Green Preparation of Antimicrobial 1D-Coordination Polymers: [Zn(4,4′-bipy)Cl2]∞ and [Zn(4,4′-bipy)2(OAc)2]∞ by Ultrasonication of Zn(II) Salts and 4,4′-Bipyridine
Abstract
:1. Introduction
2. Results and Discussion
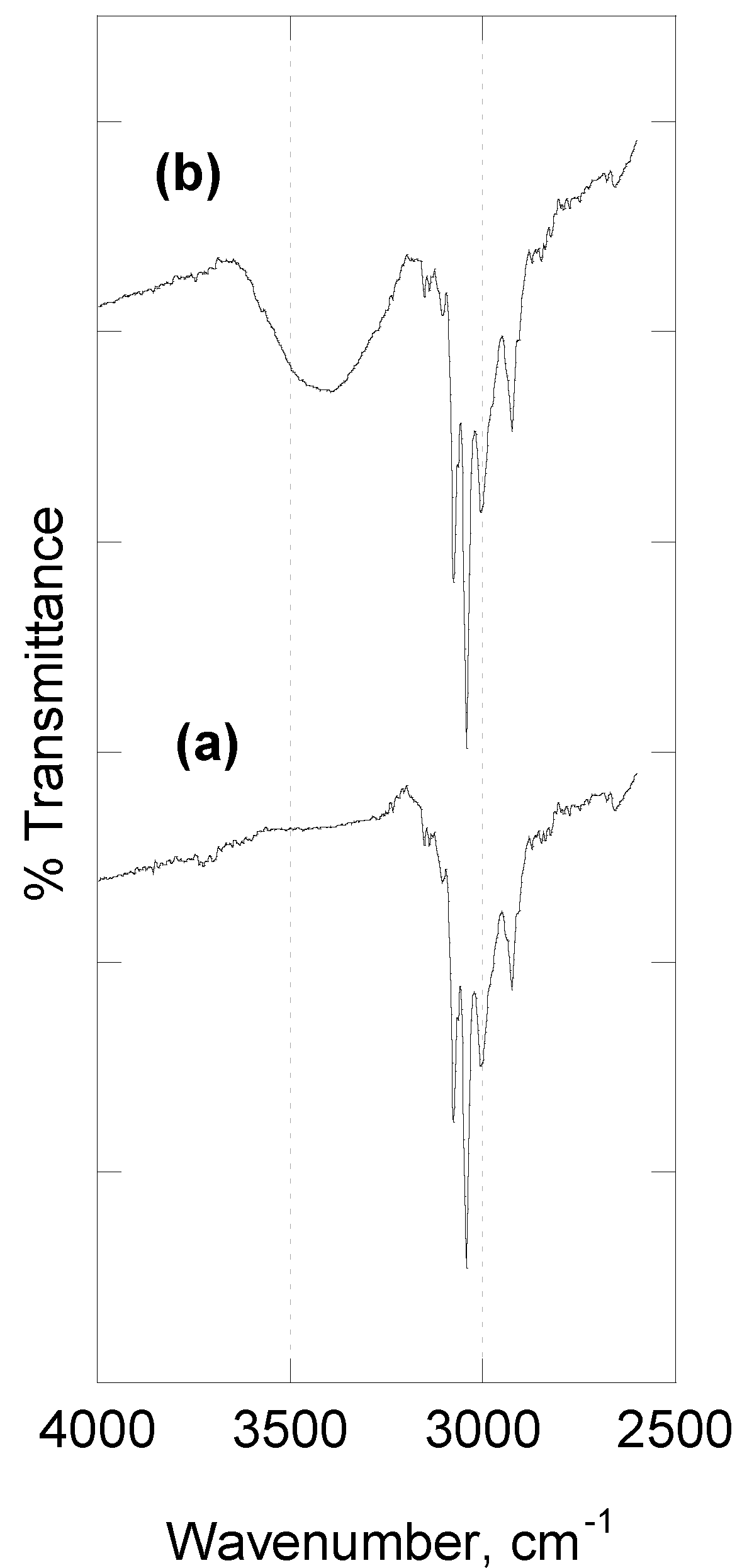
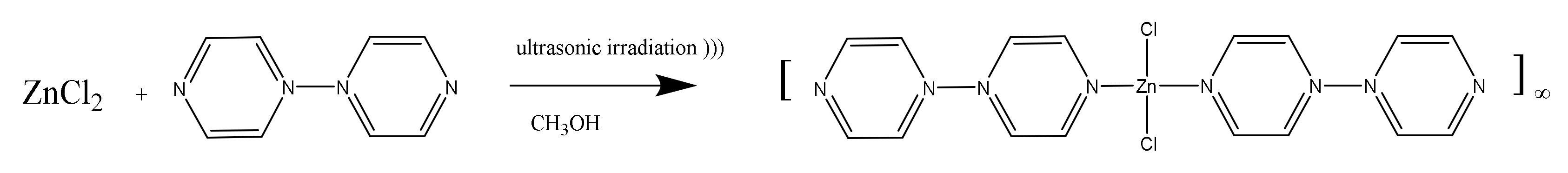
2.1. [Zn(4,4′-bipy)Cl2]∞
- -
- Polymorph I—UBOCIX02 (a = 15.821(3) Å, b = 5.109(1) Å, c = 14.612(3) Å, b = 110.33(3)°, monoclinic, C2/c);
- -
- Polymorph II—UBOCIX03 (a = 17.349(3) Å, b = 12.407(2) Å, c = 5.114(1) Å, orthorhombic, Pnma.
