Steroid Resistance Associated with High MIF and P-gp Serum Levels in SLE Patients
Abstract
:1. Introduction
2. Results
2.1. Patients and Reference Group
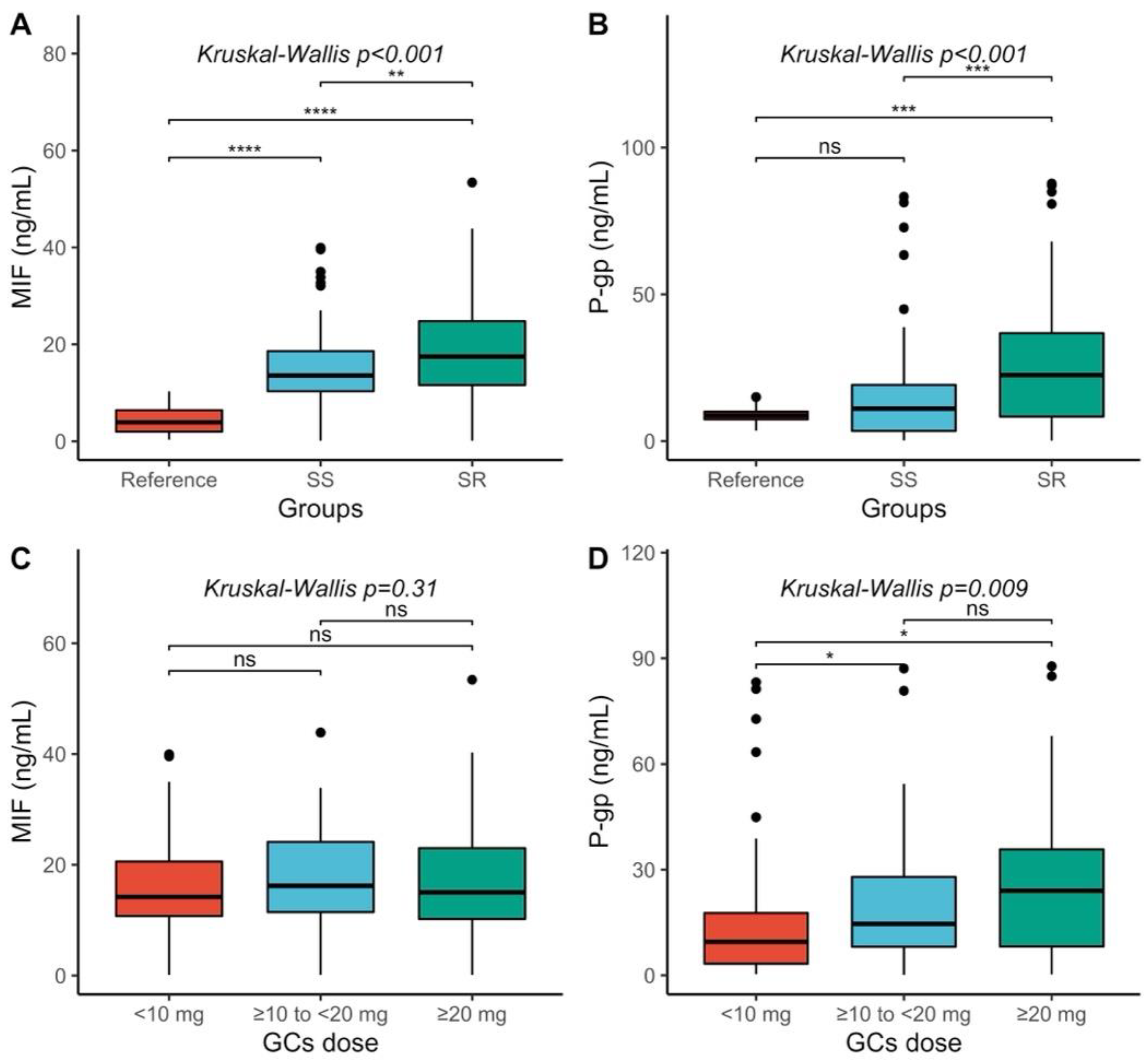
2.2. MIF and P-gp Serum Levels Comparison
2.3. Correlations between MIF, P-gp, and Clinical Variables in SLE Patients
2.4. Results of Receiver Operator Characteristic
2.5. Steroid-Resistant (SR) and Steroid-Sensitive (SS) Comparison
2.6. Unconditional Logistic Regression Models
2.7. CHAID Decision Tree
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Clinical Assessment
4.3. Determination of MIF and P-gp Serum Levels
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kaul, A.; Gordon, C.; Crow, M.K.; Touma, Z.; Urowitz, M.B.; van Vollenhoven, R.; Ruiz-Irastorza, G.; Hughes, G. Systemic Lupus Erythematosus. Nat. Rev. Dis. Prim. 2016, 2, 16039. [Google Scholar] [CrossRef] [PubMed]
- Fanouriakis, A.; Kostopoulou, M.; Alunno, A.; Aringer, M.; Bajema, I.; Boletis, J.N.; Cervera, R.; Doria, A.; Gordon, C.; Govoni, M.; et al. 2019 Update of the EULAR Recommendations for the Management of Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2019, 78, 736–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xibillé-Friedmann, D.; Pérez-Rodríguez, M.; Carrillo-Vázquez, S.; Álvarez-Hernández, E.; Aceves, F.J.; Ocampo-Torres, M.C.; García-García, C.; García-Figueroa, J.L.; Merayo-Chalico, J.; Barrera-Vargas, A.; et al. Guía de práctica clínica para el manejo del lupus eritematoso sistémico propuesta por el Colegio Mexicano de Reumatología. Reumatol. Clín. 2019, 15, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Kariburyo, F.; Xie, L.; Sah, J.; Li, N.; Lofland, J.H. Real-World Medication Use and Economic Outcomes in Incident Systemic Lupus Erythematosus Patients in the United States. J. Med. Econ. 2020, 23, 1–9. [Google Scholar] [CrossRef]
- Pons-Estel, B.A.; Bonfa, E.; Soriano, E.R.; Cardiel, M.H.; Izcovich, A.; Popoff, F.; Criniti, J.M.; Vásquez, G.; Massardo, L.; Duarte, M.; et al. First Latin American Clinical Practice Guidelines for the Treatment of Systemic Lupus Erythematosus: Latin American Group for the Study of Lupus (GLADEL, Grupo Latino Americano de Estudio Del Lupus)–Pan-American League of Associations of Rheumatology (PANLAR). Ann. Rheum. Dis. 2018, 77, 1549–1557. [Google Scholar] [CrossRef] [Green Version]
- Hardy, R.S.; Raza, K.; Cooper, M.S. Therapeutic Glucocorticoids: Mechanisms of Actions in Rheumatic Diseases. Nat. Rev. Rheumatol. 2020, 16, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef]
