5,5,5-Trichloropent-3-en-one as a Precursor of 1,3-Bi-centered Electrophile in Reactions with Arenes in Brønsted Superacid CF3SO3H. Synthesis of 3-Methyl-1-trichloromethylindenes
Abstract
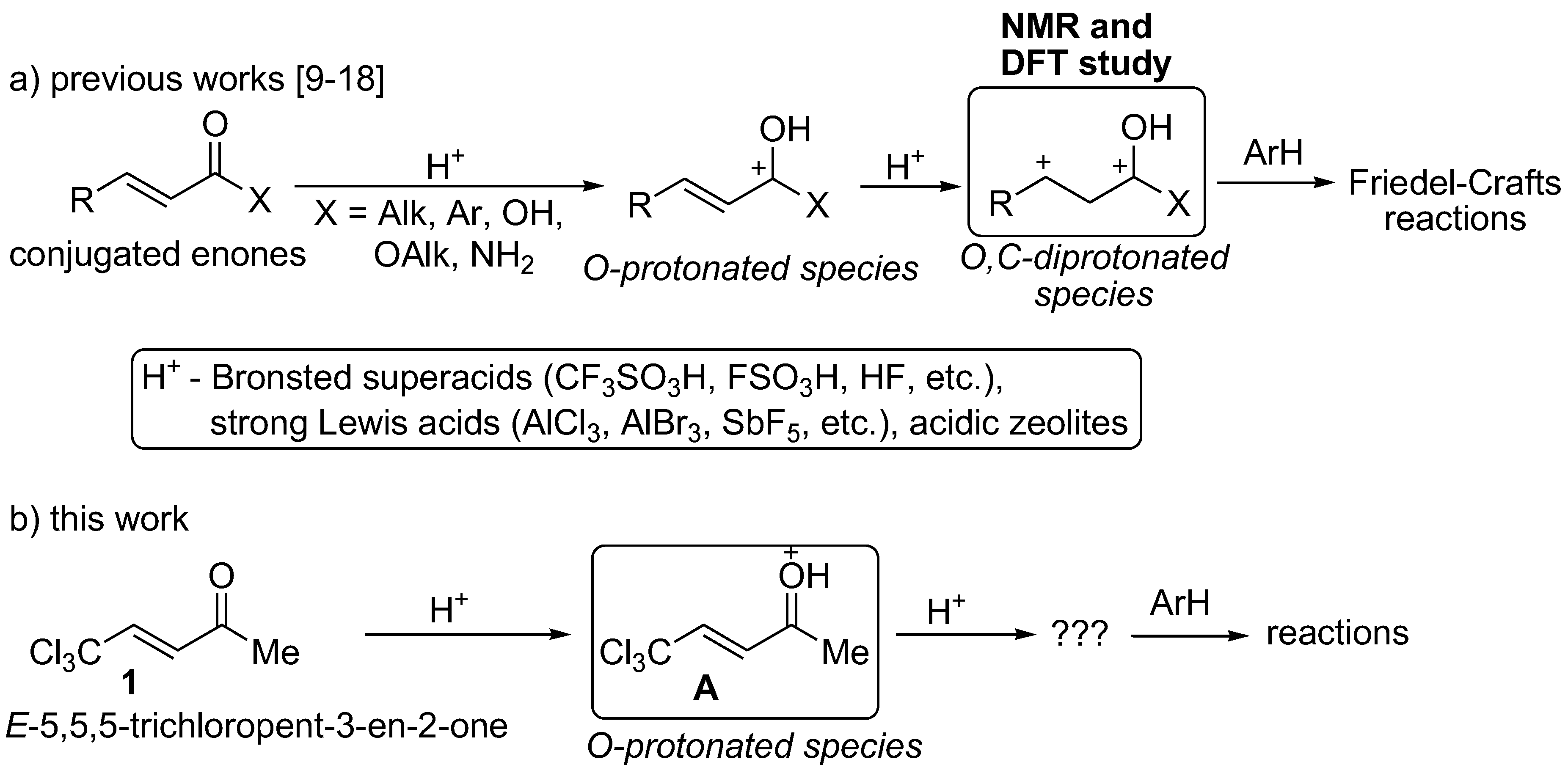
:1. Introduction
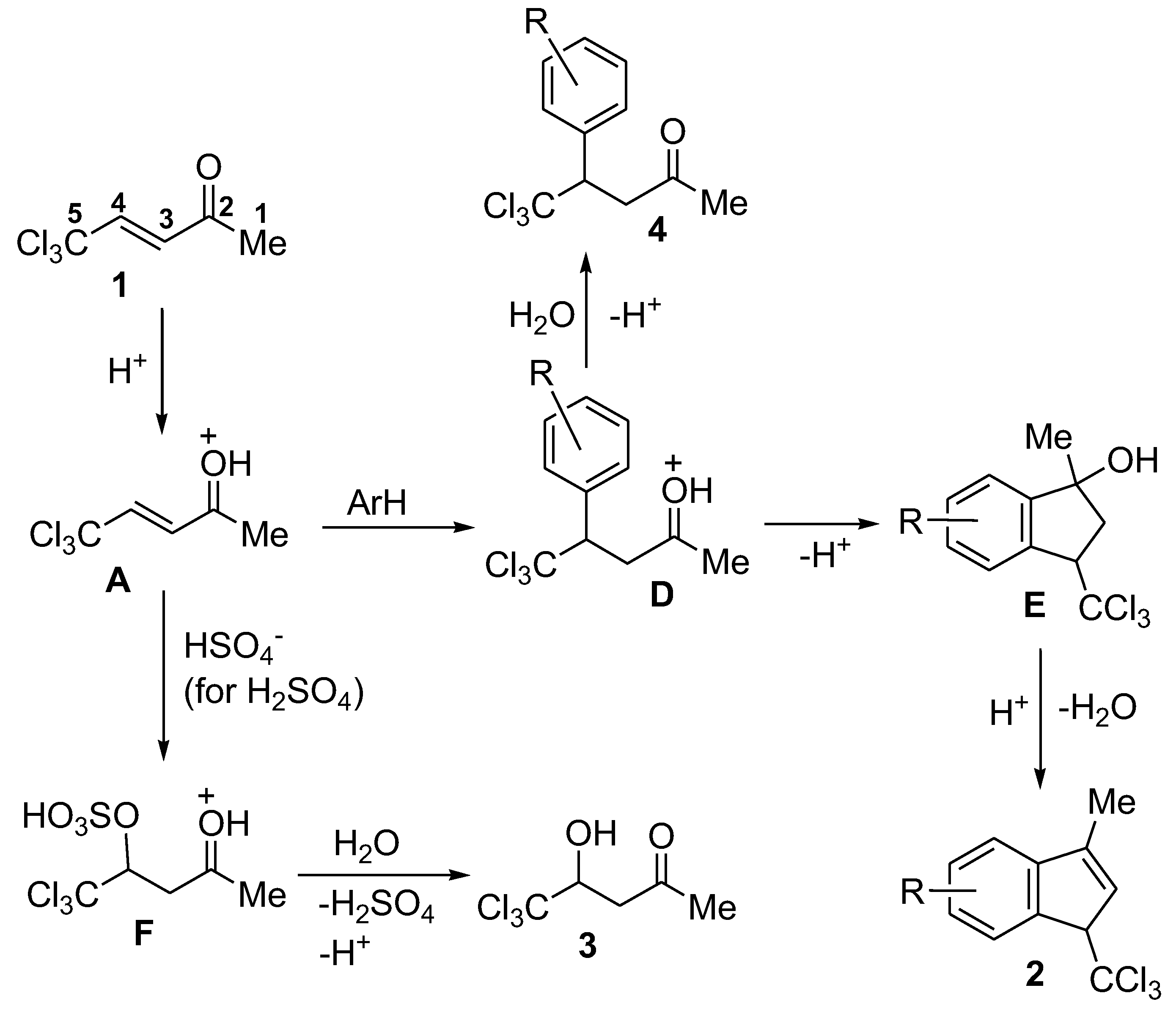
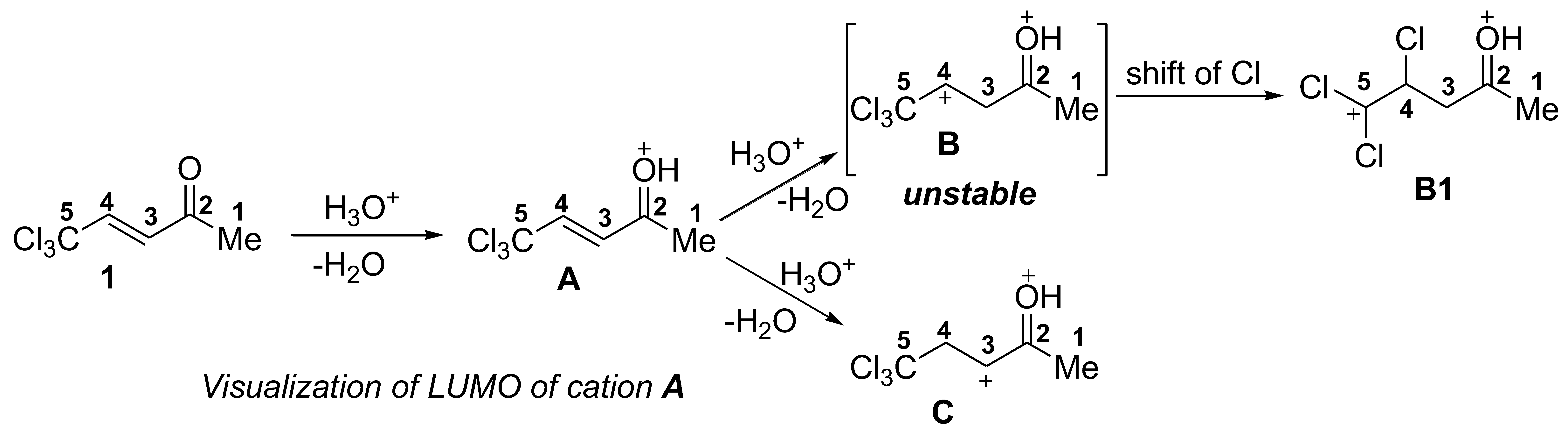
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. DFT Calculations
3.3. Preparation and Characterization of Compounds 1–4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olah, G.A.; Prakash, G.K.S.; Molnár, Á.; Sommer, J. Superacid Chemistry, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2008. [Google Scholar]
- Olah, G.A.; Klumpp, D.A. Superelectrophiles and Their Chemistry; Wiley: New York, NY, USA, 2008. [Google Scholar]
- Naredla, R.R.; Klumpp, D.A. Contemporary carbocation chemistry: Applications in organic synthesis. Chem. Rev. 2013, 113, 6905–6948. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, A.N.; Vasilyev, A.V. Trifluoromethanesulfonic acid in organic synthesis. Russ. J. Org. Chem. 2017, 53, 485–509. [Google Scholar] [CrossRef]
- Klumpp, D.A.; Kennedy, S. Superelectrophiles in ring-forming reactions. Arkivoc 2018, 87, 215–232. [Google Scholar] [CrossRef]
- Vasilyev, A.V. Superelectrophilic activation of alkynes, alkenes, and allenes. Adv. Org. Synth. 2018, 8, 81–120. [Google Scholar]
- Zhao, W.; Sun, J. Triflimide (HNTf2) in Organic Synthesis. Chem. Rev. 2018, 118, 10349–10392. [Google Scholar] [CrossRef]