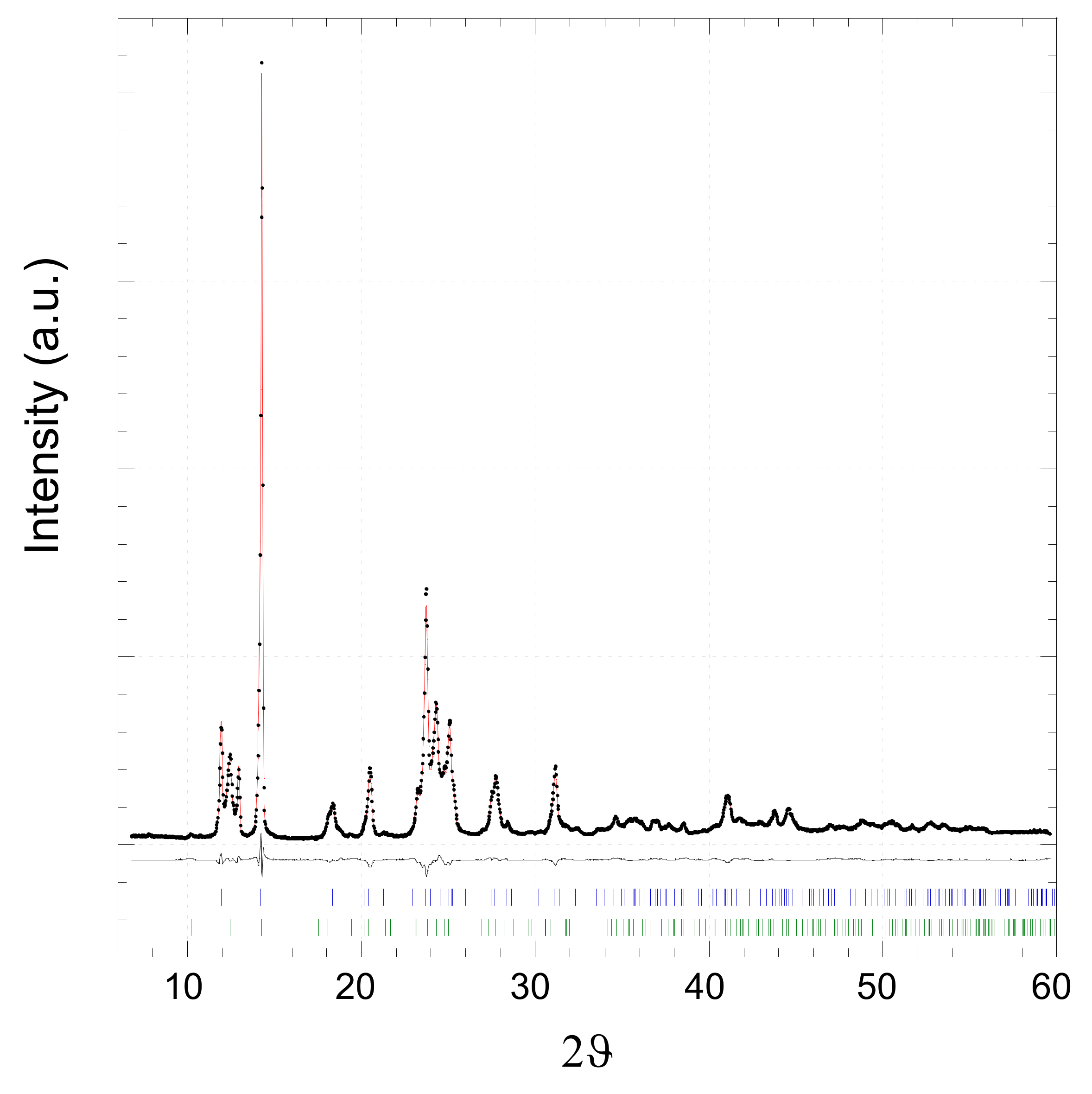
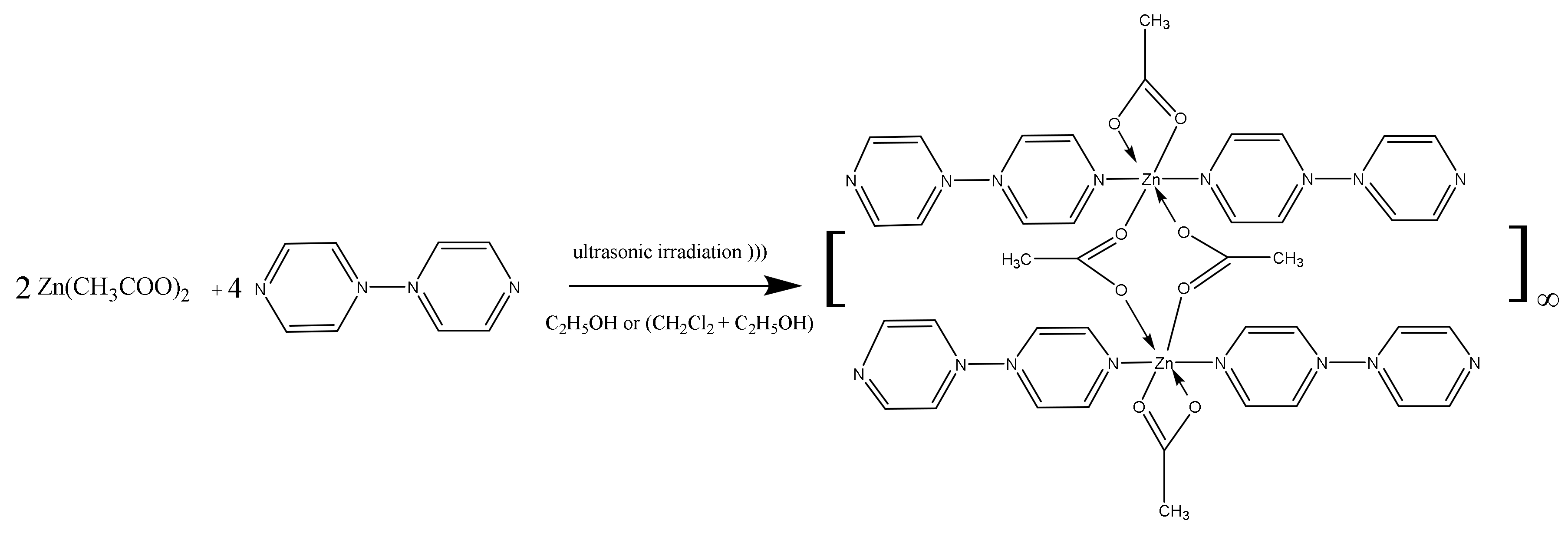
2.2. [Zn(4,4′-bipy)2(OAc)2]∞
2.3. Antimicrobial and Antibiofilm Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of [Zn(4,4′-bipy)Cl2]∞
3.3. Synthesis of [Zn(4,4′-bipy)2(OAc)2]∞
3.4. Physico-Chemical Characterization
3.4.1. X-ray Powder Diffraction (XRPD)
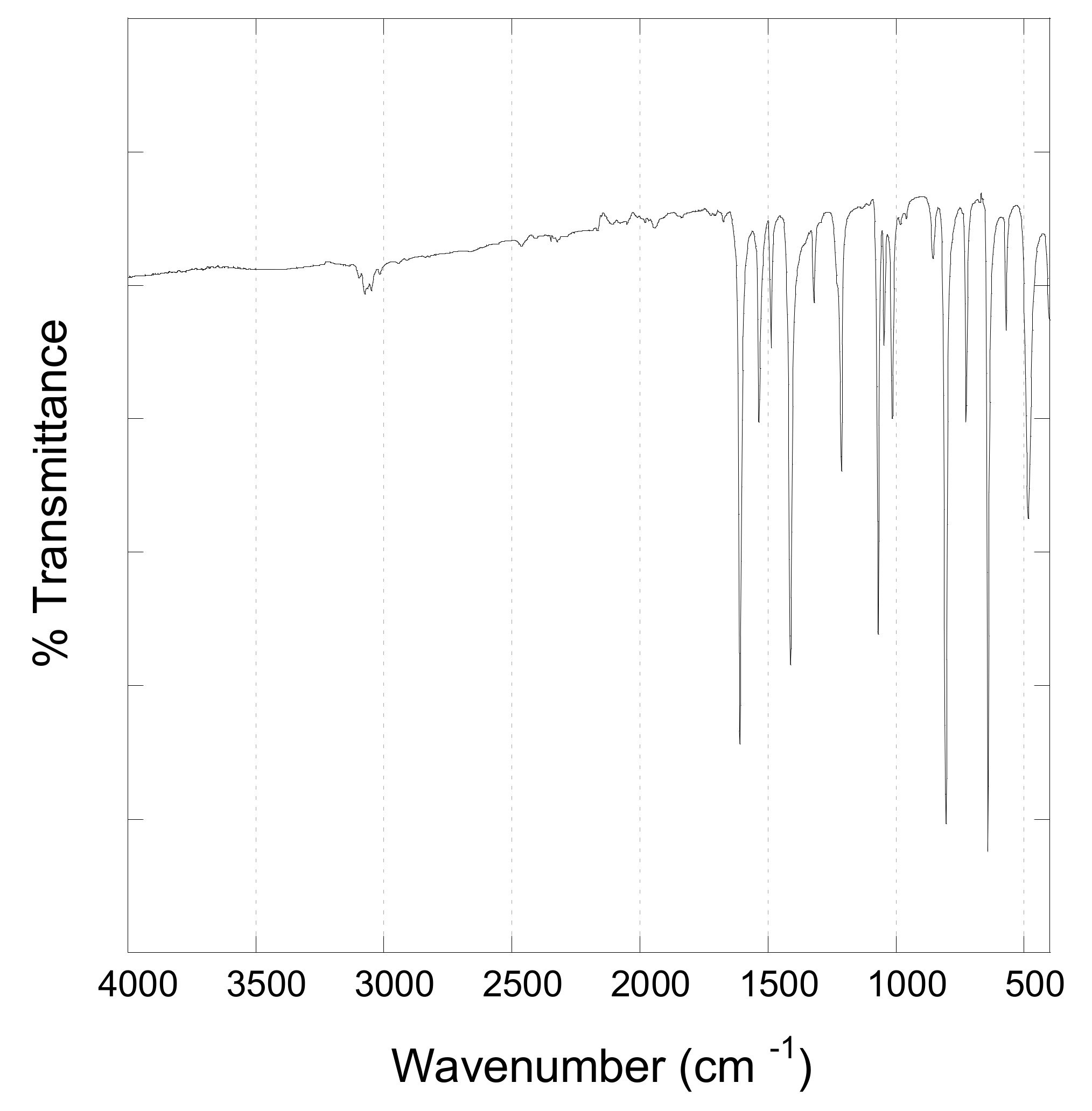
3.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
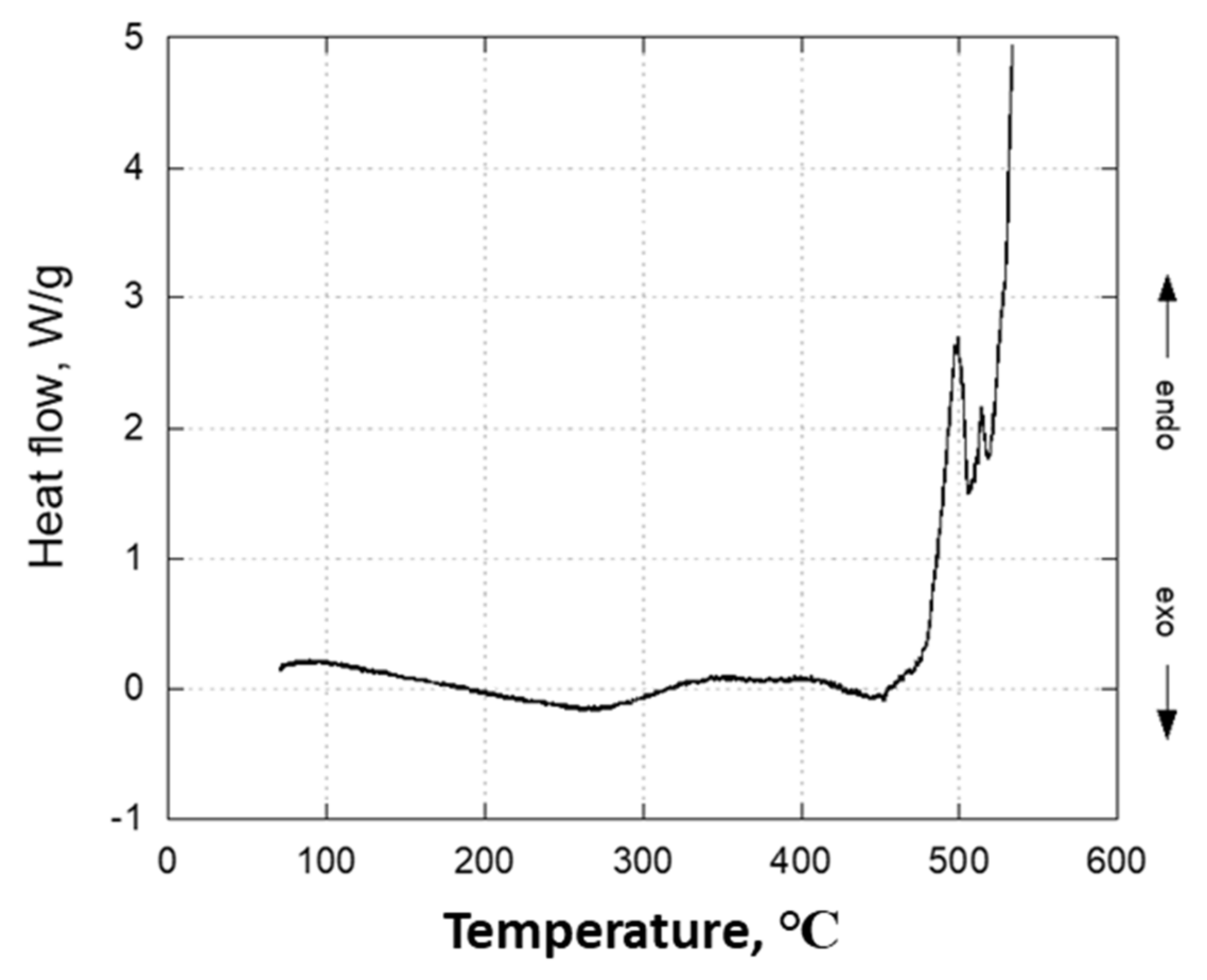
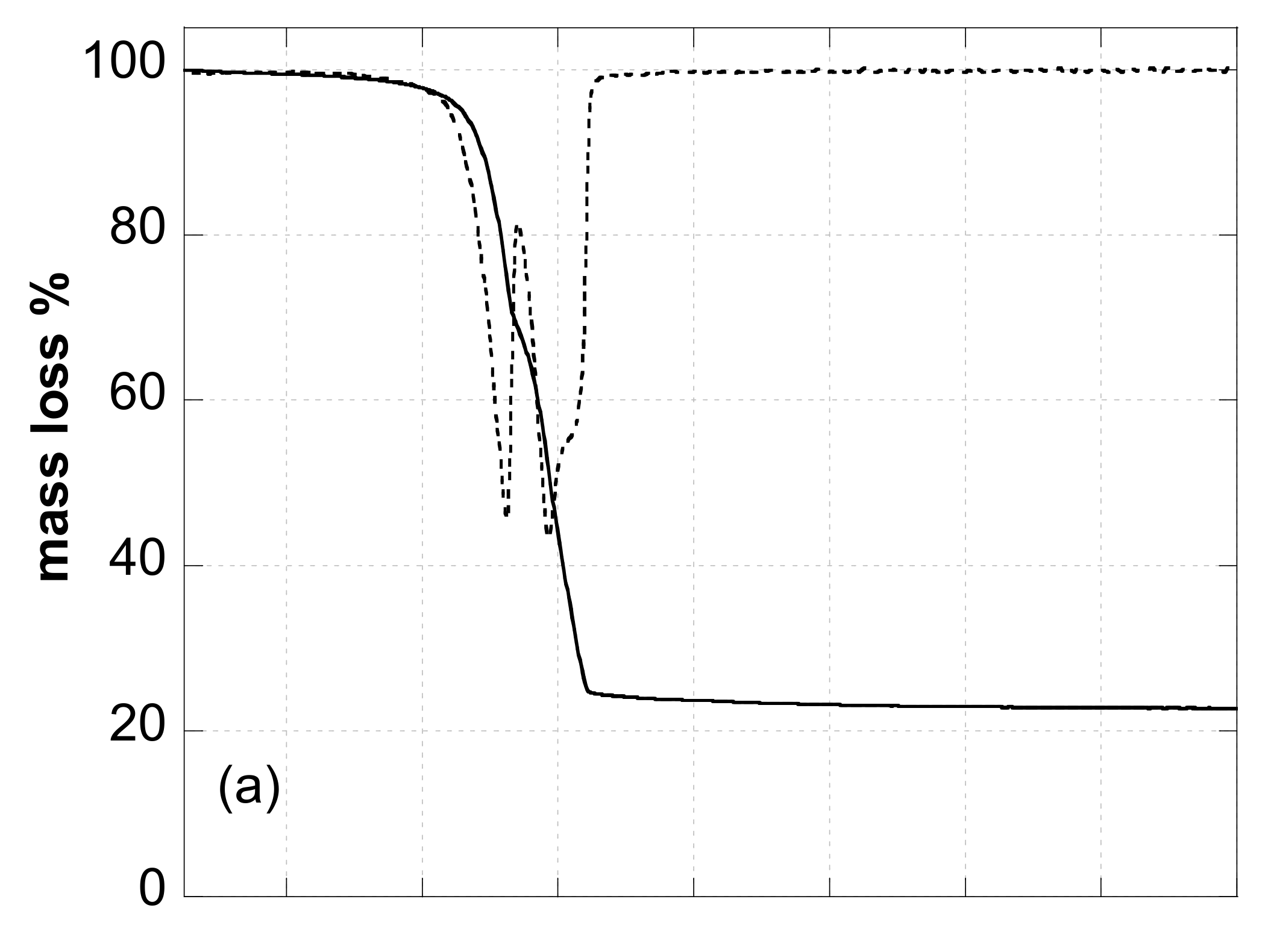
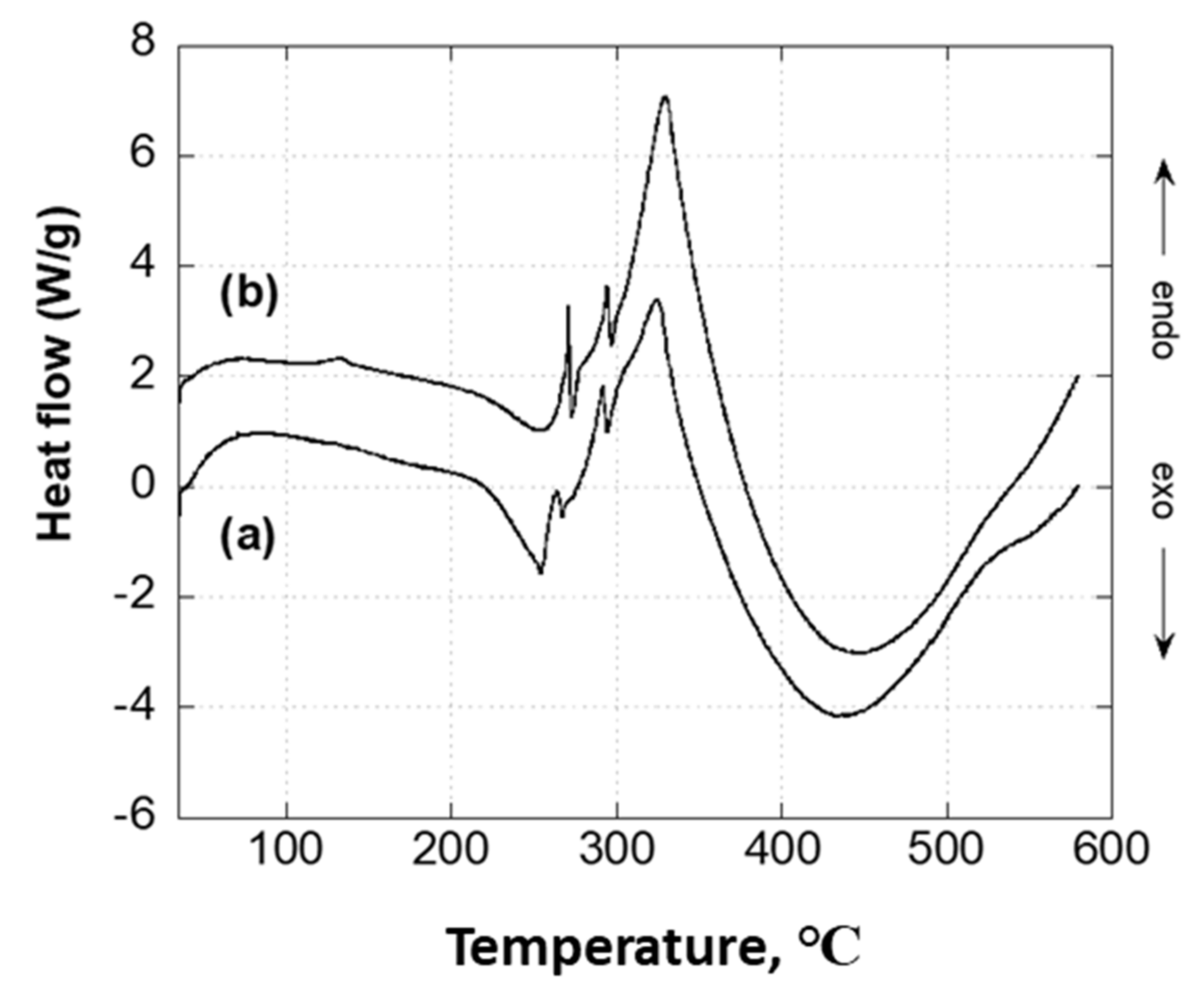
3.4.3. Thermal Analysis
3.4.4. Elemental Analysis
3.5. Antimicrobial Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batten, S.R.; Turner, D.R.; Neville, M.S. Coordination Polymers: Design, Analysis and Application; Royal Society of Chemistry (RSC): Cambridge, UK, 2009. [Google Scholar]
- Loukopoulos, E.; Kostakis, G.E. Recent advances of one-dimensional coordination polymers as catalysts. J. Coord. Chem. 2018, 71, 371–410. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Shen, K.; Li, Y. Greening the Processes of Metal–Organic Framework Synthesis and their Use in Sustainable Catalysis. ChemSusChem 2017, 10, 3165–3187. [Google Scholar] [CrossRef] [PubMed]
- Ammari, Y.; Baaalla, N.; Hlil, E.K.; Abid, S. Structure, optical and magnetic properties of a novel homometallic coordination polymers: Experimental and Computational studies. Sci. Rep. 2020, 10, 1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.L.; Bai, Y.; Pan, H.; Zheng, G.S.; Dang, D.B. Long-range magnetic ordering in a metal–organic framework based on octanuclear nickel(ii) clusters. Dalton Trans. 2017, 46, 12771–12774. [Google Scholar] [CrossRef]
- Horike, S.; Ma, N.; Fan, Z.; Kosasang, S.; Smedskjaer, M.M. Mechanics, Ionics, and Optics of Metal–Organic Framework and Coordination Polymer Glasses. Nano Lett. 2021, 21, 6382–6390. [Google Scholar] [CrossRef]
- Song, M.; Dang, Y.; Dong, J.; Zhang, X.; Lei, S.; Hu, W. Eu-based coordination polymer microrods for low-loss optical waveguiding application. Nanoscale 2019, 11, 21061–21067. [Google Scholar] [CrossRef]
- Deng, J.; Wu, F.; Yu, P.; Mao, L. On-site sensors based on infinite coordination polymer nanoparticles: Recent progress and future challenge. Appl. Mater. Today 2018, 11, 338–351. [Google Scholar] [CrossRef]
