Polyoxometalate/Ionic Liquid Desulfurization System for Hydrogen Sulfide Removal from High-Temperature Gas Stream
Abstract
:1. Introduction
2. Results and Discussion
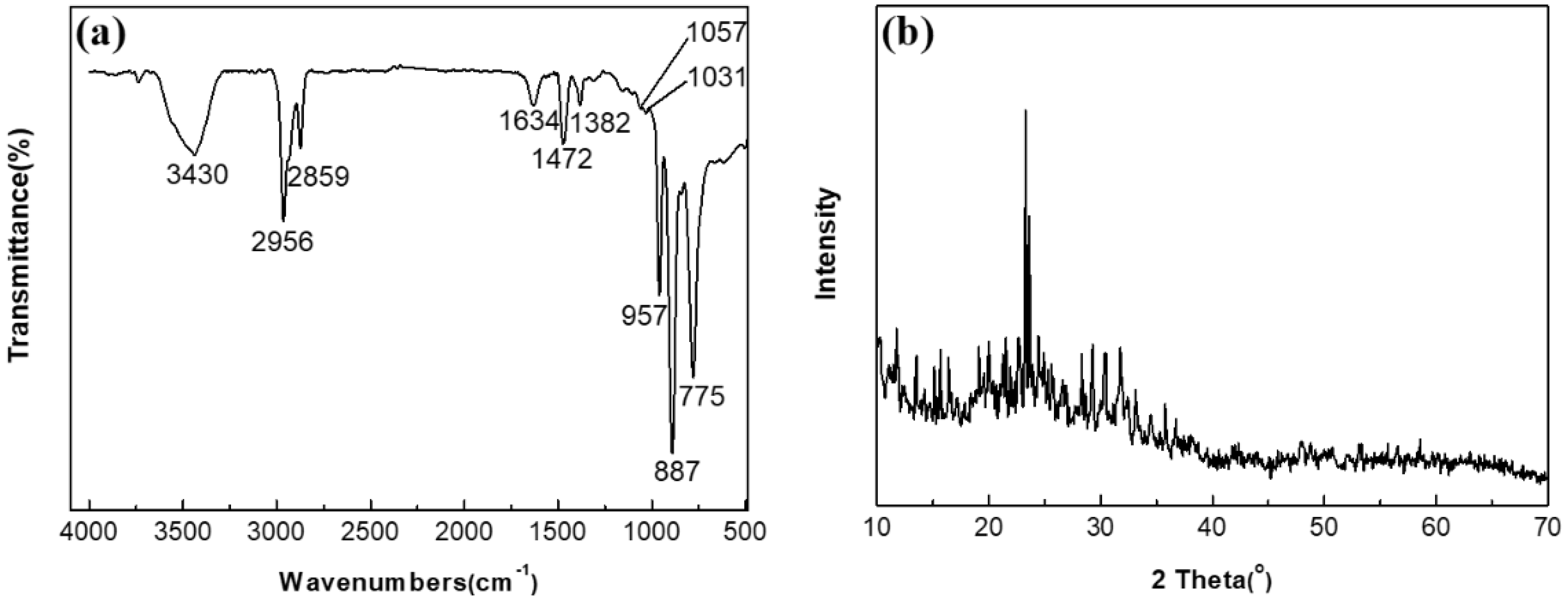
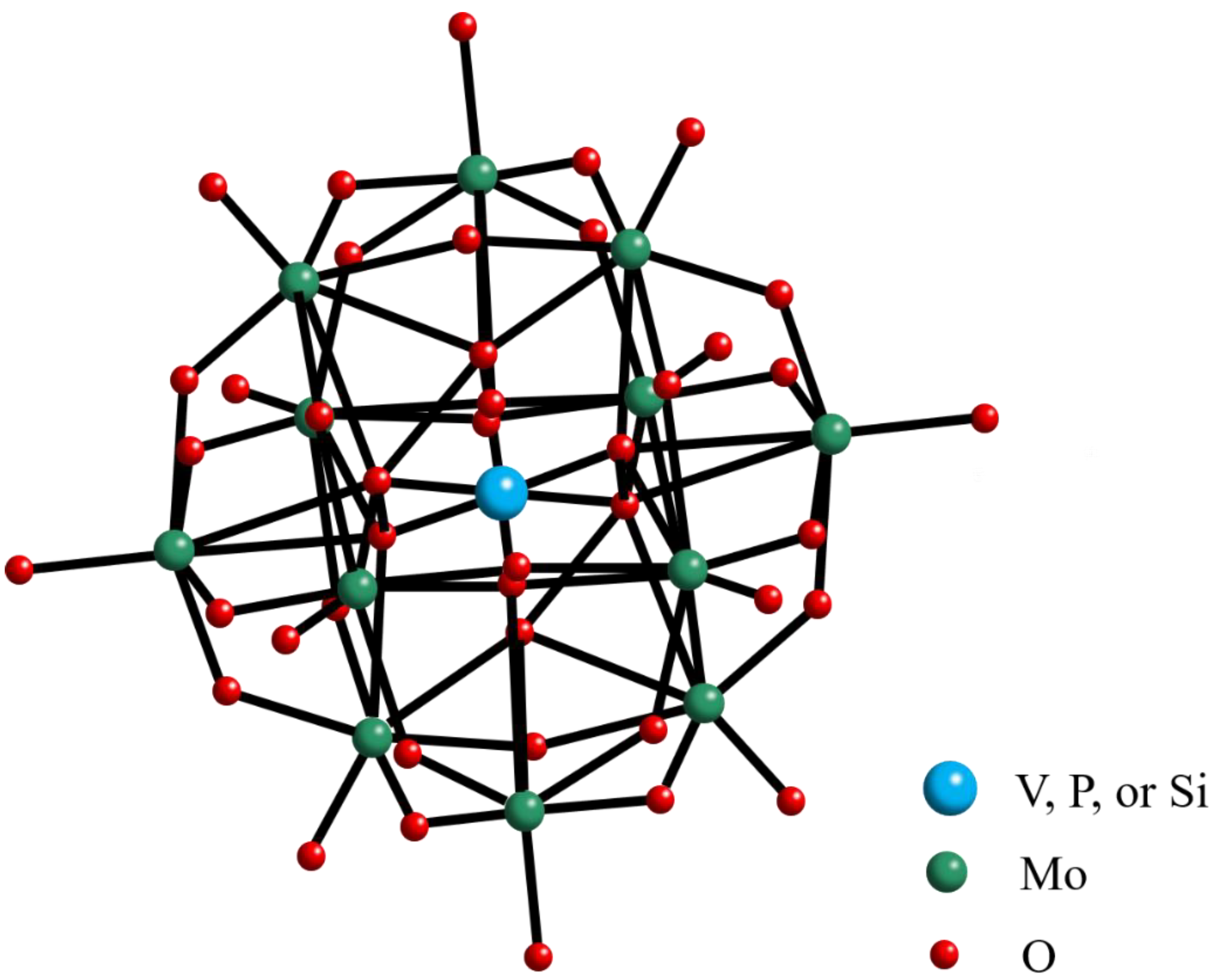
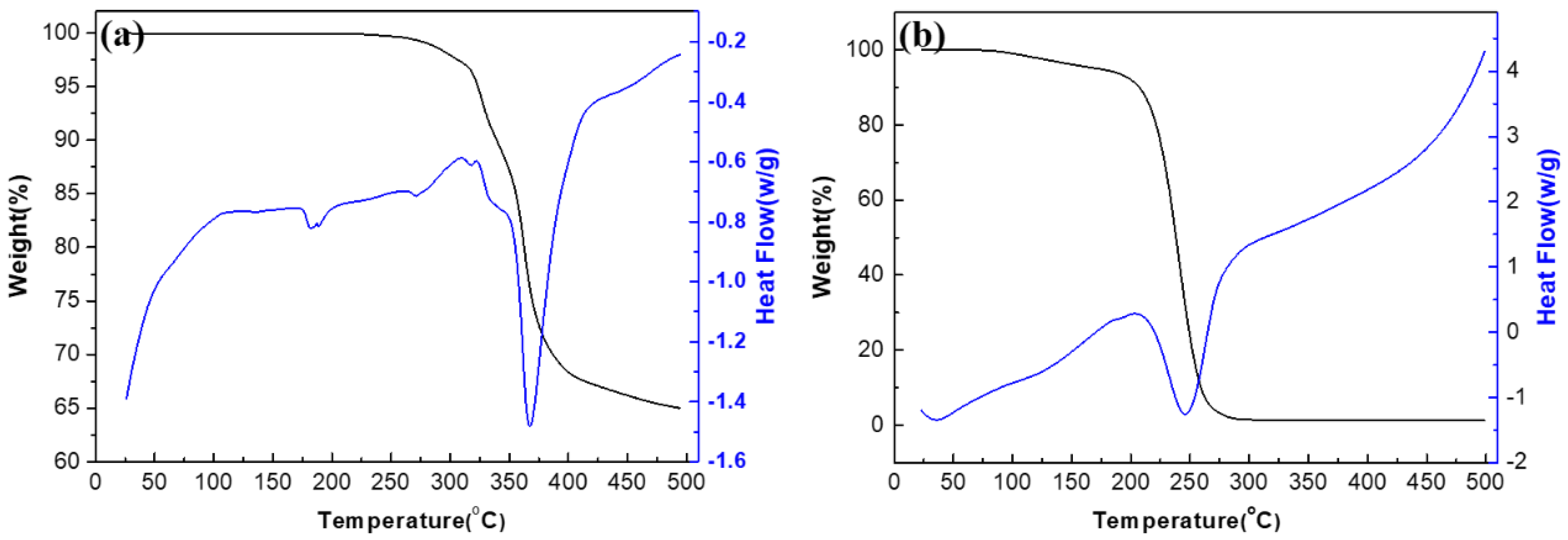
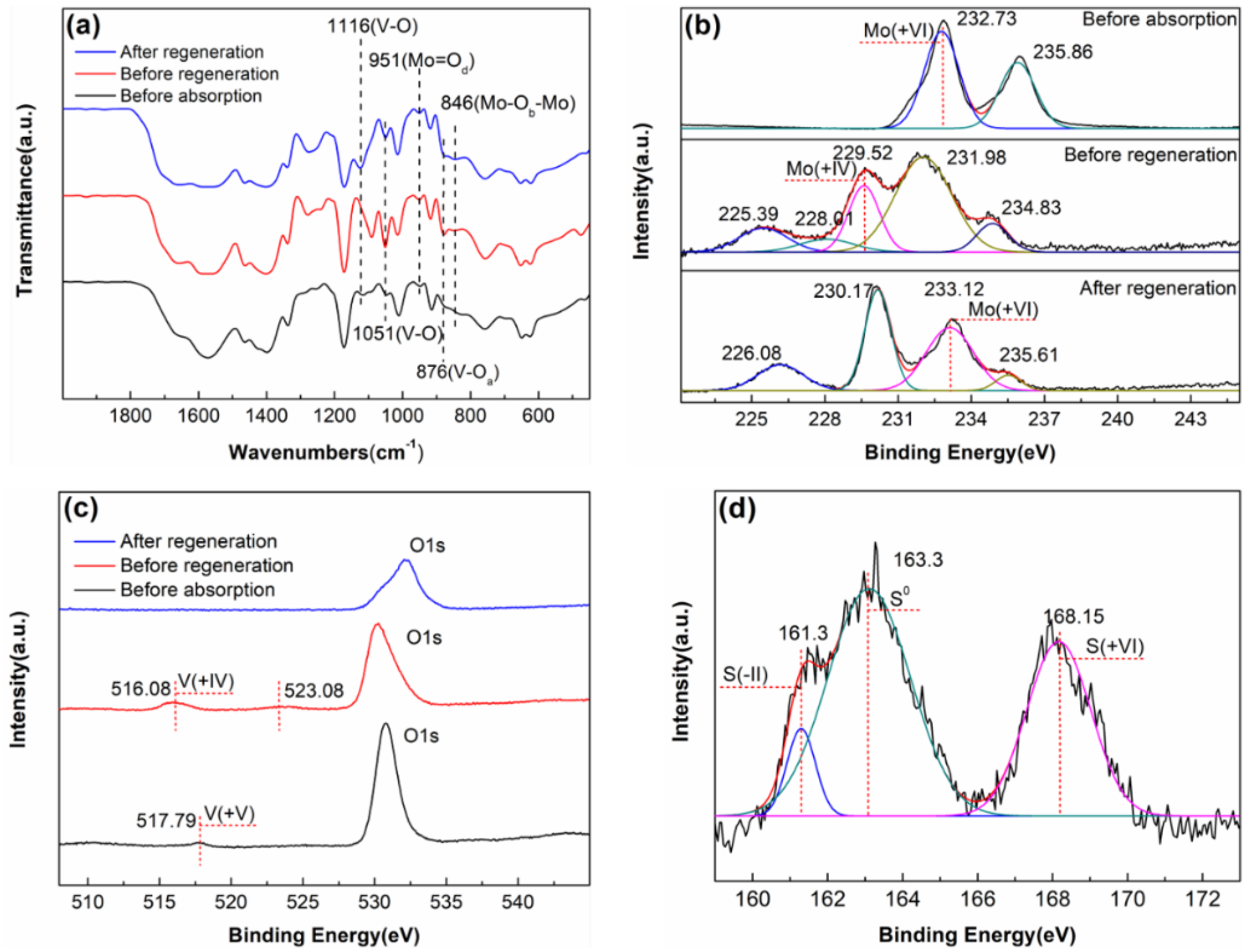
2.1. Polyoxometalate and Ionic Liquid Characterization
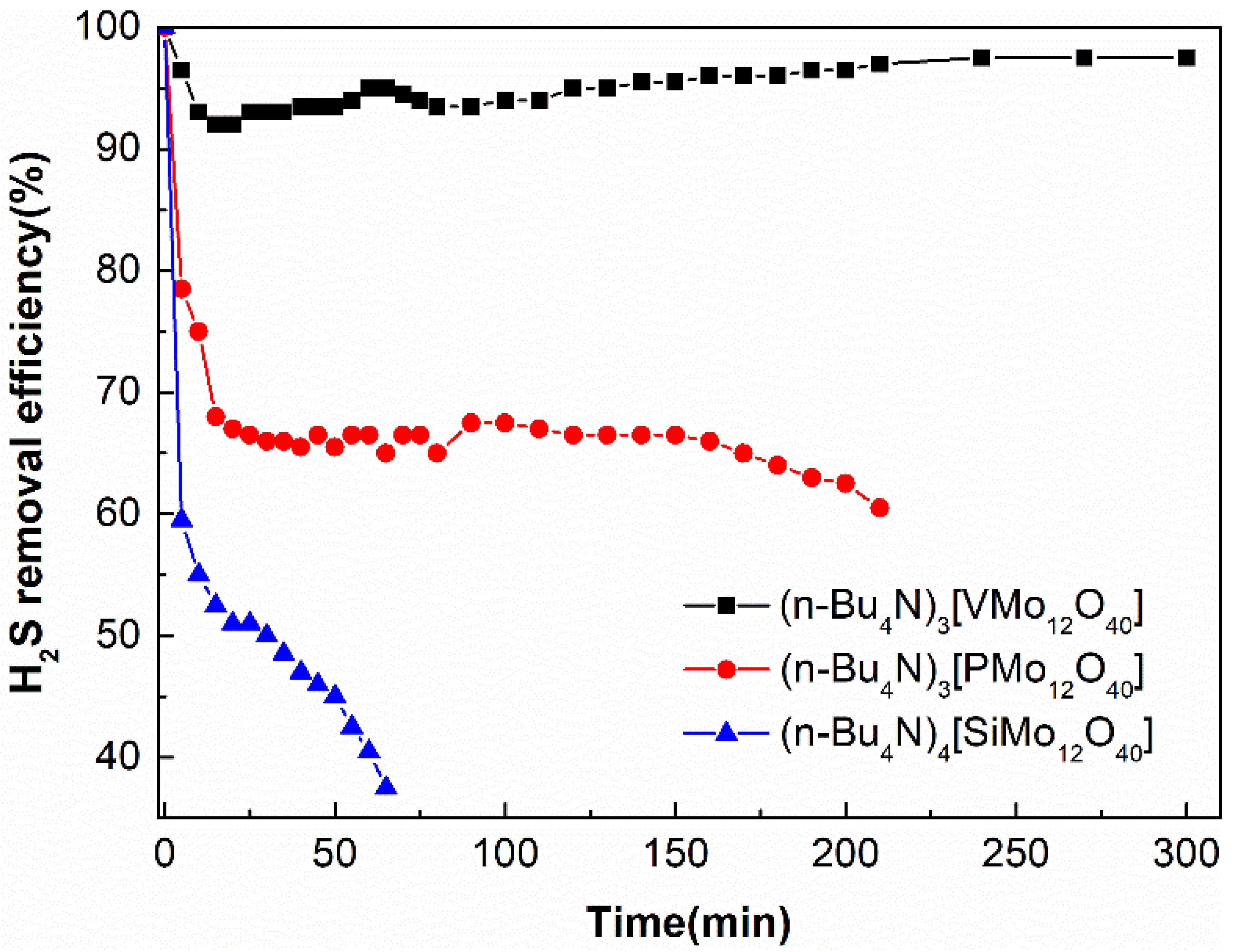
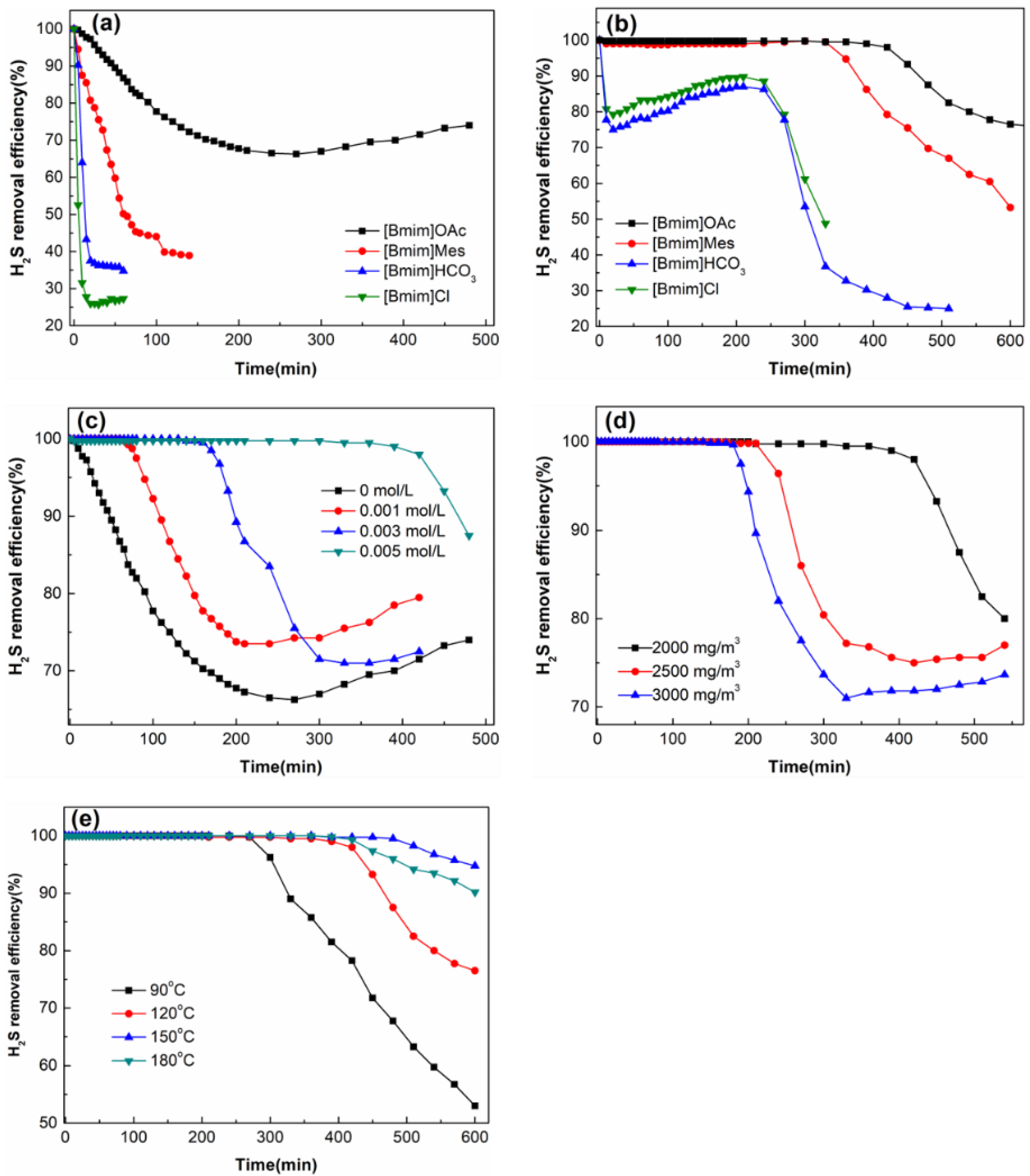
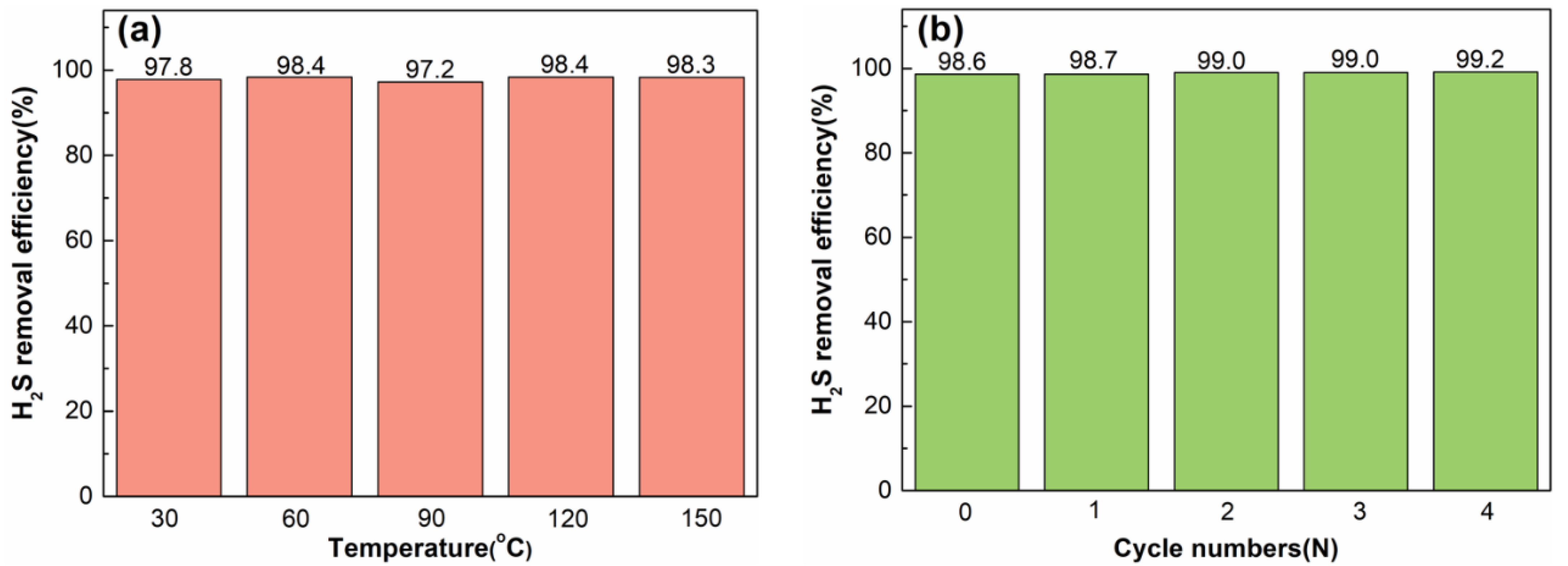
2.2. Desulphurization Performance
2.3. Regeneration Performance
2.4. Desulphurisation Product
2.5. Mechanism
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Polyoxometalates and Ionic Liquids
3.3. Characterization Techniques
3.4. H2S Removal, Desulfurization Solution Regeneration, and Desulfurization Product Recovery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tengku Hassan, T.N.A.; Shariff, A.M.; Mohd Pauzi, M.M.; Khidzir, M.S.; Surmi, A. Insights on cryogenic distillation technology for simultaneous CO2 and H2S removal for sour gas fields. Molecules 2022, 27, 1424. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Wang, R. Desulfurization and regeneration performance of heteropoly compound/ionic liquid solutions at high temperature. Chem. Eng. J. 2017, 316, 171–178. [Google Scholar] [CrossRef]