- Fernandes, A.J.; Panossian, A.; Michelet, B.; Martin-Mingot, A.; Leroux, F.R.; Thibaudeau, S. CF3-substituted carbocations: Underexploited intermediates with great potential in modern synthetic chemistry. Beilst. J. Org. Chem. 2021, 17, 343–378. [Google Scholar] [CrossRef]
- Koltunov, K.Y.; Repinskaya, I. Interaction of phenols and their derivatives with aromatic compounds in the presence of acidic agents XIV. Protonation of α,β-enones in the HF-SbF5-SO2FCl superacid system. Russ. J. Org. Chem. 1994, 30, 82–85. [Google Scholar]
- Allen, J.M.; Johnston, K.M.; Jones, J.F.; Shotter, R.G. Friedel-crafts cyclisation—VI: Polyphosphoric acid-catalysed reactions of crotonophenomes and chalcones. Tetrahedron 1977, 33, 2083–2087. [Google Scholar] [CrossRef]
- Suzuki, T.; Ohwada, T.; Shudo, K. Superacid-Catalyzed Electrocyclization of Diphenylmethyl Cations to Fluorenes. Kinetic and Theoretical Revisit Supporting the Involvement of Ethylene Dications. J. Am. Chem. Soc. 1997, 119, 6774–6780. [Google Scholar] [CrossRef]
- Walspurger, S.; Vasilyev, A.V.; Sommer, J.; Pale, P. Chemistry of 3-arylindenones: Behavior in superacids and photodimerization. Tetrahedron 2005, 61, 3559–3564. [Google Scholar] [CrossRef]
- Koltunov, K.Y.; Walspurger, S.; Sommer, J. Superacidic Activation of α,β-Unsaturated Amides and Their Electrophilic Reactions. Eur. J. Org. Chem. 2004, 4039–4047. [Google Scholar] [CrossRef]
- Koltunov, K.Y.; Walspurger, S.; Sommer, J. Friedel–Crafts alkylation of benzene with α,β-unsaturated amides. Tetrahedron Lett. 2004, 45, 3547–3549. [Google Scholar] [CrossRef]
- Rendy, R.; Zhang, Y.; McElrea, A.; Gomez, A.; Klumpp, D.A. Superacid-Catalyzed Reactions of Cinnamic Acids and the Role of Superelectrophiles. J. Org. Chem. 2004, 69, 2340–2347. [Google Scholar] [CrossRef] [PubMed]