- Liu, J.Q.; Luo, Z.D.; Pan, Y.; Singh, A.K.; Trivedi, M.; Kumar, A. Recent developments in luminescent coordination polymers: Designing strategies, sensing application and theoretical evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Clark, J.H. Green chemistry: Challenges and opportunities. Green Chem. 1999, 1, 1–8. [Google Scholar] [CrossRef]
- Suárez-García, S.; Solórzano, R.; Novio, F.; Alibés, R.; Busqué, F.; Ruiz-Molina, D. Antitumour activity of coordination polymer nanoparticles. Coord. Chem. Rev. 2020, 432, 213716. [Google Scholar] [CrossRef]
- Cai, G.; Cui, P.; Shi, W.; Morris, S.; Lou, S.N.; Chen, J.; Ciou, J.-H.; Paidi, V.K.; Lee, K.-S.; Li, S.; et al. One-Dimensional π–d Conjugated Coordination Polymer for Electrochromic Energy Storage Device with Exceptionally High Performance. Adv. Sci. 2020, 7, 1903109. [Google Scholar] [CrossRef]
- Kan, W.-Q.; He, Y.-C.; Wen, S.-Z.; Zhao, P.-S. A series of host–guest coordination polymers containing viologens: Syntheses, crystal structures, thermo/photochromism and factors influencing their thermo/photochromic behaviors. Dalton Trans. 2019, 48, 17770–17779. [Google Scholar] [CrossRef]
- Wei, P.; Yan, X.; Huang, F. Supramolecular polymers constructed by orthogonal self-assembly based on host–guest and metal–ligand interactions. Chem. Soc. Rev. 2015, 44, 815–832. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Kumar, A.; Zaworotko, M.J. Metal–Organic Framework-Based Carbon Capture and Purification Technologies for Clean Environment. Metal Organic Frameworks (MOFs) for Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 5–61. [Google Scholar]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Duan, J.; Jin, W.; Kitagawa, S. Water-resistant porous coordination polymers for gas separation. Coord. Chem. Rev. 2017, 332, 48–74. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Yang, W. 2D Metal-Organic Framework Materials for Membrane-Based Separation. Adv. Mater. Interfaces 2020, 7, 1901514. [Google Scholar] [CrossRef]
- Lin, R.B.; Xiang, S.; Xing, H.; Zhou, W.; Chen, B. Exploration of porous metal–organic frameworks for gas separation and purification. Coord. Chem. Rev. 2019, 378, 87–103. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Jiang, X.; Urban, J.J.; Lau, C.H.; Liu, S.; Shao, L. The stability of a graphene oxide (GO) nanofiltration (NF) membrane in an aqueous environment: Progress and challenges. Mater. Today 2020, 1, 554–568. [Google Scholar]