- Nikolic, M.; Caceres Najarro, M.; Johannsen, I.; Iruthayaraj, J.; Ceccato, M.; Feilberg, A. Copper adsorption on lignin for the removal of hydrogen sulfide. Molecules 2020, 25, 5577. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Wang, R. Study on the desulfurization performance of hydramine/ionic liquid solutions at room temperature and atmospheric pressure. Fuel Process. Technol. 2017, 167, 382–387. [Google Scholar] [CrossRef]
- Wang, R. Investigation on a new liquid redox method for H2S removal and sulfur recovery with heteropoly compound. Sep. Purif. Technol. 2003, 31, 111–121. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic liquids—New “solutions” for transition metal catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, B.; Zhang, Y.; Li, M.; Sun, Y.; Zhao, J.; Li, X.; Zhang, H. Regulation of the liquid-solid interface of Cs catalysts for the synthesis of 1,1-Dichloroethylene from 1,1,2-Trichloroethane. Appl. Surf. Sci. 2022, 599, 154033. [Google Scholar] [CrossRef]
- Wang, B.; Jin, C.; Shao, S.; Yue, Y.; Zhang, Y.; Wang, S.; Chang, R.; Zhang, H.; Zhao, J.; Li, X. Electron-deficient Cu site catalyzed acetylene hydrochlorination. Green Energy Environ. 2022. In press. [Google Scholar] [CrossRef]
- Zhu, W.; Li, H.; Jiang, X.; Yan, Y.; Lu, J.; He, L.; Xia, J. Commercially available molybdic compound-catalyzed ultra-deep desulfurization of fuels in ionic liquids. Green Chem. 2008, 10, 641–646. [Google Scholar] [CrossRef]
- Sanchez-Badillo, J.; Gallo, M.; Alvarado, S.; Glossman-Mitnik, D. Solvation thermodynamic properties of hydrogen sulfide in [C4mim][PF6], [C4mim][BF4], and [C4mim][Cl] ionic liquids, determined by molecular simulations. J. Phys. Chem. B 2015, 119, 10727–10737. [Google Scholar] [CrossRef]
- Jalili, A.H.; Rahmati-Rostami, M.; Ghotbi, C.; Hosseini-Jenab, M.; Ahmadi, A.N. Solubility of H2S in ionic liquids [bmim][PF6], [bmim][BF4], and [bmim][Tf2N]. J. Chem. Eng. Data 2009, 54, 1844–1849. [Google Scholar] [CrossRef]
- Huang, K.; Wu, Y.-T.; Hu, X.-B. Effect of alkalinity on absorption capacity and selectivity of SO2 and H2S over CO2: Substituted benzoate-based ionic liquids as the study platform. Chem. Eng. J. 2016, 297, 265–276. [Google Scholar] [CrossRef]
