sartorius-data.pdf (accessed on 23 July 2022).
- GraphPad. Interpreting Results: Repeated Measures Two-Way ANOVA. Available online: https://www.graphpad.com/guides/prism/latest/statistics/stat_interpreting_results_repeated_.htm#:~:text=If%20only%20one%20factor%20is,of%20the%20repeated%20measures%20factor. (accessed on 27 September 2022).
| Extract | Batches | Processing | Product Description |
|---|---|---|---|
| CX01 | 2016014 a,b 2016011 b 2016007 b 2015016 b 2015007 b | Freeze dried and decolorized | Not defined |
| CX02 | 20140402 a,b 20160603 b 20160606 b 20170902 b | Freeze dried and decolorized | Not defined |
| CX03 | 20150326 a | Freeze dried and decolorized | Not defined |
| CX04 | 20151203 a,b 20151107 b 20171003 b | Spray dried and decolorized | Not defined |
| CX05 | 20160601 a,b 20171101 b 20170502 b | Dehydrated, no decolorization | High molecular weight, Majority polysaccharides 50 kDa |
| Component | Origin of Component | IASC Aloe Standard † Content by Dry Matter | Content % in Aloe Extracts | ||||
|---|---|---|---|---|---|---|---|
| CX01 | CX02 | CX03 | CX04 | CX05 | |||
| Acemannan | Fresh Aloe | ≥5% | 19.9 | 10.9 | 91.4 | 10.8 | 9.8 |
| Glucose | Fresh Aloe | Present | 25.1 | 27.7 | Not detected | 30.9 | 5.3 |
| Fructose | Fresh Aloe | Present | 3.8 | 7.3 | Not detected | 5.5 | Detected |
| Malic acid | Fresh Aloe | Present | 17.2 | 17.9 | Not detected | 19.8 | 10.9 |
| Mg | Fresh Aloe | Present | Traces | Traces | 0.1 | 0.8 | 0.1 |
| Ca | Fresh Aloe | Present | 4.4 | 4.1 | 0.8 | 3.0 | 2.5 |
| WLM | Whole leaf marker | ≤5% | 2.5 | Not detected | Not detected | Not detected | Not detected |
| Aloin | Anthraquinone | ≤10ppm | <0.1 ppm | <0.1 ppm | <0.1 ppm | <0.1 ppm | <10 ppm |
| Citric acid | WLM or added acidifier | Limit <5% (undeclared) | 2.7 | 1.3 | Not detected | 0.6 | 1.6 |
| Lactic acid | Degradation (bacterial) | Limit <5% | 1.9 | 2.7 | Traces | 2.2 | 0.6 |
| Acetic acid | Degradation (chemical) | Limit <5% | Traces | Traces | Traces | Not detected | Not detected |
| Succinic acid | Degradation (enzymatic) | Limit <5% | Not detected | Not detected | Not detected | Not detected | Not detected |
| Fumaric acid | Degradation (enzymatic) | Limit <5% | Not detected | Not detected | Not detected | Not detected | Not detected |
| Maltodextrin | Adulterant/Additive | Absent (undeclared) | Not detected | Not detected | Not detected | Not detected | Not detected |
| Sodium benzoate | Added Preservative | Absent (undeclared) | Not detected | Not detected | Not detected | Not detected | Not detected |
| Potassium sorbate | Added Preservative | Absent (undeclared) | Not detected | Not detected | Not detected | Not detected | Not detected |
| Metabolites | T Cell Activity 0.1 mg/mL | T Cell Proliferation 0.1 mg/mL | T Cell Activity 0.5 mg/mL | T Cell Proliferation 0.5 mg/mL |
|---|---|---|---|---|
| Aspartic Acid | −0.45 | −0.62 | −0.28 | −0.30 |
| Noradrenaline | −0.58 | −0.62 | −0.46 | −0.38 |
| 2-Deoxy-Ribose | −0.52 | −0.60 | −0.37 | −0.35 |
| Maleic acid | −0.67 | −0.56 | −0.56 | −0.35 |
| Sorbitol | −0.51 | −0.55 | −0.43 | −0.36 |
| Glucaric Acid | −0.37 | −0.54 | −0.37 | −0.38 |
| 2-Deoxy-Glucose | −0.53 | −0.54 | −0.18 | −0.21 |
| Ribose-5-phosphate | −0.50 | −0.52 | −0.18 | −0.21 |
| Glucosamine | −0.47 | −0.50 | −0.52 | −0.40 |
| N-acetyl-glucosamine | −0.53 | −0.48 | −0.19 | −0.18 |
| Glyceric acid | −0.49 | −0.47 | −0.16 | −0.17 |
| Gluco-gluo-heptose | −0.19 | −0.45 | −0.10 | −0.27 |
| Malic acid | −0.61 | −0.44 | −0.50 | −0.28 |
| Ascorbic acid | −0.36 | −0.43 | −0.20 | −0.23 |
| Tartaric acid | −0.52 | −0.42 | −0.19 | −0.13 |
| Pyroglutamic acid | −0.15 | −0.42 | −0.14 | −0.28 |
| Inositol | −0.54 | −0.41 | −0.19 | −0,13 |
| Sucrose | −0.55 | −0.35 | −0.25 | −0.11 |
| Isoerythritol | −0.42 | −0.34 | −0.04 | −0.05 |
| 1,5-anhydro Glucitol | −0.49 | −0.31 | −0.18 | −0.07 |
| Octadecanoic acid | −0.51 | −0.30 | 0.44 | 0.20 |
| Adenosine | 0.45 | 0.23 | 0.44 | 0.18 |
| 2-Amino-2-Methyl-1-Propanol | 0.41 | 0.28 | 0.10 | 0.05 |
| 9,12,15-Octadecatrienoic acid | 0.48 | 0.28 | −0.36 | −0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahluwalia, B.; Magnusson, M.K.; Larsson, F.; Savolainen, O.; Ross, A.B.; Öhman, L. Differences in Metabolite Composition of Aloe barbadensis Mill. Extracts Lead to Differential Effects on Human Blood T Cell Activity In Vitro. Molecules 2022, 27, 6643. https://doi.org/10.3390/molecules27196643
Ahluwalia B, Magnusson MK, Larsson F, Savolainen O, Ross AB, Öhman L. Differences in Metabolite Composition of Aloe barbadensis Mill. Extracts Lead to Differential Effects on Human Blood T Cell Activity In Vitro. Molecules. 2022; 27(19):6643. https://doi.org/10.3390/molecules27196643
Chicago/Turabian StyleAhluwalia, Bani, Maria K. Magnusson, Fredrik Larsson, Otto Savolainen, Alastair B. Ross, and Lena Öhman. 2022. "Differences in Metabolite Composition of Aloe barbadensis Mill. Extracts Lead to Differential Effects on Human Blood T Cell Activity In Vitro" Molecules 27, no. 19: 6643. https://doi.org/10.3390/molecules27196643
APA StyleAhluwalia, B., Magnusson, M. K., Larsson, F., Savolainen, O., Ross, A. B., & Öhman, L. (2022). Differences in Metabolite Composition of Aloe barbadensis Mill. Extracts Lead to Differential Effects on Human Blood T Cell Activity In Vitro. Molecules, 27(19), 6643. https://doi.org/10.3390/molecules27196643