- Msahel, A.; Galiano, F.; Pilloni, M.; Russo, F.; Hafiane, A.; Castro-Muñoz, R.; Kumar, V.B.; Gedanken, A.; Ennas, G.; Porat, Z.; et al. Exploring the Effect of Iron Metal-Organic Framework Particles in Polylactic Acid Membranes for the Azeotropic Separation of Organic/Organic Mixtures by Pervaporation. Membranes 2021, 11, 1–18. [Google Scholar] [CrossRef]
- Pilloni, M.; Padella, F.; Ennas, G.; Lai, S.; Bellusci, M.; Rombi, E.; Sini, F.; Pentimalli, M.; Delitala, C.; Scano, A.; et al. Liquid-assisted mechanochemical synthesis of an Iron Carboxylate Metal Organic Framework and its evaluation in diesel fuel desulfurization. Micropor. Mesopor. Mater. 2015, 213, 14–21. [Google Scholar] [CrossRef]
- Engel, E.R.; Scott, J.L. Advances in the green chemistry of coordination polymer materials. Green Chem. 2020, 22, 3693. [Google Scholar] [CrossRef]
- Julien, P.A.; Mottillo, C.; Friščić, T. Metal–organic frameworks meet scalable and sustainable synthesis. Green Chem. 2017, 19, 2729. [Google Scholar] [CrossRef]
- Deacon, G.B.; Felder, P.W.; Junk, P.C.; Müller-Buschbaum, K.; Ness, T.J.; Quitmann, C.C. The X-ray crystal structures of [Hg(C6F4OH-p) 2OH2] and [Hg(C6H4OMe-p)(O 2CC6F4OMe-p)]-Factors influencing supramolecular Hg-O interactions. Inorg. Chim. Acta 2005, 358, 4389–4393. [Google Scholar] [CrossRef]
- Shur, V.B.; Tikhonova, I.A.; Yanovsky, A.I.; Struchkov, Y.T.; Petrovskii, P.V.; Panov, S.Y.; Furin, G.G.; Vol’pin, M.E. Crown compounds for anions. Unusual complex of trimetric perfluoro-o-phenylenemercury with the bromide anion having a polydecker sandwich structure. J. Organomet. Chem. 1991, 418, 29–32. [Google Scholar] [CrossRef]
- Chen, W.T.; Wang, M.S.; Liu, X.; Guo, G.C. Investigations of Group 12 (IIB) Metal Halide/Pseudohalide-Bipy Systems: Syntheses, Structures, Properties, and TDDFT Calculations (Bipy = 2,2′-bipyridine or 4,4′-bipyridine). Cryst. Growth Des. 2006, 6, 2289–2300. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Cintas, P.; Luche, J.-L. Green chemistry. The sonochemical approach. Green Chem. 1999, 1, 115–125. [Google Scholar] [CrossRef]
- Ennas, G.; Gedanken, A.; Mannias, G.; Kumar, V.B.; Scano, A.; Porat, Z.; Pilloni, M. Formation of Iron (III) Trimesate Xerogel by Ultrasonic Irradiation. Eur. J. Inorg. Chem. 2022, e202101082. [Google Scholar] [CrossRef]