- Stadler, D.; Goeppert, A.; Rasul, G.; Olah, G.A.; Prakash, G.K.S.; Bach, T.J. Chiral Benzylic Carbocations: Low-Temperature NMR Studies and Theoretical Calculations. Org. Chem. 2009, 74, 312–318. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Yan, P.; Török, B.; Olah, G.A. Superacidic Trifluoromethanesulfonic Acid-Induced Cycli-Acyalkylation of Aromatics. Cat. Lett. 2003, 87, 109–112. [Google Scholar] [CrossRef]
- Genaev, A.M.; Salnikov, G.E.; Koltunov, K.Y. DFT insight into superelectrophilic activation of α,β-unsaturated nitriles and ketones in superacids. Org. Biomol. Chem. 2022, 20, 6799–6808. [Google Scholar] [CrossRef]
- Kalyaev, M.V.; Ryabukhin, D.S.; Borisova, M.A.; Ivanov, A.Y.; Boyarskaya, I.A.; Borovkova, K.E.; Nikiforova, L.R.; Salmova, J.V.; Ulyanovskii, N.V.; Kosyakov, D.S.; et al. Synthesis of 3-aryl-3-(furan-2-yl)propanoic acid derivatives and study of their antimicrobial activity. Molecules 2022, 27, 4612. [Google Scholar] [CrossRef]
- Kochurin, M.A.; Ismagilova, A.R.; Zakusilo, D.N.; Khoroshilova, O.V.; Boyarskaya, I.A.; Vasilyev, A.V. Reactions of linear conjugated dienone structures with arenes under superelectrophilic activation conditions. An experimental and theoretical study of intermediate multicentered electrophilic species. New J. Chem. 2022, 46, 12041–12053. [Google Scholar] [CrossRef]
- Ismagilova, A.R.; Zakusilo, D.N.; Osetrova, L.V.; Vasilyev, A.V. Reaction of 5-phenylpent-2,4-dienoic acid in trifluoromethanesulfonic acid. Russ. Chem. Bull. Int. Ed. 2020, 69, 1928–1932. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Giri, S.; Duley, S. Update 2 of: Electrophilicity index. Chem. Rev. 2011, 111, PR43–PR75. [Google Scholar] [CrossRef] [PubMed]