- Silverman, M.N.; Sternberg, E.M. Neuroendocrine-Immune Interactions in Rheumatoid Arthritis: Mechanisms of Glucocorticoid Resistance. Neuroimmunomodulation 2008, 15, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, C.; Faria, R. Therapeutic Resistance in Autoimmune Diseases. Lupus 2013, 22, 3–5. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.-H.; Chen, Z.-S. Overcoming ABC Transporter-Mediated Multidrug Resistance: Molecular Mechanisms and Novel Therapeutic Drug Strategies. Drug Resist. Updates 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.; Wei, W. The Effects of DMARDs on the Expression and Function of P-Gp, MRPs, BCRP in the Treatment of Autoimmune Diseases. Biomed. Pharmacother. 2018, 105, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, S.; Saito, K.; Nakayamada, S.; Nakano, K.; Tanaka, Y. Clinical Relevance of the Expression of P-Glycoprotein on Peripheral Blood Lymphocytes to Steroid Resistance in Patients with Systemic Lupus Erythematosus. Arthritis Rheum. 2005, 52, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, S.; Tanaka, Y. Treatment Strategy Based on Targeting P-Glycoprotein on Peripheral Lymphocytes in Patients with Systemic Autoimmune Disease. Clin. Exp. Nephrol. 2012, 16, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shi, Y.; Lei, T.-C. Detection of Active P-Glycoprotein in Systemic Lupus Erythematosus Patients with Poor Disease Control. Exp. Ther. Med. 2012, 4, 705–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kansal, A.; Tripathi, D.; Rai, M.K.; Agarwal, V. Persistent Expression and Function of P-Glycoprotein on Peripheral Blood Lymphocytes Identifies Corticosteroid Resistance in Patients with Systemic Lupus Erythematosus. Clin. Rheumatol. 2016, 35, 341–349. [Google Scholar] [CrossRef]
- Perez-Guerrero, E.E.; Gamez-Nava, J.I.; Muñoz-Valle, J.F.; Cardona-Muñoz, E.G.; Bonilla-Lara, D.; Fajardo-Robledo, N.S.; Nava-Zavala, A.H.; Garcia-Cobian, T.A.; Rincón-Sánchez, A.R.; Murillo-Vazquez, J.D.; et al. Serum Levels of P-Glycoprotein and Persistence of Disease Activity despite Treatment in Patients with Systemic Lupus Erythematosus. Clin. Exp. Med. 2018, 18, 109–117. [Google Scholar] [CrossRef]
- Perez-Guerrero, E.E.; Gonzalez-Lopez, L.; Muñoz-Valle, J.F.; Vasquez-Jimenez, J.C.; Ramirez-Villafaña, M.; Sanchez-Rodriguez, E.N.; Gutierrez-Ureña, S.R.; Cerpa-Cruz, S.; Aguilar-Chavez, E.A.; Cardona-Muñoz, E.G.; et al. Serum P-Glycoprotein Level: A Potential Biomarker of DMARD Failure in Patients with Rheumatoid Arthritis. Inflammopharmacology 2018, 26, 1375–1381. [Google Scholar] [CrossRef]
- Lang, T.; Foote, A.; Lee, J.P.W.; Morand, E.F.; Harris, J. MIF: Implications in the Pathoetiology of Systemic Lupus Erythematosus. Front. Immunol. 2015, 6, 577. [Google Scholar] [CrossRef] [Green Version]
- Kang, I.; Bucala, R. The Immunobiology of MIF: Function, Genetics and Prospects for Precision Medicine. Nat. Rev. Rheumatol. 2019, 15, 427–437. [Google Scholar] [CrossRef]