- Suslick, K.S.; Didenko, Y.; Fang, M.M.; Hyeon, T.; Kolbeck, K.J.; McNamara, W.B., III; Mdleleni, M.M.; Wong, M. Acoustic cavitation and its chemical consequences. Philos. Trans. R. Soc. A 1999, 357, 335–353. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Pilloni, M.; Ennas, G.; Cabras, V.; Denotti, V.; Kumar, V.B.; Musinu, A.; Porat, Z.; Scano, A.; Gedanken, A. Thermal and structural characterization of ultrasonicated Bi-Sn alloy in the eutectic composition. J. Therm. Anal. Calorim. 2015, 120, 1543–1551. [Google Scholar] [CrossRef]
- Pilloni, M.; Kumar, V.B.; Ennas, G.; Porat, Z.; Scano, A.; Cabras, V.; Gedanken, A. Formation of metallic silver and copper in non-aqueous media by ultrasonic radiation. Ultrason. Sonochem. 2018, 47, 108–113. [Google Scholar] [CrossRef]
- Gedanken, A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef]
- Son, W.J.; Kim, J.; Kim, J.; Ahn, W.S. Sonochemical synthesis of MOF-5. Chem. Commun. 2008, 6336–6338. [Google Scholar] [CrossRef]
- Carson, C.G.; Brown, A.J.; Sholl, D.S.; Nair, S. Sonochemical Synthesis and Characterization of Submicrometer Crystals of the MetalOrganic Framework Cu[(hfipbb)(H2hfipbb)0.5]. Cryst. Growth Des. 2011, 11, 4505–4510. [Google Scholar] [CrossRef]
- Yang, D.A.; Cho, H.Y.; Kim, J.; Yang, S.T.; Ahn, W.S. CO2 capture and conversion using Mg-MOF-74 prepared by a sonochemical method. Energy Environ. Sci. 2012, 5, 6465–6473. [Google Scholar] [CrossRef]
- Cho, H.Y.; Kim, J.; Kim, S.N.; Ahn, W.S. High yield 1-L scale synthesis of ZIF-8 via a sonochemical route. Microporous Mesoporous Mater. 2013, 169, 180–184. [Google Scholar] [CrossRef]
- Erxleben, A. Structures and properties of Zn(II) coordination polymers. Coord. Chem. Rev. 2003, 246, 203–228. [Google Scholar] [CrossRef]
- Lee, T.W.; Lau, J.P.K.; Wong, W.T. Synthesis and characterization of coordination polymers of Zn(II) with 1,3-bis(4-pyridyl)propane and 4,4′-pyridine ligands. Polyhedron 2004, 23, 999–1002. [Google Scholar] [CrossRef]
- Ragasa, C.; Nacpil, Z.; Penalosa, B.; Rideout, J. Antimutagen and antifungal compounds from Cosmos caudatus. Philipp. J. Sci. 1997, 126, 199–206. [Google Scholar]
- Amiri, M.G.; Morsali, A. Synthesis and characterization of [Zn2(4,4′-bipy)n(AcO)4]-One-dimensional chain (n = 1) and one-dimensional double-chain (n = 2) coordination polymers. Z. Anorg. Allg. Chem. 2006, 632, 2491–2494. [Google Scholar] [CrossRef]