- Sakhaeinia, H.; Taghikhani, V.; Jalili, A.H.; Mehdizadeh, A.; Safekordi, A.A. Solubility of H2S in 1-(2-hydroxyethyl)-3-methylimidazolium ionic liquids with different anions. Fluid Phase Equilibria 2010, 298, 303–309. [Google Scholar] [CrossRef]
- Li, J.; Wang, R.; Dou, S. Electrolytic cell–assisted polyoxometalate based redox mediator for H2S conversion to elemental sulphur and hydrogen. Chem. Eng. J. 2021, 404, 127090. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Yang, F.; Wang, R. Synthesis, desulfurization and microwave assisted air regeneration performance of Dawson-type molybdovanadophosphoric heteropolyacid. Chin. J. Inorg. Chem. 2012, 28, 2179–2185. [Google Scholar]
- Thouvenot, R.; Fournier, M.; Franck, R.; Rocchiccioli-Deltcheff, C. Vibrational investigations of polyoxometalates. 3. isomerism in molybdenum(VI) and tungsten(VI) compounds related to the Keggin structure. Inorg. Chem. 1984, 23, 598–605. [Google Scholar] [CrossRef]
- Himeno, S.; Takamoto, M.; Higuchi, A.; Maekawa, M. Preparation and voltammetric characterization of Keggin-type tungstovanadate [VW12O40]3- and [V(VW11)O40]4- complexes. Inorg. Chim. Acta 2003, 348, 57–62. [Google Scholar] [CrossRef]