- Wang, F.-F.; Zhu, L.-A.; Zou, Y.-Q.; Zheng, H.; Wilson, A.; Yang, C.-D.; Shen, N.; Wallace, D.J.; Weisman, M.H.; Chen, S.-L.; et al. New Insights into the Role and Mechanism of Macrophage Migration Inhibitory Factor in Steroid-Resistant Patients with Systemic Lupus Erythematosus. Arthritis Res. 2012, 14, R103. [Google Scholar] [CrossRef]
- Sam, N.B.; Guan, S.-Y.; Wang, P.; Li, X.-M.; Wang, D.-G.; Pan, H.-F.; Ye, D.-Q. Levels of the Macrophage Migration Inhibitory Factorand Polymorphisms in Systemic Lupus Erythematosus: A Meta-Analysis. Arch. Med. Sci. 2021, 17, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, Q.; Yu, X.; Liu, J.; Bai, S.; Feng, J.; Wu, B. Molecular Mechanisms of Glucocorticoid Resistance in Systemic Lupus Erythematosus: A Review. Life Sci. 2018, 209, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Adcock, I.M. Glucocorticoid Resistance in Inflammatory Diseases. Lancet 2009, 373, 13. [Google Scholar] [CrossRef]
- Leech, M.; Metz, C.; Hall, P.; Hutchinson, P.; Gianis, K.; Smith, M.; Weedon, H.; Holdsworth, S.R.; Bucala, R.; Morand, E.F. Macrophage Migration Inhibitory Factor in Rheumatoid Arthritis: Evidence of Proinflammatory Function and Regulation by Glucocorticoids. Arthritis Rheum. 1999, 42, 1601–1608. [Google Scholar] [CrossRef]
- Zhu, W.-Y.; Jin, X.; Ma, Y.-C.; Liu, Z.-B. Correlations of MIF Polymorphism and Serum Levels of MIF with Glucocorticoid Sensitivity of Sudden Sensorineural Hearing Loss. J. Int. Med. Res. 2020, 48, 030006051989387. [Google Scholar] [CrossRef] [Green Version]
- Bilsborrow, J.B.; Doherty, E.; Tilstam, P.V.; Bucala, R. Macrophage Migration Inhibitory Factor (MIF) as a Therapeutic Target for Rheumatoid Arthritis and Systemic Lupus Erythematosus. Expert Opin. Ther. Targets 2019, 23, 733–744. [Google Scholar] [CrossRef]
- Brennan-Bourdon, L.M.; De la Cruz-Mosso, U.; Reyes-Castillo, Z.; Martínez-Bonilla, G.E.; Ramírez-Dueñas, M.G.; Islas-Carbajal, M.C.; Rincón-Sánchez, A.R.; Salazar-Páramo, M.; Muñoz-Valle, J.F. MIF and TNF α Serum Levels in Rheumatoid Arthritis Patients Treated with Disease-Modifying Antirheumatic Drugs: A Cross-Sectional Study. Immunopharmacol. Immunotoxicol. 2015, 37, 207–213. [Google Scholar] [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment: Miniperspective. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef]
- Picchianti-Diamanti, A.; Rosado, M.; Scarsella, M.; Laganà, B.; D’Amelio, R. P-Glycoprotein and Drug Resistance in Systemic Autoimmune Diseases. IJMS 2014, 15, 4965–4976. [Google Scholar] [CrossRef] [Green Version]
- Tsujimura, S.; Saito, K.; Nawata, M.; Nakayamada, S.; Tanaka, Y. Overcoming Drug Resistance Induced by P-Glycoprotein on Lymphocytes in Patients with Refractory Rheumatoid Arthritis. Ann. Rheum. Dis. 2007, 67, 380–388. [Google Scholar] [CrossRef]