- Koca, M.; Yerdelen, K.O.; Anil, B.; Kasap, Z.; Sevindik, H.; Ozyurek, I.; Gunesacar, G.; Turkaydin, K. Design, synthesis and biological activity of 1H-indene-2-carboxamides as multi-targeted anti-Alzheimer agents. J. Enzyme Inhib. Med. Chem. 2016, 31, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Zhang, R.; Meng, Q.; Zhang, X.; Sun, Y. Discovery of new protein kinase CK2 inhibitors with 1,3-dioxo-2,3-dihydro-1H-indene core. Med. Chem. Commun. 2016, 7, 1352–1355. [Google Scholar] [CrossRef]
- Huffman, J.W.; Padqett, L.W. Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indene. Current Med. Chem. 2005, 12, 1395–1411. [Google Scholar] [CrossRef]
- Sui-Seng, C.; Castonguay, A.; Chen, Y.; Gareau, D.; Groux, L.F.; Zargarian, D. Catalytic Reactivities of Indenyl–nickel, Indenyl–palladium, and PCsp3P–nickel Complexes. Top. Catal. 2006, 37, 81–90. [Google Scholar] [CrossRef]
- Deck, P.A. Perfluoroaryl-substituted cyclopentadienyl complexes of transition metals. Coord. Chem. Rev. 2006, 250, 1032–1055. [Google Scholar] [CrossRef]
- Leino, R.; Lehmus, P.; Lehtonen, A. Heteroatom-Substituted Group 4 Bis(indenyl)metallocenes. Eur. J. Inorg. Chem. 2004, 2004, 3201–3222. [Google Scholar] [CrossRef]
- Zargarian, D. Group 10 metal indenyl complexes. Coord. Chem. Rev. 2002, 233–234, 157–176. [Google Scholar] [CrossRef]