- Zafar, W.; Sumrra, S.H.; Chohan, Z.H. A review: Pharmacological aspects of metal based 1,2,4-triazole derived Schiff bases. Eur. J. Med. Chem. 2021, 222, 113602. [Google Scholar] [CrossRef]
- Colinas, I.R.; Rojas-Andrade, M.D.; Chakraborty, I.; Oliver, S.R.J. Two structurally diverse Zn-based coordination polymers with excellent antibacterial activity. CrystEngComm 2018, 20, 3353. [Google Scholar] [CrossRef]
- Sumrra, S.H.; Zafar, W.; Imran, M.; Chohan, Z.H. A review on the biomedical efficacy of transition metal triazole compounds. J. Coord. Chem. 2022, 75, 293–334. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Hong, M.-C.; Cao, R.; Su, W.-P.; Zhao, Y.-J.; Weng, J.-B. Infinite chain structures of [Zn(4,4-bipy)Cl2]n and [Cu(H2PO4)(4,4′-bipy)·2H2O]n via hydrothermal synthesis. Polyhedron 2001, 20, 2477–2481. [Google Scholar] [CrossRef]
- Fu, A.-H.; Lu, J.Y.; Huang, X.-Y.; Li, J. ZnCl2(4,4′-bpy): A One-dimensional Coordination Polymer with Two Isomorphic Phases. J. Alloy Comp. 2001, 319, 89. [Google Scholar] [CrossRef]
- Kraft, S.; Hanuschek, E.; Beckhaus, R.; Haase, D.; Saak, W. Titanium-Based Molecular Squares and Rectangles: Syntheses by Self-Assembly Reactions of Titanocene Fragments and Aromatic N-Heterocycles. Chem. Eur. J. 2005, 11, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Czakis-Sulikowska, D.; Kaluzna, J. 4, 4 ′-Bipyridyl and 2, 4 ′-Bipyridyl Complexes of Co (lI), Ni (II) and Cu (II) Thiocyanates. J. Therm. Anal. Calorim. 1996, 47, 1763–1776. [Google Scholar] [CrossRef]
- Conerney, B.; Jensen, P.; Kruger, P.E.; Moubaraki, B.; Murray, K.S. Synthesis and structural characterisation of two coordination polymers (molecular ladders) incorporating [M(OAc)2]2 secondary building units and 4,4′-bipyridine [M = Cu(ii), Zn(ii)]. CrystEngComm 2003, 5, 454. [Google Scholar] [CrossRef]
- Kim, J.; Lee, U.; Koo, B.K. One-Dimensional Network of Zinc(II) Coordination Polymer with 4,4′-Bipyridine (4,4′-bipy). Bull. Korean Chem. Soc. 2006, 27, 918–920. [Google Scholar]
- Ishioka, T.; Shibata, Y.; Takahashi, M.; Kanesaka, I. Vibrational spectra and structures of zinc carboxylates I. Zinc acetate dihydrate. Spectrochim. Acta 1998, 54, 1827–1836. [Google Scholar] [CrossRef]
- McMurdie, B.H.F.; Morris, M.C.; Evans, E.H.; Paretzkin, B.; Wong-ng, W. Standard X-Ray Diffraction Powder Patterns. Powder Diffraction. 1986, 1, 64–77. [Google Scholar] [CrossRef]
- Available online: https://www.eucast.org/clinical_breakpoints (accessed on 1 January 2022).