- Silva, R.; Vilas-Boas, V.; Carmo, H.; Dinis-Oliveira, R.J.; Carvalho, F.; de Lourdes Bastos, M.; Remião, F. Modulation of P-Glycoprotein Efflux Pump: Induction and Activation as a Therapeutic Strategy. Pharmacol. Ther. 2015, 149, 1–123. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.M.; Lin, T.H.; Kawinski, E. Detection of Soluble P-Glycoprotein in Culture Media and Extracellular Fluids. Biochem. Biophys. Res. Commun. 1994, 203, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-C.; Xie, F.; Yu, X.-H.; Deng, Z.-Y.; Wang, Y.; Liang, P.; Sun, L.; Zhang, F.-X. Expression Levels of P-Glycoprotein in Peripheral Blood CD8+ T Lymphocytes from HIV-1-Infected Patients on Antiretroviral Therapy. Int. J. Mol. Med. 2014, 33, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Chiampanichayakul, S.; Anuchapreeda, S.; Chruewkamlow, N.; Mahasongkram, K.; Thanaratanakorn, P.; Kasinrerk, W. Production of Monoclonal Antibodies to P-Glycoprotein: Its Application in Detection of Soluble and Surface P-Glycoprotein of Leukemia Patients. Int. J. Hematol. 2010, 92, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Nocera, A.L.; Meurer, A.T.; Singleton, A.; Simons, C.; BuSaba, J.; Gass, N.T.; Han, X.; Bleier, B.S. Intact Soluble P-Glycoprotein Is Secreted by Sinonasal Epithelial Cells. Am. J. Rhinol. Allergy 2016, 30, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Naka, R.; Sasaki, Y.; Nishimoto, S.; Yumoto, R. Effect of Cigarette Smoke Extract on P-Glycoprotein Function in Primary Cultured and Newly Developed Alveolar Epithelial Cells. Drug Metab. Pharmacokinet. 2016, 31, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.E.; Chung, K.F.; Clarke, C.J.; Durham, A.L.; Mallia, P.; Footitt, J.; Johnston, S.L.; Barnes, P.J.; Hall, S.R.; Simpson, K.D.; et al. The MIF Antagonist ISO-1 Attenuates Corticosteroid-Insensitive Inflammation and Airways Hyperresponsiveness in an Ozone-Induced Model of COPD. PLoS ONE 2016, 11, e0146102. [Google Scholar] [CrossRef] [Green Version]
- List, A.F.; Kopecky, K.J.; Willman, C.L.; Head, D.R.; Slovak, M.L.; Douer, D.; Dakhil, S.R.; Appelbaum, F.R. Cyclosporine Inhibition of P-Glycoprotein in Chronic Myeloid Leukemia Blast Phase. Blood 2002, 100, 1910–1912. [Google Scholar] [CrossRef]
- Tsujimura, S.; Tanaka, Y. Potential of B-Cell-Targeting Therapy in Overcoming Multidrug Resistance and Tissue Invasiveness Associated with P-Glycoprotein Expressing-B Cell Compartments. Immunol. Med. 2021, 44, 142–151. [Google Scholar] [CrossRef]
- De la Cruz-Mosso, U.; García-Iglesias, T.; Bucala, R.; Estrada-García, I.; González-López, L.; Cerpa-Cruz, S.; Parra-Rojas, I.; Gámez-Nava, J.I.; Pérez-Guerrero, E.E.; Muñoz-Valle, J.F. MIF Promotes a Differential Th1/Th2/Th17 Inflammatory Response in Human Primary Cell Cultures: Predominance of Th17 Cytokine Profile in PBMC from Healthy Subjects and Increase of IL-6 and TNF-α in PBMC from Active SLE Patients. Cell. Immunol. 2018, 324, 42–49. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, F.; Chen, Q.; Kang, A.; Lu, M.; Liu, W.; Zang, X.; Wang, G.; Zhang, J. Chronic Inflammation Up-Regulates P-Gp in Peripheral Mononuclear Blood Cells via the STAT3/Nf-Κb Pathway in 2,4,6-Trinitrobenzene Sulfonic Acid-Induced Colitis Mice. Sci. Rep. 2015, 5, 13558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; Mcshane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 Revised Criteria for the Classification of Systemic Lupus Erythematosus: REVISED CRITERIA FOR SLE. Arthritis Rheum. 1982, 25, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Bombardier, C.; Gladman, D.D.; Urowitz, M.B.; Caron, D.; Chang, C.H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992, 35, 630–640. [Google Scholar] [CrossRef] [PubMed]

| SLE Patients (n = 188) | Reference Group (n = 35) | p-Value | |
|---|---|---|---|
| Age (years) | 40 (18–68) | 49 (22–67) | <0.001 |
| Women, n (%) | 181 (96.28) | 35 (100) | 0.52 |
| Alcohol use, n (%) | 31 (16.49) | 5 (14.28) | 0.94 |
| Tobacco use, n (%) | 30 (15.96) | 5 (14.28) | 0.99 |
| Comorbidities, n (%) | 66 (35.11) | 11 (31.42) | 0.17 |
| Disease evolution (years) | 4 (1–28) | - | - |
| SLEDAI, active, n (%) | 90 (47.87) | - | - |
| Glucocorticoids, n (%) | 188 (100) | - | - |
| Prednisone, n (%) | 166 (88.30) | - | - |
| Deflazacort, n (%) | 22 (11.70) | - | - |
| Immunosuppressive agents, n (%) | 165 (88.77) | - | - |
| Azathioprine, n, (%) | 75 (39.89) | - | - |
| Mycophenolate, n (%) | 56 (29.79) | - | - |
| Cyclophosphamide, n (%) | 10 (5.32) | - | - |
| Chloroquine, n (%) | 79 (42.02) | - | - |
| Methotrexate, n (%) | 15 (7.98) | - | - |
| MIF | P-gp | |||
|---|---|---|---|---|
| Rho | p-Value | Rho | p-Value | |
| MIF, ng/mL | -- | -- | 0.411 | <0.001 |
| P-gp, ng/mL | 0.411 | <0.001 | -- | -- |
| Age, years | −0.036 | 0.627 | 0.013 | 0.855 |
| Disease evolution, year | 0.021 | 0.774 | 0.132 | 0.073 |
| GCS doses | 0.042 | 0.564 | 0.242 | 0.001 |
| SLEDAI score | 0.161 | 0.028 | 0.245 | 0.001 |
| SLICC score | −0.116 | 0.113 | 0.001 | 0.987 |
| C3 (mg/dL) | −0.038 | 0.661 | 0.124 | 0.145 |
| C4 (mg/dL) | −0.062 | 0.468 | 0.027 | 0.753 |
| ESR (mg/dL) | 0.111 | 0.192 | 0.125 | 0.142 |
| CRP (mm/hr) | −0.039 | 0.644 | 0.047 | 0.581 |
| Protein in urine | 0.118 | 0.166 | 0.099 | 0.245 |
| Steroid-Sensitive (n = 98) | Steroid-Resistant (n = 90) | p | |
|---|---|---|---|
| Age (years) | 42 (18–68) | 38 (18–68) | 0.66 |
| Women, n (%) | 93 (94.90) | 88 (97.78) | 0.51 |
| Alcohol use, n (%) | 14 (14.29) | 17 (18.89) | 0.51 |
| Tobacco use, n (%) | 10 (10.20) | 20 (22.22) | 0.04 |
| Comorbidities, n (%) | 26 (26.5) | 40 (44.4) | <0.001 |
| Disease evolution (years) a | 4 (1–20) | 4 (1–28) | 0.53 |
| SLEDAI, active, n (%) | 0 (0.00) | 90 (100) | - |
| C3 (mg/dL) a | 106 (54–152) | 104 (40–199) | 0.39 |
| C4 (mg/dL) a | 26.91 (8–72.69) | 23.75 (8–275) | 0.44 |