- Stradiotto, M.; McGlinchey, M.J. η1-indenyl derivatives of transition metal and main group elements: Synthesis, characterization and molecular dynamics. Coord. Chem. Rev. 2001, 219–221, 311–378. [Google Scholar] [CrossRef]
- McGlinchey, M.J.; Nikitin, K. Palladium-catalysed coupling reactions en route to molecular machines: Sterically hindered indenyl and ferrocenyl anthracenes and triptycenes, and biindenyls. Molecules. 2020, 25, 1950. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, F.R.; Qi, F.; Heumüller, T.; Distler, A.; Egelhaaf, H.-J.; Li, N.; Chow, P.C.Y.; Brabec, C.J.; Jen, A.K.-Y. Renewed prospects for organic photovoltaics. Chem. Rev. 2022, 122, 14180–14274. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, J.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Schlegel, H.B.; Scalmani, G.; Barone, V.; Mennucci, B. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Nakatsua, S.; Gubaidullin, A.T.; Mamedov, V.A.; Tsuboi, S.A. convenient synthesis of olefins via deacylation reaction. Tetrahedron 2004, 60, 2337–2349. [Google Scholar] [CrossRef]
- Dias, L.C.; de Marchi, A.A.; Ferreira, M.A.B.; Aguilar, A.M. The influence of a β-electron withdrawing substituent in aldol reactions of methylketone boron enolates. Org. Lett. 2007, 9, 4869–4872. [Google Scholar] [CrossRef] [PubMed]


| Compound 1 and Cation A | Solvent | 1H NMR, δ, ppm | 13C NMR, δ, ppm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| H1 | H3 | H4 | C1 | C2 | C3 | C4 | 5CCl3 | ||
 | CDCl3 | 2.40 | 6.61 | 7.05 | 28.9 | 196.7 | 128.0 | 144.4 | 92.6 |
 | CH3CO2H a ∆δ b | 2.29 | 6.56 | 7.06 | 27.6 | 198.4 | 128.1 | 144.3 | 92.4 |
| −0.11 | −0.05 | 0.01 | −1.3 | 1.7 | 0.1 | −0.1 | −0.2 | ||