- Lutterotti, L.; Matthies, S.; Wenk, H.R. MAUD: A friendly Java program for material analysis using diffraction. Newsl. CPD 1999, 21, 14–15. [Google Scholar]
- Young, R. The Rietveld Method; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Barry, A.L.; Coyle, M.B.; Thornsberry, C.; Gerlach, E.H.; Hawkinson, R.W. Methods of measuring zones of inhibition with the Bauer-Kirby disk susceptibility test. J. Clin. Microbiol. 1979, 10, 885–889. [Google Scholar] [CrossRef]
- Barberis, A.; Deiana, M.; Spissu, Y.; Azara, E.; Fadda, A.; Serra, P.A.; D’hallewin, G.; Pisano, M.; Serreli, G.; Orrù, G.; et al. Antioxidant, Antimicrobial, and Other Biological Properties of Pompia Juice. Molecules 2020, 25, 3186. [Google Scholar] [CrossRef]
- Podda, E.; Aragoni, C.M.; Arca, M.; Atzeni, G.; Coles, S.J.; Ennas, G.; Isaia, F.; Lippolis, V.; Orru, G.; Scano, A.; et al. Morpholine- and Thiomorpholine-Based Amidodithiophosphonato Nickel Complexes: Synthesis, Characterization, P-N Cleavage, Antibacterial Activity and Silica Nano-Dispersion. J. Nanosci. Nanotechnol. 2021, 21, 2879–2891. [Google Scholar] [CrossRef]
- Available online: http://www.biofilm.montana.edu (accessed on 1 January 2022).
- Lachowicz, J.I.; Szczepski, K.; Scano, A.; Casu, C.; Fais, S.; Orrù, G.; Pisano, B.; Piras, M.; Jaremko, M. The best peptidomimetic strategies to undercover antibacterial peptides. Int. J. Mol. Sci. 2020, 21, 7349. [Google Scholar] [CrossRef]
- Orrù, G.; Piras, V.; Ciusa, M.L.; Taccori, F.; Pisano, M.B.; Montaldo, C.; Cosentino, S.; Fadda, M.E. Azole Resistance and ERG11 464 Polymorphism in Oral Candida albicans Clinical Strains Isolated in Sardinia. Open Mycol. J. 2008, 2, 82–85. [Google Scholar] [CrossRef] [Green Version]
- Podda, E.; Arca, M.; Atzeni, G.; Coles, S.J.; Ibba, A.; Isaia, F.; Lippolis, V.; Orrù, G.; Orton, J.B.; Pintus, A.; et al. Antibacterial Activity of Amidodithiophosphonato Nickel(II) Complexes: An Experimental and Theoretical Approach. Molecules 2020, 25, 2052. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Pfaller, M.A.; Bustamante, B.; Canton, E.; Fothergill, A.; Fuller, J.; Gonzalez, G.M.; Lass-Flörl, C.; Lockhart, S.R.; Martin-Mazuelos, E.; et al. Multilaboratory study of epidemiological cutoff values for detection of resistance in eight Candida species to fluconazole, posaconazole, voriconazole. Antimicrob. Agents Chemother. 2014, 58, 2006–2012. [Google Scholar] [CrossRef] [Green Version]
- Brito, G.N.; Inocêncio, A.C.; Querido, S.M.; Jorge, A.O.; Koga-Ito, C.Y. In Vitro Antifungal Susceptibility of Candida spp. Oral Isolates from HIV-Positive Patients and Control Individuals. Braz. Oral Res. 2011, 25, 28–33. Clinical and Laboratory Standards Institute. Available online: https://clsi.org/ (accessed on 1 October 2022). [CrossRef]