- Himeno, S.; Saito, A. Preparation of dodecamolybdovanadate(V). Inorg. Chim. Acta 1990, 171, 135–137. [Google Scholar] [CrossRef]
- Wang, D.; Fang, Z.; Wei, X. Preparation and properties of the heteropolyoxometalates of large organic cation with molybdotungstosilicic acids. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2008, 23, 198–203. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, M.; Ge, H.; Wang, J. A new method for the synthesis of molybdovanadophosphoric heteropoly acids and their catalytic activities. Front. Chem. Eng. China 2007, 1, 296–299. [Google Scholar] [CrossRef]
- Shen, Q.; Pang, H.; Zhang, C. A vanadium-centered tungstovanadate modified by copper-azole: Synthesis, structure and electrocatalytic property. J. Mol. Struct. 2021, 1233, 130109. [Google Scholar] [CrossRef]
- Maeda, K.; Katano, H.; Osakai, T.; Himeno, S.; Saito, A. Charge dependence of one-electron redox potentials of Keggin-type heteropolyoxometalate anions. J. Electroanal. Chem. 1995, 389, 167–173. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V.; Matveev, K.I. Heteropolyacids in catalysis. Russ. Chem. Rev. 1982, 51, 1075–1088. [Google Scholar] [CrossRef]