| CF3CO2H a ∆δ b | 2.62 | 6.85 | 7.34 | 31.7 | 210.8 | 131.9 | 153.4 | 96.2 | |
| 0.22 | 0.24 | 0.29 | 2.8 | 14.1 | 3.9 | 9.0 | 3.6 | ||
| H2SO4 a ∆δ b | 3.03 | 7.11 | 7.86 | 26.4 | 221.8 | 123.9 | 158.5 | 89.3 | |
| 0.63 | 0.5 | 0.81 | −2.5 | 25.1 | −4.1 | 14.1 | −3.3 | ||
| CF3SO3H a ∆δ b | 3.22 | 7.31 | 8.14 | 27.5 | 226.7 | 124.3 | 163.2 | 89.9 | |
| 0.82 | 0.7 | 1.09 | −1.4 | 30.0 | −3.7 | 18.8 | −2.7 | ||
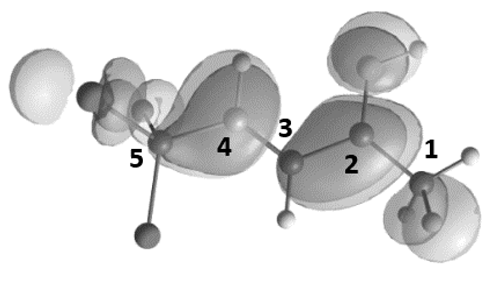
| Entry | Species | EHOMO, eV | ELUMO, eV | ω, a eV | q(C2), b e | q(C3), b e | q(C4), b e | k(C2)LUMO, c % | k(C3)LUMO, c % | k(C4)LUMO, c % | ΔG, d kJ/mol |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 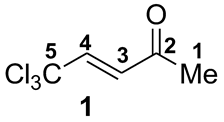 | −7.62 | −2.61 | 2.6 | 0.57 | −0.26 | −0.18 | 9 | 10 | 14 | - |
| 2 | 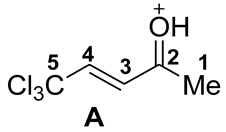 | −8.87 | −4.21 | 4.6 | 0.66 | −0.31 | −0.06 | 28 | 4 | 21 | 1→A −35 |
| 3 | 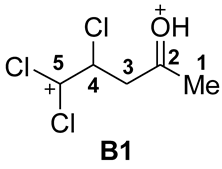 | −9.73 | −5.51 | 6.9 | 0.75 | −0.53 | −0.29 | 5.4 | 8.9 | 15.3 | A→B1 196 |
| 4 | 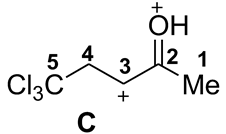 | −9.25 | −6.98 | 14.5 | 0.65 | 0.26 | −0.56 | 15 | 41 | 7 | A→C 268 |
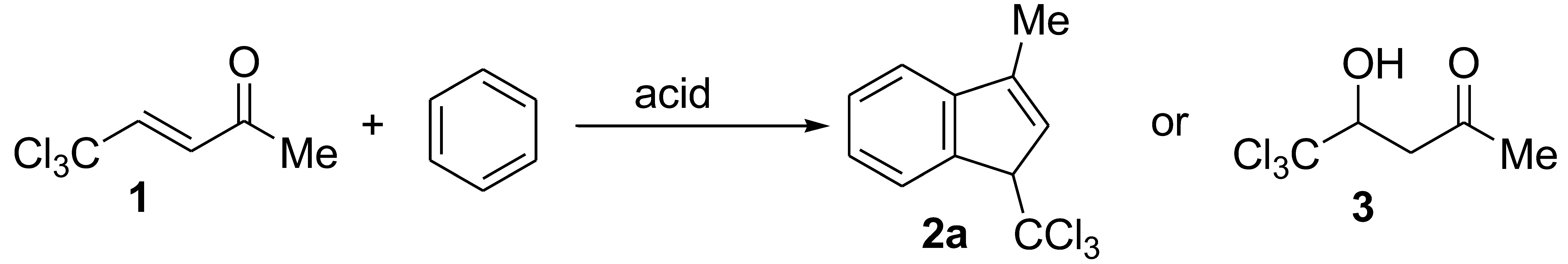
| Entry | Acid | Temperature | Time | Reaction Product, Yield, % |
|---|---|---|---|---|
| 1 | AlCl3 | room temperature | 1 h | oligomeric material a |
| 2 | AlBr3 | room temperature | 1 h | oligomeric material b |
| 3 | AlBr3 | room temperature | 2 h | oligomeric material a |
| 4 | H2SO4 | room temperature | 6 days | 3, 75% |
| 5 | CF3SO3H | room temperature | 0.5 h | quantitative isolation of starting compound 1 |
| 6 | CF3SO3H | room temperature | 3 days | 2a, 20% b |