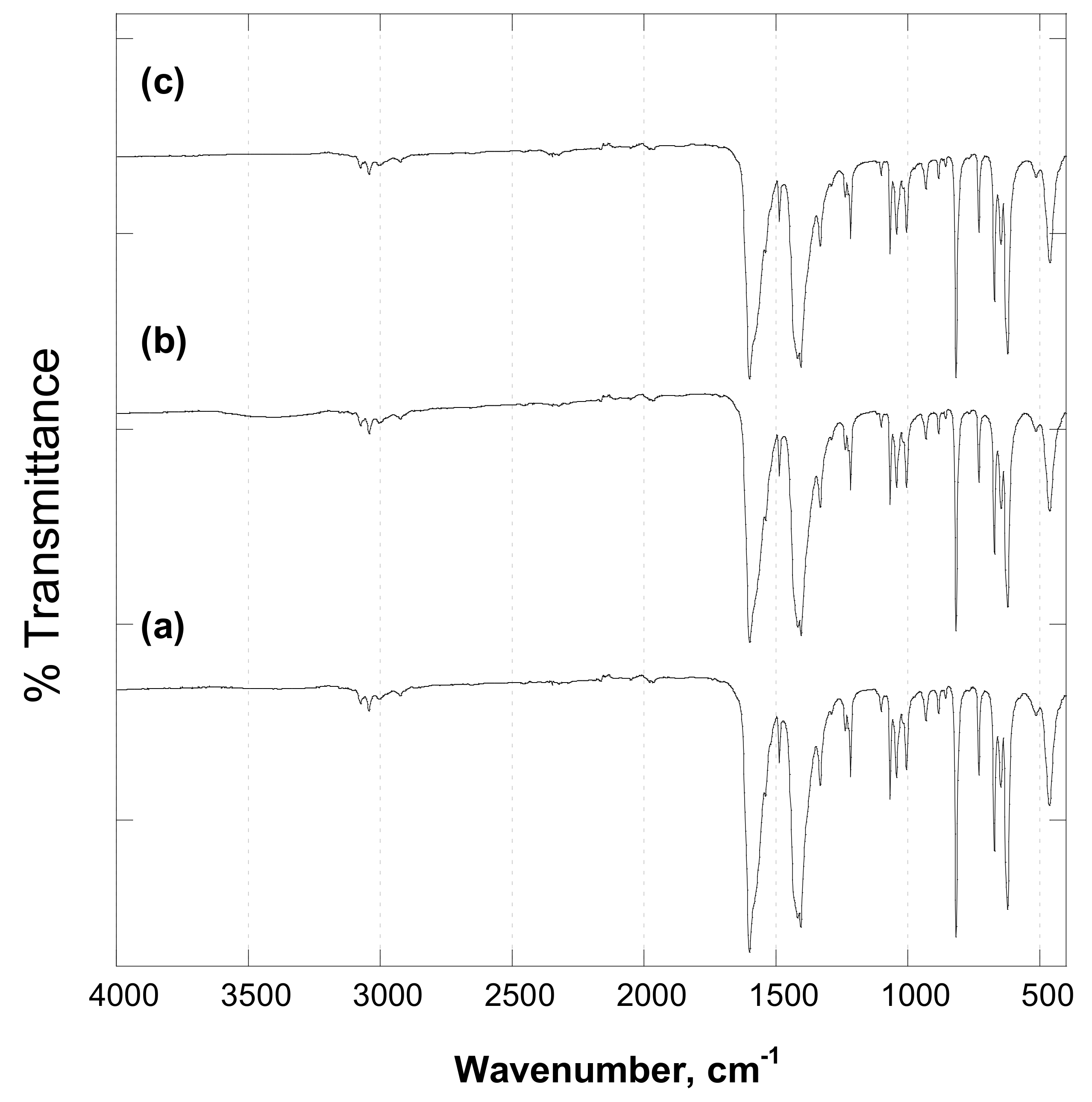

| Compounds | [C10 H8 Cl2 N2 Zn]∞—Polymorph I | [C10 H8 Cl2 N2 Zn]∞—Polymorph II |
|---|---|---|
| Crystal system | monoclinic | orthorhombic |
| Space group | C2/c (n. 15) | Pnma (n. 62) |
| a/Å | 15.80(1) | 17.37(1) |
| b/Å | 5.11(1) | 12.49(1) |
| c/Å | 14.58(1) | 5.10(1) |
| a/° | 90 | 90 |
| b/° | 110.24(1) | 90 |
| g/° | 90 | 90 |
| Z | 4 | 4 |
| wt% | 48.0(1) | 52.0(1) |
| Compound | [Zn(4,4′-bipy)2(OAc)2]∞ | [Zn(4,4′-bipy)2(OAc)2]∞ |
|---|---|---|
| XRD method | powder | single crystal |
| Crystal system | triclinic | triclinic |
| Space group | P-1 | P-1 |
| a/Å | 8.10(1) | 8.088(1) |
| b/Å | 9.18(1) | 9.190(1) |
| c/Å | 10.64(1) | 10.676(1) |
| α/° | 109.56(5) | 109.70(1) |
| β/° | 100.13(5) | 100.03(1) |
| γ/° | 101.82(5) | 101.78(1) |
| Z | 2 | 2 |
| T/K | 298 | 283–303 |
| ϑ min-max/° | 5–80 | 9.5–10.5 |
| <D>/nm | 102(5) | - |
| σ/% | < 0.1 | - |
| Wavelength/Å | 1.54056 (CuKα) | 0.71069 (MoKα) |
| R indices (all data) | Rwp% = 10.0 | R1 = 0.0278; wR2 = 0.0732 |
| ; | ||
| ; | ||
| ; | ||
| . | ||
| Strain | [Zn(4,4′-bipy)Cl2]∞ | [Zn(4,4′-bipy)2(OAc)2]∞ | ||||
|---|---|---|---|---|---|---|
| * MIC | * MBC | * MBIC | * MIC | * MBC | * MBIC | |
| Candida albicans (DSM 1386) | 25 | >50 | 25 | 6.25 | >50 | 6.25 |
| Candida krusei (DSM 70075) | >50 | >50 | >50 | >50 | >50 | >50 |
| Candida glabrata (DSM 6425) | >50 | >50 | >50 | 25 | >50 | 25 |
| Klebsiella pneumoniae (DSM 681) | 25 | >50 | 25 | 25 | >50 | 25 |
| Staphylococcus aureus (DSM 1104) | 25 | >50 | 25 | 25 | >50 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scano, A.; Mereu, E.; Cabras, V.; Mannias, G.; Garau, A.; Pilloni, M.; Orrù, G.; Scano, A.; Ennas, G. Green Preparation of Antimicrobial 1D-Coordination Polymers: [Zn(4,4′-bipy)Cl2]∞ and [Zn(4,4′-bipy)2(OAc)2]∞ by Ultrasonication of Zn(II) Salts and 4,4′-Bipyridine. Molecules 2022, 27, 6677. https://doi.org/10.3390/molecules27196677
Scano A, Mereu E, Cabras V, Mannias G, Garau A, Pilloni M, Orrù G, Scano A, Ennas G. Green Preparation of Antimicrobial 1D-Coordination Polymers: [Zn(4,4′-bipy)Cl2]∞ and [Zn(4,4′-bipy)2(OAc)2]∞ by Ultrasonication of Zn(II) Salts and 4,4′-Bipyridine. Molecules. 2022; 27(19):6677. https://doi.org/10.3390/molecules27196677
Chicago/Turabian StyleScano, Alessandra, Elisabetta Mereu, Valentina Cabras, Giada Mannias, Alessandra Garau, Martina Pilloni, Germano Orrù, Alessandra Scano, and Guido Ennas. 2022. "Green Preparation of Antimicrobial 1D-Coordination Polymers: [Zn(4,4′-bipy)Cl2]∞ and [Zn(4,4′-bipy)2(OAc)2]∞ by Ultrasonication of Zn(II) Salts and 4,4′-Bipyridine" Molecules 27, no. 19: 6677. https://doi.org/10.3390/molecules27196677