| CRP (mg/dL) a | 7.67 (3.19–263.52) | 8.9 (0.42–70.09) | 0.71 |
| ESR (mm/h) a | 23.59 (2–46) | 29.38 (5–52.30) | 0.004 |
| Proteins in urine (mg/dL/24 h) a | 0.30 (0.04–10.77) | 0.35 (0.01–10.77) | 0.01 |
| ANA, positive, n (%) | 36 (36.70) | 47 (52.20) | 0.04 |
| Anti-dsDNA, n (%) | 18 (18.35) | 24 (26.60) | 0.23 |
| Glucocorticoids, n (%) | 98 (100) | 90 (100) | 1.00 |
| Prednisone, n (%) | 88 (89.90) | 78 (86.67) | 0.66 |
| Deflazacort, n (%) | 10 (10.20) | 12 (13.33) | 0.66 |
| Glucocorticoid doses (mg/24 h) a | 7.2 (2.5–25) | 17.5 (10–75) | <0.001 |
| Immunosuppressive agents, n (%) | 83 (84.69) | 82 (91.11) | 0.26 |
| Azathioprine, n, (%) | 47 (47.96) | 28 (31.11) | 0.02 |
| Mycophenolate, n (%) | 22 (22.45) | 34 (37.78) | 0.03 |
| Cyclophosphamide, n (%) | 2 (2.04) | 8 (8.89) | 0.05 |
| Chloroquine, n (%) | 42 (42.86) | 37 (41.11) | 0.92 |
| Methotrexate, n (%) | 8 (8.16) | 7 (7.78) | 1.00 |
| High MIF serum levels, n (%) | 35 (18.6) | 52 (27.7) | 0.003 |
| High P-gp serum levels, n (%) | 33 (17.5) | 54 (28.7) | <0.001 |
| Model A | Model B | |||
|---|---|---|---|---|
| Predictors | OR | 95% CI | OR | 95% CI |
| MIF serum levels | 1.11 | 1.03–1.20 | 2.29 | 1.20–7.62 |
| P-gp serum levels | 1.11 | 1.04–1.19 | 5.17 | 2.08–13.5 |
| Tobacco use | 3.27 | 11.2–5.92. | 3.44 | 1.40–9.10 |
| Comorbidities | 3.07 | 1.37–7.27 | 2.91 | 1.48–5.92 |
| ESR | - | - | - | - |
| Age | - | - | - | - |
| Disease evolution | - | - | - | - |
| Immunosuppressive agents | - | - | - | - |
| CRP | - | - | - | - |
| Anti-dsDNA (positive) | - | - | - | - |
| ANA (positive) | - | - | - | - |
| SLICC ACR score | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán-Ramírez, A.; Muñoz-Valle, J.F.; Gamez-Nava, J.I.; Saldaña-Cruz, A.M.; Gonzalez-Lopez, L.; Padilla-Ortega, A.; Arias-García, F.I.; Sánchez-Zuno, G.A.; Nava-Valdivia, C.A.; Ponce-Guarneros, J.M.; et al. Steroid Resistance Associated with High MIF and P-gp Serum Levels in SLE Patients. Molecules 2022, 27, 6741. https://doi.org/10.3390/molecules27196741
Beltrán-Ramírez A, Muñoz-Valle JF, Gamez-Nava JI, Saldaña-Cruz AM, Gonzalez-Lopez L, Padilla-Ortega A, Arias-García FI, Sánchez-Zuno GA, Nava-Valdivia CA, Ponce-Guarneros JM, et al. Steroid Resistance Associated with High MIF and P-gp Serum Levels in SLE Patients. Molecules. 2022; 27(19):6741. https://doi.org/10.3390/molecules27196741
Chicago/Turabian StyleBeltrán-Ramírez, Alberto, José Francisco Muñoz-Valle, Jorge I. Gamez-Nava, Ana Miriam Saldaña-Cruz, Laura Gonzalez-Lopez, Alejandro Padilla-Ortega, Francisco I. Arias-García, Gabriela Athziri Sánchez-Zuno, Cesar Arturo Nava-Valdivia, Juan Manuel Ponce-Guarneros, and et al. 2022. "Steroid Resistance Associated with High MIF and P-gp Serum Levels in SLE Patients" Molecules 27, no. 19: 6741. https://doi.org/10.3390/molecules27196741