| 7 | CF3SO3H | room temperature | 5 days | 2a, 29% a |
| 8 | CF3SO3H | 60 °C | 0.5 h | oligomeric material a |
| 9 | CF3SO3H | 80 °C | 1 h | oligomeric material a |
| 10 | FSO3H | −78 °C | 2 h | quantitative isolation of starting compound 1 |
| 11 | CH3CO2H | room temperature | 2 days | quantitative isolation of starting compound 1 |
| 12 | CH3CO2H | 80 °C | 1 h | quantitative isolation of starting compound 1 |
| 13 | CF3CO2H | room temperature | 2 days | quantitative isolation of starting compound 1 |
| 14 | CF3CO2H | 80 °C | 1 h | quantitative isolation of starting compound 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shershnev, I.A.; Boyarskaya, I.A.; Vasilyev, A.V. 5,5,5-Trichloropent-3-en-one as a Precursor of 1,3-Bi-centered Electrophile in Reactions with Arenes in Brønsted Superacid CF3SO3H. Synthesis of 3-Methyl-1-trichloromethylindenes. Molecules 2022, 27, 6675. https://doi.org/10.3390/molecules27196675
Shershnev IA, Boyarskaya IA, Vasilyev AV. 5,5,5-Trichloropent-3-en-one as a Precursor of 1,3-Bi-centered Electrophile in Reactions with Arenes in Brønsted Superacid CF3SO3H. Synthesis of 3-Methyl-1-trichloromethylindenes. Molecules. 2022; 27(19):6675. https://doi.org/10.3390/molecules27196675
Chicago/Turabian StyleShershnev, Ivan A., Irina A. Boyarskaya, and Aleksander V. Vasilyev. 2022. "5,5,5-Trichloropent-3-en-one as a Precursor of 1,3-Bi-centered Electrophile in Reactions with Arenes in Brønsted Superacid CF3SO3H. Synthesis of 3-Methyl-1-trichloromethylindenes" Molecules 27, no. 19: 6675. https://doi.org/10.3390/molecules27196675