- Tian, G. Applications of green solvents in toxic gases removal. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 149–201. [Google Scholar] [CrossRef]
- Shokouhi, M.; Adibi, M.; Jalili, A.H.; Hosseini-Jenab, M.; Mehdizadeh, A. Solubility and diffusion of H2S and CO2 in the ionic liquid 1-(2-Hydroxyethyl)-3-methylimidazolium tetrafluoroborate. J. Chem. Eng. Data 2010, 55, 1663–1668. [Google Scholar] [CrossRef]
- Huang, K.; Cai, D.; Chen, Y.; Wu, Y.; Hu, X.; Zhang, Z. Thermodynamic validation of 1-alkyl-3-methylimidazolium carboxylates as task-specific ionic liquids for H2S absorption. AIChE J. 2013, 59, 2227–2235. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.; Zhao, J.; Ma, P.; Zhang, X.; Niu, J. Hydrothermal syntheses and crystal structures of two organic–inorganic hybrid molybdovanadates based on [V2Mo6(OH)2O24]4- and [VMo12O40]3- polyoxoanions. J. Coord. Chem. 2009, 62, 3754–3762. [Google Scholar] [CrossRef]
- Lede, E.J.; Requejo, F.G.; Pawelec, B.; Fierro, J.L.G. XANES Mo L-edges and XPS study of Mo loaded in HY zeolite. J. Phys. Chem. B 2002, 106, 7824–7831. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron. Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Alov, N.; Kutsko, D.; Spirovová, I.; Bastl, Z. XPS study of vanadium surface oxidation by oxygen ion bombardment. Surf. Sci. 2006, 600, 1628–1631. [Google Scholar] [CrossRef]
- Tomaszewicz, E.; Kurzawa, M. Use of XPS method in determination of chemical environment and oxidation state of sulfur and silver atoms in Ag6S3O4 and Ag8S4O4 compounds. J. Mater. Sci. 2004, 39, 2183–2185. [Google Scholar] [CrossRef]
- Hampton, M.A.; Plackowski, C.; Nguyen, A.V. Physical and chemical analysis of elemental sulfur formation during galena surface oxidation. Langmuir 2011, 27, 4190–4201. [Google Scholar] [CrossRef] [PubMed]
- Kartio, I.; Wittstock, G.; Laajalehto, K.; Hirsch, D.; Simola, J.; Laiho, T.; Szargan, R.; Suoninen, E. Detection of elemental sulphur on galena oxidized in acidic solution. Int. J. Min. Process. 1997, 51, 293–301. [Google Scholar] [CrossRef]
- Sanchez, C.; Livage, J.; Launay, J.P.; Fournier, M.; Jeannin, Y. Electron delocalization in mixed-valence molybdenum polyanions. J. Am. Chem. Soc. 1982, 104, 3194–3202. [Google Scholar] [CrossRef]
- Liu, X.; Wang, R. H2S removal by peroxo heteropoly compound/ionic liquid solution. Fuel Processing Technol. 2017, 160, 78–85. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y. Synergistic effect of an ionic liquid catalyst with two kings of basic sites on knoevenagel condensation. Chin. J. Catal. 2008, 29, 772–776. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, R. Polyoxometalate/Ionic Liquid Desulfurization System for Hydrogen Sulfide Removal from High-Temperature Gas Stream. Molecules 2022, 27, 6723. https://doi.org/10.3390/molecules27196723
Li J, Wang R. Polyoxometalate/Ionic Liquid Desulfurization System for Hydrogen Sulfide Removal from High-Temperature Gas Stream. Molecules. 2022; 27(19):6723. https://doi.org/10.3390/molecules27196723
Chicago/Turabian StyleLi, Junpeng, and Rui Wang. 2022. "Polyoxometalate/Ionic Liquid Desulfurization System for Hydrogen Sulfide Removal from High-Temperature Gas Stream" Molecules 27, no. 19: 6723. https://doi.org/10.3390/molecules27196723
APA StyleLi, J., & Wang, R. (2022). Polyoxometalate/Ionic Liquid Desulfurization System for Hydrogen Sulfide Removal from High-Temperature Gas Stream. Molecules, 27(19), 6723. https://doi.org/10.3390/molecules27196723